Abstract
Prey have evolved a number of defenses against predation, and predators have developed means of countering these protective measures. Although caterpillars of the monarch butterfly,Danaus plexippus L., are defended by cardenolides sequestered from their host plants, the Chinese mantidTenodera sinensis Saussure guts the caterpillar before consuming the rest of the body. We hypothesized that this gutting behavior might be driven by the heterogeneous quality of prey tissue with respect to toxicity and/or nutrients. We conducted behavioral trials in which mantids were offered cardenolide-containing and cardenolide-freeD. plexippus caterpillars and butterflies. In addition, we fed mantids starved and unstarvedD. plexippus caterpillars from each cardenolide treatment and nontoxicOstrinia nubilalis Hübner caterpillars. These trials were coupled with elemental analysis of the gut and body tissues of bothD. plexippus caterpillars and corn borers. Cardenolides did not affect mantid behavior: mantids gutted both cardenolide-containing and cardenolide-free caterpillars. In contrast, mantids consumed bothO. nubilalis and starvedD. plexippus caterpillars entirely.Danaus plexippus body tissue has a lower C:N ratio than their gut contents, whileO. nubilalis have similar ratios; gutting may reflect the mantid’s ability to regulate nutrient uptake. Our results suggest that post-capture prey processing by mantids is likely driven by a sophisticated assessment of resource quality.
Keywords: Danaus plexippus, Ostrinia nubilalis, Tenodera sinensis, cardenolide, prey processing
Prey utilize an array of defenses against predation (reviewed inLima and Dill 1990). Organisms can, for instance, avoid detection via crypsis or disruptive coloration that makes it difficult for predators to identify the boundaries of the prey’s body. Prey can also use behavioral measures to decrease their likelihood of attracting a predator: Veeries,Catharus fuscescens Stephens, respond to predation risk by decreasing the rate and length of their songs (Schmidt and Belinsky 2013). Once detected, prey can use secondary defenses such as aggressive or escape behaviors as well as morphological and/or chemical defenses (Ruxton et al. 2004). The presence of trout, for example, can cause macroinvertebrates to alter their drift rates and foraging activity (Simon and Townsend 2003,Eby et al. 2006), as well as their microhabitat use (Lima 1998). Morphological changes are also possible:Daphnia pulex Leydig respond to predator cues by producing fewer, but larger, offspring with prominent neck spines (Luening 1994) that make the prey more difficult for predators to attack.
Organisms that lack behavioral and/or morphological defenses may instead deter predation via the production or sequestration of noxious chemical compounds. Prey that adopt this strategy typically possess aposematic coloration that advertises their toxicity (Duffey 1980,Nishida 2002,Ruxton et al. 2004). The nudibranchCratena peregrina Gmelin, for example, uses bright coloration to display its unpalatability to fish predators (Aguado and Marin 2007). In insects, chemical defense and aposematism occur in multiple orders, including the Hemiptera, Lepidoptera, Coleoptera, and Hymenoptera. Hemipteran milkweed bugs,Oncopeltus fasciatus Dallas, feed on cardenolide-rich host plants and sequester these toxins in their bodies; their contrasting orange-and-black coloration alerts predators to their toxicity (Scudder et al. 1986). Another insect that feeds on milkweed, the Oleander aphid,Aphis nerii Boyer de Fonscolombe, also sequesters cardenolides and is brightly yellow-and-black colored (Malcolm 1990).
Although chemically based antipredator defenses are often highly effective, predators have developed a variety of techniques for overcoming them. Floodplain death adders,Acanthophis praelongus Ramsay, prey on toxic frogs by biting the prey, injecting it with toxins, and then releasing it. The adder's toxins kill the frog, whose own defensive toxins degrade after it has died; the snake can then eat the formerly toxic frog without any ill effects (Phillips and Shine 2007). Loggerhead Shrikes,Lanius ludovicianus Mearnsi, use a similar strategy for feeding on chemically defended lubber grasshoppers,Romalea guttata Beauvois. Grasshoppers captured by the birds are impaled on thorns or barbed wire; the shrikes only return to feed on them once the grasshoppers' defensive toxins have been degraded and their aposematic coloration fades (Yosef 1992). Other predators process prey to feed selectively on the most palatable portion of the prey (Glendinning 2007) or regulate their toxicity burden (Skelhorn and Rowe 2007).
The monarch butterfly,Danaus plexippus L., is chemically defended and aposematically colored in both the black-and-yellow larval and black-and-orange adult stage. Their caterpillars sequester toxins when feeding on cardenolide-containing host plants in the genusAsclepias (Aponcynaceae) (Agrawal et al. 2012). Despite this generally effective chemical defense,D. plexippus is susceptible to predation across all life stages. Its invertebrate predators include ants,Formica montana Wheeler, ladybird beetles,Harmonia axyridis Pallas (Koch et al. 2003,Prysby 2004), and predatoryPolistes (Rayor 2004) andVespula wasps (Leong et al. 1990). Birds such as Orioles,Icterus spp., Grosbeaks,Pheucticus spp. (Nishida 2002), and other vertebrate predators such asPeromyscus mice also feed onD. plexippus (Glendinning 1990).
