Abstract
Purpose of the review
Advances in computing power and wireless technologies have reshaped our approach to patient monitoring. Medical grade sensors and apps that were once restricted to hospitals and specialized clinic are now widely available. Here, we review the current evidence supporting the use of connected health technologies for the prevention and management of cardiovascular disease in an effort to highlight gaps and future opportunities for innovation.
Recent findings
Initial studies in connected health for cardiovascular disease prevention and management focused primarily on activity tracking and blood pressure monitoring but have since expanded to include a full panoply of novel sensors and pioneering smartphone apps with targeted interventions in diet, lipid management and risk assessment, smoking cessation, cardiac rehabilitation, heart failure, and arrhythmias. While outfitting patients with sensors and devices alone is infrequently a lasting solution, monitoring programs that include personalized insights based on patient-level data are more likely to lead to improved outcomes. Advances in this space have been driven by patients and researchers while healthcare systems remain slow to fully integrate and adequately adapt these new technologies into their workflows.
Summary
Cardiovascular disease prevention and management continue to be key focus areas for clinicians and researchers in the connected health space. Exciting progress has been made though studies continue to suffer from small sample size and limited follow up. Efforts that combine home patient monitoring, engagement, and personalized feedback are the most promising. Ultimately, combining patient-level ambulatory sensor data, electronic health records, and genomics using machine learning analytics will bring precision medicine closer to reality.
Keywords: mobile health, digital medicine, innovation
INTRODUCTION
Cardiovascular disease (CVD) remains a leading global health concern with an increasingly large economic burden. With approximately one in three deaths attributable to CVD, it is the primary cause of morbidity as well as mortality and is contributing to growing health care costs [1,2]. The fact that over 90% of the risk for myocardial infarction, a major contributor to CVD morbidity and mortality, is attributable to preventable risk factors [3] offers an enormous potential to improve CVD prevention and management strategies. Despite our understanding that optimizing CVD risk factors, including smoking, obesity, physical inactivity, unhealthy diets, and uncontrolled blood pressure, cholesterol, and diabetes, leads to vast improvements in cardiovascular health outcomes, implementing these modifications remains difficult. Thus, behavioral modification requires innovative tools to support individuals with health behavior changes. The emergence of connected health technology (e.g. mobile phones, smartphones, tablets, wearable devices, smartwatches, personal health sensors) presents an opportunity to revolutionize disease prevention through personalized, convenient, and easily accessible patient education and behavior change support [4–7].
In addition to supporting personalized health changes, connected health technology also has the potential to enable early disease detection and increased convenience and efficacy of chronic disease management. For instance, personal health devices now enable point-of-care screening for cardiac conditions such as hypertension and arrhythmias. Additionally, real-time, passive, non-obtrusive remote monitoring technologies offer patients with chronic conditions requiring long-term management increased convenience and support for better self-care [4–7].
With the growing availability of a broad range of connected health devices, there is enormous potential to transform prevention and management strategies for CVD in order to improve health outcomes and reduce the economic burden of CVD. The goals of connected health technology for CVD range from prevention, disease management, and patient engagement/self-care/education to remote monitoring approaches and tools to augment providers’ ability to care for patients. One of the key objectives of this emerging field is to deliver safe, effective, personalized care at all stages, including prevention, early detection, diagnosis, treatment, and management. Here, we provide an overview of connected health technologies that empower patients with CVD prevention and management tools, with particular focus on coronary artery disease (CAD), heart failure (HF), and arrhythmia.
Coronary Artery Disease (CAD)
Coronary artery disease (CAD) is the most common type of CVD. While there are numerous risk factors for CAD, many of them are modifiable and can be managed through lifestyle changes. Since behavior change is often a complex challenge, a wide range of health technology approaches have been developed to support healthy lifestyle practices, ranging from exercise to medication management.
Blood Pressure Management
Hypertension is a known risk factor for adverse cardiovascular outcomes and present in approximately one third of American adults [1]. The benefits of blood pressure reduction in hypertensive individuals are numerous and include decreased risk for heart failure, myocardial infarction, and stroke. While it has long been demonstrated that lifestyle interventions targeting weight loss, exercise, dietary salt restriction, and minimization of alcohol intake are effective approaches for blood pressure reduction, strategies to effectively support these lifestyle practices are still challenging to implement. The use of connected health technology, which includes a range of tools for patient education, behavior modification support, home self-monitoring of blood pressure, and medication reminders, have generally been shown to be effective at achieving blood pressure reduction [8–13]. Nevertheless, uncertainty remains regarding the long-term sustainability of these interventions and their long-term clinical effects [9]. Furthermore, it is important to note that although self-monitoring of blood pressure can provide greater insight into the individual’s typical blood pressure compared with clinic measurements [14], self-tracking alone is not associated with lower blood pressure or better blood pressure control. Thus, simultaneous interventions, such as systematic medication titration, patient education, or lifestyle counseling, are essential [15–17]. As the field moves forward in attempts to optimize interventions for blood pressure control, researchers are increasingly incorporating feedback from patients in order to maximize the potential impact of digital interventions for hypertension management [18]. Self-tracking of blood pressure through watch-based devices, home blood pressure cuffs, other consumer-facing blood pressure monitors, or the recently developed cuff-less blood pressure monitoring smartphone-based device using the oscillometric finger-pressing method [19] allows for easy measurements for understanding night-time versus day-time and stressed versus calm blood pressure readings [14]. The increasing ease at which blood pressure can be self-monitored and health information can be provided offer enormous potential to prevent and manage hypertension at the population level. However, more rigorous clinical validation is still necessary [20].
Lipid Management
In addition to lifestyle modifications conducive to cardiovascular health in general, there is emphasis on lipid modifying medications as an effective and cost-effective treatment option for improving lipid levels and CVD outcomes. Nevertheless, many patients who meet the indications for statins are never prescribed the medication and those who do receive the prescription have a long-term adherence rate below 50%. As a result, connected health interventions that target both patients and clinicians have been developed [21,22].
Connected health technology to support lipid management includes provider-facing tools such as electronic health record or mobile/web-application based risk calculators using the American College of Cardiology Atherosclerotic Cardiovascular Disease Risk Estimator and Statin Intolerance App, guideline support, alerts to support medication prescribing recommendations, as well as patient-facing tools such as mobile/web-application based risk or “heart age” calculators, medication reminders, patient education, connectivity to healthcare providers, and personalized health management support. Despite the availability of these tools, their uptake in clinical practice has not been wide spread. As a result, more recent studies have incorporated novel strategies to engage both clinicians and patients. In the visualization of asymptomatic atherosclerotic disease for optimum cardiovascular prevention (VIPVIZA) randomized controlled trial to promote adherence to lifestyle changes and medications for improved clinical outcomes, ultrasound-based pictorial information about subclinical carotid atherosclerosis was provided to the intervention group. At one-year follow-up, the intervention group had a decrease in the Framingham risk score and an increase in use of lipid-lowering treatment, suggesting that providing personalized image-based information likely utilizing connected health devices can be an effective precision medicine tool that improves adherence to preventive guidelines in both patients and providers [22].
