ABSTRACT
Background
Reducing red meat intake is commonly recommended. Limited observational studies suggest that healthy eating patterns with red meat are associated with improved quality of life.
Objective
The secondary objectives of this randomized crossover controlled-feeding trial were to assess the effects of following a Mediterranean-style eating pattern (Med-Pattern) with different amounts of red meat on indexes of personal well-being (i.e., perceived quality of life, mood, and sleep) in overweight or obese adults. We hypothesized that following a Med-Pattern would improve these outcomes, independent of red meat intake amount.
Methods
Forty-one participants [aged 46 ± 2 y; body mass index (kg/m2): 30.5 ± 0.6;n = 28 women,n = 13 men) were provided Med-Pattern foods for two 5-wk periods separated by 4 wk of self-selected eating. The Med-Red Pattern contained ∼500 g/wk (typical US intake), and the Med-Control Pattern contained ∼200 g/wk (commonly recommended intake in heart-healthy eating patterns) of lean, unprocessed beef or pork compensated with mainly poultry and dairy. Baseline and postintervention outcomes measured were perceived quality of life via the MOS 36-Item Short-Form Health Survey, version 2 (SF-36v2), daily mood states via the Profile of Mood States (POMS), sleep perceptions via the Pittsburgh Sleep Quality Index, and sleep patterns via actigraphy. Data were analyzed via a doubly repeated-measures ANOVA adjusted for age, sex, and body mass at each time point.
Results
Following a Med-Pattern did not change domains of physical health, mental health, total mood disturbances, sleep perceptions, and sleep patterns but improved subdomains of physical health role limitations (SF-36v2: 93.6–96.7%;P = 0.038), vitality (SF-36v2: 57.9–63.0%;P = 0.020), and fatigue (POMS: 2.9–2.5 arbitrary units;P = 0.039). There were no differences between the Med-Red and Med-Control Patterns (time × pattern,P-interaction > 0.05).
Conclusion
Following a Med-Pattern, independent of lean, unprocessed red meat intake, may not be an effective short-term strategy to meaningfully improve indexes of personal well-being in adults who are overweight or obese. This trial was registered atclinicaltrials.gov as NCT02573129.
Keywords: pork, beef, unprocessed meat, vitality, health-related quality of life, sleep, mood, actigraphy, overweight/obese adults
Introduction
Comprehensive literature assessments show that unprocessed red meat consumption is neutral to cardiovascular disease development (1–3) and associated risk factors (4,5). Evidence supports that lean unprocessed red meat can be consumed in a healthy eating pattern, such as a Dietary Approaches to Stop Hypertension (DASH) or a Mediterranean-style pattern (Med-Pattern), to improve cardiovascular disease risk factor profiles (6–9). Yet, it is still commonly recommended to adopt an eating pattern that is low in red meat (10).
Observational research suggests that healthy eating patterns including low to moderate intakes of red meat are associated with improved quality of life (11,12) and may lower the risk of depression (13–15). Conclusions about independent associations between red meat intake and quality of life or mental health outcomes are inconsistent (16), but some research suggests a lower risk of depressive symptoms when red meat is consumed in an otherwise plant-based eating pattern (17). A systematic review of dietary counseling interventions of individuals without clinical mental health diagnoses showed that recommendations to lower red meat consumption were less likely to improve depression and anxiety symptoms than typical-intake controls (18). However, there is a paucity of controlled feeding trials that assessed effects of consuming lean unprocessed red meat in the context of healthy eating patterns on multiple indexes of global personal well-being in adults who are overweight or obese and at risk of developing chronic diseases.
We previously reported that following a Med-Pattern with ∼500 g lean, unprocessed red meat/wk improves cardiovascular disease risk factor profiles in adults who are overweight or obese (19). The secondary objectives were to assess the effects of following a Med-Pattern with red meat amounts that are typical of US residents (∼500 g/wk) (20,21) or commonly recommended in heart-healthy eating patterns (∼200 g/wk) (22,23) on multiple indexes of personal well-being. We hypothesized that following a Med-Pattern would improve indexes of personal well-being, including perceived quality of life, daily mood states, and sleep. We also hypothesized that the inclusion of higher-than-recommended amounts of red meat, as lean unprocessed beef and pork, would not influence Med-Pattern–induced improvements in personal well-being.
Methods
Ethics
The study protocol and all study documents were approved by Purdue University's Biomedical Institutional Review Board (institutional review board protocol 1501015662). Participants provided written informed consent and received monetary stipends. The study was conducted July 2015 through December 2016 and is registered atclinicaltrials.gov (NCT02573129).
Experimental design
The experimental design was a 16-wk randomized, crossover, investigator-blinded, controlled-feeding study. Participants consumed a Med-Pattern for two 5-wk interventions separated by a washout period of ≥4 wk during which the participants resumed their self-selected unrestricted eating patterns. Personal well-being outcomes, as described below, were measured during both baseline weeks while participants consumed their self-selected unrestricted eating pattern and during the last week of each Med-Pattern intervention. Participants were randomly assigned using an online randomization plan generator (http://www.randomization.com/).