Danaus plexippus caterpillars are also preyed upon by an invasive generalist predator, the Chinese mantid,Tenodera sinensis Saussure (DJ Cox, personal observation). We have previously found (Rafter et al. 2013) that mantids consuming toxicD. plexippus caterpillars actively reject the gut material, allowing it to fall from the body. However, they consume nontoxic lepidopterans such as European corn borers,Ostrinia nubilalis Hübner, and wax worms,Galleria mellonella L., in their entirety. These results suggest that the mantids' gutting behavior may be a behavioral mechanism for avoiding prey toxicity. A follow-up analysis of cardenolide levels, however, found that the mantid-discarded guts and mantid-consumed bodies ofD. plexippus caterpillars contain similar cardenolide concentrations (although the two portions were composed of different individual cardenolides). We also found that gut material has a higher C:N ratio than body material, potentially making it less nutritious for this species (although nutrient requirements are unknown). As a result, the mantids' gutting behavior may reflect either their avoidance of individual cardenolides or their preference to feed selectively on the most nitrogen-rich portions of their prey (Rafter et al. 2013). Our aim was to test these specific hypotheses by conducting a series of behavioral trials in which we observed mantid prey handling behavior when presented withD. plexippus caterpillars reared on toxic cardenolide-containing and control no-cardenolide host plants. We paired the results of this experiment with other work in which we fed mantids starved and unstarved larvalD. plexippus reared on the two host plants, adultD. plexippus reared on the two host plants, and nontoxic European corn borers. Unlike in our previous work, we reared all insects (exceptO. nubilalis) in the lab. Thus, the mantids were naive to each prey type. This allowed for further understanding of the innate behaviors exhibited by mantids when presented with a novel prey type. Our results suggest that post-capture prey processing by mantids is likely driven by an assessment of resource quality.
Materials and Methods
Mantid Rearing and Maintenance
We collected a singleTenodera sinensis egg mass in early April 2012 from an abandoned agricultural field at East Farm (Kingston, RI). It was returned to the lab and maintained at 25 °C in a 50 by 25 by 30-cm Plexiglass aquarium until the eggs began to hatch. One day after hatching, nymphs were each placed in individual 1.9-liter mason jars; the top of each jar was replaced with mosquito netting for ventilation. Because they emerged from a single egg mass, all nymphs were either full- or half-sibs; using related individuals in controlled experiments is a commonly used means for minimizing the magnitude of uncontrolled population-level variation (Beukeboom and Zwaan 2005). A single stick was provided for perching; when mantids reached the fourth instar, the stick was replaced with a mesh strip secured under the lid. Water was provided using a water wick made from capped soufflé cups and braided dental cotton inserted through a hole in the lid. The jars were held in a Percival growth chamber with a photoperiod of 16:8 (L:D) h and 60–80% humidity at 25˚C during lighted hours and 23˚C during dark hours. The remaining mantids from the egg mass were communally raised in two 50 by 25 by 30-cm aquaria. Each aquarium had several sticks arranged for perching sites. Mantids in both the jars and the aquaria were fed lab-reared apterous fruit flies,Drosophila melanogaster Meigen, for the first four instars; following this, they were fed appropriately sized crickets (Acheta domesticus L.). Because crickets will prey on mantids during the molting process, we tested for satiation by using forceps to offer each mantid a cricket before adding crickets to its jar. If the mantid refused to attack the cricket, we assumed it was preparing to molt and did not feed it that day. Mantids that accepted the cricket were fed two additional crickets; we deterred crickets from attacking the mantids by adding fruit flies to the jars for the crickets to eat. Because early-instar mantids have high mortality rates, we replaced any dead Percival-reared mantids with a communally raised sibling of similar size and developmental stage; we stopped this replacement once a majority of Percival-reared mantids reached the sixth instar. Once mantids reached adulthood, they were fed three crickets daily and no fruit flies. Jars containing adult mantids were removed from the Percival and kept in the lab at ambient room temperature with a photoperiod of 16:8 (L:D) h.
Experiment 1: Do Mantids Handle Toxic (Cardenolide-Containing Host Plant) and Nontoxic (No-Cardenolide Host Plant)D. plexippus Caterpillars Differently?
This experiment tested whether mantids varied in their behavior towardD. plexippus caterpillars raised on toxic (i.e., cardenolide-containing) and nontoxic (no-cardenolide) host plants. It tests the hypothesis that the mantids' gutting behavior is a response to the presence of cardenolides inD. plexippus gut tissue. Two hundredD. plexippus eggs were purchased from Flutterby Gardens (Bradenton, FL) and reared in 50 by 25 by 30-cm aquaria. Half of the emerging larvae were reared on a cardenolide-containing host plant, the common milkweedAsclepias syriaca L.; the other half of the emerging larvae were reared on a zero-cardenolide host plant, the swamp milkweedAsclepiasincarnata L.Asclepias syriaca plants were grown from seed, whileA. incarnata plugs were purchased from Northcreek Nursery (Landenberg, PA).
