A single skin infection in group AStreptococcus–endemic settings induced a significant immune response in 38% of children. Cross-reactive immune responses occurred after skin infections. These cross-reactive responses frequently aligned withemm clusters.
Keywords: Streptococcus pyogenes, emm-cluster, immunity, vaccine, skin infection
Abstract
Background
Group AStreptococcus (GAS) skin infections are particularly prevalent in developing nations. The GAS M protein, by which strains are differentiated into >220 differentemm types, is immunogenic and elicits protective antibodies. A major obstacle for vaccine development has been the traditional understanding that immunity following infection is restricted to a singleemm type. However, recent evidence has led to the hypothesis of immune cross-reactivity betweenemm types.
Methods
We investigated the human serological response to GAS impetigo in Fijian schoolchildren, focusing on 3 majoremm clusters (E4, E6, and D4). Pre- and postinfection sera were assayed by enzyme-linked immunosorbent assay with N-terminal M peptides and bactericidal assays using the infecting-type strain,emm cluster–related strains, and nonrelated strains.
Results
Twenty of the 53 paired sera demonstrated a ≥4-fold increase in antibody titer against the infecting type. When tested against all cluster-related M peptides, we found that 9 of 17 (53%) paired sera had a ≥4-fold increase in antibody titer to cluster-related strains as well. When grouped by cluster, the mean change to cluster-relatedemm types in E4 and E6 was >4-fold (5.9-fold and 19.5-fold, respectively) but for D4 was 3.8-fold. The 17 paired sera were tested in bactericidal assays against selected cluster-related and nonrelated strains. While the responses were highly variable, numerous instances of cross-reactive killing were observed.
Conclusions
These data demonstrate that M type–specific and cross-reactive immune responses occur following skin infection. The cross-reactive immune responses frequently align withemm clusters, raising new opportunities to design multivalent vaccines with broad coverage.
Many microbial pathogens are exceptionally proficient at immune evasion, providing numerous challenges for vaccine development. Group AStreptococcus (GAS) is a prime example, with considerable antigenic diversity and many immune-evading virulence factors. GAS is responsible for major morbidity and mortality, particularly in developing nations, and causes at least 500000 deaths per year worldwide [1]. GAS pharyngitis and impetigo comprise the majority of cases, and although they can be relatively mild conditions, they have the ability to lead to more severe invasive disease or to autoimmune sequela of the heart and kidneys. The highest prevalence of GAS impetigo is found in Oceania, with a median prevalence of 40.2% [2]. Children aged <15 years are the most commonly affected by GAS impetigo [3]. Studies of the immune response following impetigo suggest a weaker response with potentially lower induction of a lasting immune response [4,5].
Due to the high burden of disease, particularly in areas where primary healthcare is limited, there has been extensive interest in the development of a GAS vaccine. A major vaccine candidate has been the M protein, the dominant immunogenic molecule on the GAS surface [6,7]. Early studies of a restricted number of GAS M types observed that protective immunity following GAS infection was limited to bacteria of the homologous M type. This observation was attributed to type-specific antibodies arising from epitopes on the M protein [8–10]. The gene encoding M proteins,emm, is the basis for sequence typing used to differentiate between strains of GAS, based on relatively minor sequence differences in the 5ʹ regions of the genes. M protein sequence diversity exceeds 200 differentemm types [11], and therefore a broad-spectrum vaccine against this protein has been difficult to develop. The current leading type-specific GAS vaccine candidate comprises peptides from 30 different M proteins [12].
Preclinical studies of the 30-valent vaccine candidate found an unexpectedly high level of cross-opsonization of types not included in the vaccine (opsonization demonstrated in 39 of 49emm types tested) [12,13]. Recent studies observed substantially lower inter–M protein sequence diversity within the wholeemm gene among strains isolated from tropical areas, in contrast to high diversity in strains recovered from high-income settings, providing a possible explanation for cross-opsonization [14–16]. Further studies of the entireemm gene from 1086 GAS isolates representing 175emm types led to the establishment of a cluster system of typing, which groupsemm types into 48 distinctemm clusters based on sequence homology and binding capacities [17]. Sixteen clusters contain 143emm types and account for 90% of global GAS infections [18,19]. There is strong evidence of shared host protein-binding capabilities within clusters, suggesting that these clusters are functionally and immunologically relevant. An experimental vaccine developed using peptides from 5 M proteins from the E4 cluster was found to induce broad opsonization of other strains within the cluster, providing further evidence that immune recognition of GAS may be a combination of “cluster-specific” and “type-specific” responses [20].
In this study, we aimed to investigate the human serologic response to GAS impetigo in an endemic setting, and to evaluate the potential relationship betweenemm clusters and cross-opsonization. We investigated the immune response to isolates belonging to 3emm clusters (E4, E6, D4) that collectively account for 67emm types and cause around 35% of GAS infections worldwide [21,22].
MATERIALS AND METHODS
Study Samples
Samples were collected in a longitudinal cohort study of pharyngitis and impetigo in 457 children aged 5–15 years followed for 10 months in 3 schools in Fiji, as described previously [3,23]. Serum samples and GAS isolates from throat and skin cultures were collected from symptomatic children as part of the study, and sera were taken at 3 predefined time points (0, 5, and 10 months), and frozen and stored at –80°C. Isolates wereemm typed by standard methods [24]. Infections caused by bacteria from E4, E6, and D4emm clusters were selected for inclusion, as these are among the most frequently recoveredemm clusters in the Pacific region [21,22]. Children with multiple episodes of GAS impetigo caused by differentemm types between blood samples were excluded from the analysis.
