INTRODUCTION:
Gastric variceal (GV) bleeding is a feared complication of cirrhosis. Traditional endoscopic treatment with cyanoacrylate (CYA) injection can be challenging. Alternatively, endoscopic ultrasound (EUS)-guided delivery of hemostatic coils has shown high therapeutic success without the complications profile of CYA alone. Our aim was to compare the clinical outcomes of EUS-guided coil embolization with endoscopic CYA injection for the treatment of GV.
METHODS:
We performed a matched cohort study using a prospective registry involving 2 tertiary centers. A total of 10 patients undergoing EUS-based coil therapy were matched in 1:3 fashion to 30 patients who underwent CYA injection. The matching criteria included type of GV, Charlson comorbidity index, and bleeding severity. Primary outcomes were technical success and complications. Secondary outcomes were rebleeding rates, reinterventions rates, total transfusion requirements, and time-to-event analysis (rebleeding, reintervention, and transfusion).
RESULTS:
Technical success was 100% for EUS coil therapy vs 96.7% for CYA injection (P = 1.0). Complication rates were 10% in the EUS coil group vs 20% in the CYA group; P = 0.65. At 9 months, no EUS coil patient had rebled compared with 38% of the CYA group. No EUS coil patient required blood transfusion for GV rebleed, whereas over 50% of CYA patients did. Ten percent of EUS coil patients required reintervention compared with 60% of CYA patients. The EUS coil group had superior time to reintervention, GV rebleed, and transfusions (all P < 0.05).
DISCUSSION:
Compared with CYA, EUS-guided coil injection appears superior for the treatment of GV and should be considered initial endoscopic treatment of choice in centers with interventional EUS expertise.
INTRODUCTION
Gastroesophageal varices are present in at least half of the patients diagnosed with cirrhosis but account for up to 80% of acute gastrointestinal bleeding (GIB) in this population (1,2). Gastric variceal (GV) bleeding occurs less frequently compared with esophageal variceal bleeding but is associated with more severe bleeding and higher mortality rates (3). Despite recent advances in the treatment of GV bleeding, management has been associated with few well-established treatment guidelines (4).
Historically, endoscopic therapy for GV has been premised on the injection of acrylate polymers, such as histoacryl and cyanoacrylate (CYA) (5–9). Despite reassuring results in bleeding cessation, CYA injection has been associated with various adverse events, such as systemic embolization (e.g., pulmonary embolism), failed withdrawal of needle from variceal nest after injection, and deep ulceration resulting in rebleeding (10–13). More recently, endoscopic ultrasound (EUS)-guided injection of hemostatic coils has been used in combination with CYA, and our group has reported using coils in combination with absorbable gelatin sponge (AGS) as an adjunctive therapy to mitigate against these possible complications (14–19).
However, there are currently limited data available by directly comparing endoscopic coil-based therapy to CYA injection for the treatment of gastric varices. The aim of this study was to compare clinical outcomes of EUS-guided coil-based therapy with traditional endoscopic CYA injection for the treatment of GV.
METHODS
We performed a matched cohort study using data from a prospective patient registry. Registry data between January 2009 and December 2018 were collected and analyzed with local Institutional Review Board approval.
Inclusion/exclusion criteria
Inclusion/exclusion criteria were the following: age, 18 years and older; if woman, not pregnant; endoscopically confirmed gastric varices (Sarin classification (20)) that were actively bleeding or had recently bled (detailed classification described below); and treatment involving EUS-guided coil injection therapy or endoscopic CYA injection.
Matching scheme
At our institution, EUS-guided coil injection therapy for the treatment of GV was not offered until November 2017. Therefore, all patients undergoing EUS-based coil therapy (10 consecutive patients, between November 2017 and December 2018) were selected and matched in 1:3 fashion to 30 patients who underwent CYA injection for GV bleeding (2009–2017). Of note, these 10 consecutive patients were also reported in another publication focusing on procedural technique (19). Matching criteria included Charlson comorbidity index, type of gastric varix, and bleeding severity on admission. Bleeding severity was categorized into 4 groups as follows. Group 1: Active upper GIB requiring transfusions, vasopressors, and/or ICU support; group 2: active upper GIB with <2 g Hgb compared with the baseline or endoscopic findings of blood, clot, or stigmata of recent bleed without the need for higher level of care or transfusion; group 3: recent upper GIB defined as GV bleed in the past 30 days before presentation; and group 4: incidental gastric varices with high stigmata of bleeding but no active or recent bleeding.
In addition, data collection included patient demographics, etiology of liver disease, size/location of varices, and clinical outcomes.
