INTRODUCTION:
Helicobacter pylori infection is a major cause of gastrointestinal diseases. However, the pathogenesis of gastric mucosal injury by H. pylori remains unclear. Exogenous glutamate supplementation protects against gastric mucosal injury caused by H. pylori. Previously, we showed that aspirin-induced gastric injury is associated with reduction in glutamate release by inhibition of cystine–glutamate transporter (xCT) activity. We hypothesized that the xCT pathway is involved in H. pylori-induced gastric mucosal injury. In this study, we tested the activity of xCT and evaluated the regulatory effect of outer inflammatory protein (Oip) A on xCT in H. pylori-induced gastric mucosal injury.
METHODS:
In the H. pylori-infected mice and cell lines, the activity of xCT and the regulatory effect of microRNA on xCT were tested, and the effect of OipA from H. pylori on xCT activity was observed.
RESULTS:
The results of in vivo and in vitro experiments showed that H. pylori infection induced gastric mucosal injury. This was accompanied by a reduction in xCT activity, which was attenuated by exogenous glutamate treatment. Furthermore, the expression of miR-30b was upregulated, and miR-30b inhibitors significantly restored xCT activity and gastric mucosal injury caused by H. pylori infection. The OipA, a virulence protein from H. pylori, significantly upregulated the expression levels of miR-30b and inhibited xCT activity.
DISCUSSION:
OipA plays a significant role in H. pylori-induced gastric mucosal injury, and the effects are mediated by micro30b/xCT pathway.
INTRODUCTION
The development of peptic ulcers involves a variety of factors, such as dietary, chemical (smoking, alcohol, and drugs), biological (Helicobacter pylori), mental, and environmental factors (1,2). H. pylori are considered as one of the most common pathogens causing chronic gastritis and gastric cancer (3,4). However, the mechanism of H. pylori-induced gastric mucosal injury has not yet been full elucidated.
H. pylori-induced gastric mucosal injury is related to changes in prostaglandins (5,6) and nitric oxide (7,8). Glutamate, as an extracellular signal mediator in peripheral tissues by autocrine and/or paracrine (9,10), might play a protective role in gastric mucosal injury from acute aspirin irritation and cold irritation (11,12). Exogenous glutamate supplementation might have beneficial effects on gastric function. For example, dietary glutamate supplementation improves gastric 13C incorporation rates and nutrition management in postweaning pigs (13). Supplementing partial enteral nutrition with monosodium glutamate slows gastric emptying in preterm pigs (14). Moreover, an oral glutamate precursor (glutathione) and a glutamate supplementation drastically reduce Helicobacter-induced gastric pathologies (15,16). It is likely that alteration in endogenous glutamate transportation is also involved in gastric mucosal injury induced by multiple factors, such as aspirin irritation and cold stress.
Therefore, we explored endogenous glutamate transportation in Helicobacter-induced gastric pathologies. Extracellular levels of glutamate are regulated by the cystine–glutamate transporter (xCT), and its activity is affected by some inflammatory factors (17). Recently, we reported that aspirin-induced acute gastric mucosa is associated with a reduction in xCT activity (11). Thus, we hypothesized that the xCT/glutamate pathway is also involved in H. pylori-induced gastric mucosal injury.
MicroRNAs (miRNAs) are the regulators and biomarkers for human diseases on multiple levels (systemic, tissue, and cellular) (18–20). Some inflammatory factors affect miRNA profiles in stomach (21–25). Furthermore, differential expression of miRNA has been observed in H. pylori-induced gastritis in vivo, in vitro, and in clinical patients (20,26,27). Therefore, we speculated that H. pylori infection induces upregulation of certain miRNAs, which inhibits their target gene xCT, thereby reducing the glutamate transport activity. Three databases (TargetScan, Starbase, and miRanda) were used to predict the putative miRNAs targeting xCT. We screened for the expression of 4 gastro-enriched miRNAs in H. pylori-infected mouse models and cells, which showed upregulated miR-30b as a candidate miRNA marker. Therefore, the regulatory role of miR-30b on xCT was tested in a H. pylori-infected model.
H. pylori-induced gastric mucosal injury is associated with the secretion of multiple virulence proteins from H. pylori, such as, CagA oncoprotein, vacuolating cytotoxin A, and outer inflammatory protein (Oip) A (28,29). The H. pylori OipA (OipA, HopH, or OMP; 13–34 kDa), one of the most important virulence factors of H. pylori, was initially identified as a surface protein that promotes inflammatory cytokine secretion and heightens gastric inflammation in vivo (30–33). More recently, OipA is believed to affect intracellular signaling and modulate host signaling pathways (34,35). To our knowledge, miRNAs regulation by OipA has not been elucidated to date. Thus, we explored the regulatory effect of OipA on the miRNA/glutamate pathway in H. pylori-induced gastric mucosal injury.
METHODS
Animal and cell experiments
H. pylori Sydney strain (SS) 1 was purchased from the American Type Culture Collection and cultured on agar plates containing 10% sheep blood under microaerophilic conditions at 37°C. We used a rapid urease test, Gram staining, and polymerase chain reaction (PCR) amplification of specific urease genes for identification of certain virulence markers. Then, the purified bacteria were used to establish an experimentally infected model. The mice were perfused with H. pylori for 6 weeks. C57BL/6 male mice (8 weeks old, 19–22 g) were randomly divided into 4 groups (n = 12): (i) The control group was perfused with phosphate-buffered saline (PBS) daily. (ii) The H. pylori-infected group was perfused with H. pylori dissolved in PBS, 2 × 109 colony-forming units/mouse/d for 6 weeks, (iii) The l-glutamate group was perfused with H. pylori bacteria solution after 30 minutes of lavage with either of 2 different doses of glutamate (3 mg/kg/d or 6 mg/kg/d) and defined as l-glutamate (L) group and l-glutamate (H) group. The administration of l-glutamate began 2 weeks after the start of infection period. All animals were killed at 6-weeks postinfection. Age-matched uninfected mice were included as controls in all experiments.
