Abstract
Context
Clinical applications of genomic assessment of thyroid cancers are rapidly evolving.
Objectives, Design, and Setting
We studied tumor samples from patients with imminently threatening and rare thyroid cancers to identify genomic alterations that might correlate with outcomes and/or be productively therapeutically targetable.
Patient Context
Progressive and metastatic, and/or rare, thyroid cancers were studied, 2012 to 2016, at Mayo Clinic sites.
Intervention
The intervention was Foundation One tumor interrogation.
Main Outcome Measures
Main outcome measures included genomic alterations, patient characteristics, and overall survival.
Results
Samples from 55 patients were evaluated: 20 anaplastic thyroid cancers (ATCs) (36%), 25 radioactive iodine–refractory differentiated thyroid cancers (DTCs)/poorly differentiated thyroid cancers (PDTCs) (45%; 14 papillary thyroid cancer [PTCs], 6 PDTCs, 5 Hürthle cell cancers), 8 medullary thyroid cancers (MTCs) (15%), and 2 others (a spindle epithelial tumor with thymus-like differentiation, and a primary thyroid sarcoma). Overall, 72% of DTCs, 79% of ATCs, and 75% of MTCs were deemed to have potentially productively targetable alterations. The most commonly encountered mutation was of TERT promoter (56% of DTCs, 68% of ATCs)—but this is not presently targetable. Targetable BRAFV600E mutations were found in 40% of DTCs/PDTCs (83% of PTCs) and 32% of ATCs; of MTCs, 75% had targetable RET mutations, and 25% HRAS mutations. Of patient tumors with nonmutated BRAFV600E, 53% of DTC/PDTCs and 69% of ATCs had other potentially productively targetable mutations. Genomic alterations in our series of poor prognosis metastatic DTC/PDTCs also closely resembled those seen in ATC.
Conclusions
Whereas genomic interrogation of favorable prognosis thyroid cancer seems ill advised, potentially productively targetable mutations were demonstrated in the majority of tumors from patients with metastatic thyroid cancers requiring systemic therapy, suggesting a rationale for the selective application of this technology.
Keywords: mutation, genomic sequencing, anaplastic thyroid cancer, poorly differentiated thyroid cancer, medullary thyroid cancer
More than 80% of all thyroid malignancies are papillary thyroid cancers (PTCs), a generally curable form of differentiated thyroid cancer (DTC) derived from follicular thyroid cells; 5-year survival is greater than 95% (1, 2). PTCs have a low density of somatic mutations relative to other solid tumors (3), perhaps reflecting their generally indolent clinical behavior. Occasionally, however, DTCs dedifferentiate into aggressive and even lethal cancers. In particular, overall survival (OS) is approximately 40% at 10 years from the detection of metastases in PTC; 10-year survival is less than 15% in patients older than 40 years with lung or multiple bone metastases (4). Such patients most often require systemic therapy beyond radioactive iodine (RAI), with the multikinase inhibitors (MKIs) sorafenib and lenvatinib now U.S. Food and Drug Administration (FDA) approved for this specific clinical indication. However, MKIs are not curative, are costly, and can be quite toxic; moreover, this subset of patients eventually requires other systemic therapies, prompting this study of the extent to which such cancers might harbor potentially productively targetable somatic mutations.
It is already known that up to 60% of PTCs harbor BRAF mutations, usually BRAFV600E, coding for a constitutively activated kinase that confers downstream MEK–extracellularly regulated kinase (ERK) activation causally implicated in PTC initiation and with progression to poorly differentiated (PDTC) and anaplastic thyroid cancers (ATC), especially when co-occurring with other mutations such as of TP53 or TERT promoter (3, 5). Nonoverlapping alternations within RAS and RET fusions account for the many remaining alterations, indicating the importance of MEK-ERK in the pathogenesis of PTC.
Alternatively, alterations within P13K/AKT/mammalian target of rapamycin (mTOR) along with MEK-ERK are characteristic of Hürthle cell cancers (HCCs), and in DTC can be associated with progression to PDTCs. HCCs, moreover, are associated with mutations in the mitochondrial genome and with unique chromosomal landscape/genetic alterations (6). In ATCs, overlapping alterations within TERT as well as MEK-ERK and P13K/AKT/mTOR pathways reflect a common evolution from DTC/PDTC, with mutations in TP53, the SWI/SNF complex, and histone methyltransferases predominately present in ATCs (5, 7). Although descriptive comprehensive genomic studies have been conducted using retrospective tumor biobanks, few genomic studies have been undertaken prospectively in patients with advanced or refractory incurable disease requiring salvage therapies also linked to longitudinal outcome data—the subject of this report.
Multikinase inhibitors (MKIs) have already shown promise in the treatment of advanced radioiodine-refractory (RAIR) DTCs and medullary thyroid cancers (MTCs), heralding a new era in thyroid cancer (TC) therapeutics. In particular, RECIST (response evaluation criteria in solid tumors) response rates from completed randomized trials are 12.2% and 64.8% from sorafenib or lenvatinib respectively in DTC; and 45% and 28% from vandetanib or cabozantinib in MTCs (8-11). The FDA moreover has recently also approved dabrafenib (BRAF inhibitor) and trametinib (MEK inhibitor) for the treatment of unresectable or metastatic BRAFV600E-mutated ATC based on limited data from a single trial (12). However, MKIs are not uniformly effective—and no cures result. Consequently, patients require multiple serial salvage treatments following failure of initially selected agents—and most still succumb to their cancers. We therefore reasoned that, regardless of response to initial therapy in a patient with widely metastatic TC, among patients wishing aggressive approaches to their care, additional later therapies will be required with potential to be informed by somatic genomic interrogation. Moreover, those patients treated with a mutationally agnostic therapy who do not benefit are subject to a) delays in effective therapy, b) increased risks from exposure to ineffective drugs, and 3) greater costs from the use of ineffective, costly drugs. The promise of effective mutationally informed therapy therefore has appeal for multiple reasons.
An evolving approach of consideration in the selection of salvage—and perhaps eventually even initial—therapeutics for patients with metastatic and imminently threatening TCs is thus to assess for the presence of potential actionable oncogenic drivers and to individualize therapy on that basis. In this context, many patients with advanced and/or rare TCs at Mayo Clinic sites have had their tumors interrogated using Foundation One analyses in hopes of identifying candidate salvage therapies; some have been treated based on this knowledge in a truly individualized/precision fashion. In this study, we analyzed results from interrogation of 55 consecutive patients with metastatic poor-prognosis RAIR DTC/PDTC, MTC, ATC, and other atypical TCs deemed candidates for systemic therapy, assessing how results might correlate with outcomes or suggest salvage approaches not otherwise apparent.