Procedural steps for EUS-guided coil-based therapy
As previously described (17–19), all procedures were performed under general anesthesia and by 2 endoscopists trained in interventional gastroenterology. All patients underwent upper endoscopy (GIF-HQ190; Olympus America Inc, Center Valley, PA) before EUS and were administered ciprofloxacin 400 mg intravenous during the procedure. The linear echoendoscope (GF-UCT180; Olympus America Inc) was positioned in the distal esophagus or the gastric cardia to evaluate the gastric fundus, intramural varices, and feeder vessels. Water was instilled into the gastric fundus with the patient shifted to the left lateral decubitus to optimize acoustic coupling and sonographic assessment of the GV vessels. The area of the feeder vessel was preferentially selected for initial puncture with a standard fine needle aspiration (FNA) needle (19 G or 22 G Expect needle; Boston Scientific, Natick, MA). Given the serpiginous path of the GV vessels, oftentimes, the needle punctured the vessel wall multiple times en route to its target. Coils (Nester Embolization coils, Cook Medical, Bloomington, IN) were deployed using the stylet as a “pusher” under both EUS and fluoroscopic guidance. Initially, the coil diameter was selected according to the short axis diameter of the varix (at least 30% larger); later, the coil diameters of 14–20 mm were used indiscriminately. Initial coil length was usually 21 cm. The coil was deployed into the vessel lumen with 3 criteria in mind: (i) reduction/cessation of the Doppler flow, (ii) dense “packing” achieved on fluoroscopy, and (iii) resistance to stylet advancement. The needle was then withdrawn into an adjacent vessel compartment to continue with coil deployment. Additional coils (21, 14, or 7 cm) were injected as necessary. Once the Doppler flow was sufficiently reduced, a test contrast was injected to confirm the absence of runoff and exclude a shunt, at which point, 1–3 cc of AGS (Gelfoam; Pfizer, New York, NY or Surgiflo; Johnson & Johnson Wound Management, Somerville, NJ) was injected as a liquid slurry for adjunctive treatment (mixed with a 1:1 solution of saline and contrast). (17,18); CYA was not injected in combination with coils because of reported adverse events with CYA injection known in the previous literature (10–13).
Patients underwent surveillance EUS at 1 month, 6 months, and 12 months by the same endoscopist performing the index procedure with a plan for repeat coil injection if large residual GV were present.
Procedural steps for endoscopic CYA injection
All procedures were performed under general anesthesia and by 3 endoscopists trained in interventional gastroenterology. All patients underwent evaluation with upper endoscopy (GIF-HQ190; Olympus America Inc). After identification of target GV for treatment, the glue was injected using one to 2 mL of 2-octyl CYA or histoacryl with or without lipiodol (1:1 mixture). Injection through the varix was performed by using EUS guidance and a 22 G FNA needle (EchoSense, Cook Endoscopy, Bloomington, IN) or a combination of EUS and direct endoscopic injection. For GV treated with standard upper endoscopy, injection was continued until the target GV nest was felt to have solidified and hardened for which the needle injector was then retracted. For GV treated with EUS guidance, injection was continued until the Doppler flow diminished. Contrast was not mixed with CYA or histoacryl, and fluoroscopic guidance was not used. Additional injections were performed at the discretion of the endoscopist and as deemed necessary.
Patients underwent repeat surveillance endoscopy without EUS by the same endoscopist performing the index procedure at the 1-month follow-up, with plans for repeat injection if large residual GV were present.
Outcome measures
The primary outcomes were technical success and adverse events. Technical success was defined as uncomplicated injection of CYA or coils with concomitant reduction or cessation in the Doppler flow within the gastric varix when EUS was available. Adverse events were defined as procedural-related complications or postprocedural-related events including pulmonary embolism, abdominal pain, and bleeding. Secondary outcomes were rebleeding rates, reinterventions rates, total transfusion requirements, and time-to-event analysis (rebleeding, reintervention, and transfusion).
Statistical analysis
Statistical analysis was performed using Student t test and Fisher exact test for categorical variables and Wilcoxon signed-rank test for continuous variables. A time-to-event analysis with Kaplan-Meier curves and a log-rank test were used to compare the following between study groups: (i) time to GV rebleeding, (ii) time to composite GV and esophageal varices (EV) rebleeding, (iii) time to postprocedure transfusion for GV bleeding, (iv) time to postprocedure transfusion for combined GV and EV bleeding, and (v) time to GV reintervention. A Cox proportional hazards regression analysis was used to adjust for potential confounders (age, sex, Child-Pugh [CP] score, size of varices, and the presence of EV) for the outcomes of time to postprocedure transfusion for combined GV and EV bleeding and time to GV reintervention. Cox proportional hazards regression analysis was not performed on the outcomes of time to GV rebleeding, time to composite GV and EV rebleeding, and time to postprocedure transfusion for GV bleeding because of the absence of outcome events in the EUS coiling arm. P values of 0.05 or lower were considered significant. Statistical analyses were performed using Statistical Analysis Software 9.4 (SAS Institute, SAS, Arlington, VA).
