Abstract
Objective
To review and analyze the literature to determine whether wearable technologies can predict health outcomes.
Materials and methods
We queried Ovid Medline 1946 -, Embase 1947 -, Scopus 1823 -, the Cochrane Library, clinicaltrials.gov 1997 – April 17, 2018, and IEEE Xplore Digital Library and Engineering Village through April 18, 2018, for studies utilizing wearable technology in clinical outcome prediction. Studies were deemed relevant to the research question if they involved human subjects, used wearable technology that tracked a health-related parameter, and incorporated data from wearable technology into a predictive model of mortality, readmission, and/or emergency department (ED) visits.
Results
Eight unique studies were directly related to the research question, and all were of at least moderate quality. Six studies developed models for readmission and two for mortality. In each of the eight studies, data obtained from wearable technology were predictive of or significantly associated with the tracked outcome.
Discussion
Only eight unique studies incorporated wearable technology data into predictive models. The eight studies were of moderate quality or higher and thereby provide proof of concept for the use of wearable technology in developing models that predict clinical outcomes.
Conclusion
Wearable technology has significant potential to assist in predicting clinical outcomes, but needs further study. Well-designed clinical trials that incorporate data from wearable technology into clinical outcome prediction models are required to realize the opportunities of this advancing technology.
Keywords: predictive modeling, mortality, readmission, wearable technology, emergency department
INTRODUCTION
Predicting clinical outcomes after hospital discharge remains a significant challenge. Strategies to predict and prevent post-discharge mortality, readmissions, and emergency department (ED) visits have had limited success.1 If improvements in predictive models were possible, morbidity, mortality, readmissions, and ED visits might be prevented by early interventions on modifiable risk factors. Potentially modifiable risk factors for these clinical outcomes include activity levels, sleep patterns, and tachy- or bradyarrhythmias, parameters that can be tracked with wearable technology.2–6
Wearable technology that tracks health-related parameters is increasing in popularity in the lay market for fitness monitoring. These technologies have been used for healthcare insurance incentives and discounts, but the extent to which the data they collect can be used in predictive models for healthcare outcomes is not well studied. Our goal was to review and analyze the currently available literature to determine whether wearable technologies can predict health outcomes and which outcomes have been tracked. We defined our primary outcome — models using wearable technology data to predict clinical outcomes — as any study that derived a model predicting mortality, readmissions, and/or ED visits that incorporated data from any wearable technology.
METHODS
Data sources and searches
The published literature was searched using strategies created by a medical librarian (L.H.Y.) for the concepts of activity trackers and hospital readmission, emergency room visits, emergency departments, and mortality. The search strategies were established using a combination of standardized terms and key words and implemented in Ovid Medline 1946 -, Embase 1947 -, Scopus 1823 -, the Cochrane Library, and clinicaltrials.gov 1997 — all through October 10, 2017, then updated on April 17, 2018. IEEE Xplore Digital Library and Engineering Village were searched and included as updated on April 18, 2018. Duplicate records were identified using Endnote’s automatic duplication finder and manual review. For the eight papers (six unique studies) initially included in our analysis, we reviewed all references to identify additional studies that might have been missed by our initial search criteria. Two additional studies were identified and incorporated into the final analysis.