Gastric tissues of all mice were separated by cutting along greater curvature of the stomach, washed, and photographed. H. pylori infection was confirmed using Giemsa staining and a polyclonal rabbit anti-H. pylori antibody. In addition, the expression of 23S rRNA was detected, which can be used to measure H. pylori colonization in the stomach. Samples of gastric tissue proteins were extracted for western blot assays. A portion of gastric tissue was fixed in 4% paraformaldehyde for morphological analysis as reported earlier (36).
Human gastric mucosa epithelial (GES-1) cells were serum starved before treatment for 24 hours in Dulbecco's Minimum Essential Medium containing 1% fetal bovine serum. Cells were prepared by seeding 2 × 106 cells on plates. We used rapid urease tests, Gram staining, and PCR amplification of specific urease genes for identification of certain virulence markers. To cause infection, bacteria were harvested in PBS (pH 7.4) and added to the host cells at a multiplicity of infection of 100 (37). The cell viability assay was measured by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide dye reduction assays and lactate dehydrogenase assays. To evaluate apoptosis, we used Hoechst staining and caspase-3 activity.
RNA expression analysis
Total RNA was extracted according to the manufacturer's instructions, and 500 ng RNA was reverse transcribed using a reverse transcription-PCR kit according to the manufacturer's protocol (TaKaRa, Kusatsu, Japan). The expression levels of messenger RNAs (mRNAs) were measured using an ABI 7300 real-time PCR system (Applied Biosystems, Foster City, CA) with the SYBR Green PCR Master Mix (TOYOBO, Osaka, Japan). GAPDH was used as an internal control in quantitative analysis. The gene expression levels were normalized to GAPDH. For miRNA detection, in situ hybridization detection of miRNAs was performed using the miRCURY LNA miRNA ISH Kit (Qidgen, Germany).
Protein detection
For western blot analysis, primary antibodies against xCT (1:1,000; Abcam, England), GAPDH (1:2,000, Abcam), and Helicobacter (1:2,000, Abcam) and a secondary HRP-anti-rabbit (1:5,000; Sangon, Shang hai) antibody were used. The relative optical density of each band was analyzed, and the results were expressed in relation to GAPDH levels. For immunofluorescence detection, cells were washed with PBS and fixed with paraformaldehyde for 15 minutes at room temperature (∼25°C), before being incubated with 0.25% Triton X-100 in PBS at room temperature for 20 minutes. The cells were blocked with 1% bovine serum albumin at 37°C for 1 hour and rinsed 3 times with PBS before incubation with anti-xCT (1:200) antibody at 4°C overnight. This was followed by incubation with an anti-rabbit secondary antibody (1:200, FITC488) for 1 hour before immunofluorescence detection. Nuclei were stained with 4',6-diamidino-2-phenylindole 2HCI for 1 minute. Images were acquired using an immunofluorescence microscope (Olympus, Japan) equipped with a ×20 objective lens and were analyzed and processed with a Nikon camera with a SPOT image acquisition system. For immunohistochemistry staining, acetate buffer (pH 6.0) was used as the immersion solution for heat antigen pretreatment step. Rabbit polyclonal antibody and the Envision (DAKO, Germany) polymer detection system were used with diaminobenzidine acting as the chromogen.
Dual luciferase reporter assay
GES-1 cells were transfected in 12-well plates with Renilla luciferase-based SLC7A11 (xCT) 3′UTR reporter constructs using Lipofectamine 2000 reagent (Invitrogen, Beijing). After 24 hours, the cells were supertransfected with miRNA mimics at 50 nM final concentration using Lipofectamine 2000. The cells were harvested 24 hours after reporter transfection and analyzed using a Dual-Luciferase Reporter Assay System (Promega). Renilla luciferase signal of each sample was normalized for differences in transfection efficiency using the activity of the cotransfected pCI-firefly reporter as control.
Statistical analysis
TargetScan (http://www.targetscan.org/), Starbase (http://starbase.sysu.edu.cn/), miRanda (http://www.microrna.org/), and miRDB (http://www.mirdb.org/) databases were used to predict the putative miRNAs targeted to xCT (SLC7A11). All data are expressed as mean ± SE. Multiple mean comparisons were performed by ANOVA and Student-Newman-Keuls multiple comparison tests. Statistical analysis was performed using SPSS 17.0 software, and P < 0.05 was considered statistically significant.
RESULTS
Decreased xCT activity in gastric tissues of H. pylori-infected mice
H. pylori infection was confirmed using Giemsa staining and polyclonal rabbit anti-H. pylori antibody. H. pylori antigen positivity was intensely observed in the gastric mucosa of the H. pylori-infected group but was undetected in the control group. Giemsa staining showed similar results (see Supplementary Figure 1A, 1B, Supplementary Digital Content 1, http://links.lww.com/CTG/A283). The relative levels of 23S rRNA were increased up to 10-fold in H. pylori group (see Supplementary Figure 1C, Supplementary Digital Content 1, http://links.lww.com/CTG/A283).
