Abstract
Aims
To determine whether the combination of standard electrocardiographic (ECG) markers reflecting domains of arrhythmic risk improves sudden and/or arrhythmic death (SAD) risk stratification in patients with coronary heart disease (CHD).
Methods and results
The association between ECG markers and SAD was examined in a derivation cohort (PREDETERMINE; N = 5462) with adjustment for clinical risk factors, left ventricular ejection fraction (LVEF), and competing risk. Competing outcome models assessed the differential association of ECG markers with SAD and competing mortality. The predictive value of a derived ECG score was then validated (ARTEMIS; N = 1900). In the derivation cohort, the 5-year cumulative incidence of SAD was 1.5% [95% confidence interval (CI) 1.1–1.9] and 6.2% (95% CI 4.5–8.3) in those with a low- and high-risk ECG score, respectively (P for Δ < 0.001). A high-risk ECG score was more strongly associated with SAD than non-SAD mortality (adjusted hazard ratios = 2.87 vs. 1.38 respectively; P for Δ = 0.003) and the proportion of deaths due to SAD was greater in the high vs. low risk groups (24.9% vs. 16.5%, P for Δ = 0.03). Similar findings were observed in the validation cohort. The addition of ECG markers to a clinical risk factor model inclusive of LVEF improved indices of discrimination and reclassification in both derivation and validation cohorts, including correct reclassification of 28% of patients in the validation cohort [net reclassification improvement 28 (7–49%), P = 0.009].
Conclusion
For patients with CHD, an externally validated ECG score enriched for both absolute and proportional SAD risk and significantly improved risk stratification compared to standard clinical risk factors including LVEF.
Clinical Trial Registration
https://clinicaltrials.gov/ct2/show/NCT01114269. ClinicalTrials.gov ID NCT01114269.
Keywords: Sudden death, Electrocardiogram, Coronary heart disease
See page 2000 for the editorial comment on this article (doi: 10.1093/eurheartj/ehaa294)
Introduction
Sudden deaths account for 15–20% of global mortality, and coronary heart disease (CHD) is the most common underlying substrate.1 While implantable cardioverter-defibrillator (ICD) therapy effectively reduces arrhythmic mortality in patients with depressed left ventricular function and/or heart failure, more than 70% of sudden deaths occur in those who do not meet contemporary guideline criteria for ICD implantation.2 In this context, meaningful reductions in the public health burden of sudden and/or arrhythmic death (SAD) will require risk stratification in this broader population that can be easily integrated into routine clinical care at relatively low cost.
Electrocardiograms (ECG) are routinely performed during office visits in patients with CHD and several ECG measures have been individually associated with SAD. Recently, combinations of ECG measures have been found to be associated with SCD in general population cohorts.3 , 4 However, the clinical application of these important findings has been limited for three major reasons. First, many of the measures included in these ECG scores have yet to be incorporated into standard ECG interpretation and thus are not readily available in real-world clinical settings. Second, even those deemed at high risk by these ECG scores remain at a relatively low absolute risk of SAD due to the exceedingly low rate of SAD in the general population. Third, prior studies3 , 4 do not report whether ECG associations were specific for SAD vs. other types of mortality, which is critical in assessing the potential utility of a screening tool to identify those who stand to benefit most from an ICD.5–7
Therefore, in the present study, we examine the association between combinations of routinely measured ECG parameters and SAD, as well as other forms of mortality, to determine if combinations of ECG markers, which capture distinct domains of arrhythmic risk, improve (SAD) risk stratification beyond standard clinical risk factors. We focus on patients with established CHD, where the absolute risk of SAD is 10 times higher than the general population,6 and thus in whom risk enrichment would yield absolute rates of SAD that would be clinically relevant and actionable. We then demonstrate how these data might be used to design a randomized trial of ICD therapy within this population.
Methods
Study cohorts
Derivation cohort
The PRE-DETERMINE (ClinicalTrials.gov ID NCT01114269) and accompanying DETERMINE Registry (ClinicalTrials.gov ID NCT00487279) study populations are multicentre prospective cohort studies comprised of patients with coronary disease on angiography or documented history of myocardial infarction (MI). The PREDETERMINE study enrolled 5764 patients with documented MI and/or mild to moderate left ventricular dysfunction [left ventricular ejection fraction (LVEF) = 35–50%] who did not fulfil consensus guideline criteria for ICD implantation on the basis of LVEF and New York Heart Association (NYHA) class (LVEF > 35% or LVEF 30–35% with NYHA Class I HF) at study entry.6 Exclusion criteria included a history of cardiac arrest not associated with acute MI, current or planned ICD, or life expectancy < 6 months. The accompanying DETERMINE Registry included 192 participants screened for enrolment in PREDETERMINE who did not fulfil entry criteria on the basis of having an LVEF < 30% (N = 99), LVEF 30–35% with NYHA Class II–IV heart failure (N = 19), or an ICD (N = 31) or were unwilling to participate in the biomarker component of PREDETERMINE (N = 43). For the present study, we excluded participants without an interpretable ECG [N = 103 (2%)] and those with an ICD at baseline [N = 61 (1%)]. Of the remaining combined 5792 participants, we additionally excluded those with incomplete information regarding clinical covariates [N = 17 (<1%)] and those for whom derivation of ECG characteristics was not possible owing to insufficient ECG wave amplitude [N = 313 (5%)], yielding a final analytic cohort of 5462 participants. All participants provided written, informed consent and the study was approved by the institutional review board of Brigham and Women’s Hospital (BWH).
Validation cohort
The ARTEMIS prospective observational study (Innovation to Reduce Cardiovascular Complications of Diabetes at the Intersection; ClinicalTrials.gov NCT1426685)8 recruited patients with angiographically documented CAD with or without diabetes mellitus from a consecutive series of patients who had undergone coronary angiography at Division of Cardiology, Oulu University hospital. Subjects who fulfilled the guidelines criteria for prophylactic implantation of cardioverter defibrillator or those with a life expectancy less than 1 year were excluded from the study. A total of 1946 subjects were recruited between 2008 and 2013. For this study, 46 (2%) participants without an interpretable ECG were excluded, yielding a final analytic cohort of 1900. All patients gave their informed consent and the study was approved by the local ethics committee. The study complies with declaration of Helsinki.
