Abstract
Glucagonlike peptide 1 (GLP-1) receptor agonists have been efficacious for the treatment of type 2 diabetes due to their ability to reduce weight and attenuate hyperglycemia. However, the activity of glucagonlike peptide 1 receptor–directed strategies is submaximal, and the only potent, sustainable treatment of metabolic dysfunction is bariatric surgery, necessitating the development of unique therapeutics. GLP-1 is structurally related to glucagon and glucose-dependent insulinotropic peptide (GIP), allowing for the development of intermixed, unimolecular peptides with activity at each of their respective receptors. In this review, we discuss the range of tissue targets and added benefits afforded by the inclusion of each of GIP and glucagon. We discuss considerations for the development of sequence-intermixed dual agonists and triagonists, highlighting the importance of evaluating balanced signaling at the targeted receptors. Several multireceptor agonist peptides have been developed and evaluated, and the key preclinical and clinical findings are reviewed in detail. The biological activity of these multireceptor agonists are founded in the success of GLP-1-directed strategies; by including GIP and glucagon components, these multireceptor agonists are thought to enhance GLP-1’s activities by broadening the tissue targets and synergizing at tissues that express multiple receptors, such at the brain and pancreatic isletβ cells. The development and utility of balanced, unimolecular multireceptor agonists provide both a useful tool for querying the actions of incretins and glucagon during metabolic disease and a unique drug class to treat type 2 diabetes with unprecedented efficacy.
Essential Points
There is a need for new therapeutic strategies with broader activities than any currently available drugs
GLP-1, GIP, and glucagon receptors have unique tissue distribution and activity, supporting their combined use to treat diabetes
GLP-1, GIP, and glucagon have similar peptide sequences, permitting the development of single-sequence multireceptor agonists
Multireceptor agonists may be more effective than monoagonists for reducing body weight and improving metabolic dysfunction
Type 2 diabetes (T2D) is a complex, multifaceted disease that has rapidly increased in prevalence, currently affecting 1 in 11 adults worldwide, and a substantial economic burden with a global cost of $1.31 trillion US (1). Although the World Health Organization classifies diabetes as the sixth leading cause of death worldwide, the contribution of diabetes to heart disease (first leading cause of death) and stroke (second leading cause of death) significantly increases the impact of this disease on overall mortality rates.
Although far from understood, T2D typically arises following the development of insulin resistance, along with dysfunctional pancreatic islet action (insufficient insulin production and excessive glucagon production). Despite the recognized complexity of T2D pathogenesis, current therapeutic strategies are targeted at single disease aspects. Many therapeutic agents aim to enhance insulin levels with administration of exogenous insulin or insulin secretagogues, while others target insulin sensitivity (i.e., peroxisome proliferator–activated receptorγ agonists). Following the discovery of leptin, and later ghrelin, it became clear that hormonal signals to the brain play an important role in the development of obesity and subsequent T2D. Thus, targeting the central nervous system (CNS) to manage weight by increasing energy expenditure and restricting caloric intake emerged as an efficacious component of a successful weight loss strategy. Indeed, the mechanism for glucagonlike peptide 1 receptor (GLP-1R) agonists has been broadened beyond actions in the pancreas, to include CNS activity that reduces food intake and facilitate weight loss. Consequently, it has become clear that the multifaceted nature of T2D demands a therapeutic strategy that is equally sophisticated, and thereby provides synergistic avenues to target the many pathogenic origins of the disease.
Bariatric surgery has proven to be both efficacious and sustainable for the treatment of T2D. Originally developed specifically for morbidly obese individuals, independent of metabolic dysfunction, it quickly became clear that bariatric surgery can induce rapid improvements in hyperglycemia. Available pharmaceutical interventions are not able to replicate the magnitude of the benefits associated with surgery. On the other hand, it is not economically feasible to perform bariatric surgery as a primary treatment option for the number of people with T2D, creating a significant need for the development of pharmaceutical agents that achieve similar efficacy as surgical options, working noninvasively to target several tissues in a complimentary, synergistic manner.
Incretin hormones, glucagonlike peptide-1 (GLP-1), and glucose-dependent insulinotropic peptide (GIP) have recently garnered attention for their broad effects on target tissues of diabetes. Most notably, these factors are crucial for postprandial rises in insulin secretion. In fact, 50% to 70% of insulin secretion following a meal is a result of incretin action. Yet in T2D, the incretin effect is significantly blunted, suggesting that lesions in this axis may be key in the development of the disease and that restoration of incretin signaling could be particularly effective as a treatment strategy (2,3). In addition to incretin peptides, glucagon has reemerged as a significant contributor to glucose homeostasis and energy metabolism. Physiologically, glucagon opposes insulin action and increases glycemia, sparking numerous efforts to inhibit its action and lower glucose (4). However, the impact of glucagon on energy metabolism is becoming more evident, with increasing efforts now being put forth to agonize the glucagon receptor in an effort to treat metabolic dysfunction (5). What is clear is that both incretin peptides and glucagon are substantially increased following bariatric surgery (6–9), igniting interests in these peptides as the mechanism for the improvements in metabolic dysfunction. This has also made clear that our understanding of how these gut-derived peptides influence systemic metabolism is not completely understood. This review will discuss the controversies currently presiding over these peptides, and attempt to reconcile their contribution to both the pathogenesis of T2D and their potential to contribute to the next generation of therapeutic agents for the treatment of the disease.
Current Incretin-Directed Therapeutic Strategies
GLP-1R agonists and dipeptidyl peptidase-4 inhibitors
Dipeptidyl peptidase-4 (DPP4) is an exopeptidase that cleaves a wide range of peptide targets, including GLP-1 and GIP, thereby limiting their activity (10). Whereas DPP4 cleaves many peptides, the DPP4 inhibitors (DPP4i) lower glycemia solely by enhancing the biological activity of GLP-1 and GIP (11). The DPP4 inhibitor sitagliptin was approved for clinical use in 2006, and additional DPP4 inhibitors have since been introduced into the clinic. Although generally considered safe, these inhibitors have modest effects on hemoglobin A1c (HbA1c) and are weight neutral when administered as monotherapy. An important limitation of this drug class is that it requires endogenous production of incretins. Indeed, supraphysiologic levels of active GLP-1, beyond the concentrations typically achieved with DPP4i, are necessary to achieve robust effects on glycemia and stimulate a decrease in body weight.
There are currently several GLP-1 receptor agonists available for the treatment of T2D, which are more efficacious in controlling glycemia. These GLP-1 analogs are modified to prevent inactivation by DPP4 and substantially elongate the half-life of active GLP-1 in circulation. In addition to providing glucose control, several large-scale cardiovascular outcome trials have demonstrated that certain GLP-1R agonists reduce cardiovascular events (12–16). Thus, GLP-1 has many target tissues, and benefits of its use are observed across several aspects of the metabolic syndrome. Despite the broad range of benefits from GLP-1R monoagonism, there are limitations to its use that prevent maximal achievable benefits. Most notably, gastrointestinal side effects such as nausea limit the tolerability of GLP-1R agonists. As a consequence, these treatments are administered at submaximal doses in terms of their effect on weight loss and have to be dose titrated during initial administrations to patients (17), highlighting the potential for additional benefits beyond what is currently being achieved in the clinic.
Bariatric surgery
The single most effective therapy for T2D is bariatric surgery, predominantly manifested as Roux-en-Y and vertical sleeve gastrectomy procedures. It is now appreciated that the benefits of bariatric surgery are not solely dependent on dietary limitations and subsequent weight loss. Both Roux-en-Y and vertical sleeve gastrectomy yield immediate, weight-independent metabolic benefits and have since been termed “metabolic surgeries” (18). Although the molecular mechanisms underlying these effects are incompletely understood, several gastrointestinal hormones, including GLP-1, GIP, glucagon, oxyntomodulin, peptide YY, and ghrelin, are altered as a result and have been implicated as part of the mechanism. Attempts to isolate a singular peptide or metabolite as the primary stimulus for the improvement in metabolism following bariatric surgery have largely been unsuccessful (19–23). A takeaway from these studies is that the metabolic changes following bariatric surgery are multifaceted and complex, and therefore may require more than manipulation of a singular metabolic pathway to recapitulate the full efficacy of the surgery. Moreover, a key attribute to the success of bariatric surgery is that it causes negative energy balance while simultaneously decreasing appetite, a feat that no other weight loss strategy can achieve, and this is likely due to collaboration of multiple hormonal factors to manipulate these opposing behaviors coincidently.
Need for Novel Therapeutics
Despite the unprecedented benefits of bariatric surgeries, their application is not widespread enough to manage the T2D epidemic. The surgeries are expensive and invasive and are accompanied by several complications, including dumping syndrome, diarrhea, and hypoglycemia, among others. Furthermore, there are physical constraints for providing this surgery, including insufficient numbers of available surgeons with technical expertise and lack of appropriate infrastructure to perform the surgeries (24). These important limitations of bariatric surgery provide the impetus to develop pharmaceutical mimetics that provide broad systematic targeting to maximize efficacy while minimizing invasiveness. Several groups have approached this need by developing multitarget agonists largely centered on hormonal gut peptides. Although many combinatorial therapies have been pursued, and their development discussed in other reviews (24–26), this review will focus on incretin-based multiagonists. The basis for these compounds is anchored in the success and safety of GLP-1R agonists. This particularly pertains to the outcomes regarding neurologic and cardiovascular side effects, while still activating several tissue targets to improve metabolism. Incretins also improve glycemia without carrying hypoglycemic risk because their insulin-inducing activities are glucose dependent, further providing safety compared with other drug classes, such as sulfonylureas. Lastly, recent studies have demonstrated synergistic effects on glycemia and body weight when receptor agonism is balanced (27,28), which provides the basis for the development and translation of several multiagonist compounds discussed herein.
Incretin/Glucagon Biology
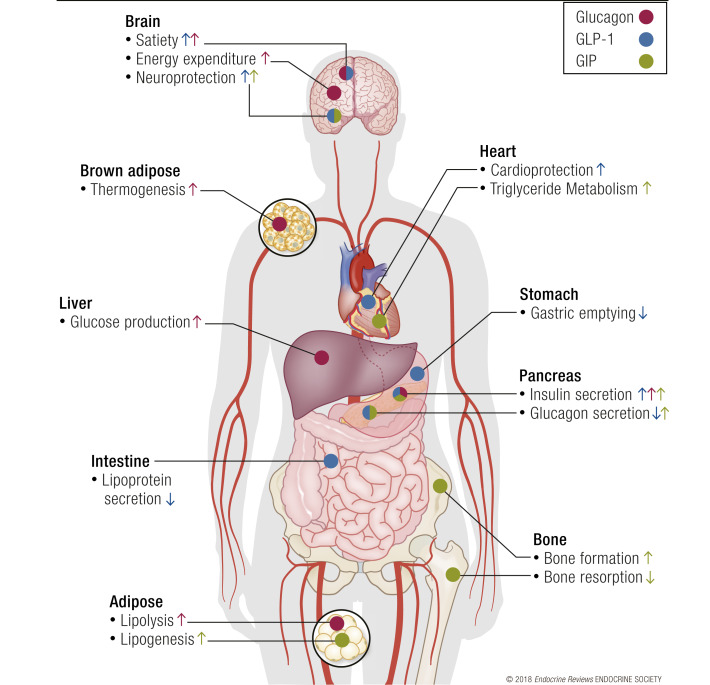
The purpose of this chapter is to provide a cursory review of relevant pathways modulated by GLP-1, GIP, and glucagon receptor activation to support their potential for use in multireceptor agonism. The review is limited to these three peptides due to their complimentary activities and similar structures at the N-terminal region that have allowed for the development of sequence-intermixed peptide therapies. GLP-1, GIP, and glucagon exert their effects through similar mechanisms of action, all acting on class B G protein–coupled receptors (GPCRs) of the Gs family and typically mediating signal transduction through the stimulation of cAMP. However, the tissue-specific distribution of the incretin/glucagon cognate receptors is unique, and likely plays a role in the variation in their respective functions (Fig. 1). For example, whereas GLP-1, GIP, and glucagon all have insulinotropic effects on the pancreaticβ cell, only glucagon can stimulate hepatic glucose production, whereas the GLP-1R and GIP receptor (GIPR) are not expressed in hepatocytes. Furthermore, even within a tissue type that expresses all three receptors (e.g.,β cells), there appear to be unique characteristics for each receptor. Together, this raises the intriguing possibility for synergistic interactions, both at the cellular, tissue, and systemic levels. Herein, we describe the relevant activities of each peptide, highlighting areas of activity that are more appropriately addressed through combinatorial treatment strategies.
Figure 1.
Glucagon, GLP-1, and GIP have unique activities at different tissue beds. Blue arrows show the effects of glucagon, green arrows show GLP-1 effects, and red arrows show GIP effects. Although there are overlapping activities of these peptides in the brain and pancreas, the majority of tissues have nonoverlapping receptor expression, yielding unique activities of each peptide at different tissues. Therefore, multireceptor agonists demonstrate additive effects, in part, by engaging a broader range of activities.
GLP-1
GLP-1 is secreted following posttranslational processing of proglucagon by prohormone convertase 1/3 in enteroendocrinel-cells and possibly pancreaticα cells (29,30). The secretion of GLP-1 from the intestine comprises the classic incretin response to ingested nutrients, while the significance ofα cell–derived GLP-1 was first identified in the early 1980s and was subsequently found to stimulate insulin secretion in a glucose-dependent manner in 1987 (31,32). GLP-1 offers added metabolic benefits beyond its effect on insulin secretion, also slowing gastric emptying, suppressing appetite, and lowering body weight. Recent insights have begun to describe anti-inflammatory actions of GLP-1R agonists (33,34), and although GLP-1R therapies have shown beneficial impact on cardiovascular events, the mechanisms by which this occurs are largely unknown (35). Comprehensive reviews of the metabolic actions of GLP-1 have been discussed previously (36–39).
Recent advances in optimizing GLP-1R agonist therapies to improve efficacy and adherence include enhancing the bioavailability for longer duration and allowing for oral delivery. Protracted GLP-1R agonists have not only improved convenience for patients by lessening the frequency of administration but have also shown unprecedented glycemic and body weight efficacy in clinical trials (14,40–47). After 10 years of clinical use, GLP-1R agonists and DPP4i are generally considered safe therapies. Compared with other therapeutic options, GLP-1R agonists present low risk of hypoglycemia, good efficacy for glycemic control and weight loss, and improvements in cardiometabolic health. These attributes have positioned GLP-1 as a favored candidate for the foundation of multireceptor agonists.
GIP
GIP, a 42–amino acid peptide, is secreted by K cells in the intestine in response to nutrients. GIP was discovered in 1973 and originally termed gastric inhibitory peptide due to its effects on gastric acid secretion. Shortly after its discovery, it was found to stimulate insulin in a glucose-dependent manner (48), and thus its role as an incretin hormone has been the primary focus for characterization of its activities. GIP exerts its actions through the GIPR, a seven-transmembrane heterotrimeric GPCR of the Gs family. GIPR is widely expressed, including but not limited to its appearance in the pancreas, stomach, bone, small intestine, adipose tissue, lung, and multiple regions of the CNS (49). The most well-defined activity of GIP is its glucose-dependent insulinotropic activity in the pancreas. GIP stimulates insulin secretion through GIPR on theβ cell, activating cAMP/protein kinase A- and cAMP/Epac2-dependent pathways in theβ cell (50). Consistent with its role as an incretin,Gipr–/– mice exhibit impaired oral glucose tolerance, although intraperitoneal glucose tolerance is unaffected (51). Interestingly, DPP4i retained their ability to stimulate insulin secretion and lower glycemia inβ-cell–specificGlp1r knockout mice (52), but notβ-cell–specificGipr knockout mice (53), under chow-fed conditions. This raises the intriguing possibility that enhanced GIPR activity may constitute the majority of the glucoregulatory actions of DPP4 inhibitors under normal conditions. Whether this relationship is altered by metabolic stress remains to be seen, which is important to understand given that GIP-stimulated insulin secretion is substantially blunted in people with T2D, even at pharmacological levels (54).
