Abstract
Aim:
To understand the potential harmful effects of dose escalation among patients with chronic, non-cancer pain (CNCP) on chronic opioid therapy.
Design:
Retrospective cohort study
Setting:
United States Veterans Healthcare Administration
Participants:
Veterans with CNCP and on chronic opioid therapy were identified using data from fiscal years 2008–2015. The Veteran sample was approximately 90% male and 70% white..
Measurements:
Dose escalators (increase of ˃20% average morphine milligram equivalent (MME) daily dose) were compared with dose maintainers (change of ±20% average MME daily dose). A composite measure of subsequent substance use disorders (SUDs: opioid, non-opioid, and alcohol use disorders) and opioid-related adverse outcomes (AOs: accidents resulting in wounds/injuries, opioid-related and alcohol and non-opioid medication-related accidents and overdoses, self-inflicted injuries) as well as the individual SUDs and AOs was examined. The primary analyses were conducted among a 1:1 matched sample of escalators and maintainers matched on propensity score and index date. Propensity scores were generated using demographic characteristics, medical comorbidities, medication and healthcare utilization characteristics. Subgroup analyses were conducted by quartile of the propensity score. Sensitivity analyses were conducted using adjusted logistic regression, logistic regression using stabilized inverse probability of treatment weighting (SIPTW), and instrumental variable (IV) models using geographic variation in opioid dose escalation as the IV.
Findings:
There were 32,420 maintainers and 20,767 escalators resulting in 19,358(93.2%) matched pairs. Composite AOs (OR=1.31,95%CI:1.23,1.40), composite SUDs (OR=1.31,95%CI:1.22,1.41), and individual SUD and AO subtypes were higher among dose escalators except for opioid-related accidents and overdoses and violence-related injuries. Subgroup analyses within the propensity score quartiles found similar results. Sensitivity analyses with the adjusted and SIPTW logistic regressions found similar results to the primary analyses for all outcomes except for opioid-related accidents and overdoses, which were found to be significantly higher among escalators. Sensitivity analyses with IV models provided mixed results with SUDs and the individual types of AOs.
Conclusion:
Escalating the opioid dose for those with chronic, non-cancer pain is likely associated with increased risks of substance use disorder and opioid-related adverse outcomes.
Keywords: opioids, dose escalation, substance use disorders, adverse outcomes
Introduction
Opioid therapy for chronic pain, especially for moderate to severe chronic pain, is common with some prevalence estimates close to 20%(1). While opioid therapy is well accepted for some indications,(2) the effectiveness of opioids for all forms of chronic pain has not been well established. A recent 12 month, pragmatic, randomized trial comparing non-opioid to opioid medication therapy among Veterans with osteoarthritis or chronic back pain found no differences in pain-related function between those randomized to opioid and non-opioid strategies; however, improved pain intensity and lower medication-related symptoms were observed among non-opioid users(3).
For patients already taking opioids chronically for chronic pain, opioid dose escalation is common due to insufficient pain relief or tolerance(4,5). Opioid tolerance is the declining responsiveness of the opioid receptor to the opioid agonist thereby creating a need for dose escalation to achieve continued analgesia(6). Opioid-induced hyperalgesia can also arise as opioid doses are escalated, creating a conundrum for prescribers(7).
The literature is limited on the harms of opioid dose escalation. A study of patients within a university health system found opioid dose escalation to be associated with higher rates of substance use disorder (SUD) development(8). Studies from Malaysia and Canada found that men were more likely to dose escalate.(9,10) The Canadian study also found that men were also more likely to die of opioid-related causes.(10) Other US studies found an increased risk of opioid misuse and mortality with dose escalation.(11,12) Other outcomes potentially related to opioid dose escalation, such as car accidents, self-inflicted injuries, and other drug related overdoses, have not been evaluated.
The purpose of this study was to provide insights into the impact of opioid dose escalation compared with dose maintenance on opioid-related adverse outcomes (AOs). We hypothesized that SUDs, accidents resulting in wounds or injuries, self-inflicted injuries, opioid-related accidents and overdoses, alcohol and non-opioid drug-related accidents and overdoses, and violence-related injuries would be significantly higher among opioid dose escalators compared with dose maintainers.
Methods
Data Source
Data were obtained from the Corporate Data Warehouse (CDW) of the Veterans Health Administration (VHA), the largest integrated health system in the United States(13). Inpatient, outpatient, demographic, pharmacy, and vital sign files for the fiscal years of 2008–2015 were obtained. The study was approved by the Central Arkansas Veterans Healthcare System Institutional Review Board. The aims and general analytic approach were pre-specified in the application to the Institutional Review Board as well as in a grant application to the National Institute On Drug Abuse; however, these aims and analysis plan were not registered in a publicly available trial registry prior to executing the study so the results could be considered exploratory.
Study Design and Subjects
Using a retrospective cohort study design, Veterans with chronic, non-cancer pain (CNCP) were identified. CNCP was defined as having at least one diagnosis for one of the following 5 major conditions: arthritis, back pain, neck pain, neuropathic pain, or headache/migraine from 10/1/2008 to 9/30/2015(14). Among those with CNCP, Veterans were further required to be to receive at least a 90 days’ supply of non-parenteral opioids without a 30 day or more gap in supply within two consecutive 180 day periods(15). The first period served as the baseline period, and the second was used to determine if the Veteran escalated their dose or maintained their dose. Opioids were defined by the VA Drug Class Code CN101 corresponding to ‘Opioid Analgesics’ (Appendix 1).
Main Independent Variable
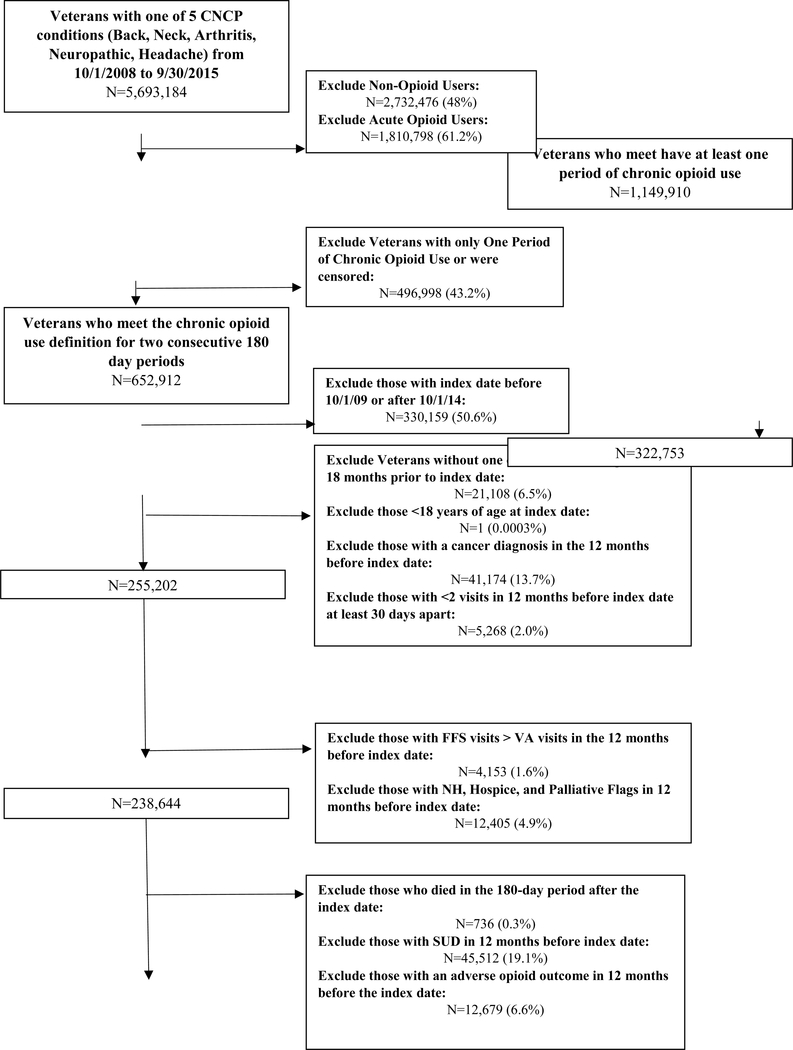
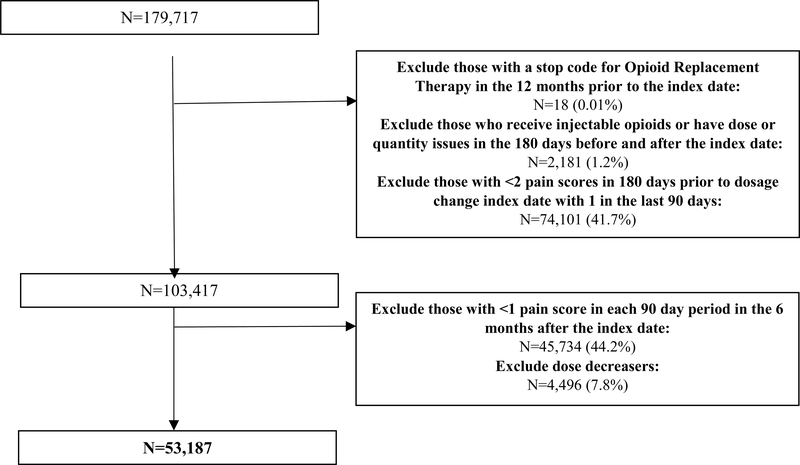
Veterans were classified into 2 mutually exclusive groups: dose escalators and dose maintainers. The initial 180-day period of chronic opioid therapy was used as the denominator for calculating the change in opioid dose expressed in morphine milligram equivalents (MME) in the second 180-day period. Dose escalation was defined as at least a 20% increase in average daily MME dose in the second 180-day period compared to the initial 180-day period. Dose maintenance was defined as an average daily dose in the second period that was within +/− 20% of the average daily dose of the initial period(16,17). Those that experienced a dose decrease of 20% or greater were excluded. The index date was defined as the first day following the initial 180-day period in which chronic opioid use was first detected which coincides with the first day of the second 180-day period in which dose changes were assessed. See eFigure 1 for visual representation of the cohort identification.
