NORMAL ESOPHAGEAL ANATOMY
The esophagus is a tubular organ, approximately 25 cm long, which connects the hypopharynx to the stomach. Along its course, it traverses 3 body areas: the neck, the chest, and the abdomen (Fig. 1A). Accordingly, it is subdivided into 3 anatomic segments: cervical, thoracic, and abdominal.
Fig. 1.
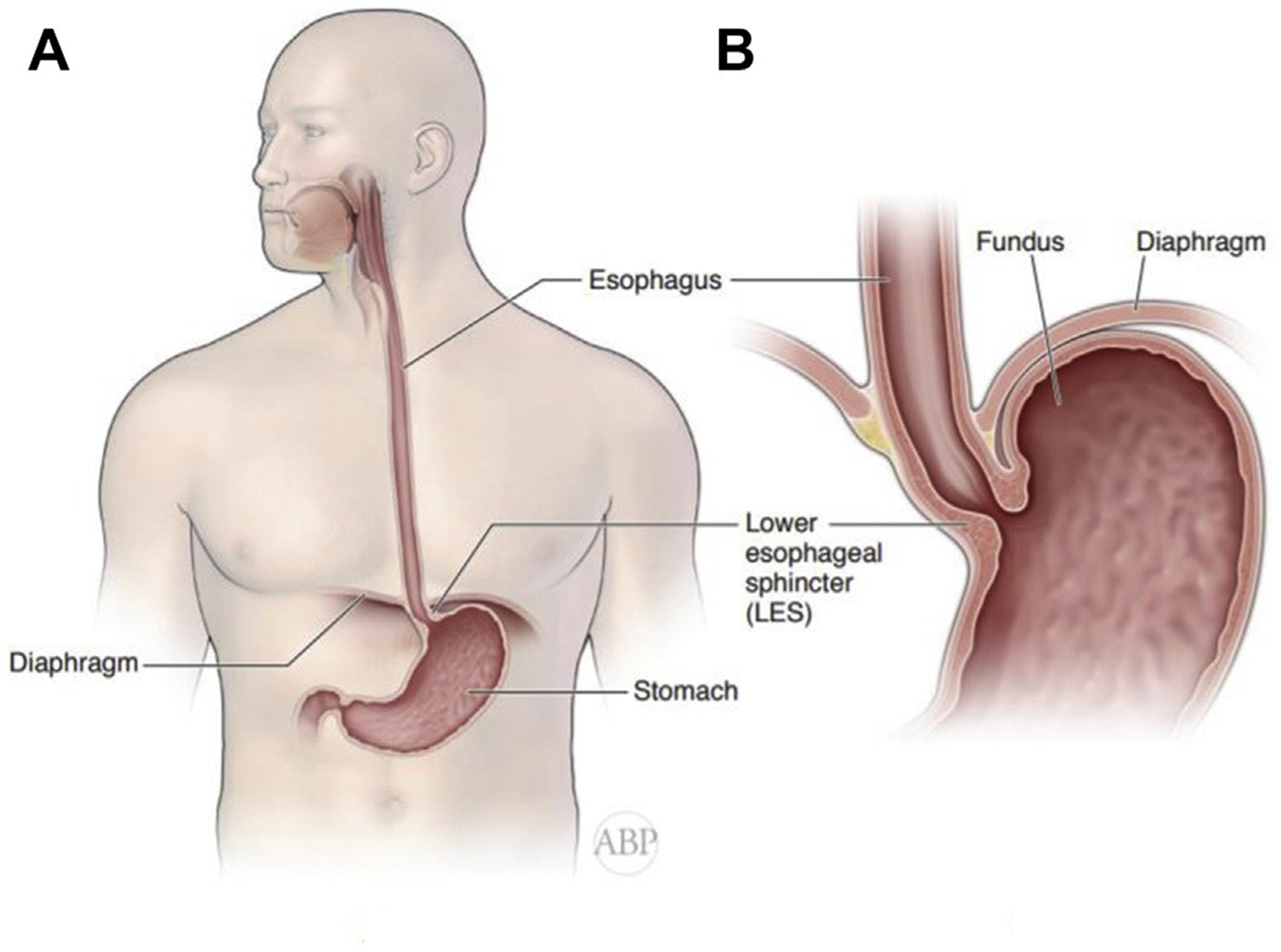
Anatomy of the esophagus and GEJ. (A) Position of the esophagus, with its locations in the neck, chest, and abdomen. (B) Diaphragm, esophageal hiatus and gastroesophageal junction.
The cervical segment of the esophagus starts at the hypopharynx, from which it is separated by the upper esophageal sphincter, at the level of the sixth cervical vertebra. The cervical esophagus extends down to the level of suprasternal notch. It is located directly behind and partially to the left of the trachea, in front of C6 and C7 vertebral bodies. Laterally, the esophagus is surrounded by the lower poles of the thyroid gland and the carotid sheaths. On the left side, the termination of the thoracic duct can be found entering the venous angle at the junction of the left internal jugular and subclavian veins.
The thoracic esophagus extends from the suprasternal notch to the diaphragmatic hiatus. It enters the chest through the thoracic inlet and is located initially in the superior and then the posterior mediastinum. The midesophagus deviates slightly to the right, usually passing behind the left mainstem bronchus and pericardium anterior to the prevertebral fascia of T1 though T10 vertebral bodies. Both vagus nerves accompany the esophagus throughout its course on each side. The descending thoracic aorta is located posteriorly and to the left, whereas the mediastinal pleura drapes over both sides of the esophagus.
The abdominal esophagus is a short segment of the organ, extending from the diaphragmatic hiatus at the level of T10 to the gastric cardia at the level of T11. After passing through the hiatus, the abdominal esophagus deviates to the left and enters the stomach, forming the gastroesophageal junction (GEJ). The descending aorta is located posterior to the GEJ, and the inferior vena cava is posterior and to the right. It is covered anteriorly by the left lobe of the liver.
In cross-section, the esophagus has 3 layers (mucosa, submucosa, and muscularis propria) surrounded by the adventitia. After crossing through the hiatus, the esophagus is enveloped by the visceral peritoneum, forming a serosa. The muscularis propria, formed in the proximal third of the esophagus by striated muscle, is replaced by smooth muscle in the distal esophagus. It consists of an inner circular layer and outer longitudinal layer. The circular muscle fibers are arranged in a helical fashion, sometimes giving rise to a corkscrew appearance in many motility disorders.1
The blood supply to the esophagus is segmental. The cervical esophagus is supplied by branches of the inferior thyroid arteries, whereas the thoracic esophagus is supplied by small aortoesophageal arteries, In addition to branches from the bronchial and superior phrenic arteries. The abdominal esophagus receives blood supply from branches of the left gastric artery, the splenic artery, and the left inferior phrenic artery. Venous drainage forms a submucosal plexus, draining the cervical segment through the inferior thyroid veins and the thoracic esophagus through the azygos system into the superior vena cava. The lower thoracic and abdominal esophagus is drained into the portal system via the gastric veins. In cases of portal hypertension, these lower esophageal veins can dilate, forming esophageal varices.
