Abstract
Objective
Unintentional medication discrepancies contribute to preventable adverse drug events in patients. Patient engagement in medication safety beyond verbal participation in medication reconciliation is limited. We conducted a pilot study to determine whether patients’ use of an electronic home medication review tool could improve medication safety during hospitalization.
Materials and Methods
Patients were randomized to use a toolbefore orafter hospital admission medication reconciliation to review and modify their home medication list. We assessed the quantity, potential severity, and potential harm of patients’ and clinicians’ medication changes. We also surveyed clinicians to assess the tool’s usefulness.
Results
Of 76 patients approached, 65 (86%) participated. Forty-eight (74%) made changes to their home medication list [before: 29 (81%),after: 19 (66%),p = .170].Before group participants identified 57 changes that clinicians subsequently missed on admission medication reconciliation. Thirty-nine (74%) had a significant or greater potential severity, and 19 (36%) had a greater than 50-50 chance of harm.After group patients identified 68 additional changes to their reconciled medication lists. Fifty-one (75%) had a significant or greater potential severity, and 33 (49%) had a greater than 50-50 chance of harm. Clinicians reported believing that the tool would save time, and patients would supply useful information.
Discussion
The results demonstrate a high willingness of patients to engage in medication reconciliation, and show that patients were able to identify important medication discrepancies and often changes that clinicians missed.
Conclusion
Engaging patients in admission medication reconciliation using an electronic home medication review tool may improve medication safety during hospitalization.
Keywords: patient engagement, medication reconciliation, medication safety, patient-centered care, information technology
BACKGROUND AND SIGNIFICANCE
Unintentional medication discrepancies, defined as differences in documented medication regimens across different care sites, contribute substantially to adverse drug events (ADEs) in hospitalized patients.1–4 The most common cause of preventable ADEs is unintentional discrepancies in the admission medication list.1,5,6 Studies demonstrate that 48% to 87% of emergency department (ED) patients’ medication lists contain one or more discrepancies,7,8 and 22% to 54% still contain discrepancies on hospital admission.1,6,9 To avoid unintentional discrepancies and prevent ADEs, the Joint Commission has designatedmedication reconciliation at admission, transfer, and discharge, a National Patient Safety Goal since 2005.10 Medication reconciliation is the process of systematically reviewing a patient’s complete medication regimen to ensure its accuracy.11
Medication reconciliation is challenging to implement successfully in general practice.12 The reconciliation process is complex and error prone, particularly if patients’ medication histories are unavailable, located in different systems, or contradictory.13 While the process to take a standardized medication history has been established,14 studies suggest that a thorough approach to medication reconciliation is time consuming, and may take an hour or more per patient.15 The process relies heavily on verbally confirming the medication list with the patient, and 40% to 60% of in-hospital medication errors result from poor communication during reconciliation.5,16,17
In the ambulatory care setting, the positive impact of medication management interventions through online patient portals is well documented.18–31 Previous studies have provided patients with their medication lists,18–29 empowered patients to communicate medication-related information to their providers,18–28 and allowed patients to refill prescriptions online.19,30 Such interventions have reduced medication discrepancies,19,27,28 prevented ADEs,22,26,28,29 and improved medication safety.19,22,26–29
Despite the success of patient engagement in medication safety in the ambulatory setting, patient engagement beyond verbal participation in medication reconciliation remains limited in the acute care setting. Interventions to improve medication reconciliation in the hospital generally focus on providers’ practices, rather than patients.32–34 Interventions that do facilitate accurate collection of medication data from patients generally focus on the home setting.32,33,35–38
OBJECTIVE
In this work, we conducted a pilot study to investigate whether an electronic home medication review tool can engage patients in the medication reconciliation process and allow them to contribute information to their home medication lists upon hospital admission. Patients with varied health and technology literacies reviewed their home medication lists either before or after the admitting team completed medication reconciliation. We evaluated the quantity, potential severity, and potential harm of the changes that patients suggested, in comparison with their clinicians’ changes. Using surveys, we assessed the tool’s potential usefulness to the admitting clinicians.
MATERIALS AND METHODS
Study design
We recruited patients from the ED of a large urban academic medical center. First, we identified and consented patients designated for hospital admission who had not yet completed admission medication reconciliation. Participants completed a baseline patient survey to assess their demographic characteristics, technology literacy, health literacy, patient activation level, and illness severity. Then, we randomly assigned participants to use the electronic home medication review toolbefore orafter their admitting team completed the admitting medication reconciliation. Afterwards, we accessed participants’ medical records to determine what changes the admitting team made to their home medication lists. Clinicians who cared forbefore group participants completed a survey about the intervention’s usefulness. The medical center’s Institutional Review Board approved the study.
Intervention
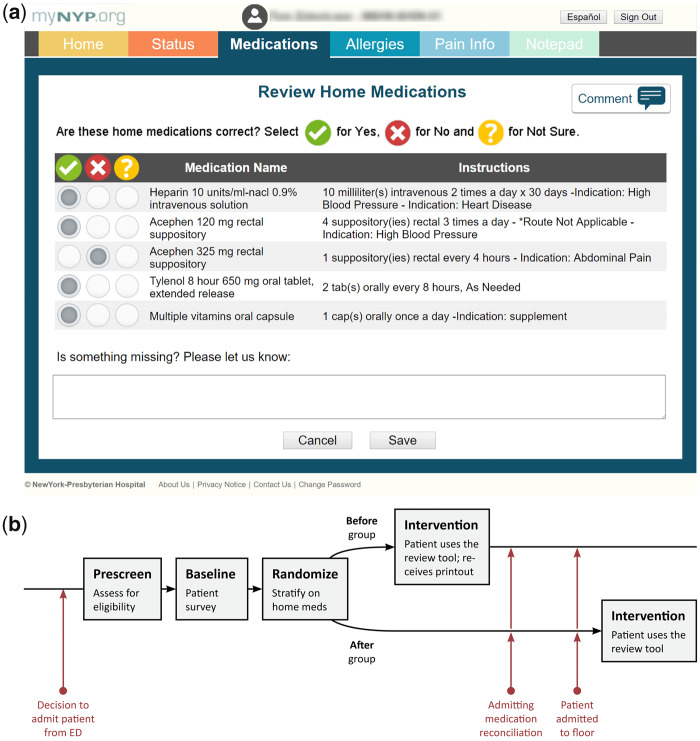
To access the internally developed home medication review tool and complete their medication reconciliation process, participants used an Apple iPad (Wi-Fi 16GB, Apple Inc., Cupertino, California) provided by the research coordinator. The tool displayed their home medication list, including the medication name, dose, route, and frequency (Figure 1a), automatically populated from the medical center’s live electronic health record (EHR) (Allscripts Sunrise Clinical Manager). The EHR system is unified across the hospital system, allowing for inpatient and ED visits to access the same medication lists. Participants selectedYes,No, orNot Sure as to whether they were currently taking each medication listed. In a free-text box, participants could record any medications missing from their lists. The system interface was designed simply, with patients of all health literacy levels in mind. The design implemented was the same used in an application developed for a randomized clinical trial that focused on patient engagement, which was successfully used by patients of all literacy levels.39 Additionally, the research coordinators were available throughout the process to answer any questions.