Study selection
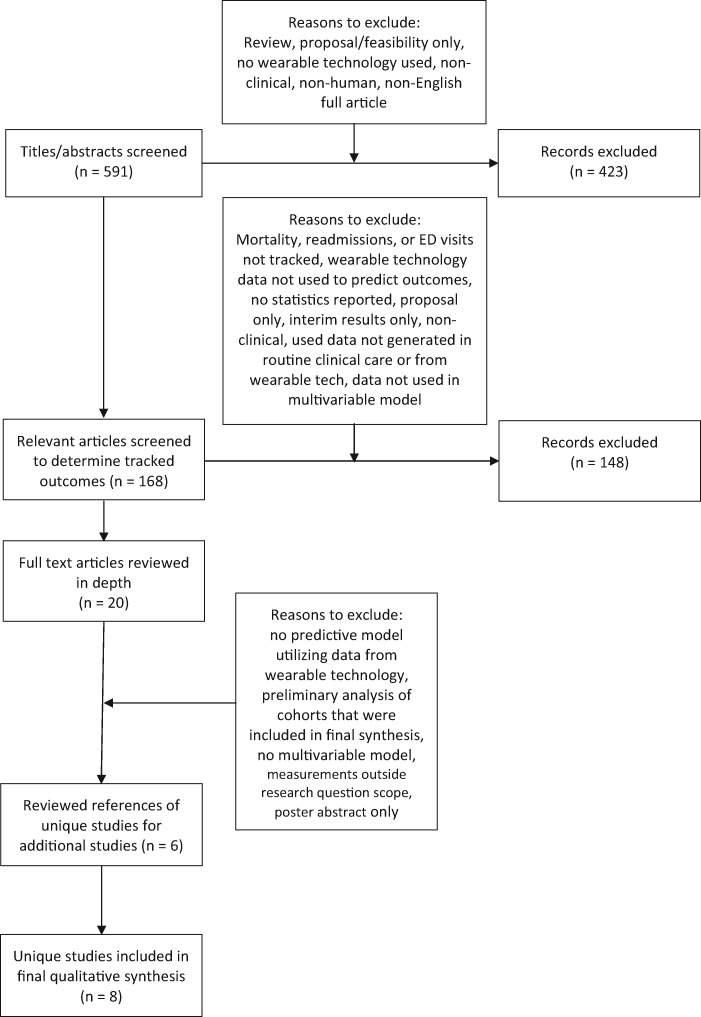
After removal of all duplicate results, all titles and abstracts were reviewed independently for relevance to the research question by J.P.B. SeeFigure 1 for a schema of the phases of application of inclusion/exclusion criteria. An additionalpost hoc review of all titles and abstracts was performed by M.H.K., which resulted in one additional study being included in the final quantitative synthesis. For the first line of review, articles were considered relevant to the research question if they involved human subjects, made use of wearable technology that tracked a health-related parameter, and tracked some outcome (ie not a feasibility study). The list of wearable technology search terms can be found in the appendix. Health-related parameters could include anything related to a patient’s health including vital signs, surrogates for physical activity such as movements or step counts, sleep quality, or electrocardiogram tracings. After determining that an article was relevant to the research question, articles were reviewed to determine what outcomes were tracked.
Figure 1.
Phases of application of exclusion/inclusion criteria as used by J.P.B. and M.H.K.
Studies that tracked mortality, readmission, and ED visits were reviewed independently in depth by J.P.B. and M.H.K. Only studies that used data from wearable technology in a predictive model for these outcomes were considered to be directly related to the research question.
Data extraction and quality and bias assessment
Full-text articles were independently reviewed by J.P.B. and M.H.K. for studies directly related to the research question. Methods and results were reviewed in depth to determine which studies incorporated data from wearable technology. Data were extracted independently by J.P.B. and M.H.K. in a standardized format and included predictive model type, patient population, enrollment, attrition rates, outcome predictor characteristics, differences in groups with and without the tracked outcome, data capture rates for wearable technology, and wearable technology type. Discrepancies were resolved by a third party (T.C.B.).
Studies were considered of high quality if model development was fully described and the full breadth of data collected by wearable technology was incorporated into a predictive model. Moderate quality studies fully described model development, but data from wearable technology were simplified or dichotomized. There were no low-quality studies (studies that did not fully describe model development and simplified or dichotomized wearable technology data). All studies were reviewed for the following biases using established guidelines7: selection, performance, detection, attrition, reporting, and publication bias.
Data synthesis and analysis
Results are descriptive in nature and categorized by the outcome that was tracked (ED visits, readmissions, and/or mortality).
RESULTS
A total of 736 results were found using our initial search strategy completed on October 10, 2017. Three-hundred-sixty-three duplicate records were identified using Endnote’s automatic duplication finder, and an additional 55 duplicate records were discovered and removed, leaving 456 unique citations in the project library. We updated our search on April 17, 2018, and added the IEEE Xplore Digital Library and Engineering Village databases on April 18, 2018. With these updates and additions, we identified an additional 153 results, 18 of which were duplicates, leaving an additional 135 records to be added to the project library. Fully reportable searches can be found inAppendix 1.
After review of the remaining titles and abstracts, 168 search results were deemed relevant to the search question. Of these 168 search results, nine included mortality as an outcome, 13 included readmission as an outcome, seven included ED visits as an outcome, and 137 included other outcomes (seeAppendix 2 for studies that looked at other clinical outcomes). Of the studies including mortality as an outcome, two included both readmissions and ED visits as outcomes. Of the other 11 articles including readmission as an outcome, an additional five included ED visits as an outcome. No articles looked only at ED visits. The 20 articles including one or more of mortality, readmissions, and ED visits as an outcome were included in the initial qualitative synthesis and are discussed below under the sections “Mortality” and “Readmissions.”
