OBJECTIVES:
Strong evidence links obesity to esophageal cancer (EC), gastric cancer (GC), colorectal cancer (CRC), and pancreatic cancer (PC). However, national-level studies testing the link between obesity and recent temporal trends in the incidence of these cancers are lacking.
METHODS:
We queried the Surveillance, Epidemiology, and End Results (SEER) to identify the incidence of EC, GC, CRC, and PC. Cancer surgeries stratified by obesity (body mass index ≥30 kg/m2) were obtained from the National Inpatient Sample (NIS). We quantified trends in cancer incidence and resections in 2002–2013, across age groups, using the average annual percent change (AAPC).
RESULTS:
The incidence of CRC and GC increased in the 20–49 year age group (AAPC +1.5% and +0.7%, respectively, P < 0.001) and across all ages for PC. Conversely, the incidence of CRC and GC decreased in patients 50 years or older and all adults for EC. According to the NIS, the number of patients with obesity undergoing CRC resections increased in all ages (highest AAPC was +15.3% in the 18–49 year age group with rectal cancer, P = 0.047). This trend was opposite to a general decrease in nonobese patients undergoing CRC resections. Furthermore, EC, GC, and PC resections only increased in adults 50 years or older with obesity.
DISCUSSION:
Despite a temporal rise in young-onset CRC, GC, and PC, we only identify a corresponding increase in young adults with obesity undergoing CRC resections. These data support a hypothesis that the early onset of obesity may be shifting the risk of CRC to a younger age.
INTRODUCTION
Obesity is a rapidly growing global health problem with a steep rise occurring in individuals younger than 50 years (1). This rise is not restricted to adults because obesity affects up to 18.5% of US children and adolescents (2). Furthermore, the current trends project a 33% increase in the prevalence of obesity in the next 2 decades (3,4). Because the rates of obesity have increased, a parallel rise in the risk of obesity-related malignancies has occurred (5). Certainly, the link between obesity and risk for various gastrointestinal malignancies is well established and include adenocarcinoma of the esophagus, stomach, pancreas, and colon/rectum (6).
Chief among gastrointestinal cancers is colorectal cancer (CRC) for which obesity is a strong risk factor (7–10). Although the overall incidence of CRC has decreased, recent reports indicate a rising CRC incidence in individuals younger than 50 years (11). Some emerging data suggest that this temporal increase in the young-onset CRC incidence may be attributable to obesity; however, validation of this finding is currently lacking (12). In this regard, nationally representative databases are a useful tool to examine incidence trends of various cancers according to age and relation to obesity. Consequently, in this study, we examine the age-specific temporal changes in the incidence of esophageal cancer (EC), gastric cancer (GC), CRC, and pancreatic cancer and their corresponding surgical resections in obese and nonobese patients between the years 2002–2013.
METHODS
Data source
Cancer incidence data were obtained from the Surveillance, Epidemiology, and End Results (SEER) database, a population-based program of the US National Cancer Institute, which includes cancer registries covering 28% of the US population (13). Yearly counts and granular characteristics of patients undergoing cancer surgical resections were derived from the National Inpatient Sample (NIS), Healthcare Cost and Utilization Project, and Agency for Healthcare Research and Quality. The NIS is the largest publicly available all-payer database of national hospital discharges and was designed to approximate a 20% stratified probability sample of all discharges from nonfederal acute care hospitals nationally, weighted to enable national estimates (14). Patient-level variables included age, gender, median household income quartile by zip code, and health insurance status. The data contained within the SEER and NIS databases are neither identifiable nor private and thus do not meet the federal definition of “subject,” and this study was exempt from the Institutional Review Board oversight.
The cohort and outcomes
Age-adjusted incidence rates per 100,000 for EC, GC, CRC, and PC between the years 2002 and 2013 were obtained from the SEER Explorer tool (15). Incidence rates were then stratified into the following age groups: 20–49, 50–64, 65–74, and 74 years and older as used in the SEER. The 2002–2013 NIS–Healthcare Cost and Utilization Project databases were queried using the ICD-9-CM codes to identify patients who had both a diagnosis code for EC, GC, CRC, and PC and a procedure code for surgical resection corresponding to the cancer diagnosis. Using the ICD-9-CM classification, we then defined patients with obesity as those with a body mass index ≥30 kg/m2, whereas patients not having any diagnosis codes indicating obesity were considered nonobese. These methods and the ICD-9-CM codes were used similar to previously published studies (16–24). Patients in the NIS were then stratified into age brackets similar to the SEER. Exclusions included the following: (i) patients younger than 18 years and (ii) patients who underwent cancer surgical resection or cancer diagnosis of more than one organ, as done previously (16). The specific ICD-9-CM codes that were used in this study are listed in Supplementary Table 1, http://links.lww.com/CTG/A227. The primary outcome of this study was to investigate age-stratified incidence trends of EC, GC, CRC, and PC during the years 2002–2013. We also compared the number of surgical resections for these cancers among patients with and without obesity during the study period.
