OBJECTIVES:
Obesity is a risk factor for several phenotypes such as gallstones, metabolic syndrome (MS), and nonalcoholic fatty liver disease (NAFLD). It has been suggested that cholecystectomy is a risk factor for metabolic abnormalities and NAFLD. We aimed to determine whether cholecystectomy is associated with MS or NAFLD in a Dutch population-based study.
METHODS:
The Rotterdam Study is an ongoing prospective population-based cohort. We included participants who underwent a liver ultrasound between 2009 and 2014 to assess steatosis. The prevalence of MS and NAFLD was calculated, and we performed regression analyses relating cholecystectomy with MS and NAFLD and adjusted for age, sex, study cohort, education level, physical activity, energy intake, time since cholecystectomy, body mass index, presence of hypertension, diabetes mellitus, and steatosis/MS.
RESULTS:
We included 4,307 participants (57.5% women, median age 66.0 years [interquartile range 58–74]). In total, 265 participants (6.2%) underwent a cholecystectomy. The median age at the time of cholecystectomy was 57.0 years (47.5–66.5), and the median time from cholecystectomy to imaging of the liver was 10.0 years (0.5–19.5). The prevalence of MS in participants with cholecystectomy was 67.2% and 51.9% in participants without cholecystectomy (P < 0.001). Ultrasound diagnosed moderate/severe NAFLD was present in, respectively, 42.7% and 34.2% of the participants (P = 0.008). After multivariable adjustments for metabolic factors, cholecystectomy was no longer associated with the presence of MS or NAFLD.
DISCUSSION:
The prevalence of MS and NAFLD is higher in participants after cholecystectomy. However, our trial shows that cholecystectomy may not be independently associated with the presence of MS and NAFLD after correction for metabolic factors.
INTRODUCTION
To date, a cholecystectomy is considered a relatively innocuous procedure. However, Ruhl and Everhart showed an independent association of nonalcoholic fatty liver disease (NAFLD) with cholecystectomy, in a large population-based study in the Unites States (1). It has been hypothesized that gallstones may be an early biomarker signaling the development of metabolic syndrome (MS) and liver steatosis even before (morbid) obesity is present. On the other hand, it is conceivably that cholecystectomy actually drives the development of NAFLD (2), which would increase the unknown medical and financial burden of this presumably low-risk operation significantly.
Inflammation associated with NAFLD may result in liver damage, liver fibrosis, and eventually cirrhosis (3). Aggravation of steatosis is multifactorial; multiple risk factors such as insulin resistance, MS, and interaction between cytokines and adipokines eventually cause cirrhosis (4,5). Several preclinical studies showed that cholecystectomy affects the enterohepatic bile salt circulation and attendant signaling via the bile salt receptors engaged in metabolic homeostasis (6–8). This may suggest that cholecystectomy is a biomarker for metabolic abnormalities via bile acid–induced changes in the enterohepatic circulation and is indeed another “fellow traveler” with, or another biomarker for NAFLD. The US study showed a stronger association of NAFLD with cholecystectomy compared with the presence of gallstones alone and suggests that cholecystectomy may be a risk factor for NAFLD itself (1).
In the Netherlands, the prevalence of gallstone disease, MS, and NAFLD is, respectively, 5%–30%, 14%, and 22% and similar with other western countries (9–14). To date, the exact association between cholecystectomy, MS, and NAFLD has not been clarified; that is, current research is conflicting and reported results needs validation. We therefore aimed to determine whether cholecystectomy is associated with MS and NAFLD in a large ongoing Dutch prospective population cohort.
METHODS
Study population
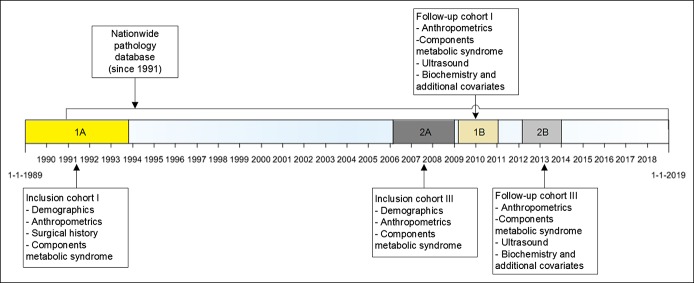
This study is an analysis of the Rotterdam Study—a large ongoing population-based cohort of participants aged 45 years and older living in a suburb of Rotterdam, the Netherlands. The rationale and design of this population-based study are previously described (15). For our study, all participants in the Rotterdam Study cohort I, visit 5 (participants ≥75 years) and cohort III, visit 2 (participants ≥50 years) were included. These participants visited the research center between March 2009 and June 2014. They had their first visit to the study center between 1989–1993 and 2006–2008, respectively (Figure 1). To exclude participants with secondary steatosis, participants with viral hepatitis B or C, alcoholic misuse (in man >3 and women >2 glasses per day), or participants who use steatogenic medication (i.e., methotrexate, tamoxifen, amiodarone, and systemic corticosteroid), for example, for autoimmune hepatitis, were excluded from the NAFLD-group. Participants with a diagnosed autoimmune hepatitis were excluded as well. The Rotterdam Study has been approved by the institutional review board (Medical Ethics Committee) of the Erasmus MC University Medical Center Rotterdam and by the review board of the Dutch Ministry of Health, Welfare and Sports. Written informed consent was obtained from all participants. This research was performed in accordance with the ethical standards of the updated tenets of the Declaration of Helsinki of 2013. The results will be presented according to the Strengthening the Reporting of Observational studies in the Epidemiology (STROBE) guidelines.
