Abstract
Background: Excessive use of antibiotics accelerates the acquisition/spread of antimicrobial resistance. A systematic review was conducted to identify the components of successful communication interventions targeted at the general public to improve antibiotic use.
Methods: The databases MEDLINE, EMBASE, CINAHL, Web of Science and Cochrane Library were searched. Search terms were related to the population (public, community), intervention (campaign, mass media) and outcomes (antibiotic, antimicrobial resistance). References were screened for inclusion by one author with a random subset of 10% screened by a second author. No date restrictions were applied and only articles in the English language were considered. Studies had to have a control group or be an interrupted time-series. Outcomes had to measure change in antibiotic-related prescribing/consumption and/or the public’s knowledge, attitudes or behaviour. Two reviewers assessed the quality of studies. Narrative synthesis was performed.
Results: Fourteen studies were included with an estimated 74–75 million participants. Most studies were conducted in the United States or Europe and targeted both the general public and clinicians. Twelve of the studies measured changes in antibiotic prescribing. There was quite strong (P < 0·05 to ≥ 0·01) to very strong (P < 0·001) evidence that interventions that targeted prescribing for RTIs were associated with decreases in antibiotic prescribing; the majority of these studies reported reductions of greater than −14% with the largest effect size reaching −30%.
Conclusion: Multi-faceted communication interventions that target both the general public and clinicians can reduce antibiotic prescribing in high-income countries but the sustainability of reductions in antibiotic prescribing is unclear.
Introduction
Even since the 1940s, shortly after the discovery of penicillin, the ability of bacteria to develop resistance to antibiotics has been known.1 The process of antimicrobial resistance (AMR) is a natural phenomenon but there is evidence that the excessive and unnecessary use of antibiotics accelerates the acquisition/spread of resistance.2,3
AMR is a major threat to health and jeopardizes many of the treatments that are now routinely performed in healthcare settings.4–6 Patients with drug-resistant infections often need a longer duration of treatment coupled with an increased length of hospital stay.4,7 As treatments are less effective patients remain infectious for a longer period of time, thereby increasing the risk of spreading resistant microorganisms to others.
Interventions to prevent the inappropriate use of antibiotics have been directed at clinicians, patients and the wider public. Clinician-directed interventions include educational materials (e.g. guidelines, lectures, workshops), audit and feedback on antibiotic prescribing practices, electronic or paper reminders, computer-aided clinical decision support systems and point-of-care testing (e.g. C-reactive protein).8
A 2005 Cochrane review examined the effectiveness of professional interventions in improving the prescription of antibiotics in ambulatory care.8 The authors determined that multifaceted interventions where educational interventions occur on multiple levels may be effective if local barriers to change are also addressed. A more recent review assessed the effectiveness of interventions to reduce outpatient antibiotic prescribing, concluding that interventions using active clinician education may lead to larger reductions in antibiotic prescribing.9
Interventions to improve patient antibiotic-related knowledge, attitudes and behaviour often involve educational components and are usually delivered in clinical settings, such as practice waiting rooms, consultation rooms or pharmacies.9,10 Targeting patients as well as clinicians is important as patient expectations and demands for antibiotics are often suggested as key reasons why clinicians inappropriately prescribe antibiotics.11,12
In addition to targeting interventions at doctors and patients, tackling the unnecessary use of antibiotics requires interventions that reach the general public.13 Misperceptions about antibiotic resistance are common worldwide.14,15 A systematic review of quantitative and qualitative studies examining public knowledge and beliefs about antibiotic use concluded that the public have an inadequate understanding of antibiotic resistance and believe that antibiotic resistance poses a minor risk to themselves.16 Raising public awareness and understanding to change these misconceptions before they become patients may play a key role in tackling antibiotic resistance. Interventions that occur outside the clinical setting could influence the antibiotic-related knowledge, attitudes and behaviour of those yet to become patients and the future carers of patients. This may range from national campaigns that employ mass media to more local interventions targeted at smaller communities.
Huttneret al. conducted a focused review in 2010 on public campaigns that aimed to improve the use of antibiotics.17 Multifaceted campaigns repeated over several years appeared to have the greatest effects, however, it remained unclear exactly what elements constituted a successful campaign. In addition, it could not be shown whether the effects of campaigns extended beyond trends occurring in the absence of such interventions because many of the included studies did not employ a control group. Furthermore, the review excluded community-level campaigns, randomized clinical trials that had recently been reviewed by other groups and studies from low and middle-income countries (LMICs). Our aim was to provide an up-to-date systematic review of the effectiveness of public-targeted communication interventions to improve the use of antibiotics that overcomes the limits of this previous review. We conducted the review in line with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement (PRISMA).
Methods
Search strategy
A systematic search was carried out in July 2015 using a predefined search protocol. No date restrictions were applied but only articles in the English language were considered. The following seven databases were searched: MEDLINE, EMBASE, CINAHL, Cochrane Library, Web of Science, The Trials Register of Promoting Health Interventions and BiblioMap. All titles and abstracts retrieved from the searches were imported into Mendeley referencing software. Duplicates were removed.
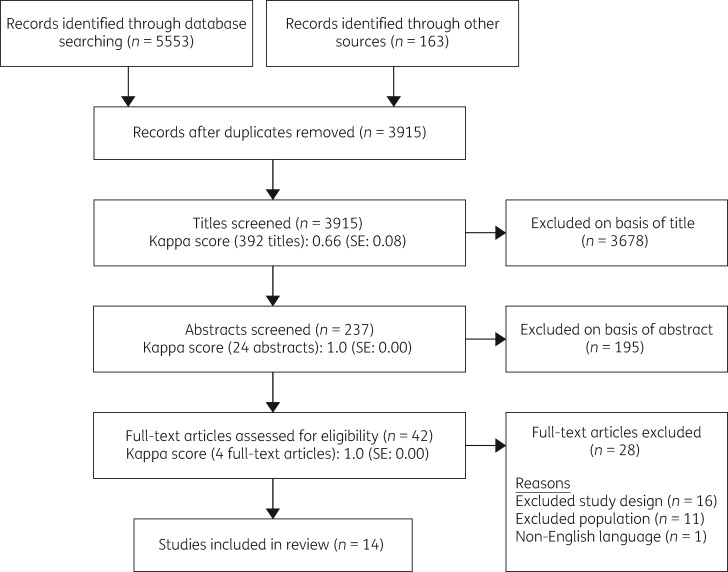
Titles, abstracts and full-text references were screened for inclusion by one author (E.C.) with a random subset of 10% screened by a second author (R.T.) at each stage. Inter-rater reliability scores were calculated using Cohen’s kappa; substantial agreement was found at the title screen stage and perfect agreement was found at abstract screen and full-text review stages (Figure 1).18 Discrepancies between reviewers were resolved by discussion and any further discrepancies were resolved by a third party (R.K.). In addition to the database search, manual searches of the bibliographies of all of the included studies were performed to identify additional relevant citations.
