Abstract
Background
Osteoarthritis (OA) is the most common joint disease, but its association with mortality is unclear.
Methods
We analysed data on adult participants in the 1988–94 and 1999–2010 National Health and Nutrition Examination Surveys, followed for mortality through 2011. OA was defined by self-report, and in a subset of participants 60 years or older with knee X-rays, radiographic knee OA (RKOA) was defined as Kellgren–Lawrence score ≥2. Cox proportional hazards were used to determine the mortality hazard ratio (HR) associated with self-reported OA and RKOA, adjusting for covariates.
Results
The sample included 51 938 participants followed for a median 8.9 years; 2589 of them had knee X-rays and were followed for a median of 13.6 years. Self-reported OA and RKOA prevalences were 6.6% and 40.6%, respectively. Self-reported OA was not associated with mortality. RKOA was associated with an increased risk of mortality from cardiovascular diseases (CVD) {HR 1.43 [95% confidence interval (CI): 1.32, 1.64]}, diabetes [HR 2.04 (1.87, 2.23)] and renal diseases [HR 1.14 (1.04, 1.25)], but with a reduced risk of cancer mortality [HR 0.88 (0.80, 0.96)]. Participants with early RKOA onset (diagnosed before age 40) had a higher risk of mortality from all causes [HR 1.53 (1.43, 1.65)] and from diabetes [HR 7.18 (5.45, 9.45)]. Obese participants with RKOA were at increased risk of mortality from CVD [HR 1.89 (1.56, 2.29)] and from diabetes [HR: 3.42 (3.01, 3.88)].
Conclusions
Self-reported OA was not associated with mortality. RKOA was associated with higher CVD, diabetes and renal mortality, especially in people with early onset of the disease or with obesity.
Keywords: Osteoarthritis, arthritis, mortality, prognosis, all-cause mortality, cause-specific mortality
Key Messages
Osteoarthritis is a common joint disease, but research on its association with mortality has produced conflicting results. We aimed to examine the association between osteoarthritis and the risk of all-cause and cause-specific mortality in a large cohort followed for up to 20 years, and to identify subgroups of participants with osteoarthritis who might be at higher risk of mortality.
In this study, which comprised 51 938 North Americans including 2589 who had knee X-rays, we found no association between self-reported osteoarthritis and mortality. However, radiographic knee osteoarthritis was associated with a higher risk of mortality from cardiovascular disease, diabetes and renal disease. There was an inverse relationship between radiographic knee osteoarthritis and cancer mortality.
Participants with early onset of radiographic knee osteoarthritis, or those with radiographic knee osteoarthritis who were obese, were at higher risk of mortality from all-cause, cardiovascular disease, and/or diabetes than those diagnosed after the age of 40 years and those who did not have obesity.
Because radiographic knee osteoarthritis is associated with an increased risk of mortality from cardiovascular diseases, diabetes and renal diseases, the management of patients with radiographic knee osteoarthritis should include the prevention and treatment of these conditions.
Introduction
Osteoarthritis (OA) is the most common joint disease and affects more than 30 million North Americans.1 It is defined by the Osteoarthritis Research Society International (OARSI) as ‘a disorder involving movable joints characterized by cell stress and extracellular matrix degradation initiated by micro- and macro-injury that activates maladaptive repair responses including proinflammatory pathways of innate immunity’.2,3 OA is characterized by a loss of cartilage, with concomitant changes to the articular structures that include the synovium, meniscus, ligaments and subchondral bone.2 Although OA affects all joints due to its systemic nature, the joints that present most symptoms are the spine, the hands, the hips, the knees and the feet, causing significant disability and a great loss in quality-adjusted life years.4,5 The lifetime risk for developing OA is 25% to 40%, and people with obesity are at higher risk of developing the condition.6
The prevalence of OA has increased sharply because of longer life expectancy, the rise in obesity rates and the lack of curative treatment.2,7 Despite the high prevalence and morbidity of OA, whether it is associated with increased mortality is still unclear. Research on the topic is recent and has yet to produce conclusive results.8 Most of the studies had small sample sizes resulting in limited generalizability.9 Moreover, no investigation has examined the relationship between OA and mortality, considering individual characteristics.10 OA is a complex and heterogeneous condition with several characteristics affecting its manifestation and prognosis.11 Therefore, we examined the association between OA and the risk of all-cause and cause-specific mortality in a cohort representative of the US population, followed for up to 20 years. We also identified subgroups of people with OA who might be at higher risk of mortality.
Methods
Data source
We used data from the National Health and Nutrition Examination Surveys (NHANES) conducted from 1988 to 1994 and from 1999 to 2010 by the National Center for Health Statistics (NCHS) of the Centers for Disease Control and Prevention (CDC). The NHANES is a continuous cross-sectional survey of the non-institutionalized civilian population. It collects data on the health status of participants through interviews, physical examinations and laboratory tests. It uses a complex multistage sampling design to derive a sample that is representative of the US population.
