Abstract
Context
Mutations in genes encoding for the succinate dehydrogenase (SDH) complex are linked to hereditary paraganglioma syndromes. Paraganglioma syndrome 3 is associated with mutations inSDHC and typically manifests as benign, nonfunctional head and neck paragangliomas.
Design
We describe a case of a 51-year-old woman who initially presented with diarrhea and hypertension and was found to have a retroperitoneal mass, which was resected with a pathology consistent with paraganglioma. Five years later, her symptoms recurred, and she was found to have new retroperitoneal lymphadenopathy and lytic lesions in the first lumbar vertebral body and the right iliac crest, which were visualized on CT scan and octreoscan but not on iodine-123-meta-iodobenzylguanidine (123I-MIBG) and bone scans. She had significantly elevated 24-hour urine norepinephrine and dopamine. The patient received external beam radiation and a series of different antineoplastic agents. Her disease progressed, and she eventually expired within 2 years. Genetic testing revealed a heterozygousSDHC c.43C>T, p.Arg15X mutation, which was also detected in her daughter and her grandson, both of whom have no biochemical or imaging evidence of paraganglioma syndrome yet.
Conclusion
We report a unique case of functional, metastatic abdominal paraganglioma associated withSDHC germline mutation. Our case exemplifies thatSDHC germline mutation has variable penetrance, which may manifest with an aggressive biology that could be missed by a123I-MIBG scan.
We report a unique case of functional, metastatic abdominal paraganglioma associated with SDHC germline mutation. Immunohistochemistry and sequencing analysis data were included.
Paragangliomas are rare, highly vascular neoplasms that arise from widely dispersed, specialized neural crest chromaffin cells that are associated with autonomic ganglia and have the ability to secrete catecholamines. The majority of paragangliomas are sporadic, but ∼30% of them are associated with an inherited syndrome (1). Mutations in the genes encoding different subunits of the succinate dehydrogenase (SDH) enzyme complexes, which are responsible for oxidative metabolism and electron transfer, have been linked to hereditary paragangliomas (2). There are five SDHx (SDHA, SDHB, SDHC, SDHD, and SDHAF2) genes, accounting for five distinct