Detection of Serum Antibodies
Enzyme-linked immunosorbent assays (ELISAs) were done to establish the antibody reactivity profile of pre- and postinfection patient sera to peptides from the M protein of (1) the infecting type, (2) heterologous strains from the same cluster as the infecting type, and (3) strains from different clusters to the infecting type. Assays were done with modifications of the method described by Dale et al [12,25] and Miura et al [26]. Synthetic peptides of the N-terminal 50 amino acids of M proteins were obtained commercially (GenScript, Piscataway, New Jersey) from sequences based on a representative collection of 1086 M proteins and were used to coat assay plates [11]. Pooled human sera (Sandoglobulin) were used on every plate to construct reference curves (Supplementary Methods). Pre- and postinfection samples were initially assayed against the M peptide from the infecting type only. Paired sera were diluted 2-fold serially from 1:50 to 1:3200 and added to an assay plate, coated with infecting-type M peptide. Detection of bound antibody was done using horseradish peroxidase–labeled antihuman immunoglobulin G (IgG) secondary antibody. Following incubation with substrate tetramethylbenzidine, absorbance was measured at 450 nm. Antibody titers were calculated in ELISA units from the standard curve.
Sera were included for investigation of cross-opsonization if there was sufficient sample available and they met our predefined criteria for seroconversion to the infecting peptide, which was defined as an increase in antibody titer of the postinfection sera ≥4-fold of the preinfection titer. Two serum dilutions located in the log-phase of the antibody response curves, as determined from the infecting-type ELISA, were selected for each pair of pre- and postinfection sera. These sera were further assayed against M peptides from all M proteins within theiremm cluster, as well as 6–7 M peptides from heterologousemm clusters (Supplementary Methods). To overcome the intrinsic variability in individual responses to a single GAS infection, we also grouped theemm cluster–related and the non-cluster-related responses for eachemm cluster and compared them byt test.
Functional Assays
Bactericidal assays were performed with modifications of those described by Lancefield [27] and Raz et al [28]. Various streptococcal strains were cultured overnight, diluted 1:50 in 10 mL Todd Hewitt broth with 1% yeast extract (THY), and grown to an optical density (at 600 nm) of 0.2 and diluted further to 1:1 × 10-4 before addition to assays and plating on THY agar to determine inoculum size. Heparinized whole blood was collected from healthy adult volunteers who had been prescreened by bactericidal assay to confirm nonimmune status, and added to assays within 2 hours of collection. Pre- and postinfection sera were also added to assays to a total volume of 300 µL comprising 50 µL of serum, 50 µL of bacterial culture (approximately 100 colony-forming units), and 200 µL of blood. Assays were incubated with end-over-end rotation for 3 hours at 37°C before plating on THY-triphenyl tetrazolium chloride agar and grown for 18 hours at 37°C with 5% CO2. Control assays, in which no sera were added, were performed concurrently, and all results were compared with the initial inoculum concentration to determine a replication factor.
Percentage killing was determined by calculating the difference between pre- and postinfection replication factors. Where there was less replication in the preinfection than postinfection serum, it was reported as “no killing” and was given a value of 0% as this could not be attributed to the presence or absence of cross-reactive antibodies. For each cluster, the infecting-type,emm cluster–related, and non-cluster-related bactericidal responses were grouped and compared, and the mean differences between theemm cluster–related and non-cluster-related strains were analyzed byt test. As a relatively high level of killing of the infecting strain is likely required to clearly observe cross-reactive killing, samples with ≥50% killing of the infecting type were analyzed separately [29–31]. All GAS strains used in assays were clinical isolates collected in Pacific countries, where possible from the infection event being investigated, or as part of the cohort study [3,32].
Ethical Approval
Ethical approval was obtained from the Fiji National Research Ethics Review Committee, the Fiji National Health Research Committee, the University of Melbourne Human Research Ethics Committee, and the Queensland Institute of Medical Research Human Research Ethics Committee. Children were enrolled only if written consent from a parent or guardian was obtained. Children aged 10 years or older were enrolled only if written assent by the child was also obtained. Blood donations from healthy adults were only taken if written assent was obtained.
RESULTS
Study Population
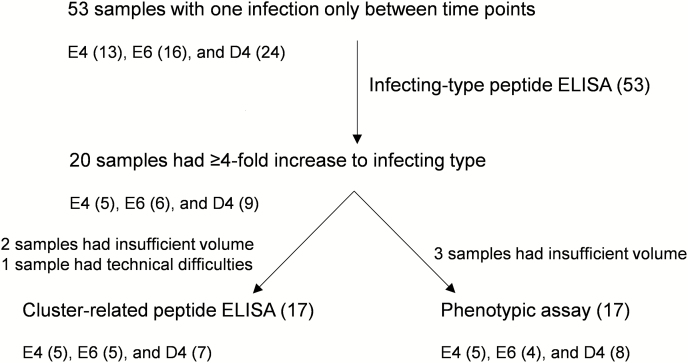
Samples from 53 children with a single skin infection from anemm type belonging to 1 of the 3 clusters were included. Of these, 20 met the seroconversion threshold for further inclusion (5 E4, 6 E6, and 9 D4) when tested against their infecting-type peptide using ELISA, and of these, 17 samples (5 E4, 5 E6, and 7 D4) were finally included for cluster-related peptide ELISA and 17 samples (5 E4, 4 E6, and 8 D4) for bactericidal assays based on volumes of available samples (Figure 1). The median time between infection and the second blood sample was 99 days (range, 43–203 days).
