Abstract
Progressive familial intrahepatic cholestasis (PFIC) is a genetically heterogeneous disorder of bile flow disruption due to abnormal canalicular transport or impaired bile acid (BA) metabolism, causing excess BA accumulation and liver failure. We previously reported an intrahepatic cholestasis mouse model based on loss of function of both farnesoid X receptor (FXR; NR1H4) and a small heterodimer partner (SHP; NR0B2) [double knockout (DKO)], which has strong similarities to human PFIC5. We compared the pathogenesis of DKO livers with that of another intrahepatic cholestasis model,Bsep−/−, which represents human PFIC2. Both models exhibit severe hepatomegaly and hepatic BA accumulation, but DKO showed greater circulating BA and liver injury, andBsep−/− had milder phenotypes. Molecular profiling of BAs uncovered specific enrichment of cholic acid (CA)–derived BAs in DKO livers but chenodeoxycholate-derived BAs inBsep−/− livers. Transcriptomic and proteomic analysis revealed specific activation of CA synthesis and alternative basolateral BA transport in DKO but increased chenodeoxycholic acid synthesis and canalicular transport inBsep−/−. The constitutive androstane receptor (CAR)/pregnane X receptor (PXR)–CYP2B/CYP2C axis is activated in DKO livers but not in other cholestasis models. Loss of this axis inFxr:Shp:Car:Pxr quadruple knockouts blockedCyp2b/Cyp2c gene induction, impaired bilirubin conjugation/elimination, and increased liver injury. Differential CYP2B expression in DKO andBsep−/− was recapitulated in human PFIC5 and PFIC2 livers. In conclusion, loss of FXR/SHP results in distinct molecular pathogenesis and CAR/PXR activation, which promotesCyp2b/Cyp2c gene transcription and bilirubin clearance. CAR/PXR activation was not observed inBsep−/− mice or PFIC2 patients. These findings provide a deeper understanding of the heterogeneity of intrahepatic cholestasis.
Activation of the CAR/PXR-CYP2B/CYP2C axis is specific for molecular pathogenesis of the PFIC5 mouse model (Fxr−/−;Shp−/−), whereas it is impaired in the PFIC2 mouse model (Bsep−/−).
Cholestasis, defined as a hereditary or an acquired disruption of bile flow, is a liver disease caused by reduced bile acid (BA) efflux from hepatocytes (intrahepatic) or bile duct obstruction (extrahepatic), resulting in BA accumulation and serious liver injury (1). Because it has been broadly defined as any condition in which substances excreted into bile such as BA and phospholipids are retained, various etiologies are associated with cholestasis, which typically causes predominant elevations of serum BA and bilirubin levels at later stages (2). Intrahepatic cholestasis occurs, not only in certain liver diseases driven by external factors such as viral, alcoholic, and drug-induced hepatitis, but also in genetic defects, including progressive familial intrahepatic cholestasis (PFIC). PFIC is currently categorized as a heterogeneous group of five autosomal recessive disorders that usually present with intrahepatic cholestasis in infancy or childhood and cause portal hypertension, cirrhosis, and liver failure (3). Initially, three main types of PFIC were known caused by mutations inATP8B1 (PFIC1),ABCB11 (PFIC2), andABCB4 (PFIC3) (4). Additional types of PFIC withTJP5 andNR1H4 mutations have recently been designated PFIC4 and PFIC5, respectively (3).
We previously reported severe neonatal cholestasis in human patients with loss of function mutations inNR1H4 (PFIC5), encoding the farnesoid X receptor (FXR) gene (3,5). FXR is a BA-activated nuclear receptor that regulates the BA metabolism, suppressing BA synthesis and stimulating enterohepatic BA circulation by inhibition of cytochrome P450 (CYP), family 7, subfamily a, polypeptide 1 (CYP7A1) and induction of the bile salt export pump (BSEP) (6). Loss of FXR function in humans causes severe cholestasis with low-to-normal serumγ-glutamyl transferase (GGT) activity, undetectable BSEP expression, and conjugated hyperbilirubinemia (5). In contrast to the severe cholestatic liver injury in human patients, ablation of the mouseNr1h4/Fxr gene results in a mild increase of BA and does not cause hepatomegaly and liver failure at a young age, suggesting the presence of potential compensatory mechanisms (5,7).