Materials and Methods
Study design and search strategy
Advanced TC patients requiring initiation of MKI therapy because of imminently threatening disease seen by providers of the Mayo Clinic Endocrine Cancers Care Team were uniformly subject to Foundation Medicine genomic interrogation beginning in 2012. A retrospective review was performed of medical records of all patients with metastatic poor-prognosis RAIR DTC, PDTC, MTC, ATC, and other atypical TCs deemed candidates for systemic therapy at Mayo Clinic, Rochester, Minnesota; Scottsdale, Arizona; and Jacksonville, Florida, United States, between November 26, 2012 through October 3, 2016, undergoing Foundation One tumor interrogation. Eligible patients were identified via interrogation of data contained in the Foundation One website and our own records, inclusive of all TC patients subject to Foundation One analysis seen by all Care Team providers.
Inclusion Criteria
Patients
All consecutive advanced TC patients deemed requiring systemic therapy for metastatic and rapidly progressing disease (emphasizing RAIR DTC, PDTC, MTC, and ATCs) whose tumors were subjected to genetic interrogation using the Foundation One platform between November 26, 2012 through October 3, 2016, were included in this study. A PDTC histology was considered if either the Turin criteria (solid/trabecular/insular growth pattern, absence of conventional nuclear features of PTC and at least one of the following: convoluted nuclei, mitotic activity ≥ 3/10 high-power microscopic fields, and tumor necrosis) or the Memorial Sloan Kettering Cancer Center criteria were identified (presence of follicular cell differentiation [on a routine microscopy and/or by immunohistochemistry, for example, thyroglobulin (Tg) positivity] with presence of ≥5 mitosis/10 high-power microscopic fields [400×] and/or tumor necrosis) (13).
Foundation One analyses
Foundation One is a next-generation sequencing assay that identifies selected genomic alterations within cancer-related genes; 315 genes, as well as introns of 28 genes involved in rearrangements, are interrogated. NTRK1 and NTRK2, but not NTRK 3, fusions are assessed, along with NTRK1-3 point alterations; ETV6 fusions are evaluated (NTRK3/ETV6 is a known PTC fusion reported per The Cancer Genome Atlas data). Foundation One somatic genomic analyses were accomplished from paraffin-embedded tissues as previously reported (14, 15); results are displayed in Fig. 1. Of primary consideration in selecting the Foundation platform among other competing platforms had been high success in assessing archived paraffin-embedded tissues and waiver of patient charges for the assessment, as well as affordable cost to patients lacking insurance coverage; limitations include absence of the ability to distinguish germline and somatic mutations because only tumor and not normal tissue is assessed.
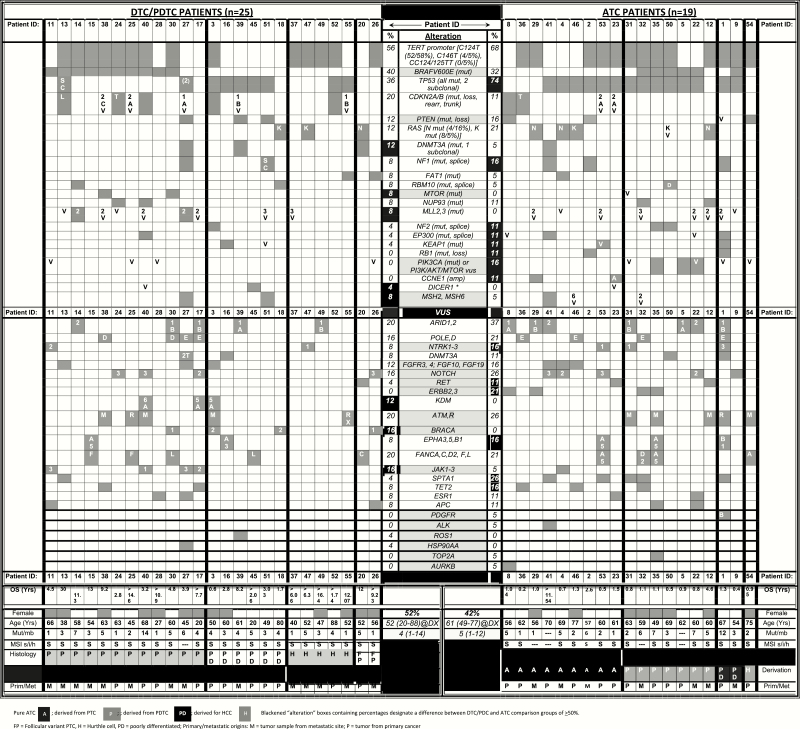
Figure 1.
Genomic alterations identified in the study cohort.
In silico assessments
NTRK fusions have recently attracted attention based on the recent cancer histology-agnostic FDA approval of larotrectinib; to date, there remains no evidence that NTRK point mutations confers sensitivity to such agents, and some reports indicate that point mutations can confer resistance to larotrectinib (16).Therefore, in efforts to assess the potential functional significances of observed NTRK1-3 mutations, we used MutPred, a web-based academic consortium application intended to predict the potential pathogenicity of the encountered point alterations in NTRK genes (http://mutpred.mutdb.org). For alterations within the kinase domain, the software tool PyMOL was used to create a 3-dimensional structure model depicting these point alterations (Schrödinger, Inc).
Efficacy measures
RECIST v1.1 was used to assess therapeutic responses. We defined potentially productively targetable mutations as those wherein drugs targeting the alteration, or the alteration-affected pathway, exist and have been used with success in at least some cancer (Table 1). In some cases, such as in NRAS/KRAS alterations, although an alteration might be considered theoretically productively targetable, when empirical clinical evidence instead indicates otherwise, we have not considered such alterations to be meaningfully targetable; in short, we have elected to be conservative in these analyses of which mutation may be productively therapeutically targetable.
Table 1.
