Abstract
Aims
To characterize the relationship between blood pressure (BP) or heart rate and mortality and morbidity in chronic obstructive pulmonary disease (COPD).
Methods and results
We performedpost hoc analysis of baseline BP or heart rate and all-cause mortality and cardiovascular events in the SUMMIT trial. SUMMIT was a randomized double-blind outcome trial of 16 485 participants (65 ± 8 years, 75% male, and 47% active smokers) enrolled at 1368 sites in 43 countries. Participants with moderate COPD with or at risk for cardiovascular disease (CVD) were randomized to placebo, long-acting beta agonist, inhaled corticosteroid, or their combination. All-cause mortality increased in relation to high systolic [≥140 mmHg; hazard ratio (HR) 1.27, 95% confidence interval (CI) 1.12–1.45] or diastolic (≥90 mmHg; HR 1.35, 95% CI 1.14–1.59) BP and low systolic (<120 mmHg; HR 1.36, 95% CI 1.13–1.63) or diastolic (<80 mmHg; HR 1.15, 95% CI 1.00–1.32) BP. Higher heart rates (≥80 per minute; HR 1.39, 95% CI 1.21–1.60) and pulse pressures (≥80 mmHg; HR 1.39, 95% CI 1.07–1.80) were more linearly related to increases in all-cause mortality. The risks of cardiovascular events followed similar patterns to all-cause mortality. Similar findings were observed in subgroups of patients without established CVD.
Conclusion
A ‘U-shaped’ relationship between BP and all-cause mortality and cardiovascular events exists in patients with COPD and heightened cardiovascular risk. A linear relationship exists between heart rate and all-cause mortality and cardiovascular events in this population. These findings extend the prognostic importance of BP to this growing group of patients and raise concerns that both high and low BP may pose health risks.
Keywords: Blood pressure, Heart rate, Pulse, COPD, Mortality
Introduction
High blood pressure (BP) is the leading risk factor for global deaths and disability.1 Measurement of this simple, yet modifiable, biomarker has proven to be invaluable in the global battle against cardiovascular disease (CV).2 Mounting evidence further supports that an elevated heart rate is an additional easily obtained haemodynamic metric independently predictive of all-cause as well as cardiovascular mortality.3
Patients with chronic obstructive pulmonary disease (COPD) die more frequently from cardiovascular than respiratory disease.4–6 Over 6% of the US population has been told by a health professional that they have COPD,7 and this prevalence appears to be similar in the 86 million patients with hypertension.8 Thus, it is likely that over 5 million patients have co-morbid COPD and hypertension. This is important because a wealth of evidence accrued over the past half-century demonstrates that BP in individuals without vascular disease is monotonically (linear-log) associated in a linear-log fashion with increased cardiovascular events in the general population.9,10 However, this relationship is more complex and perhaps non-linear among patients with established heart disease.11–13 We therefore wanted to understand the prognostic value of BP specifically among individuals with COPD at heightened cardiovascular risk because this remains largely undescribed.
The Study to Understand Mortality and MorbidITy (SUMMIT) was a multi-centre prospective, double-blind, randomized trial in patients with moderate COPD with or at risk for CVD that compared placebo with an inhaled corticosteroid (ICS)/long-acting beta agonist (LABA) combination, with inclusion of the individual components (Trial Registration: ClinicalTrials.gov, number NCT01313676).14,15 The primary (all-cause mortality) and a secondary efficacy (composite cardiovascular events) outcome did not differ between treatment arms.16 Nevertheless, this large trial with well-adjudicated outcomes provides a unique opportunity to explore in detail the nature of the relationships between BP and heart rate with all-cause mortality and cardiovascular events in a contemporary population of high-risk individuals with COPD.
Methods
SUMMIT included 16 485 participants enrolled across 1368 centres in 43 counties with an average follow-up period for on-treatment cardiovascular outcomes of 1.7 years and on and post-treatment mortality of 1.9 years. The protocol, CONSORT diagram, and trial outcomes have been described in detail previously.14,15 In brief, eligible participants included current or former smokers (≥10-pack-years) between the ages of 40 and 80 years, with a history of COPD and a post-bronchodilator forced expiratory volume in one second (FEV1) ≥50 and ≤70% of the predicted value, a ratio of post-bronchodilator FEV1 to forced vital capacity (FVC) ≤0.70, and a score ≥2 on the modified Medical Research Council dyspnoea scale. Patients were required to be at increased cardiovascular risk (defined as being ≥60 years plus receiving medications for ≥2 of the following: hypercholesterolaemia, hypertension, diabetes mellitus, or peripheral vascular disease) or have established disease (coronary artery disease, peripheral arterial disease, prior stroke or myocardial infarction (MI), or diabetes mellitus with target organ disease). The on-treatment composite secondary cardiovascular outcome included cardiovascular death, MI, stroke, unstable angina, and transient ischaemic attack (TIA).