Although GIPR and GLP-1R are structurally related and exhibit comparable activity for glucose-stimulated insulin secretion, divergent pathways have been identified related toβ-cell survival. GIP signaling through its cognate receptor promotesβ-cell survival and protects against streptozotocin-inducedβ-cell apoptosis through ERK-dependent pathways that converge onTcf7 expression, which encodes the TCF1 protein (53). Similarly,Tcf7–/– mice are more susceptible to streptozotocin-inducedβ-cell apoptosis and exhibit impaired glucose tolerance in response to aging or high-fat diet feeding (53). This pathway was unaffected by loss of GLP-1R or stimulation with Ex-4, demonstrating unique actions of these receptors. This demonstrates the benefit of activating multiple incretin receptors in theβ cell, despite certain converging activities, such as insulin secretion.
Limitations for the development of GIPR agonists
The development of GIP-directed therapies has been limited by several findings. Most notably, insulin secretion in response to GIP administration is impaired in patients with T2D, and although GLP-1-induced insulin secretion is also reduced in these patients, the amount of insulin secretion is still functionally relevant (54). This finding naturally steered the field to develop GLP-1-directed therapies. However, it is important to note that the responsiveness to GIP is reversible. Alleviation of hyperglycemia can resensitize the islet to GIP, at least in part due to restoration of GIPR expression in the islet (55,56). Hyperglycemia induced by partial pancreatectomy reduced both isletGlp1r andGipr, but receptor expression was restored by reducing hyperglycemia with phlorizin (55). Similarly, phlorizin treatment of hyperglycemic zucker diabetic fatty rats for 14 weeks restored GIPR expression from ∼20% of lean control levels to ∼80% (56). This finding is pertinent to humans; restoration of GIP-induced insulin secretion was demonstrated in T2D patients following intensive insulin therapy for 4 weeks (57). Understanding the mechanisms that facilitate a strong correlation between GIPR activity andβ -cell function may provide key insight into the etiology ofβ-cell dysfunction in T2D. Moreover, the plasticity of GIPR signaling inβ cells indicates that therapies incorporating GIPR activity may provide additional glucose-lowering effects, particularly if GIP agonism is combined with additional agents such as GLP-1.
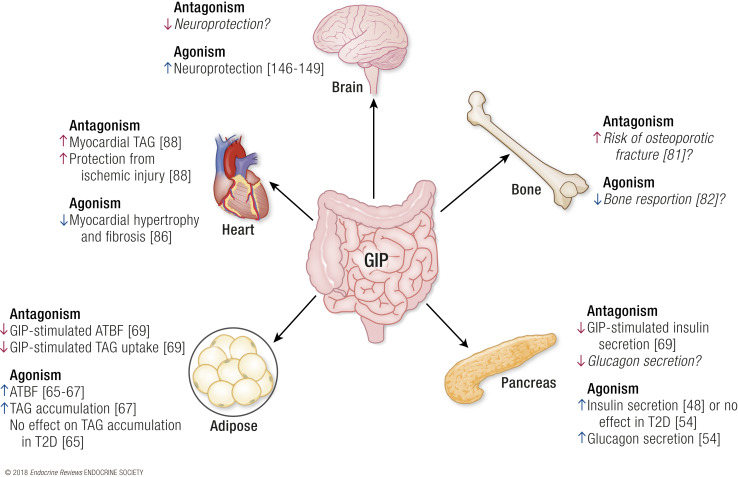
Another limitation in the pursuit of GIP-directed therapies was the observation that GIP stimulates triacylglycerol deposition in adipocytes. Initial studies utilizingGipr–/– mice revealed that germ-line deletion of GIPR prevented obesity following high-fat feeding or in the context of leptin deficiency, concluding that GIP was a lipogenic hormone (58). Notably, this effect is dependent on the type of dietary challenge;Gipr–/– mice fed a high-carbohydrate diet are not resistant to weight gain (59). Caution should be used in the interpretation of germ-line knockout models due to confounding effects of compensatory mechanisms. For instance,Glp1r–/– mice also show profound protection against diet-induced obesity (DIO) (60), yet GLP-1R agonists are now used in clinic to induce weight loss in obese individuals independent of metabolic dysfunction (61). Subsequent studies using pharmacological antagonism of GIPR with a modified GIP peptide, (Pro3)GIP, in obese animals also concluded that loss of GIPR function can limit or reduce adiposity (62,63). However, these conclusions were recently challenged by the findings that (Pro3)GIP is a full agonist at the human GIPR and a partial agonist at the rodent GIPR (64). Studies in humans demonstrate GIP agonism increases adipose tissue blood flow, events that are postulated to facilitate lipogenesis (65–67). Although establishing causality between adipose tissue blood flow and adipogenesis as a bone fide action of GIP still remains to be done, these studies did use newer GIPR antagonists that could prove to be beneficial for future efforts aimed at addressing this and other GIP actions. In fact, there are efforts to test the use of GIPR antagonism as a therapeutic strategy for limiting obesity, because loss of GIPR signaling is suggested to prevent triacylglycerol uptake (68,69). More thorough studies comparing GIPR agonism and antagonism are necessary to elucidate the impact of extrapancreatic GIPR signaling (Fig. 2). Together, the major observations leading to the conclusion that GIP is obesogenic have significant limitations that prevent clear insight into this question.
Figure 2.
Proposed activities of GIPR antagonism vs agonism. The projected activities of a GIPR antagonist at each tissue with GIPR expression is shown in red, and agonism is shown in blue. References supporting these activities are provided in brackets. Italicized text indicates a presumed effect of agonism or antagonism but does not have data to support a known activity. ATBF, adipose tissue blood flow; TAG, triacylglycerol.
Human and rodent adipocytes do express a GIPR, which has been reported to stimulate lipoprotein lipase, and mechanistic studies in adipocyte cell lines indicate a lipogenic signal for GIPR activation (70,71). Notably, the effects of GIP on isolated adipocytes were dependent on insulin, thus making it difficult to disassociate the lipogenic effects of GIP mediated through direct actions on adipocytes from those mediated indirectly through the insulinotropic effects of GIP inβ cells. Indeed, insulin appears to be a primary driver of obesity, at least in rodents (72). Even more complexity was added when subsequent studies demonstrated thatGipr–/– mice fed a high-fat diet gained less body weight than wild-type mice primarily due to differences in lean mass (73). Furthermore, rescue ofGipr specifically in the adipose tissue of the knockout mice corrected this difference in lean mass. Supporting the important role for GIPR signaling in adipose, a genome-wide association study assessing heritability of human obesity identified a unique body mass index–associated locus nearGIPR (74). Taken together, the effect of GIP on lipid deposition in adipose remains unclear and further studies utilizing adipocyte-specific knockout models or highly specific GIPR antagonists are required to better understand the significance of GIPR activity in adipose tissue.
GIPR is expressed in theα cells of both mouse and human islets, and its activation has been linked to glucagon secretion (75,76). In normal, healthy patients, GIP infusion elevated serum glucagon levels; however, this was observed in hypoglycemic and euglycemic conditions, but not during hyperglycemia (76). Notably, despite the elevated glucagon levels, glucose infusion rates in clamps were higher with GIP infusion, suggesting that at least in healthy patients, the glucagon-inducing activity of GIP does not have negative effects on glycemia. Furthermore, these findings did not correlate with insulin levels, which were significantly elevated by GIP infusion under all glycemic conditions. Whether GIP-induced glucagon secretion meaningfully impacts physiology requires further study.
Extrapancreatic effects of GIP
GIP-directed therapies may have added benefits beyond its effects on glycemia through activation of GIPR in extrapancreatic tissues. One such target is the bone, where GIP has been suggested as the key component of a posited “entero-osseous axis” (77). Proper bone health requires a balance between bone resorption by osteoclasts and bone formation by osteoblasts. Following a meal, bone resorption is suppressed, shifting the balance to bone formation and strengthening, suggesting that postprandial signals may be key mediators of this transition. Bone GIPR expression was first observed in 2000, with expression in osteoclasts, osteoblasts, and osteocytes (78). Studies performed inGipr–/– mice confirmed the importance of GIP signaling for bone homeostasis; bone length and width were decreased, with lowered bone mineral content and density inGipr–/– compared with wild-type controls (79). It remains unclear if GIP has a direct effect on osteocytes, or if the effect in the knockout mice is due to differences in circulating insulin. Indeed, insulin is known to be an integrator of nutrition and bone homeostasis (80). The role of GIPR signaling has also been confirmed in patients. The effect of a genetic variant that exhibits reduced GIP activity,GIPR Glu354Gln, was assessed in perimenopausal women, who are at increased risk for osteoporotic fractures. Women with the loss-of-function variant were about 50% more likely to experience nonvertebral fractures and had significantly lower hip and femoral neck bone mineral density (81). Additional studies assessing the circulating biomarker of bone resorption, carboxy-terminal collagen crosslinks, have demonstrated a strong relationship between GIP and nutrient-induced bone strengthening (82). Evidence for a direct effect of GIP in bone was seen in C-peptide-negative type 1 diabetic patients, where similar suppression of collagen crosslinks occurred independent of changes in circulating insulin (82). Thus, GIP may be a critical factor linking meal intake and bone formation. Notably, bariatric surgery or hypocaloric diets reduce bone mineral density in both rodents and patients (83–86). This raises the intriguing possibility that having a GIP-directed component in a mixed-agonist treatment strategy could reduce the adverse effects on bone that arise due to rapid weight loss or reduced calorie intake. However, it is important to note that GIP is also elevated following bariatric surgery (87), whereas bone mineral density is reduced; further work is necessary to elucidate the the direct and indirect effects of GIP on bone health in the context of obesity and T2D.
GIPR is expressed in both rodent and human cardiomyocytes and is linked to cardiac lipid metabolism.Gipr–/– mice were protected from ischemic cardiac injury, and this effect was independent of GIP-stimulated insulin secretion (88). The protection exerted by GIPR deletion was mediated by decreased hormone-sensitive lipase phosphorylation and increased myocardial TAG content. However, GIP agonism had no effect on cardiac ischemia, despite stimulating hormone-sensitive lipase phosphorylation and fatty acid oxidation, demonstrating the cardiac safety of GIPR agonism for use in multireceptor agonism. In fact, GIP administration limited angiotensin II–stimulated cardiac hypertrophy and fibrosis (89). However, it is unclear whether this occurs through direct effects on cardiac GIPR or indirectly through activation of insulinin vivo. Future studies are still necessary to characterize the effect of GIPR signaling in acute ischemic injuries.
Glucagon
Glucagon is a 29–amino acid peptide derived from posttranslational processing of the proglucagon protein by prohormone convertase 2. It signals through the seven-transmembrane glucagon receptor (GCGR), which is widely expressed throughout the body, including liver, brain, heart, kidney, adipose tissue, and pancreaticβ cells (90). The hyperglycemic effect of glucagon mediated through its direct stimulation of glycogenolysis in the liver was determined 70 years ago (91), and later glucagon was demonstrated to elevate glucose through the stimulation of gluconeogenesis in the liver. On the other hand, glucagon, like incretins, was found to possess glucose-dependent insulinotropic activities. Despite this behavior, the focus of glucagon has been on its elevated levels observed in diabetes, which tightly correlate with dysglycemia (92,93). As a result, much of the early efforts on glucagon-based therapies have been focused on blockade of glucagon receptor. Seminal studies demonstrated that either pharmacological or genetic blockade of glucagon action in streptozotocin-induced diabetes completely normalizes glucose (94–96). This concept appears to translate to humans, where glucagon receptor antagonists in clinical trials are efficacious in lowering HbA1c (97,98). However, these agents have stalled in their development due to a number of undesirable effects including elevated aspartate aminotransferase/alanine aminotransferase levels, accumulation of liver triglycerides, and hyperglucagonemia.
In addition to hepatocytes, glucagon receptors have been reported to be expressed in the brain, although the biological significance of GCGR activation in neuronal tissues remains unclear. Central activation of the GCGR stimulates a broad range of beneficial actions for obesity management, including satiety and energy expenditure. Glucagon levels increase in response to a protein-rich meal (99), and inhibiting glucagon’s action can increase food intake (100). Early studies also indicated an effect of glucagon on body weight, independent of food intake (101). This effect has since been attributed to the thermogenic effect of glucagon, as administration of glucagon to rats increased brown adipose tissue oxygen consumption and temperature (102). Similar effects have been observed in humans, but only during relative hypoinsulinemia (103). Induction of fibroblast growth factor 21 (FGF21) is implicated in glucagon’s weight-lowering effects in rodents and humans (104). Glucagon stimulates FGF21 secretion from hepatocytes, and FGF21 exerts similar biological effects of glucagon, including increased energy expenditure (105).
Though paradoxical, postprandial hyperglucagonemia is commonly observed following bariatric surgery (106–109). Based on studies from total pancreatectomized humans, which cannot produce islet-derived hormones, it has been confirmed that the intestine can be a source of circulating glucagon (110). Notably, glucagon was secreted in response to oral glucose administration in these patients, similar to GLP-1. Thus, it could be speculated that this intestinal source of glucagon is elevated following bariatric surgery. Moreover, the hyperglucagonemia as a result of extrapancreatic glucagon production may contribute to the beneficial effects on energy expenditure observed. Following surgery, glucagon hypersecretion would occur in the presence of postprandial insulin secretion due to a restored incretin response, thereby balancing the negative effects of glucagon on hepatic glucose production. Conversely, in T2D patients without surgery, hyperglucagonemia occurs in the absence of adequate insulin secretion, thereby promoting hepatic glucose output (111). The significance of gut-derived glucagon has yet to be elucidated, but is actively being pursued.
Current Dual Agonists and Triagonists
Although GLP-1, GIP, and glucagon have some overlapping functionality, their combined use leads to synergistic effects on diabetes and related metabolic disease. Although there is biological justification for the development of multireceptor agonist therapies, several design characteristics are important to achieve maximal efficacy. This section will focus on the following considerations for creation of multireceptor agonists: (1) structural design of effective agonists; (2) optimization of agonist activities at each receptor; and (3) efficacy of recently developed dual agonists and triagonists that use GLP-1, GIP, and glucagon receptor signaling.
Considerations for the discovery and development of multiagonist therapies
Unimolecular design: sequence-intermixed hybrids vs multivalent fusions
The most prevalent argument in favor of single-molecule multiagonists vs combination of monoagonists has centered on the pragmatic challenge of developing and registering a single compound as opposed to multiple compounds. Downstream considerations also include eventual pricing and cost of goods for production. The clinical development of combinations requires that each individual component must be studied in isolation first for clinical safety, and perhaps for efficacy, before it is studied in combination. Optimally, these combinations would also be formulated together in a single physical mixture such that it will be administered as a single injection. Formulations are finely tuned to support the storage stability and absorption following injection of a particular drug. Oftentimes this recipe is unique to a certain drug product, and not compatible with other compounds, which may limit a single formulation. Therefore, if the eventual products are aimed to be mixed together, then early chemistry efforts are necessary to ensure compatible coformulation. Furthermore, drug–drug interactions in a coformulation can influence biophysical stability, and thus the potential to alter efficacy after drug administration. One major advantage of physical mixtures or loose combinations is that it should be easier to titrate the components to achieve maximal effectiveness and safety, particularly if one of the ingredients has a narrow therapeutic window, as in the case of glucagon. Secondary to that consideration is the simpler chemical optimization of a monoagonist vs a mixed agonist. Pharmacokinetic differences between the combination products, most notably different half-lives and biodistribution, may drive differential efficacy and constitute another way to realize altered ratio ofin vivo activities, including humans. Single-molecule mixed agonists can also present different biodistribution than combinations, such that a specific component of the single molecule could be the dominant factor in tissue distribution.