Genomic alterations deemed to be potentially productively therapeutically targetable
| Mutation/ Alteration | Corresponding targeted agents | Histology: DTC/PDTC, 25 ATC, 19 MTC, 8 | No. of alterations |
|---|---|---|---|
| AURKB | Pazopanib, AMG900 | ATC (vus) | 1 |
| ALK | Crozotinib Ceritinib Alectanib | ATC (vus) | 1 |
| BRAFV600E | Vemurafenib dabrafenib | PTC ATC | 10 (40%) 6 (32%) – |
| BRCA1-2 | Olaparib Rusaparib Nuraparib | PTC (vus) PDTC (vus) HCC (vus) | 1 2 1 |
| ERBB2-3 | Trastuzumab Pertuzumab | ATC (vus) ATC/PTC (vus) | 2 2 |
| FGF/R | Pazopabib Ciritinib | PTC (vus) PDTC (vus) ATC (vus) | 2 1 3 |
| JAK | Roxolitinib Tofacitinib | PTC (vus) ATC (vus) | 4 1 |
| MSH2/6 | (Confers increased sensitivity to anti–PD-L1/PD-1 therapy) Pembrolizumab Nivolumab | PTC PDTC ATC ATC (vus) ATC/PTC (vus) | 1 1 1 1 1 |
| MTOR | Everoliums Temsirolimus | PTC ATC/PTC (vus) | 2 1 |
| PDGFRA/B | Pazopanib Lenvatinib | ATC/PDTC | 1 |
| PI3K | Alpelisib | ATC/PTC | 2 |
| RET | Vandetanib Cabozantinib, LOXO-292 BLU-667 | HCC (vus) ATC (vus) ATC/PTC (vus) MTC | 1 1 1 3 |
| ROS | Crozotinib Ciritinib Alectanib | PDTC (vus) | 1 |
| TOP2A | Etoposide Doxorubicin | ATC/PTC (vus) | 1 |
Abbreviations: ATC, anaplastic thyroid cancer; DTC, differentiated thyroid cancer; HCC, Hürthle cell cancer; MTC, medullary thyroid cancer; PDTC, progression to poorly differentiated thyroid cancer; PTC, papillary thyroid cancer; vus, variant of uncertain significance.
Statistical analysis
Descriptive summary analyses of patients’ baseline characteristics were performed using JMP13.0.0 (JMP, SAS Institute). Data are presented as frequencies (percentages) for the categorical variables and means (SD) or median (range, minimum, maximum, and or interquartile range, IQR) as appropriate for the continuous variables; for normally distributed variables, means were preferred, and for non-normal distributions, medians were used for data description. Differences between categorical variables were assessed using the chi-square test. Differences between continuous variables were assessed using t test or the Wilcoxon rank-sum test for non-normal data or analysis of variance/Kruskal-Wallis test for more than 2 groups. We also assessed OS, where OS was defined as the years from diagnosis to death from any cause. For the MKI OS analysis, OS was defined from MKI to death. Differences in OS between groups were performed using the Kaplan-Meier method and log-rank test; P values less than .05 were considered statistically significant; P values less than .1 were deemed to show a trend.
Results
Baseline characteristics
Samples from 55 patients were evaluated; 29 were men (53%). The dominant initial TC presentation was a “palpable mass” in 37 individuals (67%). Only a minority of study patients reported a family history of TC (7, 13%); some had a personal history of autoimmune thyroid disease (8, 15%). Most had no contributory radiation exposure of any source (51, 96%). Of 55 evaluated cases, 20 were ATCs (36%), 25 were DTCs/PDTCs (45%; of these, 14 were PTCs, 6 were PDTCs, and 5 were HCCs), 8 MTC (15%), and 2 other (4%; a spindle epithelial tumor with thymus-like differentiation [SETTLE] and a primary thyroid sarcoma) (Fig. 1).
The mean age at TC diagnosis for the entire cohort was 55 years (± SD 14): 62 ± 7 years for the ATC subgroup, 53 ± 16 years for the DTC/PDTC subgroup, and 55 ± 12 years for the MTC cohort. Most ATCs occurred primarily in the thyroid gland (17, 85%) but several were extrathyroidal in origin. Most in the entire cohort had undergone thyroidectomy (51, 93%), with tracheostomy required in 8 (15%), and one-third (mostly ATC patients) needed gastrostomy placement (18, 33%; 13 permanent, 24%). In terms of systemic therapies, cytotoxic chemotherapy was administered in 19 (35%, mostly in ATC patients); external radiotherapy to the neck was given to almost half the cohort (26, 47%).
The median tumor size at diagnosis of TC was 4.8 cm (IQR 3.5-6.9 cm; range, 1.4-9.4 cm); 4.6 cm (IQR 3.5-6.4 cm; range, 2.1-8.0 cm) for DTC/PDTC; 5.5 cm (IQR 3.8-7.9 cm; range, 1.5-9.4 cm) for ATC and 3.9 cm (IQR 2.5-5.2 cm; range, 1.4-7.4 cm) for MTC. Of 54 American Joint Committee on Cancer Tumor, Node, Metastasis seventh edition–evaluable tumors, most had stage IV disease (IVA 12, 22%; IVB 12, 22%; IVC 19, 35%). Most patients (49, 89%) had distant metastatic disease either at diagnosis or during follow-up. For those patients that had 18F-fluorodeoxyglucose (18F-FDG) positron emission tomography–computed tomography performed at initial diagnosis of TC (N = 33), 30 (91%) of our cohort demonstrated increased 18F-FDG avidity, consistent with poor prognosis.
Laboratory evaluations
For the DTC/PDTC group, the median thyrotropin at first evaluation was 0.42 mIU/L (IQR 0.025-2.605 mIU/L; range, 0.01-142.2 mIU/L); all patients, however, were ultimately suppressed to less than 0.1 mIU/L. Median Tg by immunoenzymatic assay was 45 ng/mL (IQR 4.45-290 ng/mL; range, 0.1-858 ng/mL) with negative Tg antibodies in the majority (23, 92%). In 3 patients Tg was assessed by liquid chromatography–tandem mass spectrometry with a median of 0.5 ng/mL (range, 0.5 to 1310 ng/mL). At last follow-up, median Tg was 197.5 ng/mL (IQR 15-2591.7 ng/mL; range, 0.1-51220 ng/mL). For the subgroup of MTC patients, the median calcitonin at first evaluation was 938 pg/mL (IQR 126-4545.8 pg/mL; range, 5-14480 pg/mL) with median carcinoembryonic antigen (available for N = 7) of 29.9 ng/mL (IQR 9.1-887 ng/mL; range, 2.9-2731.5 ng/mL); at last follow-up, the median calcitonin was 4373 pg/mL (IQR 346.7-27242 pg/mL; range, 5-60700 pg/mL) and median carcinoembryonic antigen of 759.75 ng/mL (IQR 452.8-1741.25 ng/mL; range, 2.8-15312 ng/mL).