Previously, we found that combined disruption of two nuclear receptors,Nr1h4/Fxr andNr0b2/Shp, results in severe juvenile-onset cholestasis in mice with an enlarged liver size and accelerated liver damage (8). Small heterodimer partner (SHP) is a transcriptional repressor and a direct FXR target gene. It interacts with a variety of other nuclear receptors such as hepatocyte nuclear receptor 4α, liver receptor homolog-1, and peroxisome proliferator–activated receptors (PPARs) and blocks their transactivation (9–11). Thus, SHP induction by FXR inhibitsCyp7a1 expression by inhibiting liver receptor homolog-1–mediated and hepatocyte nuclear receptor 4α–mediated transactivation, resulting in suppression ofde novo BA synthesis (6,12). In contrast toFxr orShp single knockouts, complete deletion ofFxr andShp [double knockout (DKO)] leads to severe liver damage due to uncontrolled BA overload. Similar to PFIC5 patients, histologic examination of DKO livers will show ductular reaction, hepatocyte ballooning, inflammatory cell infiltration, and hepatic fibrosis. The serum profile of DKO also indicates elevation of direct bilirubin, aspartate aminotransferase (AST), and alanine aminotransferase (ALT) levels with low-to-normal GGT levels, which fully phenocopies human patients with PFIC5. Thus, DKO mice show many similarities to PFIC5 pathology.
Clinically and pathologically, PFIC5 resembles PFIC2, with severe hepatocellular injury, low-to-normal GGT, and absent BSEP expression within the first year of life (4,5). However, targeted disruption of theAbcb11/Bsep gene (Bsep−/−) in a mixed genetic background mouse model results in a mild and nonprogressive cholestatic phenotype despite substantial hepatic BA accumulation. It has been suggested that this results from increased levels of nontoxic polyhydroxylated BAs and also by induction of an alternative BA export mechanism via the multidrug resistance (MDR) 1 transporter. In contrast, theBsep−/− allele on a pure C57BL/6 background better recapitulates human PFIC2, with increased ALT, AST, and alkaline phosphatase levels and progressive liver injury, with age-dependent hepatic fibrosis and hepatocellular damage (13).
Similar to the PFIC2 and PFIC5 pathologies, their representative animal models share many aspects of cholestatic liver injuries such as hepatomegaly and elevated hepatic BA accumulation. However, they also have distinct mechanisms beyond the overall cholestatic pathological changes. In the present study, we extensively compared the cholestatic phenotypes of DKO with those ofBsep−/− mice, using transcriptomic and proteomic analyses to investigate their common and specific molecular pathogeneses. Finally, we identified constitutive androstane receptor (CAR) and pregnane X receptor (PXR) signaling as a hallmark of DKO-specific responses to protect the liver from further BA-induced toxicity; this response is absent inBsep−/−. These results define the unique molecular pathogenesis of DKO as a representative PFIC5 animal model.
Materials and Methods
Animal studies
Male mice were used for all experiments. The C57BL/6J wild-type (WT) and C57BL/6 backgroundBsep+/− mice were obtained from the Jackson Laboratory (Bar Harbor, ME; catalog nos. 000664 and 004125). Because neonates from theBsep−/− mother will exhibit perinatal lethality (14), male and femaleBsep+/− were mated to generateBsep−/− offspring.Fxr andShp DKO (Nr1h4−/−;Nr0b2−/−),Car knockout (Nr1i3−/−), andPxr knockout (Nr1i2−/−) mice have been previously described (8,15,16). Both DKO andFxr:Shp:Car:Pxr quadruple knockout (QKO;Nr1h4−/−;Nr0b2−/−;Nr1i3−/−;Nr1i2−/−) had a lower body weight but showed normal fertility and no mortality. The institutional animal care and use committee of the Baylor College of Medicine approved all the animal studies and procedures.