NHANES protocols were approved by the Institutional Review Boards of the NCHS and the CDC, and informed consent was obtained from each participant. Details of the IRB approval are available at [http://www.cdc.gov/nchs/nhanes/irba98.htm]. Details on NHANES survey procedures and methods are available at [http://www.cdc.gov/nchs/nhanes/survey_methods.htm].
Participants
A total of 52 512 North Americans aged 20 years or older participated in the NHANES during the study periods (20 050 from 1988 to 1994 and 32 462 from 1999 to 2010). Of these, 52 444 had data on OA and 51 938 were followed for mortality until 31 December 2011. We merged the files with the corresponding mortality-linked files. Participants were matched to the National Death Index (NDI) records, and death certificates were used to confirm that deaths from the NDI corresponded to the correct participants.
OA status
Self-reported OA status was ascertained using the questions: ‘Has a doctor or other health professional ever told you that you have arthritis?’ (Then if an answer ‘yes’ was provided to the first question) ‘Which type of arthritis was it?’ Based on the answers to these questions, participants were classified as having self-reported OA, other arthritis or no arthritis. In addition, participants of the 1991 to 1996 NHANES who were 60 years old or older had non-weight-bearing antero-posterior knee X-rays done using a Centrix III X-ray unit with Kodak Lanex double screens and TML film. The radiographs were scored for osteophytes severity from 0 to 4 (0 = normal, 1 = possible osteophyte, 2 = definite osteophyte, 3 = moderate multiple osteophytes, 4 = large osteophytes/severe sclerosis) based on the Kellgren–Lawrence atlas of knee radiographs of arthritis. Two radiologists independently did the scoring, and a Kellgren–Lawrence score ≥2 in either knee was classified as radiographic knee OA (RKOA).11 Disagreement in scores between the two readers was resolved by consensus. The kappa statistic for inter-rater agreement was >71%. Additional details on the knee X-ray procedures are available at [https://wwwn.cdc.gov/nchs/data/nhanes3/11a/xray-acc.pdf]. Participants were also asked about their age at their first arthritis diagnosis, and OA or RKOA diagnosed before the age of 40 was considered to be of early onset.2
Cause-specific mortality
The specific causes of mortality were defined using a standardized list of 113 causes according to the Tenth Revision of the International Classification of Diseases (ICD-10). The causes of death included were cardiovascular (CVD) (i.e. disease of the heart and stroke), cancer, chronic lower respiratory diseases (CLRD), Alzheimer’s disease, diabetes mellitus and renal conditions (nephritis, nephrotic syndrome or nephrosis). Detailed information on the classifications is available at [http://www.cdc.gov/nchs/data/datalinkage/public_use_data_dictionary_11_17_2015].
Demographics and comorbidities
The NHANES included data on age, gender, race/ethnicity, family poverty income ratio (PIR), education level, cigarette smoking, physical activity and medical conditions. Smoking status was categorized into ‘Never smokers’, ‘Current smokers’ and ‘Past smokers’. PIR was estimated using guidelines and adjustment for family size, year and state. Participants were asked about physical activity and were classified as being physically active if they reported walking, cycling, performing moderate or vigorous work or leisure activity. Body mass index (BMI) was calculated as weight in kilograms divided by height in metres squared. Participants were considered to have a normal weight if they had a BMI <25 kg/m2, to be overweight if they had a BMI between 25 kg/m2 and 30 kg/m2, and obese if their BMI was ≥30 kg/m2. Diabetes was defined by self-reported physician diagnosis, fasting plasma glucose ≥126 mg/dl, 2-h oral glucose tolerance test ≥200 mg/dl or glycohaemoglobin ≥6.5%.12 Hypertension was defined by self-reported physician diagnosis or mean systolic blood pressure (of up to four measurements on two separate occasions) >140 mm Hg. Other comorbid conditions included self-reported diagnosis of myocardial infarction (MI) or stroke.
Data analysis
Descriptive analyses were performed to summarize the characteristics of study participants by OA status. Differences in proportions were assessed using chi-square tests for categorical variables. The Cox proportional hazards regression modelling approach was used to estimate the hazard ratio (HR) and corresponding 95% confidence interval (CI) for the risk of mortality associated with self-reported OA and RKOA. All models were adjusted for age, gender, race/ethnicity, BMI, cigarette smoking, physical activity, PIR, diabetes, hypertension and history of MI or stroke, taking into account the competing risks of mortality from other causes. The assumption of proportionality was tested by including the interaction terms (predictor*log-time) in the models; there was no violation of the assumption if none of them was significant. We tested the age of OA diagnosis, body weight, smoking and presence of diabetes for effect modification on the association of self-reported OA and RKOA with mortality risk. NHANES sample weights were used in all analyses to obtain unbiased national estimates. Standard errors (SE), CI, andP-values were determined in accordance with the complex survey design by using Taylor series linearization methods. Analyses were performed in SAS (Version 9.4) and STATA (Version 14).P-values <0.05 were considered statistically significant in all analyses.
