Abstract
Context
The effects of energy-balanced bed rest on metabolic flexibility have not been thoroughly examined.
Objective
We investigated the effects of 21 days of bed rest, with and without whey protein supplementation, on metabolic flexibility while maintaining energy balance. We hypothesized that protein supplementation mitigates metabolic inflexibility by preventing muscle atrophy.
Design and Setting
Randomized crossover longitudinal study conducted at the German Aerospace Center, Cologne, Germany.
Participants and Interventions
Ten healthy men were randomly assigned to dietary countermeasure or isocaloric control diet during a 21-day bed rest.
Outcome Measures
Before and at the end of the bed rest, metabolic flexibility was assessed during a meal test. Secondary outcomes were glucose tolerance by oral glucose tolerance test, body composition by dual energy X-ray absorptiometry, ectopic fat storage by magnetic resonance imaging, and inflammation and oxidative stress markers.
Results
Bed rest decreased the ability to switch from fat to carbohydrate oxidation when transitioning from fasted to fed states (i.e., metabolic inflexibility), antioxidant capacity, fat-free mass (FFM), and muscle insulin sensitivity along with greater fat deposition in muscle (P < 0.05 for all). Changes in fasting insulin and inflammation were not observed. However, glucose tolerance was reduced during acute overfeeding. Protein supplementation did not prevent FFM loss and metabolic alterations.
Conclusions
Physical inactivity triggers metabolic inflexibility, even when energy balance is maintained. Although reduced insulin sensitivity and increased fat deposition were observed at the muscle level, systemic glucose intolerance was detected only in response to a moderately high-fat meal. This finding supports the role of physical inactivity in metabolic inflexibility and suggests that metabolic inflexibility precedes systemic glucose intolerance.
Bed-rest–induced physical inactivity triggers metabolic inflexibility, which can be used as an early marker of metabolic disturbances developed in response to the adoption of sedentary behaviors.
Physical inactivity is responsible for 6% to 10% of major noncommunicable diseases and the second leading cause of death in the United States (1). Many epidemiological studies have provided convincing but indirect evidence of associations between daily low-level physical activity with glucose intolerance or insulin resistance and changes in the ratio of fat mass (FM) to fat-free mass (FFM). Though growing in number, data from interventional inactive studies remain scarce, and underlying mechanisms remain to be fully delineated.
Metabolic diseases share some common underlying mechanisms, such as insulin resistance, systemic inflammation, and oxidative stress (2). More recently, metabolic inflexibility, or the inability of the body to adjust nutrient oxidation to changes in nutrient availability (3), has been recognized as a core component of metabolic diseases (4–7). By reviewing data obtained during physical activity or exercise studies and bed-rest studies, we developed a theoretical construct that metabolic inflexibility is triggered by decreased physical activity (8,9). This theory is supported by a subsequent retrospective analysis of longitudinal intervention studies manipulating the level of physical activity in both lean and overweight adults, suggesting that habitual physical activity predicts metabolic flexibility (MF) (10). However, in most of these studies energy balance changed to some extent. Whether metabolic inflexibility is induced by physical inactivityper se or by the associated positive energy balance (i.e., increases in FM and loss of FFM) remains to be answered.
In that respect, although FM can be controlled by diet, FFM changes that occur during sedentary behavior are more complex. The observed loss in FFM plays a confounding role on metabolic regulation, a common feature of type 2 diabetes, obesity, and sarcopenia. Exercise, vibration, and amino acid supplementation, alone or combined, have been used to mitigate these changes during bed rest, but to date none has fully prevented the adverse effects. Whey protein has been shown to stimulate protein synthesis during bed rest (11,12) and prevent hyperinsulinemia (13), the increase in plasma lipids (14), and ectopic storage in the liver (15). It also improves insulin sensitivity in older ambulatory adults (16). It is not known whether supplementation with whey protein during bed rest could offset the decrement in MF proposed in our theoretical construct.
The purpose of this study was to prospectively determine whether physical inactivity, independent of detectable changes in energy balance, triggers metabolic inflexibility in healthy individuals and whether metabolic inflexibility precedes or follows the whole-body glucose intolerance systematically reported during bed rest. We hypothesized that after 21 days of bed rest there would be a smaller variance in substrate oxidation after an oral glucose tolerance test (OGTT) and a challenge test meal, indicative of decreased MF. We also hypothesized that the development of glucose intolerance along with metabolic inflexibility could be prevented with whey protein supplementation through preservation of FFM.
Material and Methods
Participants
Ten healthy, active men with a body mass index between 20 and 25 kg/m2 participated in a 21-day bed-rest study (Table 1). Participants were free of any history of diabetes mellitus, hyperlipidemia, hyperglycemia, hyperthyroidism, or hypothyroidism and were nonsmokers, with no excessive alcohol or drug consumption. One participant completed the control treatment but dropped out before the protein treatment; results are presented for nine participants. The study was approved by the ethics commission of the Ärztekammer Nordrhein (Düsseldorf, Germany) and conducted in accordance with ethical principles of the Declaration of Helsinki.
Table 1.