Figure 1.
Available data on time line. Participants in cohort I and cohort III of the Rotterdam Study were included. Participants in cohort I had their first visit to the study center between 1989 and 1993. We included data from visit 5 (2009–2011). Participants in cohort III had their first visit to the study center between 2006 and 2008. We included data from visit 2 (2012–2014).
Cholecystectomy
Participants in cohort I were asked for their surgical history at time of inclusion in the cohort. However, participants in cohort III were not asked for their complete surgical history. We therefore additionally linked the data of the Rotterdam study to our nationwide pathology database (PALGA), which has a nationwide coverage from 1991 onward. The database was searched for cholecystectomies in participants of cohort I and III. Of all participants with a cholecystectomy, the year of cholecystectomy was available from the surgical history or PALGA-database.
Nonalcoholic fatty liver disease
A certified and experienced technician performed the abdominal ultrasounds with a Hitachi HI VISION 900 between 2009 and 2014. All images were re-evaluated by a single hepatologist. Diagnosis of steatosis was determined dichotomously as the presence or absence of a hyperechogenic liver parenchyma, that is, moderate or severe steatosis (16). NAFLD was defined as steatosis on ultrasound with the absence of secondary causes of steatosis (as abovementioned).
Metabolic syndrome
MS was defined according to the Alberti-2009 method (17). MS was diagnosed when at least 3 of the following risk factors were present: (i) abdominal obesity, defined as WC ≥ 102 cm in men and ≥ 88 cm in women, (ii) serum triglycerides ≥150 mg/dL (1.7 mmol/L) or drug treatment for elevated triglycerides, (iii) serum high-density lipoprotein cholesterol ≤40 mg/dL (1.0 mmol/L) in men and ≤50 mg/dL (1.3 mmol/L) in women, or drug treatment for low high-density lipoprotein cholesterol, (iv) blood pressure ≥130/85 mm Hg or drug treatment for elevated blood pressure, and (v) fasting plasma glucose ≥100 mg/dL (5.6 mmol/L) or drug treatment for elevated blood glucose. Participants with missing data regarding MS or unreliable high or low values were excluded.
Diabetes mellitus (DM) was defined as a fasting plasma glucose ≥110 mg/dL or drug treatment for elevated blood glucose, and hypertension was defined as a systolic blood pressure ≥140 mm Hg, a diastolic blood pressure ≥90 mm Hg, or drug treatment for elevated blood pressure.
Biochemistry and additional covariates
Fasting blood samples were collected. Blood lipids and fasting plasma glucose were measured using automatic enzyme procedures (Roche Diagnostic GmbH, Mannheim, DE). Hepatitis B surface antigen and antihepatitis C virus were measured by an automatic immunoassay (Roche Diagnostic GmbH). Demographics, education level, medical history, comorbidities, physical activity, and smoking were obtained during a home interview. Total energy intake and alcohol use were assessed using a validated food frequency questionnaire (18). Medication use was obtained from an automatic linkage to the pharmacies. Anthropometric measurements were performed by well-trained research assistants, and weight, height, and waist circumference were measured.
Statistical analyses
Characteristics of participants were stratified by history of cholecystectomy. Continuous, normally distributed data were presented as mean with the SD and continuous non-normal distrusted data as median with the interquartile range (IQR). Dichotomous data were summarized as frequencies and proportions. Comparison of clinical characteristics between both groups was performed using the χ2 test for dichotomous data, the Student t test for normally distributed continuous data, and the Mann-Whitney U test for skewed continuous data.
Univariate logistic regression analyses were performed to examine the association between cholecystectomy and MS and the association between cholecystectomy and NAFLD. The regression analysis was first performed in a univariable fashion and second with multivariable adjustments. These adjustments were made by the inclusion of age, sex, Rotterdam Study cohort, education level, physical activity, total energy intake, time since cholecystectomy more than 10 years, presence of hypertension, presence of DM, and body mass index (BMI); in the analysis for MS, the presence of steatosis was included in the model, and in the analysis for steatosis, the presence of MS was included. Correlation and multicollinearity between cholecystectomy, MS, and NAFLD were tested. In addition, interaction between the covariables was tested. The outcome of the univariable and multivariable analyses were presented as an odds ratio (OR) and 95% confidence interval (CI).