Figure 1.
1a. Home medication review tool. 1b. Study design.
Recruitment
Inclusion and Exclusion Criteria: We included English-speaking adult patients aged 18 years or older. We excluded patients with a history of cognitive impairment and acutely ill patients unable to participate in the study.
Recruitment Protocol: The research coordinator recruited participants from new arrivals to the ED that occurred between 9 am and 6 pm. Participants agreed to use the home medication tool and granted permission to access to their medical records. After providing written informed consent, participants completed the baseline patient survey. Then, the coordinator used stratified randomization to assign participants to thebefore orafter group (Figure 1b). We used 3 strata based on participants’ home medication list: 1) no medications listed, 2) 1 to 5 medications listed, and 3) 6 or more medications listed. The coordinator conducted a brief training session to familiarize the participant with the tool. We encouraged participants to seek assistance from family, friends, or outside healthcare providers, available in person or by phone, to provide accurate information. Participants were compensated $10 for their time, typically around 15 minutes, received upon study completion.
Before group participants used the tool while in the ED. They reviewed their home medication list from previous ambulatory, emergency, or inpatient visits as documented in the medical record. After they used the tool, the coordinator provided them with a brightly colored printout detailing their responses. The coordinator encouraged participants to share the printout with their admitting team to aid medication reconciliation.
After group participants used the tool one day after hospital admission. They reviewed the home medication list their admitting team created during medication reconciliation and documented in the medical record.
Clinician Recruitment: The coordinator contacted attending physicians, fellows, residents, and physician assistants (PAs) who had cared forbefore group study participants and asked them to complete the clinician survey.
Data collection
The medication review tool stored all patient-provided and system usage data on a secure research server. The coordinator administered the patient survey via a secure online survey tool (Qualtrics LLC, Provo, Utah). Clinicians completed their survey online through the same survey tool. We accessed participant’s medical records from the clinical data warehouse at our institution. The coordinator followed specific protocols to ensure consistency in data collection.
Measurements
Patient Survey: We collected information about demographics, socioeconomic status, and technology literacy using our previously described patient survey.39 We used Chew and colleagues’ 3-item questionnaire to screen participants for inadequate health literacy.40 We used the 13-item Patient Activation Measure (PAM) to assess patient activation,41–47 a tool we have previously validated in the acute care setting.48 Patient activation refers to patients’ knowledge, skills, and confidence in managing their health and healthcare. The PAM categorizes patients into 1 of 4 activation levels: 1) disengaged and overwhelmed, 2) becoming aware, but still struggling, 3) taking action, 4) maintaining behaviors and pushing further. We assessed illness severity using the Emergency Severity Index (ESI), ranging from level 1 (most urgent) to level 5 (least urgent).49
Patient Medication Changes: We classified patients’ changes to their home medication lists as additions, deletions, and modifications. We assessed thepotential harm andpotential severity of each change if it had gone unreported.5 The potential harm scale uses 6 levels, from “little to no confidence” to “virtually certain confidence.” The potential severity scale uses 4 levels, “insignificant,” “significant,” “serious,” and “life-threatening.” We used patients’ medical record information to determine which changes wentunreconciled, or unaddressed by the admitting team.
Clinician Survey: The survey asked clinicians whether they received the brightly colored printout from patients, and clinicians answered 3 questions about the intervention’s potential usefulness. The 3 questions included 1)Do you think the tool will be useful? 2)Do you think the changes patients report through the tool will be accurate? 3)Do you think the tool will save you time? Clinicians who reported not receiving the printout were asked to report their beliefs on the potential usefulness, accuracy, and impact on time to complete admitting medication reconciliation if they had been provided with a patient-generated review first. In addition, the survey asked clinicians to report their role type (attending, fellow, resident, PA) and time in the role (<1 year, 1-2 years, ≥2 years).
Clinician Medication Changes: We used participants’ medical record information to assess the admitting team’s changes to participants’ home medication lists on admission. As with patient changes, we classified clinician changes as additions, deletions, and modifications, and assessed the potential harm and potential severity of each change.
System Usage Log: To determine how long participants needed to complete their home medication reviews, we recorded each user action in a detailed system usage log.
Data analysis
We analyzed all data using Stata SE version 14.0 and R version 3.3.3.50,51 We conducted descriptive analyses of patient and clinician survey results. We compared patients’ baseline characteristics between groups using 2-samplet tests for continuous variables and Pearson's chi-squared or Fisher’s exact tests for categorical variables.
We conducted descriptive analyses of patient and clinician medication changes, including frequency, type, and average number of changes. To evaluate the impact of the use of the tool on the medication reconciliation process at different points in time during the patients’ hospitalization, we assessed differences between thebefore andafter groups using 2-samplez tests for proportions, Wilcoxon rank-sum analyses, and Pearson’s chi-squared or Fisher’s exact tests. To determine the correlation between baseline characteristics and total number of patient medication changes, we used linear regression and Spearman rank correlation for ordinal characteristics and pairwise correlation analysis for continuous variables. Within thebefore group, we evaluated differences between patient and clinician medication changes using McNemar’s test for paired proportions, Wilcoxon signed-rank analyses, and Pearson’s chi-squared or Fisher’s exact tests. Within theafter group, we did not compare patient and clinician medication changes because the patients and clinicians modified different home medication lists. Specifically, patients in theafter group modified the list that the admitting team had already modified.