Figure 1.
The numbers of samples meeting inclusion criteria for each part of the study, indicating the numbers from each cluster. Abbreviation: ELISA, enzyme-linked immunosorbent assay.
Infecting-Type Enzyme-Linked Immunosorbent Assays
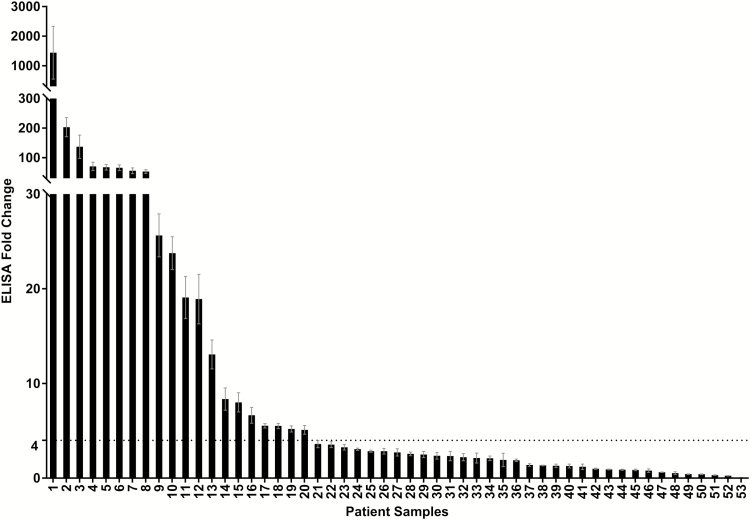
When antibody titers were determined for the 53 paired samples against the M peptide of their infecting type and overall, the geometric mean response to the infecting-type peptide resulted in a 4.1-fold increase (95% confidence interval [CI], 2.5- to 6.7-fold) (Figure 2). While some subjects exhibited little to no difference between pre- and postinfection samples (62% had <4-fold difference), others had a very large increase in antibody titer to the peptide following infection, such that the range of changes was 0.07- to 1444-fold. In each cluster, between 37% and 38% of samples had a ≥4-fold increase in antibody titer to the infecting type between pre- and postinfection samples (Figure 1).
Figure 2.
Change in antibody titer against infecting-type peptide. Average fold change between enzyme-linked immunosorbent assay (ELISA) titer from 53 pre- and postinfection sera to the N-terminal peptide from the M protein of the infecting group AStreptococcus (GAS) strain. Experiments were performed in duplicate over 7 serial dilutions and repeated, and the average values for pre- and postinfection sera were calculated from standard curves and compared. As indicated by the dotted line, the cutoff threshold for inclusion in further experiments was set at a 4-fold increase. Error bars represent the standard error of the mean.
Cluster-Related Peptide Enzyme-Linked Immunosorbent Assays
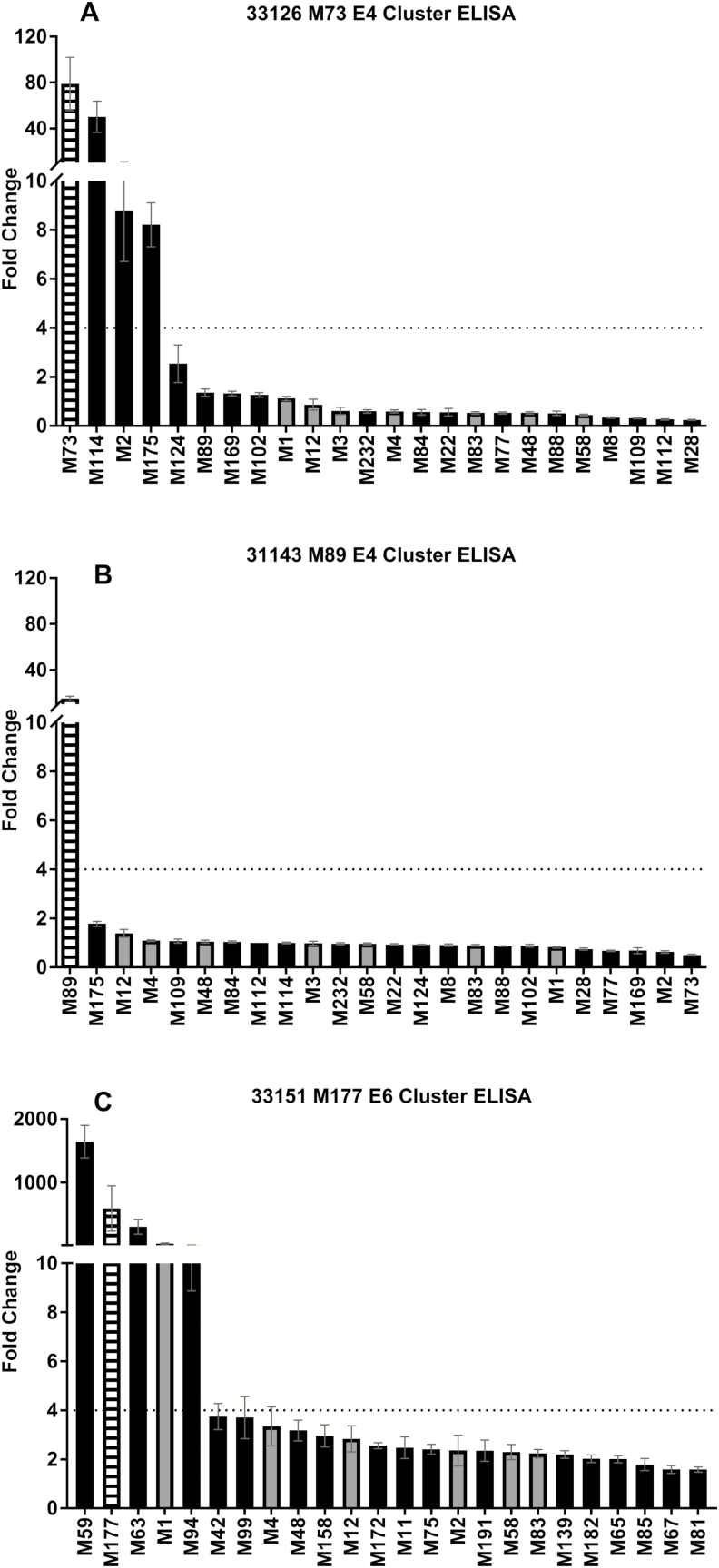