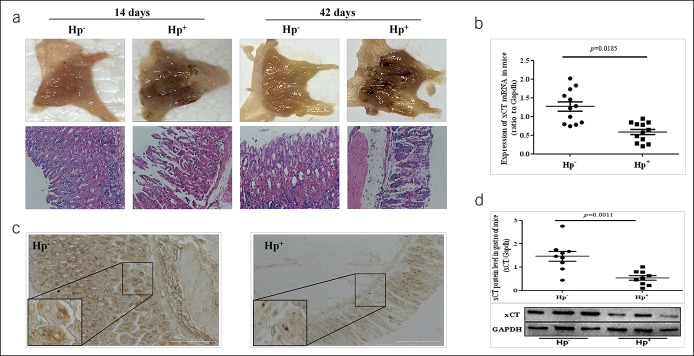
After confirming the success of the model, we detected gastric mucosal injury in mice. Photographs of gastric mucosal lesions showed sporadic hemorrhagic spots in gastric mucosa. The hematoxylin-eosin staining showed gastric mucosal damage with dilation and exfoliation of gastric epithelial cells and disruption of mucosa in H. pylori-infected mice (Figure 1a). Meanwhile, the expression of xCT mRNA was significantly downregulated (Figure 1b), and a similar result was obtained for protein expression (Figure 1c, d). These results suggest that disturbances of glutamate transporter expression might be associated with the development of gastric ulcer caused by H. pylori.
Figure 1.
Decreased cystine-glutamate transporter (xCT) activity in gastric ulcer of H. pylori-infected mice. (a) Photographs of gastric mucosal lesions showed sporadic hemorrhagic spots in gastric mucosa. Hematoxylin-eosin staining showed gastric mucosal damage with dilation and exfoliation of gastric epithelial cells and disruption of mucosal in H. pylori -infected mice with an infiltration of inflammatory cells in the mucosa and submucosa. (b) Decreased expression of xCT mRNA in Hp+ group was detected. (c) Immunohistochemical staining for xCT antigen in gastric tissue. (d) Western blot for xCT in gastric tissue. Data are means ± SEM, N = 8–12, *P < 0.05 vs Hp−, **P < 0.01 vs Hp−. GAPDH, glyceraldehyde phosphate dehydrogenase; Hp, Helicobacter pylori; mRNA, messenger RNA.
Effect of H. pylori on xCT activity in GES-1 cells
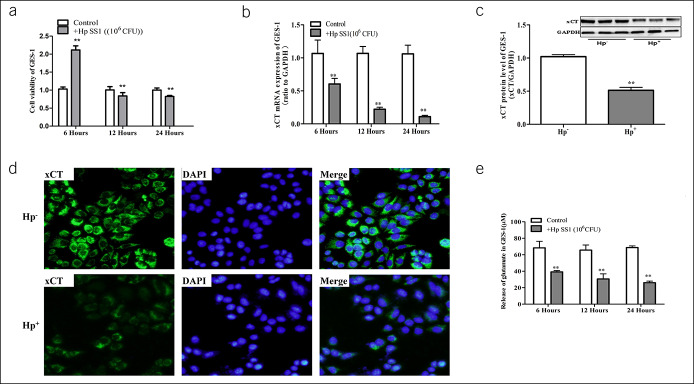
In vitro, we conducted experiments on the human gastric epithelial immortalized cell line (GES-1). When GES-1 cells were cocultured with bacteria for 24 hours, cavity lesions and prolonged degeneration could be seen in the cytoplasm of cells, and some cells gradually peeled from the surface and were suspended in the medium. Reduced cell viability and increased apoptosis were detected after exposure to H. pylori SS1 (Figure 2a, see Supplementary Figure 2, Supplementary Digital Content 1, http://links.lww.com/CTG/A283). These experimental results were consistent with previous results of H. pylori-infected cells (37).
Figure 2.
Effect of H. pylori on cystine-glutamate transporter (xCT) activity in GES-1 cells. (a) Cell viability of GES-1 were detected when coculturing with H. pylori SS1. (b) Decreased expression of xCT mRNA in H. pylori SS1 group. Western blot (c) and immunofluorescence (d) for xCT were detected in GES-1 cells. (e) The concentration of glutamate in cell culture supernatant of GES-1. Data are means ± SEM, N = 3, *P < 0.05 vs H. pylori control, **P < 0.01 vs control. CFU, colony-forming units; DPAI, 4',6-diamidino-2-phenylindole 2HCI; GES-1, gastric mucosa epithelial; GAPDH, glyceraldehyde phosphate dehydrogenase; Hp, Helicobacter pylori; mRNA, messenger RNA; SS1, Sydney strain 1.
In addition, we found that xCT mRNA levels changed over time when GES-1 cells were cocultured with H. pylori SS1 (Figure 2b). In agreement with the results of quantitative polymerase chain reaction, western blots and cell immunofluorescence results also showed a significant reduction in xCT protein levels (Figure 2c, d). Xc− system transports 1 molecule of cystine into the cell and simultaneously releases 1 molecule of glutamate from it. To further observe the activity of xCT, we tested glutamate concentration in the extracellular fluid (Figure 2e). These results suggested that the decreased release of endogenous glutamate might contribute to GES-1 apoptosis caused by H. pylori infection.