Methods from behavioral economics have also been explored in research examining the impact of financial incentives on both physicians and patients for lipid management. Patients and physicians in the incentive group were eligible for financial payments when quarterly low-density lipoprotein cholesterol (LDL-C) goals were achieved. The study employed electronic pill bottles for statin medication adherence tracking along with a web-based platform where clinicians and patients could view monthly LDL-C levels. After 12 months, the study found that financial incentives for both physicians and patients in combination, but not alone, resulted in statistically significant reductions in LDL-C levels (mean decrease of 33.6 mg/dL) [23]. Because the cost-effectiveness of this strategy (i.e. financial incentives combined with electronic pill bottles) was unclear, a recent study used a cardiovascular disease microsimulation model to evaluate the cost-effectiveness of financial incentives to physicians, patients, or both on reduction of LDL-C and found that shared financial incentives for LDL-C control can be cost-effective. However, the results are dependent upon assumptions concerning the duration of the intervention [21].
Since both genetic and lifestyle factors are associated with susceptibility to coronary artery disease [24], advances in genomic sequencing and analytic technologies offers further opportunity to individualize lipid management strategies. Although not currently in wide clinical use, genetic risk scores, based upon increasing understanding of how certain DNA variants are associated with susceptibility to diseases such as atherosclerotic cardiovascular disease, are an active area of research for assessing individual disease susceptibility and determining personalized strategies prior to disease onset to delay or even prevent the disease from developing altogether [25–27].
Physical Activity
Worldwide, insufficient physical activity is a major public health problem [28]. Increased physical activity is a powerful strategy to prevent and delay the onset of CVD as well as other chronic conditions [29]. To help encourage increased physical activity, options for personalized physical activity interventions through connected health technology are rapidly growing. Features such as automated activity tracking and ongoing, adaptive interventions individualized based upon the automatically collected activity data are available [30]. Furthermore, the platforms for physical activity connected health technology range from web-based / social media and smartphone applications to pedometers and other wearables, including fitness bands and smartwatches that capture activity metrics and physiological data [31].
Studies have consistently demonstrated that connected health technology can increase physical activity, at least in the short-term [32,33]. However, long-term, sustainable behavior change and impact on clinical health outcomes remain largely unknown [34]. Varying degrees of intervention complexity are available, ranging from simple step-tracking using basic built in smartphone accelerometers to sophisticated applications designed based upon behavior change techniques [35,36]. Many of the same approaches to support behavior change (i.e. self-monitoring, goal-setting, and social support) are employed in a variety of applications and wearable technology, and most of the studies demonstrating the efficacious impact of this technology also employ external behavior change strategies, including social support, financial incentives, and health coaching [37,38]. As a result, it is difficult to discern whether the connected health technology alone without additional support and methods for accountability is capable of sustaining clinically meaningful increases in physical activity. Hence, when considering the use of technology for physical activity promotion, it may be essential to consider theory-driven behavior change strategies that are integrated into the technology design or used as adjunctive tools to support sustainable increases in physical activity. Overall, the most effective interventions are ones that are based on behavior change theory, are personalized to the individual, and offer feedback on the user’s performance [31].
The wide availability of connected health technology to promote physical activity is generating enormous amount of activity and physiological data that can be integrated with other data sources within the healthcare setting to generate actionable insights and inform clinical decision making [39]. Nevertheless, challenges remain regarding how to understand this large volume of data and gain clinical utility from this information. Machine learning approaches are currently being applied to gain insights as well as predict disease onset or progression based upon activity, physiological, and clinical data. Furthermore, incorporating statistical methods and machine learning approaches can allow for increased targeting of intervention delivery through JITAIs that are triggered based upon the ideal timing and location for physical activity for a particular individual.
Diet
An unhealthy diet can contribute to many key CVD risk factors including high blood pressure, dyslipidemia, and insulin resistance. Digital tools aim to help individuals improve dietary practices through nutrition education and in the moment support for healthy dietary recommendations. Although numerous tools exist to support healthy diets, data remain limited on whether they have clinically meaningful results in the long term. As recognized in the American Heart Association (AHA) Scientific Statement on mobile health for CVD prevention, studies of dietary interventions are often also a part of more complex, multi-modal intervention designs that include physical activity promotion or support from a health coach [40]. For example, participants of the multi-modal SmartLoss program for weight loss targeted both diet and physical activity, and used not only a smartphone application and weight tracking through a linked scale but also personalized ongoing feedback in an automated, adaptive manner and from trained counselors [41]. Furthermore, many commercial tools to support healthy dietary practices have not been evaluated in high quality clinical studies, and interventions that have been studied clinically are often not commercially available [40]. Nevertheless, numerous diet-related connected health technology tools are in popular use, including MyFitnessPal and LoseIt. These tools enable users to perform diet tracking including caloric tracking through extensive linkage with nutritional databases. Additionally, food tracking is facilitated through automated food identification with barcode scanning. These applications also include features (i.e. calorie-controlled healthy eating, specific goal setting, self-monitoring, personalized feedback, and social support) the AHA Scientific Statement outlined as important for healthcare practitioners to consider when utilizing this technology as a tool to support weight loss [40].
Other applications focus on empowering users to make better informed healthy food choices. Since nutrition labels can often be difficult to understand and also be misleading, smartphone applications such as Foodswith and Eatery aim to provide easy to understand nutritional information that can inform in the moment dietary decisions. Foodswith allows users to scan food items with barcodes to access a food database with user-friendly nutritional information, three-tiered “traffic light” rating for each food item, and options for healthier alternatives [42]. Eatery employs crowd-sourcing for feedback to inform dietary choices when users share photos of their meals. This crowd-sourcing approach has been demonstrated to correlate well with expert raters’ overall assessments of nutritional value [43]. Overall, a number of connected health approaches exist to support improved dietary practices, yet their impact on clinical outcomes have not been well characterized.
Developments in the areas of automated diet tracking and increased personalization of dietary recommendations are rapidly emerging. Although many connected health strategies for dietary modifications involve diet tracking, recording food intake can be a tedious and time-consuming process, resulting in poor user satisfaction and discontinuation of use. As a result, some platforms are incorporating machine learning technology to enable automatic diet tracking through food detection software that can recognize the food and estimate caloric and nutritional content from photos users capture [44]. Another platform that avoids food logging is BagIQ, which uses passively collected data from grocery stores’ customer loyalty programs to provide an overall dietary quality score and personalized feedback on modifications for healthier diets. Approaches for individualized nutrition counselling through connected health platforms are also increasingly available. Furthermore, recent advancements of analytical tools that enable the study of large quantities of metabolic and health data are driving progress in the field of precision nutrition, which integrates data of various types to enable increased understanding of each person’s individual dietary needs [45]. With increasing amounts of individual-level data from connected health technology and the advancement of predictive machine learning algorithms, there is growing emphasis on strategies for personalized nutrition based on an individual’s physiologic response (such as using continuous glucose monitoring) rather than generalized dietary recommendations and a one-size-fits-all approach [46].
Smoking Cessation
Smoking is not only a major CVD risk factor but alone is the number one cause of preventable death in the United States. While cigarette smoking has declined over time, tobacco use remains widespread [1]. As a result, there has been a concerted effort through many connected health approaches, including short message services (SMS), smartphone applications, and social media-based or web-based interventions, for CVD focus on smoking cessation.