In assays using multiple cluster-related and non-cluster-related antigens, samples from 17 infections caused by 12 differentemm types were included, representing 4 from each of the 3 clusters. Responses to the cluster- and non-cluster-related strains were varied. Of the 17 paired samples, 8 (47%) had a ≥4-fold increase in antibody titer to peptides from the infecting type plus at least 1 cluster-related strain (Figure 3A), whereas 7 (41%) had a ≥4-fold increase in antibody titer to the peptide from the infecting type only (Figure 3B), and a further 2 (12%) had a ≥4-fold increase in antibody titer to peptides from both cluster-related and non-cluster-related strains (Figure 3C). Details of all individual children are provided in the Supplementary Materials.
Figure 3.
Representative individual antibody responses toemm cluster–related and non-cluster-related M peptides. The average fold change between enzyme-linked immunosorbent assay (ELISA) titer from pre- and postinfection sera from 3 individual patients to N-terminal peptides from the M protein of the infecting group AStreptococcus (GAS) strain, allemm cluster–related GAS, and 7 non-cluster-related GAS strains. The figures represent the 3 different antibody response patterns observed: high response to infecting-type andemm cluster–related peptides (A), high response to infecting-type peptide only (B), and high response to infecting-type,emm cluster–related, and non-cluster-related peptides (C). Error bars represent the standard error of the mean.
White striped bars = infecting-type peptide; black bars =emm cluster–related peptide; gray bars = non-cluster-related peptide.
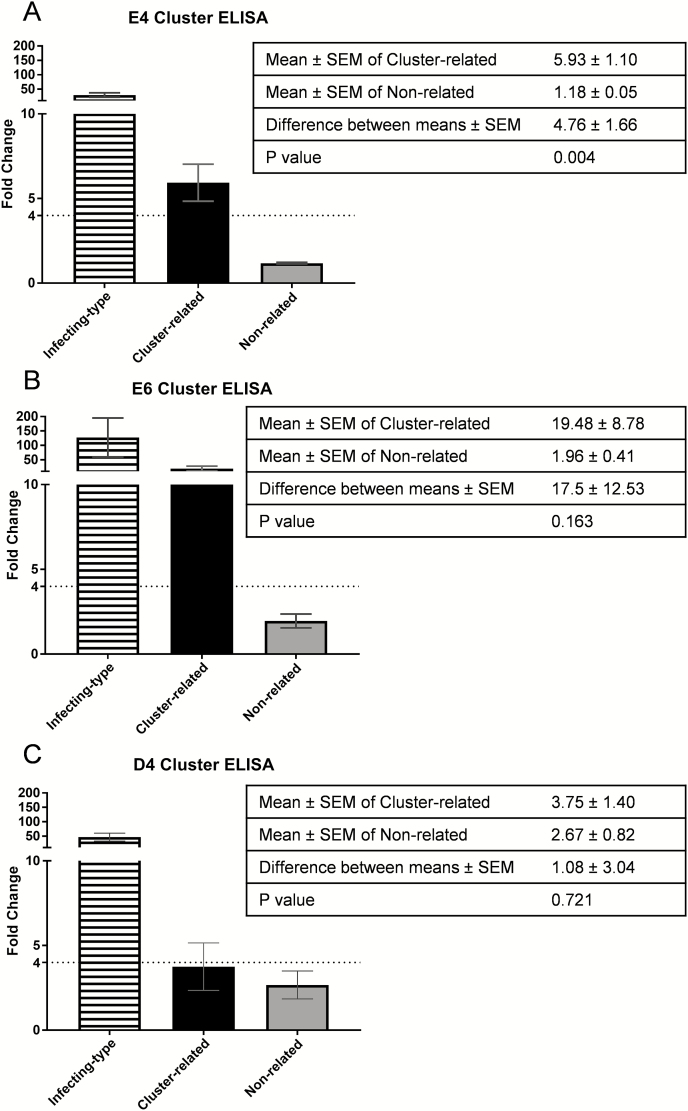
When pooling results for all the infections by cluster, the average fold change for cluster-relatedemm types was ≥4-fold for clusters E4 and E6 (Figure 4). From these pooled analyses, the mean absolute difference in fold change between cluster-related and nonrelated for patients with infections caused by E4-cluster strains was 4.8 (95% CI, 1.5–8.0), whereas for E6 was 17.5 (95% CI, –7.1 to 42.2) and for D4 was 1.1 (95% CI, –4.9 to 7.0).