Electrocardiographic and clinical risk factor ascertainment
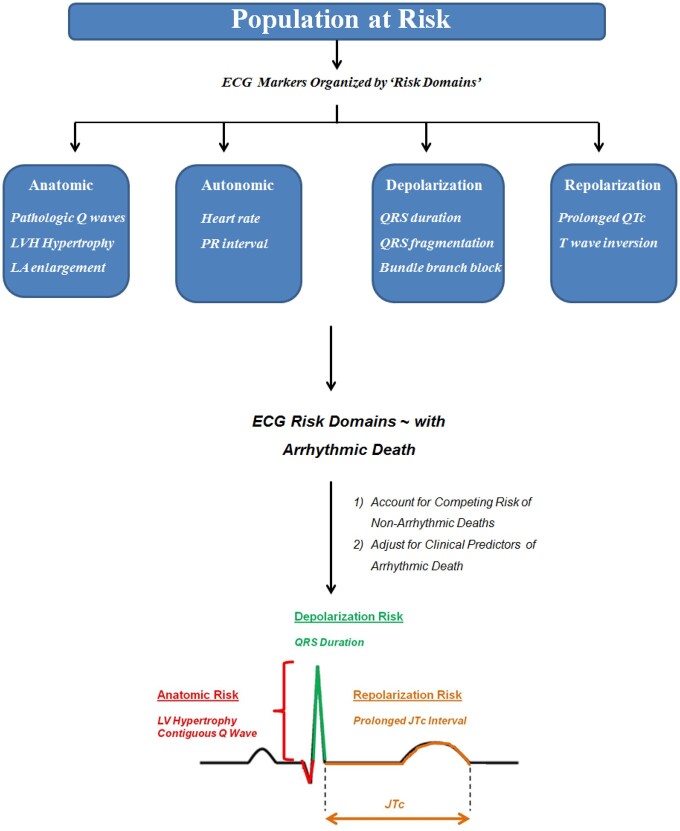
In PRE-DETERMINE, ECGs were obtained at the time of study enrolment. Electrocardiographic measures were performed at a core lab (Quintiles Cardiac Safety Services, Mumbai, India) using electronically scanned ECGs. Measurements were performed using a previously validated ECG measurement tool (Cardio Calipers v3.3; Iconico Inc., New York, USA).9 Electrocardiographic analysis included per lead assessment of amplitude and duration of the P wave, QRS complex, ST segment and T wave, measurement of the PR and QT intervals, and evaluation of morphology including QRS fragmentation, early repolarization, and T-wave inversion according to consensus guideline criteria.10–12 In order to enhance the clinical applicability of these findings, ECG measures were selected a priori on the basis of ease and routine availability of measurement on a standard 12-lead ECG. Electrocardiographic risk measures previously associated with arrhythmic mortality were selected and then organized into ‘risk domains’, which included anatomic [contiguous Q waves, left ventricular hypertrophy (LVH), and left atrial enlargement], autonomic (resting heart rate, PR interval), depolarization [QRS duration, QRS fragmentation, left bundle branch block (LBBB)], and repolarization (JTc prolongation, early repolarization, T-wave inversion in contiguous leads) risk markers (Figure 1; Supplementary material online, Table S1). Continuous measures were categorized according to previously established cutpoints of arrhythmic risk including PR > 200 ms, QRS duration (≤80, 80–110, >110 ms), and JTc prolongation (≥360 ms). Left ventricular hypertrophy was defined according to Sokolow-Lyon and/or sex-specific Cornell voltage criteria, Q waves, QRS fragmentation, early repolarization, and T-wave inversions were assessed as present if documented in anatomically contiguous ECG leads (see Supplementary material online, Table S1). For the univariable PR prolongation model in the derivation cohort, patients with atrial arrhythmia [atrial fibrillation or atrial flutter; N = 330 (6%)] at the time of ECG assessment were excluded. Electrocardiographic assessment in ARTEMIS has been previously described.13
Figure 1.
A pathophysiology-based approach to electrocardiographic prediction of sudden arrhythmic death risk. In this study, we assessed the association between several easily measurable electrocardiographic markers and the risk of sudden of arrhythmic death in patients with coronary heart disease and relatively preserved left ventricular function. Electrocardiographic markers were organized into pathophysiology-based ‘risk domains’ including markers reflecting anatomic risk, autonomic function, as well as depolarization and repolarization abnormalities. In models (1) accounting for the competing risk of non-arrhythmic deaths and (2) adjusted for clinical risk factors associated with arrhythmic death (age, sex, race/ethnicity, hypertension, diabetes mellitus, atrial fibrillation, left ventricular ejection fraction, New York Heart Association functional class, and β-blocker use), four electrocardiographic markers were independently associated with arrhythmic death: left ventricular hypertrophy, contiguous Q waves, QRS duration, and a prolonged JTc interval. ECG, electrocardiographic; LA, left atrial; LVH, left ventricular hypertrophy; NYHA, New York Heart Association functional class.
In both PRE-DETERMINE and ARTEMIS, baseline data on demographics, clinical characteristics, past medical history, lifestyle habits, cardiac test results, and medications were collected via electronic data capture. The baseline LVEF was chosen to be the most recent assessment on which current medical treatment was based at the time of entry into the study.
Ascertainment and classification of incident cardiovascular events and death
Derivation cohort
After enrolment, all participants in the PREDETERMINE Study and DETERMINE Registry were followed centrally by the Clinical Coordinating Center at BWH utilizing identical methods. Medical records pertaining to all deaths, cardiac arrests, and ICD implantations were sought to confirm study endpoints. Vital status was further assessed using contact with postal authorities, obituary searches, and serial searches of the National Death Index for names of non-respondents. For those participants who died outside of the hospital, detailed interview(s) were conducted with the next-of-kin, family members, and other potential witnesses regarding the details and circumstances surrounding the death. Endpoints were confirmed utilizing information ascertained from medical records, autopsies, and witness reports. Deaths were adjudicated by two independent reviewers (N.A.C., C.M.A.), with disagreement resolved by consensus. Information from the death certificate was not utilized in the determination of the primary endpoint.
The primary endpoint was a combined endpoint of SAD. Deaths were classified according to both timing (sudden vs. non-sudden) and mechanism (arrhythmic vs. non-arrhythmic). Unexpected deaths due to cardiac or unknown causes that occurred within 1 h of symptom onset or within 24 h of being last witnessed to be symptom free were considered sudden cardiac deaths. Deaths preceded by an abrupt spontaneous collapse of circulation without antecedent circulatory or neurologic impairment were considered arrhythmic in accordance with the criteria outlined by Hinkle and Thaler.14 Deaths that were classified as non-arrhythmic were excluded from the endpoint regardless of timing. Out-of-hospital cardiac arrests due to ventricular fibrillation that were successfully resuscitated with external electrical defibrillation were considered aborted arrhythmic deaths and included in the primary SAD endpoint.
Validation cohort
In ARTEMIS, death certificates, autopsy data, hospital records and interviews with next of kin were used for mode of death adjudication. Hospital records and paramedic data of resuscitation were used to determine aborted SAD. The cause and mode of death was reviewed by two independent investigators and if needed decided as a consensus (M.J.J., H.V.H.). The pre-specified primary endpoint in ARTEMIS was SAD or resuscitation from cardiac arrest, which ever occurred first. The definition for SAD was witnessed death within 1 h of symptom onset or within 24 h of being last witnessed to be symptom free. Medicolegal autopsy is mandatory in Finland, and thus autopsy data were available in most cases for adjudication.
Statistical analysis
For all analyses in the PREDETERMINE Study populations, participants contributed person-time from the date of enrolment to the first occurrence of death, out-of-hospital cardiac arrest, loss to follow-up, ICD implantation, or 25 October 2017. In this derivation cohort, we first examined the association between ECG risk measures and the primary SAD endpoint using univariate Fine–Gray models which accounted for the competing risk of non-SAD deaths. Electrocardiographic markers demonstrating significant univariate association (P < 0.05) with SAD were then separately examined in Fine–Gray models with multivariable adjustment for established SAD risk factors which were delineated a priori (age, sex, race/ethnicity, hypertension, diabetes mellitus, atrial fibrillation, LVEF, NYHA functional class, and β-blocker use). The final multivariable model included established clinical risk factors and the four ECG measures which retained significant associations with SAD after multivariable adjustment (contiguous Q waves, LVH, JTc prolongation, QRS duration).