SMS-based smoking cessation programs include ones such as the National Cancer Institute’s SmokefreeTXT program, the Text2Quit from the American Cancer Society’s and Alere Wellbeing’s Quit for Life program, and the UK’s National Health Service Quitkit’s Txt2Stop. Studies of SMS-based smoking cessation interventions have demonstrated proven benefit for improving long-term quit rates [47]. For example, participants with the Text2Quit intervention were two times more likely than the control group to achieve smoking cessation at 6 months post-enrollment [48]. Additionally, the “Happy Quit” randomized controlled trial demonstrated that participants in either the high-frequency or low-frequency text messaging group for smoking cessation were more likely to quit than individuals in the control text message group receiving texts unrelated to quitting [49]. While there are many possible configurations of SMS-based smoking cessation programs, general features that have been found to be effective are fixed schedule messaging (in comparison to decreasing or variable frequency messaging), messages focused on preparation to quit and how to manage smoking urges, and real-time psychological support [47].
A variety of smartphone applications that employ personalized strategies are also available to support smoking cessation. These include apps that act as adjunctive tools to traditional smoking cessation programs, while others take advantage of social connectivity and behavior change strategies. Efforts to develop smartphone applications for smoking cessation that match with clinical practice guidelines have resulted in applications such as the National Cancer Institute’s QuitGuide and QuitStart as well as the Department of Veteran Affairs’ Stay Quit Coach. Although smartphone apps have shown effectiveness for smoking cessation there is room for improvement through tailoring approaches and adaptive messaging personalized to the needs of the user [50]. Considering the individual’s socioeconomic status can also be crucial, since text messages alone may not increase quit rate for those who are socioeconomically disadvantaged, but text messages used in combination with proactive telephone counseling (e.g. assistance on coping with withdrawal, maintaining commitment to smoking cessation, and prevention of relapse) can be an effective strategy [51].
The use of financial incentives, social media, and web-based strategies as well as principles from adaptive intervention designs [52,53] have been explored to further improve the ability of these apps to support smoking cessation efforts. For example, in a pragmatic trial studying electronic cigarettes, free cessation aids (nicotine-replacement therapy or pharmacologic therapy), and financial incentives for smoking cessation, financial incentives with free cessation aids resulted in an increased rate of smoking abstinence than free cessation aids alone [54]. To enhance social connectivity and support for smoking cessation, Crush the Crave includes an open, moderated Facebook group [52]. Additionally, to target and prevent smoking lapses, strategies to manage cue-induced cravings are being developed to deliver “just-in-time” interventions when the user is vulnerable to relapse into smoking. These Just-In-Time Adaptive Interventions (JITAIs) are designed to be triggered in a context-dependent manner to target situations where the user may be prone to relapse such as when the user approaches his or her typically smoking break location [53].
Cardiac Rehabilitation
Hospitalization for cardiovascular events and the following recovery process can often be scary, confusing, and challenging for patients. Despite the demonstrated benefits of cardiac rehabilitation, utilization and completion rates remain low due to barriers such as time, cost, and transportation issues [55]. Connected health solutions offers a novel approach to expanding cardiac rehabilitation services, reaching patients in the convenience of their own homes or community, and achieving comparable if not better results than traditional, center-based approaches [56]. As recently reviewed in [57], virtual cardiac rehabilitation programs utilize a variety of technologies, ranging from video conferencing to smartphone applications. For example, a randomized, controlled trial comparing traditional cardiac rehabilitation with a smartphone cardiac rehabilitation application, which provided daily text messages, multimedia education, relaxation audio, and a light-to-moderate physical activity program, found that participants in the smartphone intervention group had significantly higher adherence and completion rates as well as significantly improved 6-minute walking test distances and depression scores [56]. Hybrid models, which combine center-based sessions with home-based sessions, have also been explored where the cardiac rehabilitation staff can create a personalized plan for each patient using a dashboard that includes a home exercise prescription, educational videos, and daily medications and vital signs reminders. On the patient-facing side of the platform, the care plan is presented as a daily checklist. As patients complete tasks, their information is updated in the cardiac rehabilitation staff’s dashboard, where alerts are presented when there are abnormal vitals or non-adherence with the care plan occurs [58]. Additionally, to provide further support for lifestyle management of risk factors, some programs employ lifestyle health coaching delivered through the telephone or web/smartphone application [59].
Digital technology is also available to help increase patient empowerment at all stages (i.e. hospitalization, discharge, and self-care/recovery). Through a smartphone application, patients who suffered from a myocardial infarction can gain assistance through educational resources for skill-building for diet, exercise, and medication habits; connection to their care teams and resources to help with their recovery; mindfulness techniques to improve patient awareness and emotional strength; and data analytics and biofeedback on steps, heart rate, and blood pressure with smart tools including an Apple watch and Bluetooth blood pressure monitor [60].
Despite the positive results demonstrated in connected health approaches for cardiac rehabilitation, some studies have shown that these interventions do not improve outcomes. For example, the HeartStrong randomized controlled trial examining the effect of electronic reminders, financial incentives, and social support on outcomes following myocardial infarction concluded that the compound intervention did not significantly improve medication adherence or vascular readmission outcomes [61]. Additionally, the HONOR randomized clinical trial demonstrated that home-based exercise with a wearable activity monitor and telephone coaching did not improving walking performance at nine-month follow-up in peripheral artery disease patients [62]. While connected health strategies are emerging that empower patients and increase personalization of cardiac rehabilitation care plans tailored to individual needs, greater research is necessary to determine which approaches are effective in improving health outcomes and why certain studies have positive results while others demonstrate no improvement through the use of connected health technology. Factors to consider include the individual patient’s preferences, quality of the connected health technology, and integration of the technology with clinical care.
Heart Failure (HF)
Heart failure (HF) is a life-threatening progressive disease associated with reduced quality of life, high levels of comorbidity, and high levels of economic burden on the healthcare system [63]. With the major risk factors for HF common to those for CAD and CAD as a risk factor for HF [64], the technology described in the CAD section are also applicable to HF prevention and management. Here, we focus on recent developments specific to HF.
The high rehospitalization rates for HF exacerbations reflect the challenges associated with long-term management of HF [63,65]. As a result, connected health technology research in the area of HF has focused on chronic care of the condition through both nonpharmacological (e.g. fluid and dietary sodium restriction, physical activity and weight loss interventions) and pharmacological management as well as home-monitoring using devices such as smart scales, blood pressure cuffs, home electrocardiogram (ECG) devices [65–70]. Permanently implantable pressure measurement systems are also available through technology such as the CardioMEMS Champion Heart Failure Monitoring System designed to wirelessly monitor pulmonary artery pressure and heart rate to guide HF management and decrease hospitalization [71]. Since cognitive decline can contribute to poor self-care and non-engagement in HF management strategies, digital games are currently in development to assess cognitive function and have the potential to provide individualized information about the patient’s cognitive status which can help inform the development of the patient’s personalized care plan [72]. Additionally, various tools have been developed for automated clinical-decision support based upon home-monitoring for early detection and prediction of HF deterioration [73,74]. Nevertheless, despite some encouraging signals, uncertainty remains regarding the impact of this technology on hospitalization rates, morbidity, and mortality [65,73,75].
Arrhythmias
With an aging population, the prevalence of arrhythmias is increasing, further heightening the need for methods capable of early detection and ongoing arrhythmia monitoring [65]. Since risk factors for arrhythmia are shared with CAD, and CAD itself is a risk factor for arrhythmia [76], the connected health preventive and management strategies described in the CAD section are also applicable to the prevention and management of arrhythmia. In this section, we focus on technology specific to arrhythmia.
