Abstract
Clusters of bacterial species within the gut microenvironment, or gut enterotype, have been correlated with cardiometabolic disease risk. The metabolic products and metabolites that bacteria produce, such as short-chain fatty acids, secondary bile acids, and trimethylamine, may also affect the microbial community and disease risk. Diet has a direct impact on the gut microenvironment by providing substrates to and promoting the colonization of resident bacteria. To date, few dietary patterns have been evaluated for their effect on the gut microbiome, but the Mediterranean diet and Vegetarian diets have shown favorable effects for both the gut microbiome and cardiometabolic disease risk. This review examines the gut microbiome as a mediator between these dietary patterns and cardiometabolic disease risk.
Keywords: microbiome, Mediterranean diet, Vegetarian diet, cardiovascular disease, trimethylamine N-oxide, secondary bile acids, SCFAs
Introduction
The gut microbiome (GM) plays a central role in health (1,2). Disruption of the GM, or gut dysbiosis, is associated with an increased risk of cardiometabolic diseases, such as metabolic syndrome (MetS), cardiovascular disease (CVD), and type 2 diabetes mellitus (T2DM) (3–5). Numerous studies have reported correlations between gut bacteria and CVD risk (4,6–11) and despite the relation between the GM and cardiometabolic disease being of great scientific interest, this area of research is in its infancy, and there are many questions that await answers. At present it is proposed that specific bacterial metabolites produced in the GM vary depending on the hosts’ gut microbial environment (12), which is influenced by many factors including age, genetics, cohabitation, medication, and diet (13–15). The metabolites produced via the microbiota are also affected by diet. Therefore, the combination of foods and nutrients consumed may change the composition and functionality of the GM. Thus, the role that the GM plays in nutrient metabolism and production of primary and secondary metabolites is likely to mediate the relation between diet and cardiometabolic disease risk (12,16–18). The objective of this review is to summarize recent evidence about how dietary patterns affect the GM and, in turn, how the GM affects cardiometabolic disease risk.
There is substantial individual variation in the GM, although differences in the distinct clusters of bacterial species within the gut ecosystem (gut enterotype) exist between healthy individuals and those with chronic diseases, such as T2DM and colorectal cancer (19–21). Thus, the gut enterotype may be related to the development of disease but may also affect the response to pharmacologic and dietary interventions (7,22–27). The production of gut-derived metabolites is reliant on the bacteria present in the GM and their function within the gut ecosystem (28–30); this is a plausible explanation for how the GM may influence disease risk. Specifically, there is evidence to support an association between disease risk and gut-derived metabolites including SCFAs, trimethylamine (TMA), and secondary bile acids, which will be a focus of this review.
Greater production of SCFAs is associated with lower risk of T2DM, chronic kidney disease, and CVD (31–36). Conversely, TMA may increase disease risk (37–39). TMA can be produced in the GM through metabolism of choline, phosphatidylcholine, andl-carnitine; it is further oxidized to trimethylamineN-oxide (TMAO), a proatherogenic molecule associated with the development of CVD (40). Finally, the conversion of primary to secondary bile acids is GM-dependent (41) and the type of secondary bile acids produced depends on diet and the composition of the GM. The secondary bile acids produced may also alter the GM composition. For example, a high dietary intake of saturated fat increases bile acid secretion and bile acids in the intestine, which results in the production of hydrophobic secondary bile acids (e.g., deoxycholic acid—DCA). These hydrophobic secondary bile acids can also change the composition and structure of the GM by affecting microbial growth (42). Secondary bile acids enter the circulation and have been implicated in atherosclerosis, diabetes, and other cardiometabolic diseases (42–44).
There is substantial evidence showing that diet modifies the GM; however, less well characterized are the functional consequences of these modifications and the relation with cardiometabolic diseases (15). Recent clinical trials have reported the individual effects of dietary components on the GM (45–48). Fewer studies have examined the effect of dietary patterns on the GM. However, examination of dietary patterns takes into consideration the combinations and quantities of foods and nutrients consumed and their cumulative effects on health, and thus is a more robust way of understanding diet-disease relations (49,50). The aim of this article is to summarize how dietary patterns affect the GM and, in turn, how the GM affects cardiometabolic disease risk.Figure 1 presents an overview of proposed interactions among diet, the microbiome and cardiometabolic risk.
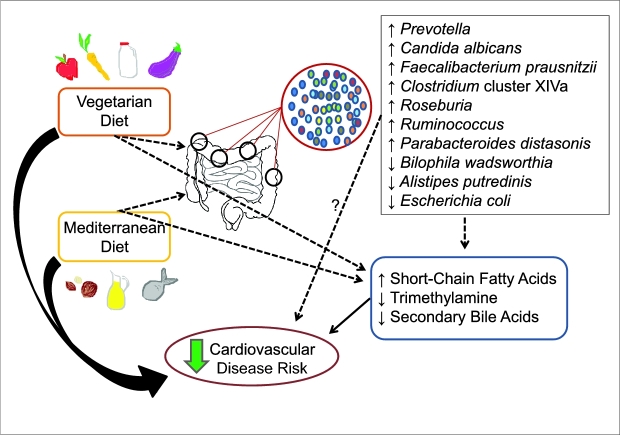
FIGURE 1.
