Summary
Severe burns lead to a high level of inflammation and high risk of infection. Inflammatory biomarkers are usually used to predict the severity of inflammation or infection and to assess the efficacy of antibiotics. The use of antibiotics in burns is still controversial. The aim of this study is to assess the effects of empirical antibiotics on level of C-reactive protein (CRP) and other inflammatory markers (leucocytes, neutrophils, lymphocytes, and ratio of neutrophils-lymphocytes) in severe burn patients. This cohort study was conducted in the burn unit of Dr. Soetomo Hospital between April and November 2019. CRP and other inflammatory markers were measured on admission, day 5, and day 7 after the administration of empirical antibiotics. Fifteen severe burn patients were enrolled in this study. All patients received Ceftazidime, 3x1 gram during seven days of hospitalization. CRP level reduced from 15,78±7,5 mg/dl to 14,98±10,29 mg/dl (p=0,705) by paired-t-test. There were no significant differences in mean decline of CRP between day 0-5 and 0-7. There was no decrease in inflammatory markers, including leucocytes, neutrophils, lymphocytes and ratio of neutrophils-lymphocytes during seven days of empirical antibiotic administration. Our conclusions are that the administration of ceftazidime as an empirical antibiotic lowers CRP level, although not significantly, while there is no decrease in several inflammatory markers.
Keywords: severe burns, c-reactive protein (CRP), inflammatory markers, empirical antibiotic
Abstract
Les brûlures étendues sont responsables d’une inflammation systémique et d’un risque élevé d’infection. Les biomarqueurs sont fréquemment utilisés pour évaluer la sévérité de l’inflammation ou de l’infection et surveiller l’efficacité de l’antibiothérapie. Le but de cette étude était d’évaluer les effets d’une antibiothérapie probabiliste sur les niveaux de CRP et d’autres marqueurs de l’inflammation (leucocytes, neutrophiles, lymphocytes, rapport neutrophiles/lymphocytes) chez des patients gravement brûlés. Il s’agissait d’une étude de cohorte conduite chez 15 patients hospitalisés dans le CTB de l’hôpital Dr Soetomo entre avril et novembre 2019. Ils recevaient 1 g x 3 de ceftazidime IV pendant 7 jours, les marqueurs de l’inflammation étant mesurés à l’entrée, à J5 et à J7 de l’antibiothérapie. La CRP passait de 157,8 +/- 75 à 149,8 +/- 10,29 mg/L (NS, test t apparié). Il n’y avait aucune baisse significative de quelque marqueur que ce soit sous ceftazidime. Nous en concluons que l’administration systématique de ceftazidime n’a pas d’effet significatif sur les marqueurs de l’inflammation.
Introduction
Burn injury is one of the most traumatic injuries.1 Burn leads to higher morbidity and mortality than any other type of traumatic injury.2 Systemic inflammatory response syndrome (SIRS) and sepsis are generally overlapped in burn patients. Sepsis is proposed as the major cause of death in burns.3 Therefore, the ideal biomarkers are needed to distinguish inflammation or infection, and even sepsis. This phenomenon causes withholding, delaying, or even overusing antibiotic therapy in burn patients.4 C-reactive protein or CRP is a common marker that is released extensively in acute inflammatory response, and elevated CRP level is associated with the clinical outcome of infection.5 A CRP level of more than 8 mg/dl can distinguish inflammatory response to infection from other types of inflammation, indicating that levels of CRP can be predictive of sepsis or infection.6
The peak of inflammatory response usually occurs within 5 to 7 days after burn.7 In burn patients, antibiotics are used immediately after burn, without waiting for the sensitivity test of antibiotics. Ceftazidime is the most widely used antibiotic in burn patients, especially in patients that are suspected of having Pseudomonas aeruginosa infection.8 Therefore, ceftazidime is commonly called upon as empirical antibiotic in burns to treat infection. Measurement of the serum level of C-reactive protein (CRP) is a simple, rapid and inexpensive procedure, and measurement of CRP level has consequently become routine clinical practice in the follow-up of patients hospitalized with severe infections.9 Burn leads to an alteration in immune response, usually characterized by an increase in neutrophil count and a decrease in lymphocyte count. A study by Zahorec et al. showed that the measurement of neutrophil-lymphocyte ratio is useful as a parameter of inflammation as well as of stress state in critically ill patients.10
However, study or evidence on the usefulness of consecutive CRP measurements as well as neutrophils, lymphocytes, and neutrophil-lymphocyte ratio for follow-up of antibiotic treatment in burn patients is lacking. The question of whether level of CRP and other inflammatory markers can decrease after the administration of antibiotics is still unclear. Therefore, the aim of this study was to examine the effects of empirical antibiotic administration on CRP levels and other inflammatory markers in severely burned patients. It is expected that knowledge of CRP levels and other inflammatory markers can be used to evaluate and shorten the use of antibiotics, and prevent side effects as well as antibiotic resistance.