RESULTS
Patient characteristics
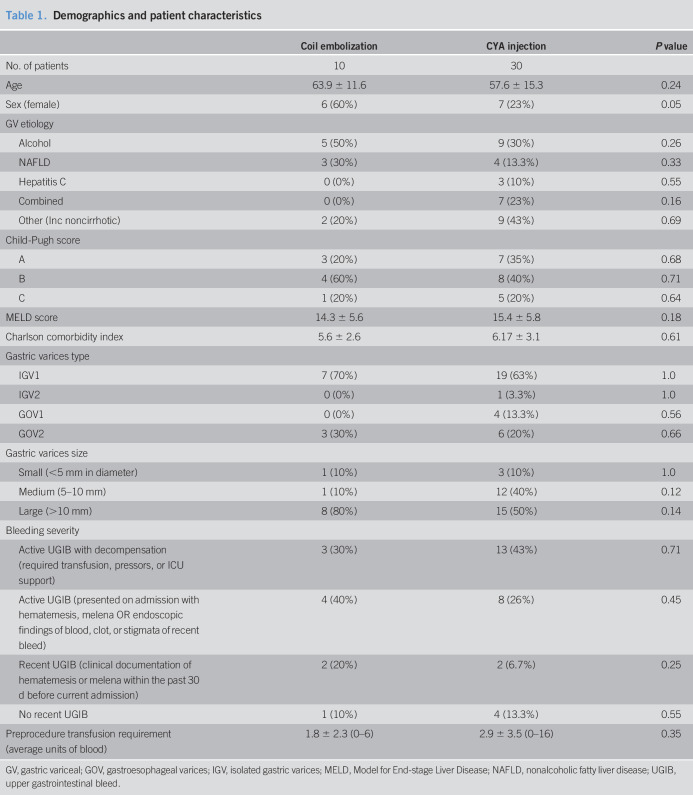
A total of 40 patients were included in this study, including 10 consecutive patients treated with coil therapy and 30 matched patients treated with CYA. There was no significant difference in baseline characteristics between the 2 treatment groups (Table 1) including age, gender, etiology of GV, MELD score, CP score, and the size of gastric varices on endoscopic evaluation.
Table 1.
Demographics and patient characteristics
Clinical outcomes
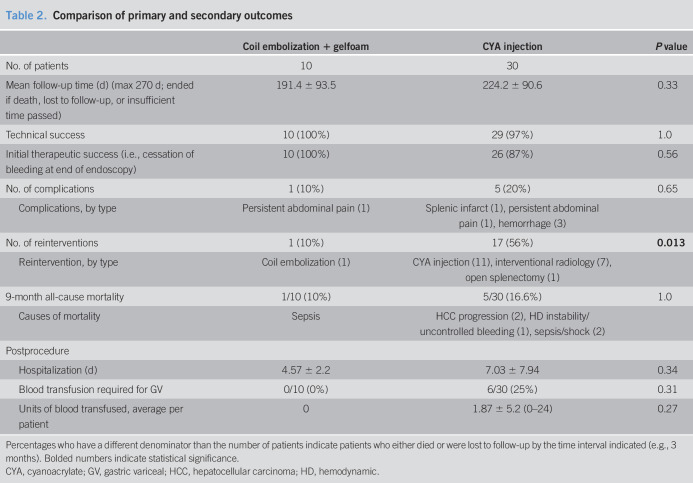
Procedural results are summarized in Table 2. Technical success was 100% (10/10) for EUS coil therapy vs 96.7% (29/30) for CYA injection (P = 1.0). Of the EUS coil patients, 100% (10/10) demonstrated Doppler-confirmed obliteration of GV. In 90% (9/10) of cases, 0.035” coils were used through a 19 G needle. A mean of 8.0 ± 2.9 coils were injected with a mean total length of 119 ± 48 cm.
Table 2.