The lymphatic drainage also starts at the submucosal plexus, forming lymphatic channels that drain into the regional lymph nodes. The deep cervical nodes receive lymph flow from the cervical and the upper thoracic esophagus. The paraesophageal lymph nodes of the superior and posterior mediastinum drain the thoracic segment. The lower thoracic segment and the abdominal esophagus drain into the celiac and perigastric lymph nodes. There is significant overlap in the lymphatic drainage between these segments.
The intrinsic nervous system is formed by the submucosal and the intramuscular neural plexi. Innervation to the esophagus is supplied by both vagus nerves, providing the parasympathetic control of esophageal wall motility, the esophageal sphincters, and glandular secretions. Sympathetic innervation is provided by the sympathetic chains and is mostly responsible for vasomotor effects and pain sensation. The vagus nerves arise in the medulla oblongata as the tenth cranial nerves and after exiting the skull base run on each side of the esophagus. They enter the chest via the thoracic inlet and travel caudally along the esophagus to the hiatus. Because of the embryologic clock-wise rotation of the lower foregut in the abdomen, the left vagus nerve becomes anterior, whereas the right vagus nerve lies posteriorly.1,2
STOMACH
The stomach is a J-shaped saccular expansion of the foregut, serving as a reservoir for the ingested food at the beginning of the digestive process (Fig. 2A). It receives the food bolus from the esophagus, via the GEJ, below the esophageal hiatus. It transitions into the duodenum at the pylorus. On the right, the concave edge of the stomach is termed the lesser curvature, whereas the left convex edge is called the greater curvature. The peritoneal reflections, connecting the lesser curvature to the liver, form the lesser omentum, also called the gastrohepatic ligament. It contains branches of the left and right gastric arteries. Peritoneal reflections on the greater curvature side form the greater omentum and are subdivided by the site of the attachment into gastrophrenic, gastrosplenic, and gastrocolic ligaments. They contain both right and left gastroepiploic branches from the pancreaticoduodenal and the splenic arteries that form an arch along the antrum and the body of the stomach about a centimeter lateral to the stomach wall. The fundus is directly supplied by the branches of the short gastric vessels.
Fig. 2.
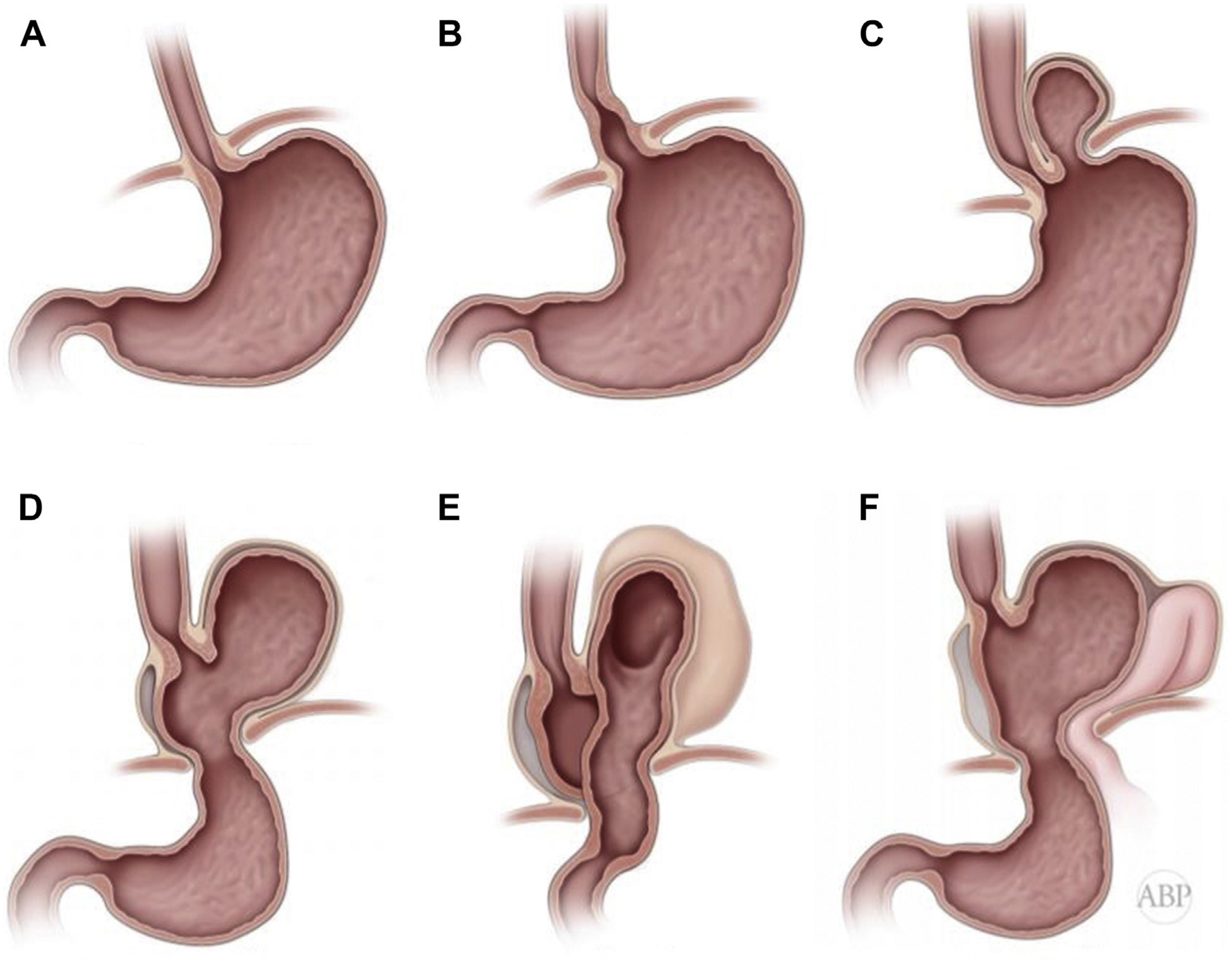
Types of paraesophageal hernias. (A) Normal anatomy of the GEJ. (B) Type I (sliding) hiatal hernia. (C) Type II paraesophageal hernia. The GEJ is retained at the hiatus and fundus has herniated into the mediastinum through a localized defect at the phrenoesophageal membrane, with formation of peritoneal hernia sac. (D) Type III paraesophageal hernia (herniation of the GEJ and various degrees of the gastric fundus and the body into posterior mediastinum, with formation of the peritoneal hernia sac). (E) Type III paraesophageal hernia with totally intrathoracic stomach, (F) Type IV paraesophageal hernia with herniation of the other intraabdominal organs in addition to the GEJ and various degrees of the gastric fundus and the body with formation of the hernia sac.
