INTRODUCTION:
Colonoscopy is an imperfect gold standard for detecting colorectal neoplasms because some proportion of adenomas may be missed, mainly small lesions. This proportion is expected to be higher in case of inadequate bowel cleansing, which is frequently seen in routine practice. We estimated the proportions of neoplasms that are in principle detectable by colonoscopy but might be missed in case of incomplete bowel preparation.
METHODS:
For 8,193 participants of screening colonoscopy in South-Western Germany, recruited between 2005 and 2016, the prevalence and numbers of different findings were extracted from colonoscopy reports and compared according to the reported bowel preparation quality.
RESULTS:
Bowel preparation quality was reported as good, poor, or was unspecified in 30.3%, 11.1%, and 58.6% of colonoscopy records. Reported prevalences of nonadvanced adenomas (NAAs) were similar among participants with poor and unspecified bowel preparation quality but substantially lower than among participants with good bowel preparation (adjusted prevalence rate ratio [RR] 0.86, 95% confidence interval [CI]: 0.77–0.96). The differences were observed for proximal but not for distal NAAs (RRs 0.82, 95% CI: 0.71–0.95 and 0.95, 95% CI: 0.82–1.10).
DISCUSSION:
Our study suggests that a significant proportion of NAAs located in the proximal colon might be missed during colonoscopy if bowel cleansing is not adequate. Major efforts should be made to further facilitate and enhance high-quality bowel preparation in routine screening practice.
INTRODUCTION
Colonoscopy is considered as the diagnostic gold standard for early detection of colorectal cancer (CRC) and its precursors. Furthermore, colonoscopy has shown great potential to reduce CRC incidence by removal of these lesions (1). Therefore, colonoscopy is widely recommended and used in several countries for CRC screening and early detection (2). However, colonoscopy is not always a true gold standard in routine practice. Although it can be assumed to detect CRC quite reliably, studies have shown that non-negligible proportions of adenomas (∼9% of advanced adenomas [AAs] and 26% of all adenomas) may be missed during the colonoscopy (3–5). The risk of missing colorectal neoplasms is expected to be particularly high in case of inadequate bowel cleansing, a problem commonly encountered in routine practice.
Although several previous studies estimated proportions of findings missed during colonoscopy because of inadequate bowel preparation with repeated colonoscopy (6–15), those studies were typically conducted in highly specialized centers, included small numbers of participants and did not consider potentially relevant characteristics of findings such as flat or sessile shape. Furthermore, per-adenoma miss rates were rarely investigated (6,9,15), and average numbers of neoplasms per subject were not yet considered in any study. To our knowledge, only one previous study has addressed the potential implications of inadequate bowel cleansing in the German screening colonoscopy program (16) but that study focused on overall adenoma detection rates (ADRs) and did not consider adenoma characteristics such as location and shape and did not differentiate between advanced and non-AAs (NAAs).
We therefore evaluated the impact of inadequate bowel cleansing on ADRs in a large study among participants of screening colonoscopy in Germany. In particular, we hypothesized that ADRs (“apparent adenoma prevalences”) would be lower in subjects with inadequate bowel preparation.
METHODS
Study design and population
Screening colonoscopy has been offered as primary screening examination to women and men aged 55 years or older in Germany since 2002. Our analyses are based on data from a large ongoing study among screening colonoscopy participants (BLITZ study) that has been described in detail elsewhere (17–25). In brief, the participants were recruited in gastroenterology practices in South-Western Germany for the evaluation of diagnostic performance of novel noninvasive tests for CRC screening, using the results of colonoscopy as reference standard. Bowel preparations used in different practices partly changed over time and differed between practices. Most recruiting centers primarily reported the use of MoviPrep. Less frequently reported substances used include CitraFleet, Eziclen, and Plenvu. By the end of the study period, practices consistently reported advising split dosing. The study was approved by the Ethics Committees of the Medical Faculty Heidelberg (178/2005) and of the responsible state physicians' chambers (Baden-Wuerttemberg, M118-05-f; Rhineland-Palatinate, 837.047.06(5145); Saarland, 217/13; Hesse, MC 254/2007) and is registered in the German Clinical Trials Register (DRKS-ID: DRKS00008737). The present analysis focuses on study participants recruited from November 2005 to June 2016. Written informed consent was obtained from each participant.