Exclusion Criteria
Eleven exclusion criteria were incorporated based on the records observed in 12 months prior to the index date unless otherwise specified: (1) classification as an opioid dose decreaser as previously described so that are comparison group consisted of persons that maintained relatively stable doses thus excluding dose decreasers who could potentially be experiencing improvements in their underlying pain etiology, (2) less than 18 years of age as of the index date due to restrictions in use of VHA data for those less than 18, (3) index date before 10-1-2009 or after 10-1-2014 to ensure a year before and after the index date to evaluate baseline covariates and outcomes, (4) diagnosis for a SUD, AO, or cancer (except for non-melanoma skin cancer) only in the 365 days prior to the index date to evaluate new onset of SUDs and AOs and ensure use of opioids for CNCP, (5) receipt of hospice/palliative care or opioid agonist therapy for addiction to exclude persons using end of life care, (6) potentially erroneous opioid prescription records [unable to calculate morphine milligram equivalents (MME), average daily dose above 1000 MMEs, or prescription quantity greater than 1000 units] in 180 days before or after the index date to ensure appropriate calculations of opioid dose, (7) more visits with providers outside the VA than with VA providers to help ensure that Veterans use the VA as their primary source of care, (8) fewer than 2 visits at least 30 days apart to any VA facility also to help ensure primary use of the VA system, (9) fewer than 2 numeric pain scores in the 180 days prior to the index date with at least 1 on or within 90 days prior to the index date to help ensure use of the VA system for care and provide a baseline assessment of changes in pain scores for adjustment, (10) fewer than 1 pain score in each 90 day periods of the 180 day period (at least 2 pain scores total in the follow-up period) after the index date to ensure continued assessment of pain scores, and (11) death in the 180 day period after the index date to ensure the ability to classify patients as dose escalators or maintainers.
Study Outcomes
Adverse Outcomes
Study outcomes were assessed over the 12-month period after the index date. Adverse opioid outcomes (AOs) were based on International Classification of Diseases, 9th Revision, Clinical Modifications (ICD-9-CM codes) for accidents resulting in wounds/injuries, opioid-related accidents and overdoses, alcohol and non-opioid drug-related accidents and overdoses, self-inflicted injuries, and violence-related injuries (Appendix 2) as defined by Seal et al.(18). AOs were assessed as a composite and separately. The composite AO was coded as a binary indicator for each patient (e.g., coded as 1 if the patient experienced any of the individual types of AOs).
Substance Use Disorders
Opioid use disorders (OUD), non-opioid drug use disorder (DUD), and alcohol use disorders (AUD), were derived from ICD-9-CM definitions from the VA Northeast Program Evaluation Center (NEPEC) (Appendix 3)(19). DUDs included use disorders for benzodiazepines, marijuana, cocaine and other non-opioid psychoactive substances. Each of the categories within both SUDs and AOs were not mutually exclusive; therefore, a Veteran could have more than one type of SUD or AO (e.g. have both OUD and AUD). Each were first evaluated as a composite (e.g. had a SUD or not) and individually (e.g. OUD).
Covariates
Baseline covariates were assessed in the 12 months before the index date. Inclusion of each of the baseline covariates were driven by previous studies evaluating substance use disorders and other opioid-related adverse outcomes(20–26). Demographic covariates included sex, age, race, marital status, and geographic region(27). Medical covariates included diagnoses for CNCP conditions, mental health conditions (schizophrenia, major depressive disorder, post-traumatic stress disorder, anxiety disorders, bipolar disorder, multiple mental health conditions), and the enhanced Charlson comorbidity score(28). Using VA Drug Class Codes, medication classes that aid in treating pain or increase the risk of opioid use were characterized as any use in the 12-month period prior to the index date (antidepressants, benzodiazepines, skeletal muscle relaxants, other non-opioid analgesics, hypnotics/other non-benzodiazepine sedatives). Opioid medication characteristics for the first 180 days of chronic opioid therapy were also assessed, including the duration of action (long acting, short acting), schedule of opioids used, as well as average morphine equivalent dose. Health care visits (physical therapy, pain clinic, chiropractic care, medicine/primary care, and mental health visits) were characterized in two ways: 1) any visit in the 12-month period prior to the index date, and 2) the number of days with each healthcare visit type. Pain score characteristics were also identified as covariates from the first 180-day period of chronic opioid use, including average pain score, last pain score, and pain score change (from initial to last pain score).
Analysis
Propensity scores for dose escalation were generated using a logistic regression with the outcome being a binary indicator for dose escalation/maintenance with all of the covariates defined above as predictors. A 1:1 greedy matching algorithm without replacement was used to match dose escalators to dose maintainers based on the propensity score and index date (within ± 180 days of each other)(29,30). The balance of covariates between dose escalators and dose maintainers was assessed by estimating standardized differences before and after matching. Covariates were considered well balanced when standardized differences were less than 10%(31). Unadjusted logistic regression among the entire sample and among the propensity score matched sample was conducted to compare the changes in estimates after matching on baseline characteristics. For the logistic regression model among the propensity score matched sample, only the dummy variable for dosage change and the counts of each type of healthcare visit in the 12 months prior to the index date were included. Veterans were balanced with the propensity score on whether they used each type of healthcare service. However, some types of healthcare services were not used by most patients making balancing on intensity of healthcare utilization difficult, so visit counts were incorporated for further adjustment. Logistic models were estimated for the two composite outcomes (SUDs, AOs) in addition to the individual components of the composite outcome measures.
Analyses were performed using SAS Enterprise Guide 7.1 using a two-sided significance level of 0.05.
Sub-group Analysis
To assess whether the effects of dose escalation were modified by background covariates, sub-group analyses were performed. Four separate series of logistic models among the propensity score matched samples were estimated by propensity score quartile.
Sensitivity Analysis
To explore the robustness of the initial findings, three alternative analytic approaches were undertaken. The first was use of traditional adjusted logistic regression among the entire sample. The second was a variant of the original propensity score approach whereas, instead of matching on the propensity score, stabilized inverse probability of treatment weighting (SIPTW) were calculated and incorporated into the logistic regression model. The SIPTW were calculated using publicly available SAS code(32). Both logistic regression techniques used the same covariates as the primary analyses and as described in the Covariates section above. Trimming of Veterans in non-overlapping regions of the propensity score distribution was conducted among the final sample.(33)
Given none of the approaches outlined above can account for potential unobserved confounding, instrumental variable (IV) models were estimated(34–36). Use of IV analyses hinge on finding a valid IV which is a variable that influences exposure, in this case, being more likely to escalate opioid dose but does not directly influence the outcome (e.g. AOs or SUDs).(36) IVs based on geographic variation of treatment are common in the literature and have been shown to be valid(35,37–39). Geographic variation in opioid prescribing, dosing, and opioid formulation is prevalent; high dose users in 2012 ranged from 1.9 per 100 persons to 8.8 per 100 persons across the US states(40–45).