DIAPHRAGM
The diaphragm is a complex flat dome-shaped fibromuscular structure that separates the chest from the abdomen. It has a central fibrous tendon and muscular fibers peripherally, attaching to the lumbar vertebrae in the back, lower 6 ribs on both sides, and xyphoid process anteriorly. Contraction of the muscular fibers results in the vertical displacement of the fibrous center of the diaphragm, providing a major contribution to the respiratory cycle. Several important structures cross the diaphragm. The inferior vena cava enters the chest through the opening in the central tendon and is accompanied by small branches of right phrenic nerve. The aorta with the accompanying thoracic duct traverses the diaphragm through aortic hiatus between the diaphragmatic crura. The splanchnic nerves, sympathetic chains, hemiazygos vein, and internal thoracic artery travel through an additional minor opening in the diaphragm. The esophagus with both vagus nerves traverses the diaphragm through the esophageal hiatus.
THE ESOPHAGEAL HIATUS
The esophageal hiatus is a slit-like opening in the diaphragm that is formed by the thickened bundles of the right crus and has a reverse teardrop shape. The undersurface of the diaphragm is covered by the thin endoabdominal fascia, which forms the phrenoesophageal ligament at the hiatus. This ligament is a fibrotic sheath of connective tissue that inserts onto the adventitia of the distal esophagus, the GEJ, as well as the intraabdominal esophagus (Fig. 1B). Thus, the GEJ is usually located below the esophageal hiatus and is secured in position by the phrenoesophageal ligament.3,4
THE LOWER ESOPHAGEAL SPHINCTER AND ANATOMIC ANTIREFLUX SYSTEM
The thickening of the circular layer of the distal esophagus above the GEJ is called the lower esophageal sphincter (LES). The LES usually measures 2.5 to 4.5 cm in length, with the upper part lying within the hiatus and the lower part in the abdomen. The LES serves as a first component of the antireflux mechanism. Although not a true anatomic sphincter, it is defined as a high-pressure zone by manometry and is tonically contracted, providing a pressure gradient between the esophagus and the stomach. The short intraabdominal segment of the distal esophagus is exposed to the positive intraabdominal pressure and serves as an additional pressure barrier, aiding the LES in its antireflux function. The crura of the esophageal hiatus are another component, exerting lateral pinching of the distal esophagus, or the so-called pinchcock effect. In deep inspiration, pressure increases due to crural contraction when transdiaphragmatic pressure gradient also increases, preventing gastroesophageal reflux.5–7 The third component of the antireflux mechanism is the angle of His, a flap valve comprising of the fundus, draping along the left side of the abdominal esophagus at an acute angle, which is held in place by the phrenoesophageal ligament.8 The final physiologic mechanism for controlling reflux is the esophageal peristalsis. Mechanical and chemical receptors in the distal esophagus mediate esophageal clearance of intraluminal contents. Poor esophageal motility, in combination with acid reflux, may lead to esophagitis, which could further impair the function and the tone of the LES.9
PATHOPHYSIOLOGY OF HIATAL HERNIA DEVELOPMENT
Hiatal hernia occurs when a portion of the gastric cardia prolapses into the posterior mediastinum through the esophageal hiatus. This leads to misalignment of the LES and the crura, thus disrupting the protective anatomic defense mechanisms. Proximal migration of the LES transposes it into the negative pressure environment of the chest, diminishing the pressure gradient at the GEJ and obliterating the flap valve function of the angle of His. Moreover, lateral traction by the stretched phrenoesophageal membrane further compromises the LES function. Misalignment of the LES and the crura leads to pressure application on a funnel-like cardia, which tends to direct intragastric contents into the esophagus with the increase of intraabdominal pressure, promoting gastroesophageal reflux.1
The transdiaphragmatic pressure gradient, formed by the negative intrathoracic pressure (due to elastic recoil of the lung) and the positive intraabdominal pressure (due to the tonic contraction of the abdominal wall muscles), exerts a constant upward push on the GEJ. The deglutition process is associated with foreshortening of the esophagus. These, in addition to large-volume meals, distend the gastric fundus and lead to stretching and weakening the LES, an effect similar to that at the neck of an inflated balloon. Additional stressors on the phrenoesophageal ligament are associated with increased intraabdominal pressure, such as obesity, constipation, urinary obstruction, chronic obstructive pulmonary disease with chronic cough, and job-related heavy exertion. Restrictive lung disease may heighten the transdiaphragmatic pressure gradient owing to increased negative intrathoracic pressures. Impaired connective tissue integrity may also play a role in developing a paraesophageal hernia. Accordingly, paraesophageal hernias frequently present in elderly patients with comorbidities and degenerative diseases. It may also be associated with familial clustering, suggesting that genetic factors may also be a causal factor.1,10,11
TYPES OF HIATAL HERNIAS
Hiatal hernias are classified into 4 types according to the degree of the intrathoracic prolapse of the GEJ and its relationship to the herniated stomach (Fig. 2).1,12,13
Type I hiatal hernia is the most common type and represents more than 95% of all cases (see Fig. 2B).1,13 It is probably the first step in the continuum of the progressive distention of the phrenoesophageal membrane and separation of the GEJ and crural diaphragm. It is frequently termed a sliding hernia. Contrary to the common misperception, it is not because it goes up and down but rather because it is a hernia that is formed by the wall of the organ itself, without a hernia sac.
Type II paraesophageal hernias form due to a local defect of the phrenoesophageal membrane, usually in the left posterior aspect. A true hernia sac forms through this defect, with intrathoracic herniation of the gastric fundus. The GEJ in a true type II hernia is below the hiatus, likely held in place by residual intact portion of the phrenoesophageal ligament (see Fig. 2C). For this reason, many of these patients may not suffer from GERD as the reflux barrier is still mostly intact. These hernias are quite infrequent.
Type III paraesophageal hernia is the most common type of paraesophageal hernias, and represent the continuous stretching of the phrenoesophageal ligament with increasing hiatal dilation and formation of the peritoneal hernia sac. The GEJ, in addition to part or all of the gastric fundus and body, migrate up (see Fig. 2D). In an extreme case, a patient may develop a totally intrathoracic stomach. As the size of the hernia sac continues to enlarge with relative fixation of the terminal esophagus to the prevertebral fascia, the greater curvature slides up, flipping over in the form of both organoaxial and mesoaxial rotation. This results in a twisted upside-down stomach in which the pylorus may be higher than the GEJ and the greater curvature is to the right of the esophagus (see Fig. 2E).
Type IV paraesophageal hernias are extreme forms of this condition in which, in addition to the majority of the stomach, other organs, such as the omentum, transverse colon, small intestines, spleen, liver, and even retroperitoneal structures (including the pancreas) can herniate (see Fig. 2F).