Anthropometry and Metabolic Variables, Inflammation, and Oxidative Stress Markers
| Control (n = 9) | Protein (n = 9) | Statistics | |||||
|---|---|---|---|---|---|---|---|
| Characteristics | Baseline | Bed Rest | Baseline | Bed Rest | BR Effect | Trt Effect | BR*Trt Effect |
| Age, y | 31.0 ± 2.1 | 31.0 ± 2.1 | |||||
| Height, m | 1.80 ± 0.02 | 1.80 ± 0.02 | |||||
| BMI, kg/m2 | 23.8 ± 0.5 | 23.6 ± 0.5 | 23.9 ± 0.5 | 23.6 ± 0.5 | 0.02 | 0.81 | 0.97 |
| BM and body composition | |||||||
| BM, kg | 77.2 ± 1.9 | 76.3 ± 1.9 | 77.3 ± 1.6 | 76.4 ± 1.5 | 0.02 | 0.91 | 0.97 |
| FM, kg | 18.6 ± 1.3 | 18.0 ± 1.1 | 17.7 ± 1.1 | 18.1 ± 1.1 | 0.72 | 0.61 | 0.56 |
| % FM | 23.8 ± 1.1 | 23.5 ± 1.0 | 22.9 ± 1.0 | 23.5 ± 1.0 | 0.49 | 0.38 | 0.47 |
| FFM, kg | 59.0 ± 1.3 | 58.4 ± 1.1 | 59.0 ± 1.0 | 58.5 ± 0.8 | 0.03 | 0.34 | 0.55 |
| Leg lean BM, kg | 19.5 ± 0.6 | 18.9 ± 0.5 | 19.3 ± 0.5 | 18.8 ± 0.4 | <0.001 | 0.91 | 0.60 |
| Liver fat content, % | 3.2 ± 0.2 | 2.8 ± 0.2 | 2.9 ± 0.2 | 2.9 ± 0.2 | <0.01 | 0.52 | 0.07 |
| Calf fat content, % | 4.5 ± 0.1 | 4.8 ± 0.2 | 4.6 ± 0.2 | 4.8 ± 0.2 | 0.04 | 0.80 | 0.59 |
| Subcutaneous adipose tissue, pixels | 8573.7 ± 980.3 | 8049.1 ± 841.7 | 8887.0 ± 1057.5 | 7646.0 ± 1021.0 | 0.01 | 0.41 | 0.34 |
| Visceral adipose tissue, pixels | 3878.0 ± 548.6 | 3538.4 ± 543.2 | 3254.7 ± 443.7 | 3477.6 ± 381.7 | 0.24 | 0.54 | 0.76 |
| Plasma fasting metabolic variables | |||||||
| Glucose, g/L | 0.84 ± 0.02 | 0.84 ± 0.02 | 0.86 ± 0.03 | 0.87 ± 0.03 | 0.68 | 0.10 | 0.76 |
| Insulin, mIU/L | 6.01 ± 0.73 | 6.09 ± 0.43 | 4.88 ± 0.49 | 5.55 ± 0.62 | 0.43 | 0.08 | 0.53 |
| QUICKI | 1.56 ± 0.13 | 1.75 ± 0.16 | 1.46 ± 0.07 | 1.56 ± 0.09 | 0.15 | 0.14 | 0.65 |
| Total glucose, mmol/L | 0.77 ± 0.08 | 0.81 ± 0.05 | 0.81 ± 0.08 | 0.77 ± 0.09 | 0.87 | 1.00 | 0.20 |
| NEFA, mmol/L | 0.33 ± 0.04 | 0.37 ± 0.05 | 0.33 ± 0.04 | 0.39 ± 0.04 | 0.07 | 0.46 | 0.83 |
| LDL/HDL cholesterol, % | 0.145 ± 0.015 | 0.151 ± 0.016 | 0.148 ± 0.014 | 0.165 ± 0.009 | 0.19 | 0.28 | 0.52 |
| Leptin, ng/mL | 8.92 ± 4.14 | 4.57 ± 0.90 | 7.55 ± 2.69 | 7.30 ± 3.06 | 0.19 | 0.71 | 0.24 |
| Total adiponectin, μg/mL | 5.03 ± 0.39 | 3.25 ± 0.31 | 5.07 ± 0.54 | 3.28 ± 0.43 | <0.001 | 0.83 | 0.99 |
| High-molecular-weight adiponectin, μg/mL | 2.87 ± 0.27 | 1.88 ± 0.18 | 2.80 ± 0.26 | 1.94 ± 0.26 | <0.001 | 0.98 | 0.60 |
| Plasma inflammation markers | |||||||
| ALT, IU/L | 14.13 ± 1.25 | 15.79 ± 1.13 | 15.53 ± 1.74 | 19.57 ± 2.41 | 0.06 | 0.12 | 0.41 |
| AST, (IU/L) | 19.23 ± 1.90 | 17.33 ± 1.27 | 19.83 ± 1.57 | 18.28 ± 1.46 | 0.04 | 0.39 | 0.83 |
| GGT, IU/L | 18.83 ± 2.94 | 18.29 ± 2.86 | 17.00 ± 2.34 | 17.69 ± 2.37 | 0.95 | 0.17 | 0.58 |
| CRP, mg/dL | 0.053 ± 0.014 | 0.054 ± 0.013 | 0.046 ± 0.011 | 0.043 ± 0.013 | 0.65 | 0.40 | 0.94 |
| Plasma oxidative stress markers | |||||||
| KRL, mmol of Trolox/L of plasma | 4.97 ± 0.20 | 4.71 ± 0.18 | 5.04 ± 0.18 | 4.53 ± 0.11 | 0.04 | 0.70 | 0.49 |
Data are means ± standard error of the mean. SignificantP values < 0.05 are indicated in bold.
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; BM, body mass; BMI, body mass index; BR, bed rest; CRP, C-reactive protein; GGT, gamma-glutamyl transpeptidase; HDL, high-density lipoprotein; KRL, Kit Radicaux Libres; LDL, low-density lipoprotein; NEFA, nonesterified fatty acid; QUICKI, quantitative insulin-sensitivity check index; Trt, treatment.
Study design
As detailed previously (17), the experimental protocol was a random crossover design where the participants completed 21 days in the control and protein supplementation conditions, separated by a minimum 4-month washout. During bed-rest periods participants remained in a supine position. In the protein treatment, participants received whey protein (0.6 g/kg body weight per day) supplementation, with potassium bicarbonate (90 mmol/d KHCO3) to offset the acidic effect of the whey protein (18). Participants resided at the facility for 7 days before and 5 days after each bed-rest period; bed resting was forbidden during the daytime, and walking was encouraged during these periods. Because the primary purpose was to address questions related to space science, the participants were at a −6° head-down tilt position for the duration of bed rest to mimic the vascular changes associated with microgravity (19). Before and at the end of the bed rest, glucose tolerance was assessed with an OGTT, MF was assessed in response to a standardized challenge test meal, the skeletal muscle insulin-signaling pathway was examined, skeletal muscle and liver fat content were measured by magnetic resonance imaging, and key plasma markers of inflammation and oxidative stress were measured (Supplemental Fig. 1). Detailed material and methods can be found in theSupplemental Materials and Methods.
Diet and energy balance
Diet was tightly controlled during the study by registered dietitians. Energy intake was 1.6 resting metabolic rate (RMR) in the baseline and recovery periods and 1.2 RMR during bed rest. RMR was measured by indirect calorimetry for 30 minutes after 30 minutes of rest. Based on the European Space Agency standardization plan, total energy intake (TEI) consisted of 56% carbohydrates, 30% lipids, and 14% protein intake (1.2 g/kg body weight/d) during baseline and recovery. Percentage of saturated fat intake was kept at 10% of total fat intake. During bed rest, protein intake was increased to 19% TEI, with an associated isocaloric reduction in carbohydrate intake to 51% TEI in the control condition, whereas protein intake was increased to 28% (1.2 g + supplementation of 0.6 g/kg body weight/d) along with an isocaloric decrease in lipid intake to 25% TEI in the protein condition. Detailed nutritional data were previously published (17).
Body mass and composition
Body mass (BM) was measured with an electronic scale after an overnight fast. FM and FFM were measured with a dual-energy X-ray absorptiometry scan. Liver and calf fat content were measured by magnetic resonance imaging (seeSupplemental Materials and Methods).