Twenty lepidopteran-naïve adult mantids were randomly assigned to consume late-instarD. plexippus larvae raised on eitherA. syriaca (10 mantids) orA. incarnata (10 mantids) host plants. All mantids were starved for 3 d prior to the trial. At the start of each trial, each mantid was weighed, placed into a pre-weighed 23.3 by 15.5 by 16.5-cm plastic container, and allowed to acclimate for 5 min. After the 5-min acclimation period, a pre-weighed caterpillar was placed into the enclosure. We video-recorded each trial from the moment the prey item was placed in the enclosure until the end of the trial. The mantid was given 10 min to orient on the prey. If the mantid did not orient within this period, the trial ended. Mantids that oriented were given an additional 10 min to attack the prey. If the mantid did not attack during this period, the trial ended. If the mantid attacked, we recorded 5 min of video following the attack. At the same time, we recorded whether or not the mantid gutted the prey. Every mantid was tested every day for 6 d during the experiment. Once an individual mantid had attacked prey in two separate trials, we disturbed the remaining trials in which the mantid attacked so that we could collect mantid-dissected gut and body material for CHN (Carbon, hydrogen, nitrogen) analysis. Gut material was collected in a 2-ml pre-weighed screw-cap tube as it fell from the caterpillar. We then pried the remaining cadaver from the mantid and placed it into a second tube. This material was frozen at −13˚C until analyzed.
Experiment 2: Does the Presence of Plant Material in the Caterpillar Gut Affect How Mantids Handle “Toxic” (Cardenolide-Containing Host Plant) and “Nontoxic” (No-Cardenolide Host Plant)D. plexippus Caterpillars?
This experiment tested whether mantid behavior varied as a function of the presence or absence of plant material in the gut ofD. plexippus caterpillars reared on cardenolide-containing and no-cardenolide host plants. It tests the hypothesis that mantid gutting behavior is driven by the presence of plant materialper se rather than by cardenolide concentrations. This experiment was conducted identically to Experiment 1 (and used the same mantids), but added an additional experimental factor: the presence (“unstarved”) or absence (“starved”) of plant material in the caterpillar gut. The 10 mantids that had previously been fed cardenolide-containingD. plexippus caterpillars were split into two groups of five mantids. Mantids in one of the five-mantid groups were fed starvedD. plexippus caterpillars whose guts were free of plant material (“starved” treatment); mantids in the other five-mantid group were fedD. plexippus caterpillars whose guts were filled with plant material (“unstarved” treatment). This design was replicated for the 10 mantids that had previously been fed no-cardenolideD. plexippus caterpillars, for a total of four five-mantid treatments: starved toxic caterpillars, unstarved toxic caterpillars, starved nontoxic caterpillars, and unstarved nontoxic caterpillars. As in Experiment 1, toxicD. plexippus caterpillars were raised onA. syriaca and nontoxicD. plexippus caterpillars were raised onA. incarnata. Starved caterpillars were kept without food for 24 h to clear their guts of any plant material; any mantid-attacked “starved” caterpillars whose guts still contained trace amounts of plant material (apparent as undigested green material within the gut) were excluded from our analysis. Mantid–D. plexippus interaction trials were conducted for 6 d following the same procedure as in the first experiment. We collected caterpillar biomass for chemical analysis once individual mantids attacked twice.
Experiment 3: Do Mantids Handle Toxic (Cardenolide-Containing Host Plant) and Nontoxic (No-Cardenolide Host Plant) AdultD. plexippus Differently?
This experiment tested whether mantids differed in their handling behavior of adultD. plexippus butterflies reared on cardenolide-containing versus no-cardenolide host plants. AdultD. plexippus are nectar feeders that no longer consume cardenolides; the experiment tested the hypothesis that this ontogenic shift affected how mantids responded toD. plexippus reared on different hosts. TwelveD. plexippus caterpillars were reared to adulthood, six onA. syriaca and six onA. incarnata. Twelve mantids used in Experiments 1 and 2 (six that were fedA. syriaca caterpillars, and six that were fedA. incarnata caterpillars) were each fed a singleA. syriaca-reared adult butterfly or a singleA. incarnata-reared adult butterfly, respectively. For each trial, we noted if the butterfly was gutted and which body parts were discarded by the mantid; all 12 trials took place on the same day.
Experiment 4: Do Mantids Handle LarvalO. nubilalis Differently ThanD. plexippus?
This experiment repeated previously published work (Rafter et al. 2013) finding that nontoxicO. nubilalis larvae were consumed in their entirety by mantids that would gutA. syriaca-rearedD. plexippus caterpillars. The current experiment was designed to confirm the results of the 2011 experiment and provide more precise information on how mantids handle prey that do not sequester toxic compounds from their host plant and that may be of higher nutritional value (i.e., lower C:N ratio). Because of the difficulty in finding sufficient late-instar caterpillars, the experiment was conducted in two stages (=trials). In the first trial of this experiment, we presented each of 16 lepidopteran-naïve mantids with one late-instarO. nubilalis caterpillar collected from organically grown flint corn,Zea mays L., growing in an experimental farm. The second trial was essentially identical to the first, but took place 2 wk later: in it, we presented each of 12 naïve mantids with one late-instarO. nubilalis. Caterpillars were always collected on the day of the trial; both trials lasted one day. Data collection procedures were as above. If mantids did not gut the caterpillars, we froze whole caterpillars and later dissected the caterpillars to isolate the gut and body portions for chemical analysis.
Chemical Analysis
All of the preserved caterpillar biomass was stored in plastic tubes and dried in a 45 °C drying oven for 5 d. After drying was complete and samples were ground and homogenized, 1.0–2.0 mg of dried material was removed from each sample and sent for CHN analysis to the Analytic Chemistry Lab at the University of Rhode Island's Graduate School of Oceanography (Narragansett, RI).