In full-text review of the 20 articles, a total of eight papers were identified that incorporated data from wearable technology into a predictive model of a target clinical outcome: four for mortality,8–11 and four for readmissions.12–15 Of the four papers predicting mortality, two were analyses of the same trial.9,10 Two papers analyzing readmissions were preliminary and final analyses of the same cohort.12,13 An additional two unique studies that included readmissions as an outcome were discovered during review of references from these six papers,16,17 bringing the number of unique studies included in the final analysis to the eight inTable 1.
Table 1.
Key aspects and limitations of studies included in the final analysis
| Study (year), # of patients | Patient population and technology used | Key findings and study quality | Limitations |
|---|---|---|---|
| Pyrkov et al. (2018), n = 745411 |
|
|
|
| Low et al (2018), n = 7115 |
|
|
|
| Joseph et al (2017), n = 10114 |
|
|
|
| Bae et al. (2016), n = 2512,13 |
|
|
|
| Takahashi et al. (2015), n = 13317 |
|
|
|
| Yates et al. (2014), n = 93069,10 |
|
|
|
| Fisher et al. (2013) n = 11116 |
|
|
|
| Walsh et al. (1997), n = 848 |
|
|
|
The included populations in these eight studies were patients with cardiovascular disease risk factors, congestive heart failure (CHF), post-operative metastatic peritoneal cancer, elderly patients admitted to medicine services, cardiac surgery patients, and elderly patients admitted to a trauma service. The studies were published between 1997 and 2018. The eight unique studies included 17 285 patients, which is skewed by the NAVIGATOR trial of patients with cardiovascular disease risk factors, which had 9306 patients, and the Pyrkov et al study with 7454 patients. The model quality of the eight unique studies was at least moderate (Table 1).
In all eight unique studies, every participant used wearable technology, minimizing selection and performance bias. In the Yates et al study,9 patients with complete pedometer data were more likely to be smokers and less likely to have congestive heart failure. However, these two particular differences would likely have biased toward the null for mortality. As our inclusion criteria required mortality, readmissions, or ED visits to be tracked, all of which are objective measures, no detection bias was found in the studies. In addition, no reporting bias was found in any of the eight unique studies. Significant associations between wearable technology data and the tracked outcomes were reported for all eight unique studies, which makes it possible that publication bias is an issue for this review. With only eight unique published studies, it is also possible that no negative studies have yet been completed. Finally, only the Yates et al study lost patients to follow-up (3.1%), which minimizes the likelihood of attrition bias.
Mortality
The four studies that incorporated data from wearable technology into a model predicting death8–11 used pedometers or accelerometers, and step or activity counts were the measures incorporated into predictive models of mortality. The Walsh et al study of 84 chronic heart failure patients found that fewer steps per week were significantly associated with mortality during the 710-day follow-up period.8 Other variables in the model that were predictive of mortality were related to cardiac disease severity. Two papers were analyses of the NAVIGATOR trial.9,10 In the study by Yates et al of 9306 participants with baseline cardiovascular disease or cardiovascular risk factors, baseline step counts and change in step counts from baseline both correlated with mortality during the average six years of follow-up.9 The other paper was a preliminary analysis of the NAVIGATOR trial.10 In the paper by Pyrkov et al, accelerometer data from participants in the National Health and Nutrition Examination Survey (NHANES) were used in various models to predict mortality.11 Their model found that activity levels predicted biological age, which in turn was a predictor of mortality.11 After incorporating biological age into their models (as calculated from activity records), activity level was a significant predictor of mortality only as the derived value of biological age. A summary of the key findings of these studies is shown inTable 1.
Of the other five studies that discussed mortality (not included inTable 1), patient populations included healthy patients, those with CHF, chronic obstructive pulmonary disease (COPD), or those on hemodialysis. These studies did not always use multivariable models, potentially falsely increasing the association of mortality with activity levels.18 Wearable technology was used in two studies to track adherence to activity programs, with adherence treated as a binary variable.19,20 Activity levels were found to be associated with reduced mortality, but data directly from wearable technology were not incorporated into the predictive models.19,20 In a study of 453 hemodialysis patients, step counts as tracked by a pedometer were not associated with mortality.21 The study by Nes et al developed an algorithm to help predict mortality that incorporated data from wearable technology.22 However, the algorithm also required the intensive step of laboratory-based VO2max measurements, which is outside the scope of our research question.