Glucose tolerance and insulin sensitivity
Glucose tolerance was assessed during a 75-g OGTT including 2.5 g of d7-glucose mixed in 300 mL of glucose solution (0.25 g/L) to measure exogenous glucose oxidation. Blood draws were collected every 30 minutes for 2 hours for glucose and insulin concentrations. The oxidation rate of exogenous glucose was inferred from the cumulative recovery of2H in water contained in blood samples, as previously described (20). A muscle insulin sensitivity index was estimated as the ratio between the rate of disappearance of glucose from the maximal glucose concentration and the average insulin concentration over the same period of time (21). The percentage of nonesterified fatty acid (NEFA) suppression divided by the total amount of insulin over the test period was used as an index of adipose tissue lipolysis.
MF
MF was assessed during a moderately high-fat, high-energy test meal (4 MJ or 52% RMR, 44% carbohydrate, 41% lipid, 15% protein). The meal consisted of a liquid meal mixed with colza and olive oil and 2.2 g d31-tripalmitic acid (99% enrichment) added to bread, butter, and raspberry jam. d31-Tripalmitic acid was used to assess changes in gastric emptying. Blood, breath, and urine samples were collected at baseline and every hour for 7 hours. Gas exchange by indirect calorimetry was performed for 30 minutes every hour and, with urinary nitrogen excretion, was used to measure nonprotein respiratory quotient (NPRQ) and total carbohydrate oxidation (22). Between 9 and 11 hours after the test meal and 2 hours after a snack (salad, 200 g bread, and a cereal bar), soleus muscle biopsies were collected. MF was assessed with different indices, as explained previously (8). In brief, we calculated the range of NPRQ and insulin over 7 hours and then calculated the mathematical variances of these parameters by using the seven postprandial time points and the fasting values. Data are presented as mean insulin variance as a function of mean NPRQ variance. Area under the curve (AUC) was calculated over 7 hours (AUC7H), over the first 3 hours (AUC0–3H), and over the last 4 hours (AUC3–7H) for plasma insulin, glucose, triglycerides, NEFA, incorporation of d31-tripalmitic acid in chylomicron triglyceride (CM-TG), and total glucose oxidation. These time periods were based on the triglyceride kinetics because the test meal was moderately high in lipids.
Gastric emptying
The incorporation of d31-tripalmitic acid in CM-TG was used to determine changes in gastric emptying potentially induced by the head-down tilt position. The incorporation involved lipoprotein separation by ultracentrifugation, extraction of lipids, separation of triglycerides, and derivation for gas chromatography–mass spectrometry measurements of both unlabeled and labeled palmitate concentration, as previously described (23).
Plasma metabolites, insulin, and inflammatory and oxidative stress markers
Plasma glucose total triglycerides, CM-TG, and NEFA concentrations were measured with colorimetric assays, whereas insulin was analyzed by enzyme-linked immunosorbent assay. The ratio between low-density lipoprotein (LDL) and high-density lipoprotein (HDL) cholesterol was measured with lipoprotein electrophoresis. Specific hepatic inflammation markers [i.e. alanine aminotransferase (ALT), aspartate aminotransferase (AST), gamma-glutamyl transpeptidase (GGT), C-reactive protein (CRP), and fibrinogen] were measured with standard techniques. Antioxidant capacity was evaluated by using the Kit Radicaux Libres (KRL) and Reserves Défenses Antioxydantes tests as previously described (24).
Gene expression
Gene expression was quantified with microfluidic real-time polymerase chain reaction (RT-PCR) for key proteins involved in the insulin signaling pathway, glucose uptake, and glycogen synthesis (IRS1, PIK3R1, PKB, GSK3B, PRKCA, GLUT4, MAP2K1, MAPK1), as previously described (25). Additional details are provided inSupplemental Materials and Methods.
Statistical analysis
Data are presented as means or AUC ± standard error of the mean (SEM). Analyses were performed with SAS software, with significance set at 0.05. After having tested the absence of carryover effect, we analyzed the data with a linear mixed effects model controlling for repeated measures over bed rest and along periods when appropriate, with period, bed rest, treatment, and bed-rest × treatment interactions as fixed effects and a compound symmetry within-subject variable. There was no statistical difference at baseline.
Associations between variable changes over bed rest were tested with a linear mixed effects model controlling for repeated measures along periods, withR2 being calculated with a likelihood test.
Results
Body composition and organ fat content
Bed rest significantly decreased BM through a significant decrease in FFM, particularly leg lean BM (Table 1). FM and the percentage of FM remained stable, indicating the maintenance of energy balance. However, a change in fat deposition was observed; whereas the percentage fat content decreased in liver, it increased in the calf. Similarly, intra-abdominal fat content did not change, whereas subcutaneous fat dropped. No treatment or interaction effects were noted. This finding suggests that muscle ectopic fat storage occurs even in energy-balanced conditions.
Cardiometabolic, inflammatory, and oxidative stress markers
No change was observed in fasting plasma triglycerides, leptin, NEFA, and LDL/HDL cholesterol ratio (Table 1).
GGT and CRP remained unchanged during bed rest. Although the ALT/AST ratio significantly increased, the ratio was far below the threshold of 2.0 commonly used to indicate clinical nonalcoholic fatty liver. KRL significantly decreased after bed rest, indicating a lower global antioxidant capacity. Altogether these data suggest that although systemic inflammation is not apparent, an onset may have been triggered despite a decrease in hepatic fat content.