We then examined the potential clinical utility of the selected ECG markers by constructing a simplified integer score, scaled to the β-coefficients of the ECG markers in the multivariable-adjusted Fine–Gray model [contiguous Q wave: 1 point, QRS duration (≤80: 0 points, 81–110: 1 point, >110: 2 points), LVH: 1 point, prolonged JTc: 1 point] (score range 0–5). Groups were stratified into low (0–1 points), moderate (2 points), and high (≥3 points) risk. Participants with ≥3 points were collapsed into a single group given the small number of participants in score levels in these strata. In both derivation and validation cohorts, Fine–Gray models with adjustment for clinical risk factors (described above) and cumulative incidence functions accounting for competing non-SAD mortality were used to examine the relative and absolute risk of SAD associated with the ECG score. To examine whether the simplified ECG score was differentially associated with SAD vs. non-SAD death, competing outcome Cox proportional hazard models with likelihood ratio comparisons were performed. To examine the incremental improvement in risk assessment for SAD when using the ECG score, we compared a baseline model comprised of clinical SAD risk factors (described above) to a model which additionally included the derived ECG score. Model calibration in the derivation cohort was assessed using the Greenwood-D’Agostino-Nam χ2. In both the derivation and validation cohorts, discrimination for SAD was assessed using the integrated discrimination improvement and reclassification of SAD was estimated using net reclassification improvement (NRI) using 5-year estimated probabilities of SAD (0–1%, 1–5%, 5–10%, >10%). In the derivation cohort, cumulative incidences of SAD and non-SAD at 5 years were assessed across ECG risk score categories stratified by quartiles of the baseline model SAD risk. To illustrate the potential impact of ICD therapy on overall mortality in high-risk subgroups, we modelled the theoretical efficacy of ICD therapy as previously described.6 Briefly, we assumed a 60% reduction in SAD and no reduction in non-SAD mortality associated with ICD therapy. The number needed to treat (NNT) to save one life, the percent reduction of total mortality, and the theoretical sample size of a randomized controlled trial to demonstrate such a mortality reduction (with 80% power) were then estimated. Statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA). A two-tailed P < 0.05 was considered to indicate statistical significance.
Results
Study populations and modes of death
Baseline characteristics for participants enrolled in the combined PRE-DETERMINE (derivation cohort; N = 5462) and ARTEMIS (validation cohort; N = 1900) studies are shown in Table 1. Baseline characteristics across the cohorts were similar including mean age (64–67 years) and prevalence of comorbidities including diabetes, hypertension, and atrial fibrillation. Due to the entry criteria, the PREDETERMINE study population had a higher prevalence of prior MI (91% vs. 48%) and LV dysfunction (proportion of patients with LVEF <50%; 38% vs. 7%). The mean LVEF was preserved but lower in the derivation compared to validation cohort (52 ± 10 vs. 64 ± 9%), whereas a greater proportion of patients in the derivation cohort had NYHA Class I HF (80 vs. 53%). Both cohorts had high rates of prior revascularization (>84%) and medical therapy for coronary disease (>88%) with aspirin, lipid-lowering therapy, and β-blockers. Over a median follow-up of 5.1 years, there were 688 deaths and 10 out-of-hospital cardiac arrests (139 SAD endpoint, 559 non-SAD deaths) in the PRE-DETERMINE cohort. In ARTEMIS, there were 50 deaths (32 SAD, 28 non-SAD) over 5-year follow-up.
Table 1.
Baseline characteristics study cohort
| Baseline characteristics | PRE-DETERMINE studies (derivation cohort; N = 5462) | ARTEMIS (validation cohort; N = 1900) |
|---|---|---|
| Age | 64 ± 11 | 67 ± 9 |
| Male gender, n (%) | 4181 (77) | 1297 (68) |
| Race/ethnicity, n (%) | ||
| White | 4866 (89) | 1900 (100) |
| Black or African-American | 295 (5) | — |
| Asian | 109 (2) | — |
| Other | 110 (2) | — |
| Unknown | 82 (1) | — |
| Hypertension | 4123 (75) | 1319 (69) |
| History of MI | 4960 (91) | 904 (48) |
| History of revascularization, n (%)a | ||
| PCI | 4360 (80) | 1175 (62) |
| CABG | 1746 (32) | 436 (23) |
| Family history SAD, n (%) | 1358 (25) | 666 (35) |
| Diabetes mellitus, n (%) | 1735 (32) | 812 (43) |
| History of AF | 746 (14) | 239 (13) |
| Ejection fraction | ||
| Continuous | 52 ± 10 | 64 ± 9 |
| Category | ||
| ≥60% | 1515 (28) | 1428 (75) |
| 50–59% | 1876 (34) | 328 (17) |
| 40–49% | 1601 (29) | 112 (6) |
| 30–39% | 411 (8) | 23 (1) |
| <30% | 59 (1) | |
| NYHA Class | ||
| I | 4354 (80) | 1001 (53) |
| II | 882 (16) | 834 (44) |
| III/IV | 226 (4) | 65 (3) |
| Medication use | ||
| Aspirin | 4817 (88) | 1700 (89) |
| β-Blocker | 4536 (83) | 1665 (88) |
| Lipid-lowering | 5090 (93) | 1736 (91) |
| ACEi/ARB/aldosterone antagonist | 3822 (70) | 1296 (68) |
ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; CABG, coronary artery bypass graft; MI, myocardial infarction; NYHA, New York Heart Association; PCI, percutaneous coronary intervention; SAD, sudden cardiac death.
Total N of PCI and CABG is greater than cohort total as some patients underwent both PCI and CABG prior to enrolment. N = 3300 for PCI alone, N = 686 for CABG alone, and N = 1060 for PCI and CABG. Therefore, N = 416 (8%) for patients without any prior revascularization.
Electrocardiographic risk domains associated with sudden and/or arrhythmic death
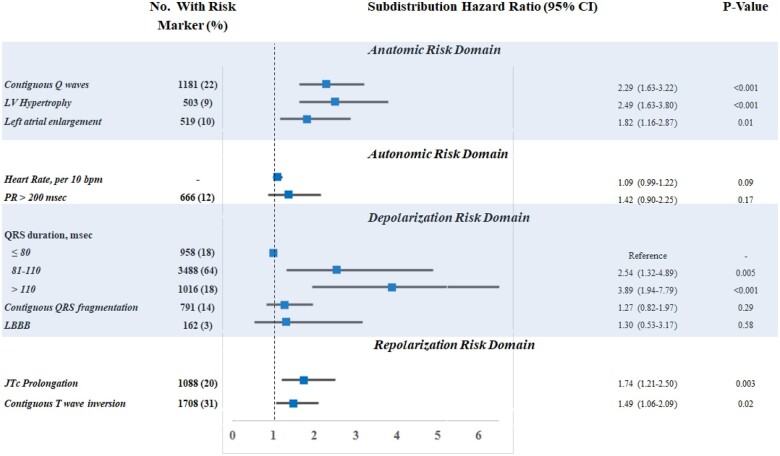
The prevalence and univariate association between ECG markers and SAD in the derivation cohort (PRE-DETERMINE) are shown in Figure 2. In univariate models accounting for the competing risk of non-SAD mortality, several ECG markers were associated with SAD including anatomic (contiguous Q waves, LVH, left atrial enlargement), depolarization (QRS duration), and repolarization (JTc prolongation, contiguous T-wave inversion) risk markers. In competing-risk models further adjusted for clinical risk factors, four ECG markers reflecting anatomic (contiguous Q waves, LVH), depolarization (QRS duration), and repolarization (JTc prolongation) risk each retained significant association with SAD (Table 2). All four ECG markers remained significantly associated with SAD in the final multivariable model inclusive of clinical and ECG measures.