Exogenous glutamate supplement attenuates H. pylori-induced gastric mucosal injury
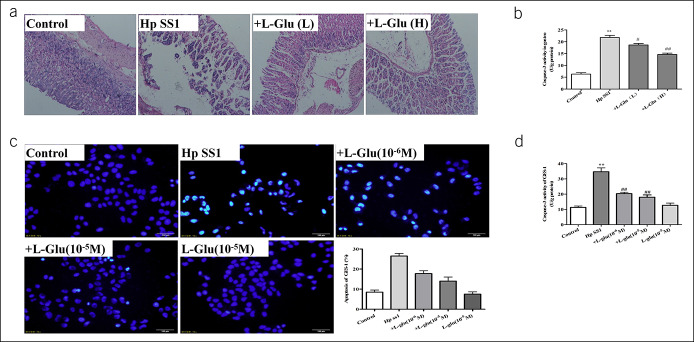
To evaluate the effects of l-glutamate on H. pylori-associated gastric mucosal ulceration, animals were pretreated with glutamate at doses of 3 mg/kg/d or 6 mg/kg/d. In morphological studies, gastric mucosal damage with dilation and exfoliation of gastric epithelial cells and disruption of mucosal layer were observed in the H. pylori group, whereas mice pretreated with glutamate had a smaller degree of loss of mucosal architecture and exfoliation than those observed in the H. pylori group (Figure 3a). In addition, lower caspase-3 activity in gastric tissues in the glutamate group was observed when compared with that in the H. pylori group (Figure 3b). Then, we verified these phenomena in cell-based experiments. We pretreated GES-1 cell line with l-glutamate. The results of caspase-3 activity and apoptosis staining suggested that glutamate treatment inhibited the process of apoptosis induced by H. pylori (Figure 3c, d). These results suggest that exogenous glutamate might be a potential supplementary therapeutic modality for the treatment of H. pylori-induced gastric ulcers.
Figure 3.
Exogenous glutamate supplement attenuates gastric mucosal injury induced by H. pylori. In vivo experiments: (a) Hematoxylin-eosin staining of gastric tissue showed the relieved dilation of gastric epithelial cells and less exfoliation of mucosal architecture in glutamate groups; (b) the effect of glutamate on apoptosis in gastric tissue was detected by Caspase-3 activity kit. N = 12, *P < 0.05 vs control, **P < 0.01 vs control, #P < 0.05 vs H. pylori SS1; ##P < 0.01 vs H. pylori SS1. In vitro experiments: (c) Hoechst staining of GES-1; (d) Caspase-3 activity detection. Data are means ± SEM, N = 4. **P < 0.01 vs Control; ##P < 0.01 vs H. pylori SS1. GES-1, gastric mucosa epithelial; GFP, greenfluorescent protein; Hp, Helicobacter pylori; NC, numerical control; SS1, Sydney strain 1.
Overexpression of the xCT (SLC7A11) gene could reduce GES-1 apoptosis induced by H. pylori
After cloning xCT gene templates, PCR product sizes were examined by running 5 μL products on 3% agarose gel. The targeted band appeared between 1.5 kb and 2 kb, as expected (1,552 bp). Positive clones of the competent Escherichia coli transformed by granulosis virus core vector with xCT gene were confirmed by PCR, and the sequence alignment showed 100% alignment with the targeted gene.
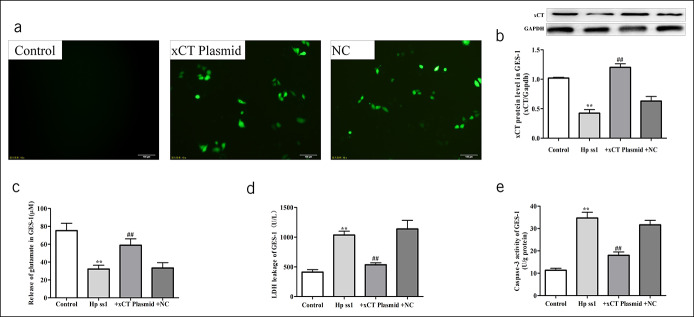
After plasmid transfection, enhanced green fluorescent protein (Figure 4a) expression in GES-1 cells was detected. We transfected GES-1 with the help of a recombinant plasmid and cocultured it with H. pylori. The results showed that xCT overexpression restored xCT protein levels (Figure 4b) and increased endogenous glutamate release to a certain degree (Figure 4c), accompanied by reductions in cellular damage (Figure 4d) and apoptosis (Figure 4e). These results further confirmed that both endogenous and exogenous glutamate are involved in H. pylori-infected gastric ulcers.
Figure 4.
Overexpression of xCT (SLC7A11) gene could reduce the GES-1 apoptosis induced by H. pylori. (a) The expression of GFP was obviously induced in plasmid transgenic cell lines. (b) Western blot for xCT in GES-1. (c) LDH was assessed for cell damage. (d) Caspase-3 activity. Data are means ± SEM, N = 4, *P < 0.05 vs control, **P < 0.01 vs control. ##P < 0.01 vs H. pylori SS1. GES-1, gastric mucosa epithelial; Hp, Helicobacter pylori; LDH, lactic dehydrogenase; SS1, Sydney strain 1; xCT, cystine-glutamate transporter.
MiRNAs expression were altered in GES-1 and mice in response to H. pylori infection, and the xCT was regulated by miRNA-30b
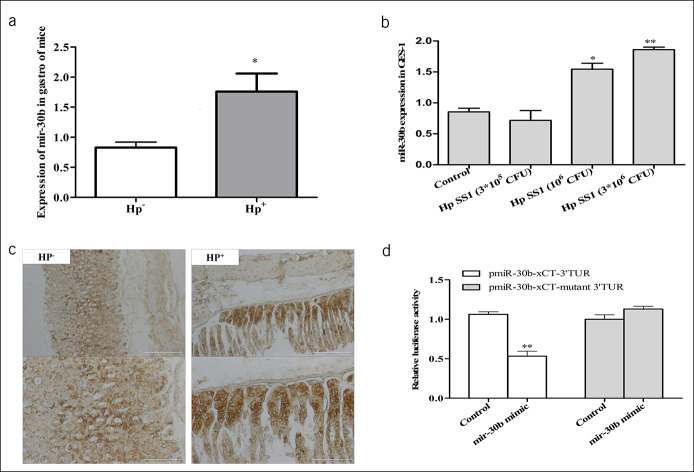
The above-mentioned results have demonstrated that reduced glutamate was closely associated with downregulation of xCT expression and abnormal glutamate transport was associated with gastric toxicity in H. pylori infection. However, it remained unknown how xCT expression was altered under H. pylori infection conditions. We screened for the expression of miRNAs enriched in stomach, which showed upregulation of miR-30b levels in both stomach tissue and cell line (Figure 5a, b). MiRNA-30b expression has been linked to xCT using bioinformatics analysis and has been shown to be associated with H. pylori infection leading to gastritis (38,39).