Materials and methods
Study design
This seven-day cohort study was conducted in the intensive care unit and burn centre of Dr. Soetomo Hospital, Surabaya. Ethical clearance for this study was granted by the Ethics Committee for Health Research of the Dr. Soetomo Hospital, with registration number 1057/KEPK/III/2019. Burn patients admitted between April and November 2019 were included in this study. The main researcher explained the study protocol, including procedures, as well as risks and benefits to patients. Written informed consent was obtained from all burned patients and/or their guardians. Then, if they agreed to take part in this study, patients or their families signed informed consent.
Inclusion criteria for patients to be enrolled in this study were: age more than 18 years old; extensive burns with severe burn criteria less than 24 hours before admission; TBSA of more than 10%; patients who received empirical antibiotics intravenously during the initial seven days of hospitalization; willingness to sign informed consent to participate. Severe burns (according to American Burn Association criteria) were: 1) Second degree or more burns in adults; 2) Second degree or more burns in children; 3) Third degree burns of 10% or more; 4) Burns on the hands, face, ears, eyes, feet and genitalia; 5) Burns with inhalation trauma, electric burns, accompanied by other trauma. Exclusion criteria were: patient had received antibiotics before admission to hospital; patient was expected to die within 48 hours; patient had history of cardiovascular disease, metabolic syndrome and autoimmune disease. Drop out criteria were: patient died during the study, withdrew from the study, or there was a change of antibiotics before the 7 days of empirical antibiotic use. All patients received standard medical burn treatments, including antibiotics, stress ulcer therapies, analgesics, total parenteral nutrition as well as debridement surgeries. Percentage of total body surface area was measured by the Wallace rule of nines. Transfusion of plasma expanders and blood components (whole blood or packed red cells) were administered if needed.
Blood measurement for markers
Serum CRP levels and other inflammatory markers were measured on first admission to the hospital, on day 5 and day 7 after burn. Two ml of venous blood samples were taken to measure CRP level and Complete Blood Count (CBC), including leucocytes, neutrophils and lymphocytes. For CBC measurement, the blood samples were taken into Vacutainer-EDTA, and for CRP measurement, the blood samples were taken into Vacutainer-non EDTA. A complete blood count was determined by a cytometry-based system. Serum CRP level was measured quantitatively by immunoturbidity methods. Level of CRP lower than 10 mg/dl was considered normal for burn patients. The neutrophil-lymphocyte ratio was calculated by dividing the neutrophil count with the lymphocyte count. The normal value of neutrophil-lymphocyte ratio was 2-4.
Statistical analysis
The sample size of this study was calculated based on previous study of CRP levels in burn patients. To assess the normality of the variables, the Shapiro-Wilk test was used due to the small number of patients. The chi-square test was performed for categorical variables. The paired-t test was used to evaluate differences in CRP level, neutrophils, lymphocytes, and ratio of neutrophil to lymphocyte on admission (day 0), day 5, and after the administration of empirical antibiotics (day 7). To assess the relationship between total body surface area (TBSA) and CRP level, and also to assess the relationship between level of CRP and leucocytes on day 7, the Pearson correlation was used. P value less than 0,05 was considered statistically significant.
Results
Eighteen severe burn patients fulfilled the inclusion criteria between April and November 2019. Three patients were dropped out due to them passing away before completing seven days of empirical antibiotic administration. The causes of burns in this study are shown in Fig. 1. The demographic data of burn patients is shown in Table I. All patients received Ceftazidime 3x1 gram intravenously over the initial seven days of hospitalization. Level of CRP and other inflammatory markers for the survivors and for the deaths group is shown in Table II. Level of CRP and other inflammatory markers over the seven days of empirical antibiotic administration is shown in Table III. A mean decline in CRP between days 0-5 and days 5-7 is shown in Table IV. Correlation between CRP and TBSA on admission is shown in Table V. Correlation between CRP and leucocytes on day 7 is shown in Table VI.
Fig. 1. Cause of burns.

Table I. Demographic data of this study.