RECURRENT PARAESOPHAGEAL HERNIAS
Recurrent paraesophageal hernias represent a particularly challenging situation because anatomic derangements occur in the previously operated field with loss of the normal tissue planes due to scarring. A clear understanding of the anatomic relationship requires extensive preoperative workup with functional and cross-sectional imaging, manometry, and direct visualization via endoscopy (Fig. 3F). Review of the previous operative record is mandatory for extrapolation of the anatomic relationships after a previous surgical procedure.
Fig. 3.
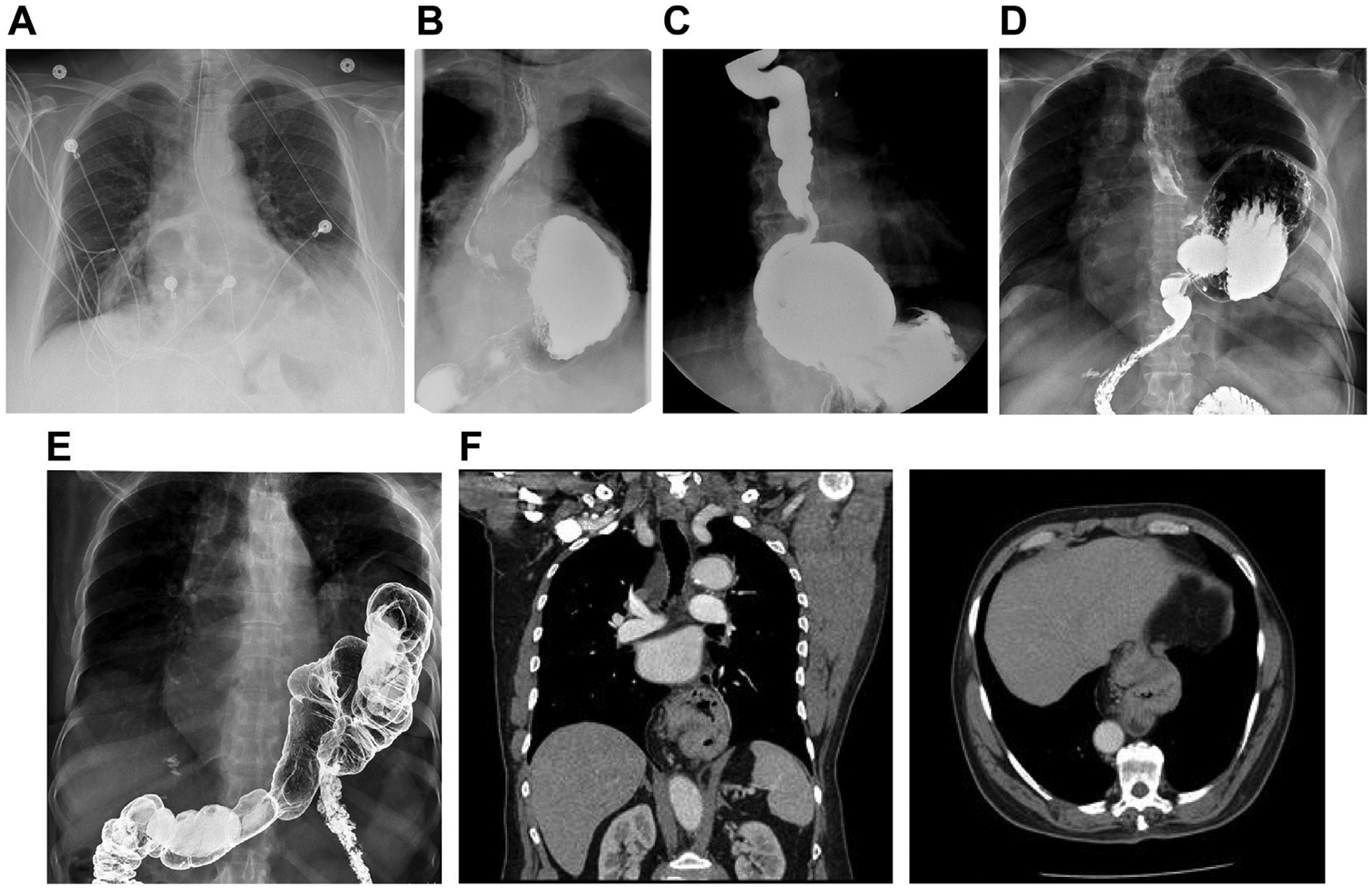
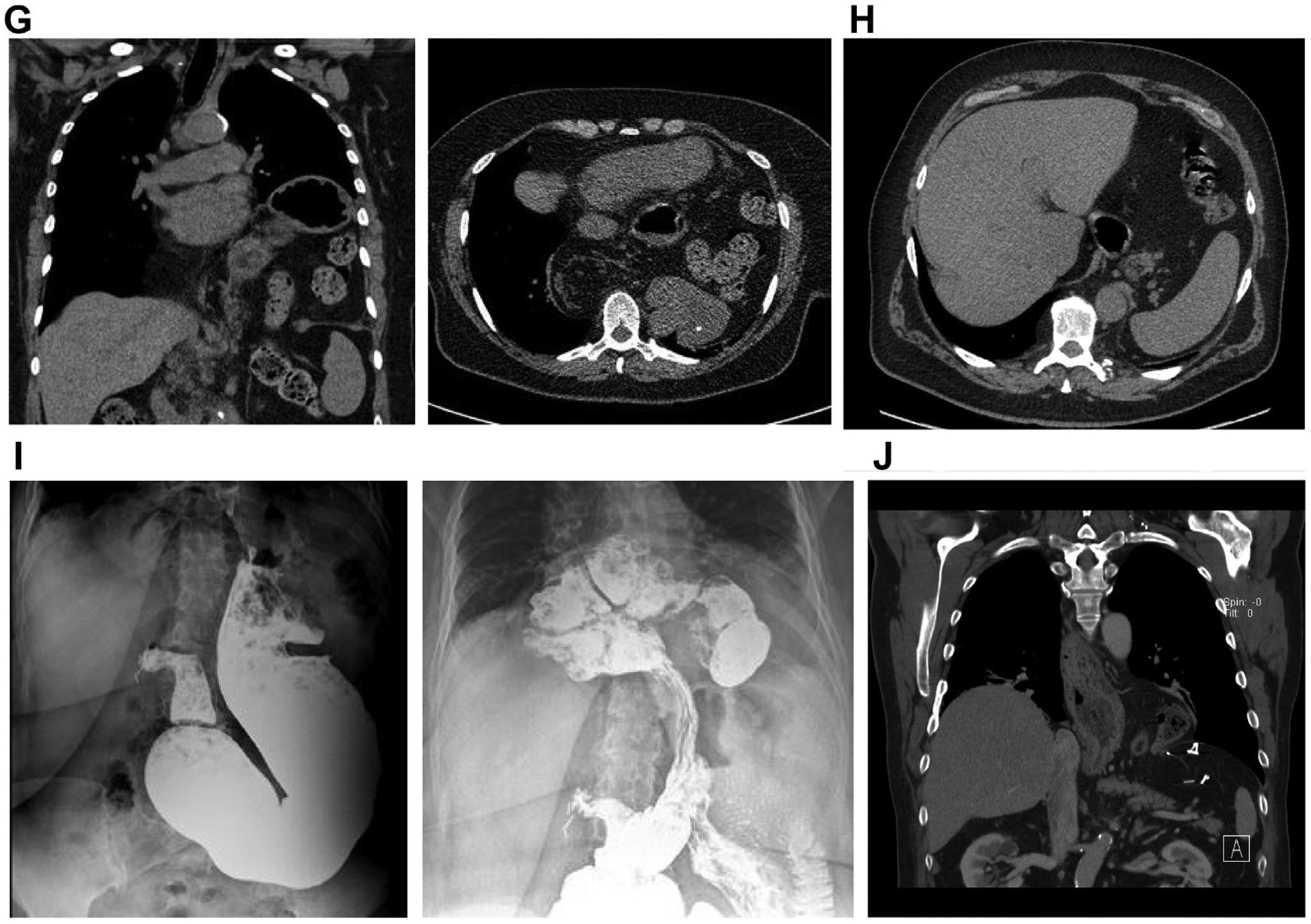
Radiologic evaluation of paraesophageal hernias. (A) Chest radiograph demonstrating retrocardiac lucency, consistent with paraesophageal hernia. (B) Esophagram, demonstrating type II paraesophageal hernia. Please note normal position of the GEJ. (C) Esophagram, demonstrating type III paraesophageal hernia. (D) Esophagram, demonstrating type III paraesophageal hernia with totally intrathoracic stomach. (E) Barium enema, demonstrating type IV paraesophageal hernia with intrathoracic colon (coronal reconstruction [left] and axial image [right]). (F) Computed tomography (CT) demonstrating type III paraesophageal hernia. (G) CT demonstrating type IV paraesophageal hernia, with totally intrathoracic stomach and colon (coronal reconstruction [left] and axial image [right]). (H) CT, demonstrating type IV paraesophageal hernia with a large posterior component and herniated retroperitoneum and pancreatic tail. (I) Esophagram, demonstrating recurrent paraesophageal hernia with posterior crural disruption, leading to small bowel herniation but normal position of the GEJ (gastric faze, showing normal intraabdominal position of the stomach [left] and intestinal faze, demonstrating transhiatal herniation of the small bowel [right]). (J) Paraconduit hernia in a patient after previous minimally invasive esophagectomy.
PARACONDUIT POSTESOPHAGECTOMY HIATAL HERNIAS
After esophagectomy, a neoesophageal conduit (tubularized stomach or colon) traverses the diaphragm. This creates a potential defect between the conduit and the edges of the hiatus, potentially leading to herniation of abdominal contents into the chest. These hernias can form on any side of the conduit, although it is the left posterior aspect of the hiatus that is more commonly herniated (Fig. 3J). Because these hernias have no hernia sac and the peritoneal cavity communicates directly with the pleural space, large segments of small and large intestines pass through the hiatus and translocate into the chest.14,15 Laparoscopic surgery is known to be associated with less fibrosis and fewer adhesions than traditional open procedures. The increasing adoption of minimally invasive techniques esophagectomy has likely led to an increase in the incidence of paraconduit hernias.