Glucose tolerance and insulin sensitivity
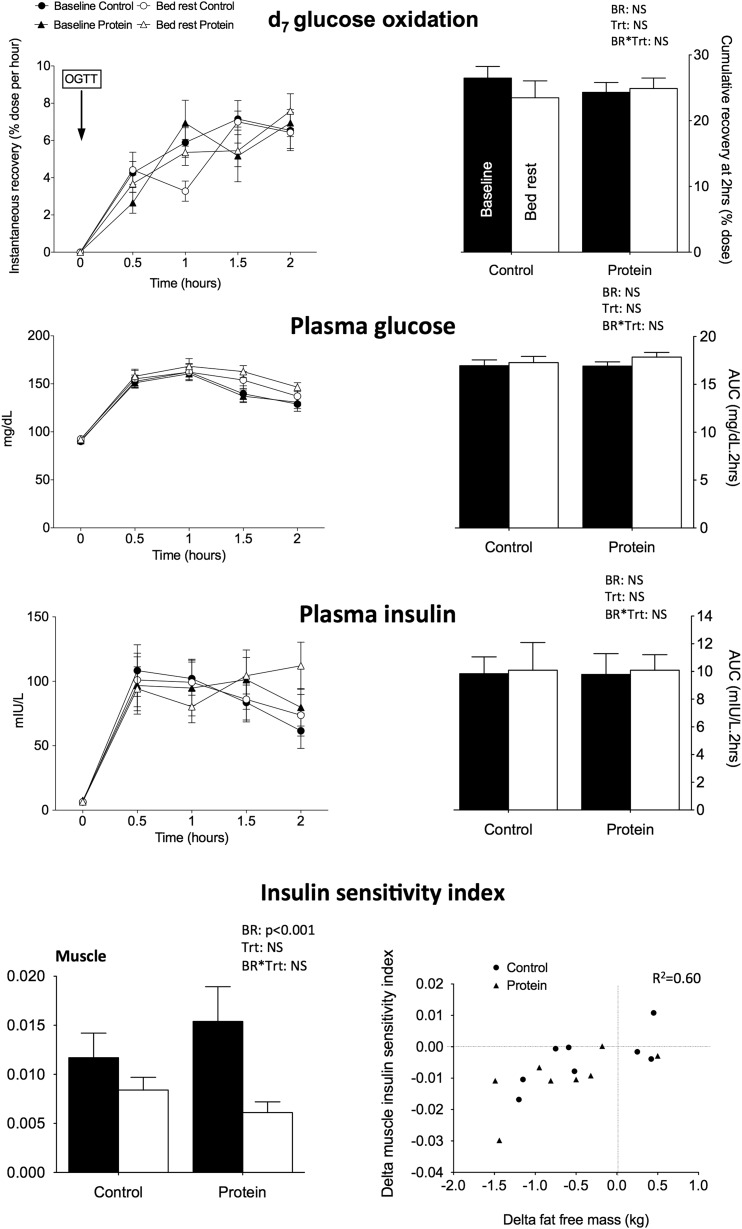
Neither fasting plasma glucose and insulin nor the quantitative insulin-sensitivity check index (QUICKI) were modified by bed rest, and no differences were noted between the two treatments (Table 1). Similarly, the 2-hour AUCs for glucose, insulin, and exogenous glucose oxidation were similar before and after bed rest (Fig. 1), indicating no significant change in whole-body glucose tolerance. The liver insulin sensitivity index (data not shown) was not decreased by bed rest. However, the decrease in both total and high-molecular-weight adiponectin (Table 1) along with the significant reduction in the muscle insulin sensitivity index suggests reduced insulin sensitivity regardless of the diet provided. No association with the changes in FM was noted. By combining data from the two conditions, we observed a positive association between bed-rest–induced changes in FFM and muscle insulin sensitivity index (R2 = 0.60;P < 0.001) (Fig. 1).
Figure 1.
Glucose tolerance after OGTT. Mean kinetics of d7 glucose oxidation and plasma insulin and glucose of the 9 participants are presented for control and protein treatment at baseline and after 21 days of bed rest after an OGTT at 75 mg. AUCs are presented on the right side of the figure. OGTT-derived muscle insulin sensitivity index derived from plasma insulin and glucose is presented as calculated from Abdul-Ghaniet al. (21) at the right bottom of the figure. Data are means ± SEM. Statistical analyses were performed with a linear mixed effects model adjusted for period effect and controlled for repeated measures. Results are presented asP values for bed rest (BR), treatment (Trt), and BR*Trt effects.P < 0.05 is considered significant. The relationship between delta insulin sensitivity index and delta FFM is presented at the left bottom of the figure. NS, nonsignificant.
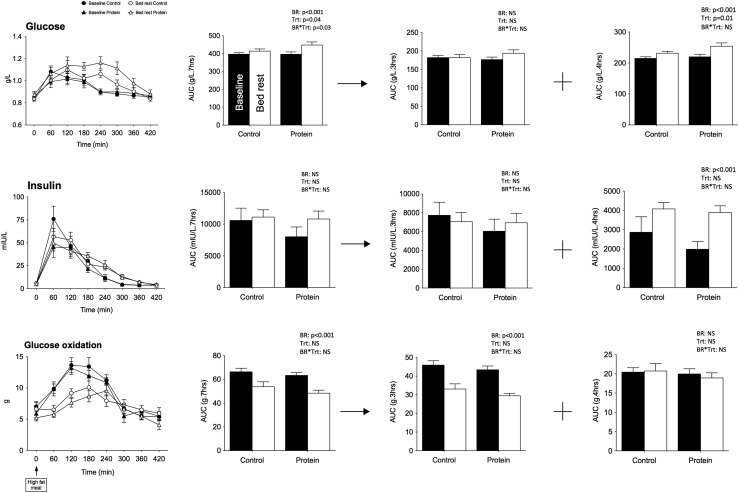
Metabolic responses to the challenge test meal
After the test meal, postprandial insulin AUC7H was not affected by bed rest (Fig. 2). However, postprandial glucose concentration significantly rose, whereas total carbohydrate oxidation dropped. There was no bed-rest × treatment interaction for carbohydrate oxidation, with postprandial glucose AUC7H increasing three times more in the protein than in the control treatment (P < 0.02), suggesting a lower glucose uptake, a greater competition between substrates with whey protein supplementation, or an increase inde novo gluconeogenesis from amino acids for oxidation.
Figure 2.
Plasma glucose, insulin, and glucose oxidation after lipid challenge. Mean kinetics of plasma glucose, insulin, and glucose oxidation and corresponding AUCs after a standardized fat meal (40% fat intake as energy) of the 9 participants are presented for the control and protein treatments at baseline and after bed rest. AUCs are presented over the 7-hour test and also for the first 3 hours and the last 4 hours of the test. Data are means ± SEM. Statistical analyses were performed with a linear mixed effects model adjusted for period effect and controlling for repeated measures. Results are presented asP values for bed rest (BR), treatment (Trt), and BR*Trt effects.P < 0.05 is considered significant. The relationship between delta insulin sensitivity index and delta FFM is presented at the left bottom of the figure. NS, nonsignificant.
Interestingly, the decrease in total carbohydrate oxidation adjusted for FFM occurred during the first 3 hours of the test only and was followed by an increase in postprandial glucose in the next 4 hours, suggesting that lower peripheral glucose uptake contributed to higher glycemia in inactive conditions. This greater glycemia over the last 4 hours of the test was concomitant with an increase in insulin AUC3–7H, suggesting the development of glucose intolerance had begun but was detectable only in late postprandial conditions when stimulated by a fat meal.
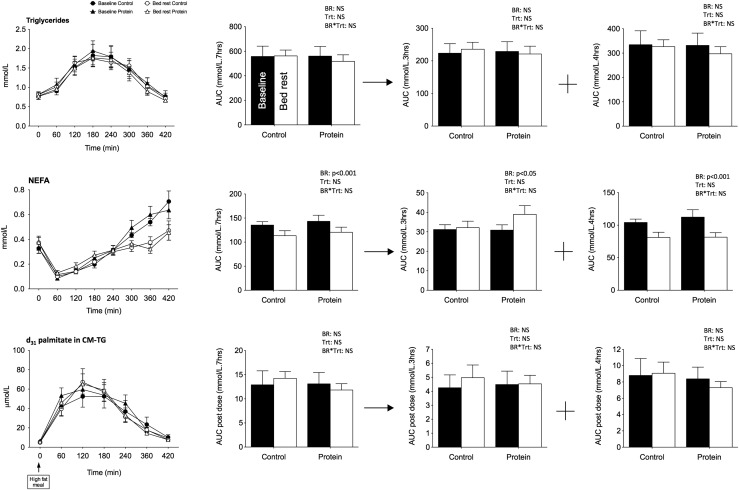
Bed rest did not change the time course of postprandial triglyceride AUC7H but led to a significant reduction in total NEFA AUC7H in both treatments (Fig. 3). In fact, postprandial NEFA AUC0–3H significantly increased, with the increase tending to be higher in the protein treatment (interaction = 0.08), whereas the NEFA AUC3–7H significantly decreased in both conditions. These changes cannot be attributed to modifications in time taken for food to enter the small intestine, given that the rate of appearance of d31-tripalmitic acid in CM-TG, a marker of gastric emptying, was not changed. Rather, they may be caused by the higher insulin concentration maintaining adipose tissue lipolysis inhibited or by a greater use of fat as fuel.