Statistical analysis
Temporal trends for age-adjusted incidence rates and resection counts of EC, GC, CRC, and PC were analyzed by segmented (Joinpoint) regression (25). For each outcome and cancer type, the average annual percent change (AAPC) was estimated for each age group. Each AAPC estimate was tested against the null hypothesis that the AAPC was equal to zero. The NIS input into the segmented regression models consisted of resection counts and standard errors. All NIS analyses, including calculations of patient and hospital characteristics, were weighted and accounted for the NIS sampling design (discharge weights, stratification, and clustering) to produce national estimates. Observations from all strata and clusters were included to obtain the correct standard errors for estimates. The recalculated NIS trend weights were used in 2002–2011 for consistency, with the sample design revised in 2012 and later. All statistical tests were evaluated at the α = 0.05 significance level. Analyses were performed with Joinpoint Regression Program version 4.6.0.0 (Statistical Methodology and Applications Branch, Surveillance Research Program, National Cancer Institute) and SAS 9.4 (SAS Institute, Cary, NC).
RESULTS
Characteristics of patients with cancer resections
Per the NIS, there were 1,272,243 CRC, GC, PC, and EC resections meeting our criteria in 2002–2013. Of those, 91,116 (7.2%) were performed in obese individuals and 1,181,127 (92.8%) in nonobese individuals (Table 1). CRC was most highly represented in both groups, making up 93.1% of all surgeries, followed by GC at 4.4%. PC and EC resections each comprised less than 1.3% of all surgeries. Obese patients undergoing resections tended to be younger, with 39.2% of them in the 50–64 year age group as opposed to 37.8% in the 74 years and older group for the nonobese population. There was also a slight predominance of women, private insurance carriers, and elective surgeries in the obese group and a greater likelihood of having multiple comorbidities. Other slight differences in characteristics did not appear clinically significant. Finally, Supplementary Table 2, http://links.lww.com/CTG/A228, provides patients' characteristics stratified by cancer type and age (<50 or ≥50 years).
Table 1.
Patient characteristics in the NIS database
Cancer incidence trends stratified by age in 2002–2013
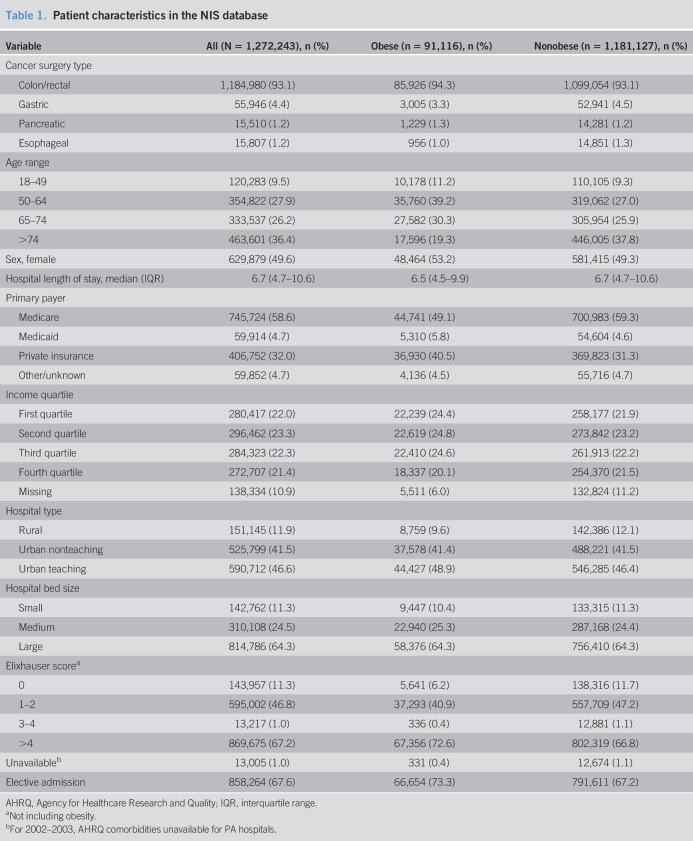
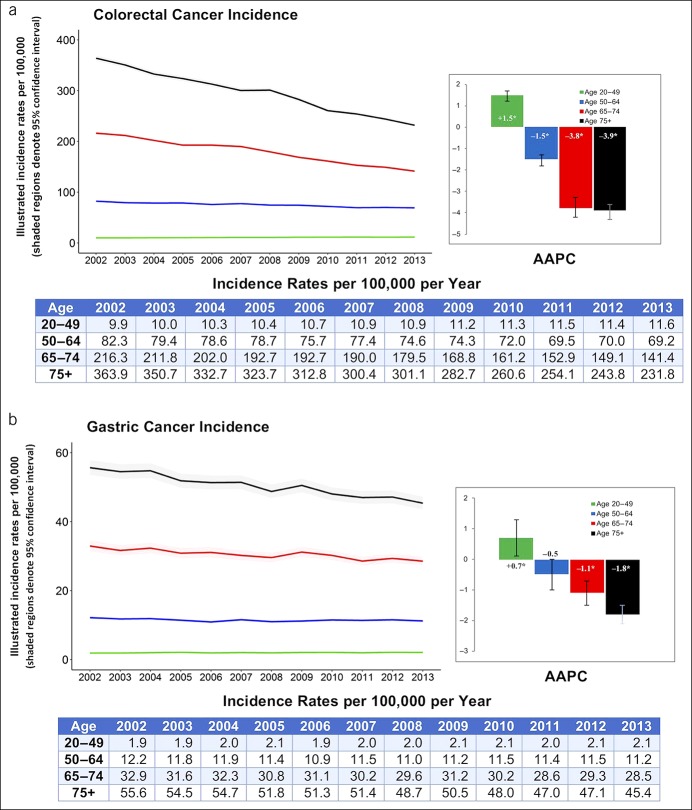
When investigating trends using the SEER, the incidence of CRC in the 20–49 year age group increased between 2002 and 2013 (AAPC +1.5%, P < 0.001) as in Figure 1. The incidence of GC in the 20–49 year age group also increased, with an AAPC of +0.7% (P = 0.02) (Figure 1). Conversely, the incidence of CRC and GC decreased in age groups older than 49 years, with a more pronounced drop as the age groups advanced (AAPC −3.9% for CRC and −1.8% for GC in the 74 years and older group, P < 0.001). In addition, the incidence of PC increased in all ages with an AAPC range of 0.7%–1.0% per year. By contrast, the incidence of EC decreased in all age groups, especially in adults aged 20–49 years (AAPC −1.8%, P = 0.002) (Figure 2).