Data collection and classification
Colonoscopy and histology records were obtained from the gastroenterology practices after completing screening colonoscopy. Adenoma shape was classified into pedunculated, flat, or sessile according to the endoscopists' assessment. Information on bowel preparation and findings at colonoscopy were extracted independently by 2 trained data extractors in a standardized manner. Based on the colonoscopy records, preparation quality was rated as “excellent, good, or adequate” (colonoscopy completed with the entire colon visualized adequately, in part, after further cleansing), “inadequate/poor” (solid stool residuals restricted the visibility during colonoscopy in part or completely), and “unspecified” (bowel preparation quality was not recorded). The most advanced findings during colonoscopy were used to assign participants to one of the following categories: CRC, AA, NAA, or no neoplasm. Adenomas were classified as AAs if they matched any of the following criteria: size ≥1 cm, villous or tubulo-villous architecture, or high-grade dysplasia. The findings were further stratified by location (proximal: cecum, ascending colon, right flexure, transverse colon, or left flexure; distal: descending colon, sigmoid colon, and rectum) and shape (pedunculated/“normal,” flat, or sessile).
Statistical analysis
The study population was described regarding age, sex, and most advanced finding at screening colonoscopy for each category of bowel preparation. Next, we determined the detection rates of CRC, advanced, nonadvanced, and any adenomas according to the quality of bowel cleansing, both for the entire colon and rectum, and specifically for the proximal colon and the distal colon and rectum.
We derived the relative detection rates of AAs, NAAs, and any adenomas adjusted for age, sex, year of colonoscopy, and gastroenterology practice, in addition to crude relative detection rates, using the log-binomial regression of relevant colonoscopic findings on bowel cleansing. From adjusted regression coefficients, the relative apparent prevalence of having relevant findings according to the bowel preparation quality was computed (26). In addition, average overall and site-specific numbers of adenomas (any, advanced, or NAAs; flat, sessile, or pedunculated) were compared according to the preparation quality. Crude and age- and sex-adjusted numbers of findings were compared using the Poisson regression models.
All statistical tests were 2-sided, and an α level of 0.05 was used to indicate statistical significance. All statistical analyses were conducted in R (27) version 3.5.3.
RESULTS
Study population
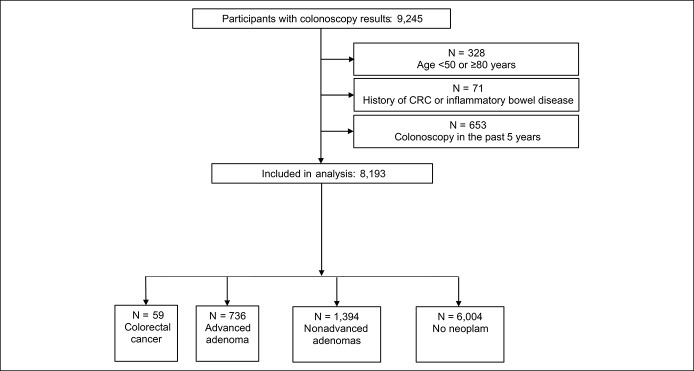
From 9,245 participants with available colonoscopy results, we excluded participants younger than 50 years of age or 80 and older (N = 328), with a history of previously diagnosed CRC or inflammatory bowel disease (N = 71) or colonoscopy in the past 5 years (N = 653), leaving 8,193 subjects for the analysis (Figure 1).
Figure 1.
Flow diagram of the study population. CRC, colorectal cancer.
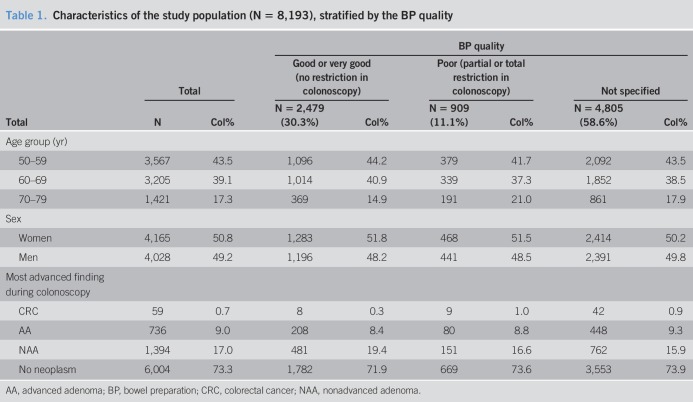
Almost equal numbers of men (49.2%) and women (50.8%) were included (Table 1). The mean age was 62.3 years. The most advanced findings at colonoscopy were CRC, AA, and NAA in 0.7%, 9.0%, and 17.0% of cases, respectively. ADRs (including CRC) met or exceeded the common performance target of ≥25% for male/female population, ≥30% for men, and ≥25% for women (28) in almost every year since the beginning of recruitment (see Supplementary Figure 1, Supplementary Digital Content 1, http://links.lww.com/CTG/A265). Gastroenterology practices used several different bowel preparation (BP) formulae. The most widely used product was MoviPrep. The quality of BP was reported as good in 2,479 participants (30.3%), poor in 909 subjects (11.1%), and not specified in 4,805 participants (58.6%). However, the proportion of colonoscopies where BP quality was not reported gradually decreased since 2011 and was 44.0% in 2016, the last included year of recruitment (see Supplementary Figure 2, Supplementary Digital Content 2, http://links.lww.com/CTG/A266).