RADIOLOGICAL ANATOMY
Imaging studies are an important step in preoperative patient evaluation. Occasionally, the presence of a paraesophageal hernia can be suspected on a chest radiograph, with demonstration of retrocardiac air-fluid level or lucency (Fig. 3A). An esophagram with fluoroscopy allows a thorough assessment of the anatomy, the configuration of the gastrointestinal tract, and the swallowing function (Fig. 3B–D, I). Computed tomography provides further assessment of the size of the hernia and hiatal defect; configuration of the stomach; size and content of the hernia sac, with the degree of the adjacent structures compression; and involvement of the other intraabdominal organs (Fig. 3F–H, J).16–18 Additional studies might be indicated to assess the involvement of other organs (Fig. 3E).
ENDOSCOPIC ANATOMY
Endoscopy is mandatory in evaluation of all paraesophageal hernias before repair. Anatomic evaluation of the paraesophageal hernias during endoscopy includes assessment of the hernia size, status of the LES, and presence of complications, such as strictures, Barret esophagus, or even tumors.19 The retroflexion endoscopic view provides important observation into the type and grading of the GEJ.8 In patients with paraesophageal hernias, a Hill type IV junction is present, with the extrinsic impression of the dilated hiatal opening along with the various degree of herniated stomach.
Measurement of the paraesophageal hernia is accomplished by subtraction of the distance from the incisors to the Z-line from that of the incisors to the hiatus. Owing to distention of the stomach by the insufflated air, most of the organ pops back into the subdiaphragmatic position. In cases of large paraesophageal hernias with most of the Intrathoracic stomach in organoaxial or mesoaxlal rotation, it is sometimes difficult to find the hiatus and the passage into the subdiaphragmatic stomach. Any attempt at accurate length measurement In these cases can be unreliable (Fig. 4A–C).
Fig. 4.
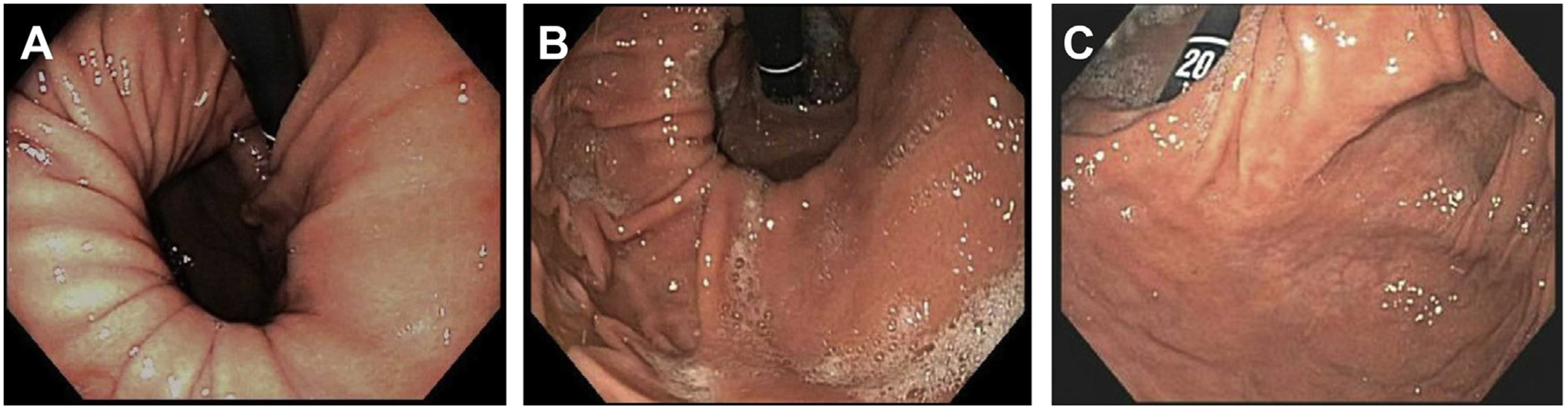
Endoscopic anatomy of paraesophageal hernias. (A) Endoscopy, demonstrating type II paraesophageal hernia on the retroflexion view. Please note normal position of the GEJ and herniation of the fundus. (B) Endoscopy, demonstrating type III paraesophageal hernia. (C) Endoscopy, demonstrating, type III paraesophageal hernia, with totally intrathoracic stomach. Please note location of gastric outlet next to GEJ due to organoaxial and mesoaxial rotation.