Participant recruitment
Male and female volunteers [BMI (kg/m2): 25–37; aged 30–69 y] were recruited from the Greater Lafayette, Indiana, community via local advertisements (paper flyers and campus e-mail listserv). Participants were excluded if they habitually consumed a Med-Pattern, as indicated by a score >5 on the 14-item Mediterranean Diet Assessment Tool (24). Possible scores range from 0 to 14 points. Inclusion criteria were nonsmokers, nondiabetic, self-reported weight stability (±4.5 kg), and consistent physical activity levels for 3 mo before starting the study. Participants were not excluded if they were previously diagnosed with a mental health disorder as long as the condition and/or all medications were stable for ≥6 mo before and throughout the study. Participant depression scores were calculated at baseline 1 using the Patient Health Questionnaire-9 (25).
Dietary intervention
All foods were prepared and provided to participants during the dietary interventions by the NIH-supported Indiana Clinical Research Center Bionutrition Facility at Purdue University. The PREDIMED protocol (26) was used to design the menus, which contained either ∼500 g (Med-Red Pattern) or ∼200 g (Med-Control Pattern) of lean, unprocessed beef or pork/wk but similar amounts of seafood and legumes. Further adjustments were required to match energy and macronutrients via manipulation of predominantly poultry and dairy. Subjects were given the option to consume 150 mL self-selected dry red wine daily. Menus were designed to maintain participants’ baseline 1 body mass. Participants completed daily menu check-off lists to record self-reported deviations from prescribed menus. Detailed descriptions of the Mediterranean patterns have been previously published (19) and are available inSupplemental Tables 1 and 2.
Perceived quality of life
Participants completed the RAND Health's Medical Outcome Study 36-Item Short-Form Health Survey, version 2 (SF-36v2), questionnaire about the previous 4 wk (28,29). The SF-36v2 measures physical health (perceived physical function, role limitations due to physical health, bodily pain, and general health) and mental health (vitality, social functioning, role limitations due to emotional health, and general mental health); 100% is optimal.
Daily mood
Participants completed the Profile of Mood States questionnaire (POMS) every waking hour for 3 d, and 3-d mean composite scores were calculated (30).
Indexes of sleep
Participants completed the Pittsburgh Sleep Quality Index (PSQI) (31,32) about the previous 4 wk. A higher global sleep score (0–21 au) indicates poorer sleep; a global sleep score ≥5 is classified as “poor sleep” (31). Analyses were performed on all participants (n = 38) and a subset of those classified as poor sleepers (n = 16).
Wrist-worn actigraphy (Actiwatch 2; Phillips Respironics) and bed-wake times were recorded for ≤4 weekdays and weeknights. Actigraphy measurements were taken at 30-s intervals. The actigraphs were scored using the manufacturer's algorithm set at medium sensitivity, with the rest intervals set manually based on1) when the indicator button was pressed,2) bed and wake times recorded in sleep logs, or3) algorithm default. Assessed variables included time spent in bed, time spent sleeping, sleep efficacy (percentage of time spent in bed sleeping), onset latency (amount of time to fall asleep once in bed), and total number of minutes awake after sleep onset (WASO). Three participants’ actigraphy data were excluded from analyses due to non–study-related circadian shifts (n = 2) or Actiwatch malfunction at >1 time point (n = 1). We measured 475 nights of actigraphy in 38 participants, but 71 (15%) nights were not usable due to Actiwatch malfunction or non–study-related activities that interfered with the participants’ regular sleep schedule. Analyses were performed on all participants (n = 38) and a subset of those with ≥3 nights of usable data (n = 15).
Power calculations
A priori power calculations (G*Power version 3.1.9.2; Heinrich-Heine-Universität, Düsseldorf, Germany) showed that 40 participants provided >90% power to detect changes in perceived vitality on the SF-36v2 from adopting a Med-Pattern similar to that observed previously (33). We hypothesized that including higher amounts of red meat would not influence changes in overall well-being. A priori power calculations showed that 40 participants provided >85% power to detect a differential response between the Med-Red Pattern and the Med-Control Pattern that is equal to half of the SD of the difference in responses (Cohen'sd = 0.5; medium effect size) (34).
Statistical analysis
A doubly repeated-measures ANOVA model was used to assess the following items:1) main effects of time (baseline compared with post; 1-tailed due to directional hypothesis),2) time × pattern (Med-Red Pattern changes compared with Med-Control Pattern changes; 2-tailed),3) post hoc pattern–specific effects (within the Med-Red Pattern and within the Med-Control Pattern; 1-tailed due to directional hypothesis) when time × patternP < 0.05,4) Med-Red Pattern compared with Med-Control Pattern postmeasurements and Med-Red Pattern compared with Med-Control Pattern baseline measurements using the slice statement to assess the cross-sectional effects of each Med-Pattern (time × pattern sliced by time; 2-tailed), and5) baseline 1 compared with baseline 2 measurements using the slice statement to determine if participants’ baseline 1 status was reestablished at baseline 2 (time × trial sliced by time; 2-tailed). This analysis was completed by invoking the Proc Mixed procedure in SAS (SAS Institute). Age, sex (due to possible confounding), and body mass at each time point [due to previously noted differences over time between the Med-Red Pattern and the Med-Control Pattern (19)] were included in the model with a random participant effect. Results are presented as least-square means ± least-square mean SEMs. AllP values were Tukey-Kramer corrected for multiple comparisons; significance was set atP < 0.05. Sleep-related outcomes were further Benjamini-Hochberg corrected to limit the family-wise error rate due to the exploratory nature of this study objective (35).