Table 1.
Characteristics of the study population (N = 8,193), stratified by the BP quality
Apparent adenoma prevalence according to the BP quality
Apparent prevalences of CRC, AA, and NAA were very similar for participants with poor or unspecified BP. In these groups, apparent prevalences of NAA were substantially lower (16.6% and 15.9%, respectively) than in the group with good BP (19.4%, P = 0.0007).
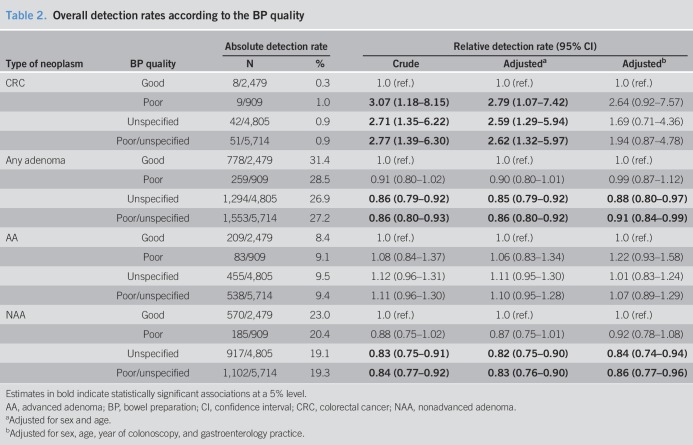
In Table 2, relative detection rates (RRs) of the various types of neoplasms are shown by BP quality. In addition to crude RRs, Table 2 shows RRs adjusted for age and sex only, and RRs adjusted for age, sex, year of colonoscopy, and gastroenterology practice. Crude and age- and sex-adjusted RRs were generally very similar. However, additional adjustment for gastroenterology practice attenuated many RRs, which is why we only refer to the most fully adjusted RRs in the following text. Using individuals with reported good bowel cleansing quality as reference, the RR of detecting any adenoma was 0.99 in participants with poor BP (95% confidence interval [CI]: 0.87–1.12). A statistically significant association was observed for unspecified BP quality (RR 0.88, 95% CI: 0.80–0.97). Reduced detection rates were seen for NAAs only (0.92 and 0.84, respectively), but not for AAs. A statistically significantly higher detection rate for CRC among participants with poor or unspecified BP compared with participants with good BP rendered statistically nonsignificant with adjustment for year of colonoscopy and gastroenterology practice.
Table 2.
Overall detection rates according to the BP quality
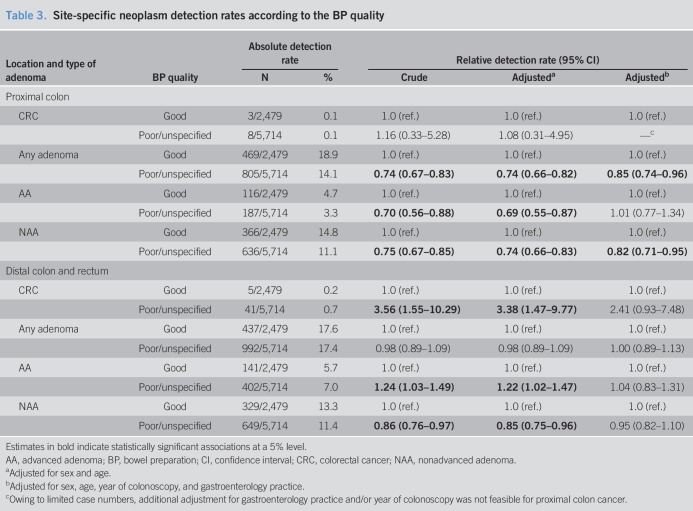
After observing that ADRs were very similar in the groups with poor BP and those whose BP was not reported, we combined them to one “poor/unspecified” category and focused on a comparison of this group to the group with good BP in the analyses of location-specific detection rates. When stratifying by location (Table 3), significantly reduced detection rates were exclusively observed for NAAs located in the proximal colon (and, as NAAs account for the largest share of adenomas, also for any adenomas located in the proximal colon). The fully adjusted RR (95% CI) for detection of NAAs and any adenomas in the proximal colon in case of poor or unspecified quality of BP was 0.82 (0.71–0.95) and 0.85 (0.74–0.96), respectively.
