Abstract
Background
Ulcerative colitis causes recurring intestinal mucosal injury and sustained inflammation, increasing the likelihood of colorectal cancer (CRC) development. Dietary red raspberry (RB) is a rich source of phytonutrients known to have anti-inflammatory activity; however, the role of RB on CRC prevention in chronic colitis has not been examined.
Objective
This study examined the effects of dietary RB supplementation on inflammation, epithelium repair, and oncogenic signaling in dextran sulfate sodium (DSS)–induced chronic colitis in mice.
Methods
Six-week-old male C57BL/6J mice were fed a control or RB (5% of dry feed weight;n = 12/group) diet for 10 wk. Starting from the fourth week, mice were administered 2 repeated cycles of 1% DSS (7-d DSS treatment plus 14-d recovery) and were monitored daily for disease activity index (DAI) score. Colonic tissues were collected at the end of the study for histochemical, immunohistochemical, and biochemical analysis of inflammation, differentiation and proliferation markers.
Results
RB supplementation reduced the DAI score and histologic damage (by 38.9%;P ≤ 0.01), expression of inflammatory mediators (by 20–70%;P ≤ 0.01), infiltration of CD4 T cells (by 50%;P ≤ 0.05), and α4β7 integrin and related adhesion molecules (by 33.3%;P ≤ 0.01). Furthermore, RB supplementation facilitated epithelium repair, as evidenced by enhanced goblet cell density, expression of transcription factors including Kruppel-like factor 4 (Klf4) and Hairy and enhancer of split 1 (Hes1), terminal differentiation markers, mucin 2 (Muc2), and intestinal alkaline phosphatase (by 20–200%;P ≤ 0.01). Conversely, proliferating cell nuclear antigen (by 70%;P ≤ 0.01), β-catenin, and signal transducer and activator of transcription 3 (STAT3) signaling (by 19–33%;P ≤ 0.05) were reduced by RB supplementation. In addition, RB supplementation enhanced p53 stability (by 53%) and reduced oncogenic gene expression (by 50–60%).
Conclusion
RB supplementation reduced DAI score and the risk of CRC development during recurring colitis in mice, suggesting that RB is a possible dietary supplement for patients with ulcerative colitis and related gut inflammatory diseases.
Keywords: colitis, colorectal cancer, inflammation, proliferation, intestine, red raspberry
Introduction
Ulcerative colitis, a common form of inflammatory bowel disease, is characterized by chronic recurring inflammation of the colon, which is known to increase the risk of colorectal cancer (CRC) development (1). CRC is the third most common cancer in the United States and Europe and is becoming common around the world (2,3). Repeated mucosal injury of the intestinal epithelium results in aberrant activation of inflammatory signaling and accumulation of immune cells, further amplifying the disease severity (1,4). Epithelium repair or mucosal healing is a process of coordinated interplay between inflammation, cell proliferation, migration, and differentiation of epithelial cells in the intestinal epithelium (5,6), which requires intricate regulatory mechanisms to prevent uncontrolled proliferation that eventually can lead to hyperplasia and CRC. Under chronic inflammation, Wingless and Int (Wnt)/β-catenin and signal transducer and activator of transcript 3 (STAT3) signaling pathways are activated and promote epithelial cell proliferation (7). On the contrary, the key tumor suppressor protein p53 (8,9) is reduced under chronic inflammation and is lost in ∼50% of malignant tumors (10).
Among cancers, CRC etiology is unique and highly affected by diet. Western high-calorie diets with low dietary fiber and polyphenolic compounds are known to increase CRC incidence. Increasing fruit consumption reduces the risk of CRC and enhances the efficacy of anticancer drugs and therapies (11,12), primarily mediated by fruit's bioactive components. Red raspberry (RB) contains ∼50 different bioactive compounds (13), including anthocyanins, ellagitannins, sanguin H-6, and lambertianin C (14,15). RB extract exerts antioxidative (16) and anti-inflammatory activities (17). Dietary RB reduces the severity of dextran sulfate sodium (DSS)–induced acute colitis in mice (18). In addition, in vitro studies showed that RB extract inhibits proliferation and induces apoptosis (19,20), but the effects of dietary whole fruit RB on CRC prevention have not been tested. We hypothesized that dietary whole RB supplementation reduces the risk of CRC development in mice with chronic colitis, likely associated with its anti-inflammatory and antiproliferative properties on intestinal epithelium.
Methods
Animal care and experimental design
Six-week-old wild-type male C57BL/6J mice were randomly assigned into 2 groups (n = 12/group) and were fed a standard rodent diet [control (CON); Research Diets, Inc.] or a CON diet supplemented with RB (5% of dry feed weight; Research Diets, Inc.) for 10 wk. The pelleted diets with or without RB were stored at −20°C under vacuum package in the dark, and fresh diets were fed to mice weekly. The composition of RB powder has been published previously (18), which includes ∼11 g gallic acid equivalents/kg dry weight. The freeze-dried RB was powdered then shipped to Research Diets, Inc., overnight for customized rodent research diet preparation. Detailed information on the diet is provided inSupplemental Tables 1 and2. The dietary RB dose is comparable to a 60-kg human dose of 29.0 g freeze-dried RB/d (21). The detailed calculation is shown in our recent publication (18), in which the same supplemental amount of RB was used.