Comparison of primary and secondary outcomes
For the glue injection cohort (n = 30), 26 (87%) were performed with CYA, whereas 4 of 30 (13%) were performed with histoacryl. Lipiodol (mixed in 1:1 fashion) was used in 4 of 30 (13%) cases. Thirteen of 30 (43%) were EUS-guided and 15 of 30 (50%) were EGD-guided, whereas 2 (7%) were guided partially with both EUS and EGD. For all injection modalities, a mean of 1.7 ± 2.9 cc of CYA was injected into the GV.
The mean length of follow-up was 191.4 ± 93.5 days for the EUS coil group vs 224.2 ± 90.6 days for the CYA group (P = 0.33). All patients in the EUS coil group had a follow-up with endoscopic ultrasonography. In the CYA group, only 3 patients were lost to follow-up (10%) with the remaining 27 having an endoscopic or clinical follow-up. Adverse event rates were less in the EUS coil group (1 case of abdominal pain with unrevealing workup and treated supportively) vs CYA group (bleeding ×2, abdominal pain ×1, and pulmonary embolism ×1) (10% vs 20%, P = 0.656). Postprocedure transfusion requirements were zero in the EUS coil group vs 1.87 ± 5.2 units of packed red blood cells in the CYA group (P = 0.27). Reinterventions at 9 months were 10% (1/10) in the EUS coil group vs 56% (17/30) in the CYA group (P = 0.013).
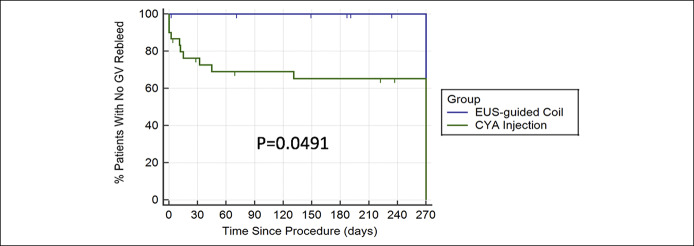
Time to gastric variceal rebleed
Kaplan-Meier analysis for time to GV rebleed was significant between the 2 groups (P = 0.0491). At 9 months, no EUS coil patient had rebled from GV compared with 38% of the CYA group (Figure 1).
Figure 1.
Kaplan-Meier analysis for time to gastric variceal rebleed. CYA, cyanoacrylate; EUS, endoscopic ultrasound; GV, gastric variceal.
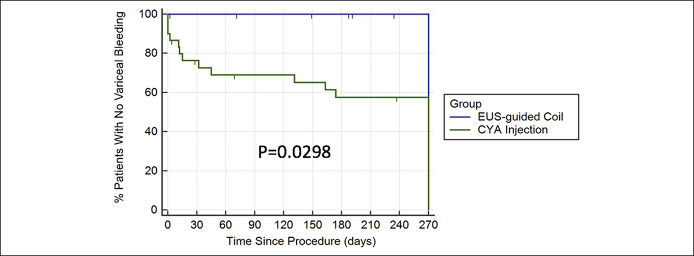
Time to gastric and esophageal variceal rebleed
Kaplan-Meier analysis for time to composite variceal rebleed was also significant between the 2 groups (P = 0.0298). At 9 months, no EUS coil patient had rebled from esophageal or gastric varices compared with 42% of the CYA group (Figure 2).
Figure 2.
Kaplan-Meier analysis for time to variceal rebleed (both gastric and esophageal). CYA, cyanoacrylate; EUS, endoscopic ultrasound.
Time to transfusion for GV rebleed
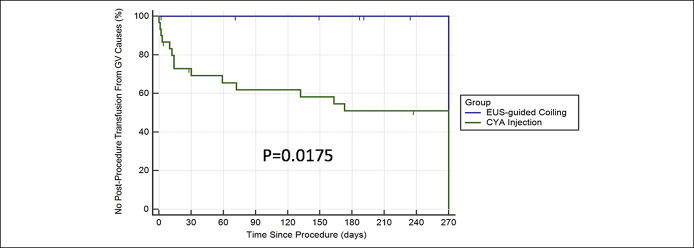
Kaplan-Meier analysis for time to transfusion for GV rebleed was also significant (P = 0.0175). Again, at 9 months, no EUS coil patient required blood transfusion for GV rebleed, whereas over 50% of CYA patients did (Figure 3).
Figure 3.
Kaplan-Meier analysis for time to transfusion (because of GV bleeding/treatment only). CYA, cyanoacrylate; EUS, endoscopic ultrasound; GV, gastric variceal.