Table 3.
Site-specific neoplasm detection rates according to the BP quality
Apparent numbers of neoplasms
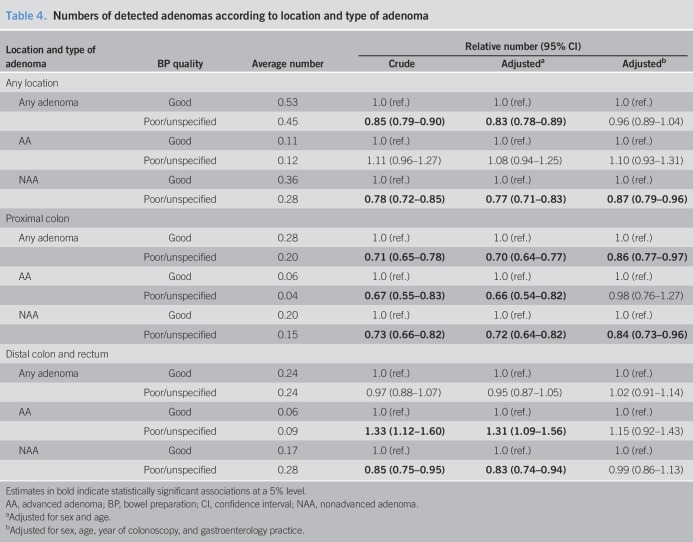
We compared the apparent average numbers of the various types of adenomas according to the BP quality (Table 4). Whereas the average overall numbers of detected AAs per participant were virtually identical irrespective of the BP quality (∼0.12), significantly fewer NAAs were detected in subjects with unreported or reported poor BP (0.28 vs 0.36 with good BP, adjusted rate ratio 0.87, 95% CI: 0.79–0.96). Again, the inverse association was found for NAAs of proximal location only (adjusted rate ratio 0.84, 95% CI: 0.73–0.96), whereas the numbers of distal NAAs were not inversely associated with good BP after adjustment for year of colonoscopy and gastroenterology practice (RR 0.99, 95% CI: 0.86–1.13).
Table 4.
Numbers of detected adenomas according to location and type of adenoma
Adenoma shape, BP, and colonoscopy findings
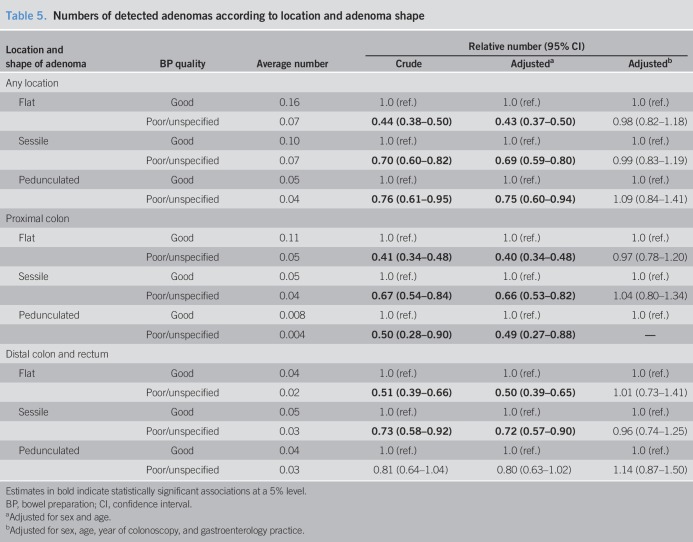
Table 5 shows the numbers of detected adenomas according to the location and adenoma shape. Flat adenomas were the most frequently detected adenomas, followed by sessile and pedunculated adenomas. The latter were very rarely detected in the proximal colon. Adenomas of all shapes were less frequently reported among participants with poor or unspecified BP compared with participants with good BP, but apparent associations entirely disappeared when adjusting for the year of colonoscopy and gastroenterology practice in addition to age and sex.
Table 5.