For chronic colitis induction, after 4 wk of the dietary treatments, mice were treated with 1% DSS (Molecular weight = 36,000–50,000 Dalton; MP Biomedicals) in drinking water for 2 repeated cycles. Each cycle consisted of 7 d of 1% DSS treatment in water, followed by 14 d of recovery by providing normal drinking water. Repeated cycles of DSS exposure were used to mimic the recurring nature of colitis in human (22). We did not include mice that were not treated with DSS, because our previous study showed that RB supplementation has no effect on mice that do not receive DSS treatment (18).
After receiving DSS treatment, the mice were monitored daily for colitis symptoms during both DSS cycles. At the end of the study, the mice were killed and colonic tissues were collected for histologic and biochemical analyses. Mice were housed in a temperature-controlled room with a 12-h light and 12-h dark cycle and had a free access to diet and drinking water. There was no difference in feed intake and water consumption. All animal procedures were approved by the Washington State University Animal Care and Use Committee (BAF 04316-001). Tissues were collected and processed according to the previously published process (18).
Colitis symptom assessment and disease activity index
During DSS induction and the recovery stages, mice under treatment were monitored for body-weight loss, fecal consistency, and blood in the feces with the use of the previously published scoring criteria (18,23). The disease activity index (DAI) score was calculated as the sum of the above 3 scores as shown inSupplemental Table 3.
Histologic assessment of colonic ulceration and goblet cells
The fixed distal colonic tissue sections were deparaffinized and subjected to hematoxylin and eosin staining. The pathological scores of the distal colon were evaluated and recorded blindly with the use of previously published scoring criteria (23,24). For goblet cell staining, the colonic sections were subjected to Alcian blue (pH 2.5) staining, examined, and quantified with the use of the Image J 1.30v software (split color channels) (National Institute of Health, USA), as described previously (25).
Immunoblotting analysis
Immunoblotting analyses were conducted per our published method (26). Band density was quantified with the use of the Odyssey Infrared Imaging System and Image Studio Lite software (Li-Cor Biosciences) and normalized to the β-actin content. Antibodies used in the study are listed in theSupplemental Material.
Immunohistochemical analysis
Immunohistochemical analyses were carried out as previously described (26). CD4 and integrin α4β7 antibodies are listed in theSupplemental Material. The CD4 T cell infiltration scores were assessed blindly according to the distribution and degree of CD4-positive cell staining per crypt, ranging from 0 to a maximum of 4 (0 = no cell staining, 1 = 0–25%, 2 = >25–50%, 3 = >50–75%, and 4 = >75–100% per crypt). The α4β7 integrin filtration score was assessed blindly with the use of the distribution of α4β7 integrin staining per crypt, ranging from 0 to 4, plus the intensity of the stain signal per whole section, ranging from 0 to 4 (0 = no staining signal and 1 = 0–25%, 2 = >25–50%, 3 = >50–75%, and 4 = >75–100% per whole section). This results in the total quantified score ranging from 0 to a maximum of 8/colonic section. Nine sections per mouse at a constant interval were used for microscopic examination and score assessment.
qRT-PCR analysis
Total RNA extraction, cDNA synthesis, and qRT-PCR were performed as previously described (18,25). 18s Ribosomal RNA was used as the reference gene. Primer sequences are listed inSupplemental Table 4.
Statistical analysis
Data were analyzed with the use of a 2-tailed Student'st test.P ≤ 0.05 was considered to be significant. Each mouse was considered as an experimental unit. Data are expressed as means ± SEMs.
Results
Dietary RB prevents colitis symptoms and histologic ulceration
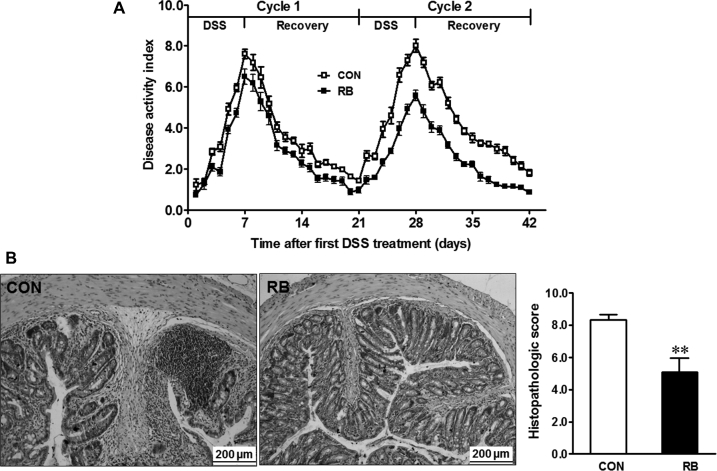
There was no difference in the weekly body weight between the treatment groups before DSS treatment (Supplemental Figure 1). RB supplementation decreased DAI scores during both DSS treatment cycles (Figure 1A). Morphologically, RB-supplemented mice showed improved colonic architecture with less inflammation and less crypt distortion than CON mice (Figure 1B), which was confirmed by histopathologic scores (Figure 1B).