Results
Description of study participants
Our total sample consisted of 51 938 participants who were followed for a median of 8.9 years [interquartile range (IQR): 4.6–17.6] (525 590 person-years). Data on RKOA were available for 2589 participants who were followed for a median time of 13.6 years (IQR: 7.4–18.2). The prevalences of self-reported OA and RKOA were 6.6% and 40.6%, respectively. Compared with participants with no arthritis, those who reported OA or had RKOA were more likely to be older, to be women, to be non-Hispanic White, to have a higher socioeconomic status (PIR), to have smoked in the past and to be less active. They also had more comorbidities (diabetes, hypertension and history of myocardial infarction or stroke) and had a higher mortality during follow-up (Table 1).
Table 1.
Characteristics of study participants by osteoarthritis (OA) status, NHANES 1988–94 and 1999–2010
| Participants with data on self-reported OA (N = 51 938) |
Participants with data on RKOA (N = 2589) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Characteristics | OA (N = 3428) | No arthritis (N = 39 473) | P-valuea | Other arthritis (N = 9037) | P-valueb | RKOA (N = 1051) | No arthritis (N = 874) | P-valuec | Other arthritis (N = 664) | P-valued |
| Age (years) median (IQR) | 62 (51-72) | 38 (28-51) | 55 (22-68) | 68 (63-74) | 67 (63-73) | 68 (64-74) | ||||
| Gender, % | <0.0001 | <0.0001 | <0.0001 | 0.002 | ||||||
| Men | 33.3 | 50.4 | 39.1 | 39.3 | 52.4 | 43.0 | ||||
| Women | 66.7 | 49.6 | 60.9 | 60.7 | 47.6 | 57.0 | ||||
| Race/ethnicity, % | <0.0001 | <0.0001 | <0.0001 | <0.0001 | ||||||
| Non-Hispanic Whites | 87.4 | 70.3 | 74.3 | 79.6 | 83.7 | 83.6 | ||||
| Non-Hispanic Blacks | 5.7 | 11.4 | 13.6 | 12.1 | 5.4 | 7.5 | ||||
| Mexican-Americans | 3.5 | 12.9 | 8.5 | 5.6 | 7.9 | 6.6 | ||||
| Other | 3.4 | 5.3 | 3.6 | 2.7 | 7.6 | 2.4 | ||||
| Poverty income ratio, % | <0.0001 | <0.0001 | <0.0001 | 0.26 | ||||||
| ≤1 | 8.7 | 12.3 | 15.9 | 11.3 | 7.4 | 11.1 | ||||
| 1< to ≤3 | 36.3 | 34.8 | 40.3 | 45.4 | 43.6 | 47.2 | ||||
| >3 | 47.9 | 45.6 | 36.6 | 35.7 | 40.3 | 34.0 | ||||
| Missing | 7.1 | 7.2 | 7.2 | 7.6 | 8.7 | 7.7 | ||||
| Body mass index, % | <0.0001 | 0.004 | <0.0001 | <0.0001 | ||||||
| Normal | 24.0 | 37.3 | 26.3 | 22.7 | 42.5 | 41.5 | ||||
| Overweight | 31.8 | 30.8 | 26.5 | 39.1 | 41.3 | 39.0 | ||||
| Obese | 37.2 | 24.2 | 39.1 | 38.2 | 16.2 | 19.5 | ||||
| Missing | 7.1 | 7.7 | 8.1 | – | – | – | ||||
| Cigarette smoking, % | <0.0001 | <0.0001 | <0.0001 | <0.0001 | ||||||
| Never smoked | 46.8 | 51.5 | 41.2 | 52.6 | 41.2 | 41.3 | ||||
| Past smoker | 37.5 | 22.1 | 31.4 | 10.9 | 16.0 | 17.4 | ||||
| Current smoker | 15.6 | 26.3 | 27.4 | 36.5 | 42.8 | 41.3 | ||||
| Physical activity, % | 29.9 | 43.4 | <0.0001 | 29.3 | 0.48 | 66.2 | 71.0 | <0.0001 | 69.3 | 0.0015 |
| Diabetes, % | 15.3 | 6.2 | <0.0001 | 18.0 | 0.021 | 19.9 | 12.5 | <0.0001 | 19.3 | 0.52 |
| Hypertension, % | 56.3 | 23.5 | <0.0001 | 50.1 | 0.0003 | 52.9 | 36.8 | <0.0001 | 46.0 | <0.0001 |
| History of MI, % | 8.0 | 2.0 | <0.0001 | 8.9 | 0.24 | 11.6 | 8.9 | <0.0001 | 18.0 | <0.0001 |
| History of stroke, % | 5.9 | 1.4 | <0.0001 | 6.7 | 0.27 | 7.1 | 4.6 | <0.0001 | 10.1 | <0.0001 |
| Mortality rate/1000 person-years | ||||||||||
| All-cause | 40.9 | 13.9 | <0.0001 | 38.9 | 0.12 | 67.0 | 56.0 | 0.0006 | 59.5 | 0.052 |
| CVD | 9.7 | 3.2 | <0.0001 | 9.5 | 0.77 | 18.7 | 14.1 | 0.006 | 13.4 | 0.007 |
| Cancer | 7.5 | 3.2 | <0.0001 | 7.9 | 0.68 | 11.1 | 13.2 | 0.15 | 10.8 | 0.84 |
| CLRD | 2.0 | 0.6 | <0.0001 | 1.8 | 0.62 | 2.5 | 2.9 | 0.63 | 4.2 | 0.052 |
| Alzheimer’s disease | 1.0 | 0.3 | <0.0001 | 1.0 | 0.77 | 1.7 | 1.1 | 0.26 | 1.3 | 0.52 |
| Diabetes | 1.4 | 0.4 | <0.0001 | 1.8 | 0.36 | 2.8 | 1.6 | 0.052 | 3.3 | 0.60 |
| Renal disease | 0.6 | 0.3 | 0.0023 | 0.9 | 0.32 | 1.3 | 1.0 | 0.53 | 1.1 | 0.74 |
CLRD, chronic lower respiratory diseases.