Numbers of detected adenomas according to location and adenoma shape
DISCUSSION
Colonoscopy is regarded as gold standard in the detection of colorectal neoplasms, and colonoscopy with polypectomy is suggested to reduce the risk of CRC considerably for many years (29). However, interval carcinomas do occur, most likely emerging from the small but significant proportion of adenomas missed during the procedure (4,5,30). This proportion is expected to be higher with poor BP than with good quality of preparation. Thus, in this study, we used the data from >8,000 participants of screening colonoscopy in South-Western Germany to estimate the proportion of findings missed because of restricted BP. We investigated the actual detection rates (apparent prevalences) for each finding and compared them between participants with adequate and those with poor or unspecified bowel cleansing. In addition, we compared the actual numbers of findings between participants with reported or adequate bowel cleansing and participants with inadequate or unreported bowel cleansing.
In this investigation from routine screening practice in 2005–2016, the BP quality was not reported in more than half of all participants. Although the share of colonoscopies lacking judgment of BP quality gradually decreased over the years, these results underline the need to enhance reporting standards in routine screening practice. Because ADRs among participants whose BP quality was not reported were similar to those among participants with reported inadequate BP, we combined the results for both groups. Whereas no differences in AA detection were observed, NAAs were detected at lower rates in individuals with poor/unspecified BP quality than in participants with good BP quality. However, this pattern was restricted to NAAs located in the proximal colon only. Our results are largely consistent with previous mostly much smaller studies, suggesting that BP is either equally or more relevant for adenoma miss rates in the proximal colon compared with miss rates in the distal colon and rectum (14). Our findings are also in line with a previous study suggesting that colonoscopy is associated with a stronger risk reduction for distal colon and rectal cancer vs proximal colon cancer (31). To our knowledge, our study is the first to report a higher detection rate for CRCs in the distal colon and rectum when the quality of BP was not reported or inadequate. Previous studies all focused on adenoma detection. Apart from a chance finding due to the small numbers of CRCs, potential explanations include reverse causality (impaired BP as a consequence of presence of distal CRC) or shared risk factors of poor BP and distal CRC. However, the association lost statistical significance after adjustment for the year of colonoscopy and gastroenterology practice, no previous studies suggested such an association, and such tumors are expected to be large and thus unlikely to remain asymptomatic.
Comparison with other studies
Several previous studies investigated the association between BP quality and ADRs during colonoscopy (6–15). A meta-analysis from 2014 (10) found significantly higher detection rates for both, any adenomas and AAs, for high-quality vs low-quality BP, but not for high- vs intermediate-quality BP. None of those studies investigated the actual numbers of detected adenomas or considered the adenoma location.
A previous study from Germany (16) among ∼12,000 participants of screening colonoscopy found that ADRs did not vary with case volume, withdrawal time, or endoscope generation. Excellent, sufficient, and even moderate BP quality all resulted in virtually identical ADRs. Only for poor and insufficient BP (“residual fecal material that cannot be [completely] cleared”), ADRs diminished considerably. No further outcomes were investigated in addition to overall ADRs. One Korean tandem colonoscopy study (7) among 277 screening participants with adenomas and polyps detected at the index colonoscopy concluded that even for AAs, miss rates were significantly higher with poor or inadequate BP (37%) than with fair (18%), good (17%), or excellent (9%) BP quality. However, those high miss rates are contrasting to the previously reported very low miss rates of colonoscopy for AAs (4,5,30). A study among 438 male veterans (13) concluded that poor BP was associated with increased miss rates for virtually all investigated outcomes (adenomas and polyps > or ≤5 mm, AAs, and sessile-serrated polyps). Even for AAs, the miss rates were estimated to reach up to 9% in the group with the lowest reported quality of BP. Case numbers in each group were very small, though (median: N = 43). To our knowledge, only one study (32) conducted in a mostly (>70%) symptomatic Chinese population investigated the miss rates of flat adenomas (N = 796 among 16,951 patients) related to poor BP. Poor (vs good) BP was associated with a ∼3.5-fold increased odds for missing a flat adenoma (odds ratio for adenoma detection vs adequate BP: 0.29). In our study, the pattern of seemingly low detection rates of flat adenomas essentially disappeared after adjustment for practice and calendar year, suggesting that practices with less often reported BP also less often specifically reported detection of flat adenomas. A meta-analysis (14) found a dose-response-like pattern of lower lesion detection rates with good, fair, poor, or insufficient BP vs excellent BP, although fair, good, and excellent BP performed very similarly. One large screening study (12) (N = 9,245), by contrast, paradoxically found higher miss rates in the group with excellent vs good BP. Overconfidence for polyp detection in a perfectly cleaned bowel was suggested as a possible explanation.