hereditary paraganglioma syndromes that are characterized by an autosomal-dominant inheritance pattern but with variable penetrance (3). Mutations in theSDHC locus are rare and usually manifest with isolated, benign, nonfunctional (parasympathetic), and asymptomatic head and neck paragangliomas (3). Our case exemplifies a distinctive variant—functional, metastatic, abdominal paraganglioma associated with anSDHC mutation.
Case
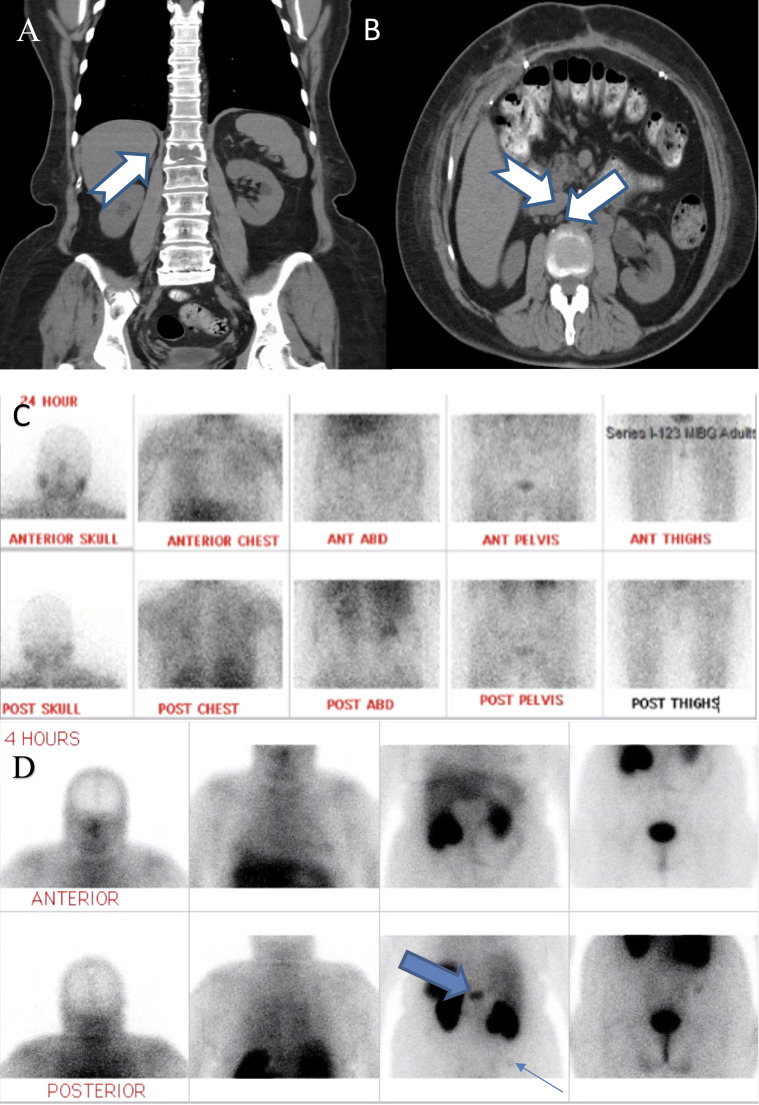
A 51-year-old woman with a medical history remarkable for hypothyroidism and cervical intraepithelial neoplasia grade 1 presented in 2005 with hypertension and diarrhea. CT scan of the abdomen revealed a 4.4-cm retroperitoneal mass. Hormonal workup is not available. Family history was positive for bladder cancer in her father but negative for paraganglioma/pheochromocytoma or gastrointestinal stromal tumors. Her mother and sister had uncontrolled hypertension diagnosed at a young age. Complete resection of the mass revealed characteristics of a paraganglioma without substantial mitotic activity or capsular and angiolymphatic invasion (Fig. 1A). Postoperative scans until 2008 showed no evidence of relapse. However, the patient developed recurrent hypertension, abdominal pain, anxiety, and diaphoresis in 2010, 5 years after the initial diagnosis. Twenty-four-hour urine catecholamines were as follows: norepinephrine 2770 μg/24 h [normal (nl) 0 to 140 μg/24 h], dopamine 3083 μg/24 h (nl 65 to 610 μg/24 h), and epinephrine 7 μg/24 h (nl 0 to 32 μg/24 h). CT scan of the abdomen/pelvis revealed lytic lesions in the first lumbar vertebral body (Fig. 2A) and right iliac crest (not pictured), as well as new retroperitoneal para-aortic and paravena caval lymphadenopathy (Fig. 2B). CT scan of the head, neck, and chest was unremarkable. Iodine-123-meta-iodobenzylguanidine (123I-MIBG) and bone scans were negative (Fig. 2C), whereas111In pentetreotide (Octreoscan) revealed foci of radiotracer activity within the first lumbar vertebral body and right iliac crest (Fig. 2D). Her hypertension improved with initiating prazosin, followed by labetalol. The patient received external beam radiation and a series of octreotide. She also received everolimus that was later switched into sunitinib and then temozolomide, which did not prevent disease progression, and she expired in 2012.
Figure 1.