FIGURE 1.
Disease activity index scores (A) and histopathologic scores (B) of male mice fed a CON diet or a 5% RB diet subjected to two 7-d cycles of 1% DSS treatment. Values are means ± SEMs,n = 12. **Different from CON,P ≤ 0.01. Panel B shows representative images of hematoxylin and eosin-stained colonic tissue. (Representative images are at 200× magnification.) CON, control; DSS, dextran sulfate sodium; RB, red raspberry.
Dietary RB reduces inflammation and associated signaling pathways
RB supplementation reduced both mRNA expression and protein levels of IL-6 and cyclooxygenase 2 (COX-2) in colonic tissues (Figure 2A). Consistently, the mRNA levels ofIl17 and interferon γ (Ifng) in colonic tissues were also reduced by RB supplementation (Figure 2B). The CD4 T cell infiltration and α4β7 integrin immunohistochemical staining, mRNA levels of intercellular adhesion molecule 1 (Icam1), vascular cell adhesion molecule 1 (Vcam1), mucosal vascular addressin cell adhesion molecule 1 (Madcam1), and chemokine (C-X-C motif) ligand 1 (Cxcl1) were all reduced in the colonic tissues of DSS-treated mice supplemented with RB (Figure 2C–F). Collectively, these data show that RB supplementation reduced DSS-induced chronic inflammation and the resultant adaptive immune responses.
FIGURE 2.
Relative protein content and mRNA expression of IL-6 and COX-2 (A), mRNA expression ofIl17 andIfng (B) and CD4-positive cells (C), mRNA expression of adhesion molecules (D, E), and α4β7 integrin filtration score (F) in male mice fed a CON diet or a 5% RB diet subjected to two 7-d cycles of 1% dextran sulfate sodium treatment. Values are means ± SEMs,n = 12. *,**Different from CON: *P ≤ 0.05, **P ≤ 0.01. C and F are representative images of CD4 and α4β7 integrin immunohistochemical stained colonic tissue, respectively. (Representative images are at 200× magnification.) CON, control; COX-2, cyclooxygenase 2;Cxcl1, chemokine (C-X-C motif) ligand 1;Icam1, intercellular adhesion molecule 1;Ifng, interferon γ;Madcam1, mucosal vascular addressin cell adhesion molecule 1; RB, red raspberry;Vcam1, vascular cell adhesion molecule 1.
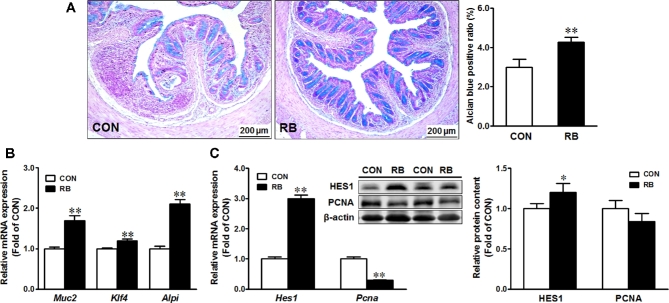
Dietary RB supplementation improves epithelium repair as indicated by enhanced differentiation of epithelial cells in chronic colitis
Dietary RB-enhanced goblet cell density in colonic tissues (Figure 3A), concomitant with elevated mRNA levels of mucin 2 (Muc2) and the goblet cell differentiation marker Kruppel-like factor 4 (Klf4), as well as intestinal alkaline phosphatase (Alpi), a differentiation marker of enterocytes in colonic tissues (Figure 3B). Furthermore, both mRNA and protein levels of Hairy and enhancer of split 1 (HES1) were enhanced, whereas those of proliferating cell nuclear antigen (PCNA), a marker of cell proliferation, were reduced by RB supplementation (Figure 3C).
FIGURE 3.
Goblet cell density (A); mRNA expression ofMuc2, Klf4, andAlpi (B); and mRNA expression and protein content of HES1 and PCNA (C) in male mice fed a CON diet or a 5% RB diet subjected to two 7-d cycles of 1% dextran sulfate sodium treatment. Values are means ± SEMs,n = 12. *,**Different from CON: *P ≤ 0.05, **P ≤ 0.01. Panel A shows representative images of Alcian blue–stained colonic tissue. (Representative images are at 200× magnification.)Alpi, intestinal alkaline phosphatase; CON, control; HES1, Hairy and enhancer of split 1;Klf4, Kruppel-like factor 4;Muc2, mucin 2; PCNA, proliferating cell nuclear antigen; RB, red raspberry.
Dietary RB supplementation suppresses β-catenin and STAT3 signaling in chronic colitis
Cytosolic β-catenin has an important role in cell adhesion and intracellular signaling, including cell proliferation (27). RB supplementation reduced the protein content of phosphorylated β-catenin (Ser 552) and β-catenin and mucin 1, and enhanced E-cadherin in colonic tissues (Figure 4A–B). In addition, mRNA level of mucin 1 was reduced (Figure 4B, C), and phosphorylation of STAT3, involved in proliferation and survival of tumor cells (7), were suppressed by RB supplementation (Figure 4D).