SURGICAL ANATOMY
Paraesophageal hernias may be approached from the abdomen or the chest. The most common modality is undoubtedly laparoscopic. However, in certain cases, an open laparotomy may be necessary. In case of multiple recurrences or a hostile abdomen from previous surgeries, the thoracic approach may be preferred. The surgeons must therefore familiarize themselves with the different appearance of the anatomy through every approach.
ANATOMIC EXPOSURE WITH LAPAROTOMY
An open hiatal hernia abdominal repair is usually performed through an upper midline laparotomy with extension above the xyphoid process. Effective elevation of the costal margin is required for adequate exposure. The left lobe of the liver covers the hiatus and is mobilized by dividing the left triangular ligament and retracting It to the right. This maneuver exposes both the central tendon and the esophageal hiatus of the diaphragm.
The hiatus is usually obviously dilated. The lesser omentum is stretched over the right crus and follows the lesser curvature into the mediastinum. After manual reduction of the stomach, the previously stretched phrenoesophageal membrane appears floppy after hernia reduction. It is grasped and opened, allowing visualization and circumferential mobilization of the esophagus. This is followed by division of the phrenoesophageal membrane and dissection of the sac.20,21
The esophagus is retracted anteriorly and to the left, exposing the posterior hiatus. The crura are approximated, leaving at least a 1-cm space between the esophageal wall and the edge of the hiatus. An antireflux procedure of choice is then performed before conclusion of the repair.22
TRANSTHORACIC ANATOMY
Transthoracic anatomy is usually accomplished through the left chest. Video-assisted thoracoscopic surgery (VATS) modification has not gained wide adoption and there are only limited reports of VATS.23,24 Robotic transabdominal modification of Belsey repair technique has also been described.25
The patient is positioned in the right lateral decubitus position and a left posterolateral thoracotomy is performed in seventh or eighth intercostal space. The inferior pulmonary ligament is divided and the lung is retracted superiorly. The descending aorta is visible in the field and the esophagus with the paraesophageal hernia is identified immediately anterior and medial to it. The left atrium is located anteriorly to the hernia sac. Opening of the hernia sac allows dissection of the crura. The esophagus is mobilized sufficiently to allow tension-free repair.
MINIMALLY INVASIVE, LAPAROSCOPIC ANATOMY
The laparoscopic approach has enjoyed wide acceptance owing to the benefits associated with minimally invasive surgery.22,26 Laparoscopy, either traditional or robotically enhanced, allows a significant advantage in visualization and magnification of the target anatomy, facilitating dissection and repair. With the camera usually placed in the left epigastric area, the viewing trajectory is right along the esophagus, clearly depicting the hiatus, gastroesophageal junction, esophagus, and structures of the posterior mediastinum.27–32
Exposure of the hiatus requires elevation of the left lobe of the liver. Either a Nathanson retractor, placed through the subxyphoid area, or a flexible retractor, placed through right flank port, is used for these purposes (Fig. 5A, B).13,33,34 The LiVac vacuum liver retractor (Livac Pty Ltd, Warrnambool, Australia) has recently been introduced into clinical practice for this purpose (Fig. 5C). Obese patients, especially men, may have a significantly enlarged and fragile left lobe of the liver due to a fatty liver and this is prone to injury and bleeding. This may complicate exposure of the relevant anatomy. Women, in contrast, more often have a thin and floppy left lobe of the liver, reaching over the spleen, which may require reapplication of the retractor throughout the procedure.
Fig. 5.
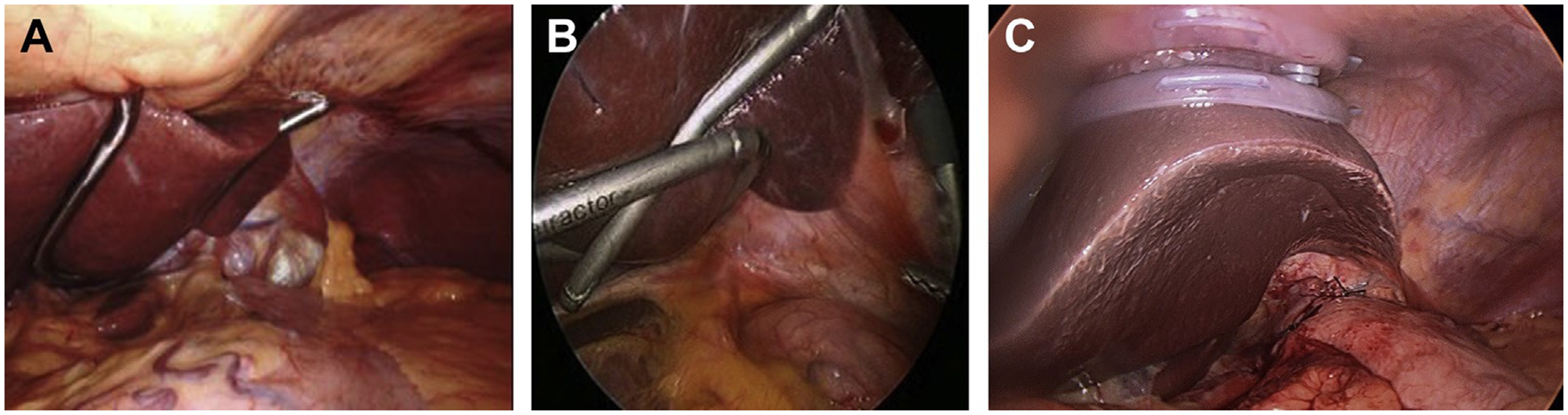
Liver retractors. (A) Nathanson liver retractor. (B) Flexible liver retractor. (C) LiVac retractor. (Courtesy of [A] Dr. Warner W. Wang, MD, Marietta, OH; and [C] Dr. Philip Gan, Livac Pty Ltd, Warrnambool, Australia.)