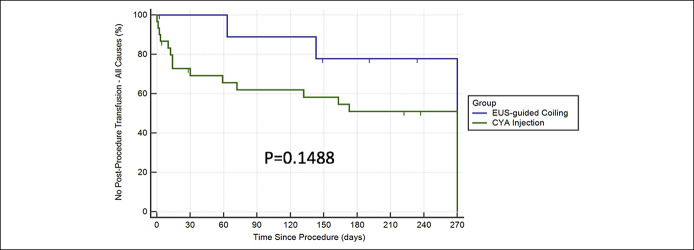
Time to transfusion (all-cause)
At 9 months, 21% of EUS coil patients required blood transfusions for any cause compared with over 50% of CYA patients (P = 0.1488). Two patients in the EUS coil arm presented back with evidence of bleeding requiring transfusion, and repeat EGD revealed a Mallory-Weiss tear in one patient and erosive esophagitis in another patient (Figure 4).
Figure 4.
Kaplan-Meier analysis for time to transfusion (all-cause bleeding). CYA, cyanoacrylate; EUS, endoscopic ultrasound.
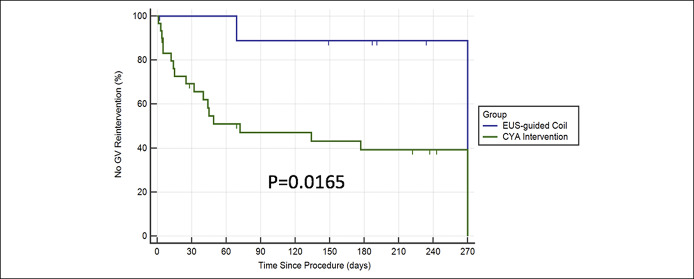
Time to Reintervention for GV
At 9 months, 10% (1/10) of EUS coil patients required reintervention (for residual large GV; asymptomatic) compared with 60% of CYA patients (P = 0.0165) (Figure 5).
Figure 5.
Kaplan-Meier analysis for time to reintervention of gastric varices. CYA, cyanoacrylate; EUS, endoscopic ultrasound; GV, gastric variceal.
Cox regression analysis
Injection of CYA for the treatment of GV was associated with shorter time to reintervention compared with EUS-guided coiling of GV after controlling for age, sex, CP score, GV size, and presence of EV (hazard ratio of 19.8, P = 0.01). There was no difference in time to transfusion for combined GV and EV bleeding after controlling for age, sex, CP score, GV size, and the presence of EV.
DISCUSSION
As one of the largest series to date comparing the EUS-guided coil embolization with traditional CYA for the treatment of GV, this matched cohort study shows that coil embolization leads to significantly lower GV rebleeding rates, lower transfusion requirements, and significantly lower 9-month reintervention rates.
As with the previous literature about EUS-guided coiling for GV (14,15,21), this study similarly reports a high technical success rate (100%) and low complication rate (10%). To our knowledge, there is one other study comparing EUS coiling with CYA injection (22), which reports similar efficacy between the 2 techniques but a significant discrepancy between the adverse event rates (57.9% for CYA vs 9.1% in the coil group; P < 0.01). This was likely because of per-protocol CT scans in all patients postprocedure, which detected asymptomatic pulmonary embolisms. By contrast, our adverse event rates were comparable (20% for CYA vs 10% for coil group; P = 0.65).
This study is unique in that it adds to the available literature by reporting important clinical outcomes between the 2 treatment groups using a time-to-event analysis. At 9 months postprocedure, no patients treated with EUS coil therapy experienced a GV rebleed or needed transfusions for GV rebleed and 10% required reintervention on their GV. Two patients from the EUS coil group re-presented with evidence of bleeding; however, endoscopy confirmed the cause as a Mallory-Weiss tear in one patient and erosive esophagitis in another patient, with collapsed and thrombosed appearing GV on endoscopy and Doppler interrogation. By contrast, approximately 40% of the CYA patients experienced a GV rebleed, almost 50% required transfusions for GV rebleeding, and more than half required reintervention for GV rebleeding.
Our recent published work on the same cohort of EUS coil patients has highlighted the detailed technique of EUS coiling and AGS injection (19). However, our technique of EUS-guided coil injection did feature some nuanced differences compared with previously published methodology. First, we used significantly more coil material—a mean of 8 coils per case (usually 0.035″ thickness), often times greater than a total of 1 m in length—compared with 1–2 coils for previous studies. Although we initially targeted the region of the feeder or perforator vessel, as suggested by others (14), our goal was to completely obliterate the Doppler flow throughout the entire GV nest, and consequently, we were more aggressive about coil insertion. However, in cases where a perforator vessel was not easily identified, the coils were used in the variceal nest with the goal of packing the coils into the network of connected varices. We did not think that there was a significant difference in technical difficulty between either scenarios. Second, the FNA needle usually punctured the serpiginous vessels at multiple points, and we often used long coil lengths (14 and 21 cm). Therefore, the deployed coils tended to occupy multiple vascular compartments anchored at points where it traversed the vessel wall. Third, we used AGS slurry as a hemostatic adjunct. We have had recent experience with AGS as adjunctive therapy and believe its potential superiority to CYA as adjunctive therapy. Although this is an off-label use, AGS is commonly used for hemostasis of GV in interventional radiology-guided therapies. AGS, a collagen-based material already widely used in multiple specialties, does not cause ulcerations and does not damage the endoscope (23,24).