Several sensitivity analyses were performed. We stratified by sex because of sex differences in the prevalence of cholecystectomy, MS, and NAFLD. To test the robustness of our data, analyses were performed stratified by cohort, and second, data were analyzed in a separate group of nonoverweighed participants (BMI <25).
Associations with a P value less than 0.05 will be considered statistically significant. All missing values were considered to be at random and were excluded from the analyses. Analyses were performed using SPSS statistics version 25.0 (IBM).
RESULTS
Study population
Between March 2009 and January 2011 2,147 participants from the Rotterdam Study cohort I and between March 2012 and June 2014 3,122 participants from Rotterdam Study cohort III visited the research center. In total, 904 participants were excluded because there were no data available regarding abdominal ultrasound. Moreover, 58 participants were excluded with missing or unreliable data regarding MS. Hence, in this analysis, 4,307 participants were included (Figure 2). Six hundred fifty-seven participants were excluded for the analyses regarding NAFLD because they fulfilled the criteria for secondary steatosis. None of the included participants had a diagnosed autoimmune hepatitis. So, all data regarding NAFLD were studied in 3,650 participants.
Figure 2.
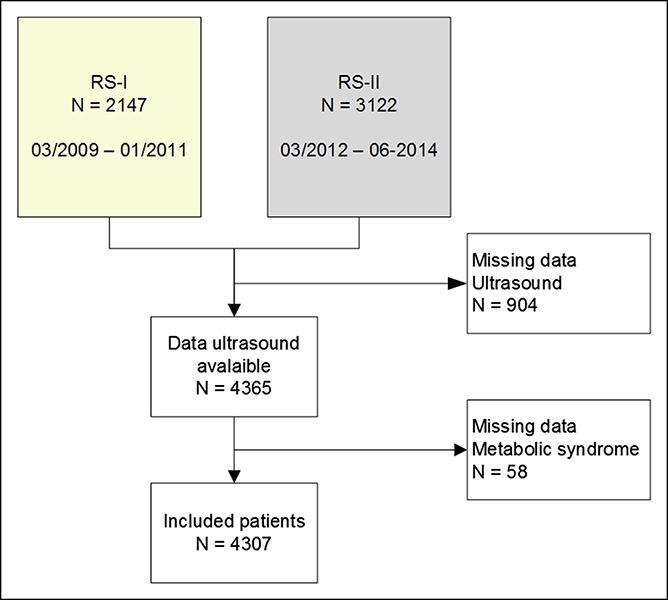
Flowchart of inclusion. Participants were included from cohort I and cohort III. Participants with missing ultrasound or metabolic syndrome data were excluded. RS, Rotterdam Study.
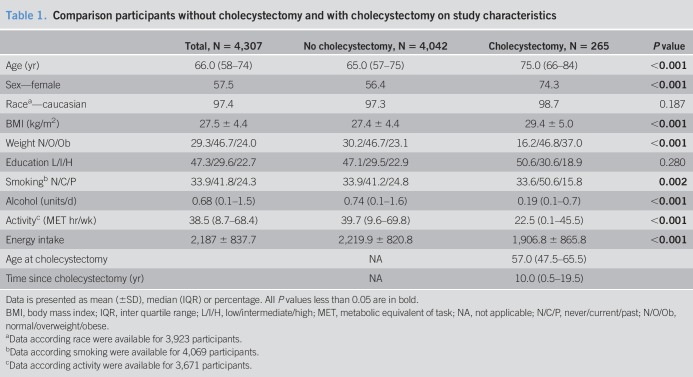
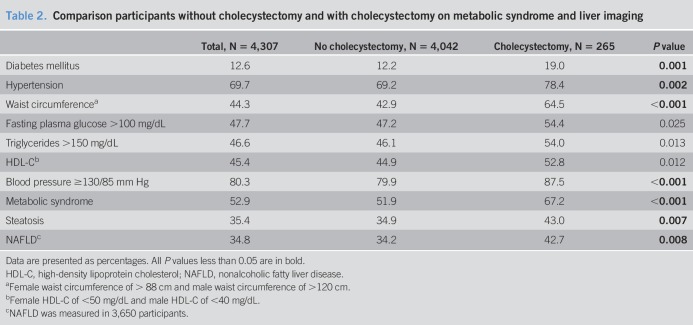
Of the included participants, 57.5% was women, median age was 66.0 years (IQR 58–74), and mean BMI was 27.5 ± 4.4 kg/m2 (Table 1). MS was present in 2,278 participants (52.9%). Steatosis was present in 35.4% (n = 1,525), and NAFLD in 34.8% (n = 1,269) (Table 2).
Table 1.
Comparison participants without cholecystectomy and with cholecystectomy on study characteristics
Table 2.