Figure 3.
Plasma triglycerides, nonesterified fatty acids, and d31 palmitate enrichment in chylomicrons after lipid challenge. Mean kinetics of plasma triglycerides and NEFA and d31 palmitate enrichment in chylomicrons observed after a standardized fat meal (40% fat intake as energy) in the 9 participants in control and protein treatments at baseline and after bed rest. AUCs are presented over 7 hours after the meal and for the first 3 hours and the last 4 hours after the meal. Data are means ± SEM. Statistical analyses were performed with a linear mixed effects model adjusted for period effect and controlled for repeated measures. Results are presented asP values for bed rest (BR), treatment (Trt), and BR*Trt effects.P < 0.05 is considered significant. NS, nonsignificant.
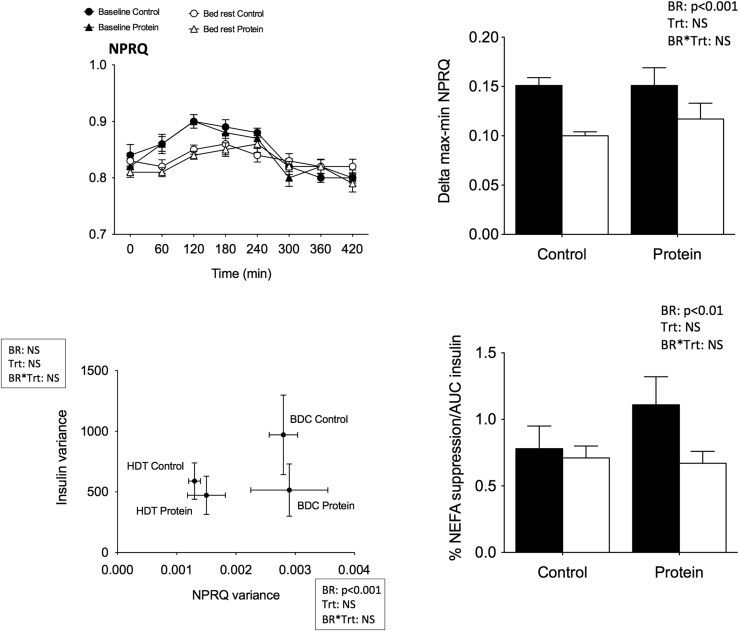
Independent of the condition, both peak to nadir values and NPRQ variances significantly decreased (Fig. 4), and insulin variance remained unchanged. This finding indicates that the lower shift from fat to carbohydrate oxidation from fasting to fed states was related mainly to metabolic disruptions at the muscle level. The percentage of NEFA suppression after meal ingestion was calculated between 0 and 60 minutes and normalized for insulin AUC7H. The values significantly decreased after bed rest in both conditions, suggesting a weaker response of adipose tissue to the effects of insulin.
Figure 4.
MF after lipid challenge. Upper left: Mean NPRQ kinetics over the 7 hours after ingestion of fat meal. Upper right: Mean delta max–min of NPRQ values. Lower left: Mean insulin variance values as a function of mean NPRQ variance calculated over the 7 hours after the meal. Lower right: Percentage NEFA suppression normalized by AUC insulin. Each graph represents the values from the 9 participants in the control and protein treatments at baseline (black) and after bed rest (white). Data are means ± SEM. Statistical analyses were performed with a linear mixed effects model adjusted for period effect and controlling for repeated measures. Results are presented asP values for bed rest (BR), treatment (Trt), and BR*Trt effects.P < 0.05 is considered significant. BDC, baseline data collection; HDT, head-down tilt bed rest; NS, nonsignificant.
Skeletal muscle insulin signaling pathway
Changes in gene expression of the key proteins were measured from skeletal muscle biopsies collected 2 hours after a snack (Table 2). In both conditions, IRS1 messenger RNA (mRNA) levels tended to decrease, whereas PIK3R2 mRNA levels tended to increase. Gene expression of all the other targeted proteins remained unchanged.
Table 2.
Microfluidic RT-PCR and Western Blot Analyses in Soleus Muscle
| Microfluidic RT-PCR Analyses (Relative mRNA Levels: Fold Change/Baseline) | Statistics | ||||
|---|---|---|---|---|---|
| Control (n = 8) | Protein (n = 9) | BR Effect | Trt Effect | BR*Trt Effect | |
| IRS1 | 0.68 ± 0.09 | 0.95 ± 0.17 | 0.06 | 0.19 | 0.15 |
| PIK3R1 | 0.95 ± 0.11 | 1.12 ± 0.11 | 0.71 | 0.28 | 0.31 |
| PIK3R2 | 1.04 ± 0.15 | 1.24 ± 0.07 | 0.11 | 0.26 | 0.26 |
| PKB | 0.96 ± 0.13 | 0.91 ± 0.06 | 0.31 | 0.79 | 0.70 |
| GSK3B | 0.96 ± 0.08 | 1.01 ± 0.07 | 0.74 | 0.58 | 0.61 |
| PRKCA | 1.08 ± 0.18 | 0.96 ± 0.09 | 0.84 | 0.74 | 0.53 |
| GLUT4 | 0.87 ± 0.15 | 0.89 ± 0.09 | 0.15 | 0.94 | 0.90 |
| MAP2K1 | 1.05 ± 0.15 | 0.96 ± 0.09 | 0.95 | 0.62 | 0.62 |
| MAPK1 | 1.02 ± 0.14 | 0.98 ± 0.07 | 0.96 | 0.96 | 0.81 |
Data are means ± SEM.
Abbreviations: GLUT4, glucose transporter type 4; GSK3B, glycogen synthase kinase 3 beta; IRS1, insulin receptor 1; MAP2K1, mitogen-activated protein kinase kinase 1; MAPK1, mitogen-activated protein kinase 1; PIK3R1, phosphoinositide-3 kinase regulatory subunit 1; PIK3R2, rhosphoinositide-3 kinase regulatory subunit 2; PKB, protein kinase B; pPKB, phosphorylated protein kinase B; PRKCA, protein kinase C-alpha.