Figure 1.
Incidence and AAPC for colorectal and gastric cancers stratified by age group: Surveillance, Epidemiology and End Result 2002–2013. AAPC, average annual percent change. *When P < 0.05.
Figure 2.
Incidence and AAPC for esophageal and pancreatic cancer stratified by age group: Surveillance, Epidemiology and End Result 2002–2013. AAPC, average annual percent change. *When P < 0.05.
Cancer resection trends stratified by age and obesity
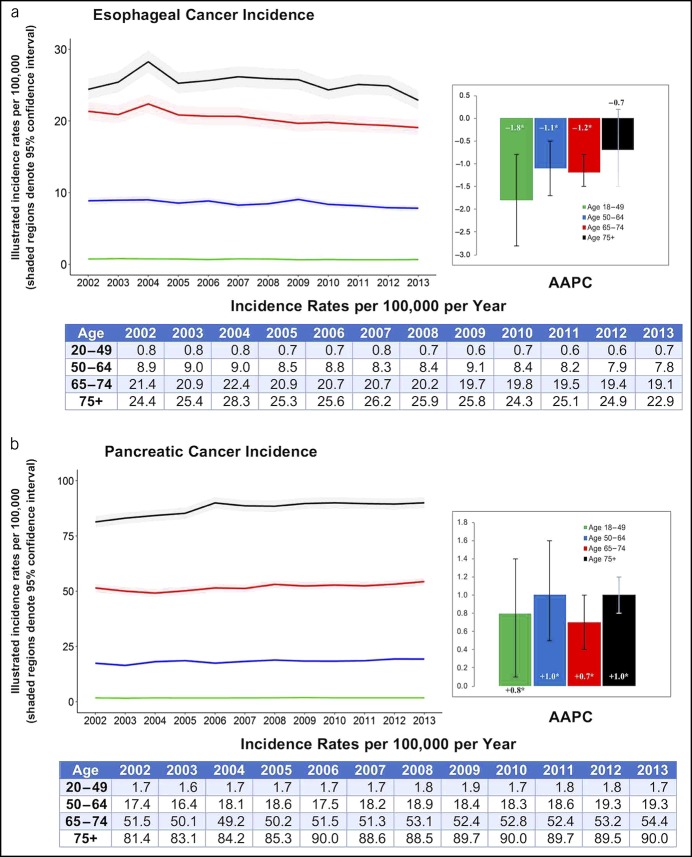
Figure 3 shows cancer resections for CRC and GC, whereas resections for PC and EC are shown in Figure 4. The number of patients with obesity undergoing CRC resections increased among all age groups, with the highest rise in the 18–49 year group (AAPC +13.1%, P < 0.001). By contrast, CRC resections decreased among all age groups for nonobese patients, with the most pronounced drop in patients 75 years or older (AAPC −4.0%, P < 0.001). We then stratified CRC into colon and rectal cancers in Supplementary Figure 1, http://links.lww.com/CTG/A226. Among obese individuals, resections for both colon and rectal cancers increased across all ages (P < 0.05); however, the highest increase was in rectal cancer resection in patients in the 18–49 year group (AAPC +15.3%, P = 0.047). Among nonobese individuals, only colon cancer resections decreased, whereas rectal cancer resection did not significantly change over time.
Figure 3.
Number of adults who underwent colorectal and gastric cancer resections with AAPC stratified by age group and obesity: NIS 2002–2013. AAPC, average annual percent change. *When P < 0.05.