Schematic of known and proposed interactions between diet and cardiometabolic risk illustrates the known risk-reducing properties of Vegetarian and Mediterranean diets on cardiometabolic diseases and the proposed interactions between the Vegetarian and Mediterranean diets with the gut microbiome and gut-derived metabolites that can reduce cardiovascular disease risk. The thick, solid-black arrows represent a large pool of evidence to support a pathway; narrow, solid-black arrows represent an intermediate amount of evidence to support a pathway; narrow, dotted black lines represent emerging evidence to support a pathway; and narrow, dotted black lines with a question mark represent other possible mechanistic pathways. Vegetarian and Mediterranean diets can alter the presence or absence of various bacteria and, in turn, also alter the gut metabolome. The direct effect of the gut microbial environment on cardiovascular disease risk is unknown. Vegetarian and Mediterranean diets can also affect the production of gut metabolites by serving as substrate for the resident bacteria.
To date, few dietary patterns have been evaluated for their effect on the GM, although several studies have examined the GM in relation to the Mediterranean diet and Vegetarian diets (22,51,52). Notably, these dietary patterns are also associated with lower CVD risk (53,54). Thus, it is reasonable to hypothesize that the GM may be an important mediator between these dietary patterns and cardiometabolic disease risk. Because studies of the effect of dietary patterns on the GM and CVD risk have predominantly investigated Mediterranean and Vegetarian diets, this review will focus on these dietary patterns.
Vegetarian Diet
A large body of evidence has demonstrated the cardiovascular benefits of following a Vegetarian diet (55–58), including lacto-ovo-vegetarian, lacto-vegetarian, ovo-vegetarian, and vegan diets, and it is plausible that the GM plays a role in the observed effects because the many plant-based constituents (including dietary fiber) of a Vegetarian diet provide substrates to microbes in the lower gastrointestinal tract.
Observational data show consumption of a Vegetarian diet compared with an omnivorous diet differentially affects the gut enterotype (22,59). de Moraes and colleagues (22) examined if gut enterotype correlated with diet and cardiometabolic risk by comparing vegans, vegetarians, and omnivores. The investigators separated individuals into 3 enterotypes based on the abundance of the most prevalent bacterial strains:Bacteroides, Prevotella, and Ruminococcaceae. The authors reported that the frequency of vegans was greater inPrevotella than in theBacteroides and Ruminococacceae enterotypes; however, the frequencies in vegetarians and omnivores did not differ (22). The authors also correlated the enterotypes with markers of CVD risk and found a favorable association between thePrevotella enterotype and LDL cholesterol; the vegans and vegetarians in thePrevotella enterotype had lower LDL cholesterol compared with omnivores. Matijašić et al. (59) reported that individuals following a Vegetarian diet had higher fecalPrevotella present and the proportion ofBacteroides + Prevotella (grouped together) relative to all other bacteria was greater, whereas the percentage ofClostridium coccoides (Clostridium cluster XIVa) was lower compared with omnivores.Prevotella bacteria have previously been associated with a carbohydrate-rich diet and produce SCFAs (22,60) whereasC. coccoides have been found in higher concentrations in individuals with irritable bowel syndrome (61). Finally, De Filippo et al. (62) reported significant, advantageous differences in gut microbial composition in children living in rural Africa compared with children residing in Europe. The authors suggested that the African children (aged 1–6 y) had a microbial advantage due to a high-fiber diet rich in cereals, legumes, and vegetables for several years.
These observational data are supported by a clinical trial showing that after short-term (5-d) consumption of an animal-based or plant-based diet,Bilophila wadsworthia, Alistipes putredinis, and aBacteroides sp. were more prevalent in fecal samples after consumption of the animal-based diet (63). These bacteria are all bile-resistant, which may be the reason they are more prevalent after the higher-fat, animal-based diet. However, a 1-mo study in obese individuals reported there were no changes in the gut enterotype after following a vegan diet, despite reduced CVD risk (64). Currently, it is unclear how long it takes for the gut enterotype to change and how stable the GM is over time. There is a need for tightly controlled studies to explore functional changes that occur in response to a vegetarian or vegan diet and how long it takes for these changes to occur.