Discussion
We have shown that, in lean healthy men, physical inactivity triggers metabolic inflexibility, even when energy balance is maintained. Although a decreased insulin sensitivity index and increased fat deposition were observed at muscle level, systemic glucose intolerance was detected only in response to a moderately high-fat, high-energy standard meal. This finding supports our hypothesis that physical inactivity and sedentary behaviors are determinants of metabolic inflexibility (8,9) and that metabolic inflexibility probably precedes the development of glucose intolerance when energy balance is maintained. This finding also suggests that subtle changes in glucose intolerance may not be apparent when energy balance is maintained unless challenged with acute overfeeding, but metabolic inflexibility may be an effective early indicator of metabolic abnormalities. Metabolic inflexibility could be a biomarker for glucose intolerance and increased risk of metabolic disease that could be used in preventive strategies.
In a review of MF and insulin resistance, Galganiet al. (26) suggested that a number of additional metabolic challenges, besides the clamp, should be considered in the assessment of MF. For example, the fuel shifts in response to dietary challenges varying in macronutrient composition could be considered. We recently extended their statement by ascribing to the view that any paradigm that aims to assess MF must have three components: stressor, regulator, and effector (9). It is the allostatic relationship between the regulator and effector in response to the stressor that is measured experimentally and informs on MF. In this study, the stressor was the moderately high-fat, high-energy test meal, the regulator was insulin, and the effector was NPRQ. By using this approach, we demonstrated that physical inactivity leads to metabolic inflexibility. These data support our previous work suggesting that habitual physical activity predicts MF (10). Because metabolic inflexibility is increasingly recognized as one of the causes of obesity and related metabolic diseases (9), our findings support the key role of physical inactivity and sedentary behaviors in weight gain.
The absence of clear evidence of whole-body glucose intolerance and insulin resistance was surprising. Insulin resistance has been repeatedly observed during bed-rest studies regardless of sex (23,27–29), age (8), duration (27,28), and phenotype (30,31). It has been detected with a number of indices and methods to assess insulin sensitivity or glucose tolerance, including insulin/glucose ratio, homeostatic model assessment, and QUICKI, during an OGTT, intravenous glucose tolerance test, or hyperinsulinemic euglycemic clamp or in response to standardized meals (see review in8). Given the observed positive association between the muscle index of insulin resistance and FFM, the modest loss of FFM in this bed rest may partly explain the discrepancy. Our participants lost on average half a kilo of FFM. By comparison, healthy men had lost 2.9 kg after 21 days (32) or 2.4 kg after 3 months of bed rest (28) in energy balance conditions. Modest decreases in FFM after only 7 days of bed rest were shown to lead to insulin resistance, but participants were in marked positive energy balance, as indicated by high levels of plasma leptin (33). In stable energy balance and in the absence of FM gain, metabolic disruption induced by bed rest (and physical inactivity) may be blunted.
Although the complex pathogenesis of glucose intolerance and insulin resistance is not fully understood, data accumulated in recent decades indicate that underlying mechanisms include systemic inflammation, oxidative stress, ectopic lipid deposition, intramuscular accumulation of lipid intermediates, vascular changes, and reduced mitochondrial content or oxidative capacity. Here, the antioxidative stress capacities of the body were altered, and the increase in ALT, known to be specific of liver damage, may represent an onset of liver metabolic dysfunction (34) despite a decrease in hepatic fat content and levels still in the normal range. The decrease in both total and high-molecular-weight adiponectin, known to play a role in regulating insulin sensitivity, despite constant whole-body FM supports a reduction in insulin sensitivity (35), even though we did not use the gold standard clamp technique. We also observed an impaired capacity to shift from fat oxidation in the fasting state to carbohydrate oxidation in the fed state, associated with a greater postprandial insulin concentration in response to a standard test meal, increased fat deposition in leg muscles, increased muscle insulin sensitivity index, and a minor alteration in the insulin-signaling pathway, as indicated by the slightly reduced muscle IRS1 mRNA. A recent report showed that 7 days of bed rest induced insulin resistance but did not lead to increased skeletal muscle lipid content (36). The repartitioning of fat we observed with an increase in muscle fat deposition suggests that muscle lipid accumulation probably takes >7 days, but direct measurements of muscle lipids are needed to confirm this assumption. Previous studies showed that bed rest induces a lower glucose disposal rate along with a decrease in glucose-mediated insulin pathway proteins GLUT4, hexokinase 2, or glycogen synthase at the muscle level (37) but also altered fatty acid transport into muscle and fat oxidation (23,28). A recent report indicated that 20 days of bed rest reduced insulin sensitivity in association with altered muscle mitochondrial respiration (32). During overfeeding, these physical inactivity–induced metabolic adaptations, (i.e., lower glucose and fatty acid uptake along with altered mitochondrial function) are probably the cause of the observed inability of adjusting nutrient oxidation to change in nutrient availability (i.e., metabolic inflexibility). Altogether these results support an ongoing development of glucose intolerance, caused by simultaneous metabolic dysfunctions, that are not detected during stable energy balance but unmasked when during acute positive energy balance.
When a person is inactive, adequate diet composition is even more critical. Because glucose uptake is impaired and oxidative capacity is compromised, maintaining low carbohydrate and fat intake in favor of high protein intake is probably preferable. In this study, we tested the effect of whey protein supplementation, because it has shown positive impact on lipid and glucose metabolism, insulin sensitivity, and muscle atrophy (13,38). Overall we observed no significant effect of the whey protein supplementation on gene expression of the key protein involved in insulin-signaling pathways, suggesting no effect on the protein anabolism pathway. Another team similarly reported no positive effect of whey protein supplementation on key proteolysis markers in the same participants (39). Overall, whey protein supplementation did not prevent adverse metabolic adaptations to bed rest. On the contrary, postprandial glycemia was even greater with protein supplementation compared with the control condition. As shown recently, this adverse effect may be explained by the decrease in insulin-mediated glucose disposal induced by whey protein supplementation (40). Based on these data, whey protein supplementation does not seem to be a good countermeasure to bed rest.
Limitations must be acknowledged. The volunteers’ habitual daily fat intake was about 34% (17), whereas 30% of energy was provided as fat in the study. This lower-fat diet in addition to a stable energy balance may explain the decrease in liver fat content (41) and the absence of fasting whole-body glucose intolerance. Muscle biopsies were not performed under controlled insulin-stimulated conditions and were limited in both number and size. Finally, insulin sensitivity was not measured by the gold standard method of the euglycemic hyperinsulinemic clamp; because insulin resistance is a metabolic adaptation constantly observed during bed rest, this procedure did not seem necessary.
In conclusion, these data support the role of low levels of physical inactivity in metabolic inflexibility status, weight gain, glucose intolerance, and associated metabolic diseases. Under inactive conditions, metabolic alterations are minor as long as energy intake matches energy expenditure. However, when the body faces metabolic challenges with acute overfeeding, metabolic dysfunctions are unmasked. Because it has been clearly shown that free-living humans are unable to spontaneously decrease their energy intake low enough to match low levels of energy expenditure in free-living conditions (42), these findings feed the debate on the respective role of energy intake and physical activity in weight gain and associated metabolic diseases. Over a lifetime, sedentary people will experience a large number of overfeeding events during which the metabolic dysfunctions are fully at play, leading to weight gain.