Table II. Comparison of inflammatory markers on admission between the survivors and the deaths groups.

Table III. Inflammatory markers over seven days of empirical antibiotic administration.

Table IV. Mean decline of CRP.

Table V. Correlation between CRP and TBSA on admission.
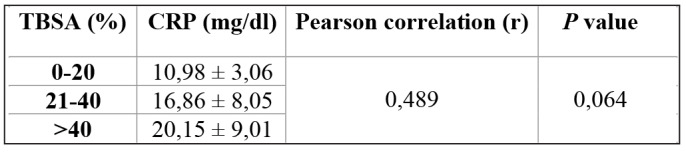
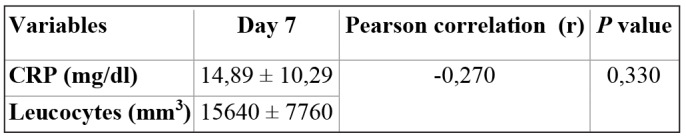
Table VI. Correlation between CRP and Leucocytes on day 7.
CRP level in the survivors group with inhalation trauma and without inhalation trauma on admission is shown in Fig. 2. CRP level between the survivors group and the deaths group with inhalation trauma on admission is shown in Fig. 3.
Fig. 2. CRP level in the survivors group with inhalation (n=7) and without inhalation trauma on admission (n=8).

Fig. 3. CRP level in the survivors group (n=7) and in the deaths group (n=3) with inhalation trauma on admission.

Discussion
Measurement of CRP is cheap, easy and rapidly available. However, the elevated level of serum CRP is supposed to be unspecific, because high level of serum CRP is also affected by several conditions, such as trauma and surgery.11 High levels of CRP are related to the severity of the disease and are often associated with the effectiveness of antibiotic therapy. Induction of CRP releasing is mediated by the stimulation of pro-inflammatory cytokines, such as interleukin-6 (IL-6), IL-1 as well as tumor necrosis factor-α (TNF-α). Therefore, the synthesis and secretion of CRP from hepatocyte usually reflects pro-inflammatory cytokine production. CRP secretion began 4–6 hours after inflammation stimulus, doubled every 8 hours and peaked at 36–50 hours. CRP has a short half-life of 19 hours, so as soon as the stimulus is stopped, it falls rapidly.12 If the main cause of the elevation persists, CRP can remain elevated for long periods. An elevated level of CRP occurs in most systemic inflammations or infections.
In our study, mean TBSA was higher in the deaths group than among the survivors, although this was not significant. Our study was similar to a study by Al-Ubady et al., which showed that mean TBSA was 63.5% in the non-survivor group, and 26.5% in the survivor group (p=0,00).12 A study by Xu et al. showed that percentage of TBSA was greater in the deaths group (92±7%) than in the survivors group (89±11%), and higher TBSA had a significant association with mortality.13 Nowadays, progressive treatments in burn patients has led to a significant reduction in mortality. A study by John et al. showed that CRP level reached a peak on the 7th day after burn (21±4,4) mg/dl, while in the control group the CRP level was lower (9,7±2,9) mg/dl. CRP reached a peak on the 7th day in moderate burn patients (<50% TBSA), and it peaked on the 5th day in severely burned patients (>50% TBSA).14
In our study, all patients received empirical antibiotics, ceftazidime as a routine prophylaxis antibiotic. All patients in our study had deeper burns, also known as partial-thickness or full thickness burns. The risks for infection are usually common in partial and full thickness burns; also complications of infection proportionally increase with the depth of the burn compared to grade I burns. One of the therapies for deeper burns is administration of systemic antibiotic, even though clinical evidence for administration of systemic antibiotic is lacking. The international society for burn injuries suggests not using systemic antibiotics as prophylaxis in acute burns, according to a meta-analysis study in adult burn patients.15
In this study, we found on admission that the greater the area of burn, the higher the CRP level. However, the correlation between total body surface area and CRP level was not significant by Pearson correlation (r=0,489; p=0,064). In addition, at day 7 post admission, CRP level was not correlated with leucocytes (r = -0,270; p=0,330). Our study is similar to a study by Jeschke et al., who reported that the larger the TBSA, the higher the level of CRP.16 Our study was also similar to a study by Lavrentieva et al., which showed that there was no significant difference in CRP level between burned patients with TBSA more than 60% (16,2±8,3) and those with less than 60% (15,1±6,3) on admission.17 A study by von Heimburg et al. showed that on admission there were no significant differences in the serum level of CRP among the no septic complications group, the septic complications group, and the patients who died in the sepsis group.18 Our study is similar to a study by Xu et al., which showed that there was no significant difference between the median of CRP (23,9) in the deaths group compared to the survivors group (8,5), and also that there were no significant differences in CRP between the deaths and the survivors group at one week post diagnosis of sepsis.13 A study by Csenkey et al. showed that developing infection, both local and systemic, was not significantly different between groups who received and did not receive systemic antibiotics. Also chance of systemic infectious complications was not different between the two groups.19