The IV explored in this analysis was the geographic variation in opioid dose escalation (percentage of escalators) across the 130 parent VA stations among the chronic opioid use sample. The parent VA station where a Veteran’s most opioid prescriptions were filled was considered his/her VA station. Two IV approaches were used: Wald estimator and two-stage least square regression models. A Wald estimator does not adjust for covariates and provides a bivariate estimate of the outcome by the IV. The Wald is estimated using the formula in Appendix 4. Yz corresponds to the outcome event (e.g., SUDs) for those in high (z=1) dose escalating stations and low (z=0) dose escalating stations. Tz corresponds to the proportion of persons that had their doses escalated in high (z=1) and low (z=0) dose escalating stations. The VA stations were classified into high and low dose escalating stations based on the median proportion of persons where doses where escalated across all VA stations. Two-stage least square regression models (2SLS) are a series of two regressions that first estimate the treatment variable (e.g., dose escalation) using the covariates, including the instrument, then, using that result, estimates the effect on the outcome (e.g., SUD development).(46)(47). To explore the validity of the IV, three steps were undertaken. First, standardized differences for all covariates were calculated between those seeking care in frequent dose-escalating VA stations compared to those in infrequent dose-escalating VA stations split into each category based on the median dose escalation rate observed. Balance of the covariates between frequent and infrequent dose-escalating VA stations suggests the instrument is unrelated to the outcome except through differences in the treatment received. Second, post estimation tests were performed including Durbin-Wu-Hausman and F-tests among the adjusted 2SLS regression models. Durbin-Wu-Hausman tests compare the 2SLS model to an ordinary least squares regression model determining if an IV is needed(48–50). The F-test evaluates the relative strength of the potential IV with an F statistic > 10 being indicative of a strong IV(51,52). Third, the IV 2SLS models were re-estimated as a biprobit model given that both the treatment variable (dose escalation) and the outcome variables (SUDs and AOs) were binary in nature(53). Biprobit models perform the same two-step process as described above with 2SLS models, but account for the binary nature of the treatment and outcome variables. IV models were estimated in STATA 15.1.
Results
Sample Derivation and Characteristics
After application of the inclusion and exclusion criteria, 53,187 Veterans were retained in the final sample of which, 32,420 (61%) maintained opioid doses and 20,767 (39%) escalated doses (Figure 1). For both dose escalators and maintainers, roughly 70% were white, 90% male, 50% between the ages of 50 and 64, and nearly 70% from urban areas. Other medication use was prevalent among this population with 56.5% and 53.4% of dose escalators and maintainers using antidepressants and 73.4% and 69.9% using other non-opioid analgesics.
Figure 1:
Derivation of Study Sample
Before matching, opioid medication characteristics differed between dose escalators and dose maintainers. More dose maintainers received short-acting opioids only and schedule IV opioids. More dose escalators received combined long and short-acting opioids and combined opioids with differing schedules. Dose escalators also had higher first, last, and average pain scores in the baseline period (Table 1). After matching, 19,358 dose escalators were matched to dose maintainers (93.2% of dose escalators and 59.7% of dose maintainers). All standardized differences were less than 10% (Table 1).
Table 1.
Baseline Demographic Characteristics of Dose Escalators and Maintainers before and after Matching
| Unmatched Sample (N=53,187) | Matched Sample (N=38,716) | |||||
|---|---|---|---|---|---|---|
| Dose Maintainer (N=32,420) | Dose Escalator (N=20,767) | Abs Std Diff | Dose Maintainer (N=19,358) | Dose Escalator (N=19,358) | Abs Std Diff | |
| N (Column %) | N (Column %) | (%) | N (Column %) | N (Column %) | (%) | |
| Race | ||||||
| White | 22715 (70.06) | 14824 (71.38) | 2.9 | 13751 (71.04) | 13729 (70.92) | 0.3 |
| Black | 5343 (16.48) | 3363 (16.19) | 0.8 | 3163 (16.34) | 3202 (16.54) | 0.5 |
| Multiracial | 1127 (3.48) | 636 (3.06) | 2.3 | 579 (2.99) | 607 (3.14) | 0.8 |
| Other | 2294 (7.08) | 1328 (6.39) | 2.7 | 1278 (6.60) | 1242 (6.42) | 0.8 |
| Unknown | 941 (2.90) | 616 (2.97) | 0.4 | 587 (3.03) | 578 (2.99) | 0.3 |
| Age | ||||||
| 58.2 ± 12.82 | 55.6 ± 13.22 | 56.21 ± 12.95 | 56.14 ± 13.06 | |||
| 18–30 | 1097 (3.38) | 1118 (5.38) | 9.8 | 905 (4.68) | 915 (4.73) | 0.2 |
| 31–49 | 5850 (18.04) | 4655 (22.42) | 10.9 | 4198 (21.69) | 4167 (21.53) | 0.4 |
| 50–64 | 16164 (49.86) | 10434 (50.24) | 0.8 | 9706 (50.14) | 9831 (50.79) | 1.3 |
| ≥65 | 9309 (28.71) | 4560 (21.96) | 15.6 | 4549 (23.50) | 4445 (22.96) | 1.3 |
| Sex | ||||||
| Male | 29342 (90.51) | 18715 (90.12) | 1.3 | 17463 (90.21) | 17458 (90.18) | 0.1 |
| Marital Status | ||||||
| Married | 16859 (52.00) | 10309 (49.64) | 4.7 | 9787 (50.56) | 9719 (50.21) | 0.7 |
| Rural-Urban Commuting Area | ||||||
| Urban | 22314 (68.83) | 14534 (69.99) | 2.5 | 13550 (70.00) | 13518 (69.83) | 0.4 |
| Large Rural | 4682 (14.44) | 2993 (14.41) | 0.1 | 2793 (14.43) | 2800 (14.46) | 0.1 |
| Isolated Small Rural | 4628 (14.28) | 2826 (13.61) | 1.9 | 2616 (13.51) | 2656 (13.72) | 0.6 |
| Missing | 796 (2.46) | 414 (1.99) | 3.1 | 399 (2.06) | 384 (1.98) | 0.6 |
| Enhanced Charlson Comorbidity Index | ||||||
| 2.6 ± 1.95 | 2.4 ± 1.95 | 2.45 ± 1.97 | 2.43 ± 1.95 | |||
| 0 | 3481 (10.74) | 2694 (12.97) | 6.9 | 2365 (12.22) | 2435 (12.58) | 1.1 |
| 1 | 7489 (23.10) | 5245 (25.26) | 5.0 | 4830 (24.95) | 4804 (24.82) | 0.3 |
| 2 | 7525 (23.21) | 4777 (23.00) | 0.5 | 4440 (22.94) | 4461 (23.04) | 0.3 |
| 3 | 5605 (17.29) | 3256 (15.68) | 4.3 | 3107 (16.05) | 3105 (16.04) | 0.0 |
| 4 | 3558 (10.97) | 2033 (9.79) | 3.9 | 1930 (9.97) | 1934 (9.99) | 0.1 |
| 5 | 2100 (6.48) | 1146 (5.52) | 4.0 | 1110 (5.73) | 1092 (5.64) | 0.4 |
| ≥6 | 2662 (8.21) | 1616 (7.78) | 1.6 | 1576 (8.14) | 1527 (7.89) | 0.9 |