To ensure that cardenolide content differed betweenA. syriaca andA. incarnata, and between caterpillars reared on these two host plants, we analyzed the cardenolide content of plant tissue from bothAsclepias species and body tissue from monarch caterpillars fed exclusively on eitherA. syriaca orA. incarnata. Fresh leaf and caterpillar tissue was stored, dried, ground, and homogenized as above. Powdered tissue was extracted at 2 °C in 95% ethanol at a ratio of 1 ml to 100 mg tissue for 48 h with occasional vortexing, and the 9,000 × g supernatant was used directly as the source of cardenolides. The commercially available 3,5-dinitrobenzoic acid (Sigma 121258;Rowson 1952,Dobler and Rowell-Rahier 1994) was used in place of 2,2′,4,4′-tetranitrodiphenyl (Brower et al. 1984). In triplicate wells of a Griner UV-Star 96-well microplate (Monroe, NC), 50 µl sample was mixed with 50 µl 2% (w:v) 3,5-dintrobenzoic acid in 100% ethanol, allowed to incubate at room temperature for 1 min, and then 100 µl 3% NaOH in 100% ethanol was added to each well. The plate was incubated at room temperature for 10 min and then the absorbance quantified at 535 nm with a Spectramax M2 Multi-Mode spectrophotometer (Molecular Devices, Sunnydale, CA). Triplicate control wells with 100% ethanol replacing 2% 3,5-dinitrobenzoic acid in 100% ethanol were used to correct for background absorbance, and cardenolide content was expressed as µg digitoxin equivalents per mg dry weight (µg mg−1 DW).
Statistical Analysis
As post-attack prey handling behavior by mantids (all of which fed multiple times in respective trials) did not vary (see results), statistical analysis was unnecessary for these data. Results from the CHN analysis were used to determine the percent carbon and nitrogen in both gut and body tissues and calculate their carbon/nitrogen (C:N) ratios. We analyzed theD. plexippus data using a two-way ANOVA that crossed the main factors toxicity (cardenolide-containing or cardenolide-free caterpillars) and body tissue (gut vs. body). We analyzed theO. nubilalis data using a one-way ANOVA with the main factor body tissue (gut vs. body). Where appropriate, we determined among-treatment differences using Tukey–Kramer HSD. All analyses were performed using JMP 9 (SAS Institute, Inc).
Results
Cardenolide Concentrations inA. syriaca, A. incarnata, and the Body Tissues of Monarch Larvae Fed Exclusively on Either Plant Species
Cardenolide content is expressed as μg digitoxin equivalents per mg of dry weight (μg mg − 1 DW).Asclepias syriaca tissue contained 5.32 ± 0.60 [SE] µg mg − 1 DW; the body tissue of larvae fed onA. syriaca also contained cardenolides (3.19 ± 0.35 µg mg − 1 DW). NeitherA. incarnata nor the body tissue of larvae fed onA. incarnata contained cardenolides at levels detectable with our assay (both 0.0 µg mg − 1 DW).
Experiment 1: Do Mantids Handle Toxic (Cardenolide-Containing Host Plant) And Nontoxic (No-Cardenolide Host Plant)D. plexippus Caterpillars Differently?
We observed 117 predator–prey interactions; predators attacked the prey in 64 of 114 cases (three caterpillars infected with a fungal pathogen were excluded from the analysis). Regardless of treatment, mantids gutted all theD. plexippus caterpillars they attacked (31/31 nontoxic and 33/33 toxic caterpillars, respectively).
Experiment 2: Does the Presence of Plant Material in the Caterpillar Gut Affect How Mantids Handle Toxic (Cardenolide-Containing Host Plant) and Nontoxic (No-Cardenolide Host Plant)D. plexippus Caterpillars?
We observed 113 predator–prey interactions; mantids attacked the prey in 20 of the 113 interactions. As in Experiment 1, mantid behavior was unaffected by toxicity and they gutted all (12/12) of the unstarved prey but none (0/8) of the starved prey.
Experiment 3: Do Mantids Handle Toxic (Cardenolide-Containing Host Plant) and Nontoxic (No-Cardenolide Host Plant) AdultD. plexippus Butterflies Differently?
We observed 12 predator–prey interactions (six for each toxicity treatment). Mantids did not gut any of the adult butterflies regardless of the larval host plant. In each case, mantids consumed the body while discarding the wings, antennae, and legs. Some mantids appeared to “taste” the wings, but stopped and returned to feeding on the body.
Experiment 4: Do Mantids HandleO. nubilalis Differently ThanD. plexippus?
We observed a total of 28 predator–prey interactions; mantids attacked the prey in 13 of the 28 interactions. In the first trial, six of seven caterpillars were not gutted, and in the remaining case, the mantid stopped feeding entirely. In the second trial, 6 of 6 caterpillars were not gutted.