Two pharmacists independently coded each patient and clinician medication change to assign potential harm and potential severity.5 We conducted 1 round of inter-rater agreement using a weighted Cohen’s Kappa. For patient medication changes, agreement was 90.0% (κ = 0.694) for potential harm and 90.1% (κ = 0.643) for potential severity. For clinician medication changes, agreement was 91.7% (κ = 0.715) for potential harm and 90.9% (κ = 0.647) for potential severity. We resolved discrepancies in assignment using averaging.
RESULTS
Study population
Of the 76 patients approached, 65 consented to and completed the study, while 11 (15%) declined to participate. Thebefore group contained 36 participants, and theafter group contained 29.Table 1 describes participants’ baseline characteristics. On average, study participants were 49 years old (range: 20-88). Participants were 38% Black and 42% Latino, and 14% preferred Spanish. Overall, 79% of participants reported access to the Internet, and 57% reported access to a desktop, laptop, or tablet.
Table 1.
Baseline characteristics of the study population
| By-group analysis |
||||
|---|---|---|---|---|
| Variable | Overall (n = 65) | Before (n = 36) | After (n = 29) | p-value |
| Demographics | ||||
| Age | 48.8 (19.0) | 52.1 (19.8) | 44.7 (17.4) | .118 |
| Female sex | 33 (50.8%) | 15 (41.7%) | 18 (62.1%) | .102 |
| Race | .956 | |||
| Asian or Pacific Islander | 3 (5.0%) | 2 (6.1%) | 1 (3.7%) | |
| Black or African American | 23 (38.3%) | 14 (42.4%) | 9 (33.3%) | |
| White | 20 (33.3%) | 10 (30.3%) | 10 (37.0%) | |
| Other or Multi-Racial | 14 (23.3%) | 7 (21.2%) | 7 (25.9%) | |
| Latino or Hispanic ethnicity | 22 (42.3%) | 13 (43.3%) | 9 (40.9%) | .567 |
| Spanish as preferred language | 9 (14.1%) | 4 (11.4%) | 5 (17.2%) | .544 |
| Country of origin | .125 | |||
| United States | 40 (62.5%) | 21 (60.0%) | 19 (65.5%) | |
| Cuba, Dominican Republic, Puerto Rico | 11 (17.2%) | 4 (11.4%) | 7 (24.1%) | |
| Other | 13 (20.3%) | 10 (28.6%) | 3 (10.3%) | |
| Socioeconomic status | ||||
| Education | .053 | |||
| Less than high school graduate or GED | 11 (17.4%) | 6 (17.6%) | 5 (17.2%) | |
| High school graduate or GED | 17 (27.0%) | 7 (20.6%) | 10 (34.5%) | |
| Associate’s degree or some college | 19 (30.2%) | 15 (44.1%) | 4 (13.8%) | |
| College graduate or higher | 16 (25.4%) | 6 (17.6%) | 10 (34.5%) | |
| Annual household income | .229 | |||
| Comfortable | 21 (35.6%) | 8 (25.8%) | 13 (46.4%) | |
| Enough to make ends meet | 27 (45.8%) | 17 (54.8%) | 10 (35.7%) | |
| Not enough to make ends meet | 11 (18.6%) | 6 (19.4%) | 5 (17.9%) | |
| Technology literacy | ||||
| Can access the Internet | 48 (78.7%) | 23 (71.9%) | 25 (86.2%) | .083 |
| Has access to desktop, laptop, or tableta | 37 (56.9%) | 18 (50.0%) | 19 (65.5%) | .365 |
| Daily Internet use in past 30 daysa | .122 | |||
| < 1 hour/day | 8 (16.0%) | 4 (16.0%) | 4 (16.0%) | |
| 1-2 hours/day | 14 (28.0%) | 9 (36.0%) | 5 (20.0%) | |
| 3-4 hours/day | 15 (30.0%) | 9 (36.0%) | 6 (24.0%) | |
| ≥ 5 hours/day | 13 (26.0%) | 3 (12.0%) | 10 (40.0%) | |
| Health literacy | ||||
| Inadequate health literacy | 20 (46.9%) | 16 (45.7%) | 14 (48.3%) | .838 |
| Patient activation | ||||
| Patient Activation Measure score | 62.8 (1.8) | 62.4 (2.6) | 63.3 (2.4) | .800 |
| Patient activation level | .452 | |||
| Level 1 | 6 (9.4%) | 5 (14.3%) | 1 (3.5%) | |
| Level 2 | 10 (15.6%) | 4 (11.4%) | 6 (20.7%) | |
| Level 3 | 36 (56.3%) | 19 (54.3%) | 17 (58.6%) | |
| Level 4 | 12 (18.8%) | 7 (20.0%) | 5 (17.2%) | |
| Clinical characteristics | ||||
| Emergency Severity Index score | 2.59 (0.53) | 2.53 (0.51) | 2.68 (0.55) | .664 |
| Emergency Severity Index level | .376 | |||
| Level 2 | 27 (42.2%) | 17 (47.2%) | 10 (35.7%) | |
| Level 3 | 36 (56.3%) | 19 (52.8%) | 17 (60.7%) | |
| Level 4 | 1 (1.6%) | 0 (0.0%) | 1 (3.6%) | |
| Median days since last visit | 31.5 (8-115) | 58.5 (8-193) | 28.0 (13-120) | .735 |
| Number of home medications | 6.65 (6.15) | 5.60 (5.28) | 7.89 (6.98) | .143 |
Continuous variables reported as mean (SD), except “median days since last visit,” reported as median (IQR).
Categorical variables reported as n (%). Percentages adjusted to account for missing data.
Abbreviations: GED: General Equivalency Diploma.
Includes only participants who reported access to the Internet.
Patient medication changes
Overall, 48 (74%) participants suggested changes to their medication lists using the electronic tool, with an average of 2.57 suggested changes per patient (range 0-13;Table 2). Participants spent a median of 1.7 minutes (IQR 0.8-4.4), or on average 3 minutes, completing their home medication reviews using the tool. Three participants (5%) reported beingNot Sure about 1 or more medications displayed on their lists. No significant differences existed between thebefore andafter groups.
Table 2.