Our study has several strengths. To our knowledge, it is one of the largest studies of its kind, including >8,000 participants of screening colonoscopy. Studies conducted in clinical settings might lack comparability to screening settings: In clinical settings, gastroenterologists might investigate the bowel more carefully if a patient was referred, e.g., because of abdominal pain or visible blood in stool compared with screenees where normal findings are expected in most subjects, potentially limiting the impact of suboptimal BP. This hypothesis is supported by a previous study (13) which found that withdrawal times were longer among the participating endoscopists from a university faculty compared with standard clinical practice. We examined different clinically relevant outcomes (advanced, nonadvanced, and any adenomas, stratified by location), and this is the first study to investigate the joint association between adenoma location and shape with apparent findings according to the quality of BP. Differences in the overall apparent prevalence (any vs no finding) and apparent number of findings were investigated in crude and multivariate-adjusted analyses using log-binomial and Poisson regression models. Adjusted results suggest that some associations, particularly those with CRC and flat adenomas, were explained by the differences in reporting quality between gastroenterology practices and point to the need for standardized reporting of BP quality.
The main limitation of our study is that no second “enhanced” gold standard was available for subjects with inadequate bowel cleansing (such as a second colonoscopy in a short time period with more adequate BP), and the analyses were performed retrospectively regarding the endpoints considered. Another potential limitation is heterogeneity among examined colonoscopists, equipment, and BP formulae. However, this heterogeneity reflects the screening reality in Germany and evidence on the potential benefit of high-definition colonoscopy is conflicting (33–35). BP quality was assessed by gastroenterologists and categorized as “poor, very poor, or no information available,” and “good or very good,” rather than the more commonly used BBPS (36,37). However, a previous meta-analysis (10) found virtually no difference in ADRs between intermediate and high-quality preparation or between excellent and good preparation and concluded that distinguishing between the latter 2 groups might be unnecessary. Similar findings were made by Hong et al. (7) where miss rates were very similar among individuals with excellent, good, and fair BP but worse with poor/inadequate BP. This is also in accordance with the findings of Clark et al. (13) where miss rates among men with BBPS segment score of 2 was virtually identical to those among men with perfect preparation (BBPS of 3), at least for adenomas and polyps ≥5 mm and for AAs.
Information on BP quality was not explicitly reported in approximately 50% of the study population. Although this proportion gradually decreased over time to 44% of examinations conducted in 2016, these results underline the need to further improve reporting standards in routine screening practice. For the vast majority of practices, information on preparation quality was lacking. In addition, also information on the preparation used, and whether or not split dosing was used was lacking for most practices. This information would be particularly useful to investigate if the observed associations with NAA detection persist with split dosing. A further limitation is that serrated lesions have unfortunately not been consistently recorded, especially in the early years of recruitment, which is why we did not perform the analyses for an association between BP and detection of serrated lesions. Finally, we could not quantify the impact of colonoscopists' efforts to improve vision despite initially poor bowel cleansing, which would be expected to have substantially reduced otherwise even much stronger impact of poor bowel cleansing.
In summary, this study implies that the quality of BP is frequently not recorded in routine screening colonoscopy practice and that poor or unreported BP goes along with lower detection rates of NAAs in the proximal colon, which may contribute to the lower effectiveness of screening colonoscopy in preventing CRC in the proximal colon. Future studies should examine the potential advantage of rating BP quality for each colonic segment, given the suggested much stronger association between BP and findings in the proximal colon compared with distal findings. Most importantly, however, major efforts should be made to further facilitate and enhance high-quality BP in routine screening practice.
CONFLICTS OF INTEREST
Guarantor of the article: Tobias Niedermaier, PhD, MPH.
Specific author contributions: H.B. designed the study. T.N. conducted the statistical analyses and drafted the manuscript. All authors contributed important intellectual content and critically revised and finally approved the manuscript.
Financial support: The BLITZ study was funded in part by a grant from the German Research Council (Deutsche Forschungsgemeinschaft, grant no. BR1704/16-1). The analyses for this study were funded in part by a grant from the German Federal Ministry of Education and Research (grant no. 01GL1712).
Potential competing interests: None to report.
Study Highlights.
WHAT IS KNOWN
✓ Colonoscopy may miss a relevant proportion of colorectal neoplasms, and this proportion is expected to be higher with inadequate BP.
✓ Studies conducted in clinical settings suggest relevant adenoma miss rates associated with inadequate bowel cleansing, but evidence from studies conducted in screening settings is still very sparse.