Blood pressure and heart rate measurement
Study sites were instructed to perform vital signs prior to spirometry at all study visits and to measure systolic and diastolic BP as well as heart rate in the seated position after 5 min of rest. The BP and heart rate values obtained at the baseline visit (study visit #2) after the screening visit were used for thesepost hoc analyses. The sphygmomanometer equipment and study staff that measured BP were consistent with the standard clinical practices of the investigators at each location.
Statistical methods
We evaluated the relationships between BP values and heart rate with health endpoints in two manners. First, we graphically explored the relationships. The rates of all-cause mortality per 100 subject years were calculated as (100 × number of deaths)/total on- and post-treatment follow-up. The rates of on-treatment cardiovascular composite events were calculated as (100 × number of events)/total on-treatment follow-up. Both rates were calculated for 5 mmHg categories of systolic and diastolic BP and for each 5 b.p.m. category of heart rate. These results and the 95% confidence intervals (CIs)17 were calculated across the range of baseline BP and heart rate values.
Second, we used clinically relevant thresholds for BP and germane cut-points for heart rate and pulse pressure to determine the health risks linked to high and low categorical values. We calculated the hazard ratios (HR) and 95% CIs using Cox regression adjusted for the covariates of randomized treatment, age, sex, body mass index (BMI), smoking habit, and beta-blocker use. We evaluated the effect in the Cox model results of both including and excluding beta-blocker use as covariates. There was marginal impact on the results; however, we retained beta-blocker use as a variable given its clinical importance. The HRs were calculated for the time to death (or first cardiovascular endpoint) compared across three ranges of BP and heart rate values. These ranges were: systolic (<120, or ≥120 to <140, or ≥140 mmHg), diastolic BP (<80, or ≥80 to <90, or ≥90 mmHg), and pulse pressure (<50, or ≥50 to <80, or ≥80 mmHg). These cut points were selected based upon clinical criteria (>140/90 mmHg being hypertension according to most guidelines and 120/80 mmHg being optimal BP per the ESC 2016 prevention guidelines and ESH 2013 hypertension guidelines.18,19 Similar models were calculated for heart rate ranges (<70, ≥70 to <80, ≥ 80 b.p.m.).
Results
Demographic and haemodynamic characteristics
The average age of study participants was 65 ± 8 years, 75% were male, and 47% remained active smokers. The mean BMI was 28 ± 6 kg/m2. Most individuals were white (81%), while 17%, and 2% were Asian or another race, respectively. By our study definition, 71% of participants had CVD. Excluding diabetes plus target organ disease from this definition, 66% of patients had ‘overt’ disease (e.g. prior MI). As previously reported, the population of patients were well-treated with contemporary medications with more than half on statins (65%) and anti-platelet (52%) therapies. In the SUMMIT intention-to-treat analysis (n = 16 485), there were 14 851 (90%) participants who had ever been diagnosed with hypertension. There were 5944 (36%) participants with systolic BP ≥140 mmHg and 3042 (18%) participants with diastolic BP ≥90 mmHg. Other characteristics of the SUMMIT study cohort (such as presence of or complications from diabetes mellitus) have been presented in the main paper.14
While there was a large overall range, the average baseline BP among participants was within the controlled category (<140/90 mmHg;Table1). The mean heart rate was 76 ± 10 b.p.m. Given the age and co-morbidities of the participants, nearly 90% were receiving some type of antihypertensive medication with the most common being a renin–angiotensin system inhibitor or antagonist (Table2).
Table 1.
Participant haemodynamic measures
| n | Mean (SD) | Minimum | Maximum | |
|---|---|---|---|---|
| Systolic BP (mmHg) | 16 482 | 133 (15) | 70 | 220 |
| Diastolic BP (mmHg) | 16 482 | 79 (9) | 40 | 140 |
| Pulse pressure (mmHg) | 16 482 | 54 (12) | 13 | 120 |
| Heart rate (b.p.m.) | 16 481 | 76 (10) | 40 | 147 |
Table 2.
Antihypertensive medications at study entry
| Number of participants | Percentage | |
|---|---|---|
| Number of medications | ||
| None | 1740 | 11 |
| 1 | 5761 | 35 |
| 2 | 4777 | 29 |
| ≥3 | 4207 | 26 |
| Medication class | ||
| Beta-blockers | 5159 | 31 |
| Calcium channel blockers | 5690 | 35 |
| Diuretics | 5449 | 33 |
| ACEi or ARB | 10 981 | 67 |
| Other | 763 | 5 |
ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker.