Figure 4.
Grouped antibody responses to infecting-type,emm cluster–related, and non-cluster-related peptides. Antibody fold-changes values from paired samples were grouped according toemm cluster of the infecting strain.emm cluster–related and non-cluster-related responses were compared byt test to determine whether cross-reactive antibodies were more commonly raised against other M peptides within a cluster. The greatest difference was observed within cluster E6 and the least difference within cluster D4. The dotted line indicates a 4-fold increase, which was the threshold for a significant increase. Error bars represent the standard error of the mean. White striped bars = infecting-type peptide; black bars =emm cluster–related peptide; gray bars = non-cluster-related peptide. Abbreviations: ELISA, enzyme-linked immunosorbent assay; SEM, standard error of the mean.
Bactericidal Assays
Sera were assayed against GAS of the infecting type and a selection of representativeemm cluster–related and non-cluster-related strains in bactericidal assays. The magnitude of percentage killing against these selected strains was variable. In all cases, we observed some level of killing against strains other than the infecting type, which could be attributed to cross-reactive antibodies, and were sometimes consistent with ELISA results (see Supplementary Results for individual bactericidal assay and parallel ELISA data).
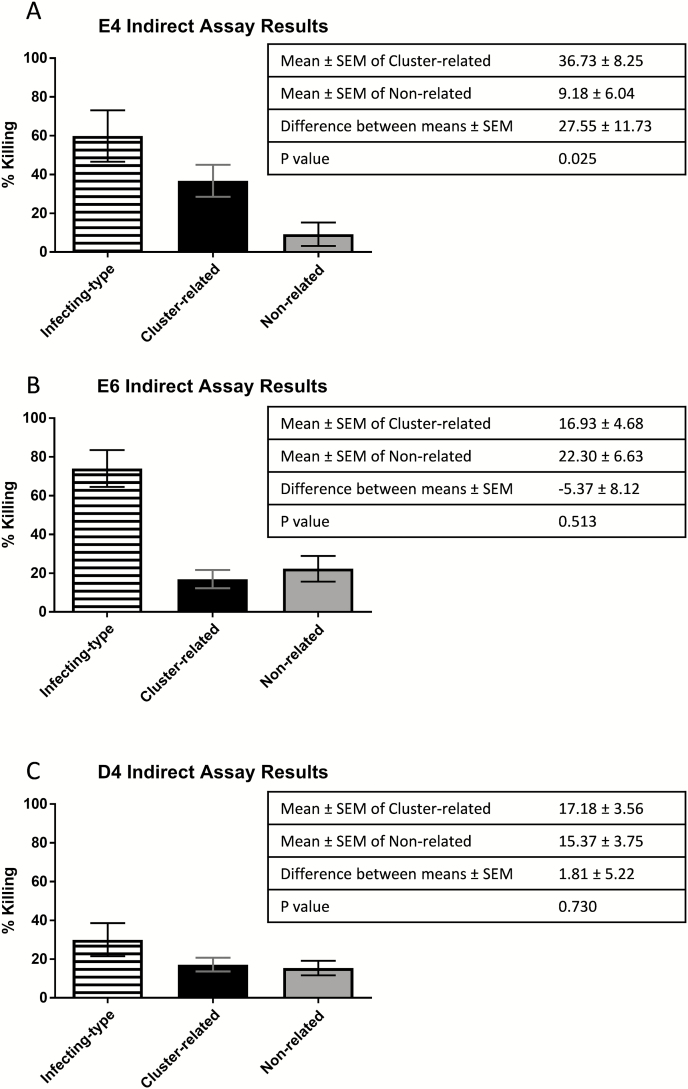
When analyzed byt test (Figure 5), the greatest difference between means of percentage killing of the cluster-related and nonrelated strains was observed in cluster E4 (27.6 [95% CI, 3.7–51.4]), compared with E6 (–5.4 [95% CI, –22.0 to 11.3]) and D4 (1.8 [95% CI, –8.6 to 12.2]).
Figure 5.