Foundation One tumor tissue interrogation
Approach to genomic interrogation
Patients seen in conjunction with the Mayo Clinic Endocrine Cancer Care Teams at Mayo Clinic sites in Rochester, Minnesota; Jacksonville, Florida; and in Scottsdale, Arizona, referred for consultation related to advanced and threatening and/or rare tumors were under the care of medical oncologists/endocrinologist TC experts who care for the most aggressive and/or rare neoplasms constituting less than 5% of all thyroid tumors, were subject to selective mutational assessments using the Foundation One platform. Our group’s approach is to initiate tumor genomic interrogation only on determination that a patient’s cancer is metastatic, progressive, and threatening—and expected to soon require systemic therapy that might be informed by such evaluation. In 2 cases (4%), however, in particular in 1 SETTLE, and in 1 thyroid sarcoma, our goals somewhat differed in that genomic interrogation was intended to seek insights into the pathogenesis of these rare tumors, with a secondary goal of potentially informing later therapy.
Most of the interrogated samples came from archived materials obtained in conjunction with each patient’s routine clinical care and did not constitute materials obtained from biopsies obtained specifically for mutational interrogation alone. Of the 55 patient tumors evaluated, primary tumors were assessed in 29 (53%), with the remaining samples obtained from metastatic sites. In one ATC patient, genomic alterations could not be assessed because of sample failure during the analytic phase of testing, leading to successful genomic interrogation in 98%. The median time from TC diagnosis to Foundation One tumor interrogation was 1.8 years (IQR 0.3-7 years; range, 0.05-29 years).
Tumor mutational burden
Observed genomic alterations are presented in Fig. 1, sometimes referred to as an “OncoPlot.” Of the 54 patients with available genomic data, 51 had microsatellite stability assessment (MSI), demonstrating 48 (94%) to be MSI stable, with 2 samples reported as ambiguous, and only 1 as MSI unstable. In terms of MSI-associated genomic predisposition, MSH6 alterations occurred in 1 (4%) DTC/PDTC patient and in 1 (5.3%) ATC patient; a point mutation in MSH2 was identified in 1 (4%) DTC/PDTC patient; moreover, variants of uncertain significance (VUSs) involving MSH2, and another involving MSH6, were observed in ATC—however, of these 5 MSH-altered cancers, none were MSI unstable. In terms of tumor mutation burden (TMB), the median number of mutations per megabase was 3.19 (IQR 2-5; range, 1-14), with TMB low in 43 (84%) and intermediate in 8 (16%). There was no difference in TMB between our interrogated ATC and DTC/PDTC tumor groups, but MTCs had numerically lower TMB. In particular, the TMB per megabase were: ATC 5 (IQR 2-6; range, 1-12), DTC/PDTC 4 (IQR 1.5-5; range, 1-14), MTC 2 (IQR 1-4; range, 1-5), and other 2 (IQR 2-2; range, 2-2).
Most frequent observed genomic alterations
The most frequently reported mutation in the DTC/PDTC group was of the TERT promoter, occurring in 14 (56%)—also representing the second most common genetic alteration observed in ATC, occurring in 13 (68%); none of the MTC patients had this mutation (P = .001). The most common mutation in the ATC group was of TP53, found in 14 (74%) samples, twice the frequency observed in DTCs/PDTCs (9, 36%); none of the MTC patients had this mutation (P < .001). BRAFV600E was the second most common mutation in the DTC/PDTC group, occurring in 10 (40%); in the ATC group it occurred in 6 (32%), and in none of the MTCs (P = .033). Hence, there was a tendency for a greater proportion of ATCs than DTCs/PDTCs to have TP53- or TERT-promoter mutations, but there was considerable overlap in mutational profiles between the 2 groups. The most common genetic alteration in the MTC group was a somatic RET mutation/alteration occurring in 6 (75%) patients, with 4 patients having an RET M918T mutation; all MTCs were screened for a germline RET mutation as part of their usual clinical care and all were negative/sporadic. Two (25%) MTC samples harbored an HRAS mutation. The patient with SETTLE had no detected pathogenic genomic alterations detected, but harbored 8 VUSs; the primary thyroid sarcoma tumor had 3 potentially pathogenic alterations: PTEN E40,*K267fs*9, RET M1109T, and TP53 I254V.