RESULTS
Participant characteristics
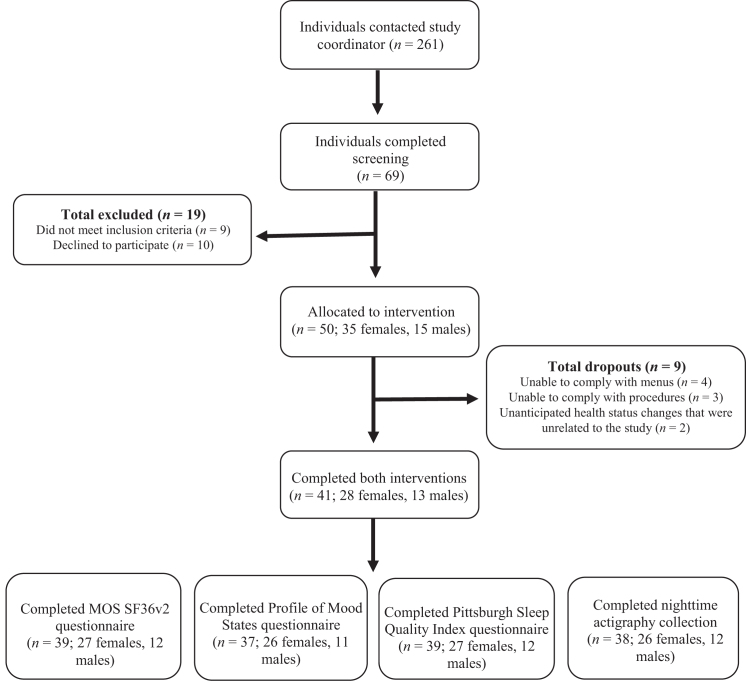
Forty-one participants completed both Med-Pattern interventions (seeFigure 1). At baseline, participants had poor perceived general health and vitality but were good sleepers, according to the PSQI, and slept ∼7 h/night (seeTable 1), on average. According to the Patient Health Questionnaire-9 at baseline 1, 36 participants were minimally to mildly depressed (3 were taking antidepressant medication), 3 participants were moderately depressed (1 was taking antidepressant medication), 1 participant was severely depressed (taking antidepressant medication), and 1 participant had missing data but reported taking antidepressant medication.
FIGURE 1.
Recruitment for a randomized, crossover, controlled trial in which adults who are overweight or obese consumed a Mediterranean-style eating pattern with different amounts of lean, unprocessed red meat. MOS; SF-36v2, 36-Item Short-Form Health Survey, version 2.
TABLE 1.
Baseline characteristics of participants following a Mediterranean-style eating pattern with different amounts of lean, unprocessed red meat1
| Variable | Med-Red Pattern | Med-Control Pattern |
|---|---|---|
| Age, y | 46 ± 10 | 46 ±10 |
| BMI, kg/m2 | 30.5 ±3.5 | 30.3 ±3.5 |
| Global sleep score,2 au | 5.2 ±3.1 | 5.0 ±2.5 |
| Time spent sleeping/night,3 min | 396.5 ±42.0 | 395.5 ±53.6 |
| Sleep efficiency,3 % | 85.3 ±5.4 | 85.1 ±6.5 |
| WASO,3 min | 40.1 ±13.8 | 39.1 ±18.7 |
| Total mood disturbances,4 au | 102.8 ±9.7 | 101.4 ±9.0 |
| Perceived general health,5 % | 71.4 ±17.7 | 74.7 ±14.7 |
| Perceived vitality,5 % | 61.7 ±20.0 | 57.0 ±19.0 |
1Values are means ± SDs,n = 41. There was no difference between baseline measurements for these outcomes (P > 0.05). Med-Control Pattern, Mediterranean-style eating pattern with ∼200 g of lean, unprocessed red meat/wk; Med-Red Pattern, Mediterranean-style eating pattern with ∼500 g of lean, unprocessed red meat/wk; MOS; SF-36v2, 36-Item Short-Form Health Survey, version 2; WASO, wake after sleep onset in minutes.
2Measured with the Pittsburgh Sleep Quality Index; ≥5 out of 21 indicates a “poor sleeper.”
3Measured by wrist-worn actigraphy.
4Measured with the Profile of Moods State questionnaire; range: 100–176, with 100 indicating no mood disturbances.
5Measured with the MOS SF-36v2; composite scores out of 100%.
Dietary intakes
Mediterranean Diet Assessment Tool scores increased from 4 au at baseline to 12 and 13 au (≥200% increase) for the Med-Red Pattern and Med-Control Pattern menus, respectively. Participants reported ≥95% compliance to prescribed menus. Details of the prescribed menus are shown inSupplemental Tables 1 and 2.