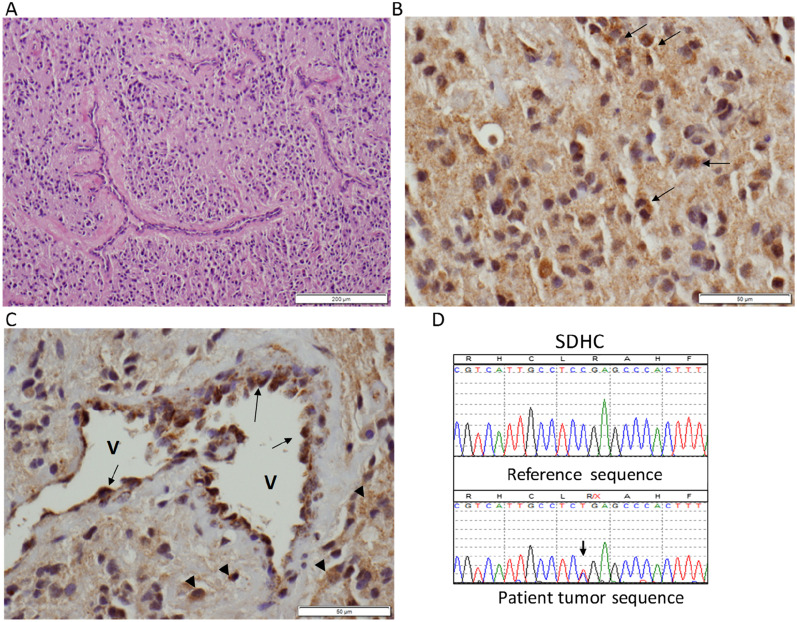
(A) Hematoxylin and eosin–stained photograph showing the paraganglioma with admixed thin-walled vessels. (B) High magnification of a representative area of the paraganglioma following SDHB immunohistochemistry (IHC): most tumor cells in the field show diffuse weak staining, and rare tumor cells have the more typical “granular” signal of SDHB staining (arrows). (C) Another high-magnification field of SDHB IHC showing negative or weak diffuse staining in the tumor, with a few scattered cells with a stronger, positive granular signal (arrowheads). Note strongly positive cells with granular appearance lining a vessel (V) wall (arrows). Original scale bars are shown at right bottom corners. (D) Sequence trace of the patient’s paraganglioma DNA (lower) showing the SDHC mutation c.43C>CT, p.R15X (arrow). There is no clear evidence of loss of heterozygosity in the tumor.
Figure 2.
(A) Coronal view of CT abdomen (ABD) and pelvis without contrast revealing first lumbar vertebral lytic bone lesion (arrow). (B) Transverse view of the same CT scan revealing extensive retroperitoneal lymphadenopathy (arrows). (C) Negative iodine-123-meta-iodobenzylguanidine (I-123 MIBG) scan despite clinical and biochemical evidence of recurrent paraganglioma. (D) Octreotide scan revealing foci of increased radiotracer activity within the first lumbar vertebral body (thick arrow) and faintly at right iliac crest (thin arrow; 300 pixels per inch). ANT, anterior; POST, posterior.
The germline mutation screen was negative for SDHB and SDHD gene mutations but was positive for a heterozygous SDHC c.43C>T (p. Arg15X) mutation. Immunohistochemistry (IHC) for SDHB, performed at ARUP Laboratories (Salt Lake City, UT), based on previously established protocols (4), revealed a heterozygous pattern, with most tumor cells showing a weakly diffuse cytoplasm staining (Fig. 1B) and few areas containing positive cells with granular cytoplasmic signal (Fig. 1C), whereas the tumor vascular network displays strong granular staining (Fig. 1C). This pattern is consistent with deficient SDH function. To investigate further the functional consequence of the SDHC mutation, we performed sequencing analysis of the SDHC exon 2, obtained from formalin-fixed, paraffin-embedded paraganglioma and processed it as we have described (5). This analysis confirmed the Arg15X mutation but revealed no evidence of loss of heterozygosity (Fig. 1D), suggesting incomplete loss of the wild-type allele as a result of tumor heterogeneity, and/or of technical limitation caused by the presence of admixed nontumor cells (Fig. 1A). The sameSDHC mutation was detected in germline DNA of her 36-year-old daughter and 9-year-old grandson, who remain clinically, biochemically, and radiologically free of paraganglioma. Of interest, the grandson has congenital hypothyroidism.
Discussion
We herein report a case of functional, metastatic abdominal paraganglioma associated with SDHC germline mutation. Paraganglioma syndrome 3 is associated with SDHC mutations and classically presents with isolated, asymptomatic, benign, nonfunctional head and neck paragangliomas (3). The first case of malignant paraganglioma with distant metastasis associated with aSDHC germline mutation, c.397>T (p.Arg133X), was described in 2014 by Bickmanet al. (6). The location of the primary tumor was in the thorax, and it was nonfunctional (6). In December 2015, Richet al. (7) reported a case of malignant sympathetic paraganglioma in a patient with a deletion of the SDHC gene. In 2016, Isaacsonet al. (8) reported a case of the SDHC mutation manifesting as a locally aggressive, catecholamine-producing, temporal bone paraganglioma associated with increased hypoxia-inducible factor 2α expression.