Overall survival by tumor histology, therapy, or mutations
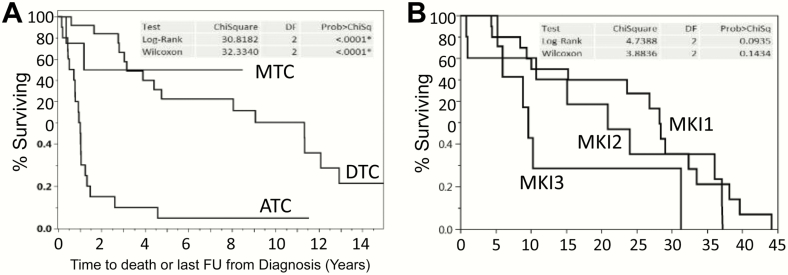
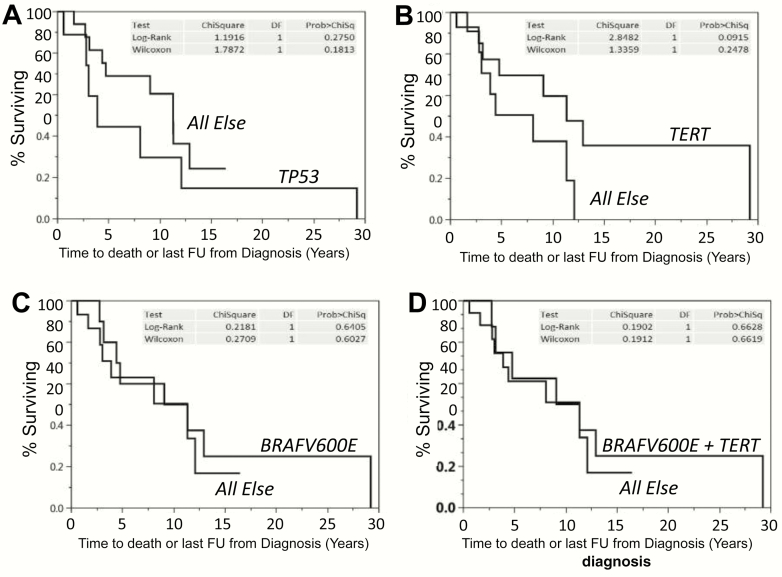
Kaplan-Meier survival data from time of diagnosis for ATC, DTC/PDTC, and MTC are depicted in Fig. 2A, demonstrating median OS of 1 year, 11.3 years, and not yet reached, respectively. Of all interrogated DTC/PDTC patients, 20 received first-line MKI, 10 second-line MKI therapy, and 8 third-line MKI therapy. Median time from MKI therapy initiation to death or last follow-up was 28 months for first-line MKI therapy, 21 months for second-line MKI therapy, and 9 months for third-line MKI therapy (Fig. 2B), demonstrating a trend (P < .1 log-rank) toward declining survival with subsequent-line MKI therapy, especially if third-line. Kaplan Meier survival data from diagnosis among the 25 DTC/PDTC patients are shown in Fig. 3, plotted as a function of the presence of mutations vs not for TP53 (Fig. 3A), TERT promoter (Fig. 3B), BRAFV600E (Fig. 3C), or BFAFV600E and TERT promoter (Fig. 3D), with no statistically significant differences observed between assessed comparison groups.
Figure 2.
Overall survival as a function of: A, thyroid cancer histology or B, line of multikinase inhibitor (MKI) therapy.
Figure 3.
Overall survival as a function of: A, TP53 mutation patients vs all other patients lacking TP53 mutations, B, TERT promoter mutation patients vs all other patients lacking TERT promoter mutations, C, BRAFVB600E mutation patients vs all other patients lacking BRAFV600E mutations, or D, BRAFVB600E plus TERT promoter mutation patients vs all other patients lacking BRAFV600E plus TERT promoter mutations.
Foundation One results and correspondingly targeted therapies with linked outcomes
Of the 54 samples generating interpretable genomic datasets, 15 patients (28%) received therapies suggested as relevant per retrospective analysis of targets observed per Foundation One assessment; of the 7 in whom RECIST response could be assessed, the median percentage of tumor tissue remaining was 84.6% of the baseline RECIST measurements (IQR 62%-102%; range, 49%-137%), equivalent to a median percentage reduction from baseline of 15.4%. In 9 patients (17%) Foundation One specifically guided the initiation of targeted therapy; of the 3 in whom RECIST response could be assessed, the median best RECIST response was similar to the earlier noted 7 responses observed in the case of the application of mutationally agnostic therapies at 82.3% of baseline (18% reduction from baseline RECIST measurements, IQR 16%-107%).
In terms of additional observations, a DICER1 point mutation was identified in a man with PDTC diagnosed at age 20 years; this represented the apparent main driver mutation identified for him. We found a very low frequency of fusions or translocations in our cohort, with only one patient having an RET translocation (RET CCDC6-RET fusion) in a relatively young (age 50 years) woman with a very aggressive PDTC from a PTC histology; although the RET fusion co-occurred in the context of other candidate drivers such as NF2, TP53, TERT promoter, CDKN2A/B mutations, and the following VUSs: BRCA2, FGFR4, and KDM5A point alterations. No other (potentially targetable) fusions were identified in the entire cohort, including of ALK or NTRK.
In silico analyses of the potential functional significances of observed NTRK point mutations
Tumors harboring NTRK1-3 translocations have high response rates to selective Trk inhibitors, with larotrectinib now FDA approved on a histology-agnostic basis for therapeutic use in this context among patients with advanced solid tumors (17) based on data from 3 multicenter, open-label, single-arm clinical trials (LOXO-TRK-14001 [NCT02122913], SCOUT [NCT02637687], and NAVIGATE [NCT02576431]), triggering scrutiny of NTRK alterations in our series. However, unlike NTRK-translocated tumors, NTRK point-mutated tumors have been reported to be unresponsive to Trk inhibitors in at least some cases (16).
None of the interrogated thyroid tumors in our series had NRTK1-3 fusions, but 5 harbored NTRK1-3 point mutations (allele frequency, AF, 15%-49%; Supplemental Table 1) (18). All 5 NTRK1-3 point-altered tumors had microsatellite stability; 4 of 5 (80%) had low TMB (2-5 mutations/megabase). NTRK alterations in the 3 ATC patients (15% prevalence) were NTRK3 I749M (AF 15%; OS, 15 months), NTRK1 R744H (AF not available; OS 9.5 months), and NTRK1 R583H (AF 20.1%; OS 5.5 months). NTRK2 S167Y (AF 49%; OS 53.5 months) or NTRK1 R6W (AF 42%; OS > 63.6 months) were found in one PTC and one HCC patient, respectively. All 3 ATC patient tumors had point alterations coding for alterations within the respective protein kinase domain, suggesting possible functional consequences. Because in silico modeling was feasible in the case of NTRK1 alterations (but not NTRK2/3 alterations), the potential for function-altering NTRK1 alterations was modeled, finding that all point alterations were predicted to be “probably damaging,” and with 2 of 3 alternatively judged “probably damaging” per the HumVar dataset (Supplemental Table 2) (18). The latter 2 alterations are within the coding region for the kinase domain. However, it remains uncertain whether such alterations will have eventual potential for productive therapeutic targeted.
Discussion
The vast majority of TC patients enjoy an excellent prognosis in response to the application of conventional approaches including surgery and (sometimes) RAI and/or thyrotropin-suppressive dosage levothyroxine (2, 19). In such patients, there is no need, established role, or rationale for somatic genomic interrogation—saving for application in the initial diagnostic evaluation of thyroid nodules. However, in the small subset of TC patients with RAI-insensitive, metastatic, and imminently threatening disease who will not be cured—the participants in this study—we posited that the situation might be different. In particular, we wondered whether potentially productively targetable genomic alterations might exist, be prevalent, and have the potential to inform therapeutic decision-making.