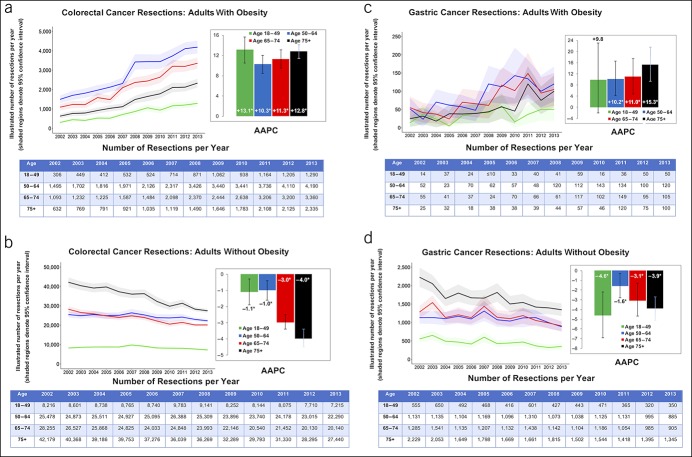
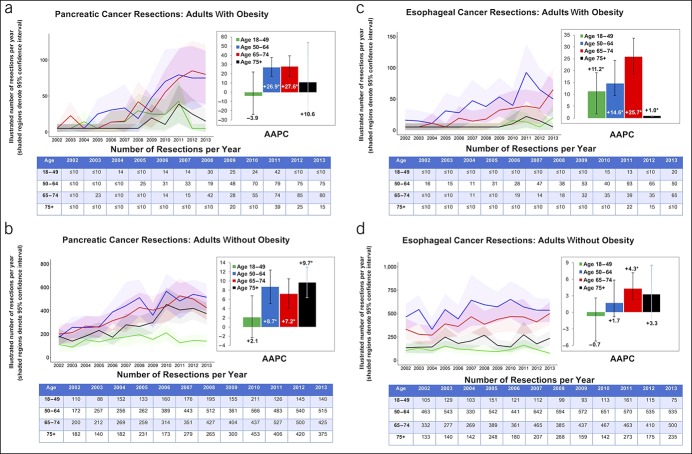
Figure 4.
Number of adults who underwent pancreatic cancer and esophageal cancer resections with AAPC stratified by age group and obesity: NIS 2002–2013. AAPC, average annual percent change. *When P < 0.05.
GC resections significantly increased among patients 50 years or older with obesity, especially in patients 74 years and older (AAPC +15.3%, P < 0.001). By contrast, GC resections decreased among all age groups for nonobese patients, especially in the younger population (AAPC for the 18–49 year group was −4.6%, P = 0.002). Interestingly, when GC is further stratified into cardia vs noncardia GCs, we do not observe a change in patients with obesity undergoing cardia cancer resections over time, although they decrease in nonobese patients (data not shown). On the contrary, the number of noncardia GCs increased in patients 50 years or older with obesity, especially for adults 74 years and older (AAPC 15.5%, P < 0.001).
PC resections increased significantly in the obese group aged 50–74 years (AAPC up to 27.6%, P < 0.05). Resections also increased in nonobese patients 50 years and older with PC but were less pronounced (highest AAPC was 9.7% for the 75 years and older group). EC resections increased among all age groups in obese patients, especially for the 65–74 year age group (AAPC +25.7%, P < 0.001). Although resections tended to increase over time for nonobese patients with EC, the trends were more modest when compared with patients with obesity.
DISCUSSION
The rates of obesity have risen in previous decades, making it a major health burden. In this study, we hypothesized that obesity is the driving force for a previously observed increase in CRC incidence in younger adults. We also investigated the incidence and surgical resections for other obesity-related gastrointestinal cancers divided by age groups. To the best of our knowledge, this study is the largest to address this question by simultaneously querying the SEER and NIS databases. Similar to previous studies, we demonstrate an increase in the incidence of young-onset CRC in the United States in 2002–2013. We also report a novel finding of increased young-onset GC and PC incidence. Using the National Inpatient Sample, our data are the first to show an increasing number of young adults with obesity who underwent surgical resections for CRC. Interestingly, this trend was not observed in young obese adults with GC and PC. These findings strengthen the role of obesity in the early-onset CRC pathogenesis.
Previous studies identify an increase in rectal cancer incidence that has driven the rise in early-onset CRC (26–29). Similarly, our data from the National Inpatient Sample show an increasing number of obese patients undergoing CRC resections, especially for young adults with rectal cancer. Interestingly, however, obesity was thought to be more related to proximal colon cancers as opposed to rectal cancers, and further data are needed to delineate the role of obesity in the pathogenesis of early-onset rectal cancer (30). One possible explanation would be the collective contribution of poor lifestyle dietary and exercise patterns to the pathogenesis of early-onset CRC, in part, by also increasing body fatness. In particular, diets rich in processed foods, red meats, added sugars, and refined grains have been strongly linked to the development of CRC (5). Specifically, such “Western diets” have been associated with an increased incidence of distal colon and rectal tumors, more so than proximal colon tumors (31,32). Notably, the association between obesity and early-onset CRC remained significant after adjusting for diet and physical activity in a previous study, suggesting an independent role of increased body fatness in the early-onset CRC carcinogenesis (12). We also observe an increasing number of obese patients 50 years or older with CRC resections, which is likely masked in the SEER data by the larger decline in resections seen among nonobese patients 50 years or older. The increased CRC resection in patients 50 years or older is likely brought by the increasing prevalence of obesity. Other potential explanation for this finding would also be less use or efficacy of the current CRC screening in patients with obesity who are 50 years or older, as was shown previously (33).