Our case is very distinctive from the classical presentation of paraganglioma syndrome 3 syndrome and the previously reported cases by the location of the primary tumor in the abdomen, its malignant and progressive nature, and its sympathetic functionality. A high false-negative rate (29% to 44%) of123I-MIBG imaging is frequently observed in metastatic tumors with unfavorable prognosis, as seen in hereditary paraganglioma syndrome, as a result of the SDHB mutation (9). Limited sensitivity is attributable to medication (labetalol, calcium channel blockers, and tricyclic antidepressants), interference of MIBG uptake, small size of tumor, and dedifferentiation with loss of norepinephrine transporters that are essential for MIBG uptake (9). Fluorodeoxyglucose-positron emission tomography and111In pentetreotide (octreoscan) appear to be more sensitive than either123I-MIBG or CT for the detection of metastatic disease in SDHB-positive paragangliomas (10). That also might be true in SDHC-positive metastatic paragangliomas, as it was in our case.
The SDHC Arg15X mutation has been previously reported in patients with paraganglioma and is predicted to be pathogenic (11). This mutation was found on the genetic screen of the index patient’s daughter and grandson, although neither has evidence of paraganglioma. Negative or weak SDHB IHC is typically associated with loss of function of any of the SDH subunit genes (4). This patient’s tumor had a predominant, weakly diffuse cytoplasmic staining, which has been reported in some tumors with SDH mutations, especiallySDHD, and is compatible with deficient SDH function, expected to occur after an early SDHC truncation (4,12). The lack of clear loss of heterozygosity in the paraganglioma may result from tumor heterogeneity with partial loss of the wild-type allele. Loss of heterozygosity detection could have been further confounded by admixed nontumor cells (e.g., from extensive vascularity). Although the germline SDHC mutation is likely the primary cause of the paraganglioma in this patient, this tumor may have acquired additional changes that contributed to its unusually aggressive behavior and poor outcome. Limitation in the amount of tumor DNA prevented a more comprehensive genetic screening to exclude the coexistence of other pathogenic mutations.
All mutation carriers of hereditary paraganglioma syndrome should undergo periodic surveillance. However, the nature of this surveillance should take into account the particular gene affected, according to the genotype-phenotype relationships, as well as considerations of penetrance and potential severity of disease. Prospective studies to guide the clinician in the frequency and type of testing are lacking. However, when an SDHx mutation (including SDHC) is identified in a relative of a proband, periodic biochemical and whole-body imaging studies are indicated. The surveillance has been negative in the patient’s daughter and grandson thus far.
Conclusion
Our case is unique in that our patient had a pathogenicSDHC germline mutation with a metastatic abdominal paraganglioma that was functional (sympathetic). Like SDHB-related paraganglioma, a false-negative123I-MIBG scan may be encountered in SDHC-related paraganglioma, especially if the tumor follows an aggressive behavior.
Acknowledgments
Financial Support: P.L.M.D. is supported by a grant from the US National Institutes of Health (GM114102), and S.K.F. is a recipient of a National Research Service Award Institutional Predoctoral Training Grant (T32CA148724).
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- 123I-MIBG
iodine-123-meta-iodobenzylguanidine
- IHC
immunohistochemistry
- nl
normal
- SDH
succinate dehydrogenase
References