Difference in characteristics between OA and no arthritis.
Difference in characteristics between OA and other arthritis.
Difference in characteristics between RKOA and no arthritis.
Difference in characteristics between OA and other arthritis.
P-values for differences in proportions or means by osteoarthritis status calculated using chi-square test for categorical variables and Student’s t test for continuous variables.
Compared with participants with other arthritis, those with self-reported OA were more inclined to be older, to be women, to be non-Hispanic White, to have a lower socioeconomic status, to be overweight and to be non-smokers. They had a higher prevalence of hypertension, but a lower prevalence of diabetes. Compared with participants with other arthritis, participants with RKOA were more likely to be women, to be non-Hispanic Black, to be obese and to be less inclined to smoke or be active. They had a higher prevalence of comorbidities such as hypertension, history of myocardial infarction or stroke (Table 1).
OA and risk of mortality
In Cox proportional regression adjusted for covariates and using participants with no arthritis as controls, self-reported OA was not associated with all-cause or any cause-specific mortality. Given the large age difference between participants who reported OA (median age = 62 years) and those who reported not having OA (median age = 38 years), we performed a subgroup analysis limited to participants aged 60 years or older, to better control for age. The subsample included 17 951 participants. The median age in participants who reported OA was 70, versus 68 years in those who reported not having OA. In adjusted Cox regression analysis, the hazard ratio (HR) for all-cause mortality in self-reported OA versus no OA in this subgroup [HR 0.95, 95% CI: 0.87, 1.05 (data not shown)] was similar to the estimate for the overall sample [odds ratio (OR) 0.96, 95% CI: 0.87, 1.06 (Table 2)].
Table 2.
Risk of mortality associated with OA diagnosis and RKOA, NHANES 1988–94 and 1999–2010
| Participants with data on self-reported OA (N = 51 938) |
Participants with data on RKOA (N = 2589) |
|||||
|---|---|---|---|---|---|---|
| Cause of mortality | Deaths | HR (95% CI) | P-value | Deaths | HR (95% CI) | P-value |
| All-cause | 10 292 | 0.96 (0.87, 1.06) | 0.41 | 1848 | 1.06 (0.99, 1.14) | 0.07 |
| CVD | 2427 | 0.93 (0.75, 1.14) | 0.47 | 481 | 1.43 (1.26, 1.62) | <0.0001 |
| Cancer | 2198 | 0.93 (0.75, 1.15) | 0.50 | 353 | 0.88 (0.80, 0.96) | 0.012 |
| CLRD | 482 | 0.85 (0.61, 1.16) | 0.30 | 90 | 1.21 (0.95, 1.54) | 0.11 |
| Alzheimer’s disease | 243 | 0.82 (0.53, 1.27) | 0.37 | 43 | 1.18 (0.67, 2.08) | 0.54 |
| Diabetes | 362 | 0.91 (0.60, 1.37) | 0.91 | 75 | 2.04 (1.87, 2.23) | <0.0001 |
| Renal disease | 195 | 0.80 (0.44, 1.46) | 0.47 | 35 | 1.14 (1.04, 1.25) | 0.0091 |
HR calculated using Cox proportional regression analysis. Models adjusted for age, gender, race/ethnicity, body mass index, cigarette smoking, physical activity, poverty income ratio, diabetes, hypertension and history of myocardial infarction or stroke.
CLRD, chronic lower respiratory disease.