Because of the highly variable and unpredictable nature of arrhythmias, management of the condition within the traditional healthcare model of regular or pre-scheduled appointments is suboptimal [77]. As recently reviewed by McConnell et al. [78] and Kotecha et al [79], and as described in the International Society for Holter and Noninvasive Electrocardiology (ISHNE) and Heart Rhythm Society (HRS) expert consensus statement [80], connected health technology has the potential to fill this great clinical need. Various connected health methods have been developed for arrhythmia detection and management, ranging from implantable devices with atrial ECG analysis capable of detecting arrhythmias to non-invasive approaches such as smartwatches and mobile applications capable of distinguishing between atrial fibrillation and normal sinus rhythm [81–84]. Wireless, single-lead real time ECG smartphone monitoring has been shown to accurately detect baseline intervals, atrial rate, and rhythm, enabling screening in diverse populations [85]. Additionally, AliveCor’s KardiaMobile smartphone application [86,87], Kardia Band paired with an Apple Watch [88], and the Apple Series 4 Watch can record ECGs for automated atrial fibrillation diagnosis. For individuals at increased risk of atrial fibrillation, clinical diagnosis can also be facilitated through the use of a home-based self-applied ECG patch [89]. For more extensive monitoring, a wireless handheld monitor capable of 12-lead ECG reconstruction for arrhythmia detection has also been developed and tested in patients following an atrial fibrillation ablation procedure. The performance of the wireless device was found to be more sensitive than periodic Holter monitoring in detecting atrial fibrillation and atrial flutter [90]. Automated, real-time analysis of ECGs and messaging to notify designated healthcare professionals when abnormalities are detected are also available through mobile devices such as HeartSaver, which can identify atrial fibrillation, myocardial infarction, and atrioventricular block using automatic detection algorithms [91].
While connected health technology for arrhythmias detection and management carries the potential to offer increased accessibility of the population to disease screening and management, most of these tools have not been integrated into routine clinical use [77,92]. Questions remain regarding issues of lead placement, electrode contact, and battery drain in cases where indefinite continuous ECG monitoring is required. Furthermore, it is uncertain whether heart rate sensors on watches and fitness bands which employ photoplethysmography (PPG) can be utilized for arrhythmia detection and monitoring [92]. Recently, a novel machine learning algorithm for automated atrial fibrillation detection has been developed using data from a series of clinical studies employing heart-rate and step count data from the Apple Watch. Nevertheless, this is a proof-of-concept study that still requires further research [93]. Of note, the Apple Heart Study is a currently ongoing trial designed to identify atrial fibrillation in up to 500,000 participants with no prior history of the condition [94]. Overall, the field of connected health for arrhythmia detection and management is generating enormous amounts of data that can be leveraged using machine learning technology, with potential to generate actionable insights and support clinical decision making. Recently, the success of machine learning for enabling automated screening has been demonstrated for detecting asymptomatic left ventricular dysfunction from ECGs and atrial fibrillation in PPG pulse waveforms through the use of convolutional neural networks [95,96]. Although additional research is necessary to determine the long-term utility of these learning systems in real-world settings and the appropriate management strategies for individuals identified to be at risk for arrhythmias using these tools, rapid progress is under way in the development, validation, and implementation of these technologies.
DISCUSSION AND CONCLUSION
CVD is a growing public health crisis which requires novel methods for prevention and management. Although there is great potential and excitement for CVD connected health technology, the field is still in an early stage and many questions remain unanswered regarding the real-world and long-term impact of these tools. While initial studies have been promising, caveats include small sample sizes, short study durations, and uncertainty about how the technology will perform in real-world settings and integrate with clinical care. Moreover, connected health devices result in enormous amounts of patient-generated data that healthcare providers currently are often unable to use clinically due to concerns about the validity of the data and challenges associated with interpreting this large volume of information in a clinically meaningful and timely manner. Since clinicians are already reporting being overworked and overwhelmed, it is necessary for connected health to help clinicians be more efficient and improve quality of care rather than adding unnecessary burden. Furthermore, safety and privacy as well as general lack of regulation of this connected health technology are other issues.
One of the biggest opportunities in digital medicine is sensor/data integration. An enormous amount of information regarding health and wellness can be learned by combining multiple signals [97]. Currently, an individual with type 2 diabetes and hypertension would likely have a continuous glucose monitor, an activity monitor, and a blood pressure monitor. Each of these would require a separate device and software application and strikingly there is a lack of an integrated dashboard where the rich data stream from each of these devices come together to provide meaningful insight to the user. Importantly, much of this information is not relayed to the electronic health record. Rather than having a single device solely measuring an individual physiologic or biologic parameter, the goal would be to utilize a single device that provides comprehensive monitoring. Although the initial growth phase for digital health technologies concentrated on the development, clinical validation, and implementation of single sensors, the field is seeing rapid growth in multi-sensor technologies. We are surely to see more efforts like the recently reported ECG and PPG sensors in neonates providing advanced, intensive care unit (ICU) level physiologic monitoring [98]. These innovations will be driven by continued miniaturization of powerful sensors but also by optimizing sensor delivery within soft, form fitting and skin-like materials [99,100]. As efforts in materials science advance with sensor platforms becoming smaller, unobtrusive, and integrated into common wearables, we are likely to see less reliance on implantable devices which risk a host of complications.
Currently, most available sensors are for physiologic parameters such as heart rate, blood pressure, ECG, activity, and SpO2. However, there has been tremendous activity in the development of wearable biosensors for the detection metabolites, proteins, bacteria, DNA, and hormones in biofluids (tears, sweat, saliva, and interstitial fluid) [100]. These novel sensors harness electrochemistry, near infrared spectroscopy, fluorescence, and radio frequency for noninvasive and continuous detection in the form of temporary tattoos, wristbands, patches and even embedded into textiles. For a patient with congestive heart failure on diuretics, a reliable at-home continuous measurement of serum sodium, potassium or NT-proBNP may have enormous clinical implications.
Overall, while further research is necessary, revolutionizing CVD prevention and management through connected health technology is well underway. A wide range of connected health tools, from those that enable population-level disease screening to those that support self-care and patient education, have been developed and offer the potential to reduce healthcare costs and increase the efficacy and personalization of care at all stages of a patient’s journey, from early diagnosis to chronic disease management. The simultaneous advancements in sensor, mobile, wearable, and computing technology as well as data analytics and machine learning algorithms make it an opportune time for connected health technology to improve health outcomes.
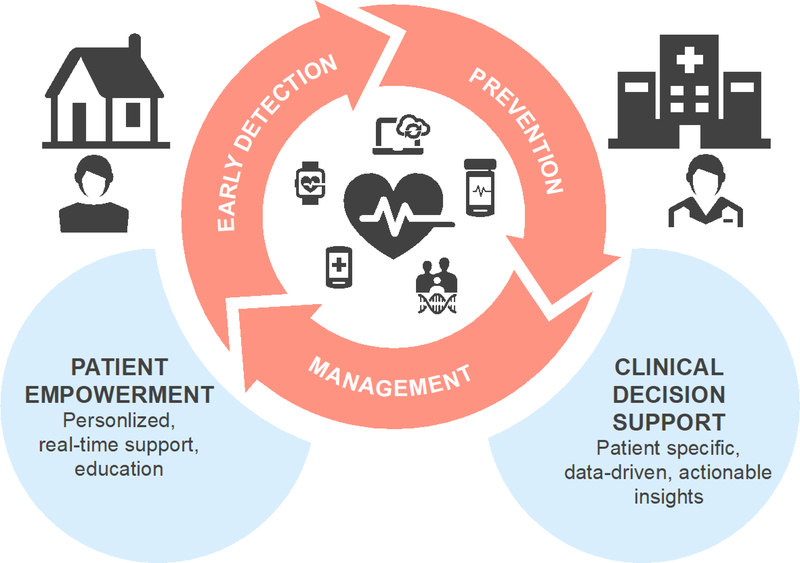
FIGURE 1.