Patient and clinician medication changes
| By-group analysis |
||||
|---|---|---|---|---|
| Variable | Overall (n = 65) | Before (n = 36) | After (n = 29) | p-value |
| Patient medication changes | ||||
| Number of patients who made changes | 48 (73.8%) | 29 (80.6%) | 19 (65.5%) | .170 |
| Average number of changes per patient | 2.57 (2.88) | 2.75 (2.73) | 2.34 (3.10) | .310 |
| Additions | 1.14 (2.39) | 1.36 (2.52) | 0.86 (2.23) | .536 |
| Deletions | 1.09 (1.43) | 1.14 (1.42) | 0.97 (1.48) | .466 |
| Modifications | 0.37 (1.04) | 0.25 (0.87) | 0.52 (1.21) | .385 |
| Unreconciled | − | 1.58 (2.49) | − | − |
| Total number of changes made | 169 | 101 | 68 | − |
| Additions | 74 (44.3%) | 49 (49.5%) | 25 (36.8%) | |
| Deletions | 71 (42.5%) | 43 (43.4%) | 28 (41.2%) | |
| Modifications | 24 (14.4%) | 9 (9.1%) | 15 (22.1%) | |
| Unreconciled | − | 57 (57.6%) | − | |
| Clinician medication changes | ||||
| Number of patients who received changes | 36 (55.4%) | 20 (55.6%) | 16 (55.2%) | .975 |
| Average number of changes per patient | 2.09 (2.95) | 2.28 (2.70) | 1.90 (3.27) | .529 |
| Additions | 0.87 (2.12) | 1.06 (2.33) | 0.69 (1.85) | .624 |
| Deletions | 1.11 (1.74) | 1.11 (1.56) | 1.10 (1.97) | .710 |
| Modifications | 0.11 (0.31) | 0.11 (0.32) | 0.10 (0.31) | .562 |
| Total number of changes received | 137 | 82 | 55 | − |
| Additions | 58 (42.3%) | 38 (46.3%) | 20 (36.4%) | |
| Deletions | 72 (52.6%) | 40 (48.8%) | 32 (58.2%) | |
| Modifications | 7 (5.1%) | 4 (4.9%) | 3 (5.5%) | |
Number of patients reported as n (%); average number of changes reported as mean (SD); total number of changes reported as n or n (%).
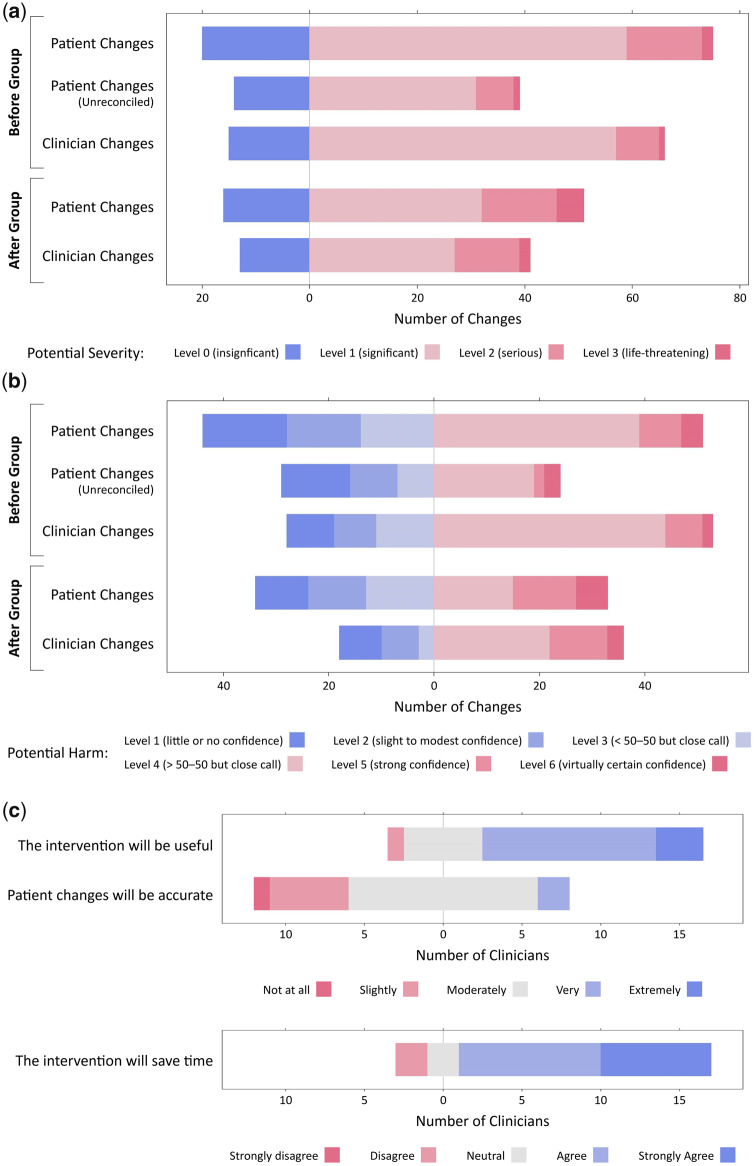
In thebefore group, 57 (58%) patient medication changes wentunreconciled, or unaddressed by the admitting team. Thirty-nine (74%) unreconciled changes had a level 2 (significant) or higher potential severity, and 24 (45%) had a level 4 (greater than 50-50 chance) or higher potential harm (Table 3;Figures 2a and b). In theafter group, patients made 68 additional changes to their already-reconciled medication lists. Fifty-one (75%) unreconciled changes had a level 2 (significant) or higher potential severity, and 33 (49%) had a level 4 (greater than 50-50 chance) or higher potential harm.
Table 3.