Perceived quality of life via SF-36v2
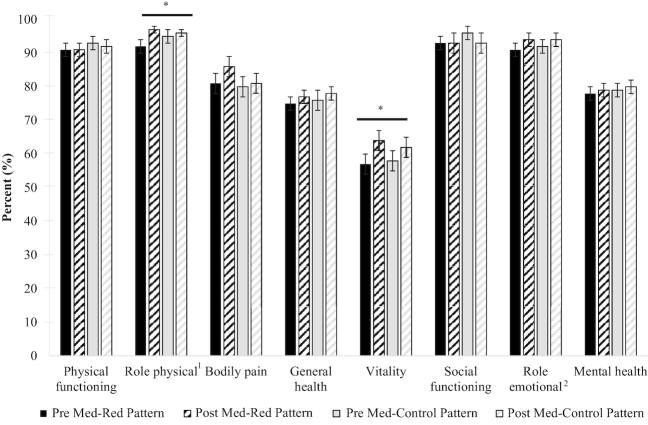
Following a Med-Pattern, independent of red meat intake, improved the physical health subdomain of role limitations due to physical health (93.6–96.7%;P = 0.038) and the mental health subdomain of vitality (57.9–63.0%;P = 0.020; seeFigure 2). There were no changes in global physical health, global mental health, or the subdomains of physical functioning, bodily pain, general health, social functioning, role limitations due to emotional health (i.e., role emotional), or mental health. Postintervention values did not differ between the Med-Red Pattern and the Med-Control Pattern for any SF-36v2 outcomes.
FIGURE 2.
Changes in perceived quality of life of adults who are overweight or obese after 5 wk of following a Mediterranean-style eating pattern with different amounts of lean, unprocessed red meat. Values are least-square means ± least-square mean SEMs,n = 39. All values were extracted from a doubly repeated-measures ANOVA adjusted for age, sex, and body mass at each time point. *Different from Pre,P < 0.05. There were no differences between the Med-Red Pattern and the Med-Control Pattern.1Meaured via MOS SF-36v2;2role limitations due to physical health;3role limitations due to emotional health. Med-Control Pattern, Mediterranean-style eating pattern with ∼200 g of lean, unprocessed red meat/wk; Med-Red Pattern, Mediterranean-style eating pattern with ∼500 g of lean unprocessed red meat/wk; MOS; SF-36v2, 36-Item Short-Form Health Survey, version 2.
Daily mood via POMS
Following a Med-Pattern, independent of red meat intake, improved fatigue (2.9 to 2.5 au;P = 0.039; seeSupplemental Table 3). There were no changes in total mood disturbances or the subdomains of tension, depression, anger, vigor, and confusion. Postintervention values did not differ between the Med-Red Pattern and the Med-Control Pattern for any POMS outcomes.
Indexes of sleep
According to the PQSI, perceived sleep quality of all participants (n = 38) improved with adoption of a Med-Pattern (1.1 au at baseline to 0.9 at postintervention out of 3 au;P = 0.003; seeSupplemental Table 4), independent of red meat intake. However, this result was not robust to Benjamini-Hochberg correction for multiple comparisons. Furthermore, this effect was not present in the “poor sleepers” subset (n = 16). Global sleep score, duration, disturbance, latency, daytime dysfunction, efficiency, and sleeping medication usage did not change in all participants or in the “poor sleepers” subset with consumption of a Med-Pattern. Postintervention values did not differ between the Med-Red Pattern and the Med-Control Pattern for any PSQI outcomes.
Nighttime actigraphy outcomes were largely unchanged with the consumption of a Med-Pattern, independent of red meat intake, for all participants (n = 38) and for the subset of those with ≥3 nights of usable data (n = 15). WASO decreased with the Med-Red Pattern but not with the Med-Control Pattern. However, the number of baseline WASO minutes was higher with the Med-Red Pattern than with the Med-Control Pattern. There were trends for time spent in bed and time spent sleeping to increase with the Med-Control Pattern but not the Med-Red Pattern, but these were not robust to Benjamini-Hochberg correction for multiple comparisons. Postintervention values did not differ between the Med-Red Pattern and the Med-Control Pattern for any actigraphy outcomes (seeTable 2).
TABLE 2.