Comparison participants without cholecystectomy and with cholecystectomy on metabolic syndrome and liver imaging
Cholecystectomy
In 265 participants (6.2%), a cholecystectomy was performed. The median age at time of cholecystectomy was 57.0 years (47.5–66.5). The median interval between cholecystectomy and liver imaging was 10.0 years (0.5–19.5). In Table 1, 265 participants with cholecystectomy were compared with the 4,042 participants without cholecystectomy. Participants with cholecystectomy were older, more often women and (ex-)smokers, used less alcohol, had a lower physical activity and energy intake, and a higher BMI as compared to participants without cholecystectomy. The prevalence of MS in participants with cholecystectomy was 67.2% (n = 178) and 51.9% in participants without cholecystectomy (n = 2,099) (P < 0.001). The prevalence of DM was 19.0% in participants with cholecystectomy and 12.2% in participants without cholecystectomy (P = 0.001); hypertension was present in 78.4% of participants with cholecystectomy and in 69.2% of participants without cholecystectomy (P = 0.002). NAFLD was present in, respectively, 42.7% and 34.2% (P = 0.008) (Table 2).
Differences in characteristics between participants with and without MS and NAFLD are shown in Table 1, Supplementary Digital Content 1, http://links.lww.com/CTG/A279, and Table 2, http://links.lww.com/CTG/A280, respectively.
Cholecystectomy and metabolic syndrome
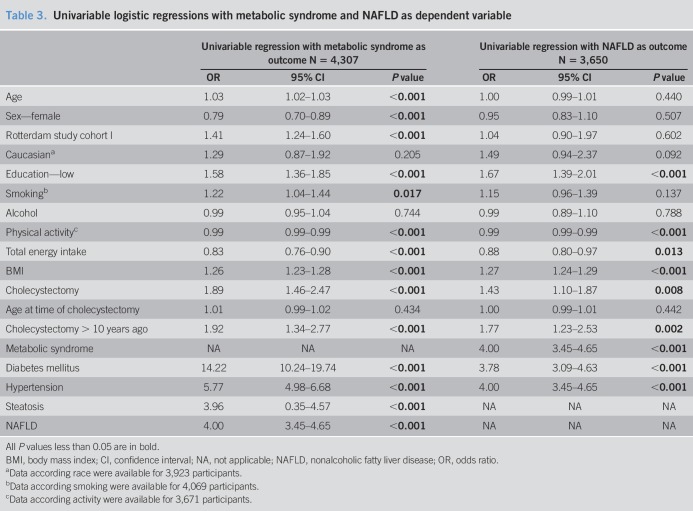
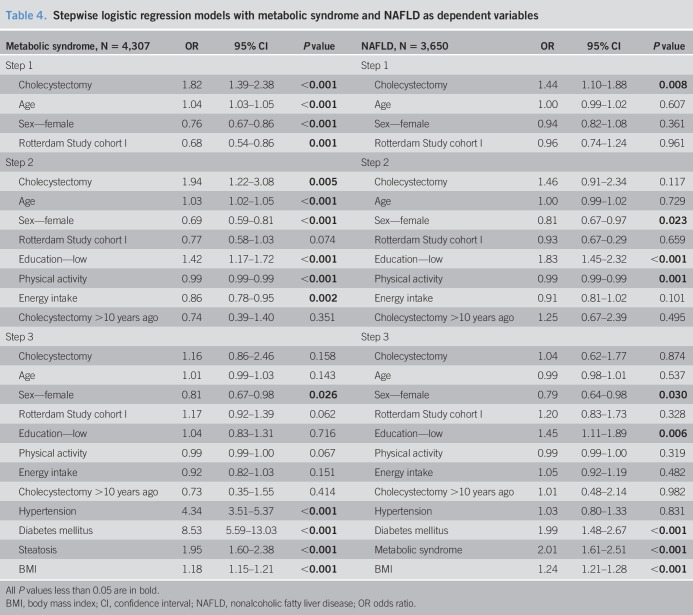
Cholecystectomy was associated with MS in univariable logistic regression analysis (Table 3). After multivariable adjustments for metabolic factors (steatosis, hypertension, and DM), cholecystectomy was no longer associated with MS (Table 4). Steatosis and MS were significantly correlated (r = 0.309, P < 0.001); however, there was no multicollinearity (VIF 1.1). Interactions between covariables and cholecystectomy were tested and found to be not significant.
Table 3.
Univariable logistic regressions with metabolic syndrome and NAFLD as dependent variable
Table 4.
Stepwise logistic regression models with metabolic syndrome and NAFLD as dependent variables
Cholecystectomy and NAFLD
Cholecystectomy was associated with NAFLD in univariable logistic regression analysis (Table 3). However, after multivariable adjustments for metabolic factors, this association was no longer significant (Table 4). DM and hypertension had a significant interaction (OR 0.422, 95% CI 0.181–0.987, P = 0.047). No other interactions between covariables and cholecystectomy were found.