| Pain Condition | ||||||
| Back and/or Neck Pain Only | 4384 (13.52) | 2929 (14.10) | 1.7 | 2597 (13.42) | 2704 (13.97) | 1.6 |
| Arthritis Only | 6094 (18.80) | 3464 (16.68) | 5.5 | 3260 (16.84) | 3332 (17.21) | 1.0 |
| Headaches Only | 276 (0.85) | 125 (0.60) | 2.9 | 149 (0.77) | 120 (0.62) | 1.8 |
| Neuropathic Pain Only | 550 (1.70) | 296 (1.43) | 2.2 | 310 (1.60) | 281 (1.45) | 1.2 |
| Arthritis and Back and/or Neck Pain Only | 9372 (28.91) | 6077 (29.26) | 0.8 | 5693 (29.41) | 5636 (29.11) | 0.7 |
| Arthritis, Back and/or Neck Pain, and Headaches Only | 1932 (5.96) | 1369 (6.59) | 2.6 | 1264 (6.53) | 1247 (6.44) | 0.4 |
| Neuropathic Pain and One or More Others | 7919 (24.43) | 5202 (25.05) | 1.4 | 4864 (25.13) | 4830 (24.95) | 0.4 |
| All Tracer Pain Conditions | 453 (1.40) | 394 (1.90) | 3.9 | 317 (1.64) | 361 (1.86) | 1.7 |
| Other Multiple Pain Conditions | 1440 (4.44) | 911 (4.39) | 0.3 | 904 (4.67) | 847 (4.38) | 1.4 |
| Other Medication Use | ||||||
| Antidepressant Use | 17322 (53.43) | 11735 (56.51) | 6.2 | 10794 (55.76) | 10809 (55.84) | 0.2 |
| Skeletal Muscle Relaxant Use | 11690 (36.06) | 8614 (41.48) | 11.1 | 7768 (40.13) | 7822 (40.41) | 0.6 |
| Benzodiazepine Use | 8768 (27.05) | 5846 (28.15) | 2.5 | 5369 (27.74) | 5408 (27.94) | 0.5 |
| Other Non-Opioid Analgesic Use | 22688 (69.98) | 15254 (73.45) | 7.7 | 14100 (72.84) | 14176 (73.23) | 0.9 |
| Hypnotics and Non-Benzodiazepine Sedative Use | 4871 (15.02) | 3405 (16.40) | 3.8 | 3052 (15.77) | 3123 (16.13) | 1.0 |
| Mental Health Conditions | ||||||
| No Mental Health Conditions | 15876 (48.97) | 9697 (46.69) | 4.6 | 9027 (46.63) | 9149 (47.26) | 1.3 |
| Schizophrenia | 271 (0.84) | 142 (0.68) | 1.8 | 133 (0.69) | 138 (0.71) | 0.3 |
| Major Depressive Disorder | 4332 (13.36) | 3066 (14.76) | 4.0 | 2751 (14.21) | 2803 (14.48) | 0.8 |
| Post-Traumatic Stress Disorder | 1854 (5.72) | 1139 (5.48) | 1.0 | 1105 (5.71) | 1068 (5.52) | 0.8 |
| Bipolar Disorder | 320 (0.99) | 244 (1.17) | 1.8 | 184 (0.95) | 229 (1.18) | 2.3 |
| 1717 (5.30) | 1069 (5.25) | 0.7 | 1045 (5.40) | 1004 (5.19) | 1.0 | |
| Multiple Mental Health Conditions | 8050 (24.83) | 5410 (26.05) | 2.8 | 5113 (26.41) | 4967 (25.66) | 1.7 |
| Percent with Each of the Following Visit Types in the 12 Months before Dosage Change Index Date | ||||||
| Physical Therapy | 10715 (33.05) | 7757 (37.35) | 9.0 | 6865 (35.46) | 7130 (36.83) | 2.9 |
| Pain Clinic | 4697 (14.49) | 3767 (18.14) | 9.9 | 3173 (16.39) | 3328 (17.19) | 2.1 |
| Chiropractic Care | 485 (1.50) | 363 (1.75) | 2.0 | 316 (1.63) | 333 (1.72) | 0.7 |
| Medicine and Primary Care | 32392 (99.91) | 20747 (99.90) | 0.3 | 19343 (99.92) | 19339 (99.90) | 0.7 |
| Mental Health Care | 15801 (48.74) | 10840 (52.20) | 6.9 | 9959 (51.45) | 9957 (51.44) | 0.0 |
| Duration of Action of Opioid Use in First 180 Days | ||||||
| Long-Acting Only | 715 (2.21) | 299 (1.44) | 5.7 | 332 (1.72) | 297 (1.53) | 1.4 |
| Short-Acting Only | 29365 (90.58) | 17792 (85.67) | 15.2 | 17219 (88.95) | 17183 (88.76) | 0.6 |
| Combination of Long and Short-Acting | 2340 (7.22) | 2676 (12.89) | 18.9 | 1807 (9.33) | 1878 (9.70) | 1.3 |
| Schedule of Opioid Use in First 180 Days | ||||||
| Schedule II Only | 18908 (58.32) | 12076 (58.15) | 0.4 | 11601 (59.93) | 11429 (59.04) | 1.8 |
| Schedule III Only | 1070 (3.30) | 457 (2.20) | 6.7 | 448 (2.31) | 457 (2.36) | 0.3 |
| Schedule IV Only | 7087 (21.86) | 2953 (14.22) | 20.0 | 2974 (15.36) | 2946 (15.22) | 0.4 |
| Schedule V Only | 0 (0.00) | 0 (0.00) | 0.0 | 0 (0.00) | 0 (0.00) | 0.0 |
| Use of Multiple Schedules | 5355 (16.52) | 5281 (25.43) | 22.0 | 4335 (22.39) | 4526 (23.38) | 2.4 |
| Duration of Action of Opioid Use in Second 180 Days | ||||||
| Long-Acting Only | 848 (2.62) | 538 (2.59) | -- | 504 (2.60) | 449 (2.32) | -- |
| Short-Acting Only | 29291 (90.35) | 16031 (77.19) | -- | 17310 (89.42) | 15424 (79.68) | -- |
| Combination of Long and Short-Acting | 2281 (7.04) | 4198 (20.21) | -- | 1544 (7.98) | 3485 (18.00) | -- |
| Schedule of Opioid Use in Second 180 Days | ||||||
| Schedule II Only | 20259 (62.49) | 13687 (65.91) | -- | 12810 (66.17) | 12701 (65.61) | -- |
| Schedule III Only | 1040 (3.21) | 248 (1.19) | -- | 485 (2.51) | 245 (1.27) | -- |
| Schedule IV Only | 6771 (20.89) | 2086 (10.04) | -- | 3199 (16.53) | 2050 (10.59) | -- |
| Schedule V Only | 0 (0.00) | 0 (0.00) | -- | 0 (0.00) | 0 (0.00) | -- |
| Use of Multiple Schedules | 4350 (13.42) | 4746 (22.85) | -- | 2864 (14.79) | 4362 (22.53) | -- |
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | |||
| Average Morphine Equivalent Dose | ||||||
| First 180 Days | 30.33 (41.44) | 27.16 (29.52) | 8.8 | 27.75 (29.29) | 26.84 (29.95) | 3.1 |
| Second 180 Days | 30.61 (41.62) | 45.76 (48.41) | -- | 28.04 (29.53) | 44.82 (48.57) | -- |
| Percent Change in Average Morphine Equivalent Dose | 1.13% | 77.29% | -- | 1.20% | 75.98% | -- |
| Pain Characteristics in 180 Days before Dosage Change Index Date | ||||||
| First Pain Score | 4.73 (3.30) | 5.26 (3.20) | 16.1 | 5.16 (3.22) | 5.17 (3.22) | 0.2 |
| Last Pain Score | 3.96 (3.28) | 4.71 (3.26) | 23.1 | 4.54 (3.24) | 4.58 (3.26) | 1.2 |
| Pain Score Average | 4.23 (2.38) | 4.88 (2.30) | 27.8 | 4.76 (2.31) | 4.76 (2.29) | 0.3 |
| Change in Pain Score | −0.77 (3.84) | −0.54 (3.83) | 6.1 | −0.62 (3.84) | −0.59 (3.86) | 0.9 |
| Service Visits in the 12 Months before Dosage Change Index Date Conditional on Use of the Visit Type | ||||||
| Physical Therapy | 3.96 (5.76) | 3.94 (5.87) | -- | 4.05 (6.08) | 3.94 (5.93) | -- |
| Pain Clinic | 3.27 (3.17) | 3.46 (3.64) | -- | 3.28 (3.21) | 3.45 (3.64) | -- |
| Chiropractic Care | 4.25 (4.62) | 4.36 (4.95) | -- | 4.18 (4.79) | 4.30 (5.05) | -- |
| Medicine and Primary Care | 10.66 (7.49) | 10.97 (7.51) | -- | 10.79 (7.51) | 10.90 (7.50) | -- |
| Mental Health Care | 8.40 (11.79) | (10.54) | -- | 8.49 (11.44) | 8.12 (10.59) | -- |
Substance Use Disorders
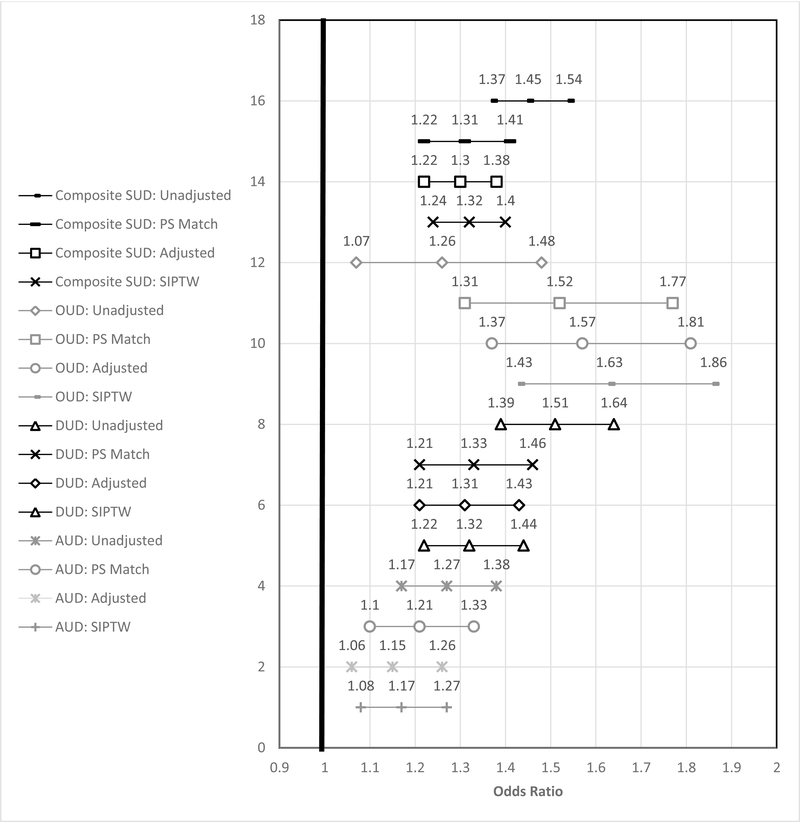
Among both unmatched and matched samples, the rates of composite SUD development and individual SUD types were higher among dose escalators than dose maintainers (Table 2). In the matched sample, dose escalators were more likely than maintainers to experience any SUD and each of the three individual SUD types (Figure 2).