Enterotypic differences appear to also affect the gut metabolome, which could contribute to the protective effects of a Vegetarian diet (22,52). TMA and secondary bile acid production are dependent on the GM, and following a Vegetarian or vegan diet can reduce concentrations of both compounds; however, it is unclear whether the alteration in substrate (dietary components) or changes to the gut enterotype are associated with changes in gut-derived metabolites. Previous data showed vegan and Vegetarian diets resulted in lower concentrations of fecal secondary bile acids (65,66) compared with omnivorous diets. Recently, David et al. (63) reported elevated secondary bile acids, including DCA, and the bacterial gene expression involved in their production, including bile salt hydrolases and sulfite reductases, after 5 d of consumption of an animal-based diet compared with a plant-based diet. DCA has been shown to reduce intestinal barrier function, increase inflammation, and promote liver cancer (67). Evidence suggests a plant-based diet may create a different enterotype compared with an omnivorous diet and promote lower production of secondary bile acids.
Reduced concentrations of TMA have also been reported subsequent to following a vegan diet compared with an omnivorous diet (52). Koeth and colleagues (52) provided vegan/vegetarian and omnivorous participants with oral carnitine and reported that vegans and vegetarians had a reduced capacity to produce TMA compared with the omnivores. Vegan and Vegetarian diets are low in choline, phosphatidylcholine, and L-carnitine owing to the absence of red meat and fish; therefore, they would be expected to produce less TMA and TMAO. However, the finding that vegans/vegetarians produce a lower amount of TMA postcarnitine challenge suggests that a Vegetarian diet may favorably alter the GM and create a microenvironment that does not metabolize carnitine into TMA.
In summary, the GM may be an important mediator in the reduced cardiometabolic disease risk associated with a Vegetarian diet, but more studies are needed to understand the mechanism. A plant-based diet can favorably affect the GM through changes in bacteria, such as increases inPrevotella taxa, and reduction of harmful metabolites, such as TMA, but the effect on gut enterotype and SCFA production requires further study. The key question that follows is mechanistically how changes in the GM modulate risk factors for cardiometabolic disease.
Mediterranean Diet
Prominent components of the Mediterranean diet include fruits, vegetables, legumes, olive oil, nuts, seafood, and wine. Evidence demonstrates that this eating pattern is associated with a lower risk of CVD and that adherence to a Mediterranean diet can affect gut microbial patterns and the fecal metabolic profile of microbial-derived phenolics (51,68–70). Thus, the GM may partly mediate the relation observed between the Mediterranean diet and reduced risk of CVD.
Emerging research suggests that following a Mediterranean diet is associated with a more-favorable GM composition (68,69). Mitsou and colleagues (68) examined the association between the Mediterranean diet and gut microbiota characteristics in an adult population. The authors reported that participants with higher Mediterranean diet scores had a lower fecalEscherichia coli count, a higher ratio ofBifidobacteria toE. coli, and increased concentrations and prevalence ofCandida albicans. Gutiérrez-Díaz et al. (69) also investigated the association between the Mediterranean diet and the GM, but reported that better adherence to a Mediterranean diet was associated with higherFaecalibacterium prausnitzii andClostridium cluster XIVa compared with lower adherence. The authors note that theClostridium cluster XIVa group contains butyrate-producing species andF. prausnitzii is a known butyrate producer, therefore suggesting the Mediterranean diet-associated GM may be made up of more beneficial bacteria. A Mediterranean-style diet has been shown to lower the risk of CVD (53), which may be partly dependent on the GM. Haro et al. (70) investigated the GM differences between individuals with coronary heart disease with and without MetS and obesity from the CORDIOPREV study, following a Mediterranean diet or low-fat diet for 2 y. This prospective randomized trial showed that consumption of a Mediterranean diet or a low-fat diet may restore some bacterial populations that are reduced in MetS patients with obesity (70). In addition, obese subjects with MetS in the Mediterranean diet group had an increased abundance ofRoseburia andRuminococcus genera,Parabacteroides distasonis, andF. prausnitzii; the low-fat diet did not change these genera (70). Cross-sectional studies suggest that the Mediterranean diet may be associated with favorable changes in the GM, and these observational data are supported by a prospective trial that showed reduced gut dysbiosis in obese individuals with MetS. However, the implications of these shifts in the GM for cardiometabolic health still need to be elucidated. Thus, more evidence is needed to understand the microbial shifts that are important for a healthy GM, and how this, in turn, affects cardiometabolic disease risk and management.
Following a Mediterranean-style diet is associated with increased concentrations of SCFAs (51). Two studies reported that individuals with a higher Mediterranean diet score had higher concentrations of fecal SCFAs (51,68). One study reported a greater ratio of acetate to other SCFAs with greater adherence to a Mediterranean diet (68) and the other study reported that individuals with higher Mediterranean diet adherence scores had increased SCFAs (51). Although some observational data show greater SCFA concentrations are associated with obesity, this is believed to be the result of an oversupply of energy, thus providing gut microbes with more substrate for SCFA production (71). In conditions of positive energy balance, excessive energy is consumed and, therefore, the GM is also provided with more energy than needed, and works to metabolize as much of the substrate as possible. This suggests SCFAs are not causally associated with obesity. Clinical trials have shown that intestinal SCFAs are correlated with improvements in blood lipid profile, glucose homeostasis, and inflammation (72–74), although it may take weeks to months for these changes to occur (73,75,76). Therefore, based on observational evidence, consumption of a Mediterranean diet could be a promising intervention to increase SCFA production and reduce cardiometabolic disease risk.