Potential severity and potential harm
| By-group analysis |
|||||
|---|---|---|---|---|---|
| Variable | Overall (n = 65) | Unreconciled (n = 36) | Before (n = 36) | After (n = 29) | p-value |
| Patient medication changes | |||||
| Average potential severity | 1.16 (0.77) | 1.01 (0.73) | 1.09 (0.69) | 1.26 (0.86) | .308 |
| Potential severity | .172 | ||||
| Level 0 (insignificant) | 36 (22.2%) | 14 (26.4%) | 20 (21.1%) | 16 (23.9%) | |
| Level 1 (significant) | 91 (56.2%) | 31 (58.5%) | 59 (62.1%) | 32 (47.8%) | |
| Level 2 (serious) | 28 (17.3%) | 7 (13.2%) | 14 (14.7%) | 14 (20.9%) | |
| Level 3 (life-threatening) | 7 (4.3%) | 1 (1.9%) | 2 (2.1%) | 5 (7.5%) | |
| Average potential harm | 3.44 (1.44) | 3.09 (1.47) | 3.38 (1.37) | 3.53 (1.54) | .558 |
| Potential harm | .100 | ||||
| Level 1 (little or no confidence) | 26 (16.0%) | 13 (24.5%) | 16 (16.8%) | 10 (14.9%) | |
| Level 2 (slight to modest confidence) | 25 (15.4%) | 9 (17.0%) | 14 (14.7%) | 11 (16.4%) | |
| Level 3 (< 50–50 but close call) | 27 (16.7%) | 7 (13.2%) | 14 (14.7%) | 13 (19.4%) | |
| Level 4 (> 50–50 but close call) | 54 (33.3%) | 19 (35.8%) | 39 (41.1%) | 15 (22.4%) | |
| Level 5 (strong confidence) | 20 (12.3%) | 2 (3.8%) | 8 (8.4%) | 12 (17.9%) | |
| Level 6 (virtually certain confidence) | 10 (6.2%) | 3 (5.7%) | 4 (4.2%) | 6 (9.0%) | |
| Clinician medication changes | |||||
| Average potential severity | 1.12 (0.69) | − | 1.10 (0.62) | 1.15 (0.78) | .905 |
| Potential severity | − | .060 | |||
| Level 0 (insignificant) | 28 (20.7%) | 15 (18.5%) | 13 (24.1%) | ||
| Level 1 (significant) | 84 (62.2%) | 57 (70.4%) | 27 (50.0%) | ||
| Level 2 (serious) | 20 (14.8%) | 8 (9.9%) | 12 (22.2%) | ||
| Level 3 (life-threatening) | 3 (2.2%) | 1 (1.2%) | 2 (3.7%) | ||
| Average potential harm | 3.64 (1.31) | − | 3.62 (1.22) | 3.69 (1.44) | .497 |
| Potential harm | − | .147 | |||
| Level 1 (little or no confidence) | 17 (12.6%) | 9 (11.1%) | 8 (14.8%) | ||
| Level 2 (slight to modest confidence) | 15 (11.1%) | 8 (9.9%) | 7 (13.0%) | ||
| Level 3 (< 50–50 but close call) | 14 (10.4%) | 11 (13.6%) | 3 (5.6%) | ||
| Level 4 (> 50–50 but close call) | 66 (48.9%) | 44 (54.3%) | 22 (40.7%) | ||
| Level 5 (strong confidence) | 18 (13.3%) | 7 (8.6%) | 11 (20.4%) | ||
| Level 6 (virtually certain confidence) | 5 (3.7%) | 2 (2.5%) | 3 (5.6%) | ||
Averages reported as mean (SD), categorical variables reported as n (%).
Percentages adjusted to account for missing data.
Figure 2.
2a. Potential severity of medication changes. 2b. Potential harm of medication changes. 2c. Clinician survey responses.
Both patients and clinicians made changes with a broad range of potential severities and potential harms. No significant differences existed in the distributions of potential severity (p = .662) or potential harm (p = .576) of patients’ versus clinicians’ changes. Patients and clinicians identified similar numbers of changes with a potential harm level of 4 to 6, but patients tended to identify more changes with a potential harm level of 1 to 3 than clinicians, although nonsignificant (p = .126).
The total number of patient medication changes was positively correlated with age (r = 0.30,p = <.001), number of home medications (rs = 0.32,p = <.001), and previous tablet use (rs = 0.366, p = .010). This suggests that older patients with more medications and tablet users make more medication changes. Patient activation and health literacy level were not associated with the number of patient medication changes.
In thebefore group, only 1 clinician viewed the patient’s suggested changes prior to admission medication reconciliation (seeClinician Survey section), and clinicians made changes to the same home medication lists as patients. Therefore, we compared patients’ and clinicians’ changes in thebefore group (Table 4). Significantly more patients than clinicians madeone or more changes to the medication list (p = .021). Twenty-nine (81%) patients changed their lists, whereas clinicians changed only 20 (56%) patients’ lists.
Table 4.
Comparison ofbefore group patient and clinician medication changes
| Variable | Patient changes (before group) | Clinician changes (before group) | p-value |
|---|---|---|---|
| Number of patients who made or received changes | 29 (80.6%) | 20 (55.6%) | .021* |
| Average number per patient | 2.75 (2.73) | 2.28 (2.70) | .140 |
| Additions | 1.36 (2.52) | 1.06 (2.33) | .546 |
| Deletions | 1.14 (1.42) | 1.11 (1.56) | .539 |
| Modifications | 0.25 (0.87) | 0.11 (0.32) | .245 |
| Average potential severity | 1.09 (0.69) | 1.10 (0.62) | .570 |
| Potential severity | .662 | ||
| Level 0 (insignificant) | 20 (21.1%) | 15 (18.5%) | |
| Level 1 (significant) | 59 (62.1%) | 57 (70.4%) | |
| Level 2 (serious) | 14 (14.7%) | 8 (9.9%) | |
| Level 3 (life-threatening) | 2 (2.1%) | 1 (1.2%) | |
| Average potential harm | 3.38 (1.37) | 3.62 (1.22) | .902 |
| Potential harm | .576 | ||
| Level 1 (little or no confidence) | 16 (16.8%) | 9 (11.1%) | |
| Level 2 (slight to modest confidence) | 14 (14.7%) | 8 (9.9%) | |
| Level 3 (< 50–50 but close call) | 14 (14.7%) | 11 (13.6%) | |
| Level 4 (> 50–50 but close call) | 39 (41.1%) | 44 (54.3%) | |
| Level 5 (strong confidence) | 8 (8.4%) | 7 (8.6%) | |
| Level 6 (virtually certain confidence) | 4 (4.2%) | 2 (2.5%) |
Number of patients reported as n (%); averages reported as mean (SD), categorical variables reported as n (%).
Percentages adjusted to account for missing data.
p-value significant at the .05 level.
Clinician medication changes
On average, clinicians made 2.11 changes to patients’ home medication lists on admission (range 0-15;Table 2). No significant differences existed between groups. In thebefore group, clinicians made 42 (42%) of the changes that patients suggested. Clinicians made an average of 1.06 changes that patients did not suggest.