Figure 5.
MicroRNAs (miRNAs) expression were regulated in GES-1 and mice under H. pylori infection, and the cystine-glutamate transporter (xCT) is regulated by miRNA-30b. (a) miR-30b expression in gastric tissue of mice, n = 10. (b) miR-30b expression in GES-1 cells, n = 4. (c) Identifying miR-30b in gastric tissue of mice using in situ hybridization. (d) The targeted regulating role of miRNA-30b on xCT gene was detected by dual luciferase reporter system. Data are means ± SEM, N = 4. **P < 0.01 vs control. CFU, colony-forming units; GES-1, gastric mucosa epithelial; Hp, Helicobacter pylori; SS1, Sydney strain 1.
To corroborate the hypothesis, we examined the expression levels of miR-30b in H. pylori-infected mice using in situ hybridization and confirmed that the levels of miR-30b were significantly elevated (Figure 5c). To validate xCT as a target of miR-30b, we constructed a luciferase expression vector containing the 3′-UTR segments of xCT along with the putative miR-30b binding sites. After cotransfection of miR-30b mimic and the xCT 3′-UTR expression vector, we found that the miR-30b mimic resulted in significant suppression of xCT luciferase activity, whereas mutating the putative miR-30b binding sites completely eliminated this inhibitory effect (Figure 5d). These data strongly indicated that upregulated miR-30b was indeed a target for xCT under H. pylori infection.
The effect of miRNA-30b loss on cystine/glutamate transporter (xCT) activity and cell damage
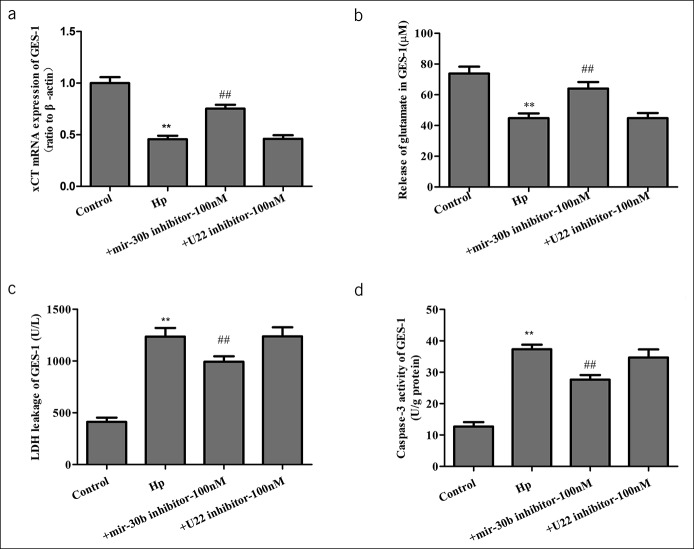
As mentioned earlier, aberrant expression of miR-30b contributes to gastric ulcer through targeting xCT, so we explored the effect of miR-30b inhibitors in gastric ulcer. GES-1 was transfected with miRNA-30b inhibitor or a negative control (U22 inhibitor) and were exposed to H. pylori for 24 hours. MiRNA-30b inhibitor upregulated xCT mRNA expression (Figure 6a), which was accompanied by recovery of glutamate release (Figure 6b) and decreased damage of GES-1 (Figure 6c, d). These results showed that miRNA-30b loss reduced cell damage and apoptosis induced by H. pylori infection.
Figure 6.
The effect of microRNA (miRNA)-30b loss on cystine-glutamate transporter (xCT) activity and cell damage. GES-1 was transfected with miRNA-30b or negative control (U22 inhibitor) and exposed to H. pylori for 24 hours. (a) xCT mRNA expression in GES-1. (b) The concentration of glutamate in cell culture supernatant of GES-1. (c) LDH was assessed for cell damage. (d) Caspase-3 activity. Data are means ± SEM, N = 4. **P < 0.01 vs control; ##P < 0.01 vs Hp. GES-1, gastric mucosa epithelial; Hp, Helicobacter pylori; LDH, lactic dehydrogenase; mRNA, messenger RNA.
OipA protein regulates the miR-30b/xCT pathway
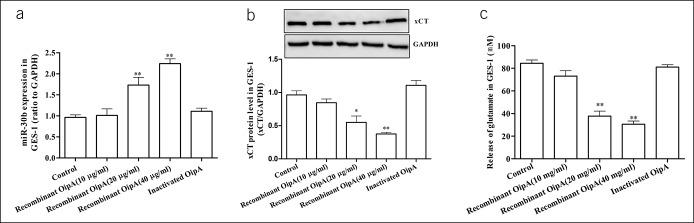
Our findings suggested that the miR-30b/xCT pathway was involved in H. pylori-induced gastric mucosal injury. However, how the host miRNAs were regulated by the bacteria toxic components remained unknown. We transfected the recombinant OipA protein encoding pET28a plasmid (see Supplementary Figure 3, Supplementary Digital Content 1, http://links.lww.com/CTG/A283). Our results showed that recombinant OipA protein increased miR-30b levels significantly (Figure 7a) and the expression of xCT and glutamate concentration was inversely related to miR-30b levels (Figure 7b, c). These results supported a regulatory effect of OipA on the miR-30b/xCT pathway.