Supplementary Material
Acknowledgments
We thank all the volunteers and the staff at the German space agency (DLR) who participated to the success of this bed-rest study.
Financial Support: The bed-rest study was funded by the European Space Agency (ESA) and the German Aerospace Center (DLR). The experiments outlined in this manuscript were supported by grants from the Centre National de la Recherche Scientifique and from the European (ESA) and French (CNES) space agencies. F.R. and A.D. are supported by PhD fellowships from CNES.
Clinical Trial Information: ClinicalTrials.gov no. NCT01655979 (registered 31 July 2012).
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- AUC
area under the curve
- BM
body mass
- CM-TG
chylomicron-triglyceride
- CRP
C-reactive protein
- FFM
fat-free mass
- FM
fat mass
- GGT
gamma-glutamyl transpeptidase
- HDL
high-density lipoprotein
- KRL
Kit Radicaux Libres
- LDL
low-density lipoprotein
- MF
metabolic flexibility
- mRNA
messenger RNA
- NEFA
nonesterified fatty acid
- NPRQ
nonprotein respiratory quotient
- OGTT
oral glucose tolerance test
- QUICKI
quantitative insulin-sensitivity check index
- RMR
resting metabolic rate
- RT-PCR
real-time polymerase chain reaction
- SEM
standard error of the mean
- TEI
total energy intake
References
- 1.The world health report 2002: reducing risk, promoting healthy life. 2002. Available at:http://www.who.int/whr/2002/en. Accessed 10 July 2009. [DOI] [PubMed]
- 2. Gratas-Delamarche A,Derbré F,Vincent S,Cillard J. Physical inactivity, insulin resistance, and the oxidative-inflammatory loop.Free Radic Res.2014;48(1):93–108. [DOI] [PubMed] [Google Scholar]
- 3. Kelley DE,Mandarino LJ. Fuel selection in human skeletal muscle in insulin resistance: a reexamination.Diabetes.2000;49(5):677–683. [DOI] [PubMed] [Google Scholar]
- 4. Astrup A.The relevance of increased fat oxidation for body-weight management: metabolic inflexibility in the predisposition to weight gain.Obes Rev.2011;12(10):859–865. [DOI] [PubMed] [Google Scholar]
- 5. Kelley DE,Goodpaster B,Wing RR,Simoneau JA. Skeletal muscle fatty acid metabolism in association with insulin resistance, obesity, and weight loss.Am J Physiol.1999;277(6 Pt 1):E1130–E1141. [DOI] [PubMed] [Google Scholar]
- 6. Kelley DE,Simoneau JA. Impaired free fatty acid utilization by skeletal muscle in non-insulin-dependent diabetes mellitus.J Clin Invest.1994;94(6):2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kelley DE,Mandarino LJ. Hyperglycemia normalizes insulin-stimulated skeletal muscle glucose oxidation and storage in noninsulin-dependent diabetes mellitus.J Clin Invest.1990;86(6):1999–2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bergouignan A,Rudwill F,Simon C,Blanc S. Physical inactivity as the culprit of metabolic inflexibility: evidence from bed-rest studies.J Appl Physiol (1985).2011;111(4):1201–1210. [DOI] [PubMed] [Google Scholar]
- 9. Rynders CA,Blanc S,DeJong N,Bessesen DH,Bergouignan A. Sedentary behaviour is a key determinant of metabolic inflexibility [published online ahead of print June 10, 2016].J Physiol. [DOI] [PMC free article] [PubMed]
- 10. Bergouignan A,Antoun E,Momken I,Schoeller DA,Gauquelin-Koch G,Simon C,Blanc S. Effect of contrasted levels of habitual physical activity on metabolic flexibility.J Appl Physiol (1985).2013;114:371–379. [DOI] [PubMed] [Google Scholar]
- 11. Stein TP,Donaldson MR,Leskiw MJ,Schluter MD,Baggett DW,Boden G. Branched-chain amino acid supplementation during bed rest: effect on recovery.J Appl Physiol (1985).2003;94(4):1345–1352. [DOI] [PubMed] [Google Scholar]
- 12. Ferrando AA,Paddon-Jones D,Wolfe RR. Alterations in protein metabolism during space flight and inactivity.Nutrition.2002;18(10):837–841. [DOI] [PubMed] [Google Scholar]
- 13. Pal S,Ellis V,Dhaliwal S. Effects of whey protein isolate on body composition, lipids, insulin and glucose in overweight and obese individuals.Br J Nutr.2010;104(5):716–723. [DOI] [PubMed] [Google Scholar]
- 14. Bortolotti M,Maiolo E,Corazza M,Van Dijke E,Schneiter P,Boss A,Carrel G,Giusti V,Lê KA,Quo Chong DG,Buehler T,Kreis R,Boesch C,Tappy L. Effects of a whey protein supplementation on intrahepatocellular lipids in obese female patients.Clin Nutr.2011;30(4):494–498. [DOI] [PubMed] [Google Scholar]
- 15. Hamad EM,Taha SH,Abou Dawood AG,Sitohy MZ,Abdel-Hamid M. Protective effect of whey proteins against nonalcoholic fatty liver in rats.Lipids Health Dis.2011;10(1):57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Turner KM,Keogh JB,Clifton PM. Dairy consumption and insulin sensitivity: a systematic review of short- and long-term intervention studies.Nutr Metab Cardiovasc Dis.2015;25(1):3–8. [DOI] [PubMed] [Google Scholar]
- 17. Buehlmeier J,Mulder E,Noppe A,Frings-Meuthen P,Angerer O,Rudwill F,Biolo G,Smith SM,Blanc S,Heer M. A combination of whey protein and potassium bicarbonate supplements during head-down-tilt bed rest: presentation of a multidisciplinary randomized controlled trial (MEP study).Acta Astronaut.2014;95:82–91. [Google Scholar]
- 18. Fettman MJ.Dietary instead of pharmacological management to counter the adverse effects of physiological adaptations to space flight.Pflugers Arch.2000;441(2–3,suppl)R15–R20. [DOI] [PubMed] [Google Scholar]
- 19. Belavý DL,Armbrecht G,Gast U,Richardson CA,Hides JA,Felsenberg D. Countermeasures against lumbar spine deconditioning in prolonged bed rest: resistive exercise with and without whole body vibration.J Appl Physiol (1985).2010;109(6):1801–1811. [DOI] [PubMed] [Google Scholar]
- 20. Beysen C,Murphy EJ,McLaughlin T,Riiff T,Lamendola C,Turner HC,Awada M,Turner SM,Reaven G,Hellerstein MK. Whole-body glycolysis measured by the deuterated-glucose disposal test correlates highly with insulin resistance in vivo.Diabetes Care.2007;30(5):1143–1149. [DOI] [PubMed] [Google Scholar]