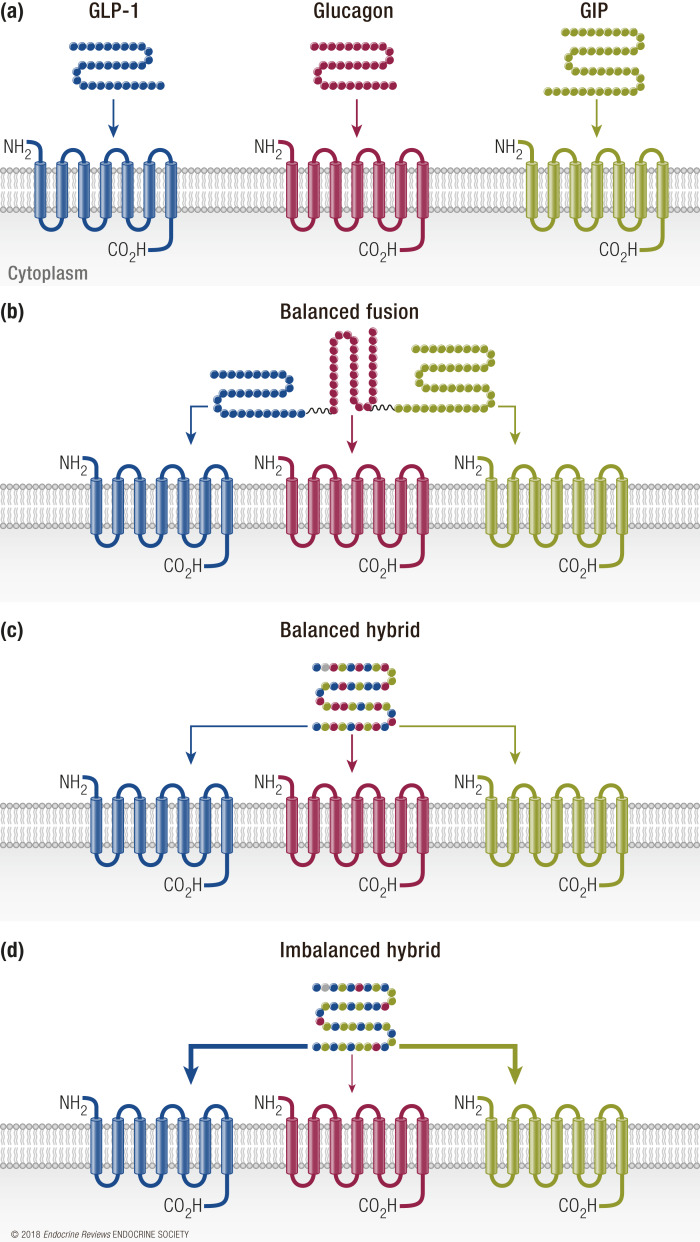
The molecular format of single-molecule mixed agonists can theoretically take two designs: a fusion molecule where monoagonist analogs are appended together in a multivalent format, or a sequence-hybridized molecule of comparable size to the native peptides (Fig. 3). The latter is the preferred approach to date for GLP-1, GIP, and glucagon mixed agonists. These three receptor targets are all class B GPCRs, and the native ligands have a high degree of sequence similarity and secondary structure. Furthermore, each of these receptors has cross-reactivity to the other hormones, with GLP-1R appearing to be the most promiscuous of the receptors. Likewise, glucagon is the ligand that shows the most cross-reactive activity at the two incretin receptors. This pattern of activity, combined with the analogous ligand structure, provides the unique foundation and opportunity to build sequence-intermixed molecules. Notably, this is unlikely the case for the reported GLP-1 fusions to amylin or gastrin (112,113). Sequence-intermixed unimolecular peptides have several advantages. First, when agonism is appropriately balanced, single peptides can only occupy one receptor at a time, unlike what could happen for multivalent fusions. Theoretically, this reduces the binding at any one receptor when delivered at equimolar concentrations, which may be of practical importance when trying to limit adverse side effects attributed to a particular receptor activity. As with all hybrids or fusions, there is a real concern of developing neutralizing antibodies to the drug itself, or worse, to the endogenous hormones. It is too early in the clinical progression of these molecules to estimate if one molecular format is preferred to limit these immunogenicity concerns.
Figure 3.
Design considerations of triagonists. (a) Each receptor (GLP-1R, blue; GCGR, red; GIPR, green) responds to its respective ligand. (b) A balanced fusion structure uses each individual peptide to provide recognition at all receptors. (c) A balanced hybrid peptide is a sequence-hybridized molecule of comparable size to the native peptides with equipotent activities at all receptors. (d) An unbalanced hybrid peptide is a sequence-intermixed molecule that preferentially activates certain receptors with higher affinity than others.
Balanced vs preferential agonism
An important consideration in the design of multiagonist therapies is the relative potency of the compound at each of the cognate receptors. Compounds can be engineered either to be balanced in mixed agonism with equal potency at each of the targeted receptors, or the agonism ratio skewed in favor of one receptor over others (Fig. 3). This is an important consideration when trying to leverage maximal efficacy and potency vs unwanted dose-dependent side effects. This is particularly evident in mixed agonists in which one of the constituent activities is aimed at GCGR. GCGR agonism is a vital component to drive body weight loss, but a steep dose response and the hyperglycemic liability must be compensated for by having appropriate incretin receptor activity. Identifying the optimum ratio to improve one metabolic endpoint, for instance body weight, may not be the same ratio that is optimum for other readouts, such as glucose or lipids. Furthermore, the optimum ratio identified in preclinical models of diabetes and obesity may not be the optimum ratio in human subjects. Moreover, the heterogeneity of these disease states in human patients complicates identifying the optimum ratio in clinical pharmacology experiments.
Another caveat is actually how to define the ratio of activity. To support high-throughput screening in structure–activity relationship (SAR) studies, oftentimes functional assays and binding assays are used to assess potency through the use of artificial cell systems in which a single receptor is overexpressed. To determine the relative potency of each constituent within a single-molecule multiagonist, receptor expression should be controlled between the various assay systems so that cross-comparisons can be accurately assessed to relate the measured potencies against each other. However, this ignores the likelihood of differing levels of expression between these receptors at a whole-body level, as well as a tissue-specific pattern. Furthermore, the endogenous peptides have slightly different binding affinities for their native receptor, as well as measurable levels of cross-reactivity at the other related receptors. So defining the activity ratio at the constituent receptors must factor in these slight differences in inherent binding characteristics.
A common approach to protract time action is to promote binding to circulating albumin or other plasma proteins, most commonly through fatty acid acylation of the peptide. Therefore, when usingin vitro assays to estimate an activity ratio, one must also consider the influence of albumin in bioassays, which is oftentimes included in low percentage to prevent compounds from sticking to assay plates. However, testing in the presence of albumin at concentrations that are consistent with circulating concentrations is another strategy to assess potency ratio in a condition that more closely resembles thein vivo situation. Thisin vitro approach essentially assesses the activity of the bound vs unbound fraction when testing in the presence or absence of albumin, and can be used as anin vitro tool to predictin vivo protraction. This should be used with caution though, as the type of fatty acylation and its placement on the backbone of the peptide can differentially influence the measured activity. All that being said, these strategies only take into account that a single receptor is expressed in a single cell. In other words, this does not take into account that receptor crosstalk can influence potency, which has been reported for this family of receptors (114).
Receptor preference is particularly important in tissues expressing multiple receptors. For example, GLP-1R/GIPR dual agonists were designed to optimize insulin secretion from theβ cell while using less pressure on GLP-1R to mitigate the dose-limiting gastrointestinal side effects of GLP-1R agonists. To assess balanced agonism in cells coexpressing incretin receptors that converge on the same signaling pathways, such asβ cells, various knockdown approaches such as viral-mediated, selective pharmacological inhibition or CRISPR/Cas9–mediated technology can be used (115). These technologies are important for high-throughput screening of candidate agonists, particularly because receptor agonism does not fully blunt receptor-mediated intracellular signaling. Use of these technologies will aid in developing appropriate agonism, particularly in cell types with overlapping receptor expression.
During the evaluation of single-molecule mixed agonists or fusions, it is important to demonstrate the multiple pharmacology within the molecule and to estimate the contribution of each component to the total efficacy. Although the use of genetically modified mice seems to be the preferred approach, genetic compensations can oftentimes substantially influence the physiology of these mice such that the pharmacological assessment of mixed agonists in these mice can be complicated (116). Therefore, it is equally advantageous to make selective chemical knockouts where the activity of a single component of the multiagonist is reduced by a slight modification to the chemical structure. Rationally, this is easier to achieve in a single-molecule fusion, as the SAR of the single molecules can pinpoint the exact amino acids that can be mutated to inactivate a single component. This is more complicated to achieve with sequence-intermixed single molecules, but it can be done (28).
Approaches to integrate activities at multiple receptors
The unimolecular GLP-1/glucagon, GLP-1/GIP, and GLP-1/GIP/glucagon peptides have been designed using various precursor hormones as the scaffold in which residues from the other hormones were introduced into these peptide backbones to introduce mixed agonism. Those precursor scaffolds include glucagon, exendin-4, and oxyntomodulin. In general, the molecular design of these multiagonists starts with a thorough understanding of the SAR of the individual hormones, particularly those sites that are crucial for individual activity and those sites that are dispensable for binding and activity. Leveraging lessons from these understandings, the swap of crucial domains between the hormones can lead to the identification of single-site substitutions that are crucial for peptide–receptor interactions at each targeted receptor. Additional mutations can be introduced into the sequence to optimize the activity balance, chemical integrity, biophysical stability, andin vivo time action.
In an approach to generate GLP-1/GCGR dual agonists, glucagon was used as the scaffold to which residues specific to GLP-1 or exendin-4, notably select amino acids derived from the proximal C-terminal domain, were introduced to gain potency at GLP-1R (117). In a different iteration of this type of coagonist, oxyntomodulin, an endogenous coagonist of weak potency and imbalanced activity, was used as the scaffold. N-terminal and distal C-terminal modifications were introduced to improve potency and activity balance with the auxiliary function to improvein vivo time action (118). Coming from the other angle, exendin-4 has been used as the scaffold to which a glucagon-derived central domain was introduced to gain GCGR potency. N-terminal modifications derived from glucagon and C-terminal modifications derived from oxyntomodulin are required to achieve higher potency and balanced activity (119). Stabilization of theα helical nature of the peptide, particularly within the mid-domain of the sequence, is a somewhat universal strategy to enhance potency across this broad class of ligands and receptors. This was achieved through covalent lactamization of the backbone (117,120), introduction of alternately charged residues in a particular register to create a salt bridge (119), or introduction of an aminoisobutyric acid (Aib) residue, which has inherent helical-promoting properties (121). Interestingly, it has also been observed that fatty acid acylation, which typically is used to promote binding to plasma proteins or membrane anchoring to protract time action, can also contribute to the pharmacophore for promoting promiscuity at GLP-1 and glucagon receptors, likely by promoting specific secondary structure of the ligand (122).
Relative to GLP-1/glucagon coagonists, much less has been reported regarding the structural aspects of GLP-1/GIP coagonists. To build GLP-1/GIP receptor coagonists, a chimeric sequence of GLP-1 and exendin-4 was used as the scaffold to which GIP-specific residues were incorporated to introduce sufficient potency at GIPR (27). The N-terminal tyrosine derived from GIP, along with isoleucine at position 12 and lysine at position 16, were pivotal for gaining GIPR activity, whereas Aib residues at positions 2 and 20 also were instrumental in gaining substantial GIPR potency without compromising GLP-1R potency. Fatty acid acylation and polyethylene glycol (PEG)ylation of these peptides resulted in analogs sufficient for once-daily administration. To generate GLP-1/GIP/glucagon triagonists, respective coagonists were used as the scaffold to which alterations were made to introduce activity at the third receptor. Starting with the aforementioned GLP-1/GIP coagonist, simple mutation at the third position to glutamine rendered a balanced triagonist byin vitro measure (28). However, uncharacterizedin vivo limitations with this backbone precluded its further pursuit. Alternatively, starting from the GLP-1/glucagon coagonist, inclusion of Aib at the second position, introduction of the C-terminal extension derived from exendin-4, site-specific acylation at position 10 of the backbone, and the selective inclusion of GIP-derived residues yielded a highly potent, and relatively balanced, triagonist (28). Multivalent fusions of GLP-1, GIP, and glucagon analogs have also been reported. In this molecular format, antibody domains were used to anchor all three constituents into a multifunctional antibody (123). The antibody fragment serves the ancillary function to protract time action as well.
Performance of various dual agonists
GLP-1R/GCGR dual agonists
Combination of GLP-1R agonists with GCGR agonists was founded in the idea that both agonists use complimentary mechanisms to induce weight loss, whereas GLP-1R activation would combat any negative effect of GCGR activation on glycemia. Ideally this treatment strategy would engage receptor agonism and subsequent weight-reducing activities in a broader range of tissues, as GCGR and GLP-1R are nonoverlapping in many cell types. This is supported by findings from human coinfusion studies, where glucagon and GLP-1 maintained their individual effects on energy expenditure and glycemia, respectively, when infused in combination (124). Although GLP-1 and glucagon both exert similar actions at theβ cell for insulin secretion, their nonoverlapping functions at other tissues are the primary benefit for their combination in treatments. Glucagon adds adipose tissue lipolysis and appetite suppression and elevates energy expenditure to the known GLP-1R agonist actions on glycemia and weight reduction. The concept for developing this dual-agonist pair is aligned with studies of the proglucagon-derived peptide oxyntomodulin. Oxyntomodulin exerts its antidiabetic effects through binding at both the GLP-1R and GCGR; it reduces glycemia through the GLP-1R while stimulating weight loss through increased energy expenditure by activation of GCGR (125). However, oxyntomodulin is not as potent an activator of GLP-1 and GCGR as either of their cognate ligands, having 10- to 100-fold less activity at the receptors than their respective ligands (126). Additionally, oxyntomodulin, like GLP-1, is a substrate of DPP4, and as such is labilein vivo, exhibiting a half-life of ∼12 minutes (127). Another study used a dual agonist similar to oxyntomodulin, but with cholesterol added to a Cys side chain near the C-terminal that allowed for enhanced duration of action (118). In the same study, this group compared an agonist with a single residue change that no longer exhibited any activity at the GCGR to analyze the benefits of compounding GCGR activation onto GLP-1R activation. Dual agonism significantly reduced body weight compared with vehicle or GLP-1R activation alone. The dual agonist yielded similar effects on food intake compared with GLP-1R agonism alone, suggesting that GCGR agonism has complementary actions to GLP-1R agonism for weight reduction. Activities at both receptors were confirmed usingGlp1r–/– andGcgr–/– mice. An important finding was that compared with GLP-1R agonism alone, there was no significant difference on glucose tolerance for the combination with GCGR agonism. Thus, glucagon receptor agonism is successfully balanced by the activation of the GLP-1R. These studies provided the impetus to develop more potent dual activators of GLP-1R and GCGR for more effective weight loss and glycemic improvements.
The combination of GLP-1 and glucagon has been thoroughly proven as an effective weight loss strategy. For example, acute administration of subanorectic doses of individual peptides (GLP-1 at 0.4 pmol/kg/min and glucagon at 2.8 pmol/kg/min) to nondiabetic, overweight patients yielded reduced food intake and increased satiety compared with either peptide alone (128). Notably, this occurred in the absence of nausea, demonstrating the potential for increasing weight loss with diminished adverse side effects from increasing the dose of GLP-1R agonists. In this study, neutral results were observed for glycemia, whereas glucagon infusion alone elevated glycemia. This suggests that higher doses of glucagon may be more effective than low doses, as glucagon could exert its effect at theβ-cell GLP-1R at higher circulating levels, compared with low levels where glucagon’s effect on hepatic glucose output may prevail. Based on the superior outcomes of combination therapy for weight loss, several groups developed coagonist therapies. Development of these stable coagonists have allowed for better understanding of glucagon biology, as glucagon is rapidly degraded, and therefore, primarily acute activities of glucagon have been reported. One group generated a balanced 40-kDa polyethylene glycol (PEG)ylated coagonist peptide for once-weekly administration (117). This compound, Aib2 C24 lactam 40k, when administered subcutaneously at a high dose, reduced body weight in a mouse DIO model by 25.8% in 1 week as a result of reduced food intake and decreased fat mass. In the longer term, low-dose studies demonstrated comparable weight loss to the short-term administration of high doses, but weight loss was associated with increased energy expenditure and thermogenesis, with no differences observed in caloric intake or locomotor activity. The balanced coagonist also demonstrated benefits for lipid metabolism, leading to reduced cholesterol levels and subsequent reduction of hepatic steatosis. Notably, this balanced agonist compound outperformed preferential agonists and maintained its weight-lowering activity in mice lacking the GLP-1R, demonstrating the utility of glucagon-directed strategies. In a study assessing balanced vs unbalanced agonism, GCGR agonism was efficacious at lowering weight loss, but was most effective when paired with appropriate GLP-1R agonism to mitigate effects on glycemia (129). Thus, balance of receptor agonism, as determined usingin vitro systems to evaluate respective activities, should be considered to maximize weight loss while considering hyperglycemic risks that are associated with glucagon action on hepatic glucose output.
“Balanced triagonists are similar in length to endogenous glucagon and are about as close in sequence as exendin-4 to GLP-1.”