Secondary analysis
In the multivariable regression analyses, sex was independently associated with the presence of MS (sex, women: OR 0.810, 95% CI 0.672–0.975, P = 0.026) and also for with the presence of NAFLD after correction for the metabolic factors (sex, women: OR 0.794 95% CI 0.644–0.978, P = 0.030). We performed the multivariable regression analyses separately for men and women. In men, a trend toward an association between cholecystectomy and MS was found in multivariable logistic regressions (OR 2.239, 95% CI 0.923–5.434, P = 0.075). Multivariable regressions in women showed no significant association between cholecystectomy and MS (OR 1.320, 95% CI 0.686–2.537, P = 0.450). In line with the main analysis, no significant association was found in multivariable analyses between cholecystectomy and NAFLD stratified by sex (men: OR 0.912, 95% CI 0.317–2.624, P = 0.864, women: OR 1.070, 95% CI 0.572–1.999, P = 0.833).
Sensitivity analysis
Separate multivariable analyses for cohort I (age ≥75 years) and cohort III (age ≥50 years) demonstrated no significant association between cholecystectomy and MS in cohort I (OR 1.146, 95% CI 0.547–2.402, P = 0.718); however, the association between cholecystectomy and MS in cohort III showed a trend toward association (OR 2.029, 95% CI 0.958–4.295, P = 0.064). For NAFLD, no significant associations were found (cohort I OR 1.126, 95% CI 0.505–2.511, P = 0.772; cohort III OR 1.003 95% CI 0.489–2.057, P = 0.994).
Analyzing only participants with a normal BMI (≤25 kg/m2), resulted in similar findings, no significant association between cholecystectomy and MS, or NAFLD, was found in multivariable regression analyses (MS: OR 0.811, 95% CI 0.230–2.862, P = 0.745, NAFLD: OR 1.026, 95% CI 0.604–1.744 P = 0.924).
DISCUSSION
The results of this study show that cholecystectomy is not independently associated with the presence of MS and NAFLD after correction for metabolic factors. Although the prevalence of NAFLD in this population is high (34.8%) and is even higher in participants with a previous cholecystectomy (42.7%), we found that after adjustment for multiple metabolic factors cholecystectomy was not associated with the presence of NAFLD nor with the presence of MS.
These results are at odds with the findings of population-based study with more than 12,000 participants in the United States (1). These researchers found an independent association of NAFLD with cholecystectomy after adjustment for multiple metabolic factors in a large population-based study of the NHANES. Although their study was methodologically different, it screened all patients for gallstones with ultrasound and compared participants with cholecystectomy with participants without gallstone disease (no gallstones, no cholecystectomy). Second, they analyzed participants with gallstones separately and found a significant association with NAFLD in men only. The association between gallstone disease and NAFLD has been confirmed in a systematic review including 12, mostly cross-sectional, studies (19). This study showed the wide range in prevalence of gallstones disease and NAFLD from 5% to 50%. Six studies compared patients with gallstones or cholecystectomy with patients with no gallstone disease at all. Of these studies, 2 did not show that gallstone disease was independently associated with NAFLD (20,21), whereas 4 did (22–25). All studies adjusted for different covariates as potential confounders. None of the 12 included studies compared subjects with cholecystectomy to subjects without cholecystectomy. Only one other study, comparable with ours, did comparing cholecystectomy to without cholecystectomy and found similar results; a significant association between cholecystectomy and NAFLD was found, but after adjustment for factors such as BMI, glucose, lipid spectrum, blood pressure, and liver function, cholecystectomy was not independently associated with NAFLD (26). Several studies showed an association between gallstone disease and MS; however, they all used different confounder correction (27,28).
MS and NAFLD are highly prevalent in patients with gallstones or cholecystectomy; these conditions share common risk factors such as overweight, hypertension, high cholesterol, and DM and are all related to lifestyle (14,29). General practitioners and surgeons should be aware of these lifestyle-related conditions.
As our research counters previous reports on the association between cholecystectomy and NAFLD, prospective clinical data are needed. In such a longitudinal study, incidence of NAFLD and MS could be assessed in patients with gallstones before and after cholecystectomy. The alterations in the dynamics of bile salt recirculation could underly the metabolic anomalies in cholecystectomized patients and needs to be tested (8,30). The presence of cholesterol gallstones is, similar to NAFLD, a hepatic manifestation of MS (31,32). Because it is shown that cholecystectomy is a mediocre solution for the treatment of patients with abdominal pain and gallstones, we may need to rethink our practice in patients with symptoms all related to the same metabolic changes (33).
The pathophysiology of steatosis is multifactorial, in which insulin resistance is an important factor (5). The risk of DM type 2 is higher in patients with NAFLD than in those without NAFLD (Supplementary Digital Content 2, http://links.lww.com/CTG/A280) (34). Insulin resistance contributes to the development and progression of NAFLD, by increasing de novo lipogenesis, by decreasing insulin suppression of lipolysis, and subconsequently indirectly by increasing the free fatty acids flux to the liver (35–37). More recently, an increasing number of studies are performed to investigate the influence of insulin resistance on the development of cholesterol gallstones. A study in pregnant women concluded that insulin resistance is a risk factor for incident gallbladder sludge and stones, even after adjustment for BMI (38). Experimental studies describe that insulin resistance decreases cholesterol absorption in the intestine and increases cholesterol synthesis and production of very low-density lipoprotein (39,40). A recent study concludes that hepatic insulin resistance also decreases expression of enzymes for the bile acid synthesis and produces partial resistance to the Farnesoid-X receptor, resulting in a lithogenic bile salt profile (41). Finally, research shows that insulin resistance causes abnormal motility of the gallbladder (42).