Changes in sleep patterns of adults who are overweight or obese after 5 wk of following a Mediterranean dietary pattern with different amounts of lean, unprocessed red meat1
| Med-Red Pattern | Med-Control Pattern | P | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline (n = 34) | Post (n = 35) | Change | Within-patternP2 | Baseline (n = 36) | Post (n = 37) | Change | Within-patternP2 | Time | Time × pattern | |
| Time in bed, min | 442 ± 10 | 431 ± 9 | −12 ± 10 | 0.023 | 427 ± 10 | 447 ± 9 | 20 ± 10 | 0.012 | 0.31 | 0.006 |
| Time sleeping, min | 401 ± 9 | 393 ± 9 | −8 ± 9 | 0.21 | 388 ± 9 | 409 ± 9 | 21 ± 9 | 0.016 | 0.21 | 0.008 |
| Sleep efficiency, % | 85 ± 1 | 85 ± 1 | 0 ± 1 | N/A | 84 ± 1 | 85 ± 1 | 1 ± 1 | N/A | 0.34 | 0.40 |
| WASO | 37 ± 2† | 33 ± 2 | −4 ± 1 | 0.005 | 34 ± 2 | 35 ± 2 | 1 ± 1 | 0.17 | 0.15 | <0.001* |
| Onset latency, min | 17 ± 2 | 17 ± 3 | 0 ± 3 | N/A | 15 ± 2 | 16 ± 3 | 1 ± 3 | N/A | 0.41 | 0.77 |
1Values are least-square means ± least-square mean SEMs. All values were extracted from a doubly repeated-measures ANOVA adjusted for age, sex, and body mass at each time point. An average of 3 nights of data were analyzed at each time point. *P value robust to Benjamini-Hochberg correction for multiple comparisons.†Difference in baseline values robust to Benjamini-Hochberg correction for multiple comparisons. Med-Control Pattern, Mediterranean-style eating pattern with ∼200 g of lean, unprocessed red meat/wk; Med-Red Pattern, Mediterranean-style eating pattern with ∼500 g of lean, unprocessed red meat/wk; N/A, not assessed; WASO, wake after sleep onset in minutes.
2Post hoc pattern-specific effects were analyzed when time × patternP < 0.05.
Discussion
Following healthy eating patterns that are low in red meat are commonly recommended to reduce cardiometabolic disease risk in the United States. However, our previous results (19), supported by other research (6–9,36), showed that healthy eating patterns with lean, unprocessed red meat have cardiometabolic benefits for adults who are overweight or obese. It is important to consider how the recommendation to reduce red meat intake influences outcomes beyond indexes of cardiometabolic health. In particular, personal well-being ramifications should be considered given the associations between red meat–containing healthy eating patterns and high quality of life (11,12) and better mental health symptoms (13). The current results show that adopting a Med-Pattern, independent of red meat intake, may not meaningfully influence indexes of personal well-being in the short term.
Our present results suggest that the adoption of a Med-Pattern, independent of red meat intake, increases participants’ energy level (i.e., increased vitality, decreased role limitations due to physical health, and decreased fatigue). These results support observational research that suggests that higher adherence to a Med-Pattern allowing up to 150 g total red meat/d is associated with higher vitality scores than is lower adherence (11,37). However, vitality differences reported between the lowest- and highest-adherence quintiles were modest (<10%) and the vitality scores of the highest-adherence quintile were poor (<70 out of 100%). Similarly, results from longer-term (12-wk) randomized controlled trials using similar Med-Patterns showed improvements of ∼11–15% in vitality scores and postintervention scores remained <70% (33,38). The vitality scores reported in these studies are similar to the changes (5% increase from baseline) and end of intervention values (63% at postintervention) documented in our study. Vitality scores are commonly lowest and have the most room for improvement out of all quality-of-life parameters, as shown in the mentioned studies as well as ours. Although significant, the clinical relevancy of these modest improvements should be interpreted with caution when making dietary recommendations.
The effects of adopting healthy eating patterns on mental health are inconsistent. A systematic review of randomized controlled trials noted that various healthy eating patterns were effective at improving depressive symptoms in ∼50% of studies (18). Dietary interventions that were focused on reducing red meat, fat, or cholesterol intake were less likely to improve depressive symptoms than typical-intake control interventions (18). Our results showed that adopting a Med-Pattern did not improve mental health (except for slight improvements in vitality) and depression scores in the short-term, with or without reductions in red meat intake. The majority of studies included in the previously mentioned systematic review (18), as well as our study, assessed indexes of personal well-being as secondary outcomes in participants who were mentally healthy. At baseline, our participants had good mental health (∼80% out of 100% on the SF36v2) with low levels of depressive mood states (<2 out of 32 au on the POMS). Therefore, our null results are likely attributable to there being little room for improvement in these outcomes. There is need for investigation with regard to how healthy eating patterns, particularly with recommendations to reduce red meat intake, affect individuals with poor mental health [1 in 6 US adults (39)].
Poor sleep quantity and quality are independently associated with an increased risk of obesity (40), cardiometabolic disease (41,42), and depression (43). Over the past few decades, sleep duration of individuals living in the United States has decreased and approximately one-third report sleeping <6 h/night (44). The 2015 Dietary Guidelines Advisory Committee emphasized the need for more research on how dietary intakes affect sleep in concert with cardiometabolic heath (45). Therefore, we explored this paradigm within our tightly controlled feeding trial using survey-measured and machine-measured indexes of sleep. We reported little effect of adopting a Med-Pattern on short-term changes in indexes of sleep, possibly due to the short intervention duration or lack of statistical power to detect changes. Our post hoc power calculations showed that we were >95% powered to detect a large effect size (Cohen'sd = −0.83) between baseline and after measurements of perceived sleep quality (PSQI) but <20% powered to detect small to medium effect sizes in global sleep scores (PQSI) or sleep efficiency (actigraphy). Adequately powered, longer-term randomized controlled trials are needed to investigate whether healthy eating pattern interventions, such as a Med-Pattern, can meaningfully improve indexes of sleep, particularly in individuals with poor sleep patterns.