Figure 1.
Flow diagram of systematic review search.
Eligibility criteria
Inclusion and exclusion criteria that were used for all stages of the screening process are stated inTable 1. Any communication intervention that targeted the general public was considered for inclusion. Studies had to be one of randomized controlled trials (RCTs), cluster-RCTs, quasi-RCTs, interrupted times series (ITS) or controlled before-and-after studies. Outcomes consisted of antibiotic prescribing/consumption and/or public antibiotic-related knowledge, attitudes and behaviour.
Table 1.
Inclusion and exclusion criteria
| Inclusion criteria | Exclusion criteria | |
|---|---|---|
| Language | English | non-English |
| Time period | inception of databases to 2015 | none |
| Population | general public |
|
| Intervention | interventions employing some form of communication | interventions that targeted only prescribing of: antivirals, antimalarials, antifungal agents or antituberculosis agents |
| Comparison | studies employing a control group | studies that did not employ a control group |
| Outcome | change in: antibiotic prescribing and/or consumption and/or the public’s antibiotic-related knowledge, attitudes or behaviour | outcomes that were not changes in antibiotic prescribing or consumption and/or changes in antibiotic-related knowledge, attitudes and behaviour |
| Study |
|
|
ITS, interrupted time series.
Studies targeting solely clinicians or other healthcare staff or based only in a clinical setting were excluded. This was to create a distinction between interventions directed at patients rather than the general public. Studies that specifically measured changes in antibiotic prescribing for children or residents in nursing homes or other long-term care facilities were excluded. This was because recent reviews concerning antibiotic use in these populations have been published and interventions are likely to differ from those targeted at the general public.19–22 Interventions that targeted prescribing of antivirals, antimalarials, antifungal agents or anti-tuberculosis agents as opposed to antibiotic agents were also excluded.
Search terms
The main search terms used were related to the population (public, community, population, neighbourhood), intervention (communication, campaign, mass media) and outcomes (antibiotic, antimicrobial resistance). Synonyms were determined for each key search term by referring to a thesaurus, search strategies from other relevant systematic reviews and the controlled vocabulary of databases. Subject headings were also identified for databases that employ these. Appropriate syntax was used to cover various spellings and truncations of search terms. All free-text terms and subject headings for each key search term were combined using OR and the results of these combinations were then combined using AND to produce the final set of results. Full details of the searches used can be accessed in theSupplementary data (available atJAC Online).
Data extraction
Data extraction forms were based on the ‘Checklist of items to consider in data collection or data extraction’ from the Cochrane Handbook for Systematic Reviews.23 The forms were modified after piloting on a sample of studies. Data were extracted on the key study characteristics, methods of data collection, participant characteristics, intervention (target illness, elements, duration), results and conclusions drawn by authors. Where there was not a clear primary or main outcome measure, data on all relevant outcome measures was collected.
Quality assessment
Two reviewers assessed the quality of studies using the Effective Public Health Practice Project’s (EPHPP) Quality Assessment Tool for Quantitative Studies.24 This tool was recommended in the Cochrane Handbook of Systematic Reviews for assessing public health interventions.25 In a systematic review concerning tools for assessing methodological quality and risk of bias of non-randomized studies the tool was one of six, out of 182 identified, that was judged to be useful for systematic reviews, as it forces reviewers to be objective and systematic with their judgements of quality.26
The EPHPP tool can be used for any quantitative study design. It includes 21 items separated into eight components; selection bias, study design, confounders, blinding, data collection methods, withdrawals or dropouts, intervention integrity and analysis. For each of the first six components a rating of weak, moderate or strong is given and these scores contribute to a global rating for the study. The tool has been evaluated for content and construct validity, through comparison with another validated instrument and an iterative process of an expert group, and meets standards for both.26 The instrument also meets standards for inter-rater and intra-rater reliability. Cohen’s Kappa was used to determine intra-rater reliability.
Results
The search yielded 5553 results through database searching and an additional 163 were identified through bibliography searches. After de-duplication 3915 references were screened of which 42 references were assessed in full text. Fourteen studies (representing thirteen interventions) met inclusion criteria for the review. A flow diagram of the study selection process is shown inFigure 1. We found substantial heterogeneity in the studies therefore narrative synthesis was employed and the assessment of evidence was informed by the method recommended by Kirkwood and Sterne.27
Study characteristics
Population
Half of the fourteen studies were conducted in the US,28–34 six in Europe35–40 and one in Thailand.41 Only one of the interventions was targeted at a specific population group (village grocery owners).41Table 2 provides a summary of the key characteristics of each included study.
Table 2.