To the best of our knowledge, our analysis is the first to indicate a rising incidence of GC among adults younger than 50 years in the United States, a trend that correlates with the mounting prevalence of the early-onset obesity. Interestingly, our study does not show a concomitant rise in young adults with obesity undergoing GC resections. Instead, we document an increasing frequency of GC resections among adults 50 years or older with obesity, especially for patients 74 years and older. Furthermore, this rise was mainly because of noncardia GCs that are typically not associated with obesity (6,34,35). Similarly, a recent report does not show an increase in the early-onset incidence of cardia adenocarcinoma, a type of GC that is associated with obesity (36,37). Thus, more data are needed to validate the increase in early-onset GC and investigate the etiologies and the increased noncardia GC resections in obese adults older than 50 years.
When examining trends for PC, we found that the incidence increased between 0.7% and 1.0% annually across all age groups in 2002–2013. Multiple modifiable risk factors have been established for PC, the 2 most common being tobacco use and diabetes mellitus (38). Recent studies have found sufficient evidence linking obesity to PC (6). Given tobacco use has declined (39), the rising incidence of PC we document can likely be attributed, at least in part, to the increasing prevalence of obesity and diabetes mellitus (40). This hypothesis is supported by our study's novel finding of greater PC resections among obese patients 50 years or older as compared with nonobese patients. Notably, we did not see a conclusive rise in PC resections in patients younger than 50 years with obesity. Given that PC resection is typically implemented for locoregional disease, this could be due to later PC diagnosis in young adults leading to a more advanced disease and thus lesser surgical resections (41,42). However, other factors may be at play, and further research regarding the early-onset PC etiology and treatment will be important to improving these patients' outcomes in the future.
In this study, we observed a decline in the incidence of EC across all age groups in 2002–2013. This is in stark contrast to the steep increase in the annual incidence rates documented from the 1970s to early 2000s. This recent decline in the EC incidence may be attributable to a greater awareness of modifiable risk factors. For example, reductions observed in the consumption of tobacco as well as the greater use of proton pump inhibitor therapy and Barrett's esophagus screening and surveillance could all explain the decline in the EC incidence. Interestingly, although we see a decline in the incidence of EC, we find an increase in the incidence of EC surgical resection during the same period, particularly among the population suffering from obesity. Given this finding, we postulate that improvements in anesthesia, surgical technique, and postoperative care are making EC surgery less risky, so people with obesity are more frequently undergoing EC surgery compared with previous years.
Although the size of our national data sets provides a reasonable estimation of the relationship between obesity and cancer resections, certain limitations still apply. First, the National Inpatient Sample is a claim-based database that is susceptible to inaccurately entered or missing codes, which could introduce misclassification bias (43). We attempted to compensate for that limitation by having stringent exclusion criteria and using the specific ICD-9-CM codes. For instance, administrative data are generally accurately coded for serious conditions, such as cancer (44). Furthermore, the sensitivity and specificity of the colectomy procedure codes was previously noted to be 87.5 and 99.6%, respectively (45). By using codes for cancers and resections, we were able to identify unique patients undergoing resections and reduce the possibility of overcounting. Alternatively, obesity codes are usually specific but underused in administrative data sets, which can explain the smaller overall percentile of patients with obesity undergoing cancer resections in our results (46). However, despite the undercoding of obesity, we were able to show an increasing trend in the specific age groups with obesity that correspond with CRC incidence. Second, we could not identify patients with cancer who did not undergo surgical resections. This includes patients who had nonoperative management of rectal cancer, the so-called watch and wait who may have been missed. Although the “watch-and-wait” treatment concept was initially proposed in 2004, results were viewed with caution during our study timeline because of the scarcity of data, and the approach was not popular until recent years (47–50). Furthermore, up to 30% of patients managed nonoperatively for rectal cancer do ultimately require salvage surgery and therefore would have been included in our analysis (51,52). In addition, despite the increasing popularity of the “watch-and-wait” approach, we witnessed an increase in rectal cancer resections in adults with obesity during the period. For these reasons, we do not believe that the increase in the number of patients treated nonoperatively was large enough to compromise our results. Third, approximately 50% of our data do come from urban nonteaching and rural hospitals, which may affect the coding quality. Interestingly, rural and urban nonteaching hospitals are shown to possibly have better coding accuracy compared with teaching hospitals (53,54). Furthermore, hospital characteristics were evenly distributed among the obese and nonobese cohorts and relatively similar across cancer subtypes, thus limiting any impact from misclassification bias on a population or cancer type. Fourth, we could not adjust for the confounding impact of a specific diet, physical activity, or the microbiome that may be contributing to obesity and ultimately an increased risk of cancer. Finally, the NIS uses the body mass index to define obesity. Although the body mass index has a high specificity for excess body fat (55), it may not accurately reflect the burden of obesity-related comorbidities or metabolic syndrome (56). Despite these limitations, however, we are confident in our results because of the overall power from large national databases.