Table 2.
Rates of SUD and AO Development comparing Opioid Dose Escalators to Maintainers for the Unmatched Sample and Matched Sample
| Unmatched Sample | Matched Sample | |||
|---|---|---|---|---|
| Dose Maintainer N=32,420 N (%) | Dose Escalator N=20,767 N (%) | Dose Maintainer N=19,358 N (%) | Dose Escalator N=19,358 N (%) | |
| Composite SUDs | 2439 (7.52) | 2194 (10.57) | 1563 (8.07) | 1990 (10.28) |
| Opioid Use Disorder (OUD) | 410 (1.27) | 493 (2.37) | 280 (1.45) | 423 (2.19) |
| Non-Opioid Drug Use Disorder (DUD) | 1306 (4.03) | 1238 (5.96) | 861 (4.45) | 1120 (5.79) |
| Alcohol Use Disorder (AUD) | 1383 (4.27) | 1112 (5.36) | 860 (4.44) | 1025 (5.30) |
| Composite AOs | 2876 (8.87) | 2409 (11.60) | 1757 (9.08) | 2235 (11.55) |
| Wounds/Injuries | 2292 (7.07) | 1787 (8.61) | 1372 (7.09) | 1671 (8.63) |
| Opioid-Related Overdoses | 78 (0.24) | 86 (0.41) | 60 (0.31) | 78 (0.40) |
| Alcohol and Non-Opioid Medication Related Overdoses | 323 (1.00) | 370 (1.78) | 198 (1.02) | 329 (1.70) |
| Self-Inflicted Injuries | 402 (1.24) | 420 (2.02) | 279 (1.44) | 387 (2.00) |
| Violence-Related Injuries | 36 (0.11) | 43 (0.21) | 24 (0.12) | 39 (0.20) |
Figure 2. Substance Use Disorder Development comparing Opioid Dose Escalators to Maintainers with Primary and All Sensitivity Analyses.
*SUD=Substance Use Disorder; OUD=Opioid Use Disorder; ✠DUD=Non-Opioid Drug Use Disorder; ‡AUD=Alcohol Use Disorder; PS=Propensity Score; SIPTW=Stabilized Inverse Probability of Treatment Weighting
Adverse Outcomes
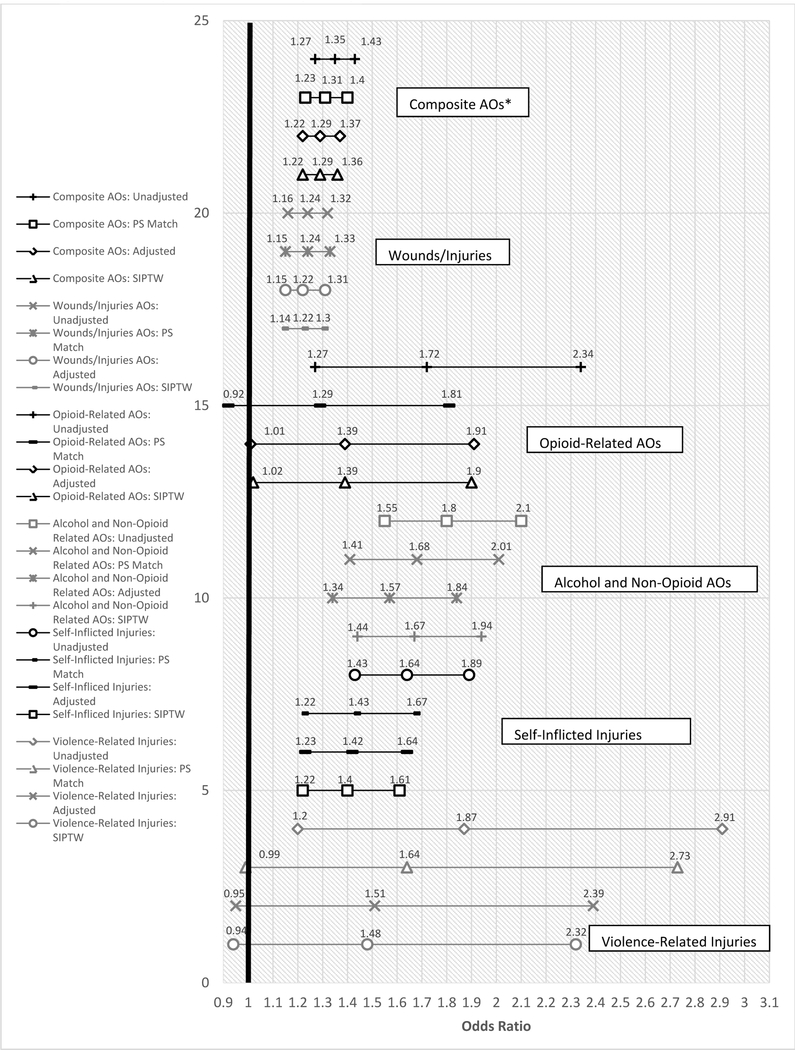
Rates of composite AOs and most individual AOs types were higher for dose escalators than dose maintainers among the unmatched and matched samples (Table 2). Wounds and injuries were the most common AO, occurring among more than 8% of opioid escalators. Opioid-related overdoses and violence-related injuries were the least common AOs (< 0.4%). In the matched sample, dose escalators were more likely than maintainers to experience any AO (Figure 3). Dose escalators were also more likely than maintainers to experience accidents resulting in wounds/injuries, alcohol and non-opioid medication related AOs, and self-inflicted injuries (Figure 3). Opioid-related AOs and violence-related injuries were not significantly different between the two groups (Figure 3).
Figure 3.
Adverse Outcome Development comparing Opioid Dose Escalators to Maintainers with Methods from Primary and All Sensitivity Analyses
Sub-group Analyses
Testing Effect Modification: SUDs and AOs
Escalators had higher rates of composite SUDs and AOs than maintainers in each of the PS quartiles, and the rate of composite SUDs and AOs rose with each increasing quartile (corresponding with increasing probability of being an escalator) (eTable 1). The odds of composite SUDs and AOs were all significantly higher for dose escalators than maintainers across the quartiles with ORs between 1.19 and 1.45 for composite SUD and between 1.18 and 1.42 for composite AOs, suggesting minimal effect modification.
Sensitivity Analyses
Adjusted and SIPTW Logistic Regressions
After trimming of the SIPTW, 53,157 of the 53,187 Veterans were used in the SIPTW analysis. Both adjusted and SIPTW analyses provided similar results to the matched sample analyses for rates of composite and individual types of SUDs (Figure 2). Adjusted and SIPTW analyses provided similar results to the matched sample for composite and individual AOs, except for opioid-related AOs. Whereas the matched sample results were null, the adjusted and SIPTW analyses found a significant increased risk of opioid-related AOs with dose escalation (Figure 3).
IV Analyses
The distribution of most covariates was well balanced among Veterans getting care at VAMCs above the median dose escalation rate (39%) versus those getting care at VA stations below the median dose escalation rate indicating balance of most measured covariates and potential balance of unmeasured covariates (eTable 2). However, patients of VA stations with higher escalation rates were more often white, from urban areas, and treated with short-acting and Schedule II opioids only than those at VA stations with lower escalation rates.
The Wald estimator (similar to a bivariate analysis between exposure and outcome) for percentage of escalators per VA station was positive and significant for both SUDs and AOs, indicating that escalating chronic opioid doses increased the risk for SUDs and AOs (eTable 3). When incorporating all covariates, the 2SLS models showed an insignificant association between dose escalation and SUDs and a significantly positive association between dose escalation and AOs. As with the 2SLS models, the biprobit models found an insignificant association between opioid dose escalation and the development of SUDs and a significant positive association between opioid dose escalation and the development of AOs, indicating an increased development of AOs with dose escalation. Bivariate probit models for the individual types of SUDs and AOs also found insignificant findings between dose escalation and development of each of the individual SUD and AO types. The Durbin and Wu-Hausman tests were insignificant indicating a lack of endogeneity with the treatment variable, dose escalation, calling into question the need for IV analyses. However, the F-statistic for the strength of percentage of escalators per VA station was quite large indicating a strong instrument.
Discussion
Substance Use Disorders
In the matched sample, escalating the average MME dose from approximately 27 MME to 45 MME was associated with an increased risk of approximately 30% for developing any SUD. For development of an OUD, the increased risk was approximately 50%. In this sample of dose escalators without a diagnosis of SUD or AO in the prior year, the one-year risk of developing any SUD was more than 10% and the risk of OUD exceeded 2%, demonstrating the non-trivial risk of these complications for opioid treated patients. The increased risk of SUD and OUD development was consistent with the primary analysis and sensitivity analyses using both adjusted and SIPTW logistic regression models.