FIGURE 4.
Protein contents of β-catenin and p-β-catenin (Ser 552) (A), mucin 1 and E-cadherin (B), mRNA expression ofMuc1 (C), and protein contents of total STAT3, p-STAT3 (Tyr 705), and Ac-STAT3 (Lys 685) (D) in male mice fed a CON diet or a 5% RB diet subjected to two 7-d cycles of 1% dextran sulfate sodium treatment. Values are means ± SEMs,n = 12.#,*,**Different from CON:#P ≤ 0.10, *P ≤ 0.05, **P ≤ 0.01. Ac-STAT3, acetylated STAT3; CON, control;Muc1, mucin 1; p-β-catenin, phosphorylated-β-catenin; p-STAT3, phosphorylated STAT3; RB, red raspberry; STAT3, signal transducer and activator of transcription 3.
Dietary RB supplementation suppresses oncogenic signaling via enhancing tumor suppressor p53 expression
Dietary RB enhanced p53 and its phosphorylation at Ser 20 and Ser 15, as well as the content of p19ARF, a small tumor suppressor protein that stabilizes p53, in colonic tissues (Figure 5A). The mRNA expression ofp21, a cyclin-dependent kinase inhibitor that is a downstream target of p53, was higher in the RB-supplemented group (Figure 5B). Consistently, mRNA expression of thep53 downstream targets, antiapoptotic B cell lymphoma 2 (Bcl2), myeloid cell leukemia 1 (Mcl1), proliferative cyclin D1 (Ccnd1), and oncogenic myelocytomatosis (Myc) was reduced in the colonic tissues of RB-supplemented mice (Figure 5C, D).
FIGURE 5.
Protein contents of total p53 and p-p53 and p19ARF (A), mRNA expression ofp21 (B), and antiapoptotic and oncogenic genes (C, D) in male mice fed a CON diet or a 5% RB diet subjected to two 7-d cycles of 1% dextran sulfate sodium treatment. Values are means ± SEMs,n = 12.#,*,**Different from CON:#P ≤ 0.10, *P ≤ 0.05, **P ≤ 0.01.Bcl2, B cell lymphoma 2;Ccdn1, proliferative cyclin D1; CON, control;Mcl1, myeloid cell leukemia 1;Myc, myelocytomatosis oncogene; p-p53, phosphorylated p53; RB, red raspberry.
Discussion
Chronic colitis is characterized by active inflammation during relapse separated by remissions, frequently with a progressive increase in wound region and duration of the disease, which might eventually lead to CRC occurrence (1). The consumption of fresh fruit, vegetables, and dietary fiber is associated with a reduced risk of CRC development (28); phytonutrients in plant foods potentially reduce the risk of cancer via multiple mechanisms (11,12). RB is a rich source of polyphenolic compounds, vitamins B and C, folate, and dietary fibers (13–15,29). Dietary RB reduced the severity of DSS-induced acute colitis (18). In the current study, we examined the protective effects of dietary RB against chronic colitis, which causes colonic mucosal inflammation and associated epithelial dysplasia, predisposing to CRC (22). We found that RB supplementation reduced the severity of chronic colitis and inflammation, modulated immune responses, enhanced epithelium repair, and reduced oncogenic signaling, suggesting that dietary RB can reduce the risk of CRC development in subjects with chronic colitis.
Chronic colitis is associated with increased production of inflammatory cytokine IL-6 (30) and other inflammatory mediators, including COX-2, IFN-γ, and CXCL1 (31,32). IL-17–expressing CD4 T cells are present in the intestinal lamina propria and produce IL-17 (33). IL-17 stimulates IL-6, which activates STAT3, mediating CD4 T cell maturation, proliferation, and perpetuation into inflammatory intestinal tissues (33,34). As a result, CD4 T cells are aberrantly activated in colitis (35,36), and express α4β7 integrin to further attract leucocytes (37). Vedolizumab, an integrin antagonist antibody, significantly induces mucosal healing and enhances remission in patients with ulcerative colitis (38). In agreement, RB supplementation reduced the expression of inflammatory mediators, CD4 T cell density, the content of α4β7 integrin, and adhesion molecules in chronic colitis, clearly showing protective effects of RB on inflammation and pathological changes of DSS-induced colitis.
Intestinal epithelium wound healing is dependent on the homeostasis of 3 cellular processes, including restitution, proliferation, and differentiation of epithelial cells into functional cells in the injured epithelium (6). Goblet cells are one of the differentiated functional lineages of epithelial cells that secrete mucin 2, the major mucin of the mucosal layer shielding the underlying epithelium from pathogenic microbes and toxic luminal content (39). The depletion of goblet cells and mucin 2 is a characteristic of intestinal inflammation in inflammatory bowel disease (40–42). Goblet cell density and mRNA expression ofMuc2 and the lineage-specific differentiation factors such asKlf4 andHes1, along withAlpi, were heightened in RB-supplemented mice, indicating enhanced epithelial cell differentiation. RB supplementation also reduced cell proliferation, thus decreasing the chance of CRC development. The total content of polyphenols could be the main contributor for these protective effects. Indeed, polyphenolic-rich grape seed extract protects epithelial integrity by reducing proliferation and enhancing differentiation in the intestine of IL-10–deficient mice (41,42); berry extracts reduce the proliferation of LNCaP prostate cancer cells and HT-29 and HCT116 colon cancer cells (43).