Baseline haemodynamic parameters, all-cause mortality, and cardiovascular risk
Table3 shows the risks for all-cause mortality and cardiovascular composite events in association with BP and heart rate levels measured at baseline among SUMMIT participants. Compared with the middle range, the risks of an event were higher in those with systolic and diastolic BP levels in either the higher or lower ranges (Supplementary material online,Figures S1–S4). The HRs for mortality for systolic BP were: high range vs. middle range HR = 1.27, low range vs. middle range HR = 1.36. The HRs for mortality for diastolic BP were: high range vs. middle range HR = 1.35, low range vs. middle range HR = 1.15 (Table3). Conversely, there appeared to be an increasing risk of death with increasing heart rate only (Supplementary material online,Figure S5) (high range vs. middle range HR = 1.39, low range vs. middle range HR = 0.83,Table3), with a similar trend for cardiovascular events (Supplementary material online,Figure S6). Like heart rate, only high (but not low) pulse pressure was associated with increased risk of an event (Supplementary material online, Figures S7 andS8) (for mortality high range vs. middle range HR = 1.39,Table3). In models including additional covariates (e.g. other antihypertensive medications), there were no consistent significant changes to the HRs.
Table 3.
Study events by baseline blood pressures and heart rates
| (A) Systolic blood pressure | <120 mmHg (n = 2041) | ≥120 mmHg to <140 mmHg (n = 8497) | ≥140 mmHg (n = 5944) |
|---|---|---|---|
| All-cause mortality | |||
| Number of deaths | 155 (8%) | 463 (5%) | 419 (7%) |
| Hazard ratio (vs. ≥120 mmHg to <140 mmHg) | 1.36 | REF | 1.27 |
| 95% CI | (1.13, 1.63) | (1.12, 1.45) | |
| CVD event | |||
| Number with at least one event | 90 (4%) | 322 (4%) | 276 (5%) |
| Hazard ratio (vs. ≥120 mmHg to <140 mmHg) | 1.2 | REF | 1.18 |
| 95% CI | (0.95, 1.52) | (1.01, 1.39) | |
| (B) Diastolic blood pressure | <80 mmHg (n = 6411) | ≥80 mmHg to <90 mmHg (n = 7029) | ≥90 mmHg (n = 3042) |
| All-cause mortality | |||
| Number of deaths | 437 (7%) | 386 (5%) | 214 (7%) |
| Hazard ratio (vs. ≥80 mmHg to <90 mmHg) | 1.15 | REF | 1.35 |
| 95% CI | (1.00, 1.32) | (1.14, 1.59) | |
| CVD event | |||
| Number with at least one event | 291 (5%) | 238 (3%) | 159 (5%) |
| Hazard ratio (vs. ≥80 mmHg to <90 mmHg) | 1.34 | REF | 1.58 |
| 95% CI | (1.13, 1.59) | (1.29, 1.93) | |
| (C) Pulse pressure | <50 mmHg (n = 4993) | ≥50 mmHg to <80 mmHg (n = 10 847) | ≥80 mmHg (n = 642) |
| All-cause mortality | |||
| Number of deaths | 292 (6%) | 681 (6%) | 64 (10%) |
| Hazard ratio (vs. ≥50 mmHg to <80 mmHg) | 0.98 | REF | 1.39 |
| 95% CI | (0.85, 1.12) | (1.07, 1.80) | |
| CVD event | |||
| Number with at least one event | 197 (4%) | 455 (4%) | 36 (6%) |
| Hazard ratio (vs. ≥50 mmHg to <80 mmHg) | 1 | REF | 1.25 |
| 95% CI | (0.84, 1.18) | (0.89, 1.76) | |
| (D) Heart rate | <70 b.p.m. (n = 3896) | ≥70 b.p.m. to <80 b.p.m. (n = 6658) | ≥80 b.p.m. (n = 5927) |
| All-cause mortality | |||
| Number of deaths | 199 (5%) | 377 (6%) | 461 (8%) |
| Hazard ratio (vs. ≥70 to <80 b.p.m.) | 0.83 | REF | 1.39 |
| 95% CI | (0.70, 0.99) | (1.21, 1.60) | |
| CVD event | |||
| Number with at least one event | 154 (4%) | 262 (4%) | 272 (5%) |
| Hazard ratio (vs. ≥70 to <80 b.p.m.) | 0.93 | REF | 1.22 |
| 95% CI | (0.76, 1.13) | (1.02, 1.44) | |
The hazard ratio represents the risks of time to death or time to first CVD event compared to the middle ranges of values for each parameter.