Readmissions
There were five unique studies (seeTable 1) that incorporated wearable technology data into predictive models for readmissions.13–17 In a well-done study of 25 postsurgical oncology patients by Bae et al, Fitbit data were used to predict readmissions.13 They found that duration of sedentary bouts and the total number of steps were associated with readmissions.13 Their multifactorial model of Fitbit collected step counts and other patient activity was able to predict readmission in 88.3% of cases.13 The model that used only Fitbit collected step counts predicted readmission accurately only 67.1% of the time.13 In a follow-up study, the authors found that in 71 patients with metastatic peritoneal cancer, higher mean daily step counts were predictive of 30- and 60-day readmissions even after adjusting for other risk factors.15
In the Joseph et al study of 101 elderly trauma patients, a wearable sensor was used to collect data about upper extremity function.14 The derived upper extremity function score was used as a proxy for frailty, and patients with scores indicative of lesser functioning upper extremities had higher rates of readmission as assessed in a multivariable model.
Fisher et al tracked post-discharge daily step counts in 111 elderly medicine patients and found that in an unadjusted model, mean daily step count was associated with an increased risk of 30-day readmission.16 However, when incorporated into a multivariable logistic regression model, daily step count was not a statistically significant predictor of readmission.16
In a study of 133 cardiac surgery patients, Takahashi et al found that low mean step counts prior to discharge was a strong predictor of cardiac re-hospitalization after cardiac surgery.17
The remainder of the studies that included readmissions as an outcome were not included in the final analysis (norTable 1) for various reasons, discussed below. Though readmissions were a tracked outcome in the studies by Evangelista et al and Paneroni et al.,19,20 these data were not incorporated into a model to predict outcomes. In a poster abstract, Berry et al reported the results of a prospective interventional trial aimed at preventing readmissions and ED visits in patients with heart failure by using wearable technology.23 However, only 24 patients were enrolled, and the study was not adequately powered to determine whether the wearable technology intervention led to a reduction in readmissions.23 In a study of 26 patients with COPD using wearable technology to track activity, increased step counts from baseline did not correlate with reduction in days of hospitalization, although data were not used in a predictive model, nor was the study adequately powered to detect a difference.24 In a randomized controlled trial of COPD patients, Katsaras et al report reductions in readmissions and ER visits with a wearable technology, but provide no statistical analysis to support this.25 In a similar publication by the same authors, they again report that their wearable technology resulted in reductions in readmissions and ED visits without providing any statistical analyses.26
In a study of 108 post bariatric surgery patients who were given an activity tracker, patients who had fewer initial steps were more likely to be readmitted or have ED visits with aP value of 0.06 (effect size not reported), though this study did not use steps to predict readmissions or ED visits.27 Though not used to predict readmissions, Fitbit data from post-op neurosurgery patients demonstrated that readmitted patients took fewer steps and had a decline in steps over time after discharge.28 In a case report, a patient wearing an activity tracker was found to have atrial fibrillation and was cardioverted, thereby averting a hospital admission.29
ED visits
There were no studies mentioning ED visits that did not also look at mortality or readmissions. None of the studies mentioning ED visits as an outcome used wearable technology data to predict the occurrence of ED visits.19,20,23–27
CONCLUSIONS
In this literature review, we identified only eight unique studies that directly incorporated data from wearable technology into models associating wearable technology data with clinical outcomes. Given the small number of studies, we can only speculate on the utility of wearable technology for predicting clinical outcomes. However, there are several promising findings from this review that suggest that further research on wearable technology for predicting clinical outcomes is needed. The studies by Yates et al of over 9306 patients and Pyrkov of et al of 7454 patients demonstrate feasibility and biologic plausibility of tracking patient data with wearable technology. Yates et al were able to associate data collected by wearable technology with clinical outcomes, despite dichotomizing complex step count data.
Utilizing more features of complicated wearable technology data would likely improve predictive models, which was demonstrated by Bae et al.13 With a sample size of only 25 patients, Bae et al used 89 features of Fitbit data and were able to predict readmission with 88.3% accuracy. Their model was significantly better at predicting readmission than previously reported models that use traditional retrospective clinical data.30 The strategy of using the full breadth of wearable technology data in clinical outcome prediction is promising,13 as model accuracy was high despite a small sample size. This strategy warrants study in larger and more diverse populations to assure generalizability and minimize the likelihood of model overfitting.