In conclusion, we demonstrate a rising incidence of early-onset GC, CRC, and PC in the United States. This rise of CRC in younger adults may be related to an increasing prevalence of the early-onset obesity, as suggested by increased CRC resections in patients with obesity. Further research is needed to validate our findings and determine the etiology of early-onset GC and PC. In addition, future studies are required to ameliorate the increasing cancer incidence in the young adult population. This could be achieved by implementing early screening programs and active measures to ameliorate the increasing obesity prevalence early in life.
CONFLICTS OF INTEREST
Guarantor of the article: Hisham Hussan, MD.
Specific author contributions: H.H. was involved in the conception, design, and interpretation of the data and the manuscript's drafting and critical revision. K.P. was involved in the study's design, acquired and statistically analyzed the data, and provided a critical revision of the manuscript. The above authors had full access to all the data in the study and take responsibility for the data's integrity and the data analysis accuracy. A.P., Z.C.-M., S.K.C., J.M.C., K.S.C., and M.L.R. were involved in the design, data interpretation, and critical revision of the manuscript. All authors gave the final approval of the submitted manuscript and take responsibility for the integrity of the work.
Financial support: H.H. work on this project was supported by the NIH National Cancer Institute–funded Transdisciplinary Research in Energetics and Cancer (TREC) Training Workshop for Early Career (R25CA203650).
Potential competing interests: None to report.
Previous presentation: This research was presented as an oral presentation at the American College of Gastroenterology (ACG) 2018 National Meeting and awarded the 2018 ACG Obesity Award for the Best Scientific Paper.
Study Highlights.
WHAT IS KNOWN
✓ Obesity is strongly associated with cancers of the esophagus, stomach, pancreas, and colorectum.
✓ The incidence of colorectal cancer, especially rectal cancer, is rising in young adults (younger than 50 years).
✓ Limited data exist on whether a similar trend is observed for esophageal, pancreatic, and gastric cancers and relation to obesity.
WHAT IS NEW HERE
✓ In addition to colorectal cancer, the incidence of young adults with gastric and pancreatic cancers increased by 0.7% and 0.8% per year, respectively, in 2002–2013.
✓ Obese young adults who had colorectal cancer resections increased in numbers between 2002 and 2013, especially rectal cancer (+15.3% per year).
✓ This trend was not observed for gastric and pancreatic cancers, suggesting that obesity may play a larger role in driving early-onset colorectal cancer.
TRANSLATIONAL IMPACT
✓ This study provides supports for translational studies testing the role of body fatness and metabolic syndrome in early-onset colorectal cancer.
Supplementary Material
ACKNOWLEDGEMENT
We thank Dr. Rishi Jain and Dr. Ravi Babu Pavurala for their involvement in the initial stages of study.
Footnotes
SUPPLEMENTARY MATERIAL accompanies this paper at http://links.lww.com/CTG/A226, http://links.lww.com/CTG/A227, http://links.lww.com/CTG/A228
REFERENCES
- 1.Fryar CD, Carroll MD, Ogden CL. Prevalence of Overweight, Obesity, and Extreme Obesity Among Adults Aged 20 and over: United States, 1960–1962 through 2013–2014. National Center for Health Statistics Data, Health E-Stats; 2016, pp 2016. [Google Scholar]
- 2.Centers for Disease Control and Prevention. Childhood Obesity Facts. (https://www.cdc.gov/obesity/data/childhood.html). Accessed May 23, 2017.
- 3.Finkelstein EA, Khavjou OA, Thompson H, et al. Obesity and severe obesity forecasts through 2030. Am J Prev Med 2012;42(6):563–70. [DOI] [PubMed] [Google Scholar]
- 4.Sturm R, Hattori A. Morbid obesity rates continue to rise rapidly in the United States. Int J Obes (Lond) 2013;37(6):889–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steele CB Thomas CC Henley SJ et al. Vital signs: Trends in incidence of cancers associated with overweight and obesity—United States, 2005‐2014. MMWR Morb Mortal Wkly Rep. 2017;66(39):1052–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lauby-Secretan B, Scoccianti C, Loomis D, et al. Body fatness and cancer—viewpoint of the IARC working group. N Engl J Med 2016;375(8):794–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ben Q, An W, Jiang Y, et al. Body mass index increases risk for colorectal adenomas based on meta-analysis. Gastroenterology 2012;142(4):762–72. [DOI] [PubMed] [Google Scholar]
- 8.Ma Y, Yang Y, Wang F, et al. Obesity and risk of colorectal cancer: A systematic review of prospective studies. PLoS One 2013;8(1):e53916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Okabayashi K, Ashrafian H, Hasegawa H, et al. Body mass index category as a risk factor for colorectal adenomas: A systematic review and meta-analysis. Am J Gastroenterol 2012;107(8):1175–85; quiz 1186. [DOI] [PubMed] [Google Scholar]
- 10.Larsson SC, Wolk A. Obesity and the risk of gallbladder cancer: A meta-analysis. Br J Cancer 2007;96(9):1457–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dwyer AJ, Murphy CC, Boland CR, et al. A summary of the fight colorectal cancer working meeting: Exploring risk factors and etiology of sporadic early-age onset colorectal cancer. Gastroenterology 2019;157(2):280–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu PH, Wu K, Ng K, et al. Association of obesity with risk of early-onset colorectal cancer among women. JAMA Oncol 2019;5(1):37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hankey BF, Ries LA, Edwards BK. The surveillance, epidemiology, and end results program: A national resource. Cancer Epidemiol Biomarkers Prev 1999;8(12):1117–21. [PubMed] [Google Scholar]
- 14.Agency for Healthcare Research and Quality. Healthcare cost and utilization project (HCUP). (http://www.qualityindicators.ahrq.gov/Downloads/Modules/PSI/V41/TechSpecs/PSI%2015%20Accidental%20Puncture%20or%20Laceration.pdf). Accessed August 10, 2016. [PubMed]
- 15.(https://seer.cancer.gov/explorer/application.php). Accessed September 1, 2019.