Grouped phenotypic responses to infecting-type,emm cluster–related, and non-cluster-related strains. All percentage killing results from included paired samples were grouped according toemm cluster of the infection. Killing ofemm cluster–related and non-cluster-related strains were compared byt test to determine whether there was any difference in the overall capacity of postinfection sera to kill these strains. The greatest difference was observed within cluster E4 and there was no difference within clusters E6 and D4. White striped bars = infecting-type peptide; black bars =emm cluster–related peptide; gray bars = non-cluster-related peptide. Abbreviation: SEM, standard error of the mean.
Samples with >50% killing of the infecting type had greater cross-reactive killing againstemm cluster–related compared with non-cluster-related strains in all 3 clusters, with this difference being most pronounced in E4 cluster samples (75% of sera demonstrated >50% killing of cluster-related strains compared to 20% of non-cluster-related strains;Table 1).
Table 1.
Summary of Assays With >50% Killing in Postinfection Sera
| Infecting Type | emm Cluster Related | Non–Cluster Related | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Tested | Killing | % | Tested | Killing | % | Tested | Killing | % | |
| E4 | 5 | 3 | 60.0 | 8 | 6 | 75.0 | 5 | 1 | 20.0 |
| E6 | 4 | 3 | 75.0 | 6 | 4 | 66.7 | 6 | 3 | 50.0 |
| D4 | 8 | 2 | 25.0 | 6 | 2 | 33.3 | 6 | 1 | 16.7 |
| Total | 17 | 8 | 47.1 | ||||||
The samples with >50% killing of their infecting type strain were further examined for their ability to kill >50% of heterologous group AStreptococcus strains. In all 3 clusters, a higher proportion of theemm cluster–related strains had >50% killing than the non-cluster-related strains, with the greatest difference in E4.
DISCUSSION
We found that GAS impetigo elicits a systemic antibody response against the M protein, with a single episode resulting in a ≥4-fold increase in anti–M protein IgG antibodies in 38% of cases. The functional nature of this antibody response is demonstrated by the fact that 8 of 17 cases had high anti-M peptide IgG antibody titers that induced >50% killing of the infecting-type strain of GAS. Furthermore, these antibodies also cross-reacted with and cross-opsonized heterologous strains.
Early studies of the serological response to superficial GAS infection suggested that pharyngitis was capable of eliciting type-specific immunity against the infecting GAS strain, but infection of the skin did not induce the same level of antibody response [4,33]. Among the 38% of participants in our study who had a ≥4-fold increase in anti-M peptide IgG antibodies, nearly half induced type-specific killing. The variable serological responses observed in this study are consistent with previous prospective studies of diagnostic markers in sera following GAS infection [34] which are typically relatively lower following skin infections compared with pharyngeal infections [4]. Antibody responses to M peptides following pharyngitis with the homologous strains have also been shown to be variable, with anti-M immune responses observed in only 63% of participants in a recent study [35]. It is interesting to note that, the majority of these responses occurred following asymptomatic GAS acquisition in the pharynx, and only half of the patients with symptomatic pharyngitis had a positive immune response to the M peptide from the infecting type [35].
Theemm cluster typing system, based on fullemm gene sequences, provides a framework for the investigation of potential immune cross-reactivity. While the clusters are able to partially predict virulence potential based on shared ligand-binding profiles [17], there are few data relating to immunity. Bacteria from the 3 clusters investigated in our study induced differing immune responses after skin infections. Higher antibody titers were observed against cluster-relatedemm types than non-cluster-related antigens in the E6 and E4 clusters; however, this was only statistically significant for the E4 cluster. There was essentially no difference observed for strains belonging to the D4 cluster. The E4 cluster elicited high titers of cross-reactive antibodies that were capable of cross-opsonization of cluster-relatedemm types. It may be that M proteins belonging to the E6 cluster are capable of eliciting high titer antibody responses, but these antibodies have a variable cross-opsonizing capacity. M proteins belonging to the D4 cluster appear to be overall less immunogenic (average of 31% killing for the infecting strain;Figure 5C), and no difference was observed in antibody function between cluster and noncluster strains. Of note, cross-opsonization observed during preclinical studies of the 30-valent vaccine was notably lower in D4 strains than other clusters, with opsonization of 4 of 9 strains [12,13]. The detection of antibodies that do not translate into killing may have a number of explanations. Immunity may be specific to each infection-patient pair, or influenced by other specific and conformational epitopes, or associated with an increase in specific IgG isotypes.
The existence of cross-protective immunity would suggest that multivalent vaccines have the potential to induce broader protection than type-specific immunity would predict. This hypothesis was first raised several years ago based on genetic analyses of Brazilian strains [15,16]. It is also supported by a recent study in a rabbit model using an experimental multivalent vaccine for the E4emm cluster [20]. Our study provides evidence that some cross-protection can in fact occur in vivo following clinical infection. Indeed, in one of the participants, antibody responses to the different cluster-related strains were consistent with antibody responses in rabbits to the experimental E4 vaccine [20]. While this may be the case for E4, it may not be applicable for D4 and thus a complementary antigen may be required for protection against these strains, such as, for example, the M-related protein [36]. Further investigation of antibody responses to the E6 cluster is required to fully elucidate the potential of a cross-protection to be exploited for vaccine development.
Epidemiological evidence suggests repeated GAS skin infection may immunize against throat infection, and also implicates a role for skin infection in the development of acute rheumatic fever [37–40]. The production of a broad immune response following impetigo is consistent with this hypothesis, but not conclusive. Follow-up studies on the duration of protection to subsequent infection or development of autoimmune conditions is required to further investigate any potential association.