CI, confidence interval; CVD, cardiovascular disease (secondary composite outcome).
Relationships between haemodynamic variables and health endpoints
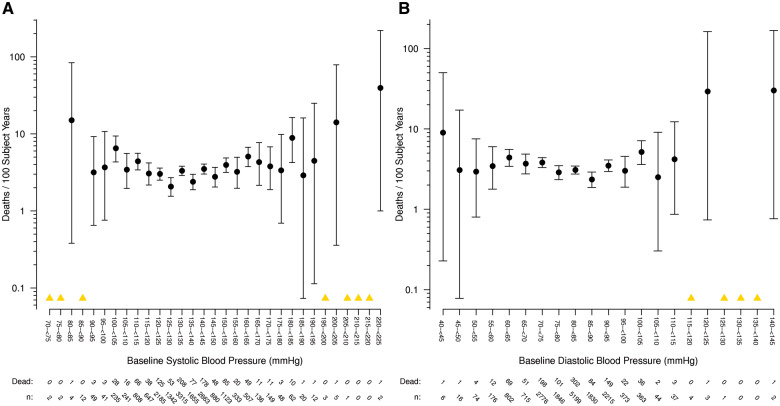
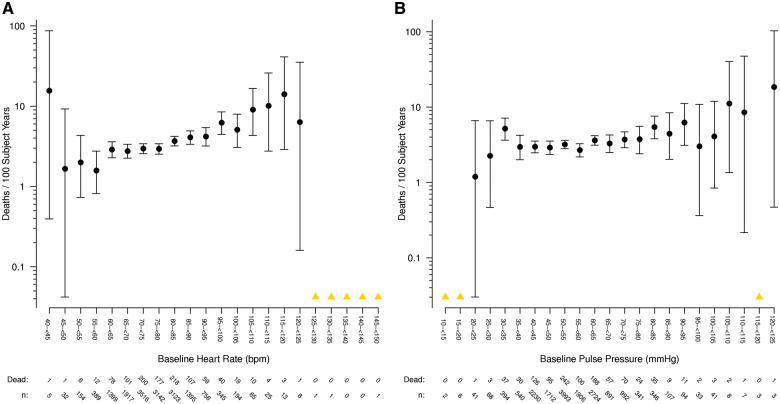
The associations between all-cause mortality and systolic and diastolic BP across the entire range of baseline study values were consistent with a generally ‘U’-shaped relationship: progressively higher and lower values outside optimal ranges being associated with increasing mortality rates (Figures1A,B). The nadir of risk visually appears to be among patients with a BP from 125–135/75–90 mmHg. There appears to be more of a linear relationship between heart rate and mortality [particularly disregarding the extreme outliers with heart rates <45 b.p.m., (Figure2A)]. However, as anticipated from the results inTable3 showing no excess risk with low levels, there appears to be more of a linear (or semi-linear) relationship between pulse pressure and mortality (Figure2B). Only high pulse pressure levels (>80 mmHg) were related to risk suggesting a possible threshold effect. When similarly plotted, the risks associated with cardiovascular events followed a similar pattern (Supplementary material online,Figures S9–S12).
Figure 1.
(A) All-cause mortality and systolic blood pressure. Rate of death per 100 subject years is shown, grouped in 5 mmHg categories according to baseline systolic blood pressure. The number of study participants at risk and the number who died are shown below the x-axis. (B) All-cause mortality and diastolic blood pressure. Rate of death per 100 subject years is shown, grouped in 5 mmHg categories according to baseline diastolic blood pressure. The number of study participants at risk and the number who died are shown below the x-axis. Note: A yellow triangle represents categories where there were zero deaths, so a rate could not be calculated.
Figure 2.
(A) All-cause mortality and heart rate. Rate of death per 100 subject years is shown, grouped in 5 b.p.m. categories according to baseline heart rate. The number of study participants at risk and the number who died are shown below the x-axis. (B) All-cause mortality and pulse pressure. Rate of death per 100 subject years is shown, grouped in 5 mmHg categories according to baseline pulse pressure. The number of study participants at risk and the number who died are shown below the x-axis. Note: A yellow triangle represents categories where there were zero deaths, so a rate could not be calculated.