In the future, we expect that enhancements in wearable technology will overcome many of the existing hurdles to its use in routine clinical care. Wearable technology is likely to support additional sensing modalities (eg, pulse oximetry, blood pressure, electrocardiography, glucose), last longer on battery power, adopt new form factors (eg hearing aid, contact lens, and generally smaller size), and achieve FDA approvals. Efforts on many of these advancements exist as prototypes or are under development. Clinical studies need to advance hand in hand with the evolution of hardware and software of wearable technology.
One potential source of bias in our study was the method of title and abstract review in which the second reviewer was not blinded to the determinations made by the first reviewer. However, strict inclusion criteria were utilized, as all studies had to use wearable technology and track mortality, readmission, or ED visits, which likely minimizes any possible bias introduced by a lack of blinding.
In conclusion, wearable technology has significant potential to assist in predicting clinical outcomes, but needs further study. Well-designed clinical trials that incorporate data from wearable technology into clinical outcome prediction models are required to realize the opportunities of this advancing technology.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Competing interest
Dr. Kollef was supported by the Barnes-Jewish Hospital Foundation. Dr. Burnham reports that, “This work was supported by the Washington University Institute of Clinical and Translational Sciences grant UL1TR000448 from the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health (NIH). The content is solely the responsibility of the authors and does not necessarily represent the official view of the NIH.” The remaining authors have no conflicts of interest to report.
Contributors
JPB, CL, LHY, TCB, and MKH made substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work; drafted the work or revised it critically for important intellectual content; will provide final approval of the version to be published; and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Supplementary Material
REFERENCES
- 1. Ziaeian B,Fonarow GC.. The prevention of hospital readmissions in heart failure.Prog Cardiovasc Dis 2016;584:379–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Muscedere J,Waters B,Varambally A, et al. The impact of frailty on intensive care unit outcomes: A systematic review and meta-analysis.Intensive Care Med 2017;438:1105–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hodgson CL,Udy AA,Bailey M, et al. The impact of disability in survivors of critical illness.Intensive Care Med 2017;437:992–1001. [DOI] [PubMed] [Google Scholar]
- 4. Courtney M,Edwards H,Chang A, et al. Fewer emergency readmissions and better quality of life for older adults at risk of hospital readmission: A randomized controlled trial to determine the effectiveness of a 24-week exercise and telephone follow-up program.J Am Geriatr Soc 2009;573:395–402. [DOI] [PubMed] [Google Scholar]
- 5. Violi F,Cangemi R,Falcone M, et al. Cardiovascular complications and short-term mortality risk in community-acquired pneumonia.Clin Infect Dis 2017;6411:1486–93. [DOI] [PubMed] [Google Scholar]
- 6. McKinley S,Fien M,Elliott R, et al. Health-related quality of life and associated factors in intensive care unit survivors 6 months after discharge.Am J Crit Care 2016;251:52–8. [DOI] [PubMed] [Google Scholar]
- 7. Ryan R,Hill S,Prictor M,McKenzie J. Cochrane Consumers and Communication Review Group. Study Quality Guide.2013http://cccrg.cochrane.org/authorresources. Accessed September 6, 2017.
- 8. Walsh JT,,Charlesworth A,Andrews A, et al. Relation of daily activity levels in patients with chronic heart failure to long-term prognosis.Am J Cardiol 1997;7910:1364–9. [DOI] [PubMed] [Google Scholar]
- 9. Yates T,Haffner SM,Schulte PJ, et al. Association between change in daily ambulatory activity and cardiovascular events in people with impaired glucose tolerance (NAVIGATOR trial): A cohort analysis.Lancet 2014;3839922:1059–66. [DOI] [PubMed] [Google Scholar]
- 10. Kraus RM,Tuomilehto J,Sun JL, et al. Relations between baseline physical activity by pedometer counts and future cardiovascular events in the NAVIGATOR study.Circulation 2012;126:A12297. [Google Scholar]
- 11. Pyrkov TV,Slipensky K,Barg M, et al. Extracting biological age from biomedical data via deep learning: Too much of a good thing? Sci Rep 2018;81:5210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Low DH,Jenkins FJ,Ahrendt S, et al. Patient-reported and fitbit-assessed physical activity: Associations with inflammation and risk of readmission after metastatic cancer surgery.Psychosomatic Med 2016;78:A127. [Google Scholar]
- 13. Bae SD,Low CA. Using passively collected sedentary behavior to predict hospital readmission. In: UbiComp 2016—proceedings of the 2016 ACM International Joint Conference on Pervasive and Ubiquitous Computing2016; 616–21.