There are several limitations to this study. First, the small sample size needs to be considered when drawing conclusions. Second, samples were assayed against all cluster-related strains but only a selection of nonrelated strains. Finally, we used 50 amino acid peptides as ELISA antigens, which represent the portion of M proteins believed to be the most immunogenic but do not account for the binding of conformational antibodies that may be highly important, particularly for cross-opsonization. Of note, the monomeric and short nature of the peptides used in our study may have led to an underestimation of the presence of cross-reactive antibodies due to loss of conformational epitopes present in the dimeric fully mature M protein.
Our study also has strengths including the use of a population at high risk of GAS impetigo for investigating the serological response to this condition. We were also able to compare the postinfection response to an internal baseline in the preinfection sera from the same patient, which allows more robust analyses than in cross-sectional studies. Furthermore, by grouping results byemm cluster, we were able to analyze the data at a population level rather than at the individual level, minimizing interference from the well-characterized intrinsic variation between individuals.
This study provides a population-based description of M protein immune response after GAS skin infection in an endemic setting. Our study confirms the existence ofemm-type specific immunity, but suggests that this is an incomplete picture and that a combination of “cluster-specific” and “type-specific” responses occur. Our study suggests that cross-reactive immune responses occur following skin infection and raises hope for the development of a broadly protective multivalent vaccine.
Supplementary Data
Supplementary materials are available atClinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Notes
Acknowledgments. We thank Jonathan Carapetis and Roy Robins Browne for their collaboration on this study.
Financial support. This work was supported by an National Health and Medical Research Council (NHMRC) project grant (grant number APP1051297) and the Murdoch Childrens Research Institute of Melbourne, Australia.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Carapetis JR,Steer AC,Mulholland EK,Weber M. The global burden of group A streptococcal diseases.Lancet Infect Dis 2005;5:685–94. [DOI] [PubMed] [Google Scholar]
- 2. Bowen AC,Mahé A,Hay RJ et al. The global epidemiology of impetigo: a systematic review of the population prevalence of impetigo and pyoderma.PLoS One 2015;10:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Steer AC,Jenney AWJ,Kado J et al. High burden of impetigo and scabies in a tropical country.PLoS Negl Trop Dis 2009;3:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kaplan EL,Anthony BF,Chapman SS,Ayoub EM,Wannamaker LW. The influence of the site of infection on the immune response to group A streptococci.J Clin Invest 1970;49:1405–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pandey M,Ozberk V,Calcutt A et al. Streptococcal immunity is constrained by lack of immunological memory following a single episode of pyoderma.PLoS Pathog 2016;12:e1006122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Steer AC,Carapetis JR,Dale JB et al. Status of research and development of vaccines forStreptococcus pyogenes.Vaccine 2016;34:6–11. [DOI] [PubMed] [Google Scholar]
- 7. Smeesters PR,McMillan DJ,Sriprakash KS. The streptococcal M protein: a highly versatile molecule.Trends Microbiol 2010;18:275–82. [DOI] [PubMed] [Google Scholar]
- 8. Dochez AR,Avery OT,Lancefield RC. Studies on the biology of streptococcus: i. antigenic relationships between strains ofStreptococcus haemolyticus.J Exp Med 1919;30:179–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lancefield RC.Current knowledge of type-specific M antigens of group A streptococci.J Immunol 1962;89:307–13. [PubMed] [Google Scholar]
- 10. Jones KF,Fischetti VA. The importance of the location of antibody binding on the M6 protein for opsonization and phagocytosis of group A M6 streptococci.J Exp Med 1988;167:1114–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. McMillan DJ,Drèze PA,Vu T et al. Updated model of group AStreptococcus M proteins based on a comprehensive worldwide study.Clin Microbiol Infect 2013;19:E222–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dale JB,Penfound TA,Chiang EY,Walton WJ. New 30-valent M protein-based vaccine evokes cross-opsonic antibodies against non-vaccine serotypes of group A streptococci.Vaccine 2011;29:8175–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dale JB,Penfound TA,Tamboura B et al. Potential coverage of a multivalent M protein-based group A streptococcal vaccine.Vaccine 2013;31:1576–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Smeesters PR,Vergison A,Campos D,de Aguiar E,Deyi VYM,Van Melderen L. Differences between Belgian and Brazilian group AStreptococcus epidemiologic landscape.PLoS One 2006;1:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Smeesters PR,Mardulyn P,Vergison A,Leplae R,Van Melderen L. Genetic diversity of group AStreptococcus M protein: implications for typing and vaccine development.Vaccine 2008;26:5835–42. [DOI] [PubMed] [Google Scholar]
- 16. Smeesters PR,Dramaix M,Van Melderen L. Theemm-type diversity does not always reflect the M protein genetic diversity—is there a case for designer vaccine against GAS.Vaccine 2010;28:883–5. [DOI] [PubMed] [Google Scholar]