The finding that opioid dose escalation is associated with subsequent SUD development is corroborated in several other studies. Henry et al, in a study of new, year-long opioid users with musculoskeletal pain, found that 17% of opioid dose escalators developed a SUD versus 1% of dose maintainers(8). Another study among Veterans and commercially insured patients with musculoskeletal pain and long-term opioid use found higher opioid doses were associated with higher self-reported hazardous alcohol and substance use(54).
Adverse Outcomes
Dose escalation also increased the risk of developing composite AOs and most individual types of AOs, except for opioid-related accidents and overdoses and violence-related injuries. Except for opioid-related overdoses and violence, the risk of escalating opioid dose conferred a 23%–68% increase in relative risk in the other adverse events, with the highest risk observed for alcohol or non-opioid medication-related AOs. Sensitivity analyses using both adjusted and SIPTW logistic regression models found similar results to the primary analysis for all AOs except for opioid-related accidents and overdoses. Unlike with the propensity score matched analyses, opioid-related accidents and overdoses were significantly higher among dose escalators using both adjusted and SIPTW logistic regression models, which used the full sample.
This may in part be due to the relatively rare occurrence of these outcomes, which would be more likely to reach significance with larger samples. Despite the conflicting statistical significance between the matched propensity score analysis and the two sensitivity analyses, all of the analyses showed an increasing trend across opioid AOs among dose escalators. Previous studies also demonstrate that opioid-related overdoses increases with escalating opioid daily doses(5,55–57). Non-opioid drug related overdoses have also been found to be higher among patients with higher opioid doses, particularly for those with concomitant high dose opioids and benzodiazepine use(58–60). New onset depression has also been associated with opioid dose escalation(61). Recent evidence also report that suicide involving opioids is increasing(62–64). Opioid involved self-inflicted injuries is a particular concern for persons being prescribed increasing doses as they are in possession of higher doses or number of dosage units which increases the lethality of opioid involved suicide attempts.
Effect Modification
The results of the sub-group analyses by propensity score quartile suggest that the relationships reported between dose escalation and AOs and SUD are not meaningfully influenced by background covariates that are collectively prognostic in determining whether or not someone escalates their opioid dose. In other words, there do not appear to be groups that are spared or are especially vulnerable to the increased risk associated with escalating opioid doses. Until evidence is reported that identifies specific subgroups with different risks or AOs or SUD when doses are escalated, clinicians should be equally cautions when considering escalating anyone’s chronic opioid dose.
Limitations
Several limitations exist with this study. First, propensity score methods can only adjust for measured confounders(31,65). Though our study included and controlled for some clinical measures such as pain scores, several other potential confounders were not captured including improvement or worsening of the underlying pain condition, drug use behaviors, and social determinants. IV models are less sensitive to unmeasured confounders as compared to propensity score approaches. IV 2-SLS models in this study, unlike the propensity score models, found no association between dose escalation and composite SUDs and the SUD and AO subtypes. However, the Durbin and Wu-Hausman tests were insignificant indicating a lack of endogeneity in the treatment variable, dose escalation. While the Durbin and Wu-Hausman tests are sensitive to model specifications (66), they remained insignificant regardless of the model specifications used. Re-estimating these models using ordinary least squares (OLS) or probit, then, is suggested. Both OLS and probit models showed positive associations between opioid dose escalation and SUD development. Second, results may not be generalizable to the civilian population. Third, these data did not include information on opioid dispensing outside VA. We tried to minimize the effect of unmeasured non-VA healthcare use by excluding Veterans with more non-VA visits than VA visits before the index date and those without sufficient pain scores after the index date. Fourth, we did not evaluate marijuana use, which could be used by some Veterans in the treatment of their CNCP. Fifth, 90% of Veterans in this sample were male; therefore, conclusions on the impact of opioid dose escalation from this study may only be generalizable to men. An additional study is warranted among women. Lastly, VA medical centers are not typically Level 1 Trauma Centers; therefore, trauma-related AOs (e.g., accidents resulting in wounds/injuries, self-inflicted injuries, violence-related injuries) are likely under-represented in the data, Furthermore, opioid-related and non-opioid, medication related overdoses included both fatal and non-fatal overdoses and many fatal overdoses are not captured within claims data since many of these patients may not present for care. When comparing the rate of opioid overdoses reported in this study to another published rate among chronic opioid users(67) we find fairly similar rates of opioid overdose suggesting that for at least detecting opioid overdoses, this may not be a major concern.
Conclusion
Escalating opioid doses among persons prescribed chronic opioids appears to increase the risk of subsequent opioid and non-opioid substance use disorders as well as other potential adverse outcomes, including wounds and injuries and self-inflicted injuries.
Supplementary Material
Acknowledgments
Funding: Research reported in this publication was supported by the National Institute On Drug Abuse of the National Institutes of Health under Award Number R36DA046717. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Dr. Hayes was also supported by the National Institute on Drug Abuse under the Translational Training in Addiction Grant [1T32 DA 022981]. This material is the result of work supported with resources and the use of facilities at the Veterans Health Administration.
Footnotes
Disclaimer: The contents do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
Conflicts of Interest: Dr. Martin receives royalties from TrestleTree LLC for the commercialization of an opioid risk prediction tool, which is unrelated to the current study. Dr. Li is a paid consultant for eMaxHealth Systems for unrelated projects.
References
- 1.Nahin RL, Sayer B, Stussman BJ, Feinberg TM. Eighteen-Year Trends in the Prevalence of, and Health Care Use for, Noncancer Pain in the United States: Data from the Medical Expenditure Panel Survey. J Pain [Internet]. 2019. July [cited 2019 Jul 22];20(7):796–809. Available from: http://www.ncbi.nlm.nih.gov/pubmed/30658177 [DOI] [PubMed] [Google Scholar]
- 2.Ballantyne JC. Chronic pain following treatment for cancer: the role of opioids. Oncologist [Internet]. 2003. December 1 [cited 2018 Aug 21];8(6):567–75. Available from: http://www.ncbi.nlm.nih.gov/pubmed/14657535 [DOI] [PubMed] [Google Scholar]
- 3.Krebs EE, Gravely A, Nugent S, Jensen AC, DeRonne B, Goldsmith ES, et al. Effect of Opioid vs Nonopioid Medications on Pain-Related Function in Patients With Chronic Back Pain or Hip or Knee Osteoarthritis Pain. JAMA [Internet]. 2018. March 6 [cited 2018 Mar 7];319(9):872 Available from: http://jama.jamanetwork.com/article.aspx?doi=10.1001/jama.2018.0899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Han H, Kass P, Wilsey B, Li C. Age, gender, and earlier opioid requirement associations with the rate of dose escalation in long-term opioid therapy. J Opioid Manag [Internet]. 2013;Mar-Apr(9(2)):129–38. Available from: https://www.ncbi.nlm.nih.gov/pubmed/23709322 [DOI] [PubMed] [Google Scholar]
- 5.Kaplovitch E, Gomes T, Camacho X, Dhalla IA, Mamdani MM, Juurlink DN. Sex Differences in Dose Escalation and Overdose Death during Chronic Opioid Therapy: A Population-Based Cohort Study. PLoS One [Internet]. 2015. [cited 2017 Feb 6];10(8):e0134550. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26291716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morgan MM, Christie MJ. Analysis of opioid efficacy, tolerance, addiction and dependence from cell culture to human. Br J Pharmacol [Internet]. 2011. October [cited 2018 Aug 7];164(4):1322–34. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21434879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hayes PharmD, MPH CJ Painter, PharmD MBA, PhD JT. A comprehensive clinical review of opioid-induced allodynia: Discussion of the current evidence and clinical implications. J Opioid Manag [Internet]. 2017. March 1 [cited 2018 Aug 21];13(2):95 Available from: http://www.ncbi.nlm.nih.gov/pubmed/28829524 [DOI] [PubMed] [Google Scholar]