Several compounds have been clinically pursued in this class of drug candidates. The earliest was LY2944876 or TT-401, created by Transition Therapeutics in collaboration with Eli Lilly. This compound was an oxyntomodulin analog that was formulated for weekly administration, and imbalanced in activity, in favor of GLP-1R activity relative to GCGR activity. At the highest dose, 50 mg, HbA1c was decreased by 1.4% and body weight reduced by 3.3 kg after 24 weeks of administration (130). However, LY2944876/TT-401 has not been continued into phase 3 trials, but is being reassessed by OPKO Health, Inc. Sanofi has also developed a dual-agonist compound, SAR425899, which has ongoing phase 2 clinical trials. SAR425899 is formulated for daily administration, and preliminary results have shown that at the highest dose, a 5.5-kg reduction in body weight was observed with a modest reduction in HbA1c after 4 weeks of treatment (131). This compound is also imbalanced in relative activity with higher GLP-1R potency. Despite positive preliminary results from the phase 2 proof-of-concept studies, gastrointestinal tolerability issues prevented the continuation of the study, which was likely the result of the dose escalation schedule. Another compound, MEDI0382, developed by MedImmune and AstraZeneca, demonstrated robust efficacy in rodent and cynomologus monkey models for weight loss and glucose control when administered in comparable doses to liraglutide (132) and is now in phase 2b trials. Several other companies have dual agonists in the pipeline, including Janssen with Hanmi Pharmaceuticals (HM12525A/JNJ-64565111; phase 1), Zealand Pharma (ZP2929/BI 456906; phase 1), Merck (MK-8521; phase 2), and Novo Nordisk (NN9277; phase 1) (133).
GLP-1R/GIPR dual agonists
The pursuit of GLP-1R/GIPR dual agonists lagged behind the development of GLP-1/glucagon agonists, largely due to the limitations of using GIP, as discussed above. Conceivably, these dual agonists would exert their activities in two steps. First, GLP-1R agonism would elicit glycemic control, among its effects on body weight, allowing GIPR sensitivity, and thus secondary modulation of glycemia and body weight by GIP. This strategy is supported by the finding that in diabetic patients, who are unresponsive to GIP alone, there was additive, increased first-phase insulin secretion in response to coinfusion of GLP-1 and GIP at physiologic levels (134). However, several additional acute human coinfusion studies found no further benefit of GIP addition to GLP-1 infusion (135–137). Thus, the effect of long-term administration of GIP, when added to GLP-1, could not be inferred from these patient studies. As such, when compared with equimolar doses of either incretin alone, the combination of GLP-1 and GIP in a rodent model of DIO was more efficacious at reducing body weight, food intake, and adiposity (27). No additive or synergistic effect of the incretin combination was observed for lowering glycemia in nondiabetic rodents, suggesting redundancy of action at the level of GPCR-mediated potentiation of insulin secretion inβ cells. Notably, GIPR agonism alone had no effect on fat mass or body weight yet when combined with GLP-1R agonists was able to outperform GLP-1R agonism alone. These findings provided the rationale to develop a balanced, unimolecular dual-agonist peptide for the treatment of metabolic syndrome and T2D (27). The acylated GLP-1R/GIPR dual agonist, which had an optimal pharmacokinetic profile to decrease dosing frequency to once daily, outperformed liraglutide in a mouse DIO model in all parameters tested; at the highest dose (equivalent to the tested dose of liraglutide), the coagonist more potently reduced body weight, food intake, fat mass, and blood glucose by 1 week posttreatment initiation. These effects persisted with biweekly injections in DIO mice and, in fact, amplified after 30 days with the added benefit of significantly lowered total cholesterol levels compared with liraglutide. Notably, the coagonist was able to reduce weight gain independent of an effect on energy expenditure or locomotion, and was not solely due to decreased food intake, as pair-fed mice did not lose body weight at the same magnitude as coagonist-treated mice. This finding demonstrates unique synergistic effects of incretins when dosed together that have yet to be described. Though fatty acid–acylated and PEGylated versions of the coagonist were developed, the former compound outperformed the latter compound for lowering body weight, while performing similarly for all other metabolic endpoints tested. Comparable findings were observed across several models, including db/db mice and cynomolgus monkeys. Importantly, this compound reduced the gastrointestinal side effects associated with monoagonism of GLP-1R.
Further supporting the concept of dual-incretin agonism, coadministration of fatty acid–acylated analogs of GLP-1 and GIP improved body weight and hyperglycemia in metabolically compromised mice (138). Additionally, Zealand Pharma recently developed a unique GIP analog, ZP4165, which in physical combination potentiated the effects of liraglutide (139). ZP4165 is more stable than native GIP, allowing for long-term assessment of GIPR activation alone in the db/db model of T2D. This GIP analog was stabilized through multiple modifications that include position 2 Aib to prevent DPP4 degradation, and a C16 fatty acid was added to Lys17 to increase the peptide half-life by increasing albumin binding. Twice-daily injections of ZP4165 reduced glycemia and increased insulin secretion in a DIO mouse and db/db model of T2D. Interestingly, when ZP4165 was coadministered with liraglutide, the profile observed was similar to that previously observed with the unimolecular dual agonist (27), demonstrating synergistic lowering of HbA1c and body weight. Combination treatment of the two incretin receptor agonists also corroborated the effects of dual agonism on total cholesterol, as lowering was only observed when GLP-1R and GIPR were both activated, with no change observed with either treatment alone.
GLP-1/GIP coagonism has recently demonstrated benefits in models of Alzheimer’s and Parkinson disease. Alzheimer’s disease increases the risk of developing T2D, and both Alzheimer’s and Parkinson diseases are associated with insulin resistance and inflammation (140,141). The fatty acid–acylated dual GLP-1R/GIPR agonist described above has recently been tested in animal models of Alzheimer’s and Parkinson disease, as well as traumatic brain injury (142). The coagonist demonstrated decreased brain inflammation, insulin resensitization, and reduced cognitive impairment in an Alzheimer’s mouse model using intracerebroventricular streptozotocin, a Parkinson mouse model using 6-hydroxydopamine lesion, and a Parkinson mouse model using 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (143–145). Though not all of these studies performed direct comparisons to GLP-1R or GIPR monoagonists, promoting either GLP-1 or GIP signaling alone has yielded benefits for cognitive impairment in either clinical or preclinical studies of Alzheimer’s and Parkinson disease, suggesting the potential for additive or synergistic benefits of dual agonists (146–151). Further studies are necessary to determine the full array of benefits from multireceptor agonism in the brain.
Although the results from the studies mentioned above are from preclinical studies, the clinical effects of a dual GLP-1/GIP receptor agonist developed by Novo Nordisk, NNC0090-2746, have recently been described from phase 2a trials (152). NNC0090-2746, previously called RG7697, is the balanced, unimolecular, fatty acid–acylated GLP-1R/GIPR agonist that has been tested in several preclinical models and safety verified in patients (27). NNC0090-2746 is thought to bind only one receptor at a time, and this concept guided the dosing in clinical trials; the study based dosing on high doses of liraglutide with the expectation that gastrointestinal side effects would be curbed due to decreased GLP-1R occupancy. In phase 1 studies, NNC0090-2746 displayed similar adverse effects as GLP-1R agonists, only at the highest doses (153,154). NNC0090-2746 was found to reach steady-state levels in patients after daily dosing for 1 week during phase 1 studies. In the recently described phase 2a studies, the dual-agonist compound was administered to 37 patients once daily for 12 weeks, with HbA1c as the primary endpoint (152). Dual GLP-1R/GIPR agonism was highly efficacious, significantly reducing HbA1c by 0.96% and fasting plasma glucose by 38.2% after 12 weeks of dosing when compared with placebo. Body weight was significantly reduced by 1.8% after 8 weeks of dosing, and, although not statistically significant, a similar absolute reduction was observed after 12 weeks of treatment. Total cholesterol was 8% lower than that observed in the placebo group, whereas liraglutide alone had no effect, suggesting a potential benefit of dual-incretin use that requires further investigation. Despite the clear efficacy of NNC0090-2746, insulin sensitivity could not be established, and will need to be further studied in larger scale trials.
It is important to note that all combination therapies demonstrate beneficial effects of GIP, particularly when coupled with GLP-1R agonism. This is counter to early genetic studies that suggested loss of GIPR signaling was protective for DIO (51,58). Taken together, the data from multireceptor agonist studies utilizing GIPR engagement supports further development of GIP agonists as a viable aspect of a comprehensive treatment strategy for T2D. Certainly, GIPR agonism in the presence of GLP-1R agonism demonstrates consistent, synergistic effects on targets of obesity and T2D and, as such, supports its incorporation into triagonist therapies, as will be discussed in the following section.
Effects of GLP-1R/GIPR/GCGR triagonism
GLP-1R, GIPR, and GCGR triagonism is supported by findings from successful dual agonists and clinically effective monoagonists. Conceptually, its use is founded in a three-prong approach; GLP-1R agonism supports weight loss and insulin secretion, GCGR agonism activates independent, complimentary weight loss mechanisms, and GIPR agonism further buffers glucagon-mediated hepatic glucose production via amplified potentiation of insulin secretion. Balanced triagonists are similar in length to endogenous glucagon and are about as close in sequence as exendin-4 to GLP-1 (24).
“The synergistic effects observed by mutlireceptor agonists have provided new lines of inquiry for understanding incretin/glucagon biology.”
An early triagonist approach was the development of a hybrid GIP-oxyntomodulin hybrid peptide, DA2GIP-Oxm. DA2GIP-Oxm was developed by replacing the first 11 residues from the N-terminal of oxyntomodulin with D-Ala-GIP (155). Thus, this peptide was resistant to DPP4 degradation and effective at stimulating cAMP through GIPR, GLP-1R, and GCGR. Although this unimolecular peptide was effective, it was less potent at each receptor (GIPR, GLP-1R, and GCGR) compared with the respective native ligand. DA2GIP-Oxm more potently reduced glycemia and decreased body weight compared with any individual peptide; however, it is important to note that comparison with the clinical standard, liraglutide, was not performed and, based on percentage weight loss, would have performed similarly.
Other triagonists were developed utilizing the peptide sequence of the three endogenous peptides. First, [dA2]GLP-1/glucagon was identified as the most promising candidate from iterative changes of peptide sequences containing components of each incretin/glucagon peptide to form several hybrid peptide candidates (156). [dA2]GLP-1/glucagon was administered twice daily for 3 weeks and demonstrated significant decreases in body weight and glucose, with concomitant elevation in plasma insulin. This peptide also performed similarly to exendin-4 in glucose tolerance tests and had similar potency for cAMP stimulation in receptor transfected cells. Another agonist was developed by manipulating the native glucagon sequence and termed Y1-dA2-I12-N17-V18-I27-G28,29-glucagon (157). Although this peptide did use all three receptors to elicit its maximal response, it was less potent than any native peptide for its cognate receptor. Twice-daily administration had no effect on body weight, but significantly improved glycemia in high-fat-fed mice (157). Notably, this study did not compare with effective monoagonist or dual-agonist strategies, so the relative effectiveness of this strategy could not be assessed. However, this was the only multireceptor-agonist strategy that had no effect on body weight, suggesting unbalanced agonism, as monoagonism of the GLP-1R or GCGR consistently elicits weight loss. Both [dA2]GLP-1/glucagon and Y1-dA2-I12-N17-V18-I27-G28,29-glucagon displayed an apparent lack of synergism, indicative of unbalanced agonism and submaximal potency.
A highly effective, balanced triagonist structure was published by Finanet al. (28) in 2015. The triagonist sequence was developed from iterative residue changes that yielded a sequence-intermixed peptide with multireceptor activity. This peptide was resistant to DPP4 cleavage, and thus exhibits an extended half-life, similar to liraglutide. The resultant triagonist was at least as potent as the native peptide at each respective rodent and human receptor. When administered at equimolar concentrations against a proven incretin dual agonist, the triagonist decreased body weight of DIO mice by 26.6% in 20 days, outperforming the dual-incretin receptor agonist by 9.9%. This enhanced weight loss occurred independent of food intake, gastric emptying, or changes in lean mass compared with the dual-incretin receptor agonist, suggesting no adverse effect of glucagon receptor agonism in this peptide. The triagonist offered additional benefits for lipid metabolism; the triagonist significantly reduced plasma cholesterol and reduced markers of hepatic steatosis. Most preclinical studies validate their compounds in male mice because female mice are relatively resistant to DIO and maintain normal glucose tolerance. Thus, this triagonist was recently tested in both age-matched and weight-matched female mice to validate its effects independent of sex (158). The triagonist performed similarly in both male and female mice, further supporting its translation to the clinic for treatment of obesity and T2D. Of the tested triple-agonist compounds developed, this GLP-1/GIP/glucagon triagonist compound was the most efficacious, likely due to its balanced receptor agonism and superior potency to native peptides at their cognate receptor. Notably, proper balance of the triagonist in regards to glucagon was important to achieve the full weight loss benefits of triagonism. Currently the triagonist compound, MAR423, developed by Novo Nordisk, is in early clinical trials (133).
A unique long-acting triagonist, HM15211, was recently described by Hanmi Pharmaceuticals in preclinical models of T2D, nonalcoholic steatohepatitis (NASH), and Parkinson disease (159,160). HM15211 significantly outperformed liraglutide for decreasing body weight, reducing hyperglycemia, and increasing energy expenditure (160). This efficacy was observed with administration every other day compared with twice-daily liraglutide administration. Similar body weight loss was observed with weekly administration. HM15211 significantly lowered blood cholesterol and hepatic steatosis in high-sucrose-fed rats and methionine choline–deficient mice, two models of NASH (160). Additionally, HM15211 protected against MPTP-induced Parkinson disease in mice by attenuating microglial activation and inflammation in the striatum and substantia nigra (159). Based on the broad beneficial effects of HM15211, it is currently being tested in phase 1 clinical trials for obesity and NASH.
Summary and Perspectives
Multireceptor agonists have shown improvements over monoagonist strategies for the treatment of T2D, obesity, and several associated complications. It is clear that agonism of all three receptors work in concert to increase insulin secretion, while reducing adiposity through decreased food intake and elevated energy expenditure. However, based on the significant effects across various tissues, there is room for the exploration of these multireceptor agonists for a broad range of diseases. For example, triagonists may be efficacious at reducing obesity, even in the absence of T2D. Furthermore, several triagonist compounds have already demonstrated cholesterol-lowering effects and benefits for nonalcoholic fatty liver disease (28,158,160). Moreover, although the mechanisms are not well defined, these multireceptor agonists may be efficacious for slowing the progression of neurologic diseases associated with T2D, such as Parkinson disease (140,161). The broad benefits of multireceptor agonism should be considered in future designs of preclinical studies and clinical trials.
Despite the pursuit of several multireceptor agonists in the clinic, a question still remains regarding the molecular mechanisms underlying synergism. One potential explanation of the results is that the triagonists are just more potent activators of GLP-1R, and thus the benefits are due to increasing activity of the single receptor. However, this is unlikely the case, as the most effective multireceptor agonists have balanced activity across the three receptors. Moreover, GLP-1R agonists are currently used at submaximal doses due to gastrointestinal issues, and therefore if the multiagonist receptor was merely more effective at the single receptor, it would be expected to not be well tolerated in patients. Currently, the intracellular signaling mechanisms of multireceptor agonism are unclear. In the case of GLP-1 and GIP, GLP-1 activity removes the brakes on GIP activity found in T2D. This is supported by findings that lowering glycemia restores GIP action on insulin secretion. Thus, in terms of insulin secretion, GLP-1 and GIP may act on their cognate receptors to increase insulin secretion more than either individual peptide can achieve. This may explain the results observed with the unimolecular dual-incretin agonist, where a plateau in the effect of GLP-1R monoagonism was surpassed by the addition of GIP (27). Another potential explanation for synergism is that lateral intracellular interactions occur, in which downstream-signaling intermediates allosterically modify coincident signaling pathways within the same cell. Further work is necessary to understand the molecular basis of synergism to maximally harness its benefits as therapies for T2D.