There are some limitations of the technique and of the study at large. Limitations of the procedure include the need for fluoroscopy and the use of intravenous contrast, which requires consideration in patients with contrast allergies or renal insufficiency. However, we feel that varicealography is critically important for anatomic delineation, as described by Robeles-Miranda et al. (14) and previous adjunctive injection (e.g., AGS or CYA) to mitigate against embolic complications. Limitations of the study include but are not limited to the objective small number of cases involved (despite being one of the largest comparative studies to date), the heterogeneity of the endoscopists performing the CYA injection vs experienced endosonographer performing coil and AGS injection (limiting generalizability), and the retrospective nature of the study. It is important to note that the rate of rebleeding in the CYA arm was 38% and is higher than that mentioned in the recent literature of 20%–30% (25–28), We hypothesize that this is most likely related to the following factors: (i) nonalgorithmic approach to CYA injection by more than one provider (EUS vs non-EUS use, volume of CYA, technique of injection, etc.), (ii) less rigorous postprocedure protocol of serial evaluation with endoscopy and EUS for varicealography, and (iii) extended follow-up time of up to 9 months. We want to also highlight that 1 patient in the EUS coil group and 4 patients in the CYA group had no evidence of active or recent bleeding. Finally, we want to acknowledge that the cohort of EUS coil + AGS patients in this study have been included in a recent step-by-step procedural technique publication highlighting the use of AGS as an alternative agent for adjunctive hemostasis (19).
There are several strengths of this study to highlight. First, a standard methodology for EUS coil therapy was used and consecutive coil cases were selected. Second, coil patients were rigorously matched to CYA cases in 3:1 fashion such that there were no significant differences in important clinical baseline characteristics (Charlson comorbidity index, type of GV, and acuity of GIB presentation (90% of coil patients and 76% of CYA patients had active or recent hemorrhage). Third, we analyzed rebleeding rates from GV alone and GV + EV; this is because balloon-occluded retrograde transvenous obliteration, an interventional radiology-guided technique using coils and AGS in a similar fashion to our technique, suggests risk of worsening EV when GV is treated, with rates as high as 58% (29–31). Finally, given that bleeding GV is less common than bleeding EV, a time-to-event analysis with Cox regression analysis was used to follow clinical outcomes and offset low patient numbers. Nevertheless, it is important to highlight the experimental nature of this study with a small number of patients involved; however, we hope this study can provide the impetus for future randomized controlled trials comparing these treatment arms.
In summary, coil-based therapy for the treatment of gastric varices appears superior to traditional endoscopic therapy with CYA injection. Specifically, compared with endoscopic CYA injection, EUS-guided coil therapy exhibited high technical success rates, low adverse event rates, superior time to rebleed (both GV rebleeding and GV + EV composite rebleeding), time to repeat transfusion, and time to repeat intervention.
CONFLICTS OF INTEREST
Guarantor of the article: Marvin Ryou, MD.
Specific author contributions: A.N.B., T.J.W., and P.J. contributed to formulating, drafting and editing drafts. T.J.W., P.J. assisted with statistical analysis. C.C.T., M.R. provided direct supervision, and contributed to write up, editing, proof reading and final approval of the paper. All authors approved the final version of the manuscript.
Financial support: None to report.
Potential competing interests: A.N.B.: None to report. T.J.W.: None to report. P.J.: consultant for endogastric solutions and GI dynamics. C.C.T.: consultant for Apollo Endosurgery, Boston Scientific, Medtronic, Fractyl, GI Dynamics, Olympus America and USGI Medical. M.R.: consultant for Olympus America, Medtronic, Boston Scientific and Fujifilm medical systems.
Study Highlights.
WHAT IS KNOWN
✓ GV accounts for 10%–20% of varices but are associated with more severe bleeding and higher mortality.
✓ Historically, endoscopic management has premised on direct injection of glue or sclerosants; however, this has been associated with the risk of complications such as systemic embolization and recurrence of bleeding.