Analysis of total BA level and serum biochemistry
To determine the total BA levels in serum and liver tissue, the Total Bile Acid Assay kit (GWB-BQK090; GenWay Biotech, San Diego, CA) was used. Serum was separated from whole blood using a gel barrier collection tube (3T-MGA; CAPIJECT, Terumo Medical Corporation, Somerset, NJ). Liver tissue was homogenized in 70% ethanol and centrifuged for 10 minutes at 12,000 rpm to isolate clear supernatant. The 10 to 20 μL of each extract was used to determine the total BA concentration. The serum AST, ALT, and bilirubin levels were analyzed by the Center for Comparative Medicine (Baylor College of Medicine).
BA profiling using ultra-HPLC–mass spectrometry
For BA profiling, 20 μL of serum was mixed with 60 μL of acetonitrile (1% NH4OH), including lithocholic acid-D5 as the internal standard, and supernatant was separated by centrifugation. The liver tissue was weighed and homogenized in 6 volume of aqueous methanol (water/methanol 1:1 v/v). Next, 100 μL of each homogenate was added to 300 μL of acetonitrile (1% NH4OH v/v), including lithocholic acid-D5 as the internal standard. After centrifugation, the supernatant was transferred to a new Eppendorf vial for subsequent centrifugation. Each supernatant was transferred to a sample vial for analysis.
Next, 5 µL of the supernatant was injected into a liquid chromatography–tandem mass spectrometry (LC-MS/MS) for analysis (6490 QQQ MS; Agilent Technologies, Santa Clara, CA). BA separation was achieved using a 1260 Infinity Binary LC System (Agilent Technologies) equipped with a 100× 2.1 mm (Waters BEH C18) column. LC-MS/MS was operated in the negative mode with electrospray ionization. Mass chromatograms and spectra were acquired using MassHunter Workstation data Acquisition software (Agilent Technologies). Analysis of BAs was processed using Quantitative Analysis software (Agilent Technologies). Details are provided in theSupplemental Experimental Procedures.
Proteome profiling using LC-MS/MS
The liver tissue was homogenized by cryogenic grinding and lysed in lysis buffer (50 mM NH5CO3 and 1 mM CaCl2). After a quick freeze-thaw cycle twice, proteins were boiled, and freeze-thaw-denaturation procedures were repeated three times. Denatured protein was digested in trypsin twice (T9600; GenDEPOT, Katy, TX) and extracted by 50% acetonitrile/0.1% formic acid solution. The pellet was further extracted with 80% acetonitrile/0.1% formic acid, quantified (Pierce Quantitative Colorimetric Peptide Assay; Thermo Fisher Scientific, Waltham, MA), lyophilized, and fractionated, as previously described (17). Vacuum-dried peptide was dissolved in loading solution (5% methanol and 0.1% formic acid) and subjected to nano-LC-MS/MS analysis with a nano-LC1000 (Thermo Fisher Scientific) coupled to a Thermo Q-Exactive mass spectrometer (Thermo Fisher Scientific). Separated peptides were directly electro-sprayed. The instrument was operated in data-dependent mode, acquiring fragmentation spectra of the top 35 strongest ions and under direct control of Xcalibur software (Thermo Fisher Scientific).
Parent MS spectrum and higher energy collisional dissociation fragmented MS/MS spectrum were acquired in the Orbitrap Mass Analyzer (Thermo Fisher Scientific) with resolution of 140,000 and 17,500. The full MS range was 375 to 1300 m/z, and the trap target was 3,000,000 and 20,000. The obtained MS/MS spectra were searched against the target-decoy mouse refseq database in Proteome Discoverer 1.4 interface (PD1.4; Thermo Fisher Scientific) with the Mascot algorithm (Mascot 2.4; Matrix Science, Boston, MA). Area under the curve–based relative quantification was analyzed using the iBAQ algorithm and normalized to intensity-based fraction of the total to compare protein abundance (17). Details are provided in theSupplemental Experimental Procedures.