With reference to no arthritis, RKOA was associated with increased risk of mortality from cardiovascular disease (HR 1.43, 95% CI: 1.26, 1.62), diabetes (HR 2.04, 95% CI: 1.87, 2.23) and renal disease (HR 1.14, 95% CI: 1.04, 1.25). However, they had decreased risk of mortality from cancer (HR: 0.88, 95% CI: 0.80, 0.96) (Table 2).
RKOA and risk of mortality by age of diagnosis and body weight
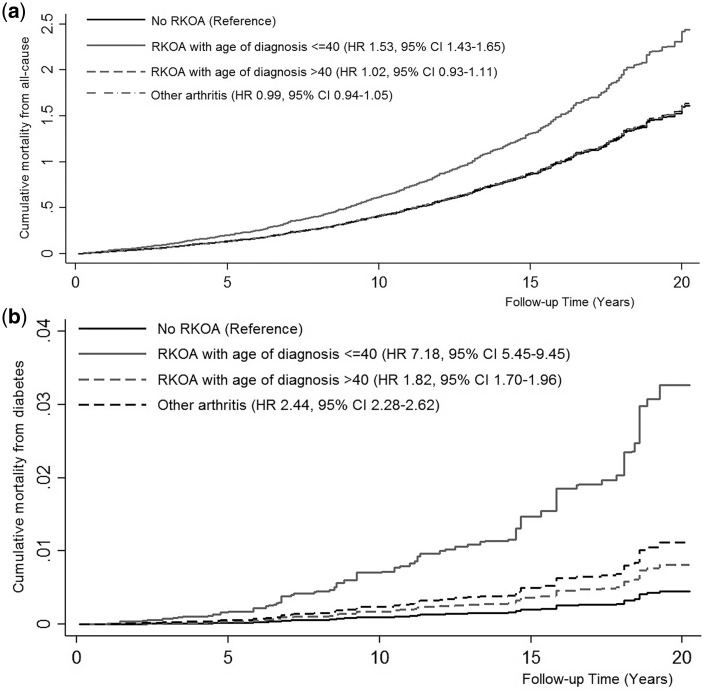
In effect modification testing, the risk of mortality differed by age of RKOA diagnosis for mortality from all-cause and diabetes (Pinteraction <0.0001). Compared with participants without RKOA, the risk of death from all causes was higher in those with RKOA diagnosed before age 40 (HR 1.53, 95% CI: 1.43–1.65), but not after age 40 (HR 1.02, 95% CI: 0.93, 1.11) (Figure 1a). When comparing the risk of all-cause mortality in RKOA by age of diagnosis, participants diagnosed before age 40 had higher all-cause mortality than those diagnosed after age 40 (HR 1.51, 95% CI: 1.41, 1.61). Likewise, compared with participants without RKOA, the risk of death from diabetes was higher in RKOA diagnosed before age 40 (HR 7.18, 95% CI: 5.45, 9.45) and after age 40 (HR 1.82, 95% CI: 1.70, 1.96) (Figure 1b). When comparing the risk of mortality from diabetes in RKOA by age of diagnosis, participants diagnosed before age 40 had higher mortality from diabetes than those diagnosed after age 40 (HR 3.93, 95% CI: 3.16, 4.91).
Figure 1.
Kaplan–Meier curves for cumulative all-cause mortality (a) and diabetes mortality (b) in participants with and without radiographic knee osteoarthritis (RKOA) by age of diagnosis. Models adjusted for age, gender, race/ethnicity, body mass index, cigarette smoking, physical activity, poverty income ratio, diabetes, hypertension and history of myocardial infarction or stroke, and taking into account competing risk of mortality from other causes.
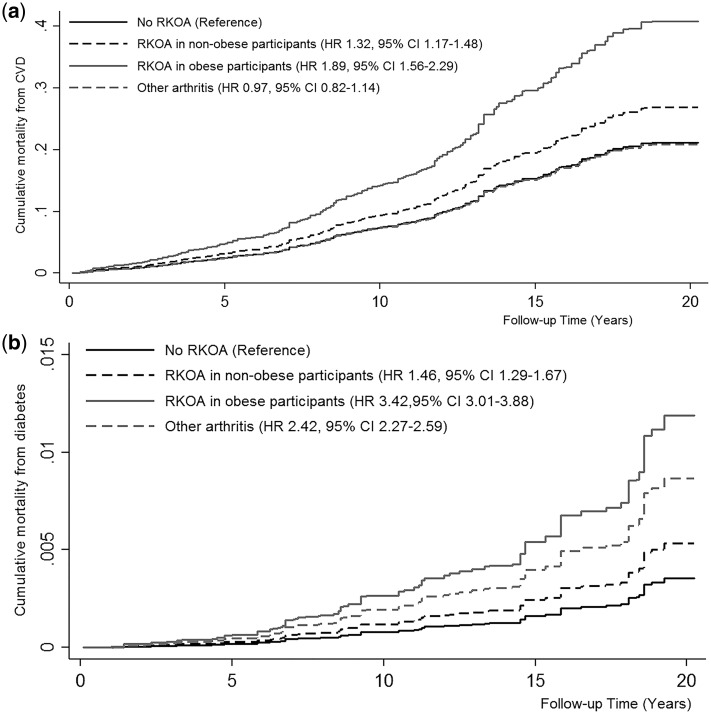
Obesity also modified RKOA-associated risk of mortality from cardiovascular disease and diabetes (Pinteraction <0.0001). Compared with participants without RKOA, obese participants had >80% more risk of mortality from cardiovascular disease (HR 1.89, 95% CI: 1.56, 2.29) (Figure 2a), and from diabetes (HR 1.83, 95% CI: 1.55, 2.15Figure 2b). When comparing the risk of mortality from all-cause and from diabetes in RKOA by body mass, obese participants were at higher risk than non-obese ones (HR 1.61, 95% CI: 1.55, 1.68 for all-cause mortality and HR 2.33, 95% CI: 1.86, 2.91 for diabetes mortality).