Clinician survey
Of the 34 clinicians contacted, 20 (59%) completed the survey. Only 1 clinician reported receiving the brightly colored printout prior to admission medication reconciliation. Among respondents, 14 (70%) described the intervention as very or extremely potentially useful, 14 (70%) thought patients’ information would be moderately, very, or extremely accurate, and 16 (80%) agreed the invention would save them time (Figure 2c). No significant differences existed based on role type or experience.
DISCUSSION
This study used an electronic home medication review tool to pilot engaging patients in the hospital admission medication reconciliation process in a high-volume urban ED. There was a high willingness of patients to participate in medication reconciliation, and participation was completed in a short amount of time. While the time commitment required was brief, we found participants could identify multiple medication discrepancies, many with a greater than 50-50 chance of harm, and were often discrepancies that clinicians did not make during their reconciliation. Clinicians reported a belief that the intervention would save them time, and that patients would report useful information. These data suggest that obtaining patient-reported medication information electronically may facilitate the medication reconciliation process and potentially improve patient safety during hospitalization, and is acceptable to both patients and clinicians.
Integrating patient-reported information into the clinical workflow remains challenging. In our study, only provider reported receiving the brightly colored printout containing patient-reported medication changes. Patients may have felt uncomfortable giving providers the printout, forgotten about it, or not known to whom it should be given. Even if providers had received the printout, it is not clear whether they would use the information it contained. Staroselsky and colleagues found that emailing physicians about patient-reported medication discrepancies had no effect.21 Future work should design and evaluate strategies to better incorporate patient-reported medication information into the clinical workflow, potentially through integration with the electronic medical record, so that providers may easily utilize it.
Successful medication reconciliation interventions often employ pharmacists.32,33,52 A 2016 systematic review found that pharmacist-led reconciliation processes prevented discrepancies and potential ADEs at hospital admission.33 However, pharmacists’ time is a limited and costly resource. Patient-centered medication reconciliation interventions, such as the intervention used in our study, may supplement pharmacist-led or physician-led interventions to save time and achieve better outcomes. The National Academy of Medicine (formerly the Institute of Medicine) promotes patient-centered interventions as central to learning health systems, or systems that generate the best evidence for patients’ and providers’ collaborative choices.53 Although some studies have already integrated pharmacist-led and patient-centered interventions,20,25 opportunity remains to investigate the impact of these multi-level, combined interventions on patient safety.
Work involving the engagement of patients is often viewed hesitantly, as providers often believe that patients do not always know enough to contribute to their clinical care. Only 2 (10%) providers thought that patients’ information would be very or extremely accurate. Providers’ belief that patients cannot accurately report their medications contrasted sharply with their patients’ actions, as the majority of participants’ suggested changes to their medication lists were often changes evaluated to be potentially severe and causing potential harm, and only 3 participants (5%) reported feeling “unsure” about 1 or more medications on their lists. Previous research at Geisinger Health System found that most patients (89%) who submitted feedback through their portals requested changes to their medication lists.27 These data suggest that patients possess the knowledge and desire to participate in medication reconciliation, and will take an active role given the opportunity. Future work should explore additional opportunities to engage patients with their medication lists in the acute care setting in general, and analyze the accuracy of patient-reported medication discrepancies and the source of providers’ concerns about accuracy.
In our study, pharmacists found that patients identified numerous medication discrepancies with potential for serious harm. This result is consistent with Schnipper and colleagues’ finding that patient portal use significantly decreased medication discrepancies with potential for serious harm.28 Heyworth and colleagues piloted the “Secure Messaging for Medication Reconciliation Tool” (SMMRT) with 60 patients after hospital discharge.26 The patients identified 23 potential ADEs, with 13 classified as serious. Tools within patient portals, such as SMMRT and our review tool, stand to improve medication safety during transitions of care. Our study focused on the outpatient-to-inpatient transition, which the Joint Commission recognizes as a critical time to complete accurate medication reconciliation.10
Interestingly, patients’ participationbefore orafter the admitting team completed medication reconciliation did not significantly impact the number of patient medication changes. Furthermore, patients made changes to their medication lists that the admitting team did not, and vice versa. As this comparison was performed using information from different time points during the patients’ hospitalization, we expected the number of medication changes to be smaller in theafter group, since the clinical team had completed the medication reconciliation. One potential reason for these unexpected results may be the admitting team’s focus on medications related to the admitting diagnosis. For example, a hospital team admitting a heart failure patient might not modify that patient’s documented psychiatric medications. Future work should explore why patients and providers make different changes to the lists. Additional data to collect include the classification of patient medication changes as related or unrelated to the admitting diagnosis, medication categories, and types (eg, blood pressure-related, diabetes management, etc.) and sub-stratification of changes by prescription, over-the-counter medications, or herbal products.
As noted, the moderate association between previous tablet use and number of changes highlights the critical importance of designing robust yet simple systems to engage populations with low technology literacy. Poor usability is patients’ top complaint about portals,54 and better attention to system design may prevent discrepancies in engagement between populations with low and high technology literacy.
Limitations
This study assessed patients’ willingness to engage with medication reconciliation, the potential impact on medication safety, and the intervention’s potential usefulness to providers. We did not demonstrate the intervention’s efficacy or compare users to a control group of nonusers. We also did not have a gold standard medication list to compare patient and provider changes against. Additionally, patients in theafter group may have been influenced by the changes that their admitting team made to their medication lists. Future work should determine the efficacy of patient-centered medication reconciliation interventions prior to hospital admission, as well as analyze the accuracy of patient-provided changes. Our study excluded patients with acute illness and cognitive impairment, despite evidence that these patient populations experience more ADEs. Future work should explore strategies to engage these patient populations, such as contacting healthcare proxies.
We conducted our study at a large academic medical center with an advanced informatics infrastructure, which may limit its generalizability. Our intervention relied on data from an EHR system, and as has been studied, EHRs can have the unintended consequence of introducing new errors, which can then influence the data available.55 Additionally, we sourced the home medication list solely from our institution’s EHR. Interfaces with outside sources, such as outpatient visits and insurance claims databases, may provide a more comprehensive list for both patients and clinicians to review.