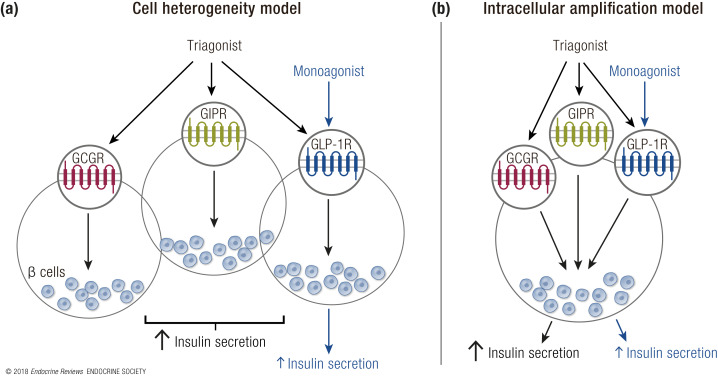
With the advent of single-cell transcriptomics, it will be necessary to understand coexpression profiles of receptors within the same tissues. For instance, it has been postulated that unique subpopulations ofβ cells exist and, potentially, GLP-1R, GCGR, and GIPR exist on unique populations of cells. Thus, the additive benefits of multireceptor agonism may result from the engagement of multiple cell populations that have unique receptor expression (Fig. 4a). Understanding expression profiles within a single cell will provide insights into the complexity and heterogeneity that might exist in various target tissues. Conversely, if receptors are expressed on the same cell, engagement of multiple receptors simultaneously may confer the benefits of multiagonists (Fig. 4b). Whether synergism is the result of activating a broader network of cells, convergence of intracellular signaling, or a combination of the two has yet to be identified. The growing technology for single-cell genomics provides the ability to ask these questions with previously unattainable precision.
Figure 4.
Two hypothesized models of synergism forβ-cell insulin secretion. (a) According to the cell heterogeneity model, unique populations ofβ cells exist that do not express all three incretin/glucagon receptors. In this model, the triagonist increases insulin secretion compared with a monoagonist by engaging multipleβ-cell subgroups to stimulate insulin secretion. (b) In the intracellular amplification model,β cells express all incretin/glucagon receptors, and the synergistic activity of the triagonist compared with the monoagonist is based on the crosstalk of downstream signaling cascades within an individualβ cell.
Neither GIP monoagonists nor antagonists have been validated clinically, but the lack of clinical investigation is largely predicated on studies using incomplete or inadequate tools to query GIP’s physiologic functions. A byproduct of GIP’s use in conjunction with GLP-1 is a better, albeit incomplete, understanding of its potential as a therapeutic. Several dual-incretin studies verified the action of their peptide’s activity at both receptors by using single-incretin receptor knockout mice. There were no reports of adverse effects on body weight or glucagon levels when dual-incretin peptides were administered toGlp1r–/– mice, suggesting safety of GIP administration alone. Furthermore, the use of these coagonists in models of neurologic disease demonstrates untapped therapeutic benefits of GIP agonism that have not been explored in relation to the central deficits associated with T2D. Additionally, the advent of specific GIPR antagonists or GIP-blocking antibodies has allowed for unique lines of investigation to query the endogenous actions of GIP, without the confounding factors associated with genetic manipulations. As such, development of stable GIPR agonists to fully understand its role remains a burgeoning area for drug development.
The incorporation of glucagon into multiagonists aimed at treating T2D patients was initially controversial because of the hyperglycemic liability, but opinion has largely changed and is supported by its effects on energy expenditure and lipolysis. Glucagon stimulates hepatic glucose output, and as such glucagon receptor agonism had not been pursued. Even the current success of coagonists utilizing glucagon biology solely focuses on the GLP-1-independent central benefits of glucagon on energy expenditure and thermogenesis. However, there are likely unappreciated roles that contribute to glucagon’s efficacy. Most notable, glucagon is a potent insulin secretagogue. One hypothesis for the primary physiologic role of glucagon is that it functions to maintain normal levels of amino acids, and its glycemic effects are a byproduct of amino acid metabolism (162). During hypoglycemia, amino acids are synthesized from muscle and fat, stimulating glucagon and promoting hepatic glucose production. Conversely, when glycemia is elevated, such as in overnutrition or in response to a protein-rich meal, elevated amino acids stimulate glucagon and in turn glucagon elevates insulin secretion in a paracrine manner. The largely beneficial effects of glucagon incorporation into unimolecular peptide agonist strategies support the notion that glucagon may have beneficial effects beyond its stimulation of GCGR in the brain.
Taken together, the similar peptide sequences of GLP-1, GIP, and glucagon, coupled with their diverging activities in a broad range of target tissues, position triagonists as a unique drug class that could recapitulate the vast benefits of bariatric surgery for T2D. Although monoagonism of GLP-1R has yielded important benefits for weight management and glucose control clinically, the potential for triagonism could vastly improve clinical outcomes for T2D patients, beyond what is even purported for dual-receptor agonists. Triagonism offers the benefit of substantial weight loss from glucagon action, with reduced hyperglycemic risk from the addition of GIP action in addition to the reported actions of GLP-1. Yet in a broader context, the synergistic effects observed by multireceptor agonists have provided new lines of inquiry for understanding incretin/glucagon biology, which opens the possibility for further optimization and translation of therapies into the clinic in the future.
Acknowledgments
Financial Support: This work was supported by American Diabetes Association Award 1-18-JDF-017 (to J.E.C.).
Disclosure Summary: B.F. and R.D.D. are current employees of Novo Nordisk. The remaining authors have nothing to disclose.
Glossary
Abbreviations
- Aib
aminoisobutyric acid
- CNS
central nervous system
- DIO
diet-induced obesity
- DPP4
dipeptidyl peptidase-4
- DPP4i
dipeptidyl peptidase-4 inhibitors
- FGF21
fibroblast growth factor 21
- GCGR
glucagon receptor
- GIP
glucose-dependent insulinotropic peptide
- GIPR
glucose-dependent insulinotropic peptide receptor
- GLP-1
glucagonlike peptide 1
- GLP-1R
glucagonlike peptide 1 receptor
- GPCR
G protein–coupled receptor
- HbA1c
hemoglobin A1c
- NASH
nonalcoholic steatohepatitis
- SAR
structure–activity relationship
- T2D
type 2 diabetes
References
- 1. Bommer C,Heesemann E,Sagalova V,Manne-Goehler J,Atun R,Bärnighausen T,Vollmer S. The global economic burden of diabetes in adults aged 20-79 years: a cost-of-illness study.Lancet Diabetes Endocrinol.2017;5(6):423–430. [DOI] [PubMed] [Google Scholar]
- 2. Nauck M,Stöckmann F,Ebert R,Creutzfeldt W. Reduced incretin effect in type 2 (non-insulin-dependent) diabetes.Diabetologia.1986;29(1):46–52. [DOI] [PubMed] [Google Scholar]
- 3. Nauck MA,Homberger E,Siegel EG,Allen RC,Eaton RP,Ebert R,Creutzfeldt W. Incretin effects of increasing glucose loads in man calculated from venous insulin and C-peptide responses.J Clin Endocrinol Metab.1986;63(2):492–498. [DOI] [PubMed] [Google Scholar]
- 4. Pearson MJ,Unger RH,Holland WL. Clinical trials, triumphs, and tribulations of glucagon receptor antagonists.Diabetes Care.2016;39(7):1075–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Müller TD,Finan B,Clemmensen C,DiMarchi RD,Tschöp MH. The new biology and pharmacology of glucagon.Physiol Rev.2017;97(2):721–766. [DOI] [PubMed] [Google Scholar]
- 6. Meek CL,Lewis HB,Reimann F,Gribble FM,Park AJ. The effect of bariatric surgery on gastrointestinal and pancreatic peptide hormones.Peptides.2016;77:28–37. [DOI] [PubMed] [Google Scholar]
- 7. Nannipieri M,Baldi S,Mari A,Colligiani D,Guarino D,Camastra S,Barsotti E,Berta R,Moriconi D,Bellini R,Anselmino M,Ferrannini E. Roux-en-Y gastric bypass and sleeve gastrectomy: mechanisms of diabetes remission and role of gut hormones.J Clin Endocrinol Metab.2013;98(11):4391–4399. [DOI] [PubMed] [Google Scholar]
- 8. Laferrère B,Heshka S,Wang K,Khan Y,McGinty J,Teixeira J,Hart AB,Olivan B. Incretin levels and effect are markedly enhanced 1 month after Roux-en-Y gastric bypass surgery in obese patients with type 2 diabetes.Diabetes Care.2007;30(7):1709–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Laferrère B.Bariatric surgery and obesity: influence on the incretins.Int J Obes Suppl.2016;6(S1,Suppl 1):S32–S36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mulvihill EE,Drucker DJ. Pharmacology, physiology, and mechanisms of action of dipeptidyl peptidase-4 inhibitors.Endocr Rev.2014;35(6):992–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hansotia T,Baggio LL,Delmeire D,Hinke SA,Yamada Y,Tsukiyama K,Seino Y,Holst JJ,Schuit F,Drucker DJ. Double incretin receptor knockout (DIRKO) mice reveal an essential role for the enteroinsular axis in transducing the glucoregulatory actions of DPP-IV inhibitors.Diabetes.2004;53(5):1326–1335. [DOI] [PubMed] [Google Scholar]
- 12. Marso SP,Daniels GH,Brown-Frandsen K,Kristensen P,Mann JF,Nauck MA,Nissen SE,Pocock S,Poulter NR,Ravn LS,Steinberg WM,Stockner M,Zinman B,Bergenstal RM,Buse JB,Committee LS,Investigators LT;LEADER Steering CommitteeLEADER Trial Investigators . Liraglutide and cardiovascular outcomes in type 2 diabetes.N Engl J Med.2016;375(4):311–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jia X,Alam M,Ye Y,Bajaj M,Birnbaum Y. GLP-1 receptor agonists and cardiovascular disease: a meta-analysis of recent cardiac outcome trials.Cardiovasc Drugs Ther.2018;32(1):65–72. [DOI] [PubMed] [Google Scholar]
- 14. Marso SP,Bain SC,Consoli A,Eliaschewitz FG,Jódar E,Leiter LA,Lingvay I,Rosenstock J,Seufert J,Warren ML,Woo V,Hansen O,Holst AG,Pettersson J,Vilsbøll T,Investigators S;SUSTAIN-6 Investigators . Semaglutide and cardiovascular outcomes in patients with type 2 diabetes.N Engl J Med.2016;375(19):1834–1844. [DOI] [PubMed] [Google Scholar]
- 15. Holman RR,Bethel MA,Mentz RJ,Thompson VP,Lokhnygina Y,Buse JB,Chan JC,Choi J,Gustavson SM,Iqbal N,Maggioni AP,Marso SP,Öhman P,Pagidipati NJ,Poulter N,Ramachandran A,Zinman B,Hernandez AF,Group ES;EXSCEL Study Group . Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes.N Engl J Med.2017;377(13):1228–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pfeffer MA,Claggett B,Diaz R,Dickstein K,Gerstein HC,Køber LV,Lawson FC,Ping L,Wei X,Lewis EF,Maggioni AP,McMurray JJ,Probstfield JL,Riddle MC,Solomon SD,Tardif JC,Investigators E;ELIXA Investigators . Lixisenatide in patients with type 2 diabetes and acute coronary syndrome.N Engl J Med.2015;373(23):2247–2257. [DOI] [PubMed] [Google Scholar]
- 17. Troke RC,Tan TM,Bloom SR. The future role of gut hormones in the treatment of obesity.Ther Adv Chronic Dis.2014;5(1):4–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Evers SS,Sandoval DA,Seeley RJ. The physiology and molecular underpinnings of the effects of bariatric surgery on obesity and diabetes.Annu Rev Physiol.2017;79(1):313–334. [DOI] [PubMed] [Google Scholar]
- 19. Wilson-Pérez HE,Chambers AP,Ryan KK,Li B,Sandoval DA,Stoffers D,Drucker DJ,Pérez-Tilve D,Seeley RJ. Vertical sleeve gastrectomy is effective in two genetic mouse models of glucagon-like peptide 1 receptor deficiency.Diabetes.2013;62(7):2380–2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ramracheya RD,McCulloch LJ,Clark A,Wiggins D,Johannessen H,Olsen MK,Cai X,Zhao CM,Chen D,Rorsman P. PYY-dependent restoration of impaired insulin and glucagon secretion in type 2 diabetes following Roux-en-Y gastric bypass surgery.Cell Reports.2016;15(5):944–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Garibay D,McGavigan AK,Lee SA,Ficorilli JV,Cox AL,Michael MD,Sloop KW,Cummings BP. β-Cell glucagon-like peptide-1 receptor contributes to improved glucose tolerance after vertical sleeve gastrectomy.Endocrinology.2016;157(9):3405–3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ryan KK,Tremaroli V,Clemmensen C,Kovatcheva-Datchary P,Myronovych A,Karns R,Wilson-Pérez HE,Sandoval DA,Kohli R,Bäckhed F,Seeley RJ. FXR is a molecular target for the effects of vertical sleeve gastrectomy.Nature.2014;509(7499):183–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hodge RJ,Nunez DJ. Therapeutic potential of Takeda-G-protein-receptor-5 (TGR5) agonists. Hope or hype?Diabetes Obes Metab.2016;18(5):439–443. [DOI] [PubMed] [Google Scholar]
- 24. Tschöp M,DiMarchi R. Single-molecule combinatorial therapeutics for treating obesity and diabetes.Diabetes.2017;66(7):1766–1769. [DOI] [PubMed] [Google Scholar]
- 25. Sadry SA,Drucker DJ. Emerging combinatorial hormone therapies for the treatment of obesity and T2DM.Nat Rev Endocrinol.2013;9(7):425–433. [DOI] [PubMed] [Google Scholar]
- 26. Tschöp MH,Finan B,Clemmensen C,Gelfanov V,Perez-Tilve D,Müller TD,DiMarchi RD. Unimolecular polypharmacy for treatment of diabetes and obesity.Cell Metab.2016;24(1):51–62. [DOI] [PubMed] [Google Scholar]
- 27. Finan B,Ma T,Ottaway N,Müller TD,Habegger KM,Heppner KM,Kirchner H,Holland J,Hembree J,Raver C,Lockie SH,Smiley DL,Gelfanov V,Yang B,Hofmann S,Bruemmer D,Drucker DJ,Pfluger PT,Perez-Tilve D,Gidda J,Vignati L,Zhang L,Hauptman JB,Lau M,Brecheisen M,Uhles S,Riboulet W,Hainaut E,Sebokova E,Conde-Knape K,Konkar A,DiMarchi RD,Tschöp MH. Unimolecular dual incretins maximize metabolic benefits in rodents, monkeys, and humans.Sci Transl Med.2013;5(209):209ra151. [DOI] [PubMed] [Google Scholar]
- 28. Finan B,Yang B,Ottaway N,Smiley DL,Ma T,Clemmensen C,Chabenne J,Zhang L,Habegger KM,Fischer K,Campbell JE,Sandoval D,Seeley RJ,Bleicher K,Uhles S,Riboulet W,Funk J,Hertel C,Belli S,Sebokova E,Conde-Knape K,Konkar A,Drucker DJ,Gelfanov V,Pfluger PT,Müller TD,Perez-Tilve D,DiMarchi RD,Tschöp MH. A rationally designed monomeric peptide triagonist corrects obesity and diabetes in rodents.Nat Med.2015;21(1):27–36. [DOI] [PubMed] [Google Scholar]