We hypothesize that cholecystectomy changes the enterohepatic circulation. Continuous secretion of bile causes a higher exposure of bile acids to peripheral which could result in a different lipid and glucose metabolism (7,43,44), resulting in increased BMI, MS, and development of NAFLD. Because BMI, glucose, and lipids change after cholecystectomy, NAFLD is not associated with cholecystectomy after correction for metabolic factors. Furthermore, a decrease of FGF-19 after cholecystectomy resulting in altered lipid, glucose, and energy metabolism could also have the outcome of an increased BMI and development of NAFLD (8).
This study comes with strengths and limitations. Strengths of our study are the large number of subjects and adjustment for many available metabolic variables, and the follow-up between cholecystectomy and ultrasound was large in many subjects. Moreover, sensitivity analyses were performed to show consistency in data. The largest limitation of this study is the cross-sectional design; ideally, variables were available before and after cholecystectomy; however, long-term longitudinal data on BMI, diabetes, and liver status in cholecystectomy patients are lacking. Furthermore, based on the available data, we could not make a difference in participants with gallstones and without gallstones. Finally, MRI for the diagnosis of NAFLD was not available in the Rotterdam Study, mainly because of the associated costs in such a large study population (45). Invasive tests (as liver biopsy) were not performed because of the ethical concerns. Therefore, NAFLD was diagnosed by ultrasound, which has limitations. Mild, moderate, and severe steatosis could not reliably be separated, that is why we have dichotomized into no steatosis/steatosis, the risk is that we exclude mild steatosis, but the question is how clinically relevant this is if you have <30% lipid droplets. Because inter-reader variability is important in the interpretation of ultrasounds, all images were re-evaluated by a single hepatologist. It could well be that many patients with mild steatosis were not included in the NALFD group. Therefore, it may be that results are only representative for moderate–severe steatosis.
In conclusion, MS and NAFLD are common in western populations and are more prevalent in patients with a history of cholecystectomy. However, in contrast to previous studies in our trial, cholecystectomy does not seem independently associated with the presence of MS or moderate/severe NAFLD, in which other metabolic factors may play a more dominant role.
CONFLICTS OF INTEREST
Guarantor of the article: Philip R. de Reuver, MD, PhD.
Specific author contributions: C.S.S.L. and L.J.M.A. substantial contributed to the conception and design of the work, contributed to acquisition, analysis, and interpretation of data for the work, drafted the manuscript, final approved the version to be published, and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. S.D.M., J.P.H.D., C.J.H.M.v.L., P.R.d.R. interpreted the data for the work, revised the manuscript critically for important intellectual content, final approved the version to be published, and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Financial support: The Rotterdam Study is supported by the Erasmus MC University Medical Centre, Erasmus University Rotterdam, the Netherlands Organization for Scientific Research (NWO), the Netherlands Organization for Health Research and Development (ZonMw), the Research Institute for Diseases in the Elderly (RIDE), the Ministry of Education, Culture and Science, the Ministry of Health, Welfare and Sports, the European Commission (DG XII), and by the Municipality of Rotterdam.
Potential competing interests: None to report.
Informed consent: Written informed consent was obtained from all participants.
Study Highlights.
WHAT IS KNOWN
✓ Gallstones, MS, and NAFLD are associated diseases.
✓ Cholecystectomy is suggested to be a risk factor for NAFLD.
WHAT IS NEW HERE
✓ MS and NAFLD are more prevalent in patients with a history of cholecystectomy, compared with patients without a history of cholecystectomy.
✓ In contrast to previous studies, cholecystectomy is not independently associated with MS or NAFLD after correction for multiple metabolic factors.
TRANSLATIONAL IMPACT
✓ FLD and gallstone disease share common metabolic risk factors such as overweight, hypertension, high cholesterol, diabetes mellitus, and are all related to lifestyle. The relationship between cholecystectomy and NAFLD is complex and needs further investigation in order to fully inform patients about the long-term consequences of a cholecystectomy.