Again, the hiatus is dilated and is oval to round in shape (Fig. 6A). The phrenoesophageal membrane is stretched and is bulging into the mediastinum, augmented by positive intraabdominal pressure of the pneumoperitoneum. The stomach, unless incarcerated by adhesions, usually freely retracts into the abdomen. Occasionally, there is a posterior crural diastasis, accompanied by significant posterior component of the hernia sac. In such scenario, a large volume of the retroperitoneal fat is prolapsed into the chest in the form of a mediastinal lipoma. This can involve prolapse of the retroperitoneal organs (pancreas and spleen) causing the type IV hernia.
Fig. 6.
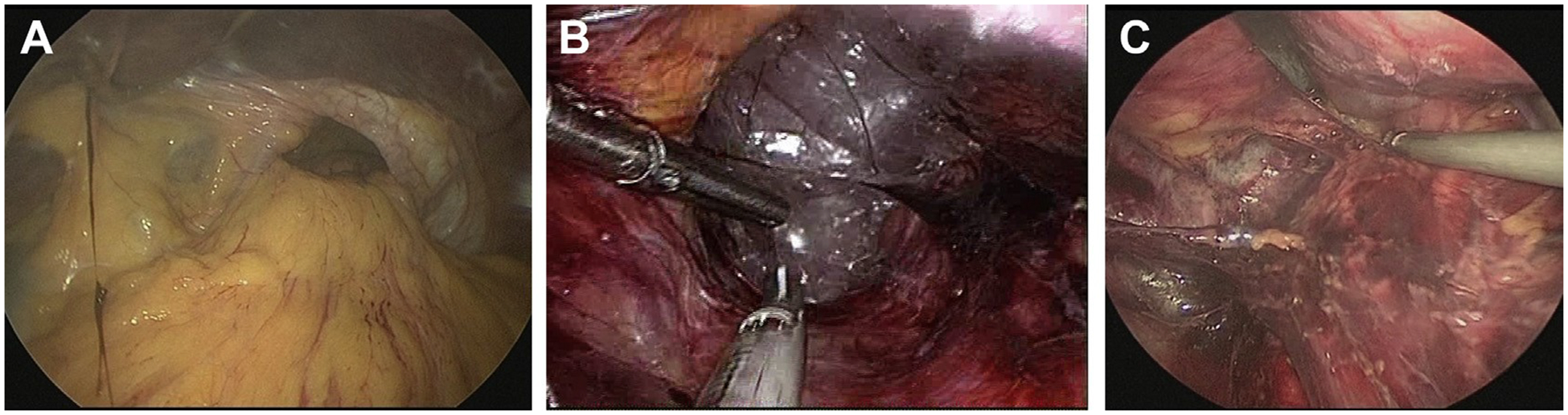
Laparoscopic anatomy of the paraesophageal hernias. (A) Dilated hiatus with totally intrathoracic stomach. Greater curvature is visible in the hernia sac and the greater omentum draping over the hiatus. (B) Foamy appearance of the mediastinal tissue signifies the correct plane of dissection. (C) Subcarinal lymph node pocket and thoracic duct exposed along the thoracic aorta are visible during the dissection.
The esophagus is mobilized high into the mediastinum with a coaxial close-up view of camera visualization. Mobilization starts with opening of the phrenoesophageal membrane and hernia sac, usually anteriorly, which allows entrance in to the mediastinal tissue.13,28,35 The CO2 creates a foamy appearance of the loose connective tissue of the mediastinum, with easy identification of any vessels (Fig. 6B). With good visualization, mobilization of the thoracic esophagus to the subcarinal area is possible. At this point, the surgeon should identify the membranous airway and minimize use of cautery to avoid an airway injury (Fig. 6C). Posteriorly, the esophagus is mobilized from the aorta, and aortoesophageal branches are controlled. Laterally, the pleural membranes can be identified and protected from injury. Premature pleural injury results in the collapse of the mediastinal space, complicating the dissection and anatomic exposure. The thoracic duct runs on the right posterior aspect of the aorta and its injury is uncommon (Fig. 6C). However, due to the variability of its course, a high index of suspicion is mandatory and, if there is concern regarding possible injury, prophylactic ligation may be advised.
SUMMARY
Paraesophageal hernias represent a complex surgical problem. Surgeons tackling this disease should become familiar with the complex anatomic relationships in this condition. Indeed, the tenets of a successful and durable repair of this disease require reconstruction of the normal anatomy, including the diaphragmatic hiatus and the abdominal esophagus. Careful identification and avoidance of the vagus nerves is essential. Comprehensive understanding of the anatomy of the diaphragm, esophagus, and stomach will allow the surgeon to be comfortable in performing this operation through the abdomen or the chest.
KEY POINTS.
Incidence of paraesophageal hernias is increasing owing to the increased proportion of advanced age patients and clinicians’ awareness.
Minimally invasive, including robotic, surgical techniques are increasing in popularity and produce excellent results.
Thorough understanding of anatomic relationship in paraesophageal hernias is required for successful surgical repair regardless of the surgical approach.
ACKNOWLEDGEMENT
This research was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA006927.
Footnotes
Dr. Abbas received honoraria from Boston Scientific and Intuitive. No disclosures for other authors.