Baseline haemodynamic characteristics, all-cause mortality, and cardiovascular risk by patient history
We investigated the effects of haemodynamic parameters on the risk of events in the subgroups of patients with and without previous coronary heart disease and in the subgroups of patients with a history of CVD (per trial definition) and those only at heightened cardiovascular risk (i.e. no overt disease) (Supplementary material online,Tables S1 andS2). In general, the patterns of outcomes were similar in each group to the main findings. The higher risks of mortality and cardiovascular events due to low systolic and diastolic BP were also observed in patients at heightened risk but without overt disease. This supports that this ‘U-shaped’ relationship was not confined only to those patients with a prior cardiovascular event or underlying disease.
Discussion
Elevated BP levels above optimal (>120/80 mmHg) are monotonically linked to increased morbidity and mortality in people without vascular disease.9,10 However, the prognostic value of high BP specifically among individuals with COPD remains poorly described. Here, we report for the first time that both high and low BPs are associated with increased all-cause mortality and cardiovascular events in patients with COPD at high risk for cardiovascular events or with CVD. This relationship was observed even among patients without a history of a prior cardiovascular event or established disease. In contrast, only a higher heart rate and pulse pressure were associated with increases in risks. These findings raise warnings that health care providers may need to be concerned about a worse prognosis in patients with COPD both with high as well as low systolic and diastolic BPs.
Outside the context of COPD, the relationship between haemodynamic measures and mortality has been determined in extremely large datasets, yielding high confidence in the results. In an analysis of over 900 000 people without vascular disease at the initiation of longitudinal study, BP was associated with outcomes in a monotonic linear-log fashion.9 A more recent analysis confirmed these findings in 1.25 million people initially free of CVD.10 Conversely, in patients with coronary heart disease, a ‘U-shaped’ relationship has been variably observed, as exemplified by a recent global observational study of over 22 000 patients.11 Similarly, systolic and diastolic BP were recently found to have U-shaped relationships with all-cause mortality among participants at high risk for cardiovascular events in the TRANSCEND and ONTARGET trials.20 Yet, earlier studies have not consistently confirmed these findings, particularly after adjusting for confounding factors.12 The topic of a ‘U-shaped’ or ‘J-curve’ relationship between BP and cardiovascular outcomes in patients with coronary heart disease has generated significant controversy over the years.12,13 The main concerns pertain to the clinical implications. Are low BPs a marker of poor underlying health (i.e. ‘reverse-causation’) or do treatment-induced reductions below a threshold (particularly of diastolic BP) lead to decreased coronary perfusion? Are additional vascular territories such as cerebral perfusion also impacted? Do these risks only pertain to patients with pre-existing coronary atherosclerosis? What is the optimal BP level to target with antihypertensive treatment in order to reduced cardiovascular risk? Despite decades of research varying opinions persist.12,13 The debate has intensified recently following the SPRINT study21 and a network meta-analysis (both suggesting that an optimal treated systolic BP level is 120–125 mmHg).22 Our observational findings cannot specifically address these issues, and BP measurements in our study are not directly comparable to those in SPRINT. However, our findings nonetheless highlight that these questions are relevant to high-risk patients with COPD.
Despite availability of robust data on relationships between haemodynamic measures and mortality, there are scant data in the population of patients with COPD. Resting heart rate was on average elevated in our study, as expected in COPD.23 Consistent with our findings and those in other populations,3 resting heart rate has been reported in the past to predict mortality in COPD23 and cardiovascular mortality in coronary artery disease.24 A fast heart rate may be a risk marker of autonomic imbalance heightening the potential for arrhythmias or sudden death, and it may directly promote myocardial ischaemia or damage. Moreover, faster heart rates might be an indicator of poor fitness or severity of illness. A meta-analysis of 15 observational studies found that use of a beta-blocker is associated with reduced mortality in patients with COPD.25 It is important to note, however, that there remains no trial evidence that pharmacologically lowering heart rate (e.g. beta blockade) directly translates into a reduction in risk in patients with COPD. In a clinical setting in which higher heart rate is associated with worse outcomes, ivabradine lowered heart rate in a trial of patients with stable coronary artery disease and left ventricular systolic dysfunction, but did not improve mortality.26 This finding informed the 2016 European Society of Cardiology heart failure guidelines. The guidelines recommend ivabradine when the heart rate is ≥70 b.p.m. in symptomatic patients with left ventricular ejection fraction ≤35% despite treatment with a beta-blocker, ACE inhibitor, and mineralocorticoid receptor antagonist.27 This recommendation cannot be assumed to be applicable in COPD. A clinical trial would be required to learn whether ivabradine improves outcomes in patients with COPD and elevated heart rate. The answer is unclear since the drug was effective in selected heart failure patients,28 but not in some other groups of patients. Although our study did not evaluate the effect of lowering the heart rate, we confirm here in high-risk patients with COPD that increases in heart rates are associated not only with mortality, but also excess cardiovascular events.