- 17. Sanderson-Smith M,De Oliveira DM,Guglielmini J et al. ;M Protein Study Group A systematic and functional classification ofStreptococcus pyogenes that serves as a new tool for molecular typing and vaccine development.J Infect Dis 2014;210:1325–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shulman ST,Tanz RR,Dale JB,Steer AC,Smeesters PR. Added value of theemm-cluster typing system to analyze the group AStreptococcus epidemiology in high-income settings.Clin Infect Dis 2014;59:1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Smeesters PR,Laho D,Beall B,Steer AC,Van Beneden CA. Seasonal, geographic, and temporal trends ofemm clusters associated with invasive group A streptococcal infections in US multistate surveillance.Clin Infect Dis 2017;64:694–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dale JB,Smeesters PR,Courtney HS et al. Structure-based design of broadly protective group a streptococcal M protein-based vaccines.Vaccine 2017;35:19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Steer AC,Law I,Matatolu L,Beall BW,Carapetis JR. Global emm type distribution of group A streptococci: systematic review and implications for vaccine development.Lancet Infect Dis 2009;9:611–6. [DOI] [PubMed] [Google Scholar]
- 22. Smeesters PR,McMillan DJ,Sriprakash KS,Georgousakis MM. Differences among group AStreptococcus epidemiological landscapes: consequences for M protein-based vaccines? Expert Rev Vaccines 2009;8:1705–20. [DOI] [PubMed] [Google Scholar]
- 23. Steer AC,Vidmar S,Ritika R et al. Normal ranges of streptococcal antibody titers are similar whether streptococci are endemic to the setting or not.Clin Vaccine Immunol 2009;16:172–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Steer AC,Magor G,Jenney AW et al. emm and C-repeat region molecular typing of beta-hemolytic streptococci in a tropical country: implications for vaccine development.J Clin Microbiol 2009;47:2502–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dale JB,Ofek I,Beachey EH. Heterogeneity of type-specific and cross-reactive antigenic determinants within a single M protein of group A streptococci.J Exp Med 1980;151:1026–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Miura K,Orcutt AC,Muratova OV,Miller LH,Saul A,Long CA. Development and characterization of a standardized ELISA including a reference serum on each plate to detect antibodies induced by experimental malaria vaccines.Vaccine 2008;26:193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lancefield RC.Differentiation of group A streptococci with a common R antigen into three serological types, with special reference to the bactericidal test.J Exp Med 1957;106:525–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Raz A,Tanasescu AM,Zhao AM et al. Streptococcus pyogenes sortase mutants are highly susceptible to killing by host factors due to aberrant envelope physiology.PLoS One 2015;10:1–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mountzouros KT,Howell AP. Detection of complement-mediated antibody-dependent bactericidal activity in a fluorescence-based serum bactericidal assay for group BNeisseria meningitidis.J Clin Microbiol 2000;38:2878–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bash MC,Lynn F,Mocca B et al. Development and use of a serum bactericidal assay using pooled human complement to assess responses to a meningococcal group A conjugate vaccine in African toddlers.Clin Vaccine Immunol 2014;21:755–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ercoli G,Baddal B,Alessandra G et al. Development of a serological assay to predict antibody bactericidal activity against non-typeableHaemophilus influenzae.BMC Microbiol 2015;15:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Baroux N,D’Ortenzio E,Amédéo N et al. The emm-cluster typing system for Group AStreptococcus identifies epidemiologic similarities across the Pacific region.Clin Infect Dis 2014;59:e84–92. [DOI] [PubMed] [Google Scholar]
- 33. Bisno AL,Nelson KE. Type-specific opsonic antibodies in streptococcal pyoderma.Infect Immun 1974;10:1356–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Johnson DR,Kurlan R,Leckman J,Kaplan EL. The human immune response to streptococcal extracellular antigens: clinical, diagnostic, and potential pathogenetic implications.Clin Infect Dis 2010;15:481–90. [DOI] [PubMed] [Google Scholar]
- 35. Hysmith ND,Kaplan EL,Cleary PP,Johnson DR,Penfound TA,Dale JB. Prospective longitudinal analysis of immune responses in pediatric subjects after pharyngeal acquisition of group A streptococci.J Pediatric Infect Dis Soc 2017;6:187–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Courtney HS,Niedermeyer SE,Penfound TA,Hohn CM,Greeley A,Dale JB. Trivalent M-related protein as a component of next generation group A streptococcal vaccines.Clin Exp Vaccine Res 2017;6:45–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. McDonald MI,Towers RJ,Andrews RM,Benger N,Currie BJ,Carapetis JR. Low rates of streptococcal pharyngitis and high rates of pyoderma in Australian aboriginal communities where acute rheumatic fever is hyperendemic.Clin Infect Dis 2006;43:683–9. [DOI] [PubMed] [Google Scholar]
- 38. Raynes JM,Frost HRC,Williamson DA et al. Serological evidence of immune priming by group A streptococci in patients with acute rheumatic fever.Front Microbiol 2016;7:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Parks T,Smeesters PR,Steer AC. Streptococcal skin infection and rheumatic heart disease.Curr Opin Infect Dis 2012;25:145–53. [DOI] [PubMed] [Google Scholar]
- 40. O’Sullivan L,Moreland NJ,Webb RH,Upton A,Wilson NJ. Acute rheumatic fever after group AStreptococcus pyoderma and group GStreptococcus pharyngitis.Pediatr Infect Dis J 2017;36:692–4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.