In contrast to our study, the Cancer Genome Atlas study (3) instead examined almost exclusively low-risk TCs, intending to define the basic genomic landscape of the majority of TCs compared to other cancers similarly studied; this was important in defining that TCs are mutationally simpler that most other cancers, that RAS and RAF alterations are early and presumed driver events in the majority of TCs and correlate also with histology, and that mutational events allow for hierarchical clustering of TCs (3). These data were reinforced by Yip and colleagues examining a large series of consecutive TC patients, of whom only 1.1% were PDTC/ATC (20). The Landa paper (5), also amplified by the Pozdeyev manuscript (21), were important also in that data on unselected PDTCs and ATCs were presented that were otherwise lacking in the Cancer Genome Atlas report, but these studies again focused on the enumeration of mutations, defining differences in the mutational landscapes of PDTC and ATC compared to better-prognosis TCs, and attempting hierarchical clustering of ATC subtypes.
By instead focusing on the genomic interrogation of tumors from patients with imminently threatening, poor-prognosis TCs requiring systemic therapy wherein detailed clinical outcome and therapy data are available and using a widely available commercial platform, the present study has an applied and practical focus examining questions including 1) how often are mutations with the potential to be productively therapeutically targeted identified in patients actually requiring otherwise mutationally agnostic therapies (lenvatinib, cytotoxic chemotherapy; Fig. 1); 2) are identified genomic alterations associated with outcomes in this very poor-risk patient population (Fig. 3); and 3) what are the outcomes from applying nonmutationally specified MKI therapies in this population when administered first, second, and third line (Fig. 2B); and in parallel providing a starting point for posing the additional question of whether there is a clinical rationale for the genomic interrogation of poor-prognosis TCs?
In the present study we accordingly obtained and examined the results of genomic interrogation of 55 TCs using the Foundation Medicine platform among patients deemed to have imminently threatening or rare neoplasms. Our goal was not to define mutational landscapes in an unbiased fashion—as has been accomplished previously by other groups—but instead to assess tumor samples from patients with a potential imminent need of systemic therapy. Our goal was to begin to define how results might correlate with clinical outcomes and perhaps have potential to inform clinical care and decision making. Our hypothesis was that selective genomic interrogation in this poor-prognosis patient cohort might be sufficiently informative to be integrated as a routine into our advanced TC practice, because it has been our experience that no therapy is curative in this clinical context and that defining next-line therapies should be undertaken proactively. From a practical standpoint, the Foundation Medicine platform was used in part because of its panel of assessed genes, but also because the vendor’s patient assistance program allowed its use at effectively no cost to our patients lacking insurance coverage for such studies—something not in our experience when using other platforms.
Several potentially clinically relevant findings arose in conjunction with the present study. First, we found 72% and 79% of the tumor samples from our DTC/PDTC and ATC cohorts, respectively, to have potentially productively therapeutically targetable alterations (Fig. 1 and Table 1). Moreover, 32% and 47% of those respective cohort tumors harbored targetable mutations even in the absence of BRAFV600E mutations. Emerging evidence from the MOSCATO1 trial provides a proof of concept for using similar somatic genomic interrogation in difficult-to-treat cancers, wherein patients with some cancers such as cholangiocarcinoma clearly derived benefit from applying this approach (22, 23).
Also deserving of comment is the potential to exploit genomic data in the context of the aspirational goal of accomplishing RAI resensitization, wherein “redifferentiation” of DTCs is intended. In particular, the use of targeted therapy matched to mutational milieu has been piloted in the hopes of redifferentiation via targeting specific driver mutations and consequent restoration of therapeutic response to RAI wherein the patients were otherwise previously found RAI insensitive. This approach has demonstrated response rates of about 20% on an intention-to-treat basis both in BRAF-mutated PTCs and in RAS-mutated follicular thyroid carcinomas (24, 25).
These data provide a rationale for undertaking genomic interrogation of tumors from patients who are candidates for systemic therapy and who will become future candidates for salvage therapies once approved mutationally agnostic therapies are applied and exhausted. Requiring further investigation, however, are the questions of how mutationally informed vs mutationally agnostic therapeutic decision making affect patient outcomes—and in parallel alternatively also affect the costs of care. Discouragingly, results from mutationally agnostic vs mutationally suggested therapies were indistinguishable in the small cohorts of corresponding patients assessable in this study, and did not demonstrate superior outcomes from the application of mutationally suggested therapies.
Although one might have expected otherwise, the mutational landscapes seen in our DTC/PDTC patients requiring systemic therapy were largely overlapping with those observed in ATC. Our poor-prognosis DTC/PDTC cohort was much more mutationally complex than that observed in the better-prognosis DTC Cancer Genome Atlas cohort (3), and yet somewhat less complex than observed in our ATC cohort, suggesting an intermediate mutational landscape. These observations lead us to speculate that there may be genomic (in addition to histologic) predictors of eventual progression to later aggressive and threatening tumor behaviors that might be identified early and useful for parsing patients into groups designated for more, vs less, intensive surveillance. This said, the median OS from diagnosis in our DTC/PDTC cohort was still quite lengthy at 11.1 years.
Other findings arising from the present study include the absence of a correlation between mutations/mutation pairs including TP53, TERT promoter, BRAFV600E, or BRAFV600E + TERT-promoter mutations and prognosis in our highly selected and already poor-prognosis DTC/PDTC cohort (Fig. 3). We speculate that one potential explanation for this observation may be that our cohort was already of such poor prognosis so as to obscure our ability to distinguish small differences in prognosis in such subset analyses. However, because our ATC and DTC/PDTC cohorts harbored similar genomic patterns but yet had greatly differing prognoses (Fig. 2A), we suspect that presently unelaborated factors beyond interrogated genes might additionally affect prognosis—something deserving of further study.
Also of note is that presented outcome data examining OS parsed by line of MKI therapy (Fig. 2B) demonstrate a trend (P < .1) toward declining survival with each successive line of MKI therapy. This is perhaps not surprising but it highlights the concern of acquired MKI resistance and is a call to action to devise alternative responsive therapeutic approaches beyond MKIs.
It is also worthy of comment that we observed a lack of clear indications for treatment with immune checkpoint inhibitors in our cohort patients in the sense that the genomically interrogated advanced and aggressive TC tumors generally lacked microsatellite instability, a known and FDA-approved indication for pembrolizumab initiation, as well as harbored a low frequency of MSH alterations, the latter without high MSI correlate. Current ongoing clinical trials with immune checkpoint inhibitors in TC patients may, however, provide greater insights as to efficacy and possible better candidate patients for these approaches (NCT03246958, NCT03914300, NCT02973997, NCT03181100).