Figure 2.
Univariate association of validated arrhythmic electrocardiographic risk markers with arrhythmic death—stratified by risk domains. Shown is the prevalence and univariate association between several electrocardiographic measures and the risk of arrhythmic death. Association was examined in subdistribution hazard models accounting for the competing risk of non-arrhythmic death. Electrocardiographic markers are organized into pathophysiology-based risk domains including measures reflecting anatomic pathology, autonomic function, as well as depolarization and repolarization abnormalities. Patients with atrial arrhythmias at the time of electrocardiographic assessment were excluded from the PR prolongation model (see Methods section). No., number; BPM, beats per minute; LBBB, left bundle branch block.
Table 2.
Multivariable-adjusted association of ECG risk markers and sudden death: validation cohort PREDETERMINE
| Multivariable-adjustment clinical risk factors sHR (95% CI) a | P-value | Multivariable-adjustment clinical + ECG risk factors sHR (95% CI) b | P-value | |
|---|---|---|---|---|
| ECG markers | ||||
| Contiguous Q waves | 1.95 (1.36–2.81) | 0.0003 | 1.88 (1.31–2.71) | <0.001 |
| LV hypertrophy | 2.09 (1.34–3.27) | 0.001 | 1.89 (1.21–2.95) | 0.005 |
| Prolonged JTc | 1.50 (1.02–2.19) | 0.04 | 1.60 (1.07–2.37) | 0.02 |
| QRS duration | ||||
| ≤80 | Reference | — | Reference | — |
| 81–110 | 2.30 (1.20–4.42) | 0.01 | 2.40 (1.24–4.67) | 0.01 |
| >110 | 2.72 (1.36–5.44) | 0.005 | 2.73 (1.33–5.61) | 0.006 |
LV, left ventricular; NYHA New York Heart Association.
Multivariable adjustment was performed for established clinical risk factors for sudden and/or arrhythmic death including age, sex, race/ethnicity, hypertension, diabetes mellitus, atrial fibrillation, left ventricular ejection fraction, NYHA functional class, and β-blocker use. Subdistribution hazard ratios (sHR) are derived from Fine–Gray competing-risk models accounting for the competing risk of non-arrhythmic deaths. Hazard ratios shown reflect adjustment of individual ECG markers for clinical risk factors.
Hazard ratios shown reflect adjustment for clinical risk factors and all ECG markers shown.
Electrocardiographic risk score and absolute incidence of sudden and/or arrhythmic death
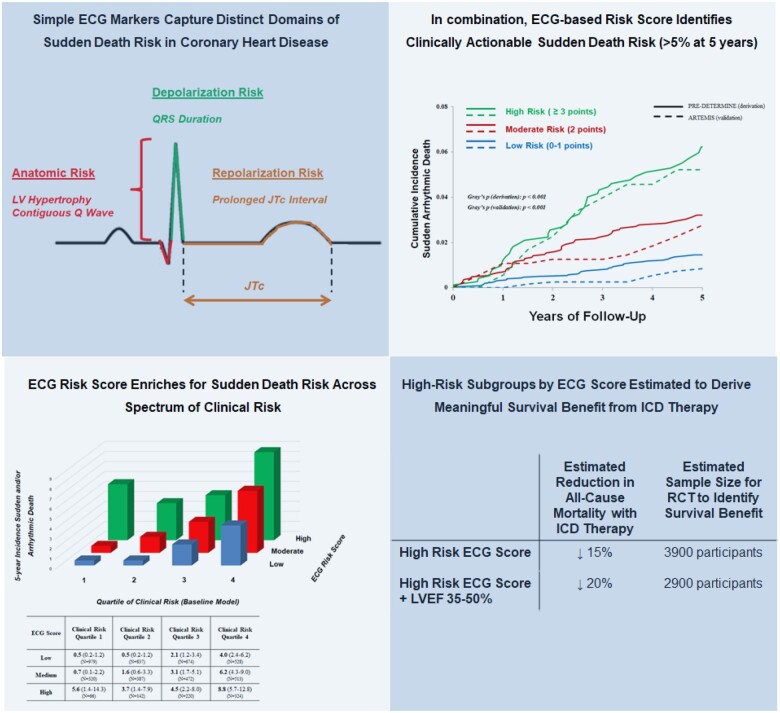
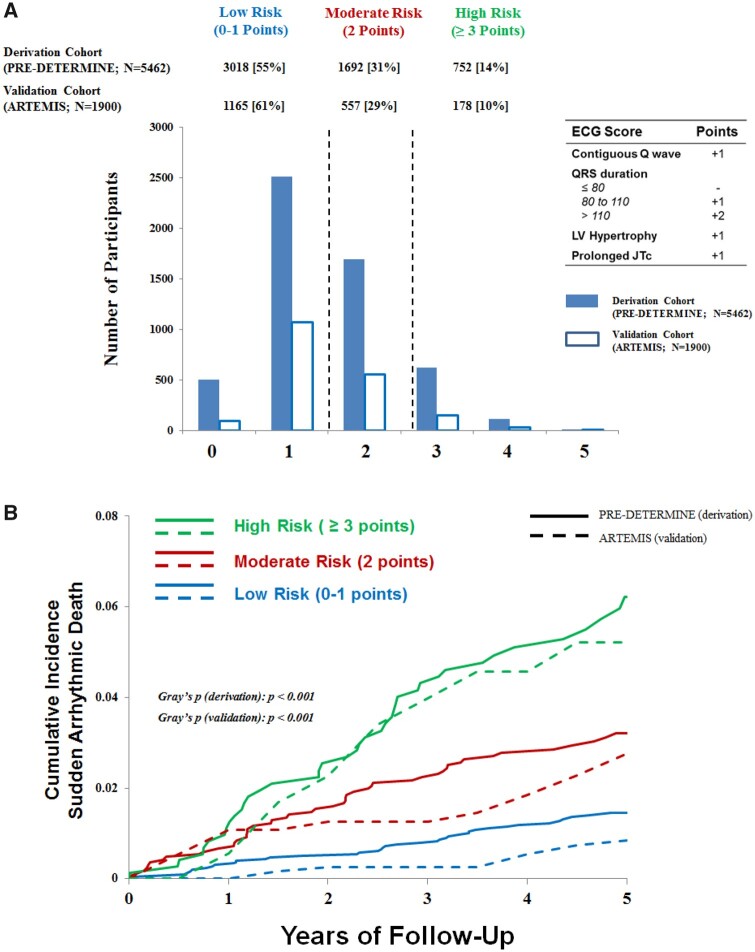
To assess the clinical utility of these ECG markers in SAD risk prediction, we constructed a simple integer-based point score reflecting the presence of the four identified ECG markers (score range 0–5). In the derivation cohort, the model with ECG and clinical risk factors was reasonably well calibrated for 5-year SAD risk (Greenwood-D’Agostino-Nam χ2 = 8.56, P = 0.04; Supplementary material online, Figure S1). Participants were stratified into low (0–1 points), moderate (2 points), or high (≥3 points) risk groups and the distribution of risk was similar in both derivation and validation cohorts (Figure 3A). The 5-year cumulative incidence of SAD, accounting for competing deaths, increased progressively across risk strata in both derivation and validation cohorts (Gray’s P < 0.001 for each cohort; Figure 3B). The 5-year SAD risk for low- and high-risk groups in the derivation cohort was 1.5% [95% confidence interval (CI) 1.1–1.9] and 6.2% (95% CI 4.5–8.3) (P for Δ < 0.001), respectively. In the validation cohort, the risk range for low- and high-risk groups was 0.9% (95% CI 0.4–1.6) to 5.2% 95% CI 2.6–9.3%) (P for Δ < 0.001).