Patients 45 years of age and older, and meeting the Global Initiative for Obstructive Lung Disease (GOLD) criteria for COPD in the Atherosclerosis Risk in Communities Study (ARIC) and the Cardiovascular Health Study (CHS) were studied by Manninoet al.29 They found an increased risk of hospitalization and mortality in patients with COPD and hypertension—considered as a dichotomous variable—compared with patients with impaired lung function. Our study is more comprehensive in several respects, including our assessment of haemodynamic variables as continuous measures, evaluating the impact of both high and low levels, and our specific analyses of systolic BP, diastolic BP, pulse pressure, and heart rate. Perhaps owing to concern for the effect of specific drug classes (e.g. beta-blockers), some prior work has been done describing the relationships between specific antihypertensive drugs and cardiovascular outcomes in COPD. Indeed, a recent meta-analysis supports the safety and overall cardiovascular protection of beta blockade in patients with COPD.25 We similarly observed no evidence of harm related to beta-blocker usage in the SUMMIT trial (manuscript in review). Herrinet al.30 reported in a retrospective analysis that two-drug antihypertensive combinations including a thiazide diuretic were more effective for preventing heart failure hospitalizations in patients with COPD. The paucity of overall evidence and lack of prospective outcome trials means that the optimal BP target and ideal antihypertensive regimen for patients with COPD must remain a matter of expert opinion.
There are several limitations to note. Foremost, this was apost hoc observational study with short follow-up, and the findings must therefore be considered hypothesis-generating. The methodology for measurement of BP in the SUMMIT trial was not governed by strict protocols and multiple aspects pertaining to its accuracy likely varied across the 1368 sites. We only evaluated the outcomes based upon BP levels at the start of the trial and not time-averaged values. These facts would tend to bias observational analyses toward the null. Nonetheless, significant health associations with a single BP reading were still detected. More rigorous standardization as well as multiple determinations of BP (or ambulatory BP monitoring) would likely have yielded an even more robust signal. We believe our findings therefore highlight the strength of the linkage between BP with health risks in patients with COPD. Our findings do not directly impugn low BP as being harmfulper se. We cannot exclude that it may simply be a marker of poor health or ‘reverse-causation’. Regardless, our study represents the first large-scale evaluation of the nature of the relationship between BP and all-cause mortality and cardiovascular events (which were well-adjudicated in a clinical trial setting) specifically in a population of patients with COPD. Cholesterol values were not available in the SUMMIT trial, and therefore we were unable to calculate absolute CV risk. However, this should not impact the nature of the associations we found between the independent risk factor BP and CV events. There is a well-known relationship between faster heart rates and increased risk of CV events in the general population. The participants in this study had faster heart rates than are typical for non-diseased patients, likely due to underlying COPD and/or beta-agonist medications. Even so, our findings confirm the prognostic importance of worse outcomes linked to faster heart rates.
Both high and low BPs are associated with increased mortality and excess cardiovascular events, whereas faster heart rates are linked to health risks, in individuals with COPD at heightened cardiovascular risk. Further studies are warranted to confirm our findings and investigate the clinical implications as well as the benefits, optimal antihypertensive strategies, and BP treatment goals in this important and growing global population of patients.
Funding
GlaxoSmithKline (study number 113782).
Conflict of interest: Drs R.D.B., P.M.A.C., B.R.C., F.J.M., J.V., and D.E.N. are external members of the SUMMIT Steering Committee. Ms J.A.A., Dr C.C., and Ms J.Y. are employed by GSK and members of the SUMMIT Steering Committee. Dr N.J.C. is an employee of a Veramed Limited, a contract research organisation that receives funding from GlaxoSmithKline.