Several major signaling pathways regulate cell proliferation and differentiation, including the Wnt/β-catenin pathway. Intestinal inflammation is known to enhance Wnt/β-catenin signaling, which further stimulates cell proliferation and CRC development (44). In the current study, we found that RB supplementation reduced the β-catenin content, which is consistent with a previous report in which dietary black raspberry reduced β-catenin concentration and colonic ulceration (45); grape seed extract supplementation downregulated the Wnt/β-catenin pathway and proliferation in the colon of IL-10–deficient mice (42). STAT3 transcriptionally regulates β-catenin expression (7) and epithelial cell proliferation and survival (30); STAT3 is activated in 50–60% of CRC (46). RB supplementation reduced STAT3 activation, which could be partially explained by the reduced production of IL-6, an upstream activator of STAT3 signaling (30). As a result, the suppression of STAT3 and β-catenin signaling pathways reduces the risk of CRC. In agreement with our results, the curcumin-derivative, small-compound FLLL32 inhibited STAT3 activity, which reduces CRC development (47). Triptolide (a diterpenoid triepoxide extracted from the traditional Chinese medicinal herb) inhibited CRC progression associated with STAT3 suppression (48).
Protein p53 is a tumor suppressor protein (8), which is reduced or lost in cancer cells (10). Phosphorylation of p53 at Ser 15 and Ser 20 stabilizes p53 by reducing its interaction with mouse double minute 2 (MDM2), which mediates p53 degradation (49). On the other hand, p14ARF (p19ARF in mice) stabilizes p53 by acting as an inhibitor of MDM2 (49). We found that RB supplementation stabilized the p53 protein correlated with increased phosphorylation at Ser 15 and Ser 20 and an upregulated level of p19ARF. These changes could be due to the biological effects of polyphenols. Green tea polyphenols, epigallocatechin-3-gallate, stabilized p53 by inducing its phosphorylation at Ser 15 and Ser 20, and p14ARF mediated downregulation of MDM2 in human prostate carcinoma LNCaP cells (49). p21 is a cyclin-dependent kinase inhibitor and one of the p53 downstream mediators, which initiates cell cycle arrest and interacts with PCNA to inhibit DNA replication (50). The p53 stabilization increases transcription of p21 and the expression of proapoptotic Bax, while reducing the antiapoptoticBCL2 (9,49). Consistently, dietary RB enhancedp21 expression and reducedBcl2, Mcl1, Ccdn1, andMyc, showing that RB suppressed epithelial cell proliferation and oncogenic signal activation in chronic colitis.
The bioactive components in whole fruit RB include vitamins, minerals, fibers, antioxidants, and polyphenols (51). RB freeze-dried powder contains anthocyanin, ellagitannins, sanguin H-6, lambertianin C, and other polyphenols (14,15). RB anthocyanin supplementation reduced the production of IL-6, IL-1β, COX-2, and inducible NO synthase, and suppressed NF-κB signaling in mice (17). RB ellagic acid showed immunoregulatory functions in various animal studies (52) and antiproliferative and apoptotic activities in Caco-2 cells (53,54). In addition, RB contains 1.6% soluble fiber and 33.5% insoluble fiber (51), which provide substrates for gut fermentation to produce SCFAs and are expected to generate additional beneficial effects (55). By using the whole-fruit approach, beneficial effects of RB could be attributed to the combined effects of the high polyphenol and fiber contents in RB.
In summary, dietary RB reduced the severity of chronic colitis, colonic ulceration, inflammation, and associated signaling. RB facilitated epithelium repair by enhancing epithelial cell differentiation and mucin 2 production, while reducing cell proliferation, which were associated with reduced β-catenin and STAT3 signaling. Moreover, dietary RB supplementation stabilized the tumor suppressor p53 and reduced oncogenic signaling, suggesting that RB is a potential dietary strategy to reduce the risk of CRC development in subjects with chronic colitis.
Supplementary Material
Acknowledgments
We thank Luís Fernando de Sousa Moraes, Yifei Kang, Xiaofei Sun, and Yansong Xue for their assistance in sample collection. The authors’ responsibilities were as follows—M-JZ and SB: designed the experiments; SB: conducted the experiments and wrote the manuscript; M-JZ and MD: edited the manuscript; and all authors: read and approved the final manuscript.
Notes
Supported by the National Processed Raspberry Council and a Washington State University Emerging Research Issues competitive grant (16-10) to MJZ.
Author disclosures: SB, MD, and MJZ, no conflicts of interest.
Supplemental Tables 1–4,Supplemental Material,Supplemental References, andSupplemental Figure 1 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents athttps://academic.oup.com/jn/.