Figure 3.
Cumulative incidence of sudden arrhythmic death according to risk-based electrocardiographic score. (A) Shown is the distribution of electrocardiographic risk score in the derivation (PRE-DETERMINE; solid blue) and validation (ARTEMIS; blue outline) cohorts. The point score was defined by the presence of contiguous Q waves (+1 point), prolonged QRS duration (80–110: 1 point, >110: 2 points), left ventricular hypertrophy (1 point), and prolonged JTc (1 point). (B) Shown are the 5-year cumulative incidences of sudden arrhythmic death in the derivation cohort (PRE-DETERMINE; solid line) and validation (ARTEMIS; dashed line), accounting for the competing risk of non-arrhythmic deaths, in the total cohort stratified by a risk-based electrocardiographic score. The equivalence of cumulative incidence functions in each cohort was assessed using Gray’s test. ECG, electrocardiographic; LV, left ventricular.
Given the broader range of LVEF in the derivation cohort, we further examined absolute risk in clinically relevant strata of LV function. In those with LVEF > 35% at baseline (i.e. those who do not qualify for primary prevention ICD therapy in contemporary consensus guidelines),15 the cumulative 5-year incidence of SAD was 1.4% (95% CI 1.0–1.9%) and 6.3% (95% CI 4.5–8.4%) in the low- and high-risk groups, respectively. For those at intermediate SAD risk based on LV function (LVEF of 35–50%), the 5-year cumulative incidence of SAD was 2.4% (95% CI 1.6–3.5%) vs. 7.4% (95% CI 5.1–10.3%) in the low- and high-risk groups, respectively.
Distinguishing sudden and/or arrhythmic death from competing modes of death with electrocardiographic score compared to left ventricular ejection fraction
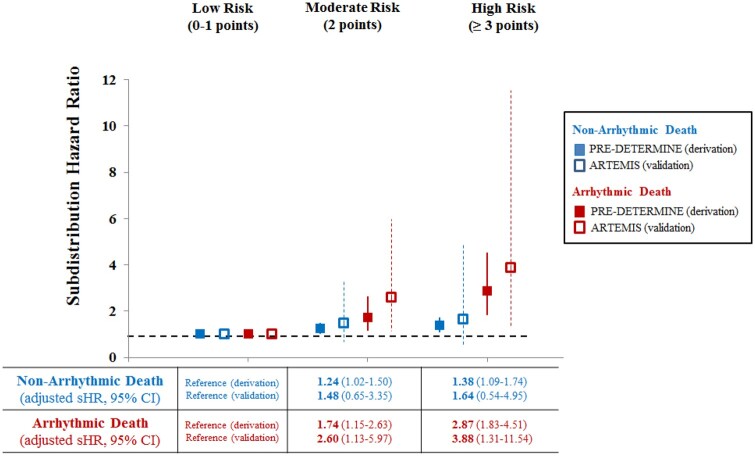
In the derivation cohort, after multivariable adjustment for clinical risk factors as well as LVEF, an increasing ECG score was associated with a progressive increase in the incidence of SAD (Figure 4). In competing outcome models examining the differential association of the ECG score with SAD vs. non-SAD death, a high-risk ECG score (≥3 points) was more strongly associated with SAD [adjusted subdistribution hazard ratio (sHR) 2.87; 95% CI 1.83–4.51] vs. non-SAD (HR 1.38; 95% CI 1.09–1.74; P for differential association = 0.003). In comparison, there was no significant difference in the association between LVEF and SAD vs. non-SAD (P for differential association = 0.34). Consistent with this more specific association of ECG score with SAD, we observed an enrichment in the proportional risk of SAD with increasing ECG score (% of deaths that were SAD by strata of ECG risk score—low risk: 16.5%, medium risk: 20.2%, high risk: 24.9%; P for trend = 0.03). In the validation cohort, increasing ECG score was likewise associated with a significant increased risk of SAD and a non-significant increased risk of competing modes of death (Figure 4).
Figure 4.
Association of risk-based electrocardiographic score with arrhythmic and non-arrhythmic death: derivation and validation cohorts. In both the derivation (PRE-DETERMINE; solid square) and validation (ARTEMIS; empty square) cohorts, we illustrate the relative increased incidence of arrhythmic and non-arrhythmic death as stratified by the risk-based electrocardiographic score. Relative incidence was assessed using multivariable-adjusted Fine–Gray models accounting for the competing risk of other deaths. Multivariable adjustment included age, sex, race/ethnicity, hypertension, diabetes mellitus, atrial fibrillation, left ventricular ejection fraction, New York Heart Association functional class, and β-blocker use. HR, hazard ratio; NYHA, New York Heart Association.
Improvement in sudden and/or arrhythmic death reclassification with electrocardiographic markers compared to clinical risk factors
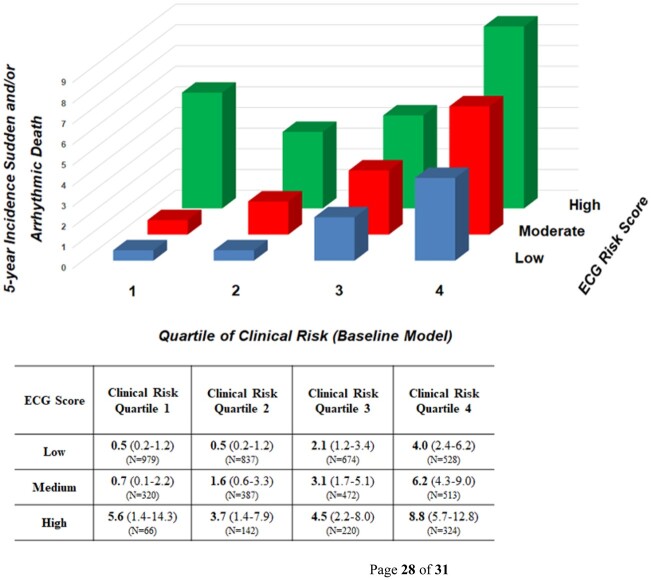
To assess the improvement in risk prediction incorporating ECG-based risk domains compared to LVEF and other clinical risk markers, we compared a baseline model of these clinical risk markers and LV function to one which additionally included the ECG risk score. Addition of the ECG score to the clinical risk factor model significantly improved reclassification indices in both the derivation [NRI 25% (15–34), P < 0.001] and validation cohorts [NRI 28% (7–49%), P = 0.009]. Addition of the ECG score also improved discrimination when assessed by integrated discrimination improvement in both derivation and validation cohorts (Supplementary material online, Table S2). To further examine the incremental value of the ECG score compared to the baseline model alone, we examined 5-year rates of SAD according to ECG risk score stratified by quartiles of baseline model SAD risk (Figure 5). Within any given strata of SAD risk defined by clinical risk factors and LVEF, an increasing ECG score enriched for absolute rates of SAD. The ECG score additionally enriched for proportional risk of SAD, particularly in groups identified as lower risk by the baseline model (Supplementary material online, Table S3).
Figure 5.