- 21. Abdul-Ghani MA,Matsuda M,Balas B,DeFronzo RA. Muscle and liver insulin resistance indexes derived from the oral glucose tolerance test.Diabetes Care.2007;30(1):89–94. [DOI] [PubMed] [Google Scholar]
- 22. Frayn KN.Calculation of substrate oxidation rates in vivo from gaseous exchange.J Appl Physiol.1983;55(2):628–634. [DOI] [PubMed] [Google Scholar]
- 23. Bergouignan A,Trudel G,Simon C,Chopard A,Schoeller DA,Momken I,Votruba SB,Desage M,Burdge GC,Gauquelin-Koch G,Normand S,Blanc S. Physical inactivity differentially alters dietary oleate and palmitate trafficking.Diabetes.2009;58(2):367–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lesgards JF,Durand P,Lassarre M,Stocker P,Lesgards G,Lanteaume A,Prost M,Lehucher-Michel MP. Assessment of lifestyle effects on the overall antioxidant capacity of healthy subjects.Environ Health Perspect.2002;110(5):479–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pagano AF,Demangel R,Brioche T,Jublanc E,Bertrand-Gaday C,Candau R,Dechesne CA,Dani C,Bonnieu A,Py G,Chopard A. Muscle regeneration with intermuscular adipose tissue (IMAT) accumulation is modulated by mechanical constraints.PLoS One.2015;10(12):e0144230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Galgani JE,Moro C,Ravussin E. Metabolic flexibility and insulin resistance.Am J Physiol Endocrinol Metab.2008;295(5):E1009–E1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yanagibori R,Suzuki Y,Kawakubo K,Makita Y,Gunji A. Carbohydrate and lipid metabolism after 20 days of bed rest.Acta Physiol Scand Suppl.1994;616:51–57. [PubMed] [Google Scholar]
- 28. Bergouignan A,Schoeller DA,Normand S,Gauquelin-Koch G,Laville M,Shriver T,Desage M,Le Maho Y,Ohshima H,Gharib C,Blanc S. Effect of physical inactivity on the oxidation of saturated and monounsaturated dietary fatty acids: results of a randomized trial.PLoS Clin Trials.2006;1(5):e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Blanc S,Normand S,Pachiaudi C,Fortrat J-O,Laville M,Gharib C. Fuel homeostasis during physical inactivity induced by bed rest.J Clin Endocrinol Metab.2000;85(6):2223–2233. [DOI] [PubMed] [Google Scholar]
- 30. Alibegovic AC,Højbjerre L,Sonne MP,van Hall G,Stallknecht B,Dela F,Vaag A. Impact of 9 days of bed rest on hepatic and peripheral insulin action, insulin secretion, and whole-body lipolysis in healthy young male offspring of patients with type 2 diabetes.Diabetes.2009;58(12):2749–2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sonne MP,Alibegovic AC,Højbjerre L,Vaag A,Stallknecht B,Dela F. Effect of 10 days of bedrest on metabolic and vascular insulin action: a study in individuals at risk for type 2 diabetes.J Appl Physiol (1985).2010;108(4):830–837. [DOI] [PubMed] [Google Scholar]
- 32. Kenny HC,Rudwill F,Breen L,Salanova M,Blottner D,Heise T,Heer M,Blanc S,O’Gorman DJ. Bed rest and resistive vibration exercise unveil novel links between skeletal muscle mitochondrial function and insulin resistance.Diabetologia.2017;60(8):1491–1501. [DOI] [PubMed] [Google Scholar]
- 33. Blanc S,Normand S,Pachiaudi C,Duvareille M,Gharib C. Leptin responses to physical inactivity induced by simulated weightlessness.Am J Physiol Regul Integr Comp Physiol.2000;279(3):R891–R898. [DOI] [PubMed] [Google Scholar]
- 34. Ampuero J,Ranchal I,Gallego-Durán R,Pareja MJ,Del Campo JA,Pastor-Ramírez H,Rico MC,Picón R,Pastor L,García-Monzón C,Andrade R,Romero-Gómez M. Oxidized low-density lipoprotein antibodies/high-density lipoprotein cholesterol ratio is linked to advanced non-alcoholic fatty liver disease lean patients.J Gastroenterol Hepatol.2016;31(9):1611–1618. [DOI] [PubMed] [Google Scholar]
- 35. Li S,Shin HJ,Ding EL,van Dam RM. Adiponectin levels and risk of type 2 diabetes: a systematic review and meta-analysis.JAMA.2009;302(2):179–188. [DOI] [PubMed] [Google Scholar]
- 36. Dirks ML,Wall BT,van de Valk B,Holloway TM,Holloway GP,Chabowski A,Goossens GH,van Loon LJ. One week of bed rest leads to substantial muscle atrophy and induces whole-body insulin resistance in the absence of skeletal muscle lipid accumulation.Diabetes.2016;65(10):2862–2875. [DOI] [PubMed] [Google Scholar]
- 37. Biensø RS,Ringholm S,Kiilerich K,Aachmann-Andersen NJ,Krogh-Madsen R,Guerra B,Plomgaard P,van Hall G,Treebak JT,Saltin B,Lundby C,Calbet JA,Pilegaard H,Wojtaszewski JF. GLUT4 and glycogen synthase are key players in bed rest-induced insulin resistance.Diabetes.2012;61(5):1090–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. McGregor RA,Poppitt SD. Milk protein for improved metabolic health: a review of the evidence.Nutr Metab (Lond).2013;10(1):46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Blottner D,Bosutti A,Degens H,Schiffl G,Gutsmann M,Buehlmeier J,Rittweger J,Ganse B,Heer M,Salanova M. Whey protein plus bicarbonate supplement has little effects on structural atrophy and proteolysis marker immunopatterns in skeletal muscle disuse during 21 days of bed rest.J Musculoskelet Neuronal Interact.2014;14(4):432–444. [PubMed] [Google Scholar]
- 40. Smith GI,Yoshino J,Stromsdorfer KL,Klein SJ,Magkos F,Reeds DN,Klein S,Mittendorfer B. Protein ingestion induces muscle insulin resistance independent of leucine-mediated mTOR activation.Diabetes.2015;64(5):1555–1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Koch M,Borggrefe J,Schlesinger S,Barbaresko J,Groth G,Jacobs G,Lieb W,Laudes M,Müller MJ,Bosy-Westphal A,Heller M,Nöthlings U. Association of a lifestyle index with MRI-determined liver fat content in a general population study.J Epidemiol Community Health.2015;69(8):732–737. [DOI] [PubMed] [Google Scholar]
- 42. Westerterp KR.Physical activity, food intake, and body weight regulation: insights from doubly labeled water studies.Nutr Rev.2010;68(3):148–154. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.