Supplementary Material
Footnotes
SUPPLEMENTARY MATERIAL accompanies this paper at http://links.lww.com/CTG/A279, http://links.lww.com/CTG/A280
References
- 1.Ruhl CE, Everhart JE. Relationship of non-alcoholic fatty liver disease with cholecystectomy in the US population. Am J Gastroenterol 2013;108(6):952–8. [DOI] [PubMed] [Google Scholar]
- 2.Amigo L, Husche C, Zanlungo S, et al. Cholecystectomy increases hepatic triglyceride content and very-low-density lipoproteins production in mice. Liver Int 2011;31(1):52–64. [DOI] [PubMed] [Google Scholar]
- 3.Estes C, Razavi H, Loomba R, et al. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology 2018;67(1):123–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buzzetti E, Pinzani M, Tsochatzis EA. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism 2016;65(8):1038–48. [DOI] [PubMed] [Google Scholar]
- 5.Friedman SL, Neuschwander-Tetri BA, Rinella M, et al. Mechanisms of NAFLD development and therapeutic strategies. Nat Med 2018;24(7):908–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Di Ciaula A, Garruti G, Wang DQ, et al. Cholecystectomy and risk of metabolic syndrome. Eur J Intern Med 2018;53:3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen Y, Wu S, Tian Y. Cholecystectomy as a risk factor of metabolic syndrome: From epidemiologic clues to biochemical mechanisms. Lab Invest 2018;98(1):7–14. [DOI] [PubMed] [Google Scholar]
- 8.Zweers SJ, Booij KA, Komuta M, et al. The human gallbladder secretes fibroblast growth factor 19 into bile: Towards defining the role of fibroblast growth factor 19 in the enterobiliary tract. Hepatology 2012;55(2):575–83. [DOI] [PubMed] [Google Scholar]
- 9.Thijs C, Knipschild P, van Engelshoven J. The prevalence of gallstone disease in a Dutch population. Scand J Gastroenterol 1990;25(2):155–60. [DOI] [PubMed] [Google Scholar]
- 10.van den Berg EH, Amini M, Schreuder TC, et al. Prevalence and determinants of non-alcoholic fatty liver disease in lifelines: A large Dutch population cohort. PLoS One 2017;12(2):e0171502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bos MB, de Vries JH, Wolffenbuttel BH, et al. The prevalence of the metabolic syndrome in the Netherlands: Increased risk of cardiovascular diseases and diabetes mellitus type 2 in one quarter of persons under 60 [in Dutch]. Ned Tijdschr Geneeskd 2007;151(43):2382–8. [PubMed] [Google Scholar]
- 12.Portincasa P, Moschetta A, Palasciano G. Cholesterol gallstone disease. Lancet 2006;368(9531):230–9. [DOI] [PubMed] [Google Scholar]
- 13.Bedogni G, Miglioli L, Masutti F, et al. Prevalence of and risk factors for nonalcoholic fatty liver disease: The dionysos nutrition and liver study. Hepatology 2005;42(1):44–52. [DOI] [PubMed] [Google Scholar]
- 14.Shaffer EA. Epidemiology and risk factors for gallstone disease: Has the paradigm changed in the 21st century? Curr Gastroenterol Rep 2005;7(2):132–40. [DOI] [PubMed] [Google Scholar]
- 15.Ikram MA, Brusselle GGO, Murad SD, et al. The Rotterdam Study: 2018 update on objectives, design and main results. Eur J Epidemiol 2017;32(9):807–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hamaguchi M, Kojima T, Itoh Y, et al. The severity of ultrasonographic findings in nonalcoholic fatty liver disease reflects the metabolic syndrome and visceral fat accumulation. Am J Gastroenterol 2007;102(12):2708–15. [DOI] [PubMed] [Google Scholar]
- 17.Alberti KG, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009;120(16):1640–5. [DOI] [PubMed] [Google Scholar]
- 18.Goldbohm RA, van den Brandt PA, Brants HA, et al. Validation of a dietary questionnaire used in a large-scale prospective cohort study on diet and cancer. Eur J Clin Nutr 1994;48(4):253–65. [PubMed] [Google Scholar]
- 19.Jaruvongvanich V, Sanguankeo A, Upala S. Significant Association between gallstone disease and nonalcoholic fatty liver disease: A systematic review and meta-analysis. Dig Dis Sci 2016;61(8):2389–96. [DOI] [PubMed] [Google Scholar]
- 20.Lu SN, Chang WY, Wang LY, et al. Risk factors for gallstones among Chinese in Taiwan. A community sonographic survey. J Clin Gastroenterol 1990;12(5):542–6. [DOI] [PubMed] [Google Scholar]
- 21.Chen JY, Hsu CT, Liu JH, et al. Clinical predictors of incident gallstone disease in a Chinese population in Taipei, Taiwan. BMC Gastroenterol 2014;14(1):83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu L, Aili A, Zhang C, et al. Prevalence of and risk factors for gallstones in Uighur and Han Chinese. World J Gastroenterol 2014;20(40):14942–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koller T, Kollerova J, Hlavaty T, et al. Cholelithiasis and markers of nonalcoholic fatty liver disease in patients with metabolic risk factors. Scand J Gastroenterol 2012;47(2):197–203. [DOI] [PubMed] [Google Scholar]