- 29. Ellingsgaard H,Hauselmann I,Schuler B,Habib AM,Baggio LL,Meier DT,Eppler E,Bouzakri K,Wueest S,Muller YD,Hansen AM,Reinecke M,Konrad D,Gassmann M,Reimann F,Halban PA,Gromada J,Drucker DJ,Gribble FM,Ehses JA,Donath MY. Interleukin-6 enhances insulin secretion by increasing glucagon-like peptide-1 secretion from L cells and alpha cells.Nat Med.2011;17(11):1481–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Marchetti P,Lupi R,Bugliani M,Kirkpatrick CL,Sebastiani G,Grieco FA,Del Guerra S,D’Aleo V,Piro S,Marselli L,Boggi U,Filipponi F,Tinti L,Salvini L,Wollheim CB,Purrello F,Dotta F. A local glucagon-like peptide 1 (GLP-1) system in human pancreatic islets.Diabetologia.2012;55(12):3262–3272. [DOI] [PubMed] [Google Scholar]
- 31. Kreymann B,Williams G,Ghatei MA,Bloom SR. Glucagon-like peptide-1 7-36: a physiological incretin in man.Lancet.1987;2(8571):1300–1304. [DOI] [PubMed] [Google Scholar]
- 32. Mojsov S,Weir GC,Habener JF. Insulinotropin: glucagon-like peptide I (7-37) co-encoded in the glucagon gene is a potent stimulator of insulin release in the perfused rat pancreas.J Clin Invest.1987;79(2):616–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Duan L,Rao X,Braunstein Z,Toomey AC,Zhong J. Role of incretin axis in inflammatory bowel disease.Front Immunol.2017;8:1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zietek T,Rath E. Inflammation meets metabolic disease: gut feeling mediated by GLP-1.Front Immunol.2016;7:154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Drucker DJ.The cardiovascular biology of glucagon-like peptide-1.Cell Metab.2016;24(1):15–30. [DOI] [PubMed] [Google Scholar]
- 36. Drucker DJ,Habener JF,Holst JJ. Discovery, characterization, and clinical development of the glucagon-like peptides.J Clin Invest.2017;127(12):4217–4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nauck MA,Meier JJ,Cavender MA,Abd El Aziz M,Drucker DJ. Cardiovascular actions and clinical outcomes with glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors.Circulation.2017;136(9):849–870. [DOI] [PubMed] [Google Scholar]
- 38. Drucker DJ.Incretin action in the pancreas: potential promise, possible perils, and pathological pitfalls.Diabetes.2013;62(10):3316–3323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Iepsen EW,Torekov SS,Holst JJ. Liraglutide for type 2 diabetes and obesity: a 2015 update.Expert Rev Cardiovasc Ther.2015;13(7):753–767. [DOI] [PubMed] [Google Scholar]
- 40. Pratley RE,Aroda VR,Lingvay I,Ludemann J,Andreassen C,Navarria A,Viljoen A. Semaglutide versus dulaglutide once weekly in patients with type 2 diabetes (SUSTAIN 7): a randomised, open-label, phase 3b trial.Lancet Diabetes Endocrinol.2018;6(4):275–286. [DOI] [PubMed] [Google Scholar]
- 41. Blundell J,Finlayson G,Axelsen M,Flint A,Gibbons C,Kvist T,Hjerpsted JB. Effects of once-weekly semaglutide on appetite, energy intake, control of eating, food preference and body weight in subjects with obesity.Diabetes Obes Metab.2017;19(9):1242–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Drucker DJ,Buse JB,Taylor K,Kendall DM,Trautmann M,Zhuang D,Porter L;DURATION-1 Study Group . Exenatide once weekly versus twice daily for the treatment of type 2 diabetes: a randomised, open-label, non-inferiority study.Lancet.2008;372(9645):1240–1250. [DOI] [PubMed] [Google Scholar]
- 43. Buse JB,Rosenstock J,Sesti G,Schmidt WE,Montanya E,Brett JH,Zychma M,Blonde L;LEAD-6 Study Group . Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6).Lancet.2009;374(9683):39–47. [DOI] [PubMed] [Google Scholar]
- 44. Pratley RE,Nauck MA,Barnett AH,Feinglos MN,Ovalle F,Harman-Boehm I,Ye J,Scott R,Johnson S,Stewart M,Rosenstock J;HARMONY 7 study group . Once-weekly albiglutide versus once-daily liraglutide in patients with type 2 diabetes inadequately controlled on oral drugs (HARMONY 7): a randomised, open-label, multicentre, non-inferiority phase 3 study.Lancet Diabetes Endocrinol.2014;2(4):289–297. [DOI] [PubMed] [Google Scholar]
- 45. Umpierrez G,Tofé Povedano S,Pérez Manghi F,Shurzinske L,Pechtner V. Efficacy and safety of dulaglutide monotherapy versus metformin in type 2 diabetes in a randomized controlled trial (AWARD-3).Diabetes Care.2014;37(8):2168–2176. [DOI] [PubMed] [Google Scholar]
- 46. Dungan KM,Povedano ST,Forst T,González JG,Atisso C,Sealls W,Fahrbach JL. Once-weekly dulaglutide versus once-daily liraglutide in metformin-treated patients with type 2 diabetes (AWARD-6): a randomised, open-label, phase 3, non-inferiority trial.Lancet.2014;384(9951):1349–1357. [DOI] [PubMed] [Google Scholar]
- 47. Ludvik B,Frías JP,Tinahones FJ,Wainstein J,Jiang H,Robertson KE,García-Pérez LE,Woodward DB,Milicevic Z. Dulaglutide as add-on therapy to SGLT2 inhibitors in patients with inadequately controlled type 2 diabetes (AWARD-10): a 24-week, randomised, double-blind, placebo-controlled trial.Lancet Diabetes Endocrinol.2018;6(5):370–381. [DOI] [PubMed] [Google Scholar]
- 48. Dupre J,Ross SA,Watson D,Brown JC. Stimulation of insulin secretion by gastric inhibitory polypeptide in man.J Clin Endocrinol Metab.1973;37(5):826–828. [DOI] [PubMed] [Google Scholar]
- 49. Baggio LL,Drucker DJ. Biology of incretins: GLP-1 and GIP.Gastroenterology.2007;132(6):2131–2157. [DOI] [PubMed] [Google Scholar]
- 50. Campbell JE,Drucker DJ. Pharmacology, physiology, and mechanisms of incretin hormone action.Cell Metab.2013;17(6):819–837. [DOI] [PubMed] [Google Scholar]
- 51. Miyawaki K,Yamada Y,Yano H,Niwa H,Ban N,Ihara Y,Kubota A,Fujimoto S,Kajikawa M,Kuroe A,Tsuda K,Hashimoto H,Yamashita T,Jomori T,Tashiro F,Miyazaki J,Seino Y. Glucose intolerance caused by a defect in the entero-insular axis: a study in gastric inhibitory polypeptide receptor knockout mice.Proc Natl Acad Sci U S A.1999;96(26):14843–14847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Smith EP,An Z,Wagner C,Lewis AG,Cohen EB,Li B,Mahbod P,Sandoval D,Perez-Tilve D,Tamarina N,Philipson LH,Stoffers DA,Seeley RJ,D’Alessio DA. The role of β cell glucagon-like peptide-1 signaling in glucose regulation and response to diabetes drugs.Cell Metab.2014;19(6):1050–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Campbell JE,Ussher JR,Mulvihill EE,Kolic J,Baggio LL,Cao X,Liu Y,Lamont BJ,Morii T,Streutker CJ,Tamarina N,Philipson LH,Wrana JL,MacDonald PE,Drucker DJ. TCF1 links GIPR signaling to the control of beta cell function and survival.Nat Med.2016;22(1):84–90. [DOI] [PubMed] [Google Scholar]
- 54. Nauck MA,Heimesaat MM,Orskov C,Holst JJ,Ebert R,Creutzfeldt W. Preserved incretin activity of glucagon-like peptide 1 [7-36 amide] but not of synthetic human gastric inhibitory polypeptide in patients with type-2 diabetes mellitus.J Clin Invest.1993;91(1):301–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Xu G,Kaneto H,Laybutt DR,Duvivier-Kali VF,Trivedi N,Suzuma K,King GL,Weir GC,Bonner-Weir S. Downregulation of GLP-1 and GIP receptor expression by hyperglycemia: possible contribution to impaired incretin effects in diabetes.Diabetes.2007;56(6):1551–1558. [DOI] [PubMed] [Google Scholar]
- 56. Piteau S,Olver A,Kim SJ,Winter K,Pospisilik JA,Lynn F,Manhart S,Demuth HU,Speck M,Pederson RA,McIntosh CH. Reversal of islet GIP receptor down-regulation and resistance to GIP by reducing hyperglycemia in the Zucker rat.Biochem Biophys Res Commun.2007;362(4):1007–1012. [DOI] [PubMed] [Google Scholar]
- 57. Højberg PV,Vilsbøll T,Rabøl R,Knop FK,Bache M,Krarup T,Holst JJ,Madsbad S. Four weeks of near-normalisation of blood glucose improves the insulin response to glucagon-like peptide-1 and glucose-dependent insulinotropic polypeptide in patients with type 2 diabetes.Diabetologia.2009;52(2):199–207. [DOI] [PubMed] [Google Scholar]
- 58. Miyawaki K,Yamada Y,Ban N,Ihara Y,Tsukiyama K,Zhou H,Fujimoto S,Oku A,Tsuda K,Toyokuni S,Hiai H,Mizunoya W,Fushiki T,Holst JJ,Makino M,Tashita A,Kobara Y,Tsubamoto Y,Jinnouchi T,Jomori T,Seino Y. Inhibition of gastric inhibitory polypeptide signaling prevents obesity.Nat Med.2002;8(7):738–742. [DOI] [PubMed] [Google Scholar]
- 59. Maekawa R,Ogata H,Murase M,Harada N,Suzuki K,Joo E,Sankoda A,Iida A,Izumoto T,Tsunekawa S,Hamada Y,Oiso Y,Inagaki N,Arima H,Hayashi Y,Seino Y. Glucose-dependent insulinotropic polypeptide is required for moderate high-fat diet- but not high-carbohydrate diet-induced weight gain.Am J Physiol Endocrinol Metab.2018;314(6):E572–E583. [DOI] [PubMed] [Google Scholar]
- 60. Hansotia T,Maida A,Flock G,Yamada Y,Tsukiyama K,Seino Y,Drucker DJ. Extrapancreatic incretin receptors modulate glucose homeostasis, body weight, and energy expenditure.J Clin Invest.2007;117(1):143–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Astrup A,Rössner S,Van Gaal L,Rissanen A,Niskanen L,Al Hakim M,Madsen J,Rasmussen MF,Lean ME,Group NNS;NN8022-1807 Study Group . Effects of liraglutide in the treatment of obesity: a randomised, double-blind, placebo-controlled study.Lancet.2009;374(9701):1606–1616. [DOI] [PubMed] [Google Scholar]
- 62. Gault VA,Irwin N,Green BD,McCluskey JT,Greer B,Bailey CJ,Harriott P,O’harte FP,Flatt PR. Chemical ablation of gastric inhibitory polypeptide receptor action by daily (Pro3)GIP administration improves glucose tolerance and ameliorates insulin resistance and abnormalities of islet structure in obesity-related diabetes.Diabetes.2005;54(8):2436–2446. [DOI] [PubMed] [Google Scholar]
- 63. Parker JC,Irwin N,Lavery KS,Green BD,O’Harte FP,Gault VA,Flatt PR. Metabolic effects of sub-chronic ablation of the incretin receptors by daily administration of (Pro3)GIP and exendin(9-39)amide in obese diabetic (ob/ob) mice.Biol Chem.2007;388(2):221–226. [DOI] [PubMed] [Google Scholar]
- 64. Sparre-Ulrich AH,Hansen LS,Svendsen B,Christensen M,Knop FK,Hartmann B,Holst JJ,Rosenkilde MM. Species-specific action of (Pro3)GIP - a full agonist at human GIP receptors, but a partial agonist and competitive antagonist at rat and mouse GIP receptors.Br J Pharmacol.2016;173(1):27–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Asmar M,Simonsen L,Arngrim N,Holst JJ,Dela F,Bülow J. Glucose-dependent insulinotropic polypeptide has impaired effect on abdominal, subcutaneous adipose tissue metabolism in obese subjects.Int J Obes.2014;38(2):259–265. [DOI] [PubMed] [Google Scholar]
- 66. Asmar M,Simonsen L,Asmar A,Holst JJ,Dela F,Bülow J. Insulin plays a permissive role for the vasoactive effect of GIP regulating adipose tissue metabolism in humans.J Clin Endocrinol Metab.2016;101(8):3155–3162. [DOI] [PubMed] [Google Scholar]
- 67. Asmar M,Simonsen L,Madsbad S,Stallknecht B,Holst JJ,Bülow J. Glucose-dependent insulinotropic polypeptide may enhance fatty acid re-esterification in subcutaneous abdominal adipose tissue in lean humans.Diabetes.2010;59(9):2160–2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Gasbjerg LS,Gabe MBN,Hartmann B,Christensen MB,Knop FK,Holst JJ,Rosenkilde MM. Glucose-dependent insulinotropic polypeptide (GIP) receptor antagonists as anti-diabetic agents.Peptides.2018;100:173–181. [DOI] [PubMed] [Google Scholar]
- 69. Asmar M,Asmar A,Simonsen L,Gasbjerg LS,Sparre-Ulrich AH,Rosenkilde MM,Hartmann B,Dela F,Holst JJ,Bülow J. The gluco- and liporegulatory and vasodilatory effects of glucose-dependent insulinotropic polypeptide (GIP) are abolished by an antagonist of the human GIP receptor.Diabetes.2017;66(9):2363–2371. [DOI] [PubMed] [Google Scholar]
- 70. Kim SJ,Nian C,McIntosh CH. Activation of lipoprotein lipase by glucose-dependent insulinotropic polypeptide in adipocytes. A role for a protein kinase B, LKB1, and AMP-activated protein kinase cascade.J Biol Chem.2007;282(12):8557–8567. [DOI] [PubMed] [Google Scholar]
- 71. Kim SJ,Nian C,McIntosh CH. Resistin is a key mediator of glucose-dependent insulinotropic polypeptide (GIP) stimulation of lipoprotein lipase (LPL) activity in adipocytes.J Biol Chem.2007;282(47):34139–34147. [DOI] [PubMed] [Google Scholar]
- 72. Mehran AE,Templeman NM,Brigidi GS,Lim GE,Chu KY,Hu X,Botezelli JD,Asadi A,Hoffman BG,Kieffer TJ,Bamji SX,Clee SM,Johnson JD. Hyperinsulinemia drives diet-induced obesity independently of brain insulin production.Cell Metab.2012;16(6):723–737. [DOI] [PubMed] [Google Scholar]