Carbon and Nitrogen Concentrations
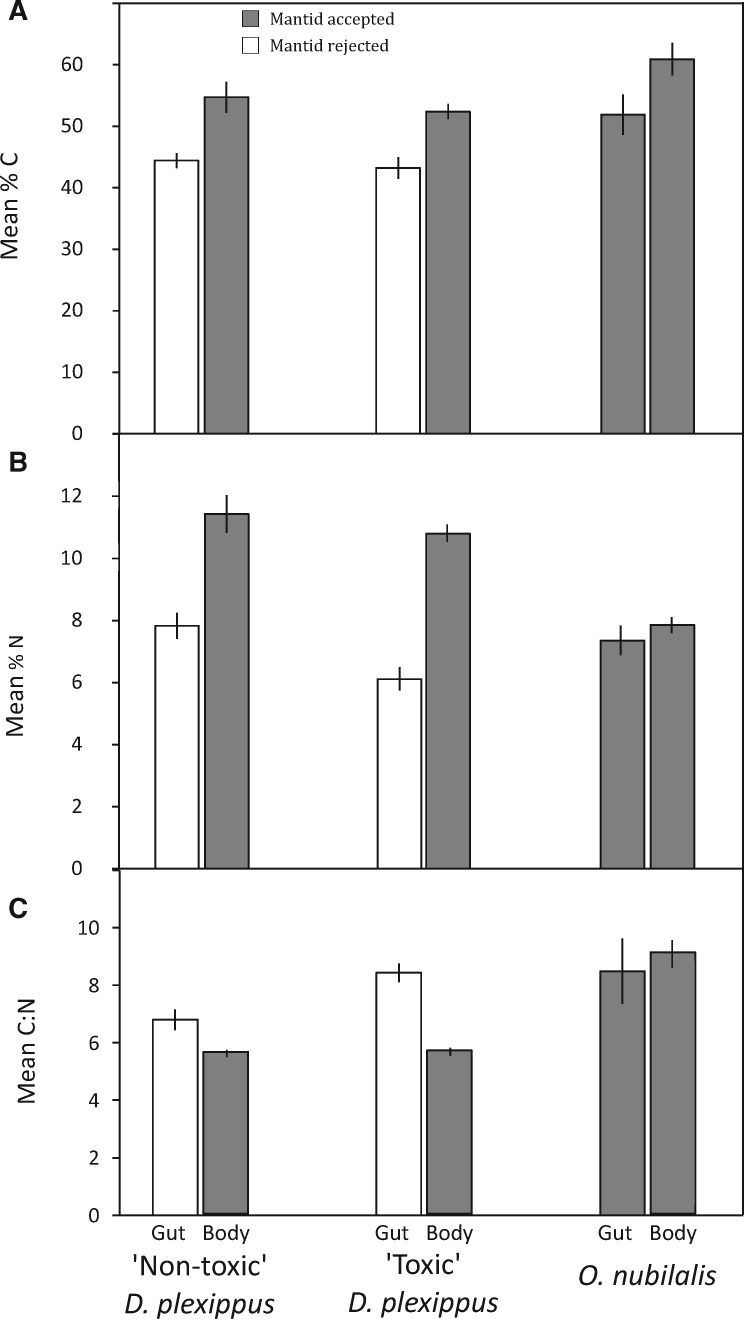
Percent carbon (Fig. 1A) was significantly higher in the mantid-consumed body tissue than in the mantid-discarded gut tissue ofD. plexippus caterpillars (F3,53 = 31.3,P < 0.001). This did not differ between toxic and nontoxicD. plexippus (F3,53 = 1.03,P = 0.31), and there was no interaction between these factors (F3,53 = 0.10,P = 0.75). Percent nitrogen (Fig. 1B) was also higher in body versus gut tissue, and in nontoxicD. plexippus (F3,53 = 94.0,P < 0.001 andF3,53 = 7.47,P < 0.001, respectively); however, the interaction was not significant (F3,53 = 1.64,P = 0.21). The resulting C:N ratio (Fig. 1C) forD. plexippus was higher in the gut versus body tissue, and higher in toxic versus nontoxic caterpillars (F3,53 = 57.3,P < 0.001 andF3,53 = 10.6,P = 0.002, respectively), and there was a significant interaction (F3,53 = 9.27,P = 0.004). In contrast, there was no difference in the percent carbon, nitrogen, and C:N ratio inO. nubilalis guts and bodies (F1,9 = 4.52,P = 0.066;F1,9 = 0.83,P = 0.39; andF1,9 = 0.24,P = 0.64, respectively). ForD. plexippus, the C:N ratio of mantid-consumed body tissue was lower than the C:N ratio of mantid-discarded gut tissue; however, mantids eagerly consumedO. nubilalis tissue with C:N ratios equal to or greater than those of theD. plexippus gut. In other words, mantids consumed tissues with both a higher and lower C:N ratio than theD. plexippus guts they rejected.
Fig. 1.
(a) Mean percent of carbon (C) present in each prey and tissue type ± 1 SE. (B) Mean percent of nitrogen (N) present in each prey and tissue type ± 1 SE. (C) Mean C:N ratio of each prey and tissue type ± 1 SE.
Discussion
We found no evidence thatD. plexippus-sequestered cardenolides affected mantid prey handling behavior. Specifically,T. sinensis behaved similarly towardD. plexippus larvae (Experiments 1–2) and adults (Experiment 3) reared on cardenolide-containingA. syriaca versus no-cardenolideA. incarnata. As these mantids were lab-reared, their inability or unwillingness to discriminate between cardenolide-containing versus no-cardenolideD. plexippus gut tissue must be innate. The lack of a behavioral response toD. plexippus adults seems appropriate given that mantids experienced no apparent ill-effects from consuming the cardenolide-laden bodies (Rafter et al. 2013) ofD. plexippus caterpillars fedA. syriaca.