Our sample included only 65 patients, which potentially limited our power to detect differences between groups. The modest difference in the level of education between the 2 groups could have impacted how they used the tool. We did not collect patient measures such as comprehensive medication knowledge or confidence with patient-provider communication, which could have provided additional insight. Our Kappa metrics for agreement on potential severity and harm lay between 0.6 and 0.7. Standardized measures for usefulness and acceptability in the clinician survey may also have provided additional insight.
CONCLUSION
This study engaged hospital patients in admission medication reconciliation using an electronic home medication review tool. Participants identified potentially serious and harmful changes that clinicians missed on admission medication reconciliation, suggesting that patient engagement in medication reconciliation may improve medication safety. Future work should explore additional opportunities to engage patients with medication safety in the acute care setting, including combined patient-centered and pharmacist-led interventions.
FUNDING
This work was supported by the Agency for Healthcare Research and Quality (R01HS21816, PI: Vawdrey) and the National Library of Medicine (T15LM007079 – Hripcsak).
Conflict of interest statement. The authors have no competing interests associated with this work.
CONTRIBUTORS
All authors contributed extensively to the work presented in this manuscript, including the generation of content for and the revision of the manuscript. J.E.P., F.P., and D.K.V. conceptualized and designed the study. J.E.P. and F.P. performed recruitment. J.E.P., F.P., and L.V.G. performed data analysis. J.E.P. wrote the original manuscript, and L.V.G contributed to significant revisions and additions for the final draft. All authors provided expertise and feedback.
REFERENCES
- 1. Cornish PL,Knowles SR,Marchesano R, et al. Unintended medication discrepancies at the time of hospital admission.Arch Intern Med 2005;1654:424–9. [DOI] [PubMed] [Google Scholar]
- 2. Schnipper JL,Kirwin JL,Cotugno MC, et al. Role of pharmacist counseling in preventing adverse drug events after hospitalization.Arch Intern Med 2006;1665:565. [DOI] [PubMed] [Google Scholar]
- 3. Schnipper JL,Liang CL,Hamann C, et al. Development of a tool within the electronic medical record to facilitate medication reconciliation after hospital discharge.J Am Med Inform Assoc 2011;183:309–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bates DW,Cullen DJ,Laird N, et al. Incidence of adverse drug events and potential adverse drug events. Implications for prevention. ADE Prevention Study Group.JAMA 1995;2741:29–34. [PubMed] [Google Scholar]
- 5. Pippins JR,Gandhi TK,Hamann C, et al. Classifying and predicting errors of inpatient medication reconciliation.J Gen Intern Med 2008;239:1414–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gleason KM,McDaniel MR,Feinglass J, et al. Results of the medications at transitions and clinical handoffs (match) study: an analysis of medication reconciliation errors and risk factors at hospital admission.J Gen Intern Med 2010;255:441–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Caglar S,Henneman PL,Blank FS, et al. Emergency department medication lists are not accurate.J Emerg Med 2011;406:613–6. [DOI] [PubMed] [Google Scholar]
- 8. Shepherd G,Schwartz RB.. Frequency of incomplete medication histories obtained at triage.Am J Health Syst Pharm 2009;661:65–9. [DOI] [PubMed] [Google Scholar]
- 9. Coffey M,Mack L,Streitenberger K, et al. Prevalence and clinical significance of medication discrepancies at pediatric hospital admission.Acad Pediatr 2009;95:360. [DOI] [PubMed] [Google Scholar]
- 10. Joint Commission.2016 National Patient Safety Goals2016. Joint Commission Resources, Inc. Retrieved fromhttps://www.jointcommission.org/assets/1/6/2016_NPSG_HAP_ER.pdf
- 11. Greenwald JL,Halasyamani L,Greene J, et al. Making inpatient medication reconciliation patient centered, clinically relevant and implementable: a consensus statement on key principles and necessary first steps.J Hosp Med 2010;58:477–85. [DOI] [PubMed] [Google Scholar]
- 12. Pevnick JM,Shane R,Schnipper JL.. The problem with medication reconciliation.BMJ Qual Saf 2016;259:726–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Plaisant C,Wu J,Hettinger AZ, et al. Novel user interface design for medication reconciliation: an evaluation of Twinlist.J Am Med Inform Assoc 2015;222:340–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mueller SK,Kripalani S,Stein J, et al. A toolkit to disseminate best practices in inpatient medication reconciliation: multi-center medication reconciliation quality improvement study (MARQUIS).Jt Comm J Qual Patient Saf 2013;398:371–AP3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Meguerditchian AN,Krotneva S,Reidel K, et al. Medication reconciliation at admission and discharge: a time and motion study.BMC Health Serv Res 2013;131:485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Barnsteiner JH. Medication reconciliation: transfer of medication information across settings-keeping it free from error.Am J Nurs 2005;105:31–6. [DOI] [PubMed] [Google Scholar]
- 17. Rozich JD,Resar RK.. Medication safety: one organization’s approach to the challenge.J Clin Outcomes Manag 2001;8:27–34. [Google Scholar]
- 18. Grant RW,Wald JS,Schnipper JL, et al. Practice-linked online personal health records for type 2 diabetes mellitus.Arch Intern Med 2008;16816:1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Staroselsky M,Volk LA,Tsurikova R, et al. Improving electronic health record (EHR) accuracy and increasing compliance with health maintenance clinical guidelines through patient access and input.Int J Med Inform 2006;75 (10–11):693–700. [DOI] [PubMed] [Google Scholar]
- 20. de Jong CC,Ros WJG,van Leeuwen M, et al. Exploring the effects of patients taking a vigilant role in collaborating on their e-medication administration record.Int J Med Inform 2016;88:18–24. [DOI] [PubMed] [Google Scholar]
- 21. Staroselsky M,Volk LA,Tsurikova R, et al. An effort to improve electronic health record medication list accuracy between visits: patients’ and physicians’ response.Int J Med Inform 2008;773:153–60. [DOI] [PubMed] [Google Scholar]
- 22. Weingart SN,Hamrick HE,Tutkus S, et al. Medication safety messages for patients via the web portal: the MedCheck intervention.Int J Med Inform 2008;773:161–8. [DOI] [PubMed] [Google Scholar]
- 23. Schnipper JL,Gandhi TK,Wald JS, et al. Design and implementation of a web-based patient portal linked to an electronic health record designed to improve medication safety: the Patient Gateway medications module.Inform Prim Care 2008;162:147–55. [DOI] [PubMed] [Google Scholar]