- 16.Neuwirth MG, Bierema C, Sinnamon AJ, et al. Trends in major upper abdominal surgery for cancer in octogenarians: Has there been a change in patient selection? Cancer 2018;124(1):125–35. [DOI] [PubMed] [Google Scholar]
- 17.Taghizadeh N, Fortin M, Tremblay A. US hospitalizations for malignant pleural effusions: Data from the 2012 national inpatient sample. Chest 2017;151(4):845–54. [DOI] [PubMed] [Google Scholar]
- 18.Sarvepalli S, Garg SK, Sarvepalli SS, et al. Inpatient burden of esophageal cancer and analysis of factors affecting in-hospital mortality and length of stay. Dis Esophagus 2018;31(9):doy022 (doi: 10.1093/dote/doy022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lapar DJ, Stukenborg GJ, Lau CL, et al. Differences in reported esophageal cancer resection outcomes between national clinical and administrative databases. J Thorac Cardiovasc Surg 2012;144(5):1152–7. [DOI] [PubMed] [Google Scholar]
- 20.Smith JK, McPhee JT, Hill JS, et al. National outcomes after gastric resection for neoplasm. Arch Surg 2007;142(4):387–93. [DOI] [PubMed] [Google Scholar]
- 21.Solsky I, Friedmann P, Muscarella P, et al. Poor outcomes of gastric cancer surgery after admission through the emergency department. Ann Surg Oncol 2017;24(5):1180–7. [DOI] [PubMed] [Google Scholar]
- 22.Hussan H, Gray DM, II, Hinton A, et al. Morbid obesity is associated with increased mortality, surgical complications, and incremental health care utilization in the peri-operative period of colorectal cancer surgery. World J Surg 2016;40(4):987–94. [DOI] [PubMed] [Google Scholar]
- 23.Shi HY, Wang SN, Lee KT. Temporal trends and volume-outcome associations in periampullary cancer patients: A propensity score-adjusted nationwide population-based study. Am J Surg 2014;207(4):512–9. [DOI] [PubMed] [Google Scholar]
- 24.Velez-Serrano JF, Velez-Serrano D, Hernandez-Barrera V, et al. Prediction of in-hospital mortality after pancreatic resection in pancreatic cancer patients: A boosting approach via a population-based study using health administrative data. PLoS One 2017;12(6):e0178757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu B, Barrett MJ, Kim H-J, et al. Estimating joinpoints in continuous time scale for multiple change-point models. Comput Stat Data Anal 2007;51(5):2420–7. [Google Scholar]
- 26.Bailey CE, Hu CY, You YN, et al. Increasing disparities in the age-related incidences of colon and rectal cancers in the United States, 1975-2010. JAMA Surg 2015;150(1):17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Joshu CE, Parmigiani G, Colditz GA, et al. Opportunities for the primary prevention of colorectal cancer in the United States. Cancer Prev Res (Phila) 2012;5(1):138–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Siegel RL, Jemal A, Ward EM. Increase in incidence of colorectal cancer among young men and women in the United States. Cancer Epidemiol Biomarkers Prev 2009;18(6):1695–8. [DOI] [PubMed] [Google Scholar]
- 29.You YN, Xing Y, Feig BW, et al. Young-onset colorectal cancer: Is it time to pay attention? Arch Intern Med 2012;172(3):287–9. [DOI] [PubMed] [Google Scholar]
- 30.Bardou M, Barkun AN, Martel M. Obesity and colorectal cancer. Gut 2013;62(6):933–47. [DOI] [PubMed] [Google Scholar]
- 31.Mehta RS, Song M, Nishihara R, et al. Dietary patterns and risk of colorectal cancer: Analysis by tumor location and molecular subtypes. Gastroenterology 2017;152(8):1944–53 e1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim MK, Sasaki S, Otani T, et al. ; Japan Public Health Center-based Prospective Study G. Dietary patterns and subsequent colorectal cancer risk by subsite: A prospective cohort study. Int J Cancer 2005;115(5):790–8. [DOI] [PubMed] [Google Scholar]
- 33.Ferrante JM, Ohman-Strickland P, Hudson SV, et al. Colorectal cancer screening among obese versus non-obese patients in primary care practices. Cancer Detect Prev 2006;30(5):459–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Petrick JL, Kelly SP, Liao LM, et al. Body weight trajectories and risk of oesophageal and gastric cardia adenocarcinomas: A pooled analysis of NIH-AARP and PLCO studies. Br J Cancer 2017;116(7):951–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang P, Zhou Y, Chen B, et al. Overweight, obesity and gastric cancer risk: Results from a meta-analysis of cohort studies. Eur J Cancer 2009;45(16):2867–73. [DOI] [PubMed] [Google Scholar]