- 24.Nervi F, Miquel JF, Alvarez M, et al. Gallbladder disease is associated with insulin resistance in a high risk Hispanic population. J Hepatol 2006;45(2):299–305. [DOI] [PubMed] [Google Scholar]
- 25.Chen CH, Huang MH, Yang JC, et al. Prevalence and risk factors of gallstone disease in an adult population of Taiwan: An epidemiological survey. J Gastroenterol Hepatol 2006;21(11):1737–43. [DOI] [PubMed] [Google Scholar]
- 26.Wang HG, Wang LZ, Fu HJ, et al. Cholecystectomy does not significantly increase the risk of fatty liver disease. World J Gastroenterol 2015;21(12):3614–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shen C, Wu X, Xu C, et al. Association of cholecystectomy with metabolic syndrome in a Chinese population. PLoS One 2014;9(2):e88189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Su PY, Hsu YC, Cheng YF, et al. Strong association between metabolically-abnormal obesity and gallstone disease in adults under 50 years. BMC Gastroenterol 2019;19(1):117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mendez-Sanchez N, Bahena-Aponte J, Chavez-Tapia NC, et al. Strong association between gallstones and cardiovascular disease. Am J Gastroenterol 2005;100(4):827–30. [DOI] [PubMed] [Google Scholar]
- 30.Barrera F, Azocar L, Molina H, et al. Effect of cholecystectomy on bile acid synthesis and circulating levels of fibroblast growth factor 19. Ann Hepatol 2015;14(5):710–21. [PubMed] [Google Scholar]
- 31.Gastaldelli A. Fatty liver disease: The hepatic manifestation of metabolic syndrome. Hypertens Res 2010;33(6):546–7. [DOI] [PubMed] [Google Scholar]
- 32.Lizardi-Cervera J, Aguilar-Zapata D. Nonalcoholic fatty liver disease and its association with cardiovascular disease. Ann Hepatol 2009;8:S40–S43. [PubMed] [Google Scholar]
- 33.van Dijk AH, Wennmacker SZ, de Reuver PR, et al. Restrictive strategy versus usual care for cholecystectomy in patients with gallstones and abdominal pain (SECURE): A multicentre, randomised, parallel-arm, non-inferiority trial. Lancet 2019;393(10188):2322–30. [DOI] [PubMed] [Google Scholar]
- 34.Mantovani A, Byrne CD, Bonora E, et al. Nonalcoholic fatty liver disease and risk of incident type 2 diabetes: A meta-analysis. Diabetes Care 2018;41(2):372–82. [DOI] [PubMed] [Google Scholar]
- 35.Utzschneider KM, Kahn SE. The role of insulin resistance in nonalcoholic fatty liver disease. J Clin Endocrinol Metab 2006;91(12):4753–61. [DOI] [PubMed] [Google Scholar]
- 36.Kitade H, Chen G, Ni Y, et al. Nonalcoholic fatty liver disease and insulin resistance: New Insights and potential new treatments. Nutrients 2017;9(4):E387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khan RS, Bril F, Cusi K, et al. Modulation of insulin resistance in nonalcoholic fatty liver disease. Hepatology 2019;70(2):711–24. [DOI] [PubMed] [Google Scholar]
- 38.Ko CW, Beresford SA, Schulte SJ, et al. Insulin resistance and incident gallbladder disease in pregnancy. Clin Gastroenterol Hepatol 2008;6(1):76–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pihlajamaki J, Gylling H, Miettinen TA, et al. Insulin resistance is associated with increased cholesterol synthesis and decreased cholesterol absorption in normoglycemic men. J Lipid Res 2004;45(3):507–12. [DOI] [PubMed] [Google Scholar]
- 40.Adiels M, Olofsson SO, Taskinen MR, et al. Overproduction of very low-density lipoproteins is the hallmark of the dyslipidemia in the metabolic syndrome. Arterioscler Thromb Vasc Biol 2008;28(7):1225–36. [DOI] [PubMed] [Google Scholar]
- 41.Biddinger SB, Haas JT, Yu BB, et al. Hepatic insulin resistance directly promotes formation of cholesterol gallstones. Nat Med 2008;14(7):778–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nakeeb A, Comuzzie AG, Al-Azzawi H, et al. Insulin resistance causes human gallbladder dysmotility. J Gastrointest Surg 2006;10(7):940–8; discussion 948–9. [DOI] [PubMed] [Google Scholar]
- 43.Kullak-Ublick GA, Paumgartner G, Berr F. Long-term effects of cholecystectomy on bile acid metabolism. Hepatology 1995;21(1):41–5. [DOI] [PubMed] [Google Scholar]
- 44.Almond HR, Vlahcevic ZR, Bell CC, Jr, et al. Bile acid pools, kinetics and biliary lipid composition before and after cholecystectomy. N Engl J Med 1973;289(23):1213–6. [DOI] [PubMed] [Google Scholar]
- 45.Schwenzer NF, Springer F, Schraml C, et al. Non-invasive assessment and quantification of liver steatosis by ultrasound, computed tomography and magnetic resonance. J Hepatol 2009;51(3):433–45. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.