Figure 7.
The regulatory of OipA on miR-30b/xCT pathway. The GES-1 cells were incubated with different concentrations of recombinant OipA protein for 24 hours. (a) The effect of recombinant OipA protein on microRNA-30b expression in GES-1. (b) The effect of recombinant OipA protein on xCT expression in GES-1. (c) The concentration of glutamate in cell culture supernatant of GES-1. Data are means ± SEM, N = 4, *P < 0.05 vs control, **P < 0.01 vs control. GAPDH, glyceraldehyde phosphate dehydrogenase; GES-1, gastric mucosa epithelial; OipA, outer inflammatory protein A.
DISCUSSION
Adequate levels of glutamate are crucial for cellular and tissue function. High concentrations of glutamate can exert toxicity effects in the brain (40); by contrast, excess glutamate can protect against gastric mucosal injury (11). Glutamate also protects against gastric mucosal injury induced by other factors, such as deoxynivalenol (41), aspirin (11), and cold stress (12). Our results confirmed earlier observations that, in cells and animal models, the exogenous glutamate pathway significantly reduces H. pylori-induced gastric mucosal injury (42). Taken together, exogenous glutamate has a protective effect on gastric mucosal injury induced by different factors. In stomach, glutamate plays protective roles as a substrate for various metabolic pathways, an energy source for intestinal mucosa, a mediator for cell signaling, and a regulator for oxidative reactions, and in immune responses.
Our earlier reports have shown that aspirin-induced gastric mucosal injury is related to reduction in endogenous glutamate levels and is accompanied by reduced activity of xCT. According to the inhibitory effect of some inflammatory factors on the xCT/glutamate pathway (43,44), we hypothesized that the xCT/glutamate pathway might be involved in H. pylori-induced gastric mucosal injury. The results of this study showed that H. pylori induced cell apoptosis with a decrease in glutamate release and xCT activity in cultured GES-1 cells, and these effects of H. pylori were attenuated by xCT (SLC7A11) overexpression. In mice, H. pylori infection induced gastric mucosal injury with downregulation of xCT expression. These results supported the hypothesis that H. pylori-induced gastric mucosal injury might be associated with a reduction in glutamate release by inhibition of xCT.
MiRNAs mediate multiple responses, including inflammatory response (45). H. pylori induce alterations in some miRNAs (46,47). Interestingly, some miRNAs regulate xCT expression (48). It is likely that the reduction in xCT activity by H. pylori might involve specific miRNA pathways. Our results based on in vivo and in vitro systems have shown that H. pylori-induced gastric mucosal injury led to an increase in miR-30b expression. MiR-30b is closely related to H. pylori-induced gastric ulcers (38). In this study, we found that miR-30b inhibitors attenuated the reduction in xCT activity caused by H. pylori, thus supporting the hypothesis that the regulatory effect of miR-30b on xCT activity plays an important role in H. pylori-induced gastric mucosal injury.
OipA is a virulence protein from H. pylori, which promotes inflammatory cytokines secretion and heightens gastric inflammation (49,50). OipA, in addition to inducing injury directly, can also weaken the protective effects of gastric tissue. Thus, the effect of OipA on the miRNA/glutamate pathway in H. pylori-induced mucosal injury was explored. The recombinant OipA protein increased miR-30b levels, accompanied by a reduction in xCT activity. Taken together, a reduction in xCT activity by H. pylori might involve the OipA/miRNA pathway.
In conclusion, decreased glutamate levels caused by reduction in xCT activity play an important role in H. pylori-induced gastric mucosal injury. Furthermore, OipA reduced the protective effects of the xCT/glutamate pathway on gastric mucosa by regulating miRNA-30b.
CONFLICTS OF INTEREST
Guarantor of the article: Yuan-Jian Li, PhD.
Specific author contributions: J.D., X.-H.L., and Y.-J.L. designed this experiment. J.D., F.L., and W.-Q.L. performed the experiment. J.D., X.-H.L., Z.-C.G., and Y.-J.L. analyzed the data and wrote this manuscript. All authors edited and approved the final manuscript.
Financial support: This work was supported by the National Natural Science Foundation of China (No. 81573486 to Y.-J.L., No. 81703592 to J.D., and No. 81770739 to Z.-C.G.). It was also supported by the Natural Science Foundation of Hunan province (No. 2019JJ50934) and by the Open Sharing Fund for the Large-scale Instruments and Equipment of Central South University.
Potential competing interests: None to report.
Study Highlights.
WHAT IS KNOWN
✓ Exogenous glutamate protected against gastric mucosal injury.
WHAT IS NEW HERE
✓ OipA plays a key role in H. pylori infection, and the effects are mediated by micro30b/xCT pathway.
TRANSLATIONAL IMPACT
✓ Our study provide a new potential target for the treatment of H. pylori infection.