- 14. Joseph BT,Orouji Jokar T,Heusser MR, et al. Upper-extremity function predicts adverse health outcomes among older adults hospitalized for ground-level falls.Gerontology 2017;634:299–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Low CA,Bovbjerg DH,Ahrendt S, et al. Fitbit step counts during inpatient recovery from cancer surgery as a predictor of readmission.Ann Behav Med 2018;521:88–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fisher SR,Kuo YF,Sharma G, et al. Mobility after hospital discharge as a marker for 30-day readmission.J Gerontol A Biol Sci Med Sci 2013;687:805–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Takahashi T,Kumamaru M,Jenkins S, et al. In-patient step count predicts re-hospitalization after cardiac surgery.J Cardiol 2015;664:286–91. [DOI] [PubMed] [Google Scholar]
- 18. Dwyer TP,Sun C,Cochrane J, et al. Objectively measured daily steps and subsequent long term all-cause mortality: The tasped prospective cohort study.PLoS ONE 2015;1011:e0141274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Evangelista LS,Hamilton MA,Fonarow GC,Dracup K.. Is exercise adherence associated with clinical outcomes in patients with advanced heart failure? Phys Sports Med 2010;381:28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Paneroni MS,Bernocchi P,Galli T.. Home telerehabilitation maintenance program for patients affected by COPD and CHF.Euro Resp J 2016;48:O268. [Google Scholar]
- 21. Winzeler RR,Kiss D,Kistler T, et al. Walking capacity improves survival in a large prospective Swiss dialysis cohort.Swiss Med Wkly 2013;143:6S. [Google Scholar]
- 22. Nes BM,Gutvik CR,Lavie CJ, et al. Personalized Activity Intelligence (PAI) for prevention of cardiovascular disease and promotion of physical activity.Am J Med 2017;1303:328–36. [DOI] [PubMed] [Google Scholar]
- 23. Berry E,Silvera A,Downer W, et al. Feasibility of integrating multiple remote monitoring technologies as a single heart failure decision support tool.Euro J Heart Failure 2017;19:36. [Google Scholar]
- 24. Chew MY,Lim TK,Chong P, et al. Effects of home oximetry on chronic obstructive pulmonary disease exacerbations.Euro Resp J 2011;38:902. [Google Scholar]
- 25. Katsaras A,Rizikari M,Saoulis N, et al. The use of the “healthwear” wearable system in chronic patients' early hospital discharge: Control randomized clinical trial. 5th International Symposium on Medical Information and Communication Technology.ISMICT 2011;2011:143–6. [Google Scholar]
- 26. Milsis A,Katsaras T,Saoulis N, et al. Clinical effectiveness of the “Healthwear” wearable system in the reduction of COPD patients’ hospitalization In:Nikita KS,Lin JC,Fotiadis DI,Arredondo Waldmeyer MT, eds.Wireless Mobile Communication and Healthcare. MobiHealth 2011. Lecture Notes of the Institute for Computer Sciences, Social Informatics and Telecommunications Engineering,2012;83:54–60. [Google Scholar]
- 27. Macht RH,Van Orden K,Wong K, et al. Utilizing activity trackers as a novel strategy to increase postoperative ambulation and evaluate the impact of mobility on bariatric surgery outcomes.Surg Endosc 2016;30:S478. [Google Scholar]
- 28. Robison T,Widemon R,Blake T, et al. Remote, continuous monitoring of patient mobility after discharge: A marker for 30-day readmission.J Neuro 2016;124:A1178–A9. [Google Scholar]
- 29. Rudner J,McDougall C,Sailam V,Smith M,Sacchetti A.. Interrogation of patient smartphone activity tracker to assist arrhythmia management.Ann Emerg Med 2016;683:292–4. [DOI] [PubMed] [Google Scholar]
- 30. Kansagara D,Englander H,Salanitro A, et al. Risk prediction models for hospital readmission: A systematic review.JAMA 2011;30615:1688–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.