- 1. Fishbein L,Merrill S,Fraker DL,Cohen DL,Nathanson KL. Inherited mutations in pheochromocytoma and paraganglioma: why all patients should be offered genetic testing.Ann Surg Oncol.2013;20(5):1444–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Astuti D,Hart-Holden N,Latif F,Lalloo F,Black GC,Lim C,Moran A,Grossman AB,Hodgson SV,Freemont A,Ramsden R,Eng C,Evans DG,Maher ER. Genetic analysis of mitochondrial complex II subunits SDHD, SDHB and SDHC in paraganglioma and phaeochromocytoma susceptibility.Clin Endocrinol (Oxf).2003;59(6):728–733. [DOI] [PubMed] [Google Scholar]
- 3. Welander J,Soderkvist P,Gimm O. Genetics and clinical characteristics of hereditary pheochromocytomas and paragangliomas.Endocr Relat Cancer.2011;18(6):R253–R276. [DOI] [PubMed] [Google Scholar]
- 4. Papathomas TG,Oudijk L,Persu A,Gill AJ,van Nederveen F,Tischler AS,Tissier F,Volante M,Matias-Guiu X,Smid M,Favier J,Rapizzi E,Libe R,Currás-Freixes M,Aydin S,Huynh T,Lichtenauer U,van Berkel A,Canu L,Domingues R,Clifton-Bligh RJ,Bialas M,Vikkula M,Baretton G,Papotti M,Nesi G,Badoual C,Pacak K,Eisenhofer G,Timmers HJ,Beuschlein F,Bertherat J,Mannelli M,Robledo M,Gimenez-Roqueplo AP,Dinjens WN,Korpershoek E,de Krijger RR. SDHB/SDHA immunohistochemistry in pheochromocytomas and paragangliomas: a multicenter interobserver variation analysis using virtual microscopy: a multinational study of the European Network for the Study of Adrenal Tumors (ENS@T).Mod Pathol.2015;28(6):807–821. [DOI] [PubMed] [Google Scholar]
- 5. Deng Y,Flores SK,Cheng Z,Qin Y,Schwartz RC,Malchoff C,Dahia PLM. Molecular and phenotypic evaluation of a novel germlineTMEM127 mutation with an uncommon clinical presentation.Endocr Relat Cancer.2017;24(11):L79–L82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bickmann JK,Sollfrank S,Schad A,Musholt TJ,Springer E,Miederer M,Bartsch O,Papaspyrou K,Koutsimpelas D,Mann WJ,Weber MM,Lackner KJ,Rossmann H,Fottner C. Phenotypic variability and risk of malignancy in SDHC-linked paragangliomas: lessons from three unrelated cases with an identical germline mutation (p.Arg133*).J Clin Endocrinol Metab.2014;99(3):E489–E496. [DOI] [PubMed] [Google Scholar]
- 7. Rich T,Jackson M,Roman-Gonzalez A,Shah K,Cote GJ,Jimenez C. Metastatic sympathetic paraganglioma in a patient with loss of the SDHC gene.Fam Cancer.2015;14(4):615–619. [DOI] [PubMed] [Google Scholar]
- 8. Isaacson B,Bullova P,Frone M,Click A,Hamplova B,Rabaglia J,Woodruff S,Nwariaku F,Kathuria A,Pacak K,Ghayee HK. An aggressive temporal bone SDHC paraganglioma associated with increased HIF-2α signaling.Endocr Pract.2016;22(2):190–195. [DOI] [PubMed] [Google Scholar]
- 9. van der Harst E,de Herder WW,Bruining HA,Bonjer HJ,de Krijger RR,Lamberts SW,van de Meiracker AH,Boomsma F,Stijnen T,Krenning EP,Bosman FT,Kwekkeboom DJ. [(123)I]metaiodobenzylguanidine and [(111)In]octreotide uptake in begnign and malignant pheochromocytomas.J Clin Endocrinol Metab.2001;86(2):685–693. [DOI] [PubMed] [Google Scholar]
- 10. Duet M,Sauvaget E,Pételle B,Rizzo N,Guichard JP,Wassef M,Le Cloirec J,Herman P,Tran Ba Huy P. Clinical impact of somatostatin receptor scintigraphy in the management of paragangliomas of the head and neck.J Nucl Med.2003;44(11):1767–1774. [PubMed] [Google Scholar]
- 11. Burnichon N,Rohmer V,Amar L,Herman P,Leboulleux S,Darrouzet V,Niccoli P,Gaillard D,Chabrier G,Chabolle F,Coupier I,Thieblot P,Lecomte P,Bertherat J,Wion-Barbot N,Murat A,Venisse A,Plouin PF,Jeunemaitre X,Gimenez-Roqueplo AP;PGL.NET network . The succinate dehydrogenase genetic testing in a large prospective series of patients with paragangliomas.J Clin Endocrinol Metab.2009;94(8):2817–2827. [DOI] [PubMed] [Google Scholar]
- 12. Santi R,Rapizzi E,Canu L,Ercolino T,Baroni G,Fucci R,Costa G,Mannelli M,Nesi G. Potential pitfalls of SDH immunohistochemical detection in paragangliomas and phaeochromocytomas harbouring germlineSDHx gene mutation.Anticancer Res.2017;37(2):805–812. [DOI] [PubMed] [Google Scholar]