Supplementary Material
Footnotes
SUPPLEMENTARY MATERIAL accompanies this paper at http://links.lww.com/CTG/A283
REFERENCES
- 1.Graham DY. History of Helicobacter pylori, duodenal ulcer, gastric ulcer and gastric cancer. World J Gastroenterol 2014;20(18):5191–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Araujo MB, Borini P, Guimaraes RC. Etiopathogenesis of peptic ulcer: Back to the past? Arq Gastroenterol 2014;51(2):155–61. [DOI] [PubMed] [Google Scholar]
- 3.Luzza F, Suraci E, Larussa T, et al. High exposure, spontaneous clearance, and low incidence of active Helicobacter pylori infection: The Sorbo San Basile study. Helicobacter 2014;19(4):296–305. [DOI] [PubMed] [Google Scholar]
- 4.Burkitt MD, Duckworth CA, Williams JM, et al. Helicobacter pylori-induced gastric pathology: Insights from in vivo and ex vivo models. Dis Model Mech 2017;10(10):89–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen Z, Wu J, Xu D, et al. Epidermal growth factor and prostaglandin E2 levels in Helicobacter pylori-positive gastric intraepithelial neoplasia. J Int Med Res 2016;44(2):241–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang T, Cai H, Zheng W, et al. A prospective study of urinary prostaglandin E2 metabolite, Helicobacter pylori antibodies, and gastric cancer risk. Clin Infect Dis 2017;64(10):1380–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ribeiro AR, Diniz PB, Pinheiro MS, et al. Gastroprotective effects of thymol on acute and chronic ulcers in rats: The role of prostaglandins, ATP-sensitive K(+) channels, and gastric mucus secretion. Chem Biol Interact 2016;244:121–8. [DOI] [PubMed] [Google Scholar]
- 8.Elfvin A, Bölin I, Lönroth H, et al. Gastric expression of inducible nitric oxide synthase and myeloperoxidase in relation to nitrotyrosine in Helicobacter pylori-infected Mongolian gerbils. Scand J Gastroenterol 2006;41(9):1013–8. [DOI] [PubMed] [Google Scholar]
- 9.Babic T, Travagli RA. Role of metabotropic glutamate receptors in the regulation of pancreatic functions. Biochem Pharmacol 2014;87(4):535–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jie D, Xiao-Hui L, Yuan-Jian L. Glutamate in peripheral organs: Biology and pharmacology. Eur J Pharmacol 2016;784:42–8. [DOI] [PubMed] [Google Scholar]
- 11.Du J, Li XH, Zhang W, et al. Involvement of glutamate-cystine/glutamate transporter system in aspirin-induced acute gastric mucosa injury. Biochem Biophys Res Commun 2015;450(1):135–41. [DOI] [PubMed] [Google Scholar]
- 12.Chen SH, Lei HL, Huang LR, et al. Protective effect of excitatory amino acids on cold-restraint stress-induced gastric ulcers in mice: Role of cyclic nucleotides. Dig Dis Sci 2001;46(10):2285–91. [DOI] [PubMed] [Google Scholar]
- 13.Assoni AD, Amorim AB, Saleh MAD, et al. Dietary glutamine, glutamic acid and nucleotide supplementation accelerate carbon turnover (δ13C) on stomach of weaned piglets. Anim Nutr 2017;3(3):225–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bauchart-Thevret C, Stoll B, Benight NM, et al. Supplementing monosodium glutamate to partial enteral nutrition slows gastric emptying in preterm pigs(1-3). J Nutr 2013;143(5):563–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Bruyne E, Ducatelle R, Foss D, et al. Oral glutathione supplementation drastically reduces Helicobacter-induced gastric pathologies. Sci Rep 2016;6:20169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amagase K, Nakamura E, Kato S, et al. Prophylactic effect of glutamate on gastrointestinal damage. Yakugaku Zasshi 2011;131(12):1711–9. [DOI] [PubMed] [Google Scholar]
- 17.Evonuk KS, Baker BJ, Doyle RE, et al. Inhibition of system Xc(-) transporter attenuates autoimmune inflammatory demyelination. J Immunol 2015;195(2):450–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mendell JT, Olson EN. MicroRNAs in stress signaling and human disease. Cell 2012;148(6):1172–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Y, Qiu C, Tu J, et al. HMDD v2.0: A database for experimentally supported human microRNA and disease associations. Nucleic Acids Res 2014;42:D1070–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cortés-Márquez AC, Mendoza-Elizalde S, Arenas-Huertero F, et al. Differential expression of miRNA-146a and miRNA-155 in gastritis induced by Helicobacter pylori infection in paediatric patients, adults, and an animal model. BMC Infect Dis 2018;18(1):463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luo XJ, Liu B, Dai Z, et al. Expression of apoptosis-associated microRNAs in ethanol-induced acute gastric mucosal injury via JNK pathway. Alcohol 2013;47(6):481–93. [DOI] [PubMed] [Google Scholar]
- 22.Liu CC, Cheng JT, Li TY, et al. Integrated analysis of microRNA and mRNA expression profiles in the rat spinal cord under inflammatory pain conditions. Eur J Neurosci 2017;46(11):2713–28. [DOI] [PubMed] [Google Scholar]
- 23.Zhai R, Wei Y, Su L, et al. Whole-miRNome profiling identifies prognostic serum miRNAs in esophageal adenocarcinoma: The influence of Helicobacter pylori infection status. Carcinogenesis 2015;36(1):87–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Santos JC, Brianti MT, Almeida VR, et al. Helicobacter pylori infection modulates the expression of miRNAs associated with DNA mismatch repair pathway. Mol Carcinog 2017;56(4):1372–9. [DOI] [PubMed] [Google Scholar]