Figure 2.
Kaplan–Meier curves for cumulative mortality from cardiovascular disease (CVD) (a) and from diabetes (b), in participants with and without radiographic knee osteoarthritis (RKOA), by body mass. Models adjusted for age, gender, race/ethnicity, body mass index, cigarette smoking, physical activity, poverty income ratio, diabetes, hypertension and history of myocardial infarction or stroke, and taking into account competing risk of mortality from other causes.
The association of self-reported OA with mortality was not modified by age of diagnosis, body weight, smoking or diabetes. The association of RKOA with mortality was not modified by smoking or diabetes.
Discussion
Self-reported OA was not associated with mortality. RKOA was associated with an increased risk of mortality from cardiovascular disease, diabetes and renal disease, but decreased risk of mortality from cancer. Participant who had been diagnosed with RKOA before the age of 40 and participants with obesity had a worse prognosis.
Consistent with these findings, other studies have reported a lack of an association between self-reported OA and mortality. Holbrooket al.13 studied 510 Americans 50 years old or older, who were followed for 15 years, and found no association between self-reported OA and all-cause mortality. An Italian study followed 2927 older people for 4.4 years, and found that OA was not associated with all-cause mortality.14 In The Netherlands, the investigators of the Genetics ARthrosis and Progression (GARP) and Osteoarthritis Care Clinic (OCC) cohorts studied mortality in participants who had been diagnosed with OA in the hand, knee, hip or spine, and found no increase in the risk of all-cause mortality associated with OA.15 The largest study on OA and mortality to date enrolled half a million Swedish participants, and followed them for a decade. The results indicated no excess in all-cause mortality in people with OA of the hip or knee (defined by health care administrative data).16 The meta-analyses combining the cohort studies similarly failed to find associations between OA and mortality.8,17 On the other hand, an association between RKOA and all-cause mortality was also found in a cohort study of 1163 participants followed for 14 years, which reported a 55% increase in all-cause mortality associated with RKOA.18 Additionally, Tsuboiet al.19 noted a 132% increase in the risk of all-causes death associated with RKOA during the 10-year follow-up of 789 older adults.
Few studies have examined the association between radiographic OA and specific-cause mortality. The only three studies on RKOA and cardiovascular disease death have produced results consistent with our findings. Two of the studies found that cardiovascular disease was the main cause of mortality associated with RKOA.18,19 The third one reported a relationship only for painful RKOA, but not for RKOA in general. Pain, regardless of the presence or absence of RKOA, was associated with a higher risk of cardiovascular disease death.20 Regarding our result of an increased risk of mortality from diabetes in people with RKOA, no study has examined this association before. Diabetes has been reported to be a risk factor for OA, but whether OA increases the risk of diabetes and mortality from diabetes is unclear.21 In animal studies, rats with diabetes were found to experience more severe OA than those without diabetes, and OA severity in diabetic rats seemed to improve partially with diabetes treatment.22 In our analysis however, we did not find diabetes to modify OA or RKOA association with mortality. Surprisingly, we found RKOA to be associated with a lower risk of cancer mortality. This is in contradiction with previous investigations that reported either a positive relationship or no association between RKOA and mortality from cancer.18–20 The exact reason for the negative association is unclear to us, but we hypothesize that it might be due to a higher use of non-steroidal anti-inflammatory drugs (NSAIDs) in participants with RKOA. NSAIDs including aspirin have been widely reported to lower the incidence and mortality from cancers including breast cancer and colorectal cancer.23–25 However, the mechanism behind this protective association is not completely understood. We hypothesize that NSAID use might also explain in part the higher risk of mortality from kidney disease observed in participants with RKOA.23–25