Summary of characteristics of included studies
| First author, year | Study design | Participants | Country | Intervention |
|||
|---|---|---|---|---|---|---|---|
| elements | clinician element | Target illness | Duration | ||||
| Nationwide interventions (n = 4) | |||||||
| Bauraind, 200438 | interrupted time series | general public, nationwide | Belgium | mass media campaign (including television); distribution of written materials for public | yes | not specified | 3 months |
| Sabuncu, 200937 | interrupted time series | general public, nationwide | France | mass media campaign (including television); training of day care workers to deliver educational messages, travelling education events and written materials | yes | RTIs | 6 months |
| Bernier, 201436 | interrupted time series | ||||||
| McNulty, 201039 | controlled before-and-after survey | 1888 persons pre- and 1830 post-intervention in 1 intervention and 1 control country | UK | mass media campaign (no television); written materials and practice-based materials | yes | RTIs | 1 month |
| Community-level interventions (n = 7) | |||||||
| Belongia, 200528 | cohort analytic | general public and 5115 primary care clinicians in 1 intervention and 1 control state | US | mass media campaign (including television); educational meetings and distribution of written materials for public | yes | not specified | not clear |
| Samore, 200533 | cluster-RCT | 407460 persons and 334 clinicians in 12 intervention and 6 control communities | US | full intervention (mass media campaign with no television; educational events, written materials, mailed household materials and clinician element) | yes (full intervention group only) | RTIs | not clear |
| partial intervention (community element alone) | |||||||
| Rubin, 200534 | controlled clinical trial | general public < 10000 and 2 family practice groups in 1 intervention community and the rest of rural Utah as a control community | US | mass media campaign (no television); educational materials for patients | yes | RTIs | ∼6 months |
| Hennessy, 200232 | controlled clinical trial | 13 villages in 1 intervention region and 2 control regions | US | community-wide educational events and meetings, educational materials in high schools, mailed written materials to households | yes | RTIs | 6 months |
| Lambert, 200740 | retrospective controlled before-and-after study | population of 16 intervention primary care organizations, number of control organizations not clear | UK | mass media campaign (including television); written materials | no | not specified | 2 months |
| Gonzales, 200831 | controlled clinical trial | 2.2 million persons in 1 intervention community and 0.53 million in 1 control community | US | mass media campaign (no television); educational events (including awareness week and ‘Antibiotics Amnesty Month’) and distribution of written educational materials for public | yes | not specified | 4 months |
| Formoso, 201335 | controlled clinical trial | 1.15 million persons in 11 intervention health districts and 3.25 million in 31 control health districts | Italy | mass media campaign (including television); educational events and distribution of written materials for public | yes | RTIs | 4 months |
| Site-based/household interventions (n= 3) | |||||||
| Gonzales, 199929 | controlled clinical trial | 2462 persons pre-, 2027 post-intervention and 93 healthcare professionals in 2 intervention practices and 2 control practices | US | full intervention (mailed educational household materials, practice-based materials and clinician elements). | yes | RTIs | not clear |
| limited intervention (practice-based element only) | |||||||
| Gonzales, 200530 | controlled clinical trial | population of 6 intervention and 362 control practices | US | mailed household and practice-based educational materials (including self-management guide) | yes (already in place) | RTIs | not clear |
| Arparsrithong-sagul, 201541 | controlled clinical trial | 48 intervention and 68 control groceries and grocery owners in 20 intervention and 20 control villages | Thailand | grocery shop-based face-to-face education by trained ‘change agents’ | no | not specified | not clear |
Intervention
Four of the studies evaluated nationwide campaigns,36–39 seven evaluated interventions conducted on a community-level28,31,32–35,40 and the remaining three studies conducted more restricted interventions where communication was limited to specific site-based and household materials.29,30,41 Mass media methods of communication, including television, radio, newspapers, magazines and billboards, were used in 10 of the studies.28,31,33–40 Nine of the studies focused on reducing antibiotic prescribing for respiratory tract infections (RTIs).29,30,32–37,39 In addition to a public-targeted element, a specific clinician-directed element was present in twelve of the included studies.28–39
Outcomes measured
Twelve of the studies measured a change in the prescribing rate or consumption of antibiotics.28–38,40 Three of the studies measured the impact of interventions on public antibiotic-related knowledge or attitudes.35,39,41 One of the studies measured the effect on antimicrobial resistance in the study population32 and one of the studies measured the change in availability of antibiotics without a prescription.41
Study design
The included studies consisted of one cluster-RCT,33 seven controlled clinical trials,29–32,34,35,41 three interrupted time series,36–38 one cohort analytic study,28 one retrospective controlled before-and-after study,40 and one controlled before-and-after survey.39
Quality of studies
A summary of quality assessment results is presented inTable 3. There were no studies of overall strong quality, seven of the studies were of overall moderate quality31,33,35–38,41 and the seven remaining studies were of overall weak quality.28–30,32,34,39,40 No studies were excluded based on their quality in order to provide an overview of all the literature.
Table 3.
Summary of quality assessment of included studies
| First author, year | Selection bias | Study design | Confounders | Blinding | Data collection methods | Withdrawals and drop-outs | Global rating |
|---|---|---|---|---|---|---|---|
| Arparsrithongsagul, 201541 | moderate | strong | moderate | moderate | weak | strong | moderate |
| Bauraind, 200438 | moderate | moderate | strong | moderate | weak | moderate | moderate |
| Belongia, 200528 | weak | moderate | moderate | moderate | weak | moderate | weak |
| Formoso, 201335 | moderate | strong | strong | moderate | weak | moderate | moderate |
| Gonzales, 199929 | moderate | strong | moderate | moderate | weak | weak | weak |
| Gonzales, 200530 | weak | strong | moderate | moderate | weak | strong | weak |
| Gonzales, 200831 | moderate | strong | strong | moderate | weak | moderate | moderate |
| Hennessy, 200232 | moderate | strong | weak | moderate | weak | strong | weak |
| Lambert, 200740 | moderate | weak | moderate | moderate | weak | moderate | weak |
| McNulty, 201039 | moderate | weak | strong | moderate | weak | moderate | weak |
| Rubin, 200534 | moderate | strong | weak | moderate | weak | moderate | weak |
| Sabuncu, 200937 | strong | moderate | strong | moderate | weak | moderate | moderate |
| Bernier, 201436 | strong | moderate | strong | moderate | weak | moderate | moderate |
| Samore, 200533 | strong | strong | strong | moderate | weak | moderate | moderate |
Changes in antibiotic prescribing rates
The findings of included studies measuring changes in antibiotic prescribing are summarized inTable 4.
Table 4.