- 25.Rossi AF, Cadamuro AC, Biselli-Périco JM, et al. Interaction between inflammatory mediators and miRNAs in Helicobacter pylori infection. Cell Microbiol 2016;18(10):1444–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang F, Xu Y, Liu C, et al. NF-κB/miR-223-3p/ARID1A axis is involved in Helicobacter pylori CagA-induced gastric carcinogenesis and progression. Cell Death Dis 2018;9(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hu G, Guo L, Ye G. Helicobacter pylori infection impairs gastric epithelial barrier function via microRNA-100-mediated mTOR signaling inhibition. Mol Med Rep 2018;18(1):587–94. [DOI] [PubMed] [Google Scholar]
- 28.Wang F, Meng W, Wang B, et al. Helicobacter pylori-induced gastric inflammation and gastric cancer. Cancer Lett 2014;345(2):196–202. [DOI] [PubMed] [Google Scholar]
- 29.Nejati S, Karkhah A, Darvish H, et al. Influence of Helicobacter pylori virulence factors CagA and VacA on pathogenesis of gastrointestinal disorders. Microb Pathog 2018;117:43–8. [DOI] [PubMed] [Google Scholar]
- 30.Garza-González E, Perez-Perez GI, Maldonado-Garza HJ, et al. A review of Helicobacter pylori diagnosis, treatment, and methods to detect eradication. World J Gastroenterol 2014;20(6):1438–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hsu WY, Lin CH, Lin CC, et al. The relationship between Helicobacter pylori and cancer risk. Eur J Intern Med 2014;25(3):235–40. [DOI] [PubMed] [Google Scholar]
- 32.Waskito LA, Salama NR, Yamaoka Y, et al. Pathogenesis of Helicobacter pylori infection. Helicobacter 2018;23(Suppl 1):e12516. [DOI] [PubMed] [Google Scholar]
- 33.Matsuo Y, Kido Y, Yamaoka Y. Helicobacter pylori outer membrane protein-related pathogenesis. Toxins (Basel) 2017;9(3):101.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kang MJ, Song EJ, Kim BY, et al. Helicobacter pylori induces vascular endothelial growth factor production in gastric epithelial cells through hypoxia-inducible factor-1α-dependent pathway. Helicobacter 2014;19(6):476–83. [DOI] [PubMed] [Google Scholar]
- 35.Teymournejad O, Mobarez AM, Hassan ZM, et al. Binding of the Helicobacter pylori OipA causes apoptosis of host cells via modulation of Bax/Bcl-2 levels. Sci Rep 2017;7(1):8036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang XL, Jiang AM, Ma ZY, et al. The synthetic antimicrobial peptide pexiganan and its nanoparticles (PNPs) exhibit the anti-Helicobacter pylori activity in vitro and in vivo. Molecules 2015;20(3):3972–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yu HJ, Liu W, Chang Z, et al. Probiotic BIFICO cocktail ameliorates Helicobacter pylori induced gastritis. World J Gastroenterol 2015;21(21):6561–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tang B, Li N, Gu J, et al. Compromised autophagy by MIR30B benefits the intracellular survival of Helicobacter pylori. Autophagy 2012;8(7):1045–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang XJ, Si RH, Liang YH, et al. Mir-30d increases intracellular survival of Helicobacter pylori through inhibition of autophagy pathway. World J Gastroenterol 2016;22(15):3978–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Egerton A, Broberg BV, Van Haren N, et al. Response to initial antipsychotic treatment in first episode psychosis is related to anterior cingulate glutamate levels: A multicentre 1H-MRS study (OPTiMiSE). Mol Psychiatry 2018;23(11):2145–55. [DOI] [PubMed] [Google Scholar]
- 41.Wu M, Xiao H, Ren W, et al. Therapeutic effects of glutamic acid in piglets challenged with deoxynivalenol. PLoS One 2014;9(7):e100591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sachs G, Marcus EA, Scott DR. The role of the NMDA receptor in Helicobacter pylori-induced gastric damage. Gastroenterology 2011;141(6):1967–9.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vartak-Sharma N, Gelman BB, Joshi C, et al. Astrocyte elevated gene-1 is a novel modulator of HIV-1-associated neuroinflammation via regulation of nuclear factor-kappaB signaling and excitatory amino acid transporter-2 repression. J Biol Chem 2014;289(28):19599–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mandolesi G, Musella A, Gentile A, et al. Interleukin-1β alters glutamate transmission at purkinje cell synapses in a mouse model of multiple sclerosis. J Neurosci 2013;33(29):12105–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu YZ, Wang YX, Jiang CL. Inflammation: The common pathway of stress-related diseases. Front Hum Neurosci 2017;11:316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ishimoto T, Izumi D, Watanabe M, et al. Chronic inflammation with Helicobacter pylori infection is implicated in CD44 overexpression through miR-328 suppression in the gastric mucosa. J Gastroenterol 2015;50(7):751–7. [DOI] [PubMed] [Google Scholar]
- 47.Cheng SF, Li L, Wang LM. miR-155 and miR-146b negatively regulates IL6 in Helicobacter pylori (cagA+) infected gastroduodenal ulcer. Eur Rev Med Pharmacol Sci 2015;19(4):607–13. [PubMed] [Google Scholar]
- 48.Drayton RM, Dudziec E, Peter S, et al. Reduced expression of miRNA-27a modulates cisplatin resistance in bladder cancer by targeting the cystine/glutamate exchanger SLC7A11. Clin Cancer Res 2014;20(7):1990–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Horridge DN, Begley AA, Kim J, et al. Outer inflammatory protein A (OipA) of Helicobacter pylori is regulated by host cell contact and mediates CagA translocation and interleukin-8 response only in the presence of a functional cag pathogenicity island type IV secretion system. Pathog Dis 2017;75(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Backert S, Tegtmeyer N. Type IV secretion and signal transduction of Helicobacter pylori CagA through interactions with host cell receptors. Toxins (Basel) 2017;9(4):E115. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.