Characteristics of connected health technology
Acknowledgements
SW is supported by the Johns Hopkins School of Medicine Medical Scientist Training Program (National Institutes of Health: Institutional Predoctoral Training Grant - T32), National Institutes of Health: Ruth L. Kirschstein Individual Predoctoral NRSA for MD/PhD: F30 Training Grant, and the Johns Hopkins Individualized Health (inHealth) Initiative. EDM and SRS are supported by UL1TR002550 from NCATS/NIH to The Scripps Research Institute.
REFERENCES
- 1.Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, et al. Heart Disease and Stroke Statistics—2018 Update: A Report From the American Heart Association. Circulation. 2018. March 20;137(12). [DOI] [PubMed] [Google Scholar]
- 2.Mokdad AH, Ballestros K, Echko M, Glenn S, Olsen HE, Mullany E, et al. The State of US Health, 1990–2016. JAMA. 2018. April 10;319(14):1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yusuf S, Hawken S, Ôunpuu S, Dans T, Avezum A, Lanas F, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004. September;364(9438):937–52. [DOI] [PubMed] [Google Scholar]
- 4.Gambhir SS, Ge TJ, Vermesh O, Spitler R. Toward achieving precision health. Sci Transl Med. 2018. February 28;10(430):eaao3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Byambasuren O, Sanders S, Beller E, Glasziou P. Prescribable mHealth apps identified from an overview of systematic reviews. npj Digit Med. 2018. December 9;1(1):12.• In an effort to identify “prescribable” health apps, this evaluation of systematic reviews illustrates the current gaps in evidence from high quality clinical studies.
- 6.Noah B, Keller MS, Mosadeghi S, Stein L, Johl S, Delshad S, et al. Impact of remote patient monitoring on clinical outcomes: an updated meta-analysis of randomized controlled trials. npj Digit Med. 2018. December 15;1(1):20172.•Updated meta-analysis of clinical trials assessing wearable sensors highlighting the importance of behavior change and coaching for targeted interventions.
- 7.Topol EJ. High-performance medicine: the convergence of human and artificial intelligence. Nat Med. 2019. January 7;25(1):44–56. [DOI] [PubMed] [Google Scholar]
- 8.Senecal C, Widmer RJ, Johnson MP, Lerman LO, Lerman A. Digital health intervention as an adjunct to a workplace health program in hypertension. J Am Soc Hypertens. 2018. October 1;12(10):695–702. [DOI] [PubMed] [Google Scholar]
- 9.Mclean G, Band R, Saunderson K, Hanlon P, Murray E, Little P, et al. Digital interventions to promote self-management in adults with hypertension systematic review and meta-analysis. 2016; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lakshminarayan K, Westberg S, Northuis C, Fuller CC, Ikramuddin F, Ezzeddine M, et al. A mHealth-based care model for improving hypertension control in stroke survivors: Pilot RCT. Contemp Clin Trials. 2018. July 1;70:24–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Green BB, Cook AJ, Ralston JD, Fishman PA, Catz SL, Carlson J, et al. Effectiveness of Home Blood Pressure Monitoring, Web Communication, and Pharmacist Care on Hypertension Control. JAMA. 2008. June 25;299(24):2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bosworth HB, Powers BJ, Olsen MK, McCant F, Grubber J, Smith V, et al. Home Blood Pressure Management and Improved Blood Pressure Control. Arch Intern Med. 2011. July 11;171(13):1173. [DOI] [PubMed] [Google Scholar]
- 13.Morawski K, Ghazinouri R, Krumme A, Lauffenburger JC, Lu Z, Durfee E, et al. Association of a Smartphone Application With Medication Adherence and Blood Pressure Control. JAMA Intern Med. 2018. June 1;178(6):802.• In this study, individuals with hypertension received home blood pressure monitors and were randomized to the use of a novel smartphone application to promote mediation adherence. Despite these interventions, no improvement in blood pressure was achieved.
- 14.Steinhubl SR, Muse ED, Barrett PM, Topol EJ. Off the cuff: rebooting blood pressure treatment. Lancet (London, England). 2016. August 20;388(10046):749. [DOI] [PubMed] [Google Scholar]
- 15.Tucker KL, Sheppard JP, Stevens R, Bosworth HB, Bove A, Bray EP, et al. Self-monitoring of blood pressure in hypertension: A systematic review and individual patient data meta-analysis. Rahimi K, editor. PLOS Med. 2017. September 19;14(9):e1002389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McManus RJ, Mant J, Franssen M, Nickless A, Schwartz C, Hodgkinson J, et al. Efficacy of self-monitored blood pressure, with or without telemonitoring, for titration of antihypertensive medication (TASMINH4): an unmasked randomised controlled trial. Lancet (London, England). 2018. March 10;391(10124):949–59.•Pivotal trial for home blood pressure monitoring illustrating benefits over traditional (clinical) blood pressure monitoring in the ambulatory setting.