Summary of findings of included studies measuring changes antibiotic prescribing outcomes
| First author, year | Primary outcome(s) | Change in intervention group | Change in control group | Effect size (95% CI) | P value | |
|---|---|---|---|---|---|---|
| Nationwide interventions (n= 3) | ||||||
| Bauraind, 200438 | change in total outpatient antibiotic sales | a | a | first campaign year: −6.5% | < 0.05 | |
| change in total outpatient antibiotic sales | a | a | second campaign year: −3.4% | > 0.05 | ||
| Sabuncu, 200937 | change in winter antibiotic prescribing rate (Oct to Mar) | a | a | −26.5% (−33.5% to −19.6%)b | < 0.0001 | |
| Bernier, 201436 | change in antibiotic prescribing rate | a | a | −30% (−36.3% to −23.8%)c | < 0.001 | |
| Community-level interventions (n = 7) | ||||||
| Belongia, 200528 | change in antimicrobial prescribing rate | −20.4%, | −19.8% | −0.6% | NR | |
| change in retail sales of antimicrobial drugs (grams per capita) | −17.3% | −27.4% | 10.1% | NR | ||
| Samore, 200533 | change in antibiotic prescribing rate per 100 person-years (partial intervention vs. control) | 1% | 6% | −5% | 0.03 (difference between three groups) | |
| change in antibiotic prescribing rate per 100 person-years (full intervention vs. control) | −10% | 6% | −16% | |||
| Rubin, 200534 | change in proportion of upper RTIs episodes treated with an antibiotic | −15.6% (P = 0.002) | −1.5% (P = 0.47) | −14.1% | NR | |
| Hennessy, 200232 | change in mean number of antibiotic courses per person | −31% (P = < 0.01) | −10% (P ≥ 0.05) | −21% | NR | |
| Lambert, 200740 | change in antibiotic prescribing rate | 21.7 fewer items prescribed per 1000 populationd | NR | −5.8% | < 0.0005 | |
| Gonzales, 200831 | net change in antibiotic dispenses per 1000 persons | – | – | −3.8% | 0.30 | |
| net change in managed care-associated antibiotic dispenses per 1000 members | – | – | −8.8% | 0.03 | ||
| Formoso, 201335 | average change in antibiotic prescribing rates for outpatient | – | – | −4.3% (−7.1% to − 1.5%) | 0.008 | |
| Site-based/household interventions (n = 2) | ||||||
| Gonzales, 199929 | change in antibiotic prescribing rate for uncomplicated acute bronchitis (limited intervention vs. control) | −5% | −2% | −3% | 0.02 (full-intervention vs. limited intervention and control) | |
| change in antibiotic prescribing rate for uncomplicated acute bronchitis (full intervention vs. control) | −26% | −2% | −24% | |||
| Gonzales, 200530 | change in antibiotic prescribing rate for adult bronchitis (intervention vs. local control) | −24% | −10% | −14% | 0.006 | |
| change in antibiotic prescribing rate for adult bronchitis (intervention vs. distal control) | −24% | −7% | −17% | < 0.002 | ||
NR, not reported.
Not reported as ITS design.
During campaign periods (Oct to Mar) 2002 to 2007.
Maximum significant decrease observed during campaign periods (Oct to Mar) 2002 to 2010.
Over winter months (Nov to Mar).
Population level
The nationwide interventions evaluated by the included studies included the French and Belgium campaigns. The French campaign consisted of the central theme ‘Antibiotics are not automatic’ and the aim was to reduce total antibiotic use in the community by 25%. There was strong to very strong evidence that the French campaign resulted in large reductions in antibiotic prescribing; between 2002 to 2010 antibiotic use during the campaign periods (October to March) decreased by −26% and reached a maximum decrease of −30%.36,37 The Belgium mass media campaign used simple messages such as ‘Use antibiotics less frequently but better’ and ‘Save antibiotics, they may save your life’. The campaign was associated with a reduction of 6.5% in outpatient antibiotic sales in the first campaign year, for which there was quite strong evidence.38 However, this effect was not sustained into the second intervention year.
Community-level interventions varied in scale, with some assigning small rural villages to intervention groups32 and others implementing interventions in larger regions31,35 or whole states.28 Belongiaet al. conducted a study on a statewide level (Wisconsin, USA) and reported no evidence for a reduction in antibiotic prescribing in the intervention state relative to the control.28 Two of the studies evaluated interventions implemented in communities with estimated populations of > 1 million people; one found no evidence for a reduction in antibiotic prescribing in metropolitan communities of Colorado31 and the other found strong evidence for an average change in prescribing rates of −4.3% (measured as defined daily doses per 1000 inhabitants per day) in the provinces of Modena and Palma, Italy.35 Two of the studies that evaluated interventions conducted on much smaller communities in the US (< 10 000 people) reported strong evidence for the largest reductions in prescribing of −14.1%34 and −21%.32
Two US studies where interventions were limited to practice-based and mailed household materials demonstrated large effect sizes. One of the studies found quite strong evidence for a reduction in antibiotic prescribing of −24% at the full intervention healthcare practice site (practice and household educational materials).29 The other study also delivered practice and household-based educational materials as part of the intervention and found reductions ranging from −14% (P = 0.006) to −18% (P ≤ 0.002), when compared with two separate control populations.30
Communication method
The use of mass media was associated with a variable effect on antibiotic prescribing. The majority of studies where mass media was used reported positive findings,35–38,40 with very strong evidence for the largest effects found in the studies by Sabuncuet al. and Bernieret al. who evaluated the French national campaign at different time periods.36,37 However, not all of the studies that employed mass media reported convincing evidence of a reduction in antibiotic prescribing; Gonzaleset al. found no evidence for a reduction in antibiotic prescribing in the general population of Colorado.31 In addition, another US campaign that made extensive use of mass media materials (including newspaper reports, radio advertising, local television news stories and television advertising) found that while the antibiotic prescribing rate decreased by −20.4% in the intervention state (Wisconsin), the control community (Minnesota) also experienced a −19.8% reduction.28 Furthermore, there was evidence that interventions that did not employ mass media still managed to achieve some of the largest reductions in prescribing.29,30,32 Similarly, the use of television in interventions was associated with reductions in antibiotic prescribing in the majority of cases, for which there was strong evidence,35–37,40 but television use was not essential for an intervention to be effective.29–30,33,34
Target illness
Eight of the studies involved interventions that aimed to specifically reduce antibiotic prescribing for RTIs.29,30,32–37 Overall these studies found evidence of reductions in antibiotic prescribing, with seven of the eight reporting effect sizes of greater than −14%.29,30,32–34,36,37 For interventions in which specific campaign slogans communicated the general message of ‘antibiotics do not work against colds and flu’ there was strong evidence that this could lead to large reductions in antibiotic prescribing.29,36,37 Studies in which interventions were not specifically aimed at reducing antibiotic prescribing for RTIs reported either no effect or evidence of a limited effect.28,31,38,40
Public element versus clinician element
Only three of the included studies did not include a specific clinician-directed element to the intervention and,33,40,41 of these, only two measured changes in antibiotic prescribing.33,40 The first study by Lambertet al. evaluated a regional mass media campaign implemented over two consecutive years in the North East of England.40 The authors found no difference in prescribing rates between the groups over the total time periods compared but did report very strong evidence for a reduction in antibiotic prescribing, equivalent to −5.8%, in the intervention communities over the winter months of the second campaign year.
The second study conducted by Samoreet al. was able to partially distinguish the separate effects of the public- and clinician-directed elements of the intervention.33 Twelve rural communities in Utah and Idaho were randomized to a full intervention group (encompassing both public and clinician-directed elements), a partial intervention group (public element alone) and a control group. There was quite strong evidence that there was a reduction in the antibiotic prescribing rate for the full intervention group compared with the partial intervention and control groups.