WHAT IS NEW HERE
✓ A significant proportion of NAAs located in the proximal colon might be missed during colonoscopy if bowel cleansing is not adequate.
✓ Quality of BP is frequently not recorded in routine screening colonoscopy practice.
TRANSLATIONAL IMPACT
✓ Future studies should examine the potential advantage of rating BP quality for each colonic segment.
✓ Major efforts should be made to further facilitate and enhance high-quality BP in routine screening practice.
Supplementary Material
Footnotes
SUPPLEMENTARY MATERIAL accompanies this paper at http://links.lww.com/CTG/A265 and http://links.lww.com/CTG/A266
REFERENCES
- 1.Brenner H, Stock C, Hoffmeister M. Effect of screening sigmoidoscopy and screening colonoscopy on colorectal cancer incidence and mortality: Systematic review and meta-analysis of randomised controlled trials and observational studies. BMJ 2014;348:g2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schreuders EH, Ruco A, Rabeneck L, et al. Colorectal cancer screening: A global overview of existing programmes. Gut 2015;64:1637–49. [DOI] [PubMed] [Google Scholar]
- 3.Zhao S, Wang S, Pan P, et al. Magnitude, risk factors, and factors associated with adenoma miss rate of tandem colonoscopy: A systematic review and meta-analysis. Gastroenterology 2019;156:1661–74.e11. [DOI] [PubMed] [Google Scholar]
- 4.Ahn SB, Han DS, Bae JH, et al. The miss rate for colorectal adenoma determined by quality-adjusted, back-to-back colonoscopies. Gut Liver 2012;6:64–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim NH, Jung YS, Jeong WS, et al. Miss rate of colorectal neoplastic polyps and risk factors for missed polyps in consecutive colonoscopies. Intest Res 2017;15:411–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chokshi RV, Hovis CE, Hollander T, et al. Prevalence of missed adenomas in patients with inadequate bowel preparation on screening colonoscopy. Gastrointest Endosc 2012;75:1197–203. [DOI] [PubMed] [Google Scholar]
- 7.Hong SN, Sung IK, Kim JH, et al. The effect of the bowel preparation status on the risk of missing polyp and adenoma during screening colonoscopy: A tandem colonoscopic study. Clin Endosc 2012;45:404–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Menees SB, Kim HM, Elliott EE, et al. The impact of fair colonoscopy preparation on colonoscopy use and adenoma miss rates in patients undergoing outpatient colonoscopy. Gastrointest Endosc 2013;78:510–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xiang L, Zhan Q, Zhao XH, et al. Risk factors associated with missed colorectal flat adenoma: A multicenter retrospective tandem colonoscopy study. World J Gastroenterol 2014;20:10927–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clark BT, Rustagi T, Laine L. What level of bowel prep quality requires early repeat colonoscopy: Systematic review and meta-analysis of the impact of preparation quality on adenoma detection rate. Am J Gastroenterol 2014;109:1714–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oh CH, Lee CK, Kim JW, et al. Suboptimal bowel preparation significantly impairs colonoscopic detection of non-polypoid colorectal neoplasms. Dig Dis Sci 2015;60:2294–303. [DOI] [PubMed] [Google Scholar]
- 12.Calderwood AH, Thompson KD, Schroy PC, III, et al. Good is better than excellent: Bowel preparation quality and adenoma detection rates. Gastrointest Endosc 2015;81:691–9.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clark BT, Protiva P, Nagar A, et al. Quantification of adequate bowel preparation for screening or surveillance colonoscopy in men. Gastroenterology 2016;150:396–5; quiz e14–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sulz MC, Kroger A, Prakash M, et al. Meta-analysis of the effect of bowel preparation on adenoma detection: Early adenomas affected stronger than advanced adenomas. PLoS One 2016;11:e0154149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang JY, Moon CM, Lee HJ, et al. Predictive factors for missed adenoma on repeat colonoscopy in patients with suboptimal bowel preparation on initial colonoscopy: A KASID multicenter study. PLoS One 2018;13:e0195709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adler A, Wegscheider K, Lieberman D, et al. Factors determining the quality of screening colonoscopy: A prospective study on adenoma detection rates, from 12,134 examinations (Berlin colonoscopy project 3, BECOP-3). Gut 2013;62:236–41. [DOI] [PubMed] [Google Scholar]