- 8.Henry SG, Wilsey BL, Melnikow J, Iosif A-M. Dose escalation during the first year of long-term opioid therapy for chronic pain. Pain Med [Internet]. 2015. April [cited 2016 Oct 16];16(4):733–44. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25529548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zin CS, Alias NE, Taufek NH, Ahmad MM. Sex differences in high opioid dose escalation among Malaysian patients with long term opioid therapy. J Pain Res [Internet]. 2019. April 24 [cited 2019 Sep 17];Volume 12:1251–7. Available from: https://www.dovepress.com/sex-differences-in-high-opioid-dose-escalation-among-malaysian-patient-peer-reviewed-article-JPR [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaplovitch E, Gomes T, Camacho X, Dhalla IA, Mamdani MM, Juurlink DN. Sex Differences in Dose Escalation and Overdose Death during Chronic Opioid Therapy: A Population-Based Cohort Study. Mintzes B, editor. PLoS One [Internet]. 2015. August 20 [cited 2018 Aug 21];10(8):e0134550. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26291716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morasco B, Smith N, Dobscha S, Deyo R, Hyde S, Yarborough B. (142) Outcomes of Prescription Opioid Dose Escalation for the Treatment of Chronic Pain: Results from a Prospective Cohort Study. J Pain [Internet]. 2019. April 1 [cited 2019 Sep 17];20(4):S12 Available from: https://linkinghub.elsevier.com/retrieve/pii/S1526590019301385 [DOI] [PubMed] [Google Scholar]
- 12.Hser Y-I, Saxon AJ, Mooney LJ, Miotto K, Zhu Y, Yoo CK, et al. Escalating Opioid Dose Is Associated With Mortality. J Addict Med [Internet]. 2019. [cited 2019 Sep 17];13(1):41–6. Available from: http://insights.ovid.com/crossref?an=01271255-201902000-00008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.About VHA - Veterans Health Administration [Internet]. [cited 2018 Aug 24]. Available from: https://www.va.gov/health/aboutvha.asp
- 14.Edlund MJ, Austen MA, Sullivan MD, Martin BC, Williams JS, Fortney JC, et al. Patterns of opioid use for chronic noncancer pain in the Veterans Health Administration from 2009 to 2011. Pain [Internet]. 2014. November [cited 2015 Sep 17];155(11):2337–43. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4252255&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vanderlip ER, Sullivan MD, Edlund MJ, Martin BC, Fortney J, Austen M, et al. National study of discontinuation of long-term opioid therapy among veterans. Pain [Internet]. 2014. December [cited 2016 Dec 7];155(12):2673–9. Available from: http://content.wkhealth.com/linkback/openurl?sid=WKPTLP:landingpage&an=00006396-201412000-00031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen L, Vo T, Seefeld L, Malarick C, Houghton M, Ahmed S, et al. Lack of correlation between opioid dose adjustment and pain score change in a group of chronic pain patients. J Pain [Internet]. 2013. April [cited 2016 Aug 20];14(4):384–92. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23452826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DiBenedetto DJ, Wawrzyniak KM, Finkelman M, Kulich RJ, Chen L, Schatman ME, et al. Relationships Between Opioid Dosing, Pain Severity, and Disability in a Community-Based Chronic Pain Population: An Exploratory Retrospective Analysis. Pain Med [Internet]. 2019. January 17 [cited 2019 Aug 8]; Available from: https://academic.oup.com/painmedicine/advance-article/doi/10.1093/pm/pny240/5290259 [DOI] [PubMed] [Google Scholar]
- 18.Seal KH, Shi Y, Cohen G, Cohen BE, Maguen S, Krebs EE, et al. Association of mental health disorders with prescription opioids and high-risk opioid use in US veterans of Iraq and Afghanistan. JAMA [Internet]. 2012. March 7 [cited 2016 May 14];307(9):940–7. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22396516 [DOI] [PubMed] [Google Scholar]
- 19.Greenberg G, Pilver L, Hoff R. 2012 National Mental Health Fact Sheet: National, VISN, and VAMC Tables [Internet]. 2012. Available from: http://vaww.nepec.mentalhealth.va.gov/NMHPPMS/nmhFaSAC.pdf
- 20.Edlund MJ, Steffick D, Hudson T, Harris KM, Sullivan M. Risk factors for clinically recognized opioid abuse and dependence among veterans using opioids for chronic non-cancer pain. Pain [Internet]. 2007. June [cited 2016 Feb 25];129(3):355–62. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17449178 [DOI] [PubMed] [Google Scholar]
- 21.Sullivan MD, Edlund MJ, Fan M-Y, Devries A, Brennan Braden J, Martin BC. Risks for possible and probable opioid misuse among recipients of chronic opioid therapy in commercial and medicaid insurance plans: The TROUP Study. Pain [Internet]. 2010. August [cited 2015 Sep 19];150(2):332–9. Available from: http://www.sciencedirect.com/science/article/pii/S0304395910003234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Edlund MJ, Martin BC, Fan M-Y, Devries A, Braden JB, Sullivan MD. Risks for opioid abuse and dependence among recipients of chronic opioid therapy: Results from the TROUP Study. Drug Alcohol Depend [Internet]. 2010. November 1 [cited 2019 Apr 9];112(1–2):90–8. Available from: https://www.sciencedirect.com/science/article/pii/S0376871610002048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Edlund MJ, Martin BC, Devries A, Fan M-Y, Braden JB, Sullivan MD. Trends in use of opioids for chronic noncancer pain among individuals with mental health and substance use disorders: the TROUP study. Clin J Pain [Internet]. 2010. January [cited 2016 Aug 10];26(1):1–8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20026946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cochran G, Gordon AJ, Lo-Ciganic W-H, Gellad WF, Frazier W, Lobo C, et al. An Examination of Claims-based Predictors of Overdose from a Large Medicaid Program. Med Care [Internet]. 2017. March [cited 2019 Aug 23];55(3):291–8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27984346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dilokthornsakul P, Moore G, Campbell JD, Lodge R, Traugott C, Zerzan J, et al. Risk Factors of Prescription Opioid Overdose Among Colorado Medicaid Beneficiaries. J Pain [Internet] 2016. April [cited 2019 Oct 15];17(4):436–43. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26721613 [DOI] [PubMed] [Google Scholar]
- 26.Webster LR. Risk Factors for Opioid-Use Disorder and Overdose. Anesth Analg [Internet]. 2017. November [cited 2019 Apr 9];125(5):1741–8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29049118 [DOI] [PubMed] [Google Scholar]
- 27.Rural Urban Commuting Area Codes Data [Internet]. [cited 2017 Dec 6]. Available from: http://depts.washington.edu/uwruca/ruca-uses.php
- 28.Charlson ME, Charlson RE, Peterson JC, Marinopoulos SS, Briggs WM, Hollenberg JP. The Charlson comorbidity index is adapted to predict costs of chronic disease in primary care patients. J Clin Epidemiol [Internet]. 2008. December [cited 2017 Dec 7];61(12):1234–40. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18619805 [DOI] [PubMed] [Google Scholar]
- 29.Parsons LS. Performing a 1:N Case-Control Match on Propensity Score. [cited 2017 Dec 12]; Available from: http://www2.sas.com/proceedings/sugi29/165-29.pdf
- 30.Austin PC. Goodness-of-fit diagnostics for the propensity score model when estimating treatment effects using covariate adjustment with the propensity score. Pharmacoepidemiol Drug Saf [Internet]. 2008. December [cited 2017 Jul 31];17(12):1202–17. Available from: http://doi.wiley.com/10.1002/pds.1673 [DOI] [PubMed] [Google Scholar]
- 31.Austin PC. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivariate Behav Res [Internet]. 2011. May [cited 2014 Jul 9];46(3):399–424. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3144483&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Layton B Estimation and Use of Propensity Score. 2013. [Google Scholar]
- 33.Stürmer T, Wyss R, Glynn RJ, Brookhart MA. Propensity scores for confounder adjustment when assessing the effects of medical interventions using nonexperimental study designs. J Intern Med [Internet]. 2014. June [cited 2019 Sep 13];275(6):570–80. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24520806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sargan JD. The Estimation of Economic Relationships using Instrumental Variables. Econometrica [Internet]. 1958. July [cited 2018 Aug 24];26(3):393 Available from: https://www.jstor.org/stable/1907619?origin=crossref [Google Scholar]
- 35.Lousdal ML. An introduction to instrumental variable assumptions, validation and estimation. Emerg Themes Epidemiol [Internet]. 2018. December 22 [cited 2018 Sep 8];15(1):1 Available from: https://ete-online.biomedcentral.com/articles/10.1186/s12982-018-0069-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baiocchi M, Cheng J, Small DS. Instrumental variable methods for causal inference. Stat Med [Internet]. 2014. June 15 [cited 2018 Aug 24];33(13):2297–340. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24599889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fang G, Brooks JM, Chrischilles EA. A New Method to Isolate Local-Area Practice Styles in Prescription Use as the Basis for Instrumental Variables in Comparative Effectiveness Research. Med Care [Internet]. 2010. August [cited 2018 Sep 8];48(8):710–7. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20613655 [DOI] [PubMed] [Google Scholar]