This study is the first to examine the difference in the risk of mortality associated with OA in participants with certain characteristics. One study reported that pain in knee OA (and pain in the absence of knee OA) was associated with the increased risk of mortality.20 Nüeschet al. found that disability and history of cancer, diabetes and cardiovascular disease were the risk factors for mortality in OA. However, whether these factors modified the relationship between RKOA and mortality OA was unclear from the study.18 Moreover, no study has examined whether the age of OA diagnosis is associated with mortality. OA is typically a disease of the older age, but can occur prematurely (age ≤40) in those with genetic predisposition (i.e. heritable osteochondrodysplasias) and as OA secondary to trauma (e.g. of the knee).26,27 Early onset of OA develops after an inflammatory disease or due to biomechanical defects and often affects more than one joint. It can be part of a syndrome or systemic condition, which could explain its association with excess mortality.26 We found RKOA to be associated with a higher mortality in participants who were obese. To our knowledge, our study is also the first to report a difference in the risk of mortality associated with OA by body mass. Using the NHANES, Losinaet al.28 reported an important loss in quality-adjusted life-years due to both obesity and knee OA in the USA, especially in Black and Hispanic participants. Other reports have suggested that obesity might increase the risk of complications and premature mortality in patients who had knee arthroplasty, the treatment for advanced OA.29 Obesity is a known risk factor for OA, and the pathophysiology is believed to be mediated by mechanical factors (overloading of joints). However, the production of adipokines and chronic inflammation resulting from obesity might also play a role, as shown in the association between obesity and OA of the hand.30
Our study had limitations. Self-reported OA could not be verified. The results for the association between self-reported OA and mortality might be biased towards the null because of potential misclassification in self-reported OA. For instance, participants who had the condition but were not diagnosed because of limited access to health care were classified as not having OA. Different sites for OA might be associated with different mortality risks which could not be calculated in the study because of lack of data on the sites of self-reported OA. It is worth mentioning, however, that participants with RKOA all had self-reported OA. RKOA was defined using non-weight-bearing radiographs and relying on osteophytes, which may have resulted in some misclassification if the joint space narrowing would only be detected on standing. Also, the specific causes of death were defined using death certificates that should preferably be confirmed by from autopsy if feasible. On the other hand, our study had major strengths such as the fact that knee OA was defined using radiological examination and two radiologists independently scored the films, and the fact that the sample was drawn from the general US population, which contributes to the generalizability of findings and reduces the risk of selection bias. Our study was the first to examine mortality in OA considering age at OA diagnosis and obesity.
In conclusion, self-reported OA diagnosis was not associated with mortality, whereas RKOA was associated with an increase in the risk of mortality from CVD, diabetes or kidney diseases. However, participants with RKOA were less likely to die from cancer. RKOA diagnosed before or at age 40, as well as obesity in participants with RKOA, seemed to be associated with a worse prognosis. Future studies should evaluate whether preventing obesity could reduce mortality in RKOA. The management of patients with RKOA should include the prevention and treatment of CVD, diabetes and renal diseases.
Authors Contributions
All authors contributed to the study design, to the interpretation of the results and to the writing and critical review of the manuscript. A.M. analysed the data and had complete access to the dataset and takes full responsibility for its integrity. Therefore A.M. will act the guarantor for the paper and will vouch for its validity.
Conflict of interest: The authors have no conflict of interest to disclose.
References
- 1. CDC.Osteoarthritis2017https://www.cdc.gov/arthritis/basics/osteoarthritis.htm (11 November 2017, date last accessed).
- 2. Arden N,Blanco F,Cooper C,Guermazi A,Hayashi D,Hunter D.. Atlas of Osteoarthritis. London: Springer,2014. [Google Scholar]
- 3. Parker DA. (ed).Management of Knee Osteoarthritis in the Younger, Active Patient: An Evidence-Based Practical Guide for Clinicians. London: Springer,2016. [Google Scholar]
- 4. Johnson VL,Hunter DJ.. The epidemiology of osteoarthritis.Best Pract Res Clin Rheumatol 2014;28:5–15. [DOI] [PubMed] [Google Scholar]
- 5. Cleveland RJ,Callahan LF.. Can osteoarthritis predict mortality? N C Med J 2017;78:322–25. [DOI] [PubMed] [Google Scholar]