In our study, CRP level was persistently high from day 0 until day 7 post burns. A study by Menendez et al. showed that a persistently high level of serum CRP on days 1 and 3 in the follow-up of patients with mild-to-severe pneumonia was directly related to a high risk of therapy failure.20 A study by Bruns et al. showed that severe community-acquired pneumonia patients with low decline in CRP also had a higher risk of therapy failure, although this was not statistically significant.21 Our study showed that delayed decrease in CRP levels was associated with inappropriate empirical antibiotic treatment. In contrast, CRP level that returns to the normal range might indicate that duration of antibiotic treatment is sufficient, making it possible to discontinue antibiotics or switch to oral antibiotics. Administration of systemic antibiotics was useful in severe burn patients with mechanical ventilation and patients who required skin graft procedures.22 In our study, burn injury resulted in persistent elevation of leucocytes, neutrophils, and neutrophil-lymphocyte ratio on admission until day 7. A study by Dinsdale et al. showed that in thermal injury, level of leucocytes and neutrophils was elevated significantly on day 1 post burns compared to the control cohort.23 A study by Stoilova et al. showed that there was a significant increase in leukocytes on day 0 (10792 ± 990.89) and on the seventh day (12113 ± 1219,7) compared to the controls (7000 ± 3500).24
The high neutrophil-lymphocyte ratio over the seven days post burn indicates higher levels of neutrophils and lower levels of lymphocytes. Our results are consistent with a study by Fuss et al., which showed that the neutrophil-lymphocyte ratio was significantly higher in burn sepsis patients than in SIRS burn patients.25 Another study by Surbatovic et al. showed that the median of NLR in the non survivors group is significantly higher - 12,26 (7,72-18,25) - than that of the survivors group - 9,91 (6,17-13,78) - in critically ill and injured patients.26 Lymphocytes play an important role in regulating inflammatory response, and in decreasing lymphocytes due to persistent sepsis-induced apoptosis which may lead to suppressing the immune system and unresolved inflammation. 27,28 Therefore, an increase in NLR may indicate patients with persistent inflammation, together with concomitant decreased survival rates.
The pharmacokinetics of ceftazidime are mainly eliminated by renal excretion via glomerular filtration. More than 88% of the initial dose of ceftazidime is eliminated in the urine over 24 hours as unchanged drug. The hypermetabolic phase after burn, characterized by reduced renal blood flow, affects the pharmacokinetics of ceftazidime. As a consequence, a 2-3 gram dose of ceftazidime every eight hours may be inadequate in burn patients or in critically ill patients. In our study, ceftazidime was administered by IV bolus injection. Continuous infusion of ceftazidime may optimize the pharmacodynamics with adequate antibacterial activity for 24 hours. A study by Conil et al. suggested that doses between 3 and 16 g per day are sufficient to treat infection in burn patients.29
The other findings in our study are that in the survivors group with inhalation trauma, CRP level was higher (19,37 ± 8,87) mg/dl than in those without inhalation trauma (12,63 ± 4,80 mg/dl with p value 0,085), and also burn size in the deaths group was higher than that of the survivors group. In our study, we found that in the deaths group, all patients had inhalation trauma. Morbidity associated with inhalation trauma is produced by exposure to heat and inhaled toxin. Mortality increases to a maximum of 20% by inhalation trauma alone and 60% when inhalation trauma and pneumonia occur in burn patients.30 Inhalation trauma has been suggested to be an independent factor in mortality in burn patients and can worsen the condition of burn patients even with the same age and burn size.31
The results of this study indicate that shortly after the patient is hospitalized, a culture examination should be carried out, especially in burn patients with a high suspicion of infection. In this way, the bacterial isolate and antibiotic sensitivity can be identified. Thus, the use of antibiotics can be more appropriate. The limitation of this study is the small number of samples, so our results may not represent the overall condition of burn patients. Another limitation was that some factors that can affect CRP level were not controlled, such as surgery or debridement, analgesic and steroid use.