- 73. Ugleholdt R,Pedersen J,Bassi MR,Füchtbauer EM,Jørgensen SM,Kissow HL,Nytofte N,Poulsen SS,Rosenkilde MM,Seino Y,Thams P,Holst PJ,Holst JJ. Transgenic rescue of adipocyte glucose-dependent insulinotropic polypeptide receptor expression restores high fat diet-induced body weight gain.J Biol Chem.2011;286(52):44632–44645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Speliotes EK,Willer CJ,Berndt SI,Monda KL,Thorleifsson G,Jackson AU,Lango Allen H,Lindgren CM,Luan J,Mägi R,Randall JC,Vedantam S,Winkler TW,Qi L,Workalemahu T,Heid IM,Steinthorsdottir V,Stringham HM,Weedon MN,Wheeler E,Wood AR,Ferreira T,Weyant RJ,Segrè AV,Estrada K,Liang L,Nemesh J,Park JH,Gustafsson S,Kilpeläinen TO,Yang J,Bouatia-Naji N,Esko T,Feitosa MF,Kutalik Z,Mangino M,Raychaudhuri S,Scherag A,Smith AV,Welch R,Zhao JH,Aben KK,Absher DM,Amin N,Dixon AL,Fisher E,Glazer NL,Goddard ME,Heard-Costa NL,Hoesel V,Hottenga JJ,Johansson A,Johnson T,Ketkar S,Lamina C,Li S,Moffatt MF,Myers RH,Narisu N,Perry JR,Peters MJ,Preuss M,Ripatti S,Rivadeneira F,Sandholt C,Scott LJ,Timpson NJ,Tyrer JP,van Wingerden S,Watanabe RM,White CC,Wiklund F,Barlassina C,Chasman DI,Cooper MN,Jansson JO,Lawrence RW,Pellikka N,Prokopenko I,Shi J,Thiering E,Alavere H,Alibrandi MT,Almgren P,Arnold AM,Aspelund T,Atwood LD,Balkau B,Balmforth AJ,Bennett AJ,Ben-Shlomo Y,Bergman RN,Bergmann S,Biebermann H,Blakemore AI,Boes T,Bonnycastle LL,Bornstein SR,Brown MJ,Buchanan TA,Busonero F,Campbell H,Cappuccio FP,Cavalcanti-Proença C,Chen YD,Chen CM,Chines PS,Clarke R,Coin L,Connell J,Day IN,den Heijer M,Duan J,Ebrahim S,Elliott P,Elosua R,Eiriksdottir G,Erdos MR,Eriksson JG,Facheris MF,Felix SB,Fischer-Posovszky P,Folsom AR,Friedrich N,Freimer NB,Fu M,Gaget S,Gejman PV,Geus EJ,Gieger C,Gjesing AP,Goel A,Goyette P,Grallert H,Grässler J,Greenawalt DM,Groves CJ,Gudnason V,Guiducci C,Hartikainen AL,Hassanali N,Hall AS,Havulinna AS,Hayward C,Heath AC,Hengstenberg C,Hicks AA,Hinney A,Hofman A,Homuth G,Hui J,Igl W,Iribarren C,Isomaa B,Jacobs KB,Jarick I,Jewell E,John U,Jørgensen T,Jousilahti P,Jula A,Kaakinen M,Kajantie E,Kaplan LM,Kathiresan S,Kettunen J,Kinnunen L,Knowles JW,Kolcic I,König IR,Koskinen S,Kovacs P,Kuusisto J,Kraft P,Kvaløy K,Laitinen J,Lantieri O,Lanzani C,Launer LJ,Lecoeur C,Lehtimäki T,Lettre G,Liu J,Lokki ML,Lorentzon M,Luben RN,Ludwig B,Manunta P,Marek D,Marre M,Martin NG,McArdle WL,McCarthy A,McKnight B,Meitinger T,Melander O,Meyre D,Midthjell K,Montgomery GW,Morken MA,Morris AP,Mulic R,Ngwa JS,Nelis M,Neville MJ,Nyholt DR,O’Donnell CJ,O’Rahilly S,Ong KK,Oostra B,Paré G,Parker AN,Perola M,Pichler I,Pietiläinen KH,Platou CG,Polasek O,Pouta A,Rafelt S,Raitakari O,Rayner NW,Ridderstråle M,Rief W,Ruokonen A,Robertson NR,Rzehak P,Salomaa V,Sanders AR,Sandhu MS,Sanna S,Saramies J,Savolainen MJ,Scherag S,Schipf S,Schreiber S,Schunkert H,Silander K,Sinisalo J,Siscovick DS,Smit JH,Soranzo N,Sovio U,Stephens J,Surakka I,Swift AJ,Tammesoo ML,Tardif JC,Teder-Laving M,Teslovich TM,Thompson JR,Thomson B,Tönjes A,Tuomi T,van Meurs JB,van Ommen GJ,Vatin V,Viikari J,Visvikis-Siest S,Vitart V,Vogel CI,Voight BF,Waite LL,Wallaschofski H,Walters GB,Widen E,Wiegand S,Wild SH,Willemsen G,Witte DR,Witteman JC,Xu J,Zhang Q,Zgaga L,Ziegler A,Zitting P,Beilby JP,Farooqi IS,Hebebrand J,Huikuri HV,James AL,Kähönen M,Levinson DF,Macciardi F,Nieminen MS,Ohlsson C,Palmer LJ,Ridker PM,Stumvoll M,Beckmann JS,Boeing H,Boerwinkle E,Boomsma DI,Caulfield MJ,Chanock SJ,Collins FS,Cupples LA,Smith GD,Erdmann J,Froguel P,Grönberg H,Gyllensten U,Hall P,Hansen T,Harris TB,Hattersley AT,Hayes RB,Heinrich J,Hu FB,Hveem K,Illig T,Jarvelin MR,Kaprio J,Karpe F,Khaw KT,Kiemeney LA,Krude H,Laakso M,Lawlor DA,Metspalu A,Munroe PB,Ouwehand WH,Pedersen O,Penninx BW,Peters A,Pramstaller PP,Quertermous T,Reinehr T,Rissanen A,Rudan I,Samani NJ,Schwarz PE,Shuldiner AR,Spector TD,Tuomilehto J,Uda M,Uitterlinden A,Valle TT,Wabitsch M,Waeber G,Wareham NJ,Watkins H,Wilson JF,Wright AF,Zillikens MC,Chatterjee N,McCarroll SA,Purcell S,Schadt EE,Visscher PM,Assimes TL,Borecki IB,Deloukas P,Fox CS,Groop LC,Haritunians T,Hunter DJ,Kaplan RC,Mohlke KL,O’Connell JR,Peltonen L,Schlessinger D,Strachan DP,van Duijn CM,Wichmann HE,Frayling TM,Thorsteinsdottir U,Abecasis GR,Barroso I,Boehnke M,Stefansson K,North KE,McCarthy MI,Hirschhorn JN,Ingelsson E,Loos RJ;MAGICProcardis Consortium . Association analyses of 249,796 individuals reveal 18 new loci associated with body mass index.Nat Genet.2010;42(11):937–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Chia CW,Carlson OD,Kim W,Shin YK,Charles CP,Kim HS,Melvin DL,Egan JM. Exogenous glucose-dependent insulinotropic polypeptide worsens post prandial hyperglycemia in type 2 diabetes.Diabetes.2009;58(6):1342–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Christensen M,Vedtofte L,Holst JJ,Vilsbøll T,Knop FK. Glucose-dependent insulinotropic polypeptide: a bifunctional glucose-dependent regulator of glucagon and insulin secretion in humans.Diabetes.2011;60(12):3103–3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Holst JJ,Windeløv JA,Boer GA,Pedersen J,Svendsen B,Christensen M,Torekov S,Asmar M,Hartmann B,Nissen A. Searching for the physiological role of glucose-dependent insulinotropic polypeptide.J Diabetes Investig.2016;7(Suppl 1):8–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Bollag RJ,Zhong Q,Phillips P,Min L,Zhong L,Cameron R,Mulloy AL,Rasmussen H,Qin F,Ding KH,Isales CM. Osteoblast-derived cells express functional glucose-dependent insulinotropic peptide receptors.Endocrinology.2000;141(3):1228–1235. [DOI] [PubMed] [Google Scholar]
- 79. Xie D,Cheng H,Hamrick M,Zhong Q,Ding KH,Correa D,Williams S,Mulloy A,Bollag W,Bollag RJ,Runner RR,McPherson JC,Insogna K,Isales CM. Glucose-dependent insulinotropic polypeptide receptor knockout mice have altered bone turnover.Bone.2005;37(6):759–769. [DOI] [PubMed] [Google Scholar]
- 80. Ferron M,Wei J,Yoshizawa T,Del Fattore A,DePinho RA,Teti A,Ducy P,Karsenty G. Insulin signaling in osteoblasts integrates bone remodeling and energy metabolism.Cell.2010;142(2):296–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Torekov SS,Harsløf T,Rejnmark L,Eiken P,Jensen JB,Herman AP,Hansen T,Pedersen O,Holst JJ,Langdahl BL. A functional amino acid substitution in the glucose-dependent insulinotropic polypeptide receptor (GIPR) gene is associated with lower bone mineral density and increased fracture risk.J Clin Endocrinol Metab.2014;99(4):E729–E733. [DOI] [PubMed] [Google Scholar]
- 82. Christensen MB,Lund A,Calanna S,Jorgensen NR,Holst JJ,Vilsboll T,Knop FK. Glucose-dependent insulinotropic polypeptide (GIP) inhibits bone resorption independently of insulin and glycemia.J Clin Endocrinol Metab.2018;103(1):288–294. [DOI] [PubMed] [Google Scholar]
- 83. Coates PS,Fernstrom JD,Fernstrom MH,Schauer PR,Greenspan SL. Gastric bypass surgery for morbid obesity leads to an increase in bone turnover and a decrease in bone mass.J Clin Endocrinol Metab.2004;89(3):1061–1065. [DOI] [PubMed] [Google Scholar]
- 84. Mahdy T,Atia S,Farid M,Adulatif A. Effect of Roux-en Y gastric bypass on bone metabolism in patients with morbid obesity: Mansoura experiences.Obes Surg.2008;18(12):1526–1531. [DOI] [PubMed] [Google Scholar]
- 85. Stemmer K,Bielohuby M,Grayson BE,Begg DP,Chambers AP,Neff C,Woods SC,Erben RG,Tschöp MH,Bidlingmaier M,Clemens TL,Seeley RJ. Roux-en-Y gastric bypass surgery but not vertical sleeve gastrectomy decreases bone mass in male rats.Endocrinology.2013;154(6):2015–2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Shapses SA,Sukumar D. Bone metabolism in obesity and weight loss.Annu Rev Nutr.2012;32(1):287–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Moran-Atkin E,Brody F,Fu SW,Rojkind M. Changes in GIP gene expression following bariatric surgery.Surg Endosc.2013;27(7):2492–2497. [DOI] [PubMed] [Google Scholar]
- 88. Ussher J,Campbell J,Mulvhill E,Baggio L,Bates H,McLean B,Gopal K,Capozzi M,Yusta B,Cao X,Ali S,Kim M,Kabir M,Seino Y,Suzuki J,Drucker D. Inactivation of the glucose-dependent insulinotropic polypeptdie receptor improves outcomes following experimental myocardial infarction.Cell Metab.2018;27(2):450–460.e6. [DOI] [PubMed] [Google Scholar]
- 89. Hiromura M,Mori Y,Kohashi K,Terasaki M,Shinmura K,Negoro T,Kawashima H,Kogure M,Wachi T,Watanabe R,Sato K,Kushima H,Tomoyasu M,Nakano Y,Yamada Y,Watanabe T,Hirano T. Suppressive effects of glucose-dependent insulinotropic polypeptide on cardiac hypertrophy and fibrosis in angiotensin II-infused mouse models.Circ J.2016;80(9):1988–1997. [DOI] [PubMed] [Google Scholar]
- 90. Campbell JE,Drucker DJ. Islet α cells and glucagon--critical regulators of energy homeostasis.Nat Rev Endocrinol.2015;11(6):329–338. [DOI] [PubMed] [Google Scholar]
- 91. Sutherland EW,Cori CF. Influence of insulin preparations on glycogenolysis in liver slices.J Biol Chem.1948;172(2):737–750. [PubMed] [Google Scholar]
- 92. Müller WA,Faloona GR,Aguilar-Parada E,Unger RH. Abnormal alpha-cell function in diabetes. Response to carbohydrate and protein ingestion.N Engl J Med.1970;283(3):109–115. [DOI] [PubMed] [Google Scholar]
- 93. Unger RH,Aguilar-Parada E,Müller WA,Eisentraut AM. Studies of pancreatic alpha cell function in normal and diabetic subjects.J Clin Invest.1970;49(4):837–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Lee YH,Wang MY,Yu XX,Unger RH. Glucagon is the key factor in the development of diabetes.Diabetologia.2016;59(7):1372–1375. [DOI] [PubMed] [Google Scholar]
- 95. Wang MY,Yan H,Shi Z,Evans MR,Yu X,Lee Y,Chen S,Williams A,Philippe J,Roth MG,Unger RH. Glucagon receptor antibody completely suppresses type 1 diabetes phenotype without insulin by disrupting a novel diabetogenic pathway.Proc Natl Acad Sci USA.2015;112(8):2503–2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Lee Y,Wang MY,Du XQ,Charron MJ,Unger RH. Glucagon receptor knockout prevents insulin-deficient type 1 diabetes in mice.Diabetes.2011;60(2):391–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Kazierad DJ,Bergman A,Tan B,Erion DM,Somayaji V,Lee DS,Rolph T. Effects of multiple ascending doses of the glucagon receptor antagonist PF-06291874 in patients with type 2 diabetes mellitus.Diabetes Obes Metab.2016;18(8):795–802. [DOI] [PubMed] [Google Scholar]
- 98. Kazda CM,Ding Y,Kelly RP,Garhyan P,Shi C,Lim CN,Fu H,Watson DE,Lewin AJ,Landschulz WH,Deeg MA,Moller DE,Hardy TA. Evaluation of efficacy and safety of the glucagon receptor antagonist LY2409021 in patients with type 2 diabetes: 12- and 24-week phase 2 studies.Diabetes Care.2016;39(7):1241–1249. [DOI] [PubMed] [Google Scholar]
- 99. Muller WA,Faloona GR,Unger RH. The influence of the antecedent diet upon glucagon and insulin secretion.N Engl J Med.1971;285(26):1450–1454. [DOI] [PubMed] [Google Scholar]
- 100. Langhans W,Zeiger U,Scharrer E,Geary N. Stimulation of feeding in rats by intraperitoneal injection of antibodies to glucagon.Science.1982;218(4575):894–896. [DOI] [PubMed] [Google Scholar]
- 101. Salter JM,Ezrin C,Laidlaw JC,Gornall AG. Metabolic effects of glucagon in human subjects.Metabolism.1960;9:753–768. [PubMed] [Google Scholar]
- 102. Doi K,Kuroshima A. Modified metabolic responsiveness to glucagon in cold-acclimated and heat-acclimated rats.Life Sci.1982;30(9):785–791. [DOI] [PubMed] [Google Scholar]
- 103. Nair KS.Hyperglucagonemia increases resting metabolic rate in man during insulin deficiency.J Clin Endocrinol Metab.1987;64(5):896–901. [DOI] [PubMed] [Google Scholar]
- 104. Habegger KM,Stemmer K,Cheng C,Müller TD,Heppner KM,Ottaway N,Holland J,Hembree JL,Smiley D,Gelfanov V,Krishna R,Arafat AM,Konkar A,Belli S,Kapps M,Woods SC,Hofmann SM,D’Alessio D,Pfluger PT,Perez-Tilve D,Seeley RJ,Konishi M,Itoh N,Kharitonenkov A,Spranger J,DiMarchi RD,Tschöp MH. Fibroblast growth factor 21 mediates specific glucagon actions.Diabetes.2013;62(5):1453–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Coskun T,Bina HA,Schneider MA,Dunbar JD,Hu CC,Chen Y,Moller DE,Kharitonenkov A. Fibroblast growth factor 21 corrects obesity in mice.Endocrinology.2008;149(12):6018–6027. [DOI] [PubMed] [Google Scholar]
- 106. Falkén Y,Hellström PM,Holst JJ,Näslund E. Changes in glucose homeostasis after Roux-en-Y gastric bypass surgery for obesity at day three, two months, and one year after surgery: role of gut peptides.J Clin Endocrinol Metab.2011;96(7):2227–2235. [DOI] [PubMed] [Google Scholar]
- 107. Salehi M,Prigeon RL,D’Alessio DA. Gastric bypass surgery enhances glucagon-like peptide 1-stimulated postprandial insulin secretion in humans.Diabetes.2011;60(9):2308–2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Jørgensen NB,Jacobsen SH,Dirksen C,Bojsen-Møller KN,Naver L,Hvolris L,Clausen TR,Wulff BS,Worm D,Lindqvist Hansen D,Madsbad S,Holst JJ. Acute and long-term effects of Roux-en-Y gastric bypass on glucose metabolism in subjects with Type 2 diabetes and normal glucose tolerance.Am J Physiol Endocrinol Metab.2012;303(1):E122–E131. [DOI] [PubMed] [Google Scholar]
- 109. Salehi M,Gastaldelli A,D'Alessio DA. Blockade of glucagon-like peptide 1 receptor corrects postprandial hypoglycemia after gastric bypass.Gastroenterology.2014;146(3):669–680.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Lund A,Bagger JI,Wewer Albrechtsen NJ,Christensen M,Grøndahl M,Hartmann B,Mathiesen ER,Hansen CP,Storkholm JH,van Hall G,Rehfeld JF,Hornburg D,Meissner F,Mann M,Larsen S,Holst JJ,Vilsbøll T,Knop FK. Erratum. Evidence of extrapancreatic glucagon secretion in man. Diabetes 2016;65:585-597.Diabetes.2016;65(6):1752. [DOI] [PubMed] [Google Scholar]
- 111. Baron AD,Schaeffer L,Shragg P,Kolterman OG. Role of hyperglucagonemia in maintenance of increased rates of hepatic glucose output in type II diabetics.Diabetes.1987;36(3):274–283. [DOI] [PubMed] [Google Scholar]
- 112. Sun C,Trevaskis JL,Jodka CM,Neravetla S,Griffin P,Xu K,Wang Y,Parkes DG,Forood B,Ghosh SS. Bifunctional PEGylated exenatide-amylinomimetic hybrids to treat metabolic disorders: an example of long-acting dual hormonal therapeutics.J Med Chem.2013;56(22):9328–9341. [DOI] [PubMed] [Google Scholar]
- 113. Skarbaliene J,Secher T,Jelsing J,Ansarullah,Neerup TS,Billestrup N,Fosgerau K. The anti-diabetic effects of GLP-1-gastrin dual agonist ZP3022 in ZDF rats.Peptides.2015;69:47–55. [DOI] [PubMed] [Google Scholar]
- 114. Roed SN,Nøhr AC,Wismann P,Iversen H,Bräuner-Osborne H,Knudsen SM,Waldhoer M. Functional consequences of glucagon-like peptide-1 receptor cross-talk and trafficking.J Biol Chem.2015;290(2):1233–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Naylor J,Suckow AT,Seth A,Baker DJ,Sermadiras I,Ravn P,Howes R,Li J,Snaith MR,Coghlan MP,Hornigold DC. Use of CRISPR/Cas9-engineered INS-1 pancreatic β cells to define the pharmacology of dual GIPR/GLP-1R agonists.Biochem J.2016;473(18):2881–2891. [DOI] [PubMed] [Google Scholar]