Abbreviations used:Bcl2, B cell lymphoma 2; CON, control; COX-2, cyclooxygenase 2; CRC, colorectal cancer; DAI, disease activity index; DSS, dextran sulfate sodium; MDM2, mouse double minute 2; RB, red raspberry; STAT3, signal transducer and activator of transcription 3; Wnt, Wingless and Int.
References
- 1. Itzkowitz SH,Yio X. Inflammation and cancer IV. Colorectal cancer in inflammatory bowel disease: the role of inflammation.Am J Physiol Gastrointest Liver Physiol 2004;287:G7–17. [DOI] [PubMed] [Google Scholar]
- 2. Ferlay J,Soerjomataram I,Dikshit R,Eser S,Mathers C,Rebelo M,Parkin DM,Forman D,Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012.Int J Cancer 2015;136:E359–386. [DOI] [PubMed] [Google Scholar]
- 3. Siegel R,Ma J,Zou Z,Jemal A. Cancer statistics, 2014.CA Cancer J Clin 2014;64:9–29. [DOI] [PubMed] [Google Scholar]
- 4. Naito Y,Saito K,Shiiba K,Ohuchi A,Saigenji K,Nagura H,Ohtani H. CD8+ T cells infiltrated within cancer cell nests as a prognostic factor in human colorectal cancer.Cancer Res 1998;58:3491–4. [PubMed] [Google Scholar]
- 5. Paclik D,Lohse K,Wiedenmann B,Dignass AU,Sturm A. Galectin-2 and -4, but not galectin-1, promote intestinal epithelial wound healing in vitro through a TGF-beta-independent mechanism.Inflamm Bowel Dis 2008;14:1366–72. [DOI] [PubMed] [Google Scholar]
- 6. Dignass AU. Mechanisms and modulation of intestinal epithelial repair.Inflamm Bowel Dis 2001;7:68–77. [DOI] [PubMed] [Google Scholar]
- 7. Ibrahem S,Al-Ghamdi S,Baloch K,Muhammad B,Fadhil W,Jackson D,Nateri AS,Ilyas M. STAT3 paradoxically stimulates β-catenin expression but inhibits β-catenin function.Int J Exp Pathol 2014;95:392–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bates S,Vousden KH. p53 in signaling checkpoint arrest or apoptosis.Curr Opin Genet Dev 1996;6:12–18. [DOI] [PubMed] [Google Scholar]
- 9. Yu J,Wang Z,Kinzler KW,Vogelstein B,Zhang L. PUMA mediates the apoptotic response to p53 in colorectal cancer cells.Proc Natl Acad Sci USA 2003;100:1931–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Agarwal ML,Taylor WR,Chernov MV,Chernova OB,Stark GR. The p53 network.J Biol Chem 1998;273:1–4. [DOI] [PubMed] [Google Scholar]
- 11. Stoner GD,Mukhtar H. Polyphenols as cancer chemopreventive agents.J Cell Biochem Suppl 1995;22:169–80. [DOI] [PubMed] [Google Scholar]
- 12. Martin MA,Goya L,Ramos S. Potential for preventive effects of cocoa and cocoa polyphenols in cancer.Food Chem Toxicol 2013;56:336–51. [DOI] [PubMed] [Google Scholar]
- 13. Carvalho E,Franceschi P,Feller A,Palmieri L,Wehrens R,Martens S. A targeted metabolomics approach to understand differences in flavonoid biosynthesis in red and yellow raspberries.Plant Physiol Biochem 2013;72:79–86. [DOI] [PubMed] [Google Scholar]
- 14. Borges G,Degeneve A,Mullen W,Crozier A. Identification of flavonoid and phenolic antioxidants in black currants, blueberries, raspberries, red currants, and cranberries.J Agric Food Chem 2010;58:3901–9. [DOI] [PubMed] [Google Scholar]
- 15. Gasperotti M,Masuero D,Vrhovsek U,Guella G,Mattivi F. Profiling and accurate quantification of Rubus ellagitannins and ellagic acid conjugates using direct UPLC-Q-TOF HDMS and HPLC-DAD analysis.J Agric Food Chem 2010;58:4602–16. [DOI] [PubMed] [Google Scholar]
- 16. de Souza VR,Pereira PA,da Silva TL,de Oliveira Lima LC,Pio R,Queiroz F. Determination of the bioactive compounds, antioxidant activity and chemical composition of Brazilian blackberry, red raspberry, strawberry, blueberry and sweet cherry fruits.Food Chem 2014;156:362–8. [DOI] [PubMed] [Google Scholar]
- 17. Li L,Wang L,Wu Z,Yao L,Wu Y,Huang L,Liu K,Zhou X,Gou D. Anthocyanin-rich fractions from red raspberries attenuate inflammation in both RAW264.7 macrophages and a mouse model of colitis.Sci Rep 2014;4:6234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bibi S,Kang Y,Du M,Xue Y,Zhu M-J. Dietary red raspberries attenuate dextran sulfate sodium-induced acute colitis.J Nutr Biochem 2018;51:40–46. [DOI] [PubMed] [Google Scholar]