✓ EUS-guided therapy with the use of hemostatic coils has recently emerged as a novel therapy for the treatment of GV.
✓ EUS-guided coil injection is associated with reduced risk of recurrent bleeding and low risk of complications.
✓ Data comparing this EUS-guided coil injection with direct endoscopic injection is limited.
WHAT IS NEW HERE
✓ EUS-guided coil therapy for the treatment of GV is safe and associated with high technical success rate.
✓ EUS-guided coil therapy results in lower re-GV bleeding and need for reintervention
✓ Time to rebleeding and reintervention for GV is longer for patients treated with EUS-guided therapy compared with patients treated with standard endoscopic injection
TRANSLATIONAL IMPACT
✓ This study confirms the safety and efficacy of EUS-guided coil therapy for the treatment of gastric varices. This modality is associated less GV rebleeding and less need for reintervention. Centers with EUS expertise should consider adopting this modality for the treatment of bleeding GV.
Supplementary Material
REFERENCES
- 1.Sharara AI, Rockey DC. Gastroesophageal variceal hemorrhage. N Engl J Med 2001;345:669–81. [DOI] [PubMed] [Google Scholar]
- 2.Guadalupe GT, Bosch J. Management of varices and variceal hemorrhage in cirrhosis. N Engl J Med 2010;362:823–32. [DOI] [PubMed] [Google Scholar]
- 3.Wani ZA, Bhat RA, Bhadoria AS, et al. Gastric varices: Classification, endoscopic and ultrasonographic management. J Res Med Sci 2015;20(12):1200–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hwang JH, Shergill AK, Acosta RD, et al. The role of endoscopy in the management of variceal hemorrhage. Gastrointest Endosc 2014;80:221–7. [DOI] [PubMed] [Google Scholar]
- 5.Soehendra N, Nam VC, Grimm H, et al. Endoscopic obliteration of large esophagogastric varices with bucrylate. Endoscopy 1986;18(1):25–6. [DOI] [PubMed] [Google Scholar]
- 6.Ramond MJ, Valla D, Gotlib JP, et al. [Endoscopic obturationof esophagogastric varices with bucrylate. I. Clinical study of 49 patients]. Gastroenterol Clin Biol 1986;10(8–9):575–9. French. [PubMed] [Google Scholar]
- 7.Iwase H, Maeda O, Shimada M, et al. Endoscopic ablation with cyanoacrylate glue for isolated gastric variceal bleeding. Gastrointest Endosc 2001;53(6):585–92. [DOI] [PubMed] [Google Scholar]
- 8.Sarin SK, Jain AK, Jain M, et al. A randomized controlled trial of cyanoacrylate versus alcohol injection in patients with isolated fundic varices. Am J Gastroenterol 2002;97(4):1010–5. [DOI] [PubMed] [Google Scholar]
- 9.Cheng LF, Wang ZQ, Li CZ, et al. Treatment of gastric varices by endoscopic sclerotherapy using butyl cyanoacrylate: 10 years' experience of 635 cases. Chin Med J (Engl) 2007;120(23):2081–5. [PubMed] [Google Scholar]
- 10.Fry LC, Neumann H, Olano C, et al. Efficacy, complications and clinical outcomes of endoscopic sclerotherapy with N-butyl-2-cyanoacrylate for bleeding gastric varices. Dig Dis 2008;26(4):300–3. [DOI] [PubMed] [Google Scholar]
- 11.Dhiman RK, Chawla Y, Taneja S, et al. Endoscopic sclerotherapy of gastric variceal bleeding with N-butyl-2-cyanoacrylate. J Clin Gastroenterol 2002;35(3):222–7. [DOI] [PubMed] [Google Scholar]
- 12.Kok K, Bond RP, Duncan IC, et al. Distal embolization and local vessel wall ulceration after gastric variceal obliteration with N-butyl-2-cyanoacrylate: A case report and review of the literature. Endoscopy 2004;36(5):442–6. [DOI] [PubMed] [Google Scholar]
- 13.Rickman OB, Utz JP, Aughenbaugh GL, et al. Pulmonary embolization of 2-octyl cyanoacrylate after endoscopic injection therapy for gastric variceal bleeding. Mayo Clin Proc 2004;79(11):1455–8. [DOI] [PubMed] [Google Scholar]
- 14.Robles-Medranda C, Valero M, Nebel JA, et al. Endoscopic-ultrasound guided coil and cyanoacrylate embolization for gastric varices and the roles of endoscopic Doppler and endosonographic varicealography in vascular targeting. Dig Endosc 2019;31:283–90. [DOI] [PubMed] [Google Scholar]