Five year incidence of sudden and/or arrhythmic death according to electrocardiographic score stratified by clinical risk. In the derivation cohort, 5-year cumulative incidence (with 95% confidence intervals) of sudden and/or arrhythmic death are shown according to electrocardiographic risk score stratified by quartiles of baseline model (clinical risk factors, left ventricular ejection fraction) risk. Subgroup sample size (N) are highlighted.
Take home figure.
Simple electrocardiographic markers predict actionable sudden death risk in patients with coronary heart disease. Our study demonstrates that simple electrocardiographic markers, reflecting distinct domains of risk, can specifically identify sudden arrhythmic death risk in patients with coronary heart disease. In combination, these electrocardiographic markers identify clinically actionable sudden death at 5 years in derivation and validation cohorts. The electrocardiographic risk score enriches for sudden death risk across the spectrum of risk defined by clinical risk factors. The highest risk subgroups in our study were estimated to derive significant survival benefit from implantable cardioverter-defibrillator (ICD) therapy. We show that randomized controlled trials (RCT) needed to identify this survival benefit are feasible.
Implications of electrocardiographic risk score for implantable cardioverter-defibrillator efficacy and clinical trial design
To examine the implications of SAD risk enrichment by ECG score, we estimated the theoretical efficacy of ICD therapy in high-risk subgroups, including examining the relative risk reduction in overall mortality and NNT to save one life. We further estimated the required sample size for a randomized controlled trial to demonstrate these mortality reductions with 80% power. In the overall study population, those with the highest risk ECG score would have a ∼15% relative risk reduction in all-cause mortality with ICD therapy with a NNT to save one life of 27. A randomized controlled trial of 3900 participants would be required to demonstrate this mortality risk reduction. When focusing further on those with the highest risk ECG score and moderate LV dysfunction (LVEF 35–50%), we would estimate a ∼20% relative risk reduction in all-cause mortality (NNT of 22) with an estimated randomized trial sample size of 2900 participants to identify this mortality benefit.
Discussion
In two contemporary cohorts of more than 7000 patients with CHD, easily measured ECG markers specifically predicted SAD as opposed to other modes of death and improved multiple measures of SAD risk stratification compared to standard clinical risk factors alone. After accounting for LVEF, clinical risk factors, and competing mortality risk, ECG markers of anatomic pathology (Q waves, LVH), depolarization abnormality (QRS duration), and repolarization abnormality (JTc prolongation) were each individually associated with SAD. When present together, a high-risk combination of these ECG markers was associated with a three-fold increase in SAD risk in a derivation cohort and a four-fold elevation in SAD risk in an independent validation cohort. Elevation of SAD risk was specific with minimal elevation in the competing risk of non-SAD mortality. The presence of high-risk ECG markers identified clinically meaningful absolute risk, with 5-year SAD event rates of 5.2 and 6.2% in the derivation and validation cohorts, respectively. The addition of the ECG score to a risk model of standard clinical risk factors including LVEF significantly improved indices of reclassification and discrimination for SAD, correctly reclassifying one-third of patients in a validation cohort.
Contemporary approaches to electrocardiographic sudden and/or arrhythmic death risk stratification
In the contemporary era of primary percutaneous intervention and secondary prevention pharmacotherapy, less than 10% of patients with MI develop an LVEF ≤ 35%16 and most SADs occur in patients with an LVEF > 35%.1 To date, clinical SAD risk scores have been specifically developed in patients with severe LV dysfunction and/or heart failure,7 who represent only a minority of the global burden of SAD. Therefore, to meaningfully impact the public health burden of sudden death, pragmatic screening tools for SAD risk stratification in this broader population are needed. Screening with a standard 12-lead ECG, which has already been integrated into the routine care of patients with CHD, would be both feasible and relatively inexpensive. While previous studies have examined the association between ECG measures and SAD in the general population, the clinical applicability of these findings are limited by the low absolute rate of SAD in an unselected community cohort (e.g. 0.05% per year in the general population)17 and the non-specific association of several ECG markers with non-arrhythmic deaths.
Our study builds upon this previous literature in several significant ways. First, we combine multiple ECG markers reflecting distinct domains of arrhythmic risk, accounting for the interplay of multiple risk pathways underlying SAD. We demonstrate complementary contributions from multiple risk domains including anatomic substrate, depolarization abnormality and repolarization abnormality. The association of QRS duration, but not LBBB, may be due to the relatively limited number of patients with LBBB; however, similar findings for QRS duration and LBBB have been previously reported in patients with CHD.18 Taken together, these data suggest that for patients with CHD, QRS widening (of any qualitative type) may capture biologically relevant arrhythmic risk. The lack of association of several of the other markers previously associated with SAD in either general population samples or in patients with cardiomyopathy may be related to differences in underlying arrhythmic substrate and/or widespread use of medications known to alter the systemic autonomic response in this population.
Second, we explicitly account for the competing risk of other causes of death, thus identifying markers which enriched for SAD but not for other causes of death. While prior studies have identified several potentially promising ECG markers associated with elevated relative risks of SAD or composite endpoints that include SAD (e.g. T-wave alternans),19 these studies often did not report on whether these same markers were equally associated with non-SAD endpoints and subsequently were not demonstrated to prospectively identify patients who benefit from ICD implantation when tested in randomized clinical trials.20 In this study, we show that those with a higher number of ECG markers were at specific increased risk of SAD without meaningful elevations of competing deaths such that both absolute and proportional SAD risk were enriched. Third, to enhance the clinical applicability of our findings, we examined easily measured ECG markers that can be readily assessed at the bedside using a 12-lead ECG. Fourth, we studied a cardiovascular disease population at intermediate risk for SAD, where clinically meaningful enrichment of risk is more likely to be observed. While previous studies have demonstrated the potential utility of an ECG-based SAD risk score in the general population,3 , 4 given the low baseline risk of SAD in these general population cohorts, clinically relevant SAD event rates were noted only in the highest risk group after an average of 10–14 years of follow-up. In this study, we demonstrate actionable SAD risk over 5 years, reflecting real-time clinical utility in patients with CHD. Finally, the external validity of our findings was strengthened by evaluation of the derived ECG score in an independent validation cohort with comprehensive autopsy evaluation of death mechanism. While most patients in the derivation cohort had a history of MI, the ECG risk score was robust in the validation cohort where prior MI was less prevalent (48%).
Clinical utility of the electrocardiogram for sudden and/or arrhythmic death risk stratification
Effective SAD risk stratification requires the presence of sufficient baseline SAD risk. In this study, by starting with patients at intermediate baseline SAD risk, we were able to identify a high-risk subpopulation (14% of derivation cohort, 10% of validation cohort) with an absolute 5-year risk of SAD of 5.2–6.2%. This absolute risk was further enriched in patients with moderately depressed LV function (LVEF 35–50%), where the 5-year incidence of SAD was 7.4% in those at high risk by ECG score. These event rates (i.e. ∼5% SAD at 5 years) are similar to those observed in contemporary cardiomyopathy cohorts (LVEF < 35%)21 and are similar to the SAD risk threshold above which current guidelines recommend primary prevention ICD implantation in other cardiovascular disease populations such as patients with hypertrophic cardiomyopathy.22
While the absolute rate of competing (non-arrhythmic) mortality is greater than arrhythmic mortality in patients with CHD, our group6 and others7 have demonstrated that the relative benefit of ICD therapy is projected to be greatest in patients with increasing proportional risk of SAD, as identified here. In support of the incremental utility of the 12-lead ECG as a SAD risk stratification tool, the ECG markers identified in this study correctly reclassified SAD mortality risk in nearly one-third of patients in the validation cohort when compared to the use of standard clinical risk factors including LVEF. Indeed, in this study, we demonstrate that the ECG score enriched for SAD risk across all quartiles of clinical risk factor-based SAD risk. Taken together, our findings represent the validation of a method to specifically identify arrhythmic risk in the context of competing mortality. We believe these electrical markers could be used in concert with other tools including imaging and serum biomarkers, to design randomized clinical trials assessing the efficacy of ICD therapy in patients with coronary disease who are not represented in contemporary guidelines. Here, we demonstrate the feasibility of using the ECG score alone or in concert with moderate LV dysfunction for the design of an achievable randomized controlled trial demonstrating meaningful mortality reductions and clinically relevant numbers needed to treat to save one life with an ICD.