- 17.Hundt S, Haug U, Brenner H. Comparative evaluation of immunochemical fecal occult blood tests for colorectal adenoma detection. Ann Intern Med 2009;150:162–9. [DOI] [PubMed] [Google Scholar]
- 18.Brenner H, Tao S, Haug U. Low-dose aspirin use and performance of immunochemical fecal occult blood tests. JAMA 2010;304:2513–20. [DOI] [PubMed] [Google Scholar]
- 19.Brenner H, Tao S. Superior diagnostic performance of faecal immunochemical tests for haemoglobin in a head-to-head comparison with guaiac based faecal occult blood test among 2235 participants of screening colonoscopy. Eur J Cancer 2013;49:3049–54. [DOI] [PubMed] [Google Scholar]
- 20.Chen H, Werner S, Brenner H. Fresh vs frozen samples and ambient temperature have little effect on detection of colorectal cancer or adenomas by a fecal immunochemical test in a colorectal cancer screening cohort in Germany. Clin Gastroenterol Hepatol 2016;15:1547–56.e5. [DOI] [PubMed] [Google Scholar]
- 21.Werner S, Krause F, Rolny V, et al. Evaluation of a 5-marker blood test for colorectal cancer early detection in a colorectal cancer screening setting. Clin Cancer Res 2016;22:1725–33. [DOI] [PubMed] [Google Scholar]
- 22.Niedermaier T, Weigl K, Hoffmeister M, et al. Diagnostic performance of one-off flexible sigmoidoscopy with fecal immunochemical testing in a large screening population. Epidemiology 2018;29:397–406. [DOI] [PubMed] [Google Scholar]
- 23.Niedermaier T, Weigl K, Gies A, et al. Accuracy of a fecal immunochemical test according to outside temperature and travel time. Clin Epidemiol 2018;10:1203–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gies A, Cuk K, Schrotz-King P, et al. Direct comparison of diagnostic performance of 9 quantitative fecal immunochemical tests for colorectal cancer screening. Gastroenterology 2018;154:93–104. [DOI] [PubMed] [Google Scholar]
- 25.Weigl K, Thomsen H, Balavarca Y, et al. Genetic risk score is associated with prevalence of advanced neoplasms in a colorectal cancer screening population. Gastroenterology 2018;155:88–98.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spiegelman D, Hertzmark E. Easy SAS calculations for risk or prevalence ratios and differences. Am J Epidemiol 2005;162:199–200. [DOI] [PubMed] [Google Scholar]
- 27.R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria, 2016. [Google Scholar]
- 28.Rex DK, Schoenfeld PS, Cohen J, et al. Quality indicators for colonoscopy. Gastrointest Endosc 2015;81:31–53. [DOI] [PubMed] [Google Scholar]
- 29.Brenner H, Kretschmann J, Stock C, et al. Expected long-term impact of screening endoscopy on colorectal cancer incidence: A modelling study. Oncotarget 2016;7:48168–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Rijn JC, Reitsma JB, Stoker J, et al. Polyp miss rate determined by tandem colonoscopy: A systematic review. Am J Gastroenterol 2006;101:343–50. [DOI] [PubMed] [Google Scholar]
- 31.Shergill AK, Conners EE, McQuaid KR, et al. Protective association of colonoscopy against proximal and distal colon cancer and patterns in interval cancer. Gastrointest Endosc 2015;82:529–37.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xiang L, Zhan Q, Wang XF, et al. Risk factors associated with the detection and missed diagnosis of colorectal flat adenoma: A Chinese multicenter observational study. Scand J Gastroenterol 2018;53:1519–25. [DOI] [PubMed] [Google Scholar]
- 33.Jrebi NY, Hefty M, Jalouta T, et al. High-definition colonoscopy increases adenoma detection rate. Surg Endosc 2017;31:78–84. [DOI] [PubMed] [Google Scholar]
- 34.Roelandt P, Demedts I, Willekens H, et al. Impact of endoscopy system, high definition, and virtual chromoendoscopy in daily routine colonoscopy: A randomized trial. Endoscopy 2019;51:237–43. [DOI] [PubMed] [Google Scholar]
- 35.Richardson J, Thaventhiran A, Mackenzie H, et al. The use of high definition colonoscopy versus standard definition: Does it affect polyp detection rate? Surg Endosc 2018;32:2676–82. [DOI] [PubMed] [Google Scholar]
- 36.Lai EJ, Calderwood AH, Doros G, et al. The Boston bowel preparation scale: A valid and reliable instrument for colonoscopy-oriented research. Gastrointest Endosc 2009;69:620–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kluge MA, Williams JL, Wu CK, et al. Inadequate Boston Bowel Preparation Scale scores predict the risk of missed neoplasia on the next colonoscopy. Gastrointest Endosc 2018;87:744–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.