The addition of a starved or unstarved caterpillar treatment to Experiment 2 revealed that the mantids’ gutting behavior reflects the active rejection of partially digested plant material found within the gut. This suggests that rather than avoiding cardenolides, mantids may instead be avoiding the lower-quality (higher C:N ratio) plant material found in the gut tissue (Fig. 1C). This interpretation is further supported by the third experiment that found mantids did not gut adultD. plexippus, nectar feeders whose guts are free of plant material. While our threeD. plexippus experiments support the “food quality” hypothesis for the mantids’ gutting behavior, the results of our fourth experiment (O. nubilalis trials) do not. In this experiment, which was intended to confirm results first reported inRafter et al (2013), we again found that mantids readily consumeO. nubilalis gut and body tissue. The results of our first three experiments led us to hypothesize that the gut material ofO. nubilalis caterpillars would be of higher nutritional quality (as indicated by the C:N ratio) than the mantid-discarded portions ofD. plexippus caterpillars. While we found that bothO. nubilalis gut and body tissue were relatively high in C and N (Fig. 1A and 1B, respectively), the C:N ratio of mantid-acceptedO. nubilalis gut tissue equaled or exceeded those of mantid-rejectedD. plexippus gut tissue (Fig. 1C). Researchers commonly use C:N ratios as a proxy for nutritional quality of food types and have been able to relate nutrient quality to prey selectivity (Zandonà et al. 2011). However, given the inconsistency in mantid preference for tissues in relation to their respective C:N ratios, this metric does not appear to explain the gutting behavior. It may be that mantids are not responding to a specific C:N ratioper se, but rather are processing prey based on detectable differences in the nutritional quality of prey gut content versus body tissues. The gut content ofD. plexippus is largely undigested leaf material low in nutrients and high in indigestible cellulose, while that ofO. nubilalis is largely undigested corn that is higher in nutrients and lower in cellulose. The gut and body tissues ofD. plexippus differ markedly in their chemical signatures with respect to carbon, nitrogen, and the resulting C:N ratio, while those ofO. nubilalis do not (Fig. 1). Mantids may gutD. plexippus larva to maximize intake of high-quality body tissues, but consumeO. nubilalis entirely because the nutritional quality of their guts and body tissue is similar.
AlthoughT. sinensis appears to be insensitive to the presence of cardenolide inD. plexippus caterpillars, it does exhibit an adverse reaction when consuming cardenolide-sequestering milkweed bugs,Oncopeltus fasciatus. They quickly learn to reject and will eventually completely avoid this prey after few encounters (Berenbaum and Miliczky 1984,Paradise and Stamp 1991). This suggests that the Chinese mantid is tolerant of, rather than unaffected by, cardenolide consumption. Milkweed bugs uptake cardenolides more efficiently and at substantially higher concentrations than doD. plexippus (Scudder et al. 1986,Agrawal et al. 2012); mantids may be intolerant to the higher cardenolide concentrations found in milkweed bugs.
An alternate hypothesis for the mantid’s gutting behavior is that they may be responding to the presence of other secondary plant compounds found in prey biomass. AdultD. plexippus have been shown to feed on plants containing pyrrolizidine alkaloids and sequester these compounds; these compounds may play a role in defending adultD. plexippus against both vertebrate and invertebrate predators (Kelley et al. 1987,Stelljes and Seiber 1990). These compounds are sequestered during the adult stage, however, andD. plexippus butterflies were fed sugar water in this experiment. To our knowledge, there are no reports ofD. plexippus caterpillars sequestering toxins other than cardenolides. However, plants often use a suite of defenses against herbivory and maintain multiple defense strategies with little cost (Koricheva et al. 2004). Thus, there are a number of potential toxins that mantids could be responding to in the plant material found in the caterpillar’s gut. Many cardenolide-containing plants in the Apocynaceae, including genusAsclepias, also contain alkaloids (Agrawal et al. 2012). In addition, althoughA. incarnata is cardenolide-free, it is not undefended. Both the roots and aboveground biomass contain pregnane glycosides (Warashina and Noro 2000a,b) that are inducible defenses against herbivory (A. Agrawal, personal communication). If mantids are unable to tolerate compounds found in undigested plant material, they might respond by gutting the caterpillar.
Our results may also be influenced by the fact thatD. plexippus caterpillars and European corn borers feed on different parts of their respective host plants;D. plexippus feed on leaves, while corn borers feed on seeds. Corn has been selectively bred for human consumption and is thus relatively undefended compared with milkweed leaves. This further supports the idea that mantids may be guttingD. plexippus because of their intolerance to plant compounds found in the leaves ofAsclepias plants. A number of other species are able to process food items in response to toxicity. Tanagers,Pipraeida melanonota Vieillot, reduce the toxicity of ithomiine moths by chewing on them until the abdominal content is expelled; they then eat the abdominal contents while leaving the rest behind (Brown and Neto 1976). The European paper waspPolistes dominula Christ will gutPieris napi L. caterpillars that were reared on toxic host plants, but not those that were reared on nontoxic plants (Rayor et al. 2007). Herbivores such as the meadow vole will cut branches from conifers and leave them uneaten for several days until tannins and phenolic concentrations are reduced sufficiently for the vegetation to be palatable (Roy and Bergeron 1990). Mantids may be similarly reducing their toxin burden by processing prey.