The Mediterranean diet may also reduce circulating choline metabolites, such as TMAO, and therefore reduce CVD risk. De Filippis et al. (51) reported that higher adherence to a Mediterranean diet was associated with lower TMAO concentrations. This association was also observed in a subpopulation of the PREDIMED study. Guasch-Ferré et al. (77) examined the relation between plasma concentrations of 5 metabolites in the choline pathway (TMAO, betaine, choline, phosphocholine, and α-glycerophosphocholine) and major CVD endpoints in individuals at risk for CVD. The authors reported that participants classified in the highest metabolite quartile and assigned to a low-fat control diet had a higher risk (HR: 2.37; 95% CI: 1.34–4.18) of CVD than participants in the lowest metabolite quartile and assigned to a Mediterranean diet. The large proportion of plant-based foods in the Mediterranean diet may alter the GM to produce less TMA and favorably change the metabolic profile.
The dietary pattern consumed affects the composition of the GM through the provision of substrate for the gut microbes. Various bacteria prefer different energy sources and the type of foods consumed promotes the residency of bacteria that favor the energy substrate provided. This results in gut-derived metabolic products that can alter the structure and function of the GM and affect disease risk. Moreover, changes in GM that have emerged with adherence to a Mediterranean diet suggest it may provide important dietary components that can improve GM functionality with consequent benefits on the host. Future research is needed to further investigate how the Mediterranean diet affects gut enterotype. The present evidence to date suggests that the Mediterranean diet increases SCFA production, metabolites that have been shown to reduce the risk of cardiometabolic diseases.
Conclusions
Vegetarian and Mediterranean diets both reduce cardiometabolic risk and favorably change the GM. Current knowledge suggests that consumption of a Vegetarian or Mediterranean diet generates favorable shifts in the microbes in the lower gastrointestinal tract and beneficial gut-derived metabolites may be a product of these alterations. The temporality of this relation is not well understood and it may be bidirectional. The composition of the GM affects the gut-derived metabolic products and these metabolites may also affect the microbial community and gut enterotype. Examination of the gut enterotype, microbial functionality, and gut-derived metabolites could provide a better understanding of the changes that occur with specific dietary patterns. The entire gut environment, including present microbes, relative abundance of microbes, microbial gene expression, and microbial metabolites, is important to consider relative to the link between the GM and disease risk. However, the majority of evidence to support the correlations between these diets and changes in the GM composition and metabolites is primarily derived from epidemiologic research. Further research is needed to increase our understanding of how dietary patterns alter the GM and, importantly, how alterations in the GM mechanistically modulate risk of cardiometabolic diseases.
Acknowledgments
The authors' contributions were as follows—All authors (AMT, KSP, and PMK-E) were responsible for design, writing, and final content and have read and approved the final manuscript.
Notes
The authors reported no funding received for this study.
Author disclosures: AMT, KSP, and PMK-E, no conflicts of interest.
Abbreviations used: CVD, cardiovascular disease; DCA, deoxycholic acid; GM, gut microbiome; MetS, metabolic syndrome; TMA, trimethylamine; TMAO, trimethylamineN-oxide; T2DM, type 2 diabetes mellitus.