To our knowledge, this study is the first contribution to the literature about the effects of following healthy eating patterns and red meat on indexes of personal well-being. The majority of published research on this topic consist of observational cohort studies that assigned Med-Pattern scoring systems to FFQ data or randomized controlled trials that were dietary counseling based. This study, although short in duration, was a tightly controlled randomized trial in which all foods and beverages were prepared and provided to participants. This feature is strengthened by a ≥95% self-reported dietary compliance rate and a low participant drop-out percentage (<18%). The inclusion and exclusion criteria of this study were designed to achieve the primary goal of assessing cardiometabolic changes, as previously published (19), and did not include measures of quality of life, mental health, or sleep quality. This resulted in a convenience sample of participants who were fair sleepers and mentally healthy, which was likely a contributor to the largely null results.
Our results support previous literature that shows that the effects of adopting healthy eating patterns, such as a Med-Pattern, on personal well-being are modest and inconsistent. Furthermore, reducing red meat intake does not enhance improvements in indexes of personal well-being related to adopting a Med-Pattern. There is a need to assess how adopting healthy eating patterns, with and without recommendations to reduce red meat intake, influences long-term changes in perceived quality of life, daily mood, and measures of sleep in populations with poor mental health and dysregulated sleep patterns.
Supplementary Material
Acknowledgments
We thank Jan K Green for clinical support; Amy J Wright, Steven A Hulsey Jr., and Anne K Wilcox for dietary support; Jia Li for statistical support; and Richard D Sayer for grant-writing contributions. The authors’ responsibilities were as follows—LEO, DP-J, and WWC: designed the research project; LEO: was responsible for participant recruitment and conducting the research and analyzed the data; LEO and SLB: compiled and processed the data; LEO and WWC: wrote the manuscript with editorial assistance from DP-J and AJS; WWC: had primary responsibility for final content; and all authors: read and approved the final manuscript.
Notes
Supported in part by the Beef Checkoff and the Pork Checkoff with additional support from the National Institutes of Health's Ingestive Behavior Research Center at Purdue University (5T32DK076540-08) and the National Institutes of Health's Indiana Clinical and Translational Sciences Institute (UL1TR001108). These organizations had no role in the design or conduct of the study; collection, analysis, or interpretation of the data; or writing of the manuscript.
Author disclosures: LEO and SLB, no conflicts of interest. DP-J, AJS, and WWC, no conflicts of interest directly related to the research presented in this article. WWC's relationships over the past 2 y include grant funding to support other research projects unrelated to the research presented in this article and/or travel reimbursements from the Beef Checkoff, the Pork Checkoff, the National Dairy Council, the North Dakota Beef Commission, the American Egg Board–Egg Nutrition Center, and Barilla International. DP-J's relationships over the past 2 y include grant funding to support other research projects unrelated to the research presented in this article and/or travel reimbursements or honoraria from the American Egg Board, Leprino Foods, the National Dairy Council, the Beef Checkoff, and the US Dairy Export Council. AJS's relationships over the past 2 y include grant funding to support other research projects unrelated to the research presented in this article and/or travel reimbursements from the National Institute of Mental Health, National Institute of Child Health and Human Development, the Kinley Trust, Purdue Research Foundation, Indiana Clinical and Translational Sciences Institute, Gadomski Foundation, and Johnson's Baby and the Center for Child and Family Well-being at the University of Wisconsin, Madison.
Supplemental Tables 1–4 are available from the “Supplementary data” link in the online posting of this article and from the same link in the online table of contents athttps://academic.oup.com/jn/.
Abbreviations used:
- Med-Control Pattern
Mediterranean-style eating pattern with ∼200 g of lean, unprocessed red meat/wk
- Med-Pattern
Mediterranean-style eating pattern
- Med-Red Pattern
Mediterranean-style eating pattern with ∼500 g of lean, unprocessed red meat/wk
- POMS
Profile of Mood States questionnaire
- PSQI
Pittsburgh Sleep Quality Index
- SF-36v2
36-Item Short-Form Health Survey, version 2
- WASO
wake after sleep onset in minutes.