- 19. Coates EM,Popa G,Gill CI,McCann MJ,McDougall GJ,Stewart D,Rowland I. Colon-available raspberry polyphenols exhibit anti-cancer effects on in vitro models of colon cancer.J Carcinog 2007;6:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. God J,Tate PL,Larcom LL. Red raspberries have antioxidant effects that play a minor role in the killing of stomach and colon cancer cells.Nutr Res 2010;30:777–82. [DOI] [PubMed] [Google Scholar]
- 21. Reagan-Shaw S,Nihal M,Ahmad N. Dose translation from animal to human studies revisited.FASEB J 2008;22:659–61. [DOI] [PubMed] [Google Scholar]
- 22. Elson CO,Sartor RB,Tennyson GS,Riddell RH. Experimental models of inflammatory bowel disease.Gastroenterology 1995;109:1344–67. [DOI] [PubMed] [Google Scholar]
- 23. Hamamoto N,Maemura K,Hirata I,Murano M,Sasaki S,Katsu K. Inhibition of dextran sulphate sodium (DSS)-induced colitis in mice by intracolonically administered antibodies against adhesion molecules (endothelial leucocyte adhesion molecule-1 (ELAM-1) or intercellular adhesion molecule-1 (ICAM-1)).Clin Exp Immunol 1999;117:462–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kang Y,Xue Y,Du M,Zhu MJ. Preventive effects of Goji berry on dextran-sulfate-sodium-induced colitis in mice.J Nutr Biochem 2016;40:70–76. [DOI] [PubMed] [Google Scholar]
- 25. Wang H,Xue Y,Zhang H,Huang Y,Yang G,Du M,Zhu MJ. Dietary grape seed extract ameliorates symptoms of inflammatory bowel disease in IL10-deficient mice.Mol Nutr Food Res 2013;57:2253–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhu MJ,Du M,Hess BW,Nathanielsz PW,Ford SP. Periconceptional nutrient restriction in the ewe alters MAPK/ERK1/2 and PI3K/Akt growth signaling pathways and vascularity in the placentome.Placenta 2007;28:1192–9. [DOI] [PubMed] [Google Scholar]
- 27. Ilyas M,Tomlinson IP. The interactions of APC, E-cadherin and beta-catenin in tumour development and progression.J Pathol 1997;182:128–37. [DOI] [PubMed] [Google Scholar]
- 28. Watson AJ,Collins PD. Colon cancer: a civilization disorder.Dig Dis 2011;29:222–8. [DOI] [PubMed] [Google Scholar]
- 29. Probst Y. A review of the nutrient composition of selected Rubus berries.Nutr Food Sci 2015;45:242–54. [Google Scholar]
- 30. Grivennikov S,Karin E,Terzic J,Mucida D,Yu GY,Vallabhapurapu S,Scheller J,Rose-John S,Cheroutre H,Eckmann L,Karin M. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer.Cancer Cell 2009;15:103–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cheng WL,Wang CS,Huang YH,Tsai MM,Liang Y,Lin KH. Overexpression of CXCL1 and its receptor CXCR2 promote tumor invasion in gastric cancer.Ann Oncol 2011;22:2267–76. [DOI] [PubMed] [Google Scholar]
- 32. Paiotti AP,Marchi P,Miszputen SJ,Oshima CT,Franco M,Ribeiro DA. The role of nonsteroidal antiinflammatory drugs and cyclooxygenase-2 inhibitors on experimental colitis.In Vivo 2012;26:381–93. [PubMed] [Google Scholar]
- 33. Atarashi K,Nishimura J,Shima T,Umesaki Y,Yamamoto M,Onoue M,Yagita H,Ishii N,Evans R,Honda K,Takeda K. ATP drives lamina propria T(H)17 cell differentiation.Nature 2008;455:808–12. [DOI] [PubMed] [Google Scholar]
- 34. Atreya R,Mudter J,Finotto S,Mullberg J,Jostock T,Wirtz S,Schutz M,Bartsch B,Holtmann M,Becker C,et al. Blockade of interleukin 6 trans signaling suppresses T-cell resistance against apoptosis in chronic intestinal inflammation: evidence in Crohn disease and experimental colitis in vivo.Nat Med 2000;6:583–8. [DOI] [PubMed] [Google Scholar]
- 35. Adams DH,Eksteen B. Aberrant homing of mucosal T cells and extra-intestinal manifestations of inflammatory bowel disease.Nat Rev Immunol 2006;6:244–51. [DOI] [PubMed] [Google Scholar]
- 36. Cheroutre H,Lambolez F,Mucida D. The light and dark sides of intestinal intraepithelial lymphocytes.Nat Rev Immunol 2011;11:445–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Behm BW,Bickston SJ. Humanized antibody to the alpha4beta7 integrin for induction of remission in ulcerative colitis.Cochrane Database Syst Rev 2009;1:CD007571. [DOI] [PubMed] [Google Scholar]
- 38. Parikh A,Leach T,Wyant T,Scholz C,Sankoh S,Mould DR,Ponich T,Fox I,Feagan BG. Vedolizumab for the treatment of active ulcerative colitis: a randomized controlled phase 2 dose-ranging study.Inflamm Bowel Dis 2012;18:1470–9. [DOI] [PubMed] [Google Scholar]