Another study investigated the additional effect of a public-targeted intervention element to a clinician-centred quality improvement intervention that was already in place in private office practices in Denver, Colorado.30 The intervention practices therefore received combined public and clinician-directed interventions, while the control practices only received the on-going clinician intervention. There was strong evidence that the addition of the public-targeted element led to substantial reductions in prescribing rates for adult bronchitis of −14% and −17%, when compared with two separate control groups.
Changes in antibiotic knowledge and attitudes
Only three of the included studies reported the effect of interventions on antibiotic-related knowledge and attitudes.35,39,41 An improvement in antibiotic-related knowledge and attitudes was found in only one of the studies; Arparsrithongsagulet al. targeted village grocery owners in Thailand through trained community ‘change agents’, including a mixture of village community leaders, village health volunteers, active villagers, consumers and government public health officers.41 The authors reported an improvement in the mean antibiotic knowledge score in the intervention group (9.04 to 10.90,P ≤ 0.01) and no change in the control group (9.22 to 9.22,P ≥ 0.05).
The two other studies that reported no improvement in antibiotic-related knowledge and attitudes were also mass media campaigns involving both public and clinician elements and targeting antibiotic prescribing for RTIs.35,39 McNultyet al. studied the effects of the English national campaign and found no evidence of a difference in the proportion of participants with incorrect responses to the main attitude the campaign attempted to change, ‘Antibiotics works on most coughs and colds’.39 In addition, there was very strong evidence of an increase in the proportion of English respondents reporting that they kept any leftover antibiotics (2.2% to 7%,P ≤ 0.001). Formosoet al. conducted a community-level controlled trial in northern Italy and reported no significant difference in the proportion of correct responses to six antibiotic-related knowledge and attitudes statements.35 However, there was an increase in the proportion of those agreeing incorrectly to the statement ‘Antibiotics are effective against viruses’ (47% to 62%,P ≤ 0.05) post-intervention.
Other outcome measures
Hennessyet al. studied the impact of an educational intervention in remote Alaskan villages on the levels of antibiotic-resistant bacteria.32 People in the intervention villages were surveyed at baseline and after the initial intervention by nasopharyngeal cultures forStreptococcus pneumoniae carriage. There was a reduction in the proportion of penicillin-non-susceptibleStreptococcus pneumoniae (PNSP) (41% to 29%,P = 0.01) and penicillin-resistantStreptococcus pneumoniae (PRSP) (25% to 11%,P ≤ 0.01) with no change in the control population. However, when the intervention was extended for a second year in both the intervention and control villages, the reduction in the carriage of PNSP and PRSP in the intervention population was not sustained.
Arparsrithongsagulet al. measured the effect of an intervention on the antibiotic availability in the village grocery stores in Thailand.41 Antibiotics in grocery stores can be purchased without a prescription and self-administered. The proportion of intervention village groceries containing antibiotics decreased from 79.2% to 22.9% (P ≤ 0.001) with little change in the control village groceries (88.2% to 85.3%). Even after controlling for confounding factors the intervention group had an 87% reduction in antibiotic availability (relative rate = 0.13; 95% CI, 0.07 to 0.23), while the control group had an 8% reduction in antibiotic availability (relative rate = 0.92; 95% CI, 0.88 to 0.97).
Discussion
Main findings of this study
This review found evidence that interventions conducted on a national, community and site-based/household level could achieve reductions in antibiotic prescribing in developed countries, in at least the short-term. No clear relationship between the use of mass media and the effect on antibiotic prescribing was found. There was evidence that interventions targeting antibiotic prescribing for RTIs were associated with substantial reductions in antibiotic prescribing. There are an inadequate number of appropriately designed studies to evaluate how effective public-targeted interventions are at independently reducing antibiotic prescribing without a clinician component. Similarly, there were only a small number of studies measuring changes in antibiotic-related knowledge and attitudes and these had mixed findings. There was only one study conducted in an LMIC. All studies were of weak to moderate quality and therefore some caution is needed in interpreting these findings.
Strengths and limitations
This study is important because it provides an up-to-date systematic review of the effectiveness of communication interventions targeted at the general public to improve the use of antibiotics. A key strength of this review is that only studies with a control group or ITS were included. Uncontrolled before and after studies do not take account of possible significant background variation and seasonal patterns to antibiotic prescribing.42 Therefore, previous research that had included such studies was unable to show whether the effects of campaigns extended beyond trends occurring in their absence.17 We can be more confident that the studies in this review have protected against secular trends and therefore are more likely to represent true changes.
There are a number of limitations to the methods employed in this review. Firstly the results may be affected by publication bias because the grey literature was not searched. The effect sizes from the included studies in this review may be misleading because published trials are more likely to demonstrate positive and larger intervention effects than evidence existing within the grey literature or unpublished evidence.43 Secondly, only studies written in the English language were included, which may have introduced language bias. Most of the studies identified were from the US or Europe, which may be suggestive of this bias, or may also reflect the current evidence base. Thirdly, the review only included articles that targeted the prescribing of antibiotics and since AMR also refers to resistance conferred to other anti-infective agents this can be considered a key limitation. During the screening process titles and abstracts of articles were not screened simultaneously and therefore some relevant studies may have been incorrectly excluded at the title screening stage. In addition to this, the reviewers were not blinded to study authors, institutions, journal name and results when conducting the quality assessment of studies.44 Furthermore, study designs of included studies were often complex and heterogeneous making the judgement of study quality challenging. In relation to this, the EPHPP quality assessment tool scored controlled clinical trials comparably with RCTs for study design. The EPHPP tool may also be criticized because studies that failed to report certain aspects (e.g. validity and reliability of data collection methods) were scored as weak, whereas this may not represent weak quality but simply poor reporting.