References
- 1. Kashyap PC,Chia N,Nelson H,Segal E,Elinav E. Microbiome at the frontier of personalized medicine.Mayo Clin Proc 2017;92:1855–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Le Chatelier E,Nielsen T,Qin J,Prifti E,Hildebrand F,Falony G,Almeida M,Arumugam M,Batto J-M,Kennedy S et al. Richness of human gut microbiome correlates with metabolic markers.Nature 2013;500:541–6. [DOI] [PubMed] [Google Scholar]
- 3. Org E,Blum Y,Kasela S,Mehrabian M,Kuusisto J,Kangas AJ,Soininen P,Wang Z,Ala-Korpela M,Hazen SL et al. Relationships between gut microbiota, plasma metabolites, and metabolic syndrome traits in the METSIM cohort.Genome Biol 2017;18:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jie Z,Xia H,Zhong S-L,Feng Q,Li S,Liang S,Zhong H,Liu Z,Gao Y,Zhao H et al. The gut microbiome in atherosclerotic cardiovascular disease.Nat Commun 2017;8:845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wen L,Duffy A. Factors influencing the gut microbiota, inflammation, and type 2 diabetes.J Nutr 2017;147:1468S–75S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Qin J,Li Y,Cai Z,Li S,Zhu J,Zhang F,Liang S,Zhang W,Guan Y,Shen D et al. A metagenome-wide association study of gut microbiota in type 2 diabetes.Nature 2012;490:55–60. [DOI] [PubMed] [Google Scholar]
- 7. Li J,Zhao F,Wang Y,Chen J,Tao J,Tian G,Wu S,Liu W,Cui Q,Geng B et al. Gut microbiota dysbiosis contributes to the development of hypertension.Microbiome 2017;5:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Navab-Moghadam F,Sedighi M,Khamseh ME,Alaei-Shahmiri F,Talebi M,Razavi S,Amirmozafari N. The association of type II diabetes with gut microbiota composition.Microb Pathog 2017;110:630–6. [DOI] [PubMed] [Google Scholar]
- 9. Heianza Y,Ma W,Manson JE,Rexrode KM,Qi L. Gut microbiota metabolites and risk of major adverse cardiovascular disease events and death: a systematic review and meta-analysis of prospective studies.J Am Heart Assoc 2017;6:e004947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cui L,Zhao T,Hu H,Zhang W,Hua X. Association study of gut flora in coronary heart disease through high-throughput sequencing.Biomed Res 2017:3796359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Emoto T,Yamashita T,Sasaki N,Hirota Y,Hayashi T,So A,Kasahara K,Yodoi K,Matsumoto T,Mizoguchi T et al. Analysis of gut microbiota in coronary artery disease patients: a possible link between gut microbiota and coronary artery disease.J Atheroscler Thromb 2016;23:908–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sharon G,Garg N,Debelius J,Knight R,Dorrestein PC,Mazmanian SK. Specialized metabolites from the microbiome in health and disease.Cell Metab 2014;20:719–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang F,Yu T,Huang G,Cai D,Liang X,Su H,Zhu Z,Li D,Yang Y,Shen P et al. Gut microbiota community and its assembly associated with age and diet in Chinese centenarians.J Microbiol 2015;25:1195–204. [DOI] [PubMed] [Google Scholar]
- 14. O'Toole PW,Jeffery IB. Gut microbiota and aging.Science 2015;350:1214–15. [DOI] [PubMed] [Google Scholar]
- 15. Rothschild D,Weissbrod O,Barkan E,Kurilshikov A,Korem T,Zeevi D,Costea PI,Godneva A,Kalka IN,Bar N et al. Environment dominates over host genetics in shaping human gut microbiota.Nature 2018;555:210–15. [DOI] [PubMed] [Google Scholar]
- 16. Brown DG,Borresen EC,Brown RJ,Ryan EP. Heat-stabilised rice bran consumption by colorectal cancer survivors modulates stool metabolite profiles and metabolic networks: a randomised controlled trial.Br J Nutr 2017;117:1244–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hazim S,Curtis PJ,Schär MY,Ostertag LM,Kay CD,Minihane A-M,Cassidy A. Acute benefits of the microbial-derived isoflavone metabolite equol on arterial stiffness in men prospectively recruited according to equol producer phenotype: a double-blind randomized controlled trial.Am J Clin Nutr 2016;103:694–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sheflin AM,Borresen EC,Kirkwood JS,Boot CM,Whitney AK,Lu S,Brown RJ,Broeckling CD,Ryan EP,Weir TL. Dietary supplementation with rice bran or navy bean alters gut bacterial metabolism in colorectal cancer survivors.Mol Nutr Food Res 2017;61:1500905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhu A,Sunagawa S,Mende DR,Bork P. Inter-individual differences in the gene content of human gut bacterial species.Genome Biol 2015;16:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Upadhyaya S,Banerjee G. Type 2 diabetes and gut microbiome: at the intersection of known and unknown.Gut Microbes 2015;6:85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Allali