Limitations
Our study has several strengths including significant sample size, rigorous adjudication of modes of death, use of easily measured ECG markers measured in an ECG core laboratory, explicit incorporation of competing risk, and validation of our findings in an independent cohort. However, there are also limitations. First, there are several ECG measures previously associated with SAD which were not explicitly assessed in this study, and the potential utility of a similar multi-domain strategy incorporating these more complex measures may be of interest. Our intent was to use easily and routinely assessed ECG markers in order to maximize the clinical applicability of our findings. Electrocardiographic criteria reflected previously published definitions with established associations with SAD (see Supplementary material online, Table S1 for comprehensive reference list). Second, as comprehensive autopsy and forensic evaluation was not present in all cases of death in the derivation cohort, we cannot rule out the possibility that some patients included in the primary endpoint may have died from non-arrhythmic mechanisms. As such we were careful to define the primary endpoint as SAD. Importantly, forensic autopsy was present in nearly all deaths in the validation cohort, and replication of our results was robust. While we cannot rule out the possibility of misclassification, we would expect any potential misclassification to be non-differential and thus bias our results conservatively towards the null. Third, given the relatively homogeneous ethnic background of patients in the derivation and validation cohorts, our findings warrant evaluation in cohorts with greater diversity. Finally, we only examined a single ECG reflecting arrhythmic substrate at baseline. Whether serial assessment of ECGs or continuous monitoring devices further enhance prediction of arrhythmic risk are reasonable avenues for future investigation.
Conclusion
In summary, an ECG-based score which incorporated complementary domains of arrhythmic risk specifically enriched for clinically significant SAD risk in patients with CHD. Electrocardiographic markers of anatomic pathology (Q waves, LVH), depolarization (QRS duration), and repolarization (JT interval) improved SAD risk stratification in both derivation and validation cohorts when compared to traditional risk factors, including LVEF. The standard 12-lead ECG, in combination with other SAD risk markers, is a promising tool for SAD risk stratification in CHD patients and its use in identifying those who might benefit from preventive sudden death therapies warrants further study.
Supplementary Material
Acknowledgements
We are indebted to the participants in the PRE-DETERMINE Study and their families for their outstanding commitment and cooperation. We would also like to thank Denise Barry, Julie Pester, Ivelina Darvenyashka, Samuel Attaya, Ingrid Ma, and Lucy Phillips for their expert assistance.
Funding
The PRE-DETERMINE Study was supported by a research grant from the National Heart, Lung, and Blood Institute (R01HL091069) and St Jude Medical Inc. and St. Jude Medical Foundation. The funding institutions had no role in the design, analysis, or preparation of the manuscript.
Conflict of interest: none declared.
Contributor Information
Neal A Chatterjee, Division of Cardiology, Department of Medicine, University of Washington, Seattle, USA; Division of Preventive Medicine, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, 900 Commonwealth Avenue, Boston, MA 02215, USA.
Jani T Tikkanen, Division of Preventive Medicine, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, 900 Commonwealth Avenue, Boston, MA 02215, USA; Department of Cardiology, Research Unit of Internal Medicine, University Hospital of Oulu and University of Oulu, Oulu, Finland.
Gopi K Panicker, Cardiac Safety Services, Quintiles, Mumbai, India.
Dhiraj Narula, Mountainview Hospital, Las Vegas, NV, USA.
Daniel C Lee, Department of Medicine, Division of Cardiology, Northwestern University Feinberg School of Medicine, Chicago, IL, USA.
Tuomas Kentta, Department of Cardiology, Research Unit of Internal Medicine, University Hospital of Oulu and University of Oulu, Oulu, Finland.
Juhani M Junttila, Department of Cardiology, Research Unit of Internal Medicine, University Hospital of Oulu and University of Oulu, Oulu, Finland.
Nancy R Cook, Division of Preventive Medicine, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, 900 Commonwealth Avenue, Boston, MA 02215, USA.
Alan Kadish, Department of Medicine, Division of Cardiology, Touro College and University System, New York, NY, USA.
Jeffrey J Goldberger, Department of Medicine, Division of Cardiology, University of Miami Miller School of Medicine, Miami, FL, USA.
Heikki V Huikuri, Department of Cardiology, Research Unit of Internal Medicine, University Hospital of Oulu and University of Oulu, Oulu, Finland.
Christine M Albert, Division of Preventive Medicine, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, 900 Commonwealth Avenue, Boston, MA 02215, USA; Department of Cardiology, Smidt Institute, Cedars Sinai Medical Center, Los Angeles, CA, USA.