REFERENCES
- 1.Jobe BA, Hunter JG, Watson Dl. Esophagus and diaphragmatic hernia In: Brunicardi FC, Andersen DK, Billiar TR, et al. , editors. Schwartz’s principles of surgery. 10th edition New York: McGraw-Hill Education; 2014. [Google Scholar]
- 2.Zhang X, Patil D, Odze RD, et al. The microscopic anatomy of the esophagus including the individual layers, specialized tissues, and unique components and their responses to injury. Ann N Y Acad Sci 2018; 1434(1):304–18. [DOI] [PubMed] [Google Scholar]
- 3.Botros KG, El-Ayat AA, El-Naggar MM, et al. The development of the human phreno-oesophageal membrane. Acta Anat (Basel) 1983;115(1):23–30. [DOI] [PubMed] [Google Scholar]
- 4.Eliska O Phreno-oesophageal membrane and its role in the development of hiatal hernia. Acta Anat (Basel) 1973;86(1): 137–50. [PubMed] [Google Scholar]
- 5.Mittal RK, Zifan A, Kumar D, et al. Functional morphology of the lower esophageal sphincter and crural diaphragm determined by three-dimensional high-resolution esophago-gastric junction pressure profile and CT imaging. Am J Physiol Gastrointest Liver Physiol 2017;313(3):G212–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Costa MM, Pires-Neto MA. Anatomical investigation of the esophageal and aortic hiatuses: physiologic, clinical and surgical considerations. Anat Sei Int 2004;79(1):21–31. [DOI] [PubMed] [Google Scholar]
- 7.Kahrilas PJ, Lin S, Chen J, et al. The effect of hiatus hernia on gastro-oesophageal junction pressure. Gut 1999;44(4):476–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hill LD, Kozarek RA, Kraemer SJ, et al. The gastroesophageal flap valve: in vitro and in vivo observations. Gastrointest Endosc 1996;44(5):541–7. [DOI] [PubMed] [Google Scholar]
- 9.Iwakiri K The role of excessive esophageal acid exposure in patients with gastroesophageal reflux disease. Clin J Gastroenterol 2009;2(6):371–9. [DOI] [PubMed] [Google Scholar]
- 10.Bohmer AC, Schumacher J. Insights into the genetics of gastroesophageal reflux disease (GERD) and GERD-related disorders. Neurogastroenterol Motil 2017;29(2). 10.1111/nmo.13017. [DOI] [PubMed] [Google Scholar]
- 11.Herbella FA, Patti MG. Gastroesophageal reflux disease: from pathophysiology to treatment. World J Gastroenterol 2010;16(30):3745–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hagarty G A classification of esophageal hiatus hernia with special reference to sliding hernia. Am J Roentgenol Radium Ther Nucl Med 1960;84: 1056–60. [PubMed] [Google Scholar]
- 13.Oleynikov D, Jolley JM. Paraesophageal hernia. Surg Clin North Am 2015;95(3):555–65. [DOI] [PubMed] [Google Scholar]
- 14.Kent MS, Luketich JD, Tsai W, et al. Revisionai surgery after esophagectomy: an analysis of 43 patients. Ann Thorac Surg 2008;86(3):975–83 [discussion: 967–74]. [DOI] [PubMed] [Google Scholar]
- 15.Gust L, Nafteux P, Allemann P, et al. Hiatal hernia after oesophagectomy: a large European survey. Eur J Cardiothorac Surg 2019;55(6): 1104–12. [DOI] [PubMed] [Google Scholar]
- 16.Tsunoda S, Jamieson GG, Devitt PG, et al. Early reoperation after laparoscopic fundoplication: the importance of routine postoperative contrast studies. World J Surg 2010;34(1):79–84. [DOI] [PubMed] [Google Scholar]
- 17.Burdan F, Rozylo-Kalinowska I, Szumilo J, et al. Anatomical classification of the shape and topography of the stomach. Surg Radiol Anat 2012; 34(2):171–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abbas AE. The management of gastroesophageal reflux disease In: Cameron JL, Cameron AM, editors. Current surgical therapy. 12th edition Philadelphia: Elsevier Health Sciences; 2016. p. 10–9. [Google Scholar]
- 19.Tatum JM, Samakar K, Bowdish ME, et al. Videoeso-phagography versus endoscopy for prediction of intraoperative hiatal hernia size. Am Surg 2018;84(3): 387–91. [PubMed] [Google Scholar]
- 20.Johnson AB, Oddsdottir M, Hunter JG. Laparoscopic Collis gastroplasty and Nissen fundoplication. A new technique for the management of esophageal foreshortening. Surg Endosc 1998; 12(8): 1055–60. [DOI] [PubMed] [Google Scholar]
- 21.Terry ML, Vernon A, Hunter JG. Stapled-wedge Collis gastroplasty for the shortened esophagus. Am J Surg 2004; 188(2): 195–9. [DOI] [PubMed] [Google Scholar]
- 22.Goldberg MB, Abbas AE, Smith MS, et al. Minimally invasive fundoplication is safe and effective in patients with severe esophageal hypomotility. Innovations (Phila) 2016;11(6):396–9. [DOI] [PubMed] [Google Scholar]
- 23.Nguyen NT, Schauer PR, Hutson W, et al. Preliminary results of thoracoscopic Belsey Mark IV antireflux procedure. Surg Laparosc Endosc 1998;8(3): 185–8. [PubMed] [Google Scholar]
- 24.Molena D, Mungo B, Stem M, et al. Novel combined VATS/laparoscopic approach for giant and complicated paraesophageal hernia repair: description of technique and early results. Surg Endosc 2015; 29(1):185–91. [DOI] [PubMed] [Google Scholar]
- 25.Gharagozloo F, Atiquzzaman B, Tempesta B, et al. Long-term results of robotic modified Belsey (gastroesophageal valvuloplasty) fundoplication. Surg Technol Int 2018;34:121–7. [PubMed] [Google Scholar]
- 26.Bakhos CT, Fabian T, Oyasiji TO, et al. Impact of the surgical technique on pulmonary morbidity after esophagectomy. Ann Thorac Surg 2012;93(1): 221–6 [discussion: 226–7]. [DOI] [PubMed] [Google Scholar]
- 27.Terry M, Smith CD, Branum GD, et al. Outcomes of laparoscopic fundoplication for gastroesophageal reflux disease and paraesophageal hernia. Surg Endosc 2001;15(7):691–9. [DOI] [PubMed] [Google Scholar]
- 28.Luketich JD, Nason KS, Christie NA, et al. Outcomes after a decade of laparoscopic giant paraesophageal hernia repair. J Thorac Cardiovasc Surg 2010; 139(2):395–404, 404.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lerut T, Deschamps C. Techniques for repair of paraesophageal hiatal hernia In: Sugarbaker DJ, Bueno R, Colson YL, et al. , editors. Adult chest surgery. 2nd edition New York: McGraw-Hill Education; 2015. [Google Scholar]
- 30.Sarkaria IS, Latif MJ, Bianco VJ, et al. Early operative outcomes and learning curve of robotic assisted giant paraesophageal hernia repair. Int J Med Robot 2017; 13(1). 10.1002/rcs.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vasudevan V, Reusche R, Nelson E, et al. Robotic paraesophageal hernia repair: a single-center experience and systematic review. J Robot Surg 2018; 12(1):81–6. [DOI] [PubMed] [Google Scholar]
- 32.Mertens AC, Tolboom RC, Zavrtanik H, et al. Morbidity and mortality in complex robot-assisted hiatal hernia surgery: 7-year experience in a high-volume center. Surg Endosc 2019;33(7):2152–61. [DOI] [PubMed] [Google Scholar]
- 33.Petrov R, Bakhos C, Abbas A. Robotic-assisted minimally invasive esophagectomy In: Kudsi Y, Carbonell A, Yiengpruksawan A, et al. , editors. Atlas of robotic surgery. Woodbury (CT): Cine-Med; 2018. p. 106–53. [Google Scholar]
- 34.Nason KS, Luketich JD, Witteman BP et al. The laparoscopic approach to paraesophageal hernia repair. J Gastrointest Surg 2012;16(2):417–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Petrov R, Bakhos C, Abbas A. Robotic esophagectomy In: Tsuda S, Kudsi OY, editors. Robotic-assisted minimally invasive surgery. Cham (Switzerland): Springer Nature; 2019. p. 277–93. [Google Scholar]