RCTs do not lend themselves to interventions that employ mass communication on a population level; therefore, the majority of included studies were non-randomized. It has been previously suggested that non-randomized studies report larger effect estimates because of increased susceptibility to bias and confounding.45 However, a recent review found that larger effect estimates were not always found in non-randomized studies.46 A key limitation of the evidence base is that most of the included studies did not measure outcomes at greater than 6 months post-intervention; the short length of follow-up means we are unable to judge whether interventions led to sustainable reductions in antibiotic prescribing. This is not only important for determining whether campaigns need to be repeated to remain effective, and the appropriate time interval for this, but it is also key to establishing the cost-effectiveness of interventions over longer periods of time. Another major challenge of the evidence base is how the success of interventions is measured, with different studies using different metrics and data sources to do this. This is problematic because these differences can lead to substantial variation in perceived levels of antibiotic use.47 For instance, Bruyndonckxet al. found that European outpatient antibiotic use significantly increased when measured as defined daily dose per 1000 inhabitants per day but for the same time period contrasting trends were found when the data was analysed as packages per 1000 inhabitants per day.48 Moreover, a total decrease in antibiotic use does not necessarily mean an improved quality of prescribing, for example, in France during the national campaign between 2002 and 2007, there was a substantial increase in the use of fluoroquinolones, which is arguably not desirable.37 This highlights how important it is to ensure that the data collected truly reflects the desired impact and also any unintended consequences of an intervention. Inappropriate reductions in antibiotic prescribing may be associated with harms such as longer duration and severity of infection or more complications. However, the majority of studies did not attempt to measure potential harms that may be associated with reductions in antibiotic prescribing. In addition to this, antibiotic availability without a prescription is a significant problem particularly in LMICs, with a recent meta-analysis demonstrating the prevalence of antimicrobial use without a prescription to be 38.8% (95% CI, 29.5% to 48.1%).49 The current review found little evidence for interventions to target the problem of antibiotic use without a prescription but this may be partly due to a lack of high quality studies addressing this problem. Relatedly, only one of the studies included in this review was conducted in an LMIC (Thailand) and this did not measure changes in antibiotic prescribing, therefore the findings from this review cannot be generalized to LMICs.
Findings in relation to other research
Antibiotic awareness campaigns employing mass media (e.g. posters and leaflets) alone as opposed to more interactive elements (e.g. prescriber feedback) appear to be ineffective in improving prescribing rates and antibiotic-related knowledge, attitudes and behaviour.50 Indeed, while many of the successful campaigns in this review had used mass media as part of a multi-modal approach, the use of mass media was not a pre-requisite for an effective campaign. The results from this review are in line with previous findings, that multi-faceted interventions, which target both clinicians and the public through a variety of formats, are successful at reducing antibiotic prescribing.8,9,17,50 Experience from other public health campaigns also suggest the need for repeated exposure to campaign messages over a long duration in order to produce sustained effects.50–52 While this was evident in some of the studies in this review,33,36,37 this was not the case for all of the studies.38 Inappropriate prescribing most commonly occurs for RTIs and the large reductions in antibiotic prescribing that were found for interventions that targeted RTIs is consistent with this.53 In an attempt to provide more quantitative evidence on the topic, Filippiniet al. employed a differences-in-differences approach, using available observational data to model the effect of national public campaigns on antibiotic usage.54 They included data from 21 European countries and estimated that between 1997 and 2007 public campaigns substantially reduced mean level of antibiotic use by about −6.5% to −28.3%. These findings are largely in line with the effect sizes observed in our review.
There were only three studies identified in this review where the effects of an intervention that solely targeted the public could be evaluated. Ranjiet al. summarized the findings from ten trials that studied interventions in which only clinician education was delivered.9 The authors estimated that the additional reduction in antibiotic prescribing rates between the intervention and control groups ranged from −6.5% to −28.6% (median −8.9%). This suggests that clinician education alone without public involvement can produce substantial reductions in prescribing. Nonetheless, two of the studies included in this review compared a full intervention group (combined public and clinician elements) with a limited intervention group (either public or clinician element only) and both reported greater reductions in antibiotic use for the full intervention group.30,33 The authors report that there may be a synergy created between the public and clinician-directed components when used together. As a variety of factors may influence the prescribing of antibiotics such as patient expectations, colour of secretions and even clinician pay,11,55,56 it could be reasoned that interventions that target multiple behaviours of all involved may be more successful than those that target them in isolation.
For studies that measured changes in antibiotic-related knowledge and attitudes, two of the campaigns specifically included key messages about antibiotics not being useful for colds or flu.35,39 However, it appears that this message failed to improve the public’s knowledge of, or attitudes towards, antibiotics. Indeed, previous campaign evaluations have demonstrated the difficulty with educating the public about the differences between viral and bacterial infections.17,57 While Formosoet al. found no improvement in public knowledge and attitudes the authors did show reductions in antibiotic prescribing.35 This, albeit an isolated finding from one study, may suggest that improving the public’s knowledge and attitudes towards antibiotic resistance is less important for reducing antibiotic use. On the other hand, Gonzaleset al. concluded that the reduction in antibiotic use that they found was largely due to a reduction in clinical consultations, which suggests a change in the public’s behaviour rather than improved prescribing behaviour by clinicians.31 Similarly, Grijalvaet al. examined US antibiotic prescribing trends and found that in children < 5 years old the reduction in antibiotic use was actually due to a decrease in the number of clinical consultations rather than improved prescribing practice (no change in proportion of visits where an antibiotic was prescribed). However, for the older age groups prescribing practice did appear to improve.58
Recommendations for future research
No studies of high quality were identified; therefore future research should aim to be of greater quality by employing randomized or cluster-randomized designs to ensure baseline comparability of study groups and adequate control of confounding factors. Studies should clearly report on blinding of investigators and participants, the validity and reliability of data collection tools and the extent of withdrawals and dropouts. To distinguish the separate impacts of public and clinician intervention components, three-armed trials are required in which a combined intervention (public and clinician elements) is compared with each separate component. Studies should measure the sustainability of reductions in antibiotic prescribing and potential adverse harms of reductions in prescribing. More research is needed to assess the impact of communication interventions on the public’s antibiotic-related knowledge and attitudes. Research concerning interventions to tackle antibiotic availability without a prescription in LMICs should be undertaken as this unregulated use poses a serious concern and AMR is ultimately a global problem.
Conclusions
Communication interventions conducted on a national, community or practice/household-level should be considered as part of policy to reduce antibiotic use in high-income countries. Interventions that target prescribing for RTIs may yield the largest reductions in antibiotic use. The use of mass media is not a prerequisite for an effective intervention and a multi-faceted approach is likely to prove more successful. There is an inadequate amount of evidence to determine how effective public-targeted interventions are at independently reducing antibiotic prescribing without a clinician component. Further gaps in the literature exist with regard to the impact of communication interventions on the public’s antibiotic-related knowledge and attitudes and the use of antibiotics (both regulated and unregulated) in LMICs.
Supplementary Material
Funding
This work was supported by the South West Public Health Training Programme through funding E.L.A.C. and R.T.’s posts. The work was also undertaken with the support of The Centre for the Development and Evaluation of Complex Interventions for Public Health Improvement (DECIPHer), a UK Clinical Research Collaboration Public Health Research Centre of Excellence. Joint funding (MR/KO232331/1) from the British Heart Foundation, Cancer Research UK, Economic and Social Research Council, Medical Research Council, the Welsh Government and the Wellcome Trust, under the auspices of the UK Clinical Research Collaboration, is gratefully acknowledged.