- 17.Margolis KL, Asche SE, Dehmer SP, Bergdall AR, Green BB, Sperl-Hillen JM, et al. Long-term Outcomes of the Effects of Home Blood Pressure Telemonitoring and Pharmacist Management on Blood Pressure Among Adults With Uncontrolled Hypertension. JAMA Netw Open. 2018. September 7;1(5):e181617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bradbury K, Morton K, Band R, van Woezik A, Grist R, McManus RJ, et al. Using the Person-Based Approach to optimise a digital intervention for the management of hypertension. Aslani P, editor. PLoS One. 2018. May 3;13(5):e0196868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chandrasekhar A, Kim C-S, Naji M, Natarajan K, Hahn J-O, Mukkamala R. Smartphone-based blood pressure monitoring via the oscillometric finger-pressing method. Sci Transl Med. 2018. March 7;10(431):eaap8674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Plante TB, Urrea B, MacFarlane ZT, Blumenthal RS, Miller ER, Appel LJ, et al. Validation of the Instant Blood Pressure Smartphone App. JAMA Intern Med. 2016;176(5):700–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pandya A, Asch DA, Volpp KG, Sy S, Troxel AB, Zhu J, et al. Cost-effectiveness of Financial Incentives for Patients and Physicians to Manage Low-Density Lipoprotein Cholesterol Levels. JAMA Netw Open. 2018. September 14;1(5):e182008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Näslund U, Ng N, Lundgren A, Fhärm E, Grönlund C, Johansson H, et al. Visualization of asymptomatic atherosclerotic disease for optimum cardiovascular prevention (VIPVIZA): a pragmatic, open-label, randomised controlled trial. Lancet (London, England). 2018. December 3;393(10167):133–42. [DOI] [PubMed] [Google Scholar]
- 23.Asch DA, Troxel AB, Stewart WF, Sequist TD, Jones JB, Hirsch AG, et al. Effect of Financial Incentives to Physicians, Patients, or Both on Lipid Levels. JAMA. 2015. November 10;314(18):1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khera AV, Emdin CA, Drake I, Natarajan P, Bick AG, Cook NR, et al. Genetic Risk, Adherence to a Healthy Lifestyle, and Coronary Disease. N Engl J Med. 2016;375(24):2349–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Muse ED, Torkamani A, Topol EJ. When genomics goes digital. Lancet (London, England). 2018. June 16;391(10138):2405. [DOI] [PubMed] [Google Scholar]
- 26.Khera AV, Chaffin M, Aragam KG, Haas ME, Roselli C, Choi SH, et al. Genome-wide polygenic scores for common diseases identify individuals with risk equivalent to monogenic mutations. Nat Genet. 2018. September 13;50(9):1219–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Torkamani A, Wineinger NE, Topol EJ. The personal and clinical utility of polygenic risk scores. Nat Rev Genet. 2018. September 22;19(9):581–90. [DOI] [PubMed] [Google Scholar]
- 28.Guthold R, Stevens GA, Riley LM, Bull FC. Worldwide trends in insufficient physical activity from 2001 to 2016: a pooled analysis of 358 population-based surveys with 1·9 million participants. Lancet Glob Heal. 2018. October 1;6(10):e1077–86. [DOI] [PubMed] [Google Scholar]
- 29.Lee I-M, Shiroma EJ, Lobelo F, Puska P, Blair SN, Katzmarzyk PT, et al. Effect of physical inactivity on major non-communicable diseases worldwide: an analysis of burden of disease and life expectancy. Lancet (London, England). 2012. July 21;380(9838):219–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Franklin NC. Technology to Promote and Increase Physical Activity in Heart Failure. Heart Fail Clin. 2015. January;11(1):173–82. [DOI] [PubMed] [Google Scholar]
- 31.Murray CJL, Abraham J, Ali MK, Alvarado M, Atkinson C, Baddour LM, et al. The State of US Health, 1990–2010. JAMA. 2013. August 14;310(6):591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martin SS, Feldman DI, Blumenthal RS, Jones SR, Post WS, McKibben RA, et al. mActive: A Randomized Clinical Trial of an Automated mHealth Intervention for Physical Activity Promotion. J Am Heart Assoc. 2015. October 29;4(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Compernolle S, Vandelanotte C, Cardon G, De Bourdeaudhuij I, De Cocker K. Effectiveness of a Web-Based, Computer-Tailored, Pedometer-Based Physical Activity Intervention for Adults: A Cluster Randomized Controlled Trial. J Med Internet Res. 2015. February 9;17(2):e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Finkelstein EA, Haaland BA, Bilger M, Sahasranaman A, Sloan RA, Nang EEK, et al. Effectiveness of activity trackers with and without incentives to increase physical activity (TRIPPA): a randomised controlled trial. lancet Diabetes Endocrinol. 2016. December 1;4(12):983–95.•A large-scale evaluation of the use of positive incentive strategies (cash or charitable donations) to increase physical activity. Despite improvements in activity with either incentive, there were no changes in health outcomes.
- 35.Urrea B, Misra S, Plante TB, Kelli HM, Misra S, Blaha MJ, et al. Mobile Health Initiatives to Improve Outcomes in Primary Prevention of Cardiovascular Disease. Curr Treat Options Cardiovasc Med. 2015. December 17;17(12):59. [DOI] [PubMed] [Google Scholar]
- 36.Conroy DE, Yang C-H, Maher JP. Behavior Change Techniques in Top-Ranked Mobile Apps for Physical Activity. Am J Prev Med. 2014. June;46(6):649–52. [DOI] [PubMed] [Google Scholar]
- 37.Phillips SM, Cadmus-Bertram L, Rosenberg D, Buman MP, Lynch BM. Wearable Technology and Physical Activity in Chronic Disease: Opportunities and Challenges. Am J Prev Med. 2018. January 1;54(1):144–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chokshi NP, Adusumalli S, Small DS, Morris A, Feingold J, Ha YP, et al. Loss-Framed Financial Incentives and Personalized Goal-Setting to Increase Physical Activity Among Ischemic Heart Disease Patients Using Wearable Devices: The ACTIVE REWARD Randomized Trial. J Am Heart Assoc. 2018. June 19;7(12).•In a CV high risk cohort, this study investigated loss-framed incentives (in contrast to TRIPPA which used positive incentives) to promote healthy behaviors.
- 39.Lim WK, Davila S, Teo JX, Yang C, Pua CJ, Blöcker C, et al. Beyond fitness tracking: The use of consumer-grade wearable data from normal volunteers in cardiovascular and lipidomics research. Kirkwood T, editor. PLOS Biol. 2018. February 27;16(2):e2004285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Burke LE, Ma J, Azar KMJ, Bennett GG, Peterson ED, Zheng Y, et al. Current Science on Consumer Use of Mobile Health for Cardiovascular Disease Prevention. Circulation. 2015. September 22;132(12):1157–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martin CK, Miller AC, Thomas DM, Champagne CM, Han H, Church T. Efficacy of SmartLoss SM, a smartphone-based weight loss intervention: Results from a randomized controlled trial. Obesity. 2015. May;23(5):935–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dunford E, Trevena H, Goodsell C, Ng KH, Webster J, Millis A, et al. FoodSwitch: A Mobile Phone App to Enable Consumers to Make Healthier Food Choices and Crowdsourcing of National Food Composition Data. JMIR mHealth uHealth. 2014. August 21;2(3):e37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Turner-McGrievy GM, Helander EE, Kaipainen K, Perez-Macias JM, Korhonen I. The use of crowdsourcing for dietary self-monitoring: crowdsourced ratings of food pictures are comparable to ratings by trained observers. J Am Med Informatics Assoc. 2014. August 4;22(e1):e112–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang W, Yu Q, Siddiquie B, Divakaran A, Sawhney H. “Snap-n-Eat.” J Diabetes Sci Technol. 2015. May 21;9(3):525–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.O’Sullivan A, Henrick B, Dixon B, Barile D, Zivkovic A, Smilowitz J, et al. 21st century toolkit for optimizing population health through precision nutrition. Crit Rev Food Sci Nutr. 2017. July 5;1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bashiardes S, Godneva A, Elinav E. Towards utilization of the human genome and microbiome for personalized nutrition. Curr Opin Biotechnol. 2018. June 1;51:57–63. [DOI] [PubMed] [Google Scholar]
- 47.Spohr SA, Nandy R, Gandhiraj D, Vemulapalli A, Anne S, Walters ST. Efficacy of SMS Text Message Interventions for Smoking Cessation: A Meta-Analysis. J Subst Abuse Treat. 2015. September;56:1–10. [DOI] [PubMed] [Google Scholar]
- 48.Abroms LC, Boal AL, Simmens SJ, Mendel JA, Windsor RA. A Randomized Trial of Text2Quit. Am J Prev Med. 2014. September;47(3):242–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liao Y, Wu Q, Kelly BC, Zhang F, Tang Y-Y, Wang Q, et al. Effectiveness of a text-messaging-based smoking cessation intervention (“Happy Quit”) for smoking cessation in China: A randomized controlled trial. Degenhardt L, editor. PLOS Med. 2018. December 18;15(12):e1002713.•This study, done exclusively in China, showed clinical efficacy of a cognitive behavioral therapy based text-messaging approach for smoking cessation.