I,Delgado S,Marron PI,Astudillo A,Yeh JJ,Ghazal H,Amzazi S,Keku T,Azcarate-Peril MA. Gut microbiome compositional and functional differences between tumor and non-tumor adjacent tissues from cohorts from the US and Spain.Gut Microbes 2015;6:161–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. de Moraes AC,Fernandes GR,da Silva IT,Almeida-Pititto B,Gomes EP,Pereira AD,Ferreira SR. Enterotype may drive the dietary-associated cardiometabolic risk factors.Front Cell Infect Microbiol 2017;7:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wu GD,Chen J,Hoffmann C,Bittinger K,Chen Y-Y,Keilbaugh SA,Bewtra M,Knights D,Walters WA,Knight R et al. Linking long-term dietary patterns with gut microbial enterotypes.Science 2011;334:105–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. de la Cuesta-Zuluaga J,Mueller NT,Corrales-Agudelo V,Velásquez-Mejía EP,Carmona JA,Abad JM,Escobar JS. Metformin is associated with higher relative abundance of mucin-degrading Akkermansia muciniphila and several short-chain fatty acid–producing microbiota in the gut.Diabetes Care 2017;40:54–62. [DOI] [PubMed] [Google Scholar]
- 25. Dueñas M,Muñoz-González I,Cueva C,Jiménez-Girón A,Sánchez-Patán F,Santos-Buelga C,Moreno-Arribas MV,Bartolomé B. A survey of modulation of gut microbiota by dietary polyphenols.Biomed Res Int 2015:850902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Clayton TA,Baker D,Lindon JC,Everett JR,Nicholson JK. Pharmacometabonomic identification of a significant host-microbiome metabolic interaction affecting human drug metabolism.Proc Natl Acad Sci U S A 2009;106:14728–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Arumugam M,Raes J,Pelletier E,Le Paslier D,Yamada T,Mende DR,Fernandes GR,Tap J,Bruls T,Batto J-M et al. Enterotypes of the human gut microbiome.Nature 2011;473:174–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wu GD,Compher C,Chen EZ,Smith SA,Shah RD,Bittinger K,Chehoud C,Albenberg LG,Nessel L,Gilroy E et al. Comparative metabolomics in vegans and omnivores reveal constraints on diet-dependent gut microbiota metabolite production.Gut 2016;65:63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Palau-Rodriguez M,Tulipani S,Isabel Queipo-Ortuño M,Urpi-Sarda M,Tinahones FJ,Andres-Lacueva C. Metabolomic insights into the intricate gut microbial–host interaction in the development of obesity and type 2 diabetes.Front Microbiol 2015;6:1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lamendella R,Verberkmoes N,Jansson JK,Hazen TC,Wuertz S. ‘Omics’ of the mammalian gut – new insights into function.Curr Opin Biotechnol 2012;23:491–500. [DOI] [PubMed] [Google Scholar]
- 31. Canfora EE,Jocken JW,Blaak EE. Short-chain fatty acids in control of body weight and insulin sensitivity.Nat Rev Endocrinol 2015;11:577–91. [DOI] [PubMed] [Google Scholar]
- 32. Vinolo MAR,Rodrigues HG,Nachbar RT,Curi R. Regulation of inflammation by short chain fatty acids.Nutrients 2011;3:858–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Koh A,De Vadder F,Kovatcheva-Datchary P,Bäckhed F. From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites.Cell 2016;165:1332–45. [DOI] [PubMed] [Google Scholar]
- 34. Yan Q,Gu Y,Li X,Yang W,Jia L,Chen C,Han X,Huang Y,Zhao L,Li P et al. Alterations of the gut microbiome in hypertension.Front Cell Infect Microbiol 2017;7:381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Arora T,Bäckhed F. The gut microbiota and metabolic disease: current understanding and future perspectives.J Intern Med 2016;280:339–49. [DOI] [PubMed] [Google Scholar]
- 36. Sabatino A,Regolisti G,Cosola C,Gesualdo L,Fiaccadori E. Intestinal microbiota in type 2 diabetes and chronic kidney disease.Curr Diab Rep 2017;17:16. [DOI] [PubMed] [Google Scholar]
- 37. Savi M,Bocchi L,Bresciani L,Falco A,Quaini F,Mena P,Brighenti F,Crozier A,Stilli D,Del Rio D. Trimethylamine-N-oxide (TMAO)-induced impairment of cardiomyocyte function and the protective role of urolithin B-glucuronide.Molecules 2018;23:549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yu L,Meng G,Huang B,Zhou X,Stavrakis S,Wang M,Li X,Zhou L,Wang Y,Wang M et al. A potential relationship between gut microbes and atrial fibrillation: trimethylamine N-oxide, a gut microbe-derived metabolite, facilitates the progression of atrial fibrillation.Int J Cardiol 2018;255:92–8. [DOI] [PubMed] [Google Scholar]
- 39. Tang WHW,Wang Z,Levison BS,Koeth RA,Britt EB,Fu X,Wu Y,Hazen SL. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk.N Engl J Med 2013;368:1575–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Aron-Wisnewsky J,Clément K. The gut microbiome, diet and links to cardiometabolic and chronic disorders.Nat Rev Nephrol 2016;12:169–81. [DOI] [PubMed] [Google Scholar]