Strengths of the present study include 1) that it is a well-annotated, consecutive-patient series with confirmed pathology and associated outcome data including survival and therapies administered otherwise lacking in the largest similar reported series (20, 21); and 2) the present series includes only patients expected shortly requiring systemic therapy in whom the mutational data might be more expeditiously actionable.
Limitations of this report are several and must be appropriately highlighted. First, the Foundation Medicine platform assesses only tumor—without parallel assessment of paired normal tissue; as such, attained results reflect the convergence of somatic and germline alterations. Hence, the determination of whether a particular alteration may be somatic or germline is challenging, because all that is certain is that the alteration is contained in tumor cells; this same limitation is shared with the Pozdeyev study (21). Important also is that the sources of tumor used in this study were sometimes remotely collected, indicating that the observed alterations may not necessarily reflect the most current genomic landscape of the metastatic deposits; also, 53% of samples were also procured from the primary tumor, which might differ from what might alternatively be encountered in metastatic deposits. Additionally, although the cancer genome coverage of the Foundation Medicine platform is generally very good, there are some shortcomings. For example, coverage of NTRK3 translocations is limited, meaning that some alterations might be missed.
Perhaps a strength, as well as a constraint, of this study is that our treating clinicians tend to institute systemic therapy with MKIs only when disease is imminently threatening, symptomatic, not addressable via locoregional approaches, and/or unpredictable in terms of the random appearance of threatening metastases. Because other groups may alternatively perhaps institute MKIs earlier, results of genomic interrogation of tumors from our patient cohort may not reflect results from other groups with lower thresholds for MKI initiation, or in comparison to studies that have interrogated all unselected patients. Also, because we are assessing only TC patients with the very worst prognoses with progressing and metastatic disease, our data set is clearly biased and not comparable to data that might otherwise be attained from assessing alterations in broader contexts, perhaps accounting for differences observed between our data and those published by others (5, 20, 21). This said, there are many similarities between our results and those of Landa (5); for example, the BRAFV600E mutation frequency for our ATC and DTC/PDTC cohorts were 32% and 40%, respectively, whereas it was found in 45% of ATCs and 33% of PDTCs by Landa et al (5). The RAS mutation in our ATCs occurred at a similar frequency of approximately 20% as reported by Landa and colleagues, but our cohort had fewer DTC/PDTCs with RAS mutations (12% vs 28% per Landa). The TERT-promoter mutation was the most common mutation in our cohort, ATC (68%) and DTC/PDTC (56%); this occurred at a similar frequency for ATC per Landa (73%), but at a slightly higher frequency for the DTC/PDTC group (Landa reported 40%). The TP53 mutation was the most frequent of all in our ATC cohort (74%) consistent with published data (73% per Landa), but our DTC/PDTC group had a strikingly higher frequency of this mutation, 36%, compared to the reported frequency of 8% per Landa—perhaps a reflection of our selection bias to sequence tumors only from patients with imminently threatening disease. Moreover, NF1 mutations occurred at a higher frequency both in our ATC (16% vs 9% per Landa) and DTC/PDTC groups (8% vs 0% PDTC per Landa). The PTEN mutation occurred at a similar frequency in ATC (16% vs 15% per Landa) but in our DTC/PDTC group this mutation was 3 times higher than what has been reported (12% vs 4% per Landa). The PIK3CA mutation occurred at a similar frequency in ATC (16% vs reported 18% per Landa), but we had no patients in the DTC/PDTC with this mutation, unlike the 2% reported frequency for PDTC per Landa. Collectively, this pattern may reflect that ATCs are expected to be more uniform across institutions, and that our DTCs/PDTCs were enriched for those with poor prognosis and perhaps therefore account for higher mutational frequencies.
Conclusion and Next Steps
Genomic interrogation of tumors from patients with favorable-prognosis TC, constituting the vast majority of TC, seems unlikely to have significant potential to further improve already excellent prognoses. However, mutational tumor interrogation among patients with progressing, metastatic, and imminently threatening TCs demonstrated candidate-targetable genomic alterations in the majority with potentially productively targetable mutation found even in patients with cancers lacking BRAF alterations. We posit therefore that, because no therapies are expected curative in this patient population, genomic interrogation of this poor-prognosis population has potential clinical relevance by suggesting targeted approaches of consideration for use in the salvage setting.
However, prospective studies assessing the specific impacts of genomic interrogation on TC patient outcomes including survival are needed to add further clarity. Such studies might be devised in parallel to also assess effects on costs of care so as to define whether this approach adds value.
One attractive prospective trial design in this context could be to randomly assign second-line therapy in RAIR-DTC patients on progression through lenvatinib to receive either a) second-line MKI therapy of investigator choice or b) mutationally tailored therapy—with crossover allowed on progression on either arm. In such a design, BRAFV600E-mutated cancers in arm b) might best be analyzed as a distinct group from other pooled non-BRAF somatic mutations to define whether BRAFV600E-targeted therapy in particular might be superior to, or inferior to, second-line MKI therapy, and doing the same with other pooled mutations analyzed separately. With such prospective trial designs we can thus begin to critically access the value of mutational interrogation of patients with therapy-requiring RAIR DTCs.
Acknowledgments
Dr Iñiguez-Ariza is indebted to the Board of Trustees of the Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, Mexico City, Mexico, for generous Fellowship support enabling her studies at the Mayo Clinic. This study was also presented in part at the 2016 annual meeting of the American Thyroid Association. The NTRK 1-3 mutation data were presented at the 2017 Annual Meeting of the American Society of Clinical Oncology.
Financial Support: This work was supported by the National Center for Advancing Translational Sciences (Grant UL1 TR000135). The authors otherwise have no potential financial disclosures to report. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Author Contributions: N.M.I.-A., data collection, data analysis, manuscript writing and revision; S.J., data collection, manuscript revision; M.M.R., manuscript revision, expert advice on disease; A.V.C., manuscript revision; J.C.M. III, manuscript revision; C.R.H., manuscript revision; M.E.M., manuscript revision; R.C.S., manuscript revision; N.J.K., manuscript revision; C.A., in silico assessment, manuscript revision; K.C.B., data collection, manuscript revision, expert advice on disease, overall supervision.