References
- 1. Micha R,Michas G,Mozaffarian D. Unprocessed red and processed meats and risk of coronary artery disease and type 2 diabetes—an updated review of the evidence.Curr Atheroscler Rep 2012;14:515–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wang X,Lin X,Ouyang YY,Liu J,Zhao G,Pan A,Hu FB. Red and processed meat consumption and mortality: dose-response meta-analysis of prospective cohort studies.Public Health Nutr 2016;19:893–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kaluza J,Wolk A,Larsson SC. Red meat consumption and risk of stroke: a meta-analysis of prospective studies.Stroke 2012;43:2556–60. [DOI] [PubMed] [Google Scholar]
- 4. O'Connor LE,Kim JE,Campbell WW. Total red meat intake of ≥0.5 servings/d does not negatively influence cardiovascular disease risk factors: a systemically searched meta-analysis of randomized controlled trials.Am J Clin Nutr 2017;105:57–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Maki KC,Van Elswyk ME,Alexander DD,Rains TM,Sohn EL,McNeill S. A meta-analysis of randomized controlled trials that compare the lipid effects of beef versus poultry and/or fish consumption.J Clin Lipidol 2012;6:352–61. [DOI] [PubMed] [Google Scholar]
- 6. Roussell MA,Hill AM,Gaugler TL,West SG,Ulbrecht JS,Vanden Heuvel JP,Gillies PJ,Kris-Etherton PM. Effects of a DASH-like diet containing lean beef on vascular health.J Hum Hypertens 2014;28:600–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Roussell MA,Hill AM,Gaugler TL,West SG,Vanden Heuvel JP,Alaupovic P,Gillies PJ,Kris-Etherton PM. Beef in an optimal lean diet study: effects on lipids, lipoproteins, and apolipoproteins.Am J Clin Nutr 2012;95:9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sayer RD,Wright AJ,Chen N,Campbell WW. Dietary Approaches to Stop Hypertension diet retains effectiveness to reduce blood pressure when lean pork is substituted for chicken and fish as the predominant source of protein.Am J Clin Nutr 2015;102:302–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nowson CA,Wattanapenpaiboon N,Pachett A. Low-sodium dietary approaches to stop Hypertension-type diet including lean red meat lowers blood pressure in postmenopausal women.Nutr Res 2009;29:8–18. [DOI] [PubMed] [Google Scholar]
- 10. US Department of Health and Human Services;USDA . 2015–2020 Dietary guidelines for Americans,8th editionVersion current December2015[cited 2018 Mar 10].Available from:http://health.gov/dietaryguidelines/2015/guidelines/. [Google Scholar]
- 11. Henriquez Sanchez P,Ruano C,de Irala J,Ruiz-Canela M,Martinez-Gonzalez MA,Sanchez-Villegas A. Adherence to the Mediterranean diet and quality of life in the SUN project.Eur J Clin Nutr 2012;66:360–8. [DOI] [PubMed] [Google Scholar]
- 12. Munoz MA,Fito M,Marrugat J,Covas MI,Schroder H;Regicor, Investigators H Adherence to the Mediterranean diet is associated with better mental and physical health.Br J Nutr 2009;101:1821–7. [DOI] [PubMed] [Google Scholar]
- 13. Jacka FN,Mykletun A,Berk M,Bjelland I,Tell GS. The association between habitual diet quality and the common mental disorders in community-dwelling adults: the Hordaland Health Study.Psychosom Med 2011;73:483–90. [DOI] [PubMed] [Google Scholar]
- 14. Li Y,Lv MR,Wei YJ,Sun L,Zhang JX,Zhang HG,Li B. Dietary patterns and depression risk: a meta-analysis.Psychiatry Res 2017;253:373–82. [DOI] [PubMed] [Google Scholar]
- 15. Lai JS,Hiles S,Bisquera A,Hure AJ,McEvoy M,Attia J. A systematic review and meta-analysis of dietary patterns and depression in community-dwelling adults.Am J Clin Nutr 2014;99:181–97. [DOI] [PubMed] [Google Scholar]
- 16. Zhang Y,Yang Y,Xie MS,Ding X,Li H,Liu ZC,Peng SF. Is meat consumption associated with depression? A meta-analysis of observational studies.BMC Psychiatry 2017;17:409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Meyer BJ,Kolanu N,Griffiths DA,Grounds B,Howe PR,Kreis IA. Food groups and fatty acids associated with self-reported depression: an analysis from the Australian National Nutrition and Health Surveys.Nutrition 2013;29:1042–7. [DOI] [PubMed] [Google Scholar]
- 18. Opie RS,O'Neil A,Itsiopoulos C,Jacka FN. The impact of whole-of-diet interventions on depression and anxiety: a systematic review of randomised controlled trials.Public Health Nutr 2015;18:2074–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. O'Connor LE,Paddon-Jones D,Wright AJ,Campbell WW. A Mediterranean-style eating pattern with lean, unprocessed red meat has cardiometabolic benefits for adults who are overweight or obese in a randomized, crossover, controlled feeding trial.Am J Clin Nutr 2018;108(1):33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gifford CL,O'Connor LE,Campbell WW,Woerner DR,Belk KE. Broad and inconsistent muscle food classification is problematic for dietary guidance in the U.S.Nutrients 2017;9(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Daniel CR,Cross AJ,Koebnick C,Sinha R. Trends in meat consumption in the USA.Public Health Nutr 2011;14(4):575–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Karanja NM,Obarzanek E,Lin PH,McCullough ML,Phillips KM,Swain JF,Champagne CM,Hoben KP; DASH Collaborative Research Group.Descriptive characteristics of the dietary patterns used in the Dietary Approaches to Stop Hypertension Trial.J Am Diet Assoc 1999;99(8 Suppl):S19–27. [DOI] [PubMed] [Google Scholar]