Transparency declarations
None to declare.
Author contributions
E.L.A.C. conceived of and designed the study, conducted the searches, data extraction, quality assessment and narrative synthesis of included studies. R.T. screened a subset of articles for inclusion into the systematic review and dual-quality assessed all included studies. R.K. resolved disagreements relating to quality assessment. R.K. contributed to interpretation and critical review of the narrative synthesis and abstract.
References
- 1.Fleming A.Penicillin, Nobel lecture 1945.http://www.nobelprize.org/nobel_prizes/medicine/laureates/1945/fleming-lecture.pdf
- 2. Davies J,Davies D.. Origins and evolution of antibiotic resistance.Microbiol Mol Biol Rev 2010;74:417–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Courvalin P. Predictable and unpredictable evolution of antibiotic resistance.J Intern Med 2008;264:4–16. [DOI] [PubMed] [Google Scholar]
- 4. Review on Antimicrobial Resistance.Antimicrobial Resistance: Tackling a crisis for the health and wealth of nations London,2014https://amr-review.org/sites/default/files/AMR%20Review%20Paper%20-%20Tackling%20a%20crisis%20for%20the%20health%20and%20wealth%20of%20nations_1.pdf.
- 5. Cars O,Högberg LD,Murray M. et al. Meeting the challenge of antibiotic resistance.BMJ 2008;337:a1438. [DOI] [PubMed] [Google Scholar]
- 6. Wild SM. Antibiotic prophylaxis at caesarean section.Lancet 2002;360:724.. [DOI] [PubMed] [Google Scholar]
- 7. World Health Organisation 2014Antimicrobial resistance: global report on surveillance 2014http://apps.who.int/iris/bitstream/10665/112642/1/9789241564748_eng.pdf.
- 8. Arnold S,Straus S.. Interventions to improve antibiotic prescribing practices in ambulatory care.Cochrane Database Syst Rev 2005;4:CD003539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ranji SR,Steinman MA,Shojania KG. et al. Interventions to reduce unnecessary antibiotic prescribing: a systematic review and quantitative analysis.Med Care 2008;46:847–62. [DOI] [PubMed] [Google Scholar]
- 10. Drekonja D,Filice G,Greer N. et al. Antimicrobial stewardship programs in outpatient settings: a systematic review.Infect Control Hosp Epidemiol 2015;36:142–52. [DOI] [PubMed] [Google Scholar]
- 11. Hamm RM,Hicks RJ,Bemben DA.. Antibiotics and respriratory infections: are patients more satisfied when expectations are met? J Fam Pract 1996;43:56–62. [PubMed] [Google Scholar]
- 12. Coenen S,Michiels B,Renard D. et al. Antibiotic prescribing for acute cough: the effect of perceived patient demand.Br J Gen Pract 2006;56:183–90. [PMC free article] [PubMed] [Google Scholar]
- 13. Review on Antimicrobial Resistance.Tackling drug-resistant infections globally: Final report and recommendations London,2016https://amr-review.org/sites/default/files/160518_Final%20paper_with%20cover.pdf.
- 14. World Health Organisation 2015Multi-country public awareness surveyhttp://apps.who.int/iris/bitstream/10665/194460/1/9789241509817_eng.pdf
- 15. Wellcome Trust 2015Exploring the consumer perspective on antimicrobial resistance.http://wellcomelibrary.org/item/b24978000#?c=0&m=0&s = 0 & cv= 0
- 16. McCullough AR,Parekh S,Rathbone J. et al. A systematic review of the public's knowledge and beliefs about antibiotic resistance.J Antimicrob Chemother 2016;71:27–33. [DOI] [PubMed] [Google Scholar]
- 17. Huttner B,Goossens H,Verheij T. et al. Characteristics and outcomes of public campaigns aimed at improving the use of antibiotics in outpatients in high-income countries.Lancet Infect Dis 2010;10:17–31. [DOI] [PubMed] [Google Scholar]
- 18. Viera AJ,Garrett JM.. Understanding interobserver agreement: the kappa statistic.Fam Med 2005;37:360–3. [PubMed] [Google Scholar]
- 19. Vodicka TA,Thompson M,Lucas P. et al. Reducing antibiotic prescribing for children with respiratory tract infections in primary care: a systematic review.Br J Gen Pract 2013;63:e445–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Andrews T,Thompson M,Buckley DI. et al. Interventions to influence consulting and antibiotic use for acute respiratory tract infections in children: a systematic review and meta-analysis.PLoS One 2012;7:e30334.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Davey P,Brown E,Charani E. et al. Interventions to improve antibiotic prescribing practices for hospital inpatients.Cochrane Database Syst Rev 2013;4:CD003543. [DOI] [PubMed] [Google Scholar]
- 22. Fleming A,Browne J,Byrne S.. The effect of interventions to reduce potentially inappropriate antibiotic prescribing in long-term care facilities: a systematic review of randomised controlled trials.Drugs Aging 2013;30:401–8. [DOI] [PubMed] [Google Scholar]
- 23. Higgins JPT,Deeks J.. Chapter 7: selecting studies and collecting data In:Higgins JPT,Green S, eds.Cochrane Handbook for Systematic Reviews of Interventions Version 5.10.London:The Cochrane Collaboration,2011. [Google Scholar]
- 24. Effective Public Health Practice Project 1998Quality Assessment Tool for Quantitative Studieshttp://www.ephpp.ca/PDF/Quality%20Assessment%20Tool_2010_2.pdf.