Conclusion
The administration of ceftazidime as an empirical antibiotic lowers CRP level, although this is not significant. Level of leucocytes, neutrophils, lymphocytes, and ratio of neutrophils to lymphocytes was still increased after 7 days of ceftazidime administration. Further studies are needed with a larger sample size and other variables that can affect CRP level, or other inflammatory markers should be investigated.
References
- 1.Brusselaers N, Monstrey S, Vogelaers D, Hoste E, Blot S. Severe burn injury in Europe: a systematic review of the incidence, etiology, morbidity, and mortality. Crit. Care. 2010;14(5) doi: 10.1186/cc9300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wasiak J, Lee SJ, Paul E. Predictors of health status and health-related quality of life 12 months after severe burn. Burns. 2014;40(4):568–574. doi: 10.1016/j.burns.2014.01.021. [DOI] [PubMed] [Google Scholar]
- 3.Jeschke MG, Chinkes DL, Finnerty CC, Kulp G. Pathophysiologic response to severe burn injury. Ann Surg. 2008;248:387–401. doi: 10.1097/SLA.0b013e3181856241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garnacho-Montero J, Garcia-Garmendia JL, Barrero-Almodovar AE, Gimenez FG. Impact of adequate empirical antibiotic therapy on the outcome of patients admitted to the intensive care unit with sepsis. Crit Care Med. 2003;31:2742–2751. doi: 10.1097/01.CCM.0000098031.24329.10. [DOI] [PubMed] [Google Scholar]
- 5.Povoa P. C-reactive protein: a valuable marker of sepsis. Intensive Care Med. 2002;28:235–243. doi: 10.1007/s00134-002-1209-6. [DOI] [PubMed] [Google Scholar]
- 6.Sierra R, Rello J, Bailen MA, Benitez E. C-reactive protein used as an early indicator of infection in patients with systemic inflammatory response syndrome. Intensive Care Med. 2004;30:2038–2045. doi: 10.1007/s00134-004-2434-y. [DOI] [PubMed] [Google Scholar]
- 7.Belcher HJR: XXXXXXXXXXX. New York: Churchill Livingstone; 1996. Principles and practice of burns management; pp. 163–176. [Google Scholar]
- 8.Hancock RE, Speert DP. Antibiotic resistance in Pseudomonas aeruginosa: mechanisms and impact on treatment. Drug Resist Updat. 2000;3:247–255. doi: 10.1054/drup.2000.0152. [DOI] [PubMed] [Google Scholar]
- 9.Clyne B, Olshaker JS. The C-reactive protein. J Emerg Med. 1999;17:1019–1025. doi: 10.1016/s0736-4679(99)00135-3. [DOI] [PubMed] [Google Scholar]
- 10.Zahorec R. Ratio of neutrophil to lymphocyte counts - rapid and simple parameter of systemic inflammation and stress in critically ill. Bratisl Lek Listy. 2001;102(1):5–14. [PubMed] [Google Scholar]
- 11.Barati M, Alinejad F, Bahar MA, Tabrisi MS. Comparison of WBC, ESR, CRP and PCT serum levels in septic and nonseptic burn case. Burns. 2008;34:770–774. doi: 10.1016/j.burns.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 12.Al-Ubady AE, Al-Bahadly AB, Jasem MA, Salman IY. A comparative study between C-Reactive Protein and procalcitonin in Iraqi burn patients. Al-Muntansiriyah Journal of Science. 2017;28(1):41–46. [Google Scholar]
- 13.Yichao Xu, Xinyuan Jin, Xiaonan Shao, Feng Zheng, Hong Zhou. Valuable prognostic for severe burn sepsis with inhalation lesion: age, platelet count, and procalcitonin. Burns & Trauma. 2018;6:29. doi: 10.1186/s41038-018-0132-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.John J, Chisthi MM, Kuttanchettiyar KG. C-reactive protein: an early predictor of sepsis in patients with thermal burns. Int Surg J. 2017;4(2):628–632. [Google Scholar]
- 15.Papini RP, Wilson AP, Steer JA, McGrouther DA, Parkhouse N. Wound management in burn centres in the United Kingdom. Br J Surg. 1995;82(4):505–509. doi: 10.1002/bjs.1800820423. [DOI] [PubMed] [Google Scholar]
- 16.Jeschke MG, Finnerty CC, Kulp GA, Kraft R, Herndon DN. Can we use C-reactive protein levels to predict severe infection or sepsis in severely burned patients? Int J Burn Trauma. 2013;3(3):137–143. [PMC free article] [PubMed] [Google Scholar]