References
- 1. Hayashi M, Shimizu W, Albert CM. The spectrum of epidemiology underlying sudden cardiac death. Circ Res 2015;116:1887–1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Stecker EC, Vickers C, Waltz J, Socoteanu C, John BT, Mariani R, McAnulty JH, Gunson K, Jui J, Chugh SS. Population-based analysis of sudden cardiac death with and without left ventricular systolic dysfunction: two-year findings from the Oregon Sudden Unexpected Death Study. J Am Coll Cardiol 2006;47:1161–1166. [DOI] [PubMed] [Google Scholar]
- 3. Aro AL, Reinier K, Rusinaru C, Uy-Evanado A, Darouian N, Phan D, Mack WJ, Jui J, Soliman EZ, Tereshchenko LG, Chugh SS. Electrical risk score beyond the left ventricular ejection fraction: prediction of sudden cardiac death in the Oregon Sudden Unexpected Death Study and the Atherosclerosis Risk in Communities Study. Eur Heart J 2017;38:3017–3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Waks JW, Sitlani CM, Soliman EZ, Kabir M, Ghafoori E, Biggs ML, Henrikson CA, Sotoodehnia N, Biering-Sorensen T, Agarwal SK, Siscovick DS, Post WS, Solomon SD, Buxton AE, Josephson ME, Tereshchenko LG. Global electric heterogeneity risk score for prediction of sudden cardiac death in the general population: the Atherosclerosis Risk in Communities (ARIC) and Cardiovascular Health (CHS) Studies. Circulation 2016;133:2222–2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Elming MB, Nielsen JC, Haarbo J, Videbæk L, Korup E, Signorovitch J, Olesen LL, Hildebrandt P, Steffensen FH, Bruun NE, Eiskjær H, Brandes A, Thøgersen AM, Gustafsson F, Egstrup K, Videbæk R, Hassager C, Svendsen JH, Høfsten DE, Torp-Pedersen C, Pehrson S, Køber L, Thune JJ. Age and outcomes of primary prevention implantable cardioverter-defibrillators in patients with nonischemic systolic heart failure. Circulation 2017;136:1772–1780. [DOI] [PubMed] [Google Scholar]
- 6. Chatterjee NA, Moorthy MV, Pester J, Schaecter A, Panicker GK, Narula D, Lee DC, Goldberger JJ, Kadish A, Cook NR, Albert CM; PRE-DETERMINE Study Group. Sudden death in patients with coronary heart disease without severe systolic dysfunction. JAMA Cardiol 2018;3:591–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bilchick KC, Wang Y, Cheng A, Curtis JP, Dharmarajan K, Stukenborg GJ, Shadman R, Anand I, Lund LH, Dahlstrom U, Sartipy U, Maggioni A, Swedberg K, O’Conner C, Levy WC. Seattle heart failure and proportional risk models predict benefit from implantable cardioverter-defibrillators. J Am Coll Cardiol 2017;69:2606–2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Junttila MJ, Kiviniemi AM, Lepojarvi ES, Tulppo M, Piira OP, Kentta T, Perkiomaki JS, Ukkola OH, Myerburg RJ, Huikuri HV. Type 2 diabetes and coronary artery disease: preserved ejection fraction and sudden cardiac death. Heart Rhythm 2018;15:1450–1456. [DOI] [PubMed] [Google Scholar]
- 9. Panicker GK, Karnad DR, Joshi R, Shetty S, Vyas N, Kothari S, Narula D. Z-score for benchmarking reader competence in a central ECG laboratory. Ann Noninvasive Electrocardiol 2009;14:19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rautaharju PM, Surawicz B, Gettes LS, Bailey JJ, Childers R, Deal BJ, Gorgels A, Hancock EW, Josephson M, Kligfield P, Kors JA, Macfarlane P, Mason JW, Mirvis DM, Okin P, Pahlm O, van Herpen G, Wagner GS, Wellens H. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: Part IV: the ST segment, T and U waves, and the QT interval. J Am Coll Cardiol 2009;53:982–991. [DOI] [PubMed] [Google Scholar]
- 11. Hancock EW, Deal BJ, Mirvis DM, Okin P, Kligfield P, Gettes LS, Bailey JJ, Childers R, Gorgels A, Josephson M, Kors JA, Macfarlane P, Mason JW, Pahlm O, Rautaharju PM, Surawicz B, van Herpen G, Wagner GS, Wellens H. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: Part V: electrocardiogram changes associated with cardiac chamber hypertrophy. J Am Coll Cardiol 2009;53:992–1002. [DOI] [PubMed] [Google Scholar]
- 12. Surawicz B, Childers R, Deal BJ, Gettes LS, Bailey JJ, Gorgels A, Hancock EW, Josephson M, Kligfield P, Kors JA, Macfarlane P, Mason JW, Mirvis DM, Okin P, Pahlm O, Rautaharju PM, van Herpen G, Wagner GS, Wellens H; Heart Rhythm Society. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: part III: intraventricular conduction disturbances. Circulation 2009;119:e235–40. [DOI] [PubMed] [Google Scholar]
- 13. Perkiomaki J, Exner DV, Piira OP, Kavanagh K, Lepojarvi S, Talajic M, Karvonen J, Philippon F, Junttila J, Coutu B, Huikuri H. Heart rate turbulence and T-wave alternans in patients with coronary artery disease: the influence of diabetes. Ann Noninvasive Electrocardiol 2015;20:481–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hinkle LE Jr, Thaler HT. Clinical classification of cardiac deaths. Circulation 1982;65:457–464. [DOI] [PubMed] [Google Scholar]
- 15. Priori SG, Blomström-Lundqvist C, Mazzanti A, Blom N, Borggrefe M, Camm J, Elliott PM, Fitzsimons D, Hatala R, Hindricks G, Kirchhof P, Kjeldsen K, Kuck K-H, Hernandez-Madrid A, Nikolaou N, Norekvål TM, Spaulding C, Van Veldhuisen DJ. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur Heart J 2015;36:2793–2867. [DOI] [PubMed] [Google Scholar]
- 16. Voller H, Kamke W, Klein HU, Block M, Reibis R, Treusch S, Contzen K, Wegscheider K; PreSCD II Registry Investigators. Clinical practice of defibrillator implantation after myocardial infarction: impact of implant time: results from the PreSCD II registry. Europace 2011;13:499–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fishman GI, Chugh SS, DiMarco JP, Albert CM, Anderson ME, Bonow RO, Buxton AE, Chen P-S, Estes M, Jouven X, Kwong R, Lathrop DA, Mascette AM, Nerbonne JM, O'Rourke B, Page RL, Roden DM, Rosenbaum DS, Sotoodehnia N, Trayanova NA, Zheng Z-J. Sudden cardiac death prediction and prevention: report from a National Heart, Lung, and Blood Institute and Heart Rhythm Society Workshop. Circulation 2010;122:2335–2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Teodorescu C, Reinier K, Uy-Evanado A, Navarro J, Mariani R, Gunson K, Jui J, Chugh SS. Prolonged QRS duration on the resting ECG is associated with sudden death risk in coronary disease, independent of prolonged ventricular repolarization. Heart Rhythm 2011;8:1562–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Exner DV, Kavanagh KM, Slawnych MP, Mitchell LB, Ramadan D, Aggarwal SG, Noullett C, Van Schaik A, Mitchell RT, Shibata MA, Gulamhussein S, McMeekin J, Tymchak W, Schnell G, Gillis AM, Sheldon RS, Fick GH, Duff HJ. Noninvasive risk assessment early after a myocardial infarction the REFINE study. J Am Coll Cardiol 2007;50:2275–2284. [DOI] [PubMed] [Google Scholar]
- 20. Costantini O, Hohnloser SH, Kirk MM, Lerman BB, Baker JH 2nd, Sethuraman B, Dettmer MM, Rosenbaum DS. The ABCD (Alternans Before Cardioverter Defibrillator) Trial: strategies using T-wave alternans to improve efficiency of sudden cardiac death prevention. J Am Coll Cardiol 2009;53:471–479. [DOI] [PubMed] [Google Scholar]
- 21. Kober L, Thune JJ, Nielsen JC, Haarbo J, Videbaek L, Korup E, Jensen G, Hildebrandt P, Steffensen FH, Bruun NE, Eiskjaer H, Brandes A, Thogersen AM, Gustafsson F, Egstrup K, Videbaek R, Hassager C, Svendsen JH, Hofsten DE, Torp-Pedersen C, Pehrson S, Investigators D. Defibrillator implantation in patients with nonischemic systolic heart failure. N Engl J Med 2016;375:1221–1230. [DOI] [PubMed] [Google Scholar]
- 22. O’Mahony C, Jichi F, Ommen SR, Christiaans I, Arbustini E, Garcia-Pavia P, Cecchi F, Olivotto I, Kitaoka H, Gotsman I, Carr-White G, Mogensen J, Antoniades L, Mohiddin SA, Maurer MS, Tang HC, Geske JB, Siontis KC, Mahmoud KD, Vermeer A, Wilde A, Favalli V, Guttmann OP, Gallego-Delgado M, Dominguez F, Tanini I, Kubo T, Keren A, Bueser T, Waters S, Issa IF, Malcolmson J, Burns T, Sekhri N, Hoeger CW, Omar RZ, Elliott PM. International External Validation Study of the 2014 European Society of Cardiology Guidelines on Sudden Cardiac Death Prevention in Hypertrophic Cardiomyopathy (EVIDENCE-HCM). Circulation 2018;137:1015–1023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.