- 23. Swain JF,McCarron PB,Hamilton EF,Sacks FM,Appel LJ. Characteristics of the diet patterns tested in the Optimal Macronutrient Intake Trial to Prevent Heart Disease (OmniHeart): options for a heart-healthy diet.J Am Diet Assoc 2008;108(2):257–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Martinez-Gonzalez MA,Garcia-Arellano A,Toledo E,Salas-Salvado J,Buil-Cosiales P,Corella D,Covas MI,Schroder H,Aros F,Gomez-Gracia E et al.. A 14-item Mediterranean diet assessment tool and obesity indexes among high-risk subjects: the PREDIMED trial.PLoS One 2012;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kroenke K,Spitzer RL,Williams JB. The PHQ-9: validity of a brief depression severity measure.J Gen Intern Med 2001;16:606–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. PREDIMED Study Mediterranean diet in the primary prevention of cardiovascular disease: research protocol.Version 1. Version current October2003[cited 2018 Mar 10].Available from:http://www.predimed.es/uploads/8/0/5/1/8051451/_1estr_protocol_olf.pdf. [Google Scholar]
- 27. Lin PH,Windhauser MM,Plaisted CS,Hoben KP,McCullough ML,Obarzanek E; DASH Collaborative Research Group.The linear index model for establishing nutrient goals in the Dietary Approaches to Stop Hypertension trial.J Am Diet Assoc 1999;99:S40–44. [DOI] [PubMed] [Google Scholar]
- 28. McHorney CA,Ware JE Jr.,Raczek AE. The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs.Med Care 1993;31:247–63. [DOI] [PubMed] [Google Scholar]
- 29. McHorney CA,Ware JE Jr.,Lu JF,Sherbourne CD. The MOS 36-item Short-Form health survey (SF-36): III. Tests of data quality, scaling assumptions, and reliability across diverse patient groups.Med Care 1994;32:40–66. [DOI] [PubMed] [Google Scholar]
- 30. Torres SJ,Nowson CA. A moderate-sodium DASH-type diet improves mood in postmenopausal women.Nutrition 2012;28:896–900. [DOI] [PubMed] [Google Scholar]
- 31. Buysse DJ,Reynolds CF, Monk TH,Berman SR,Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research.Psychiatry Res 1989;28:193–213. [DOI] [PubMed] [Google Scholar]
- 32. Zhou J,Kim JE,Armstrong CL,Chen N,Campbell WW. Higher-protein diets improve indexes of sleep in energy-restricted overweight and obese adults: results from 2 randomized controlled trials.Am J Clin Nutr 2016;103:766–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Skoldstam L,Hagfors L,Johansson G. An experimental study of a Mediterranean diet intervention for patients with rheumatoid arthritis.Ann Rheum Dis 2003;62:208–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sullivan GM,Feinn R. Using effect size—or why theP value is not enough.J Grad Med Educ 2012;4:279–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Benjamin Y,Hocheberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing.J Royal Stat Soc Ser B 1995;57:289–300. [Google Scholar]
- 36. Hill AM,Harris Jackson KA,Roussell MA,West SG,Kris-Etherton PM. Type and amount of dietary protein in the treatment of metabolic syndrome: a randomized controlled trial.Am J Clin Nutr 2015;102:757–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Milte CM,Thorpe MG,Crawford D,Ball K,McNaughton SA. Associations of diet quality with health-related quality of life in older Australian men and women.Exp Gerontol 2015;64:8–16. [DOI] [PubMed] [Google Scholar]
- 38. Landaeta-Diaz L,Fernandez JM,Da Silva-Grigoletto M,Rosado-Alvarez D,Gomez-Garduno A,Gomez-Delgado F,Lopez-Miranda J,Perez-Jimenez F,Fuentes-Jimenez F. Mediterranean diet, moderate-to-high intensity training, and health-related quality of life in adults with metabolic syndrome.Eur J Prev Cardiol 2013;20:555–64. [DOI] [PubMed] [Google Scholar]
- 39. National Institute of Mental Health Mental illness.[cited 2018 Mar 10].Available from:https://www.nimh.nih.gov/health/statistics/mental-illness.shtml. [Google Scholar]
- 40. Wu Y,Zhai L,Zhang D. Sleep duration and obesity among adults: a meta-analysis of prospective studies.Sleep Med 2014;15:1456–62. [DOI] [PubMed] [Google Scholar]
- 41. Cappuccio FP,Cooper D,D'Elia L,Strazzullo P,Miller MA. Sleep duration predicts cardiovascular outcomes: a systematic review and meta-analysis of prospective studies.Eur Heart J 2011;32:1484–92. [DOI] [PubMed] [Google Scholar]
- 42. Cappuccio FP,D'Elia L,Strazzullo P,Miller MA. Quantity and quality of sleep and incidence of type 2 diabetes: a systematic review and meta-analysis.Diabetes Care 2010;33:414–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Murphy MJ,Peterson MJ. Sleep disturbances in depression.Sleep Med Clin 2015;10:17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ford ES,Cunningham TJ,Croft JB. Trends in self-reported sleep duration among US adults from 1985 to 2012.Sleep 2015;38:829–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. US Department of Health and Human Services ;USDA Scientific report of the 2015 Dietary Guidelines Advisory Committee, part D. Chapter 3, introduction, page 2. Version current February2015[cited 2018 Mar 2].Available from:https://health.gov/dietaryguidelines/2015-scientific-report/pdfs/scientific-report-of-the-2015-dietary-guidelines-advisory-committee.pdf. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.