- 25. Armstrong R,Waters E,Doyle J.. Chapter 21: reviews in health promotion and public health In:Higgins J,Green S, eds.Cochrane Handbook for Systematic Reviews of Interventions Version 5.10.London:The Cochrane Collaboration,2011. [Google Scholar]
- 26. Deeks J,Dinnes J,D’Amico R. et al. Evaluating non-randomised intervention studies.Health Technol Assess 2003;7: iii – x,1–173. [DOI] [PubMed] [Google Scholar]
- 27. Kirkwood B,Stern J.. Essential Medical Statistics,2nd edn.London:Wiley-Blackwell,2003. [Google Scholar]
- 28. Belongia EA,Knobloch MJ,Kieke BA. et al. Impact of statewide program to promote appropriate antimicrobial drug use.Emerging Infect Dis 2005;11:912–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gonzales R,Steiner JF,Lum A. et al. Decreasing antibiotic use in ambulatory practice: impact of a multidimensional intervention on the treatment of uncomplicated acute bronchitis in adults.J Am Med Assoc 1999;281:1512–9. [DOI] [PubMed] [Google Scholar]
- 30. Gonzales R,Corbett KK,Leeman-Castillo BA. et al. The "minimizing antibiotic resistance in Colorado" project: impact of patient education in improving antibiotic use in private office practices.Health Serv Res 2005;40:101–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gonzales R,Corbett KK,Wong S. et al. "Get Smart Colorado": impact of a mass media campaign to improve community antibiotic use.Med Care 2008;46:597–605. [DOI] [PubMed] [Google Scholar]
- 32. Hennessy TW,Petersen KM,Bruden D. et al. Changes in antibiotic-prescribing practices and carriage of penicillin-resistantStreptococcus pneumoniae: a controlled intervention trial in rural Alaska.Clin Infect Dis 2002;34:1543–50. [DOI] [PubMed] [Google Scholar]
- 33. Samore MH,Bateman K,Alder SC. et al. Clinical decision support and appropriateness of antimicrobial prescribing: a randomized trial.JAMA 2005;294:2305–14. [DOI] [PubMed] [Google Scholar]
- 34. Rubin M,Bateman K,Alder S. et al. A multifaceted intervention to improve antimicrobial prescribing for upper respiratory tract infections in a small rural community.Clin Infect Dis 2005;40:546–53. [DOI] [PubMed] [Google Scholar]
- 35. Formoso G,Paltrinieri B,Marata AM. et al. Feasibility and effectiveness of a low cost campaign on antibiotic prescribing in Italy: community level, controlled, non-randomised trial.BMJ 2013;347:f5391.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bernier A,Delarocque-Astagneau E,Ligier C. et al. Outpatient antibiotic use in France between 2000 and 2010: after the nationwide campaign, it is time to focus on the elderly.Antimicrob Agents Chemother 2014;58:71–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sabuncu E,David J,Bernède-Bauduin C. et al. Significant reduction of antibiotic use in the community after a nationwide campaign in France, 2002-2007.PLoS Med 2009;6:e1000084.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bauraind I,Lopez-Lozano J,Beyaert A. et al. Association between antibiotic sales and public campaigns for their appropriate use.J Am Med Assoc 2004;292:2468–70. [DOI] [PubMed] [Google Scholar]
- 39. McNulty CAM,Nichols T,Boyle PJ. et al. The English antibiotic awareness campaigns: did they change the public’s knowledge of and attitudes to antibiotic use? J Antimicrob Chemother 2010;65:1526–33. [DOI] [PubMed] [Google Scholar]
- 40. Lambert MF,Masters GA,Brent SL.. Can mass media campaigns change antimicrobial prescribing? A regional evaluation study.J Antimicrob Chemother 2007;59:537–43. [DOI] [PubMed] [Google Scholar]
- 41. Arparsrithongsagul S,Kulsomboon V,Zuckerman HI.. Multidisciplinary perspective intervention with community involvement to decrease antibiotic sales in village groceries in Thailand.Asia-Pacific J Public Heal 2015;27:NP2480–8. [DOI] [PubMed] [Google Scholar]
- 42. Eccles M,Grimshaw J,Campbell M. et al. Research designs for studies evaluating the effectiveness of change and improvement strategies.Qual Saf Health Care 2003;12:47–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hopewell S,McDonald S,Clarke M. et al. Grey literature in meta-analyses of randomized trials of health care interventions.Cochrane Database Syst Rev 2007;2:MR000010.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Jadad AR,Moore RA,Carroll D. et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials.1996;17:1–12. [DOI] [PubMed] [Google Scholar]
- 45. Kunz R,Vist G,Oxman A.. Randomisation to protect against selection bias in healthcare trials.Cochrane Database Syst Rev 2007;2:MR000012. [DOI] [PubMed] [Google Scholar]
- 46. Odgaard-Jensen J,Vist G,Timmer A. et al. Randomisation to protect against selection bias in healthcare trials.Cochrane Database Syst Rev 2011;4:MR000012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Frippiat F,Vercheval C,Layios N.. Decreased antibiotic consumption in the Belgian community: is it credible? Clin Infect Dis 2016;62:403–4. [DOI] [PubMed] [Google Scholar]
- 48. Bruyndonckx R,Hens N,Aerts M. et al. Measuring trends of outpatient antibiotic use in Europe: jointly modelling longitudinal data in defined daily doses and packages.J Antimicrob Chemother 2014;69:1981–6. [DOI] [PubMed] [Google Scholar]
- 49. Ocan M,Obuku E,Bwanga F. et al. Household antimicrobial self-medication: a systematic review and meta-analysis of the burden, risk factors and outcomes in developing countries.BMC Public Health 2015;15:742.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ashiru-Oredope D,Hopkins S.. Antimicrobial resistance: moving from professional engagement to public action.J Antimicrob Chemother 2015;70:2927–30. [DOI] [PubMed] [Google Scholar]
- 51. Bala M,Strzeszynski L,Cahill K.. Mass media interventions for smoking cessation in adults.Cochrane Database Syst Rev 2008;1:CD004704. [DOI] [PubMed] [Google Scholar]
- 52. Hornik R,Kelly B.. Communication and diet: an overview of experience and principles.J Nutr Educ Behav 2007;39:S5–12. [DOI] [PubMed] [Google Scholar]
- 53. Gonzales R,Steiner JF,Sande MA.. Antibiotic prescribing for adults with colds, upper respiratory tract infections, and bronchitis by ambulatory care physicians.JAMA 1997;278:901–4. [PubMed] [Google Scholar]
- 54. Filippini M,Ortiz LG,Masiero G.. Assessing the impact of national antibiotic campaigns in Europe.Eur J Health Econ 2013;14:587–99. [DOI] [PubMed] [Google Scholar]
- 55. Mainous A,Hueston W,Eberlein C.. Colour of respiratory discharge and antibiotic use.Lancet 1997;350:1077.. [DOI] [PubMed] [Google Scholar]
- 56. Hutchinson J,Foley R.. Method of physician remuneration and rates of antibiotic prescription.CMAJ 1999;160:1013–7. [PMC free article] [PubMed] [Google Scholar]
- 57. Curry M,Sung L,Arroll B. et al. Public views and use of antibiotics for the common cold before and after an education campaign in New Zealand.N Z Med J 2006;119:U1957.. [PubMed] [Google Scholar]
- 58. Grijalva CG,Nuorti JP,Griffin MR.. Antibiotic prescription rates for acute respiratory tract infections in US ambulatory settings.JAMA 2009;302:758–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.