- 24. Chrischilles EA,Hourcade JP,Doucette W, et al. Personal health records: a randomized trial of effects on elder medication safety.J Am Med Inform Assoc 2014;214:679–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Green BB. Effectiveness of home blood pressure monitoring, web communication, and pharmacist care on hypertension control.JAMA 2008;29924:2857.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Heyworth L,Paquin AM,Clark J, et al. Engaging patients in medication reconciliation via a patient portal following hospital discharge.J Am Med Inform Assoc 2014;21 (e1):e157–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dullabh P,Sondheimer N,Katsh E, et al. How patients can improve the accuracy of their medical records.EGEMS 2014;23:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schnipper JL,Gandhi TK,Wald JS, et al. Effects of an online personal health record on medication accuracy and safety: a cluster-randomized trial.J Am Med Inform Assoc 2012;195:728–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Weingart SN,Toth M,Eneman J, et al. Lessons from a patient partnership intervention to prevent adverse drug events.Int J Qual Health Care 2004;166:499–507. [DOI] [PubMed] [Google Scholar]
- 30. Sarkar U,Lyles CR,Parker MM, et al. Use of the refill function through an online patient portal is associated with improved adherence to statins in an integrated health system.Med Care 2014;523:194–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. de Lusignan S,Mold F,Sheikh A, et al. Patients’ online access to their electronic health records and linked online services: a systematic interpretative review.BMJ Open 2014;49:e006021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mueller SK,Sponsler KC,Kripalani S, et al. Hospital-based medication reconciliation practices: a systematic review.Arch Intern Med 2012;17214:1057–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mekonnen AB,McLachlan AJ,Brien JAE.. Pharmacy-led medication reconciliation programmes at hospital transitions: a systematic review and meta-analysis.J Clin Pharm Ther 2016;412:128–44. [DOI] [PubMed] [Google Scholar]
- 34. Pevnick JM,Nguyen C,Jackevicius CA, et al. Improving admission medication reconciliation with pharmacists or pharmacy technicians in the emergency department: a randomised controlled trial.BMJ Qual Saf 2017;27:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Murphy EM,Oxencis CJ,Klauck JA, et al. Medication reconciliation at an academic medical center: implementation of a comprehensive program from admission to discharge.Am J Health Syst Pharm 2009;6623:2126–31. [DOI] [PubMed] [Google Scholar]
- 36. Poon EG,Blumenfeld B,Hamann C, et al. Design and implementation of an application and associated services to support interdisciplinary medication reconciliation efforts at an integrated healthcare delivery network.J Am Med Inform Assoc 2006;136:581–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Vawdrey DK,Chang N,Compton A, et al. Impact of electronic medication reconciliation at hospital admission on clinician workflow.AMIA Annu Symp Proc 2010;2010:822–6. [PMC free article] [PubMed] [Google Scholar]
- 38. Polinski JM,Moore JM,Kyrychenko P, et al. An insurer’s care transition program emphasizes medication reconciliation, reduces readmissions and costs.Health Aff (Millwood) 2016;357:1222–9. [DOI] [PubMed] [Google Scholar]
- 39. Masterson Creber R,Prey J,Ryan B, et al. Engaging hospitalized patients in clinical care: study protocol for a pragmatic randomized controlled trial.Contemp Clin Trials 2016;47:165–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chew L,Bradley KA,Boyko EJ.. Brief questions to identify patients with inadequate health literacy.Health Lond Engl 1997 2004;11:12.. [PubMed] [Google Scholar]
- 41. Hibbard JH,Mahoney ER,Stock R, et al. Do increases in patient activation result in improved self-management behaviors? Health Serv Res 2007;424:1443–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hibbard JH,Greene J.. What the evidence shows about patient activation: better health outcomes and care experiences; fewer data on costs.Health Aff (Millwood) 2013;322:207–14. [DOI] [PubMed] [Google Scholar]
- 43. Hibbard JH,Mahoney ER,Stockard J, et al. Development and testing of a short form of the patient activation measure.Health Serv Res 2005;40 (6p1):1918–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hibbard JH. Using systematic measurement to target consumer activation strategies.Med Care Res Rev 2009;66 (1_suppl):9–27S. [DOI] [PubMed] [Google Scholar]
- 45. Fowles JB,Terry P,Xi M, et al. Measuring self-management of patients’ and employees’ health: further validation of the Patient Activation Measure (PAM) based on its relation to employee characteristics.Patient Educ Couns 2009;771:116–22. [DOI] [PubMed] [Google Scholar]
- 46. Greene J,Hibbard JH.. Why does patient activation matter? An examination of the relationships between patient activation and health-related outcomes.J Gen Intern Med 2012;275:520–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hibbard JH,Greene J,Tusler M.. Improving the outcomes of disease-management by tailoring care to the patient’s level of activation.Am J Manag Care 2009;156:353–60. [PubMed] [Google Scholar]
- 48. Prey JE,Qian M,Restaino S, et al. Reliability and validity of the patient activation measure in hospitalized patients.Patient Educ Couns 2016;9912:2026–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gilboy N,Tanabe P,Travers D, et al. Emergency Severity Index (ESI): A Triage Tool for Emergency Department Care, Version 4Implementation Handbook 2012 Edition. Rockville, MD: Agency for Healthcare Research and Quality;2012 [Google Scholar]
- 50. R Core Team.R: A Language and Environment for Statistical Computing Vienna, Austria: R Foundation for Statistical Computing;2013.
- 51. StataCorp.Stata Statistical Software: Release 14. College Station, TX: StataCorp LP;2015. [Google Scholar]
- 52. McNab D,Bowie P,Ross A, et al. Systematic review and meta-analysis of the effectiveness of pharmacist-led medication reconciliation in the community after hospital discharge.BMJ Qual Saf 2017; doi:10.1136/bmjqs-2017-007087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Institute of Medicine.The Learning Healthcare System: Workshop Summary. Washington, DC: National Academies Press,2007. [PubMed] [Google Scholar]
- 54. Kruse CS,Argueta DA,Lopez L, et al. Patient and provider attitudes toward the use of patient portals for the management of chronic disease: a systematic review.J Med Internet Res 2015;172:e40–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Coiera E,Ash J,Berg M.. The unintended consequences of health information technology revisited.Yearb Med Inform 2016;1:163–9. [DOI] [PMC free article] [PubMed] [Google Scholar]