Glossary
Abbreviations
- AF
allele frequency
- ATC
anaplastic thyroid cancer
- DTC
differentiated thyroid cancer
- HCC
Hürthle cell cancer
- MKI
multikinase inhibitor
- MSI
microsatellite stability assessment
- MTC
medullary thyroid cancer
- PDTC
poorly differentiated thyroid cancer
- PTC
papillary thyroid cancer
- RAI
radioactive iodine
- RAIR
radioiodine refractory
- RECIST
response evaluation criteria in solid tumors
- TC, thyroid cancer; Tg, thyroglobulin; TMB
tumor mutation burden
- Vus
variant of uncertain significance
Additional Information
Disclosure Summary. The authors have nothing to disclose.
Data Availability: The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Hay ID, Thompson GB, Grant CS, et al. Papillary thyroid carcinoma managed at the Mayo Clinic during six decades (1940-1999): temporal trends in initial therapy and long-term outcome in 2444 consecutively treated patients. World J Surg. 2002;26(8):879-885. [DOI] [PubMed] [Google Scholar]
- 2. Hay ID, Johnson TR, Kaggal S, et al. Papillary thyroid carcinoma (PTC) in children and adults: comparison of initial presentation and long-term postoperative outcome in 4432 patients consecutively treated at the Mayo Clinic during eight decades (1936-2015). World J Surg. 2018;42(2):329-342. [DOI] [PubMed] [Google Scholar]
- 3. Cancer Genome Atlas Research Network. Integrated genomic characterization of papillary thyroid carcinoma. Cell. 2014;159(3):676-–690.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Durante C, Haddy N, Baudin E, et al. Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: benefits and limits of radioiodine therapy. J Clin Endocrinol Metab. 2006;91(8):2892-2899. [DOI] [PubMed] [Google Scholar]
- 5. Landa I, Ibrahimpasic T, Boucai L, et al. Genomic and transcriptomic hallmarks of poorly differentiated and anaplastic thyroid cancers. J Clin Invest. 2016;126(3):1052-1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ganly I, Makarov V, Deraje S, et al. Integrated genomic analysis of Hürthle cell cancer reveals oncogenic drivers, recurrent mitochondrial mutations, and unique chromosomal landscapes. Cancer Cell. 2018;34(2):256-270.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Xu B, Ghossein R. Genomic landscape of poorly differentiated and anaplastic thyroid carcinoma. Endocr Pathol. 2016;27(3):205-212. [DOI] [PubMed] [Google Scholar]
- 8. Wells SA Jr, Robinson BG, Gagel RF, et al. Vandetanib in patients with locally advanced or metastatic medullary thyroid cancer: a randomized, double-blind phase III trial. J Clin Oncol. 2012;30(2):134-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Elisei R, Schlumberger MJ, Müller SP, et al. Cabozantinib in progressive medullary thyroid cancer. J Clin Oncol. 2013;31(29):3639-3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brose MS, Nutting CM, Jarzab B, et al. ; DECISION investigators Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, phase 3 trial. Lancet. 2014;384(9940):319-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schlumberger M, Tahara M, Wirth LJ, et al. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N Engl J Med. 2015;372(7):621-630. [DOI] [PubMed] [Google Scholar]
- 12. Subbiah V, Kreitman RJ, Wainberg ZA, et al. Dabrafenib and trametinib treatment in patients with locally advanced or metastatic BRAF V600-mutant anaplastic thyroid cancer. J Clin Oncol. 2018;36(1):7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ibrahimpasic T, Ghossein R, Shah JP, Ganly I. Poorly differentiated carcinoma of the thyroid gland: current status and future prospects. Thyroid. 2019;29(3):311-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Frampton GM, Fichtenholtz A, Otto GA, et al. Development and validation of a clinical cancer genomic profiling test based on massively parallel DNA sequencing. Nat Biotechnol. 2013;31(11):1023-1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Young G, Wang K, He J, et al. Clinical next-generation sequencing successfully applied to fine-needle aspirations of pulmonary and pancreatic neoplasms. Cancer Cytopathol. 2013;121(12): 688-694. [DOI] [PubMed] [Google Scholar]
- 16. Russo M, Misale S, Wei G, et al. Acquired resistance to the TRK inhibitor entrectinib in Colorectal Cancer. Cancer Discov. 2016;6(1):36-44. [DOI] [PubMed] [Google Scholar]
- 17.Mullard A. FDA approves landmark tissue-agnostic cancer drug. Nat Rev Drug Discov. 2019; 18: 7. [DOI] [PubMed] [Google Scholar]
- 18. Bible K.Bible_Suppl Table 1.pdf. figshare Deposited on 2020. 10.6084/m9.figshare.11594061.v1 [DOI] [Google Scholar]
- 19. Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26(1):1-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yip L, Nikiforova MN, Yoo JY, et al. Tumor genotype determines phenotype and disease-related outcomes in thyroid cancer: a study of 1510 patients. Ann Surg. 2015;262(3):519-5 25; discussion 524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pozdeyev N, Gay LM, Sokol ES, et al. Genetic analysis of 779 advanced differentiated and anaplastic thyroid cancers. Clin Cancer Res. 2018;24(13):3059-3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Massard C, Michiels S, Ferté C, et al. High-throughput genomics and clinical outcome in hard-to-treat advanced cancers: results of the MOSCATO 01 trial. Cancer Discov. 2017;7(6):586-595. [DOI] [PubMed] [Google Scholar]
- 23. Verlingue L, Malka D, Allorant A, et al. Precision medicine for patients with advanced biliary tract cancers: an effective strategy within the prospective MOSCATO-01 trial. Eur J Cancer. 2017;87:122-130. [DOI] [PubMed] [Google Scholar]
- 24. Ho AL, Grewal RK, Leboeuf R, et al. Selumetinib-enhanced radioiodine uptake in advanced thyroid cancer. N Engl J Med. 2013;368(7):623-632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rothenberg SM, McFadden DG, Palmer EL, Daniels GH, Wirth LJ. Redifferentiation of iodine-refractory BRAF V600E-mutant metastatic papillary thyroid cancer with dabrafenib. Clin Cancer Res. 2015;21(5):1028-1035. [DOI] [PubMed] [Google Scholar]