The results of our work illustrate the unexpectedly complex mechanisms determining how Chinese mantids process lepidopteran prey. This predator is responding to a number of chemical cues, as it consumes prey items that are heterogeneous in nutritional value and degree of toxicity. Because mantids did not respond to cardenolides inD. plexippus, it seems most likely that their gutting behavior is driven instead by other plant secondary compounds and/or the nutritional quality of prey tissue. Irrespective of mechanism, this mantid’s ability to efficiently process toxic and nontoxic prey is likely important in allowing this non-native generalist predator to utilize a wide array of prey taxa.
Acknowledgments
We gratefully acknowledge L. Hurd, whose willingness to discuss these ideas and offer suggestions led to this manuscript. R. Casagrande and L. Tewksbury assisted with insect husbandry; S. Alm, N. Castrataro, and E. Roberts assisted with plant propagation and maintenance; L. Elder, J. Hayward, C. Klein, N. Le Claire-Conway, L. Rafter, and L. Westbrook assisted with mantid rearing; A. Agrawal, R. Casagrande, M. Hickin, and C. Thornber provided comments to improve the manuscript. Funding was provided by a Sigma Xi Grant-in-Aid of Research, a University of Rhode Island Enhancement of Graduate Research Award, and NIFA 2011-67013-30142 to E.P.
References Cited
- Agrawal A. A.,Petschenka G.,Bingham R. A.,Weber M. G.,Rasmann S.. 2012. Toxic cardenolides: Chemical ecology and coevolution of specialized plant–herbivore interactions.New Phytol.194:28–45. [DOI] [PubMed] [Google Scholar]
- Aguado F.,Marin A.. 2007. Warning coloration associated with nematocyst-based defences in aeolidiodean nudibranchs.J. Molluscan Stud.73:23–28. [Google Scholar]
- Berenbaum M. R.,Miliczky E.. 1984. Mantids and milkweed bugs: Efficacy of aposematic coloration against invertebrate predators.Am. Midl. Nat.111:64–68. [Google Scholar]
- Beukeboom L. W.,Zwaan B. J.. 2005Genetics, pp.167–218.InJervis M. A. (ed.),Insects as natural enemies: A practical perspective. Springer, Dordrecht. [Google Scholar]
- Brower L. P.,Seiber J. N.,Nelson C. J.,Lynch S. P.,Hoggard M. P.,Cohen J. A.. 1984. Plant-determined variation in cardenolide content and thin-layer chromatography profiles of monarch butterflies,Danaus plexippus reared on milkweed plants in California: 3.Asclepias Calif. J. Chem. Ecol.10:1823–1857. [DOI] [PubMed] [Google Scholar]
- Brown K.S.J.,Neto J. V.. 1976. Predation on aposematic Ithomiine butterflies by tanagers,Pipraeidea melanonota.Biotropica 8:136–141. [Google Scholar]
- Dobler S.,Rowell-Rahier M.. 1994. Production of cardenolides versus sequestration of pyrrolizidine alkaloids in larvae ofOreina species (Coleoptera, Chrysomelidae).J. Chem. Ecol.20:555–568. [DOI] [PubMed] [Google Scholar]
- Duffey S. S. 1980. Sequestration of plant natural products by insects.Annu. Rev. Entomol.25:447–477. [Google Scholar]
- Eby L. A.,Roach W. J.,Crowder L. B.,Stanford J. A.. 2006. Effects of stocking-up freshwater food webs.Trends Ecol. Evol.21:576–584. [DOI] [PubMed] [Google Scholar]
- Glendinning J. I. 1990. Responses of three mouse species to deterrent chemicals in the monarch butterfly. II. Taste tests using intact monarchs.Chemoecology 1:124–130. [Google Scholar]
- Glendinning J. I. 2007. How do predators cope with chemically defended foods? Biol. Bull.213:252–266. [DOI] [PubMed] [Google Scholar]
- Kelley R. B.,Seiber J. N.,Jones A. D.,Segall H. J.,Brower L. P.. 1987. Pyrrolizidine alkaloids in overwintering Monarh butterflies (Danaus plexippus) from Mexico.Experientia 43:943–946. [Google Scholar]
- Koch R. L.,Hutchison W. D.,Venette R. C.,Heimpel G. E.. 2003. Susceptibility of immature monarch butterfly,Danaus plexippus (Lepidoptera: Nymphalidae: Danainae), to predation byHarmonia axyridis (Coleoptera: Coccinellidae).Biol. Control 28:265–270. [Google Scholar]
- Koricheva J.,Nykänen H.,Gianoli E.. 2004. Meta-analysis of trade-offs among plant antiherbivore defenses: Are plants jacks-of-all-trades, masters of all? Am. Nat.163:E64–E75. [DOI] [PubMed] [Google Scholar]
- Leong K.,Frey D.,Nagano C.. 1990. Wasp predation on overwintering monarch butterflies (Lepidoptera: Danaidae) in central California.Pan-Pacific Entomol.66:326–328. [Google Scholar]