Real-time quantitative PCR
Liver RNA was isolated by PureXtract RNAsol (R6101; GenDEPOT), and cDNA was synthesized using the qScript reverse transcription kit (95047; Quanta Bio, Beverly, MA). The relative expression level was determined using the quantitative PCR method and the LightCycler 480 real-time PCR system (Roche, Basel, Switzerland) with the SYBR FAST qPCR kit (KAPA Biosystems, Wilmington, MA) and calculated using the 2-ΔΔCT method, normalized to the glyceraldehyde 3-phosphate dehydrogenase mRNA level. Primer information is listed inSupplemental Table 1.
Analysis of Gene Expression Omnibus data set
The GSE20599 (for DKO gene expression) and GSE70179 (forBsep−/− gene expression) data sets were analyzed using the GEO2R web-based program. To compare the data sets in two different platforms (GSE20599; Illumina mouse Ref-8, version 1.1, expression bead chip;vs GSE70179; Affymetrix HT MG-430 PM Array Plate), data from each platform were first sorted by official gene symbols. For duplicated gene symbols, the most significantly changed gene (i.e., lowerP value) was selected. In total, 12,959 nonduplicated genes were shared by two microarrays (78.6% of total DKO; 68.6% of totalBsep−/−). The 662 gene symbols showing a statistically significant difference (P < 0.01) were collectively or separately analyzed using DAVID Bioinformatics Resources 6.8 Tools (available at:https://david.ncifcrf.gov; National Institute of Allergy and Infectious Diseases), and representative functional categories were displayed [Gene Ontology–Biological Pathways (GO-BP) and Kyoto Encyclopedia of Genes and Genomes Pathway (KEGG)] according to their expression patterns.
Immunohistochemistry
Immunohistochemical analysis of BSEP and CYP2B6 proteins in the mouse model and human patient liver sections was performed according to the general guidelines of immunohistochemistry. In brief, deparaffinized sections were heated in sodium citrate buffer (pH 6.0) for 15 minutes. Next, the sections were incubated with blocking buffer containing 5% normal goat serum (30 minutes), followed by diluted anti-BSEP (1:200; R31844; NSJ Bioreagents, San Diego, CA; RRID:AB_2725751) and anti-CYP2B6 (1:50; NBP2-01800; Novus Biologicals, Littleton, CO; RRID:AB_2725750) antibodies. After binding of biotin-conjugated secondary antibodies (rabbit: BA-1000; mouse: BA-2000; Vector Laboratories, Burlingame, CA; RRIDs:AB_2313606 andAB_2313581), positive signal was detected using avidin-biotin complex (ABC) method using Vectastatin Elite ABC Reagent (PK-7100; Vector Laboratories) and Vector NovaRed Peroxidase Substrate Kit (SK-4800; Vector Laboratories). Next, stained sections were mounted with Poly-Mount solution (08381-940; Polysciences, Warrington, PA). A high sequence homology is present between human CYP2B6 and mouse CYP2B10 proteins (79.4%), and anti-human CYP2B6 antibody has been known to cross-react with mouse CYP2B proteins (Novus Biologicals).
Statistical analysis
All results are presented as the mean ± SEM, and statistical significance between WTvs DKO,Bsep+/−vs Bsep−/−, DKOvs QKO, and DKO vsBsep−/− was calculated using the two-tailed Studentt test with the assumption that the sample data are in a normal distribution. In a preliminary larger data-set analysis focused onCyp7A1 mRNA expression, we confirmed that the data followed a normal distribution and no statistically significant difference in the average value was observed between this larger data set and the smaller one (n = 3;P < 0.05,P < 0.01, andP < 0.005). For the comparison of WT, DKO, and QKO, one-way ANOVA, followed by apost hoc t test with Bonferroni correction, was used. With a family of three comparisons (three groups), original significance was corrected toP < 0.0167 (= 0.05/3),P < 0.00333 (= 0.01/3), andP < 0.00167 (= 0.005/3).