- 15.Bhat YM, Weilert F, Fredrick RT, et al. EUS-guided treatment of gastric fundal varices with combined injection of coils and cyanoacrylateglue: A large U.S. experience over 6 years (with video). Gastrointest Endosc 2016;83(6):1164–72. [DOI] [PubMed] [Google Scholar]
- 16.Binmoeller KF, Weilert F, Shah JN, et al. EUS-guided transesophageal treatment of gastric fundal varices with combined coiling and cyanoacrylate flue injection (with videos). Gastrointest Endosc 2011;74(5):1019–25. [DOI] [PubMed] [Google Scholar]
- 17.Ge PS, Bazarbashi AN, Thompson CC, et al. Successful EUS-guided treatment of gastric varices with coil embolization and injection of absorbable gelatin sponge. VideoGIE 2018;4:154–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bazarbashi AN, Ryou M. Endoscopic ultrasound-guided coil injection therapy for gastric variceal bleeding not amenable to interventional radiology-guided therapies. ACG Case Rep J 2019;6(1):1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bazarbashi AN, Wang TJ, Thompson CC, et al. Endoscopic ultrasound-guided treatment of gastric varices with coil embolization and absorbable gelatin sponge: A novel alternative to cyanoacrylate. Endosc Int Open 2020;8:E221–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sarin SK, Lahoti D, Saxena SP, et al. Prevalence, classification and natural history of gastric varices: A long-term follow-up study in 568 portal hypertension patients. Hepatology 1992;16:1343–9. [DOI] [PubMed] [Google Scholar]
- 21.Romero-Castro R, Pellicer-Bautista F, Giovannini M, et al. Endoscopic ultrasound (EUS)-guided coil embolization therapy in gastric varices. Endoscopy 2010;42(Suppl 2):E35–6. [DOI] [PubMed] [Google Scholar]
- 22.Romero-Castro R, Ellrichmann M, Ortiz-Moyano C, et al. EUS-guided coil versus cyanoacrylate therapy for the treatment of gastric varices: A multicenter study (with videos). Gastrointest Endosc 2013;78(5):711–21. [DOI] [PubMed] [Google Scholar]
- 23.Council on Pharmacy and Chemistry. Absorbable gelatin sponge—New and nonofficial remedies. JAMA 1947;135:921. [Google Scholar]
- 24.Jenkins HP, Senz EH, Owen H, et al. Present status of gelatin sponge for control of hemorrhage. JAMA 1946;132:614–9. [DOI] [PubMed] [Google Scholar]
- 25.McCarty TR, Bazarbashi AN, Hathorn KE, et al. Endoscopic submucosal dissection (ESD) versus transanal endoscopic microsurgery (TEM) for treatment of rectal tumors: A comparative systematic review and meta-analysis. Surg Endosc 2020;34:1688–95. [DOI] [PubMed] [Google Scholar]
- 26.Bick B, Al-Haddad M, Liangpunsakul L, et al. EUS-guided fine needle injection is superior to direct endoscopic injection of 2-octyl cyanoacrylate for the treatment of gastric variceal bleeding. Surg Endosc 2019;33(6):1837–45. [DOI] [PubMed] [Google Scholar]
- 27.Mosli MH, Aljudaibi B, Almadi M, et al. The safety and efficacy of gastric fundal variceal obliteration using N-butyl-2-cyanoacrylate; the experience of a single canadian tertiary care centre. Saudi J Gastroenterol 2013;19(4):152–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Noophun P, Kongkam P, Gonlachanvit S, et al. Bleeding gastric varices: Results of endoscopic injection with cyanoacrylate at King Chulalongkorn Memorial Hospital. World J Gastroenterol 2005;11(47):7531–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fukuda T, Hirota S, Sugimura K. Long-term results of balloon-occluded retrograde transvenous obliteration for the treatment of gastric varices and hepatic encephalopathy. J Vasc Interv Radiol 2001;12:327–36. [DOI] [PubMed] [Google Scholar]
- 30.Chikamori F, Kuniyoshi N, Shibuya S, et al. Eight years of experience with transjugular retrograde obliteration for gastric varices with gastrorenal shunts. Surgery 2001;129:414–20. [DOI] [PubMed] [Google Scholar]
- 31.Ninoi T, Nishida N, Kaminou T, et al. Balloon-occluded retrograde transvenous obliteration of gastric varices with gastrorenal shunt: Long-term follow-up in 78 patients. AJR 2005;184:1340–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.