Supplementary Material
References
- 1. GBD 2015 Risk Factors Collaborators. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015.Lancet 2016;388:1659–1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rahimi K,Emdin CA,MacMahon S.. The epidemiology of blood pressure and its worldwide management.Circ Res 2015;116:925–936. [DOI] [PubMed] [Google Scholar]
- 3. Zhang D,Shen X,Qi X.. Resting heart rate and all-cause and cardiovascular mortality in the general population: a meta-analysis.CMAJ 2016;188:E53–E63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hansell AL,Walk JA,Soriano JB.. What do chronic obstructive pulmonary disease patients die from? A multiple cause coding analysis.Eur Respir J 2003;22:809–814. [DOI] [PubMed] [Google Scholar]
- 5. Rothnie KJ,Smeeth L,Herrett E,Pearce N,Hemingway H,Wedzicha J,Timmis A,Quint JK.. Closing the mortality gap after a myocardial infarction in people with and without chronic obstructive pulmonary disease.Heart 2015;101:1103–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rothnie KJ,Yan R,Smeeth L,Quint JK.. Risk of myocardial infarction (MI) and death following MI in people with chronic obstructive pulmonary disease (COPD): a systematic review and meta-analysis.BMJ Open 2015;5:e007824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wheaton AG,Cunningham TJ,Ford ES,Croft JB.. Employment and activity limitations among adults with chronic obstructive pulmonary disease–United States, 2013.MMWR Morb Mortal Wkly Rep 2015;64:289–295. [PMC free article] [PubMed] [Google Scholar]
- 8. Benjamin EJ,Blaha MJ,Chiuve SE,Cushman M,Das SR,Deo R,de Ferranti SD,Floyd J,Fornage M,Gillespie C,Isasi CR,Jimenez MC,Jordan LC,Judd SE,Lackland D,Lichtman JH,Lisabeth L,Liu S,Longenecker CT,Mackey RH,Matsushita K,Mozaffarian D,Mussolino ME,Nasir K,Neumar RW,Palaniappan L,Pandey DK,Thiagarajan RR,Reeves MJ,Ritchey M,Rodriguez CJ,Roth GA,Rosamond WD,Sasson C,Towfighi A,Tsao CW,Turner MB,Virani SS,Voeks JH,Willey JZ,Wilkins JT,Wu JH,Alger HM,Wong SS,Muntner P.. Heart disease and stroke statistics-2017 update: a report from the American Heart Association.Circulation 2017;135:e146–e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lewington S,Clarke R,Qizilbash N,Peto R,Collins R.. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies.Lancet 2002;360:1903–1913. [DOI] [PubMed] [Google Scholar]
- 10. Rapsomaniki E,Timmis A,George J,Pujades-Rodriguez M,Shah AD,Denaxas S,White IR,Caulfield MJ,Deanfield JE,Smeeth L,Williams B,Hingorani A,Hemingway H.. Blood pressure and incidence of twelve cardiovascular diseases: lifetime risks, healthy life-years lost, and age-specific associations in 1.25 million people.Lancet 2014;383:1899–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vidal-Petiot E,Ford I,Greenlaw N,Ferrari R,Fox KM,Tardif JC,Tendera M,Tavazzi L,Bhatt DL,Steg PG.. Cardiovascular event rates and mortality according to achieved systolic and diastolic blood pressure in patients with stable coronary artery disease: an international cohort study.Lancet 2016;388:2142–2152. [DOI] [PubMed] [Google Scholar]
- 12. Verdecchia P,Angeli F,Mazzotta G,Garofoli M,Reboldi G.. Aggressive blood pressure lowering is dangerous: the J-curve: con side of the argument.Hypertension 2014;63:37–40. [DOI] [PubMed] [Google Scholar]
- 13. Mancia G,Grassi G.. Aggressive blood pressure lowering is dangerous: the J-curve: pro side of the arguement.Hypertension 2014;63:29–36. [DOI] [PubMed] [Google Scholar]
- 14. Vestbo J,Anderson J,Brook RD,Calverley PM,Celli BR,Crim C,Haumann B,Martinez FJ,Yates J,Newby DE.. The Study to Understand Mortality and Morbidity in COPD (SUMMIT) study protocol.Eur Respir J 2013;41:1017–1022. [DOI] [PubMed] [Google Scholar]
- 15. Vestbo J,Anderson JA,Brook RD,Calverley PM,Celli BR,Crim C,Martinez F,Yates J,Newby DE.. Fluticasone furoate and vilanterol and survival in chronic obstructive pulmonary disease with heightened cardiovascular risk (SUMMIT): a double-blind randomised controlled trial.Lancet 2016;387:1817–1826. [DOI] [PubMed] [Google Scholar]