- 17.Lavrentieva A, Papadopoulou S, Kioumis J, Kaimakamis E, Bitzani M. PCT as a diagnostic and prognostic tool in burn patients. Whether time course has a role in monitoring sepsis treatment. Burns. 2012;38:356–363. doi: 10.1016/j.burns.2011.08.021. [DOI] [PubMed] [Google Scholar]
- 18.von Heimburg D, Stieghorst W, Khorram-Sefat R, Paulla N. Procalcitonin - a sepsis parameter in severe burn injuries. Burns. 1998;24:745–750. doi: 10.1016/s0305-4179(98)00109-0. [DOI] [PubMed] [Google Scholar]
- 19.Csenkey A, Jozsa G, Gede N, Pakai E, Tinusz B. Systemic antibiotic prophylaxis does not affect infectious complications in pediatric burn injury: a meta-analysis. PLoS ONE. 2019;14(9) doi: 10.1371/journal.pone.0223063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Menendez R, Cavalcanti M, Reyes S. Markers of treatment failure in hospitalized community-acquired Pneumonia. Thorax. 2008;63:447–452. doi: 10.1136/thx.2007.086785. [DOI] [PubMed] [Google Scholar]
- 21.Bruns AHW, Oosterheert JJ, Hak E, Hoepelman AIM. Usefulness of consecutive C-reactive protein measurements in followup of severe community-acquired pneumonia. Eur Respir J. 2008;32:726–732. doi: 10.1183/09031936.00003608. [DOI] [PubMed] [Google Scholar]
- 22.Ramos G, Cornistein W, Terros Cerino G, Nacil G. Systemic antimicrobial prophylaxis in burn patients: systematic review. JHI. 2017;97(2):105–114. doi: 10.1016/j.jhin.2017.06.015. [DOI] [PubMed] [Google Scholar]
- 23.Dinsdale RJ, Devi A, Hampson P, Wearn CM. Changes in novel haematological parameters following thermal injury: a prospective observational cohort study. Scientific reports. 2017 doi: 10.1038/s41598-017-03222-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stoilova YD, Haidushka IA. Immunological and microbiological investigations of patients with burn injuries. Folia Medica. 2007;XLIX:1–2. [PubMed] [Google Scholar]
- 25.Fuss J, Voloboyeva A, Poliovyj V. Prognostic value of using neutrophil-lymphocyte ratio in patients with burn injury for the diagnosis of sepsis and bacteraemia. Pol przegl chir. 2018;90(5):13–16. doi: 10.5604/01.3001.0012.0971. [DOI] [PubMed] [Google Scholar]
- 26.Djordjevic D, Rondovic G, Surbatovic M, Stanojevic I. Mediators of inflammation. Hindawi; 2018. Neutrophil-to-lymphocyte ratio, monocyte-to-lymphocyte, platelet-to-lymphocyte ratio, and mean platelet volume-to platelet count ratio as biomarkers in critically ill and injured patients: which ratio to choose to predict outcome and nature of bacteremia? [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heffernan DS, Monaghan SF, Thakkar RK, Machan JT. Failure to normalize lymphopenia following trauma is associated with increased mortality, independent of the leukocytosis pattern. Critical Care. 2012;16(1) doi: 10.1186/cc11157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Menges T, Engel J, Welters I. Changes in blood lymphocyte populations after multiple trauma: Association with posttraumatic complications. Critical Care Medicine. 1999;27(4):733–740. doi: 10.1097/00003246-199904000-00026. [DOI] [PubMed] [Google Scholar]
- 29.Conil J-M, Georges B, Ravat F, Ruiz S. Ceftazidime dosage regimentations in burn patients: from a population pharmacokinetics approach to clinical practice via Monte Carlo simulations. Clinical Therapeutics. 2013;35(10):1603–1612. doi: 10.1016/j.clinthera.2013.08.014. [DOI] [PubMed] [Google Scholar]
- 30.Dries DJ, Endorf FW. Inhalation Injury: epidemiology, pathology, treatment strategies. Scandinavian Journal of Trauma, Resuscitation and Emergency. 2013;21:31. doi: 10.1186/1757-7241-21-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.You K, Yang HT, Kym D, Yoon J. Inhalation injury in burn patients: establishing the link between diagnosis and prognosis. Burns. 2014;40:1470–1475. doi: 10.1016/j.burns.2014.09.015. [DOI] [PubMed] [Google Scholar]