- 50.BinDhim NF, McGeechan K, Trevena L. Who Uses Smoking Cessation Apps? A Feasibility Study Across Three Countries via Smartphones. JMIR mhealth uhealth. 2014. February 6;2(1):e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vidrine DJ, Frank-Pearce SG, Vidrine JI, Tahay PD, Marani SK, Chen S, et al. Efficacy of Mobile Phone–Delivered Smoking Cessation Interventions for Socioeconomically Disadvantaged Individuals. JAMA Intern Med. 2018. December 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Struik LL, Baskerville NB. The Role of Facebook in Crush the Crave, a Mobile- and Social Media-Based Smoking Cessation Intervention: Qualitative Framework Analysis of Posts. J Med Internet Res. 2014. July 11;16(7):e170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Delivering Naughton F. “Just-In-Time” Smoking Cessation Support Via Mobile Phones: Current Knowledge and Future Directions: Table 1. Nicotine Tob Res. 2016. May 28;19(3):ntw143. [DOI] [PubMed] [Google Scholar]
- 54.Halpern SD, Harhay MO, Saulsgiver K, Brophy C, Troxel AB, Volpp KG. A Pragmatic Trial of E-Cigarettes, Incentives, and Drugs for Smoking Cessation. N Engl J Med. 2018. June 14;378(24):2302–10. [DOI] [PubMed] [Google Scholar]
- 55.Dalal HM, Doherty P, Taylor RS. Cardiac rehabilitation. BMJ. 2015. September 29;351:h5000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Varnfield M, Karunanithi M, Lee C-K, Honeyman E, Arnold D, Ding H, et al. Smartphone-based home care model improved use of cardiac rehabilitation in postmyocardial infarction patients: results from a randomised controlled trial. Heart. 2014. November 15;100(22):1770–9. [DOI] [PubMed] [Google Scholar]
- 57.Lear SA. The Delivery of Cardiac Rehabilitation Using Communications Technologies: The “Virtual” Cardiac Rehabilitation Program. Can J Cardiol. 2018. October 1;34(10):S278–83. [DOI] [PubMed] [Google Scholar]
- 58.Classick-Wallace MaryAnn; Bockol Faith; Blaber R. Feasibility of a Smartphone-Delivered, Hybrid Cardiac Rehabilitation Program. J Cardiopulm Rehabil Prev. 2017. September;37(5):363–85.28858034 [Google Scholar]
- 59.Gordon NF, Salmon RD, Wright BS, Faircloth GC, Reid KS, Gordon TL. Clinical Effectiveness of Lifestyle Health Coaching: Case Study of an Evidence-Based Program. Am J Lifestyle Med. 2017;11(2):153–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Marvel FA, Wang J, Martin SS. Digital Health Innovation: A Toolkit to Navigate From Concept to Clinical Testing. JMIR Cardio. 2018. January 18;2(1):e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Volpp KG, Troxel AB, Mehta SJ, Norton L, Zhu J, Lim R, et al. Effect of Electronic Reminders, Financial Incentives, and Social Support on Outcomes After Myocardial Infarction. JAMA Intern Med. 2017. August 1;177(8):1093.•An inventive study that utilized a combination approach consisting of financial incentives, electronic pill bottles, and social support to promote medication adherence, yet resulting in no significant improvements. Highlights the complex issues regarding behavior change.
- 62.McDermott MM, Spring B, Berger JS, Treat-Jacobson D, Conte MS, Creager MA, et al. Effect of a Home-Based Exercise Intervention of Wearable Technology and Telephone Coaching on Walking Performance in Peripheral Artery Disease. JAMA. 2018. April 24;319(16):1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lee WC, Chavez YE, Baker T, Luce BR. Economic burden of heart failure: a summary of recent literature. Heart Lung. 33(6):362–71. [DOI] [PubMed] [Google Scholar]
- 64.Del Gobbo LC, Kalantarian S, Imamura F, Lemaitre R, Siscovick DS, Psaty BM, et al. Contribution of Major Lifestyle Risk Factors for Incident Heart Failure in Older Adults. JACC Hear Fail. 2015. July 1;3(7):520–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Honeyman E, Ding H, Varnfield M, Karunanithi M. Mobile health applications in cardiac care. Interv Cardiol. 2014. April;6(2):227–40. [Google Scholar]
- 66.Koehler F, Winkler S, Schieber M, Sechtem U, Stangl K, Böhm M, et al. Telemedicine in heart failure: Pre-specified and exploratory subgroup analyses from the TIM-HF trial. Int J Cardiol. 2012. November 29;161(3):143–50. [DOI] [PubMed] [Google Scholar]
- 67.Talmor G, Nguyen B, Keibel A, Temelkovska T, Saxon L. Use of software applications to improve medication adherence and achieve more integrated disease management in heart failure. Trends Cardiovasc Med. 2018. October 1;28(7):483–8. [DOI] [PubMed] [Google Scholar]
- 68.Athilingam P, Jenkins B. Mobile Phone Apps to Support Heart Failure Self-Care Management: Integrative Review. JMIR Cardio. 2018. May 2;2(1):e10057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Alnosayan N, Chatterjee S, Alluhaidan A, Lee E, Houston Feenstra L. Design and Usability of a Heart Failure mHealth System: A Pilot Study. JMIR Hum factors. 2017. March 24;4(1):e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.De La I, Díez T, Garcia-Zapirain B, Méndez-Zorrilla Amaia &, López-Coronado M. Monitoring and Follow-up of Chronic Heart Failure: a Literature Review of eHealth Applications and Systems. [DOI] [PubMed] [Google Scholar]
- 71.Abraham WT, Adamson PB, Bourge RC, Aaron MF, Costanzo MR, Stevenson LW, et al. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: a randomised controlled trial. Lancet. 2011. February 19;377(9766):658–66. [DOI] [PubMed] [Google Scholar]
- 72.Pereira VFA, Valentin LSS. The MentalPlus® Digital Game Might Be an Accessible Open Source Tool to Evaluate Cognitive Dysfunction in Heart Failure with Preserved Ejection Fraction in Hypertensive Patients: A Pilot Exploratory Study. Int J Hypertens. 2018. August 6;2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Koehler F, Koehler K, Deckwart O, Prescher S, Wegscheider K, Kirwan B-A, et al. Efficacy of telemedical interventional management in patients with heart failure (TIMHF2): a randomised, controlled, parallel-group, unmasked trial. Lancet (London, England). 2018. September 22;392(10152):1047–57.•This study combined home telemonitoring sensor technologies (BP, bodyweight, heart rate and rhythm, SpO2) with tailored, patient-specific interventions that resulted in a reduction of days lost due to CV hospitalizations and all-cause mortality.