- 41. Ridlon JM,Harris SC,Bhowmik S,Kang D-J,Hylemon PB. Consequences of bile salt biotransformations by intestinal bacteria.Gut Microbes 2016;7:22–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Brown JM,Hazen SL. Microbial modulation of cardiovascular disease.Nat Rev Microbiol 2018;16:171–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Fiorucci S,Distrutti E. Bile acid-activated receptors, intestinal microbiota, and the treatment of metabolic disorders.Trends Mol Med 2015;21:702–14. [DOI] [PubMed] [Google Scholar]
- 44. Tang WHW,Kitai T,Hazen SL. Gut microbiota in cardiovascular health and disease.Circ Res 2017;120:1183–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Singh RK,Chang H-W,Yan D,Lee KM,Ucmak D,Wong K,Abrouk M,Farahnik B,Nakamura M,Zhu TH et al. Influence of diet on the gut microbiome and implications for human health.J Transl Med 2017;15:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Fava F,Gitau R,Griffin BA,Gibson GR,Tuohy KM,Lovegrove JA. The type and quantity of dietary fat and carbohydrate alter faecal microbiome and short-chain fatty acid excretion in a metabolic syndrome “at-risk” population.Int J Obes 2013;37:216–23. [DOI] [PubMed] [Google Scholar]
- 47. Klinder A,Shen Q,Heppel S,Lovegrove JA,Rowland I,Tuohy KM. Impact of increasing fruit and vegetables and flavonoid intake on the human gut microbiota.Food Funct 2016;7:1788–96. [DOI] [PubMed] [Google Scholar]
- 48. Beaumont M,Portune KJ,Steuer N,Lan A,Cerrudo V,Audebert M,Dumont F,Mancano G,Khodorova N,Andriamihaja M et al. Quantity and source of dietary protein influence metabolite production by gut microbiota and rectal mucosa gene expression: a randomized, parallel, double-blind trial in overweight humans.Am J Clin Nutr 2017;106:1005–19. [DOI] [PubMed] [Google Scholar]
- 49. US Department of Agriculture and US Department of Health and Human Services Scientific report of the 2015 Dietary Guidelines Advisory Committee.Washington (DC):USDA, HHS;2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tapsell LC,Neale EP,Satija A,Hu FB. Foods, nutrients, and dietary patterns: interconnections and implications for dietary guidelines.Adv Nutr 2016;7:445–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. De Filippis F,Pellegrini N,Vannini L,Jeffery IB,La Storia A,Laghi L,Serrazanetti DI,Di Cagno R,Ferrocino I,Lazzi C et al. High-level adherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome.Gut 2016;65:1812–21. [DOI] [PubMed] [Google Scholar]
- 52. Koeth RA,Wang Z,Levison BS,Buffa JA,Org E,Sheehy BT,Britt EB,Fu X,Wu Y,Li L et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis.Nat Med 2013;19:576–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Estruch R,Ros E,Salas-Salvadó J,Covas M-I,Corella D,Arós F,Gómez-Gracia E,Ruiz-Gutiérrez V,Fiol M,Lapetra J et al. Primary prevention of cardiovascular disease with a Mediterranean diet.N Engl J Med 2013;368:1279–90. [DOI] [PubMed] [Google Scholar]
- 54. Orlich MJ,Fraser GE. Vegetarian diets in the Adventist Health Study 2: a review of initial published findings.Am J Clin Nutr 2014;100(Suppl 1):353S–8S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kwok CS,Umar S,Myint PK,Mamas MA,Loke YK. Vegetarian diet, Seventh Day Adventists and risk of cardiovascular mortality: a systematic review and meta-analysis.Int J Cardiol 2014;176:680–6. [DOI] [PubMed] [Google Scholar]
- 56. Dinu M,Abbate R,Gensini GF,Casini A,Sofi F. Vegetarian, vegan diets and multiple health outcomes: a systematic review with meta-analysis of observational studies.Crit Rev Food Sci Nutr 2017;57:3640–9. [DOI] [PubMed] [Google Scholar]
- 57. Patel H,Chandra S,Alexander S,Soble J,Williams KA. Plant-based nutrition: an essential component of cardiovascular disease prevention and management.Curr Cardiol Rep 2017;19:104. [DOI] [PubMed] [Google Scholar]
- 58. Melina V,Craig W,Levin S. Position of the Academy of Nutrition and Dietetics: vegetarian diets.J Acad Nutr Diet 2016;116:1970–80. [DOI] [PubMed] [Google Scholar]
- 59. Matijašić BB,Obermajer T,Lipoglavšek L,Grabnar I,Avguštin G,Rogelj I. Association of dietary type with fecal microbiota in vegetarians and omnivores in Slovenia.Eur J Nutr 2014;53:1051–64. [DOI] [PubMed] [Google Scholar]