- 6. Chaganti RK,Lane NE.. Risk factors for incident osteoarthritis of the hip and knee.Curr Rev Musculoskelet Med 2011;4:99.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Park J,Mendy A,Vieira ER.. Various types of arthritis in the United States: prevalence and age-related trends from 1999 to 2014.Am J Public Health 2018;108:256–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Xing D,Xu Y,Liu Q. et al. Osteoarthritis and all-cause mortality in worldwide populations: grading the evidence from a meta-analysis.Sci Rep 2016;6:24393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lapane KL,Liu SH.. Commentary: osteoarthritis and mortality: answering questions or questioning answers? Epidemiology 2016;27:477–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Glyn-Jones S,Palmer A,Agricola R. et al. Osteoarthritis.Lancet 2015;386:376–87. [DOI] [PubMed] [Google Scholar]
- 11. Kellgren JH. The Epidemiology of Chronic Rheumatism: A Symposium. Philadelphia: FA Davis,1963. [Google Scholar]
- 12. Khot UN,Khot MB,Bajzer CT. et al. Prevalence of conventional risk factors in patients with coronary heart disease.JAMA 2003;290:898–904. [DOI] [PubMed] [Google Scholar]
- 13. Holbrook TL,Wingard DL,Barrett-Connor E.. Self-reported arthritis among men and women in an adult community.J Community Health 1990;15:195–208. [DOI] [PubMed] [Google Scholar]
- 14. Cacciatore F,Della-Morte D,Basile C. et al. Long-term mortality in frail elderly subjects with osteoarthritis.Rheumatology 2014;53:293–99. [DOI] [PubMed] [Google Scholar]
- 15. Liu R,Kwok W,Vliet Vlieland T. et al. Mortality in osteoarthritis patients.Scand J Rheumatol 2015;44:70–73. [DOI] [PubMed] [Google Scholar]
- 16. Turkiewicz A,Neogi T,Bjork J,Peat G,Englund M.. All-cause mortality in knee and hip osteoarthritis and rheumatoid arthritis.Epidemiology 2016;27:479–85. [DOI] [PubMed] [Google Scholar]
- 17. Veronese N,Cereda E,Maggi S. et al. Osteoarthritis and mortality: a prospective cohort study and systematic review with meta-analysis.Semin Arthritis Rheum 2016;46:160–67. [DOI] [PubMed] [Google Scholar]
- 18. Nüesch E,Dieppe P,Reichenbach S,Williams S,Iff S,Jüni P.. All cause and disease specific mortality in patients with knee or hip osteoarthritis: population based cohort study.BMJ 2011;342:d1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tsuboi M,Hasegawa Y,Matsuyama Y,Suzuki S,Suzuki K,Imagama S.. Do musculoskeletal degenerative diseases affect mortality and cause of death after 10 years in Japan? J Bone Miner Metab 2011;29:217–23. [DOI] [PubMed] [Google Scholar]
- 20. Kluzek S,Sanchez-Santos MT,Leyland KM. et al. Painful knee but not hand osteoarthritis is an independent predictor of mortality over 23 years follow-up of a population-based cohort of middle-aged women.Ann Rheum Dis 2016;75:1749–56. [DOI] [PubMed] [Google Scholar]
- 21. Williams MF,London DA,Husni EM,Navaneethan S,Kashyap SR.. Type 2 diabetes and osteoarthritis: a systematic review and meta-analysis.J Diabetes Complicat 2016;30:944–50. [DOI] [PubMed] [Google Scholar]
- 22. Courties A,Sellam J.. Osteoarthritis and type 2 diabetes mellitus: What are the links? Diabetes Res Clin Pract 2016;122:198–206. [DOI] [PubMed] [Google Scholar]
- 23. Gridley G,McLaughlin JK,Ekbom A. et al. Incidence of cancer among patients with rheumatoid arthritis.J Natl Cancer Inst 1993;85:307–11. [DOI] [PubMed] [Google Scholar]
- 24. Takkouche B,Regueira-Méndez C,Etminan M.. Breast cancer and use of nonsteroidal anti-inflammatory drugs: a meta-analysis.J Natl Cancer Inst 2008;100:1439–47. [DOI] [PubMed] [Google Scholar]
- 25. Hua X,Phipps AI,Burnett-Hartman AN. et al. Timing of aspirin and other nonsteroidal anti-inflammatory drug use among patients with colorectal cancer in relation to tumor markers and survival.J Clin Oncol 2017;35:2806–3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Aury-Landas J,Marcelli C,Leclercq S,Boumédiene K,Baugé C.. Genetic determinism of primary early-onset osteoarthritis.Trends Mol Med 2016;22:38–52. [DOI] [PubMed] [Google Scholar]
- 27. Bálint G,Szebenyi B.. Hereditary disorders mimicking and/or causing premature osteoarthritis.Best Pract Res Clin Rheumatol 2000;14:219–50. [DOI] [PubMed] [Google Scholar]
- 28. Losina E,Walensky RP,Reichmann WM. et al. Impact of obesity and knee osteoarthritis on morbidity and mortality in older Americans.Ann Intern Med 2011;154:217–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kerkhoffs GM,Servien E,Dunn W,Dahm D,Bramer JA,Haverkamp D.. The influence of obesity on the complication rate and outcome of total knee arthroplasty: a meta-analysis and systematic literature review.J Bone Joint Surg Am 2012;94:1839–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Berenbaum F,Eymard F,Houard X.. Osteoarthritis, inflammation and obesity.Curr Opin Rheumatol 2013;25:114–18. [DOI] [PubMed] [Google Scholar]