- 74.Support-hf, Investigators, Committees. Home monitoring with IT-supported specialist management versus home monitoring alone in patients with heart failure: Design and baseline results of the SUPPORT-HF 2 randomized trial SUPPORT-HF 2 Investigators and Committees 1 Trial Designs. 2018. [DOI] [PubMed] [Google Scholar]
- 75.Cajita MI, Gleason KT, Han H-R. A Systematic Review of mHealth-Based Heart Failure Interventions. J Cardiovasc Nurs. 2016;31(3):E10–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lau DH, Nattel S, Kalman JM, Sanders P. Modifiable Risk Factors and Atrial Fibrillation. Circulation. 2017. August 8;136(6):583–96. [DOI] [PubMed] [Google Scholar]
- 77.Turakhia MP, Kaiser DW. Transforming the care of atrial fibrillation with mobile health. J Interv Card Electrophysiol. 2016. October 15;47(1):45–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.McConnell MV, Turakhia MP, Harrington RA, King AC, Ashley EA. Mobile Health Advances in Physical Activity, Fitness, and Atrial Fibrillation: Moving Hearts. J Am Coll Cardiol. 2018. June 12;71(23):2691–701. [DOI] [PubMed] [Google Scholar]
- 79.Kotecha D, Chua WWL, Fabritz L, Hendriks J, Casadei B, Schotten U, et al. European Society of Cardiology smartphone and tablet applications for patients with atrial fibrillation and their health care providers. EP Eur. 2018. February 1;20(2):225–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Steinberg JS, Varma N, Cygankiewicz I, Aziz P, Balsam P, Baranchuk A, et al. 2017 ISHNE-HRS expert consensus statement on ambulatory ECG and external cardiac monitoring/telemetry. Hear Rhythm. 2017. Jul;14(7):e55–96. [DOI] [PubMed] [Google Scholar]
- 81.McMANUS DD, CHONG JW, SONI A, SACZYNSKI JS, ESA N, NAPOLITANO C, et al. PULSE-SMART: Pulse-Based Arrhythmia Discrimination Using a Novel Smartphone Application. J Cardiovasc Electrophysiol. 2016. January;27(1):51–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Golzar M, Fotouhi-Ghazvini F, Rabbani H, Zakeri FS. Mobile Cardiac Health-care Monitoring and Notification with Real Time Tachycardia and Bradycardia Arrhythmia Detection. J Med Signals Sens. 7(4):193–202. [PMC free article] [PubMed] [Google Scholar]
- 83.McManus DD, Lee J, Maitas O, Esa N, Pidikiti R, Carlucci A, et al. A novel application for the detection of an irregular pulse using an iPhone 4S in patients with atrial fibrillation. Hear Rhythm. 2013. Mar;10(3):315–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chan P, Wong C, Poh YC, Pun L, Leung WW, Wong Y, et al. Diagnostic Performance of a Smartphone-Based Photoplethysmographic Application for Atrial Fibrillation Screening in a Primary Care Setting. J Am Heart Assoc. 2016. July 6;5(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.HABERMAN ZC, JAHN RT, BOSE R, TUN H, SHINBANE JS, DOSHI RN, et al. Wireless Smartphone ECG Enables Large-Scale Screening in Diverse Populations. J Cardiovasc Electrophysiol. 2015. May;26(5):520–6. [DOI] [PubMed] [Google Scholar]
- 86.William AD, Kanbour M, Callahan T, Bhargava M, Varma N, Rickard J, et al. Assessing the accuracy of an automated atrial fibrillation detection algorithm using smartphone technology: The iREAD Study. Hear Rhythm. 2018. October 1;15(10):1561–5. [DOI] [PubMed] [Google Scholar]
- 87.Halcox JPJ, Wareham K, Cardew A, Gilmore M, Barry JP, Phillips C, et al. Assessment of Remote Heart Rhythm Sampling Using the AliveCor Heart Monitor to Screen for Atrial Fibrillation. Circulation. 2017. November 7;136(19):1784–94. [DOI] [PubMed] [Google Scholar]
- 88.Bumgarner JM, Lambert CT, Hussein AA, Cantillon DJ, Baranowski B, Wolski K, et al. Smartwatch Algorithm for Automated Detection of Atrial Fibrillation. J Am Coll Cardiol. 2018. May 29;71(21):2381–8. [DOI] [PubMed] [Google Scholar]
- 89.Steinhubl SR, Waalen J, Edwards AM, Ariniello LM, Mehta RR, Ebner GS, et al. Effect of a Home-Based Wearable Continuous ECG Monitoring Patch on Detection of Undiagnosed Atrial Fibrillation. JAMA. 2018. July 10;320(2):146.•While this study reported a creative screening strategy for individuals at high risk of atrial fibrillation, a key aspect here is the complete digitization of the clinical trial operations from start to finish.
- 90.Gussak I, Vukajlovic D, Vukcevic V, George S, Bojovic B, Hadzievski L, et al. Wireless remote monitoring of reconstructed 12-lead ECGs after ablation for atrial fibrillation using a hand-held device. J Electrocardiol. 2012. March;45(2):129–35. [DOI] [PubMed] [Google Scholar]
- 91.Sankari Z, Adeli H. HeartSaver: A mobile cardiac monitoring system for auto-detection of atrial fibrillation, myocardial infarction, and atrio-ventricular block. Comput Biol Med. 2011. April;41(4):211–20. [DOI] [PubMed] [Google Scholar]
- 92.Turakhia MP. Moving From Big Data to Deep Learning—The Case of Atrial Fibrillation. JAMA Cardiol. 2018. May 1;3(5):371. [DOI] [PubMed] [Google Scholar]
- 93.Tison GH, Sanchez JM, Ballinger B, Singh A, Olgin JE, Pletcher MJ, et al. Passive Detection of Atrial Fibrillation Using a Commercially Available Smartwatch. JAMA Cardiol. 2018. May 1;3(5):409.•This was one of the first studies to combine a smartwatch assessment of cardiac rhythm with deep learning analytics illustrating the challenges of rhythm prediction in an ambulatory setting.
- 94.Apple Heart Study: Assessment of Wristwatch-Based Photoplethysmography to Identify Cardiac Arrhythmias - Full Text View - ClinicalTrials.gov [Internet]. [cited 2018 Dec 14]. Available from: https://clinicaltrials.gov/ct2/show/NCT03335800
- 95.Attia ZI, Kapa S, Lopez-Jimenez F, McKie PM, Ladewig DJ, Satam G, et al. Screening for cardiac contractile dysfunction using an artificial intelligence–enabled electrocardiogram. Nat Med. 2019. January 7;25(1):70–4. [DOI] [PubMed] [Google Scholar]
- 96.Poh M-Z, Poh YC, Chan P-H, Wong C-K, Pun L, Leung WW-C, et al. Diagnostic assessment of a deep learning system for detecting atrial fibrillation in pulse waveforms. Heart. 2018. December 1;104(23):1921–8. [DOI] [PubMed] [Google Scholar]
- 97.Li X, Dunn J, Salins D, Zhou G, Zhou W, Schüssler-Fiorenza Rose SM, et al. Digital Health: Tracking Physiomes and Activity Using Wearable Biosensors Reveals Useful Health-Related Information. Kirkwood T, editor. PLOS Biol. 2017. January 12;15(1):e2001402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chung HU, Kim BH, Lee JY, Lee J, Xie Z, Ibler EM, et al. Binodal, wireless epidermal electronic systems with in-sensor analytics for neonatal intensive care. Science. 2019. March 1;363(6430):eaau0780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Huang Z, Hao Y, Li Y, Hu H, Wang C, Nomoto A, et al. Three-dimensional integrated stretchable electronics. Nat Electron. 2018. August 13;1(8):473–80. [Google Scholar]
- 100.Kim J, Campbell AS, de Ávila BE-F, Wang J. Wearable biosensors for healthcare monitoring. Nat Biotechnol. 2019. February 25;1. [DOI] [PMC free article] [PubMed] [Google Scholar]