- 116. Ali S,Lamont BJ,Charron MJ,Drucker DJ. Dual elimination of the glucagon and GLP-1 receptors in mice reveals plasticity in the incretin axis.J Clin Invest.2011;121(5):1917–1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Day JW,Ottaway N,Patterson JT,Gelfanov V,Smiley D,Gidda J,Findeisen H,Bruemmer D,Drucker DJ,Chaudhary N,Holland J,Hembree J,Abplanalp W,Grant E,Ruehl J,Wilson H,Kirchner H,Lockie SH,Hofmann S,Woods SC,Nogueiras R,Pfluger PT,Perez-Tilve D,DiMarchi R,Tschöp MH. A new glucagon and GLP-1 co-agonist eliminates obesity in rodents.Nat Chem Biol.2009;5(10):749–757. [DOI] [PubMed] [Google Scholar]
- 118. Pocai A,Carrington PE,Adams JR,Wright M,Eiermann G,Zhu L,Du X,Petrov A,Lassman ME,Jiang G,Liu F,Miller C,Tota LM,Zhou G,Zhang X,Sountis MM,Santoprete A,Capito’ E,Chicchi GG,Thornberry N,Bianchi E,Pessi A,Marsh DJ,SinhaRoy R. Glucagon-like peptide 1/glucagon receptor dual agonism reverses obesity in mice.Diabetes.2009;58(10):2258–2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Evers A,Haack T,Lorenz M,Bossart M,Elvert R,Henkel B,Stengelin S,Kurz M,Glien M,Dudda A,Lorenz K,Kadereit D,Wagner M. Design of novel exendin-based dual glucagon-like peptide 1 (GLP-1)/glucagon receptor agonists.J Med Chem.2017;60(10):4293–4303. [DOI] [PubMed] [Google Scholar]
- 120. Muppidi A,Zou H,Yang PY,Chao E,Sherwood L,Nunez V,Woods AK,Schultz PG,Lin Q,Shen W. Design of potent and proteolytically stable oxyntomodulin analogs.ACS Chem Biol.2016;11(2):324–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Chabenne J,Chabenne MD,Zhao Y,Levy J,Smiley D,Gelfanov V,Dimarchi R. A glucagon analog chemically stabilized for immediate treatment of life-threatening hypoglycemia.Mol Metab.2014;3(3):293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Ward BP,Ottaway NL,Perez-Tilve D,Ma D,Gelfanov VM,Tschöp MH,Dimarchi RD. Peptide lipidation stabilizes structure to enhance biological function.Mol Metab.2013;2(4):468–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Wang Y,Du J,Zou H,Liu Y,Zhang Y,Gonzalez J,Chao E,Lu L,Yang P,Parker H,Nguyen-Tran V,Shen W,Wang D,Schultz PG,Wang F. Multifunctional antibody agonists targeting glucagon-like peptide-1, glucagon, and glucose-dependent insulinotropic polypeptide receptors.Angew Chem Int Ed Engl.2016;55(40):12475–12478. [DOI] [PubMed] [Google Scholar]
- 124. Tan TM,Field BC,McCullough KA,Troke RC,Chambers ES,Salem V,Gonzalez Maffe J,Baynes KC,De Silva A,Viardot A,Alsafi A,Frost GS,Ghatei MA,Bloom SR. Coadministration of glucagon-like peptide-1 during glucagon infusion in humans results in increased energy expenditure and amelioration of hyperglycemia.Diabetes.2013;62(4):1131–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Pocai A.Action and therapeutic potential of oxyntomodulin.Mol Metab.2013;3(3):241–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Baldissera FG,Holst JJ,Knuhtsen S,Hilsted L,Nielsen OV. Oxyntomodulin (glicentin-(33-69)): pharmacokinetics, binding to liver cell membranes, effects on isolated perfused pig pancreas, and secretion from isolated perfused lower small intestine of pigs.Regul Pept.1988;21(1-2):151–166. [DOI] [PubMed] [Google Scholar]
- 127. Zhu L,Tamvakopoulos C,Xie D,Dragovic J,Shen X,Fenyk-Melody JE,Schmidt K,Bagchi A,Griffin PR,Thornberry NA,Sinha Roy R. The role of dipeptidyl peptidase IV in the cleavage of glucagon family peptides: in vivo metabolism of pituitary adenylate cyclase activating polypeptide-(1-38).J Biol Chem.2003;278(25):22418–22423. [DOI] [PubMed] [Google Scholar]
- 128. Cegla J,Troke RC,Jones B,Tharakan G,Kenkre J,McCullough KA,Lim CT,Parvizi N,Hussein M,Chambers ES,Minnion J,Cuenco J,Ghatei MA,Meeran K,Tan TM,Bloom SR. Coinfusion of low-dose GLP-1 and glucagon in man results in a reduction in food intake.Diabetes.2014;63(11):3711–3720. [DOI] [PubMed] [Google Scholar]
- 129. Day JW,Gelfanov V,Smiley D,Carrington PE,Eiermann G,Chicchi G,Erion MD,Gidda J,Thornberry NA,Tschöp MH,Marsh DJ,SinhaRoy R,DiMarchi R,Pocai A. Optimization of co-agonism at GLP-1 and glucagon receptors to safely maximize weight reduction in DIO-rodents.Biopolymers.2012;98(5):443–450. [DOI] [PubMed] [Google Scholar]
- 130. Sheetz M,Bray R,Tham LS,Jones C,Kinsella J,Violante-Ortiz R Results of a phase 2 study of the oxyntomodulin (OXM) analogue LY2944876 in patients with type 2 diabetes (T2DM). 77th Scientific Session American Diabetes Association; June 10, 2017; San Diego, CA. [Google Scholar]
- 131. Tillner J,Posch MG,Hueser A,Teichert L,Einig C,Bergmann K,Keil S,Rimbault M,Larsen PJ,Lorenz M Safety, pharmacokinetics, and pharmacodynamics of the novel dual GLP-1/glucagon agonist SAR425899 in healthy subjects and diabetes patients. 76th Scientific Sessions American Diabetes Association; June 10 2016; New Orleans, LA. [Google Scholar]
- 132. Henderson SJ,Konkar A,Hornigold DC,Trevaskis JL,Jackson R,Fritsch Fredin M,Jansson-Löfmark R,Naylor J,Rossi A,Bednarek MA,Bhagroo N,Salari H,Will S,Oldham S,Hansen G,Feigh M,Klein T,Grimsby J,Maguire S,Jermutus L,Rondinone CM,Coghlan MP. Robust anti-obesity and metabolic effects of a dual GLP-1/glucagon receptor peptide agonist in rodents and non-human primates.Diabetes Obes Metab.2016;18(12):1176–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Brandt SJ,Götz A,Tschöp MH,Müller TD. Gut hormone polyagonists for the treatment of type 2 diabetes.Peptides.2018;100:190–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Nauck MA,Bartels E,Orskov C,Ebert R,Creutzfeldt W. Additive insulinotropic effects of exogenous synthetic human gastric inhibitory polypeptide and glucagon-like peptide-1-(7-36) amide infused at near-physiological insulinotropic hormone and glucose concentrations.J Clin Endocrinol Metab.1993;76(4):912–917. [DOI] [PubMed] [Google Scholar]
- 135. Mentis N,Vardarli I,Köthe LD,Holst JJ,Deacon CF,Theodorakis M,Meier JJ,Nauck MA. GIP does not potentiate the antidiabetic effects of GLP-1 in hyperglycemic patients with type 2 diabetes.Diabetes.2011;60(4):1270–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Lund A,Vilsbøll T,Bagger JI,Holst JJ,Knop FK. The separate and combined impact of the intestinal hormones, GIP, GLP-1, and GLP-2, on glucagon secretion in type 2 diabetes.Am J Physiol Endocrinol Metab.2011;300(6):E1038–E1046. [DOI] [PubMed] [Google Scholar]
- 137. Daousi C,Wilding JP,Aditya S,Durham BH,Cleator J,Pinkney JH,Ranganath LR. Effects of peripheral administration of synthetic human glucose-dependent insulinotropic peptide (GIP) on energy expenditure and subjective appetite sensations in healthy normal weight subjects and obese patients with type 2 diabetes.Clin Endocrinol (Oxf).2009;71(2):195–201. [DOI] [PubMed] [Google Scholar]
- 138. Gault VA,Kerr BD,Harriott P,Flatt PR. Administration of an acylated GLP-1 and GIP preparation provides added beneficial glucose-lowering and insulinotropic actions over single incretins in mice with type 2 diabetes and obesity.Clin Sci (Lond).2011;121(3):107–117. [DOI] [PubMed] [Google Scholar]
- 139. Nørregaard PK,Deryabina MA,Tofteng Shelton P,Fog JU,Daugaard JR,Eriksson PO,Larsen LF,Jessen L. A novel GIP analogue, ZP4165, enhances glucagon-like peptide-1-induced body weight loss and improves glycaemic control in rodents.Diabetes Obes Metab.2018;20(1):60–68. [DOI] [PubMed] [Google Scholar]
- 140. Athauda D,Foltynie T. Insulin resistance and Parkinson’s disease: a new target for disease modification?Prog Neurobiol.2016;145-146:98–120. [DOI] [PubMed] [Google Scholar]
- 141. Arnold SE,Arvanitakis Z,Macauley-Rambach SL,Koenig AM,Wang HY,Ahima RS,Craft S,Gandy S,Buettner C,Stoeckel LE,Holtzman DM,Nathan DM. Brain insulin resistance in type 2 diabetes and Alzheimer disease: concepts and conundrums.Nat Rev Neurol.2018;14(3):168–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Tamargo IA,Bader M,Li Y,Yu SJ,Wang Y,Talbot K,DiMarchi RD,Pick CG,Greig NH. Novel GLP-1R/GIPR co-agonist “twincretin” is neuroprotective in cell and rodent models of mild traumatic brain injury.Exp Neurol.2017;288:176–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Shi L,Zhang Z,Li L,Hölscher C. A novel dual GLP-1/GIP receptor agonist alleviates cognitive decline by re-sensitizing insulin signaling in the Alzheimer icv. STZ rat model.Behav Brain Res.2017;327:65–74. [DOI] [PubMed] [Google Scholar]
- 144. Yuan Z,Li D,Feng P,Xue G,Ji C,Li G,Hölscher C. A novel GLP-1/GIP dual agonist is more effective than liraglutide in reducing inflammation and enhancing GDNF release in the MPTP mouse model of Parkinson’s disease.Eur J Pharmacol.2017;812:82–90. [DOI] [PubMed] [Google Scholar]
- 145. Jalewa J,Sharma MK,Gengler S,Hölscher C. A novel GLP-1/GIP dual receptor agonist protects from 6-OHDA lesion in a rat model of Parkinson’s disease.Neuropharmacology.2017;117:238–248. [DOI] [PubMed] [Google Scholar]
- 146. Duffy AM,Hölscher C. The incretin analogue D-Ala2GIP reduces plaque load, astrogliosis and oxidative stress in an APP/PS1 mouse model of Alzheimer’s disease.Neuroscience.2013;228:294–300. [DOI] [PubMed] [Google Scholar]
- 147. Faivre E,Hölscher C. Neuroprotective effects of D-Ala(2)GIP on Alzheimer’s disease biomarkers in an APP/PS1 mouse model.Alzheimers Res Ther.2013;5(2):20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Faivre E,Hölscher C. D-Ala2GIP facilitated synaptic plasticity and reduces plaque load in aged wild type mice and in an Alzheimer’s disease mouse model.J Alzheimers Dis.2013;35(2):267–283. [DOI] [PubMed] [Google Scholar]
- 149. Ji C,Xue GF,Li G,Li D,Hölscher C. Neuroprotective effects of glucose-dependent insulinotropic polypeptide in Alzheimer’s disease.Rev Neurosci.2016;27(1):61–70. [DOI] [PubMed] [Google Scholar]
- 150. Gejl M,Gjedde A,Egefjord L,Møller A,Hansen SB,Vang K,Rodell A,Brændgaard H,Gottrup H,Schacht A,Møller N,Brock B,Rungby J. In Alzheimer’s disease, 6-month treatment with GLP-1 analog prevents decline of brain glucose metabolism: randomized, placebo-controlled, double-blind clinical trial.Front Aging Neurosci.2016;8:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Liu W,Jalewa J,Sharma M,Li G,Li L,Hölscher C. Neuroprotective effects of lixisenatide and liraglutide in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson’s disease.Neuroscience.2015;303:42–50. [DOI] [PubMed] [Google Scholar]
- 152. Frias JP,Bastyr EJ,Vignati L,Tschop MH,Schmitt C,Owen K,Christensen RH,DiMarchi RD. The sustained effects of a dual GIP/GLP-1 receptor agonist, NNC0090-2746, in patients with type 2 diabetes.Cell Metab.2017;26(2):343–352.e2. [DOI] [PubMed] [Google Scholar]
- 153. Portron A,Jadidi S,Sarkar N,DiMarchi R,Schmitt C. Pharmacodynamics, pharmacokinetics, safety and tolerability of the novel dual glucose-dependent insulinotropic polypeptide/glucagon-like peptide-1 agonist RG7697 after single subcutaneous administration in healthy subjects.Diabetes Obes Metab.2017;19(10):1446–1453. [DOI] [PubMed] [Google Scholar]
- 154. Schmitt C,Portron A,Jadidi S,Sarkar N,DiMarchi R. Pharmacodynamics, pharmacokinetics and safety of multiple ascending doses of the novel dual glucose-dependent insulinotropic polypeptide/glucagon-like peptide-1 agonist RG7697 in people with type 2 diabetes mellitus.Diabetes Obes Metab.2017;19(10):1436–1445. [DOI] [PubMed] [Google Scholar]
- 155. Bhat VK,Kerr BD,Flatt PR,Gault VA. A novel GIP-oxyntomodulin hybrid peptide acting through GIP, glucagon and GLP-1 receptors exhibits weight reducing and anti-diabetic properties.Biochem Pharmacol.2013;85(11):1655–1662. [DOI] [PubMed] [Google Scholar]
- 156. Gault VA,Bhat VK,Irwin N,Flatt PR. A novel glucagon-like peptide-1 (GLP-1)/glucagon hybrid peptide with triple-acting agonist activity at glucose-dependent insulinotropic polypeptide, GLP-1, and glucagon receptors and therapeutic potential in high fat-fed mice.J Biol Chem.2013;288(49):35581–35591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Bhat VK,Kerr BD,Vasu S,Flatt PR,Gault VA. A DPP-IV-resistant triple-acting agonist of GIP, GLP-1 and glucagon receptors with potent glucose-lowering and insulinotropic actions in high-fat-fed mice.Diabetologia.2013;56(6):1417–1424. [DOI] [PubMed] [Google Scholar]
- 158. Jall S,Sachs S,Clemmensen C,Finan B,Neff F,DiMarchi RD,Tschöp MH,Müller TD,Hofmann SM. Monomeric GLP-1/GIP/glucagon triagonism corrects obesity, hepatosteatosis, and dyslipidemia in female mice.Mol Metab.2017;6(5):440–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kim JA, Lee SH, Choi IY, Kim YH, Lee YM, Kwon SC. Neuroprotective effects of HM15211, a novel long-acting GLP-1/glucagon/GIP triple agonist in the MPTP Parkinson’s disease mouse model. American Diabetes Association’s 77th Scientific Sessions; June 9–13, 2017; San Diego, CA. [Google Scholar]
- 160.Choi IY, Lee JS, Kim JK, Park YJ, Jung SY, Kim YH. Potent body weight loss and efficacy in a NASH animal model by a novel long-acting GLP-1/glucagon/GIP triple-agonist (HM15211). American Diabetes Association’s 77th Scientific Session; June 9–13, 2017; San Diego, CA. [Google Scholar]
- 161. Aviles-Olmos I,Limousin P,Lees A,Foltynie T. Parkinson’s disease, insulin resistance and novel agents of neuroprotection.Brain.2013;136(Pt 2):374–384. [DOI] [PubMed] [Google Scholar]
- 162. Holst JJ,Wewer Albrechtsen NJ,Pedersen J,Knop FK. Glucagon and amino acids are linked in a mutual feedback cycle: the liver-α-cell axis.Diabetes.2017;66(2):235–240. [DOI] [PubMed] [Google Scholar]