- 16. Brook RD,Anderson JA,Calverley PM,Celli BR,Crim C,Denvir MA,Magder S,Martinez FJ,Rajagopalan S,Vestbo J,Yates J,Newby DE.. Cardiovascular outcomes with an inhaled beta2-agonist/corticosteroid in patients with COPD at high cardiovascular risk.Heart 2017;103:1536–1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fleiss JL,Levin B,Paik MC,Statistical Methods for Rates and Proportions.3rd ed. Hoboken, NJ, Wiley;2003. [Google Scholar]
- 18. Piepoli MF,Hoes AW,Agewall S,Albus C,Brotons C,Catapano AL,Cooney MT,Corra U,Cosyns B,Deaton C,Graham I,Hall MS,Hobbs FDR,Lochen ML,Lollgen H,Marques-Vidal P,Perk J,Prescott E,Redon J,Richter DJ,Sattar N,Smulders Y,Tiberi M,van der Worp HB,van Dis I,Verschuren WMM,Binno S.. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: the Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts) Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR).Eur Heart J 2016;37:2315–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mancia G,Fagard R,Narkiewicz K,Redon J,Zanchetti A,Bohm M,Christiaens T,Cifkova R,De Backer G,Dominiczak A,Galderisi M,Grobbee DE,Jaarsma T,Kirchhof P,Kjeldsen SE,Laurent S,Manolis AJ,Nilsson PM,Ruilope LM,Schmieder RE,Sirnes PA,Sleight P,Viigimaa M,Waeber B,Zannad F.. 2013 ESH/ESC Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC).J Hypertens 2013;31:1281–1357. [DOI] [PubMed] [Google Scholar]
- 20. Bohm M,Schumacher H,Teo KK,Lonn EM,Mahfoud F,Mann JFE,Mancia G,Redon J,Schmieder RE,Sliwa K,Weber MA,Williams B,Yusuf S.. Achieved blood pressure and cardiovascular outcomes in high-risk patients: results from ONTARGET and TRANSCEND trials.Lancet 2017;389:2226–2237. [DOI] [PubMed] [Google Scholar]
- 21. Wright JT Jr,Williamson JD,Whelton PK,Snyder JK,Sink KM,Rocco MV,Reboussin DM,Rahman M,Oparil S,Lewis CE,Kimmel PL,Johnson KC,Goff DC Jr,Fine LJ,Cutler JA,Cushman WC,Cheung AK,Ambrosius WT.. A randomized trial of intensive versus standard blood-pressure control.N Engl J Med 2015;373:2103–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bundy JD,Li C,Stuchlik P,Bu X,Kelly TN,Mills KT,He H,Chen J,Whelton PK,He J.. Systolic blood pressure reduction and risk of cardiovascular disease and mortality: a systematic review and network meta-analysis.JAMA Cardiol 2017;2:775–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jensen MT,Marott JL,Lange P,Vestbo J,Schnohr P,Nielsen OW,Jensen JS,Jensen GB.. Resting heart rate is a predictor of mortality in COPD.Eur Respir J 2013;42:341–349. [DOI] [PubMed] [Google Scholar]
- 24. Fox K,Ford I,Steg PG,Tendera M,Robertson M,Ferrari R.. Heart rate as a prognostic risk factor in patients with coronary artery disease and left-ventricular systolic dysfunction (BEAUTIFUL): a subgroup analysis of a randomised controlled trial.Lancet 2008;372:817–821. [DOI] [PubMed] [Google Scholar]
- 25. Du Q,Sun Y,Ding N,Lu L,Chen Y.. Beta-blockers reduced the risk of mortality and exacerbation in patients with COPD: a meta-analysis of observational studies.PLoS One 2014;9:e113048.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fox K,Ford I,Steg PG,Tendera M,Ferrari R.. Ivabradine for patients with stable coronary artery disease and left-ventricular systolic dysfunction (BEAUTIFUL): a randomised, double-blind, placebo-controlled trial.Lancet 2008;372:807–816. [DOI] [PubMed] [Google Scholar]
- 27. Ponikowski P,Voors AA,Anker SD,Bueno H,Cleland JG,Coats AJ,Falk V,Gonzalez-Juanatey JR,Harjola VP,Jankowska EA,Jessup M,Linde C,Nihoyannopoulos P,Parissis JT,Pieske B,Riley JP,Rosano GM,Ruilope LM,Ruschitzka F,Rutten FH,van der Meer P.. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC.Eur J Heart Fail 2016;18:891–975. [DOI] [PubMed] [Google Scholar]
- 28. Swedberg K,Komajda M,Bohm M,Borer JS,Ford I,Dubost-Brama A,Lerebours G,Tavazzi L.. Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study.Lancet 2010;376:875–885. [DOI] [PubMed] [Google Scholar]
- 29. Mannino DM,Thorn D,Swensen A,Holguin F.. Prevalence and outcomes of diabetes, hypertension and cardiovascular disease in COPD.Eur Respir J 2008;32:962–969. [DOI] [PubMed] [Google Scholar]
- 30. Herrin MA,Feemster LC,Crothers K,Uman JE,Bryson CL,Au DH.. Combination antihypertensive therapy among patients with COPD.Chest 2013;143:1312–1320. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.