- 60. Ríos-Covián D,Ruas-Madiedo P,Margolles A,Gueimonde M,de Los Reyes-Gavilán CG,Salazar N. Intestinal short chain fatty acids and their link with diet and human health.Front Microbiol 2016;7:185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Malinen E,Rinttila T,Kajander K,Matto J,Kassinen A,Krogius L,Saarela M,Korpela R,Palva A. Analysis of the fecal microbiota of irritable bowel syndrome patients and healthy controls with real-time PCR.Am J Gastroenterol 2005;100:373–82. [DOI] [PubMed] [Google Scholar]
- 62. De Filippo C,Cavalieri D,Di Paola M,Ramazzotti M,Poullet JB,Massart S,Collini S,Pieraccini G,Lionetti P. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa.Proc Natl Acad Sci U S A 2010;107:14691–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. David LA,Maurice CF,Carmody RN,Gootenberg DB,Button JE,Wolfe BE,Ling AV,Devlin AS,Varma Y,Fischbach MA et al. Diet rapidly and reproducibly alters the human gut microbiome.Nature 2014;505:559–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kim M-S,Hwang S-S,Park E-J,Bae J-W. Strict vegetarian diet improves the risk factors associated with metabolic diseases by modulating gut microbiota and reducing intestinal inflammation.Environ Microbiol Rep 2013;5:765–75. [DOI] [PubMed] [Google Scholar]
- 65. van Faassen A,Bol J,van Dokkum W,Pikaar NA,Ockhuizen T,Hermus RJ. Bile acids, neutral steroids, and bacteria in feces as affected by a mixed, a lacto-ovovegetarian, and a vegan diet.Am J Clin Nutr 1987;46:962–7. [DOI] [PubMed] [Google Scholar]
- 66. van Faassen A,Hazen MJ,van den Brandt PA,van den Bogaard AE,Hermus RJ,Janknegt RA. Bile acids and pH values in total feces and in fecal water from habitually omnivorous and vegetarian subjects.Am J Clin Nutr 1993;58:917–22. [DOI] [PubMed] [Google Scholar]
- 67. Cao H,Xu M,Dong W,Deng B,Wang S,Zhang Y,Wang S,Luo S,Wang W,Qi Y et al. Secondary bile acid-induced dysbiosis promotes intestinal carcinogenesis.Int J Cancer 2017;140:2545–56. [DOI] [PubMed] [Google Scholar]
- 68. Mitsou EK,Kakali A,Antonopoulou S,Mountzouris KC,Yannakoulia M,Panagiotakos DB,Kyriacou A. Adherence to the Mediterranean diet is associated with the gut microbiota pattern and gastrointestinal characteristics in an adult population.Br J Nutr 2017;117:1645–55. [DOI] [PubMed] [Google Scholar]
- 69. Gutiérrez-Díaz I,Fernández-Navarro T,Salazar N,Bartolomé B,Moreno-Arribas MV,De Andres-Galiana EJ,Fernández-Martínez JL,De Los Reyes-Gavilán CG,Gueimonde M,González S. Adherence to a Mediterranean diet influences the fecal metabolic profile of microbial-derived phenolics in a Spanish cohort of middle-age and older people.J Agric Food Chem 2017;65:586–95. [DOI] [PubMed] [Google Scholar]
- 70. Haro C,Montes-Borrego M,Rangel-Zúñiga OA,Alcalá-Díaz JF,Gómez-Delgado F,Pérez-Martínez P,Delgado-Lista J,Quintana-Navarro GM,Tinahones FJ,Landa BB et al. Two healthy diets modulate gut microbial community improving insulin sensitivity in a human obese population.J Clin Endocrinol Metab 2016;101:233–42. [DOI] [PubMed] [Google Scholar]
- 71. Sonnenburg JL,Bäckhed F. Diet–microbiota interactions as moderators of human metabolism.Nature 2016;535:56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Wolever TM,Schrade KB,Vogt JA,Tsihlias EB,McBurney MI. Do colonic short-chain fatty acids contribute to the long-term adaptation of blood lipids in subjects with type 2 diabetes consuming a high-fiber diet? Am J Clin Nutr 2002;75:1023–30. [DOI] [PubMed] [Google Scholar]
- 73. Robertson MD,Bickerton AS,Dennis AL,Vidal H,Frayn KN. Insulin-sensitizing effects of dietary resistant starch and effects on skeletal muscle and adipose tissue metabolism.Am J Clin Nutr 2005;82:559–67. [DOI] [PubMed] [Google Scholar]
- 74. McLoughlin RF,Berthon BS,Jensen ME,Baines KJ,Wood LG. Short-chain fatty acids, prebiotics, synbiotics, and systemic inflammation: a systematic review and meta-analysis.Am J Clin Nutr 2017;106:930–45. [DOI] [PubMed] [Google Scholar]
- 75. Rahat-Rozenbloom S,Fernandes J,Cheng J,Gloor GB,Wolever TMS. The acute effects of inulin and resistant starch on postprandial serum short-chain fatty acids and second-meal glycemic response in lean and overweight humans.Eur J Clin Nutr 2017;71:227–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Bodinham CL,Smith L,Thomas EL,Bell JD,Swann JR,Costabile A,Russell-Jones D,Umpleby AM,Robertson MD. Efficacy of increased resistant starch consumption in human type 2 diabetes.Endocr Connect 2014;3:75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Guasch-Ferré M,Hu FB,Ruiz-Canela M,Bulló M,Toledo E,Wang DD,Corella D,Gómez-Gracia E,Fiol M,Estruch R et al. Plasma metabolites from choline pathway and risk of cardiovascular disease in the PREDIMED (Prevention With Mediterranean Diet) study.J Am Heart Assoc 2017;6:e006524. [DOI] [PMC free article] [PubMed] [Google Scholar]