- 36.Sung H, Siegel RL, Rosenberg PS, et al. Emerging cancer trends among young adults in the USA: Analysis of a population-based cancer registry. Lancet Public Health 2019;4(3):e137–e147. [DOI] [PubMed] [Google Scholar]
- 37.Rawla P, Barsouk A. Epidemiology of gastric cancer: Global trends, risk factors and prevention. Prz Gastroenterol 2019;14(1):26–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eibl G, Cruz-Monserrate Z, Korc M, et al. Diabetes mellitus and obesity as risk factors for pancreatic cancer. J Acad Nutr Diet 2018;118(4):555–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Agaku IT, King BA, Husten CG, et al. Tobacco product use among adults—United States, 2012-2013. MMWR Morb Mortal Wkly Rep 2014;63(25):542–7. [PMC free article] [PubMed] [Google Scholar]
- 40.Menke A, Casagrande S, Geiss L, et al. Prevalence of and trends in diabetes among adults in the United States, 1988-2012. JAMA 2015;314(10):1021–9. [DOI] [PubMed] [Google Scholar]
- 41.Wray CJ, Ahmad SA, Matthews JB, et al. Surgery for pancreatic cancer: Recent controversies and current practice. Gastroenterology 2005;128(6):1626–41. [DOI] [PubMed] [Google Scholar]
- 42.Neoptolemos JP, Stocken DD, Bassi C, et al. Adjuvant chemotherapy with fluorouracil plus folinic acid vs gemcitabine following pancreatic cancer resection: A randomized controlled trial. JAMA 2010;304(10):1073–81. [DOI] [PubMed] [Google Scholar]
- 43.Klabunde CN, Warren JL, Legler JM. Assessing comorbidity using claims data: An overview. Med Care 2002;40(8 Suppl):IV–26–35. [DOI] [PubMed] [Google Scholar]
- 44.Hennessy DA, Quan H, Faris PD, et al. Do coder characteristics influence validity of ICD-10 hospital discharge data?. BMC Health Serv Res 2010;10(1):99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Quan H, Parsons GA, Ghali WA. Validity of procedure codes in International Classification of Diseases, 9th revision, clinical modification administrative data. Med Care 2004;42(8):801–9. [DOI] [PubMed] [Google Scholar]
- 46.Basques BA, Miller CP, Golinvaux NS, et al. Morbidity and readmission after open reduction and internal fixation of ankle fractures are associated with preoperative patient characteristics. Clin Orthop Relat Res 2015;473(3):1133–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Glynne-Jones R, Wallace M, Livingstone JI, et al. Complete clinical response after preoperative chemoradiation in rectal cancer: Is a “wait and see” policy justified? Dis Colon Rectum 2008;51(1):10–9; discussion 19–20. [DOI] [PubMed] [Google Scholar]
- 48.O'Neill BD, Brown G, Heald RJ, et al. Non-operative treatment after neoadjuvant chemoradiotherapy for rectal cancer. Lancet Oncol 2007;8(7):625–33. [DOI] [PubMed] [Google Scholar]
- 49.Sammour T, Price BA, Krause KJ, et al. Nonoperative management or “watch and wait” for rectal cancer with complete clinical response after neoadjuvant chemoradiotherapy: A critical appraisal. Ann Surg Oncol 2017;24(7):1904–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maas M, Beets-Tan RG, Lambregts DM, et al. Wait-and-see policy for clinical complete responders after chemoradiation for rectal cancer. J Clin Oncol 2011;29(35):4633–40. [DOI] [PubMed] [Google Scholar]
- 51.Kong JC, Guerra GR, Warrier SK, et al. Outcome and salvage surgery following “watch and wait” for rectal cancer after neoadjuvant therapy: A systematic review. Dis Colon Rectum 2017;60(3):335–45. [DOI] [PubMed] [Google Scholar]
- 52.Habr-Gama A, Gama-Rodrigues J, Sao Juliao GP, et al. Local recurrence after complete clinical response and watch and wait in rectal cancer after neoadjuvant chemoradiation: Impact of salvage therapy on local disease control. Int J Radiat Oncol Biol Phys 2014;88(4):822–8. [DOI] [PubMed] [Google Scholar]
- 53.Rangachari P. Coding for quality measurement: The relationship between hospital structural characteristics and coding accuracy from the perspective of quality measurement. Perspect Health Inf Manag 2007;4:3. [PMC free article] [PubMed] [Google Scholar]
- 54.Lorence DP, Spink A, Jameson R. Manager's reports of variation in coding accuracy across U.S. oncology centers. J Oncol Manag 2002;11(6):20–6. [PubMed] [Google Scholar]
- 55.Romero-Corral A, Somers VK, Sierra-Johnson J, et al. Accuracy of body mass index in diagnosing obesity in the adult general population. Int J Obes (Lond) 2008;32(6):959–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Karelis AD. Metabolically healthy but obese individuals. Lancet 2008;372(9646):1281–3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.