- 38.Frölich M, Lechner M. Regional Treatment Intensity as an Instrument for the Evaluation of Labour Market Policies [Internet]. 2004. [cited 2018 Sep 8]. Available from: www.iza.org
- 39.Chay KY, Greenstone M, Blundell R, Card D, Case A, Deaton A, et al. THE IMPACT OF AIR POLLUTION ON INFANT MORTALITY: EVIDENCE FROM GEOGRAPHIC VARIATION IN POLLUTION SHOCKS INDUCED BY A RECESSION We are grateful to The Impact of Air Pollution on Infant Mortality: Evidence from Geographic Variation in Pollution Shocks Induc [Internet]. 1999. [cited 2018 Sep 8]. Available from: http://www.nber.org/papers/w7442
- 40.U.S. Prescribing Rate Maps | Drug Overdose | CDC Injury Center [Internet]. [cited 2017 Dec 19]. Available from: https://www.cdc.gov/drugoverdose/maps/rxrate-maps.html
- 41.Paulozzi LJ, Mack KA, Hockenberry JM, Division of Unintentional Injury Prevention, National Center for Injury Prevention and Control, CDC. Vital signs: variation among States in prescribing of opioid pain relievers and benzodiazepines - United States, 2012. MMWR Morb Mortal Wkly Rep. 2014. July;63(26):563–8. [PMC free article] [PubMed] [Google Scholar]
- 42.Webster BS, Cifuentes M, Verma S, Pransky G. Geographic variation in opioid prescribing for acute, work-related, low back pain and associated factors: A multilevel analysis. Am J Ind Med [Internet]. 2009. February 1 [cited 2018 Oct 15];52(2):162–71. Available from: http://doi.wiley.com/10.1002/ajim.20655 [DOI] [PubMed] [Google Scholar]
- 43.McDonald DC, Carlson K, Izrael D. Geographic variation in opioid prescribing in the U.S. J Pain [Internet]. 2012. October [cited 2015 Nov 21];13(10):988–96. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3509148&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Curtis LH, Stoddard J, Radeva JI, Hutchison S, Dans PE, Wright A, et al. Geographic Variation in the Prescription of Schedule II Opioid Analgesics among Outpatients in the United States. Health Serv Res [Internet]. 2006. June [cited 2015 Oct 12];41(3p1):837–55. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1713206&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Delgado MK, Huang Y, Meisel Z, Hennessy S, Yokell M, Polsky D, et al. National Variation in Opioid Prescribing and Risk of Prolonged Use for Opioid-Naive Patients Treated in the Emergency Department for Ankle Sprains. Ann Emerg Med [Internet]. 2018. October 1 [cited 2018 Oct 15];72(4):389–400.e1. Available from: http://www.ncbi.nlm.nih.gov/pubmed/30054152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Burgess S, Small DS, Thompson SG. A review of instrumental variable estimators for Mendelian randomization. Stat Methods Med Res [Internet]. 2017. October 17 [cited 2019 Sep 17];26(5):2333–55. Available from: http://journals.sagepub.com/doi/10.1177/0962280215597579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Burgess S, Small DS, Thompson SG. A review of instrumental variable estimators for Mendelian randomization. Stat Methods Med Res [Internet]. 2017. October 17 [cited 2019 Mar 1];26(5):2333–55. Available from: http://journals.sagepub.com/doi/10.1177/0962280215597579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Durbin J Errors in Variables. Rev l’Institut Int Stat / Rev Int Stat Inst [Internet]. 1954. [cited 2018 Aug 24];22(1/3):23 Available from: https://www.jstor.org/stable/1401917?origin=crossref [Google Scholar]
- 49.Hausman JA. Specification Tests in Econometrics. Econometrica [Internet] 1978. November [cited 2018 Aug 24];46(6):1251 Available from: https://www.jstor.org/stable/1913827?origin=crossref [Google Scholar]
- 50.Wu D-M. Alternative Tests of Independence between Stochastic Regressors and Disturbances. Econometrica [Internet]. 1973. July [cited 2018 Aug 24];41(4):733 Available from: https://www.jstor.org/stable/1914093?origin=crossref [Google Scholar]
- 51.Stock J, Yogo M. Testing for Weak Instruments in Linear IV Regression in Identification and Inference for Econometric Models [Internet]. New York: Cambridge University Press; 2005. [cited 2018 Aug 24]. Available from: https://scholar.harvard.edu/stock/publications/testing-weak-instruments-linear-iv-regression [Google Scholar]
- 52.Stock JH, Wright JH, Yogo M. A Survey of Weak Instruments and Weak Identification in Generalized Method of Moments [Internet] Vol. 20, Journal of Business & Economic Statistics. Taylor & Francis, Ltd.American Statistical Association; 2002. [cited 2018 Aug 24]. p. 518–29. Available from: https://www.jstor.org/stable/1392421 [Google Scholar]
- 53.Title stata.com biprobit-Bivariate probit regression [Internet]. [cited 2018 Aug 24]. Available from: https://www.stata.com/manuals13/rbiprobit.pdf
- 54.Morasco BJ, Yarborough BJ, Smith NX, Dobscha SK, Deyo RA, Perrin NA, et al. Higher Prescription Opioid Dose is Associated With Worse Patient-Reported Pain Outcomes and More Health Care Utilization. J Pain [Internet]. 2017. April 1 [cited 2018 Aug 26];18(4):437–45. Available from: https://www.sciencedirect.com/science/article/pii/S152659001630356X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dunn KM, Saunders KW, Rutter CM, Banta-Green CJ, Merrill JO, Sullivan MD, et al. Opioid prescriptions for chronic pain and overdose: a cohort study. Ann Intern Med [Internet]. 2010. January 19 [cited 2017 Mar 29];152(2):85–92. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20083827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Adewumi AD, Hollingworth SA, Maravilla JC, Connor JP, Alati R. Prescribed Dose of Opioids and Overdose: A Systematic Review and Meta-Analysis of Unintentional Prescription Opioid Overdose. CNS Drugs [Internet]. 2018. February 1 [cited 2018 Aug 26];32(2):101–16. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29498021 [DOI] [PubMed] [Google Scholar]
- 57.Gomes T, Mamdani MM, Dhalla IA, Paterson JM, Juurlink DN. Opioid Dose and Drug-Related Mortality in Patients With Nonmalignant Pain. Arch Intern Med [Internet]. 2011. April 11 [cited 2018 Aug 26];171(7):686–91. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21482846 [DOI] [PubMed] [Google Scholar]
- 58.Gressler LE, Martin BC, Hudson TJ, Painter JT. The relationship between concomitant benzodiazepine-opioid use and adverse outcomes among U.S. veterans. Pain [Internet] 2017. November 20 [cited 2017 Dec 12];1. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29189516 [DOI] [PubMed] [Google Scholar]
- 59.Jones CM, McAninch JK. Emergency Department Visits and Overdose Deaths From Combined Use of Opioids and Benzodiazepines. Am J Prev Med [Internet]. 2015. October [cited 2018 Aug 26];49(4):493–501. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26143953 [DOI] [PubMed] [Google Scholar]
- 60.Dasgupta N, Funk MJ, Proescholdbell S, Hirsch A, Ribisl KM, Marshall S. Cohort Study of the Impact of High-dose Opioid Analgesics on Overdose Mortality. Pain Med [Internet]. 2015. August [cited 2018 Aug 26];17(1):n/a – n/a. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26333030 [DOI] [PubMed] [Google Scholar]
- 61.Salas J, Scherrer JF, Schneider FD, Sullivan MD, Bucholz KK, Burroughs T, et al. New-onset depression following stable, slow, and rapid rate of prescription opioid dose escalation. Pain [Internet]. 2017. February [cited 2019 Sep 12];158(2):306–12. Available from: http://content.wkhealth.com/linkback/openurl?sid=WKPTLP:landingpage&an=00006396-201702000-00015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Braden JB, Edlund MJ, Sullivan MD. Suicide Deaths With Opioid Poisoning in the United States: 1999–2014. Am J Public Health [Internet]. 2017. March 8 [cited 2018 Aug 26];107(3):421–6. Available from: http://ajph.aphapublications.org/doi/10.2105/AJPH.2016.303591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Oquendo MA, Volkow ND. Suicide: A Silent Contributor to Opioid-Overdose Deaths. N Engl J Med [Internet]. 2018. April 26 [cited 2018 Aug 26];378(17):1567–9. Available from: http://www.nejm.org/doi/10.1056/NEJMp1801417 [DOI] [PubMed] [Google Scholar]
- 64.Johnson EM, Lanier WA, Merrill RM, Crook J, Porucznik CA, Rolfs RT, et al. Unintentional Prescription Opioid-Related Overdose Deaths: Description of Decedents by Next of Kin or Best Contact, Utah, 2008–2009. J Gen Intern Med [Internet]. 2013. April 16 [cited 2018 Aug 26];28(4):522–9. Available from: http://link.springer.com/10.1007/s11606-012-2225-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Austin PC. A Tutorial and Case Study in Propensity Score Analysis: An Application to Estimating the Effect of In-Hospital Smoking Cessation Counseling on Mortality. Multivariate Behav Res [Internet]. 2011. [cited 2017 Jul 12];46(1):119–51. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22287812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Baum CF, Schaffer ME, Stillman S. Instrumental variables and GMM: Estimation and testing [Internet]. [cited 2019 Feb 6]. Available from: http://faculty.washington.edu/ezivot/econ583/ivreg2_bcwp545.pdf
- 67.Dunn KM, Saunders KW, Rutter CM, Banta-Green CJ, Merrill JO, Sullivan MD, et al. Opioid prescriptions for chronic pain and overdose: a cohort study. Ann Intern Med [Internet]. 2010. January 19 [cited 2017 Jan 18];152(2):85–92. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20083827 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.