- 39. Birchenough GMH,Johansson MEV,Gustafsson JK,Bergström JH,Hansson GC. New developments in goblet cell mucus secretion and function.Mucosal immunol 2015;8:712–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gersemann M,Becker S,Kubler I,Koslowski M,Wang G,Herrlinger KR,Griger J,Fritz P,Fellermann K,Schwab M,et al. Differences in goblet cell differentiation between Crohn's disease and ulcerative colitis.Differentiation 2009;77:84–94. [DOI] [PubMed] [Google Scholar]
- 41. Bibi S,Kang Y,Yang G,Zhu MJ. Grape seed extract improves small intestinal health through suppressing inflammation and regulating alkaline phosphatase in IL-10-deficient mice.J Funct Foods 2016;20:245–52. [Google Scholar]
- 42. Yang G,Xue Y,Zhang H,Du M,Zhu MJ. Favourable effects of grape seed extract on intestinal epithelial differentiation and barrier function in IL10-deficient mice.Br J Nutr 2015;114:15–23. [DOI] [PubMed] [Google Scholar]
- 43. Seeram NP,Adams LS,Zhang Y,Lee R,Sand D,Scheuller HS,Heber D. Blackberry, black raspberry, blueberry, cranberry, red raspberry, and strawberry extracts inhibit growth and stimulate apoptosis of human cancer cells in vitro.J Agric Food Chem 2006;54:9329–39. [DOI] [PubMed] [Google Scholar]
- 44. Claessen MM,Schipper ME,Oldenburg B,Siersema PD,Offerhaus GJ,Vleggaar FP. WNT-pathway activation in IBD-associated colorectal carcinogenesis: potential biomarkers for colonic surveillance.Cell Oncol 2010;32:303–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wang L-S,Kuo C-T,Huang THM,Yearsley M,Oshima K,Stoner GD,Yu J,Lechner JF,Huang Y-W. Black raspberries protectively regulate methylation of Wnt pathway genes in precancerous colon tissue.Cancer Prev Res (Phila) 2013;6:1317–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Morikawa T,Baba Y,Yamauchi M,Kuchiba A,Nosho K,Shima K,Tanaka N,Huttenhower C,Frank DA,Fuchs CS,Ogino S. STAT3 expression, molecular features, inflammation patterns, and prognosis in a database of 724 colorectal cancers.Clin Cancer Res 2011;17:1452–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lin L,Deangelis S,Foust E,Fuchs J,Li C,Li P-K,Schwartz EB,Lesinski GB,Benson D,Lü J,et al. A novel small molecule inhibits STAT3 phosphorylation and DNA binding activity and exhibits potent growth suppressive activity in human cancer cells.Mol Cancer 2010;9:217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wang Z,Jin H,Xu R,Mei Q,Fan D. Triptolide downregulates Rac1 and the JAK/STAT3 pathway and inhibits colitis-related colon cancer progression.Exp Mol Med 2009;41:717–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hastak K,Gupta S,Ahmad N,Agarwal MK,Agarwal ML,Mukhtar H. Role of p53 and NF-kappaB in epigallocatechin-3-gallate-induced apoptosis of LNCaP cells.Oncogene 2003;22:4851–9. [DOI] [PubMed] [Google Scholar]
- 50. Warbrick E,Lane DP,Glover DM,Cox LS. Homologous regions of Fen1 and p21Cip1 compete for binding to the same site on PCNA: a potential mechanism to co-ordinate DNA replication and repair.Oncogene 1997;14:2313–21. [DOI] [PubMed] [Google Scholar]
- 51. Noratto GD,Chew BP,Atienza LM. Red raspberry (Rubus idaeus L.) intake decreases oxidative stress in obese diabetic (db/db) mice.Food Chem 2017;227:305–14. [DOI] [PubMed] [Google Scholar]
- 52. Burton-Freeman BM,Sandhu AK,Edirisinghe I. Red raspberries and their bioactive polyphenols: cardiometabolic and neuronal health links.Adv Nutr 2016;7:44–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Larrosa M,Tomas-Barberan FA,Espin JC. The dietary hydrolysable tannin punicalagin releases ellagic acid that induces apoptosis in human colon adenocarcinoma Caco-2 cells by using the mitochondrial pathway.J Nutr Biochem 2006;17:611–25. [DOI] [PubMed] [Google Scholar]
- 54. Losso JN,Bansode RR,Trappey A,Bawadi HA,Truax R. In vitro anti-proliferative activities of ellagic acid.J Nutr Biochem 2004;15:672–8. [DOI] [PubMed] [Google Scholar]
- 55. Jakobsdottir G,Nilsson U,Blanco N,Sterner O,Nyman M. Effects of soluble and insoluble fractions from bilberries, black currants, and raspberries on short-chain fatty acid formation, anthocyanin excretion, and cholesterol in rats.J Agric Food Chem 2014;62:4359–68. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.