- Lima S. L. 1998. Nonlethal effects in the ecology of predator-prey interactions.BioScience 48:25–34. [Google Scholar]
- Lima S. L.,Dill L. M.. 1990. Behavioral decisions made under the risk of predation: A review and prospectus.Can. J. Zool.68:619–640. [Google Scholar]
- Luening J. 1994. Anti-predator defenses inDaphnia: Are life-history changes always linked to induced neck spines? Oikos 69:427–436. [Google Scholar]
- Malcolm S. 1990. Chemical defence in chewing and sucking insect herbivores: Plant-derived cardenolides in the monarch butterfly and oleander aphid.Chemoecology 1:12–21. [Google Scholar]
- Nishida R. 2002. Sequestration of defensive substances from plants by Lepidoptera.Annu. Rev. Entomol.47:57–92. [DOI] [PubMed] [Google Scholar]
- Paradise C. J.,Stamp N. E.. 1991. Prey recognition time of praying mantids (Dictyoptera, Mantidae) and consequent survivorship of unpalatable prey (Hemiptera, Lygaeidae).J. Insect Behav.4:265–273. [Google Scholar]
- Phillips B.,Shine R.. 2007. When dinner is dangerous: Toxic frogs elicit species-specific responses from a generalist snake predator.Am. Nat.170:936–942. [DOI] [PubMed] [Google Scholar]
- Prysby M. D. 2004Natural enemies and survival of monarch eggs and larvae, pp.27–37.InOberhauser K.,Solensky M. (eds.),The monarch butterfly: Ecology and conservation. Cornell University Press, Ithaca, NY. [Google Scholar]
- Rafter J. L.,Agrawal A. A.,Preisser E. L.. 2013. Chinese mantids gut toxic monarch caterpillars: Avoidance of prey defence? Ecol. Entomol.38:76–82. [Google Scholar]
- Rayor L. S. 2004Effects of monarch larval host plant chemistry and body size onPolistes wasp predation, pp.39–46.InOberhauser K.,Solensky M. (eds.),The monarch butterfly: Ecology and conservation. Cornell University Press, Ithaca, NY. [Google Scholar]
- Rayor L. S.,Mooney L. J.,Renwick J. A.. 2007. Predatory behavior ofPolistes dominulus wasps in response to cardenolides and glucosinolates inPieris napi caterpillars.J. Chem. Ecol.33:1177–1185. [DOI] [PubMed] [Google Scholar]
- Rowson J. M. 1952. Studies in the genusDigitalis part I. The colorimetric estimation of digitoxin and of preparations ofDigitalis purpurea.J. Pharm. Pharmacol.4:814–830. [DOI] [PubMed] [Google Scholar]
- Roy J.,Bergeron J. M.. 1990. Branch-cutting behavior by the vole (Microtus pennsylvanicus).J. Chem. Ecol.16:735–741. [DOI] [PubMed] [Google Scholar]
- Ruxton G. D.,Sherratt T. N.,Speed M. P.. 2004Avoiding attack: The evolutionary ecology of crypsis, warning signals, and mimicry, vol.249, Oxford University Press, Oxford. [Google Scholar]
- Schmidt K. A.,Belinsky K. L.. 2013. Voices in the dark: Predation risk by owls influences dusk singing in a diurnal passerine.Behav. Ecol. Sociobiol.67:1837–1843. [Google Scholar]
- Scudder G.G.E.,Moore L. V.,Isman M. B.. 1986. Sequestration of cardenolides inOncopeltus fasciatus: morphological and physiological adaptations.J. Chem. Ecol.12:1171–1187. [DOI] [PubMed] [Google Scholar]
- Simon K. S.,Townsend C. R.. 2003. Impacts of freshwater invaders at different levels of ecological organisation, with emphasis on salmonids and ecosystem consequences.Freshwater Biol.48:982–994. [Google Scholar]
- Skelhorn J.,Rowe C.. 2007. Predators' toxin burdens influence their strategic decisions to eat toxic prey.Curr. Biol.17:1479–1483. [DOI] [PubMed] [Google Scholar]
- Stelljes M. E.,Seiber J. N.. 1990. Pyrrolizidine alkaloids in an overwintering population of monarch butterflies (Danaus plexippus) in California.J. Chem. Ecol.16:1459–1470. [DOI] [PubMed] [Google Scholar]
- Warashina T.,Noro T.. 2000a. Steroidal glycosides from the aerial part ofAsclepias incarnata.Phytochemistry 53:485–498. [DOI] [PubMed] [Google Scholar]
- Warashina T.,Noro T.. 2000b. Cardenolide and oxypregnane glycosides from the root ofAsclepias incarnata L.Chem. Pharm. Bull.48:516–524. [DOI] [PubMed] [Google Scholar]
- Yosef R. 1992. Predator exaptations and defensive adaptations in evolutionary balance: No defence is perfect.Evol. Ecol.6:527–536. [Google Scholar]
- Zandonà E.,Auer S. K.,Kilham S. S.,Howard J. L.,López-Sepulcre A.,O’Connor M. P.,Bassar R. D.,Osorio A.,Pringle C. M.,Reznick D. N.. 2011. Diet quality and prey selectivity correlate with life histories and predation regime in Trinidadian guppies.Funct. Ecol.25:964–973. [Google Scholar]