Results
DKO and Bsep−/− exhibit distinct phenotype of intrahepatic cholestasis
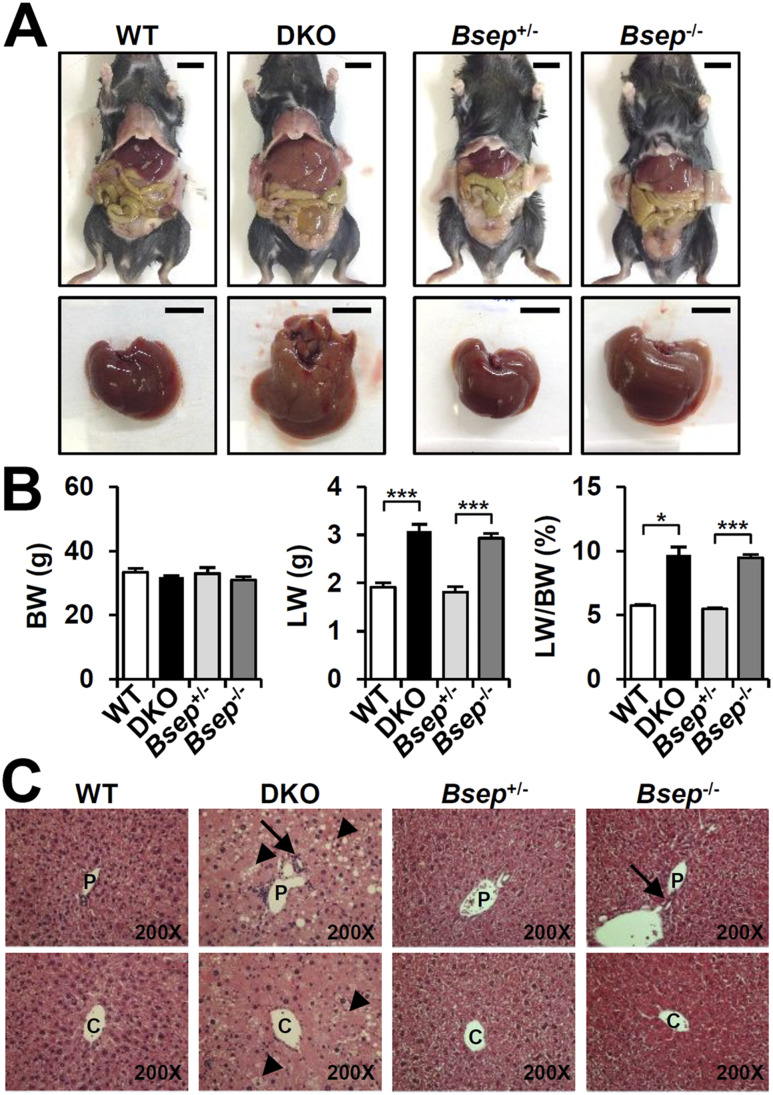
As previously reported (8), combined deletion ofFxr andShp (DKO) greatly increased the liver weight-to-body weight ratio (9%;Fig. 1A and 1B). In the C57BL/6 background, introduction of theBsep−/− allele did not change the body weight, but the liver weight-to-body weight ratio doubled to >9% compared with either WT orBsep+/− mice (5%;Fig. 1A and 1B) (13). Thus, hepatomegaly occurs to a similar extent in the two cholestatic models. Hematoxylin and eosin staining of DKO liver revealed severe structural alterations with increased hepatic steatosis (arrowhead), bile duct proliferation (arrow), immune cell infiltration, polyploid nuclei, and hepatocyte ballooning (Fig. 1C). TheBsep−/− liver showed less severe phenotypes with mild bile duct proliferation (arrow) and less lipid accumulation than in the DKO liver (Fig. 1C).
Figure 1.
DKO andBsep−/− mice showed hepatomegaly. (A) Representative view of whole body and liver at 3 months of age. Scale bar, 10 mm. (B) Body weight (BW), liver weight (LW), and LW/BW ratio at the time of death (n = 3 for each group). (C) Hematoxylin and eosin staining of liver (magnification, ×200). Arrowheads indicate hepatic steatosis; arrows indicate bile duct proliferation. Statistics: Studentt test: *P < 0.05 and ***P < 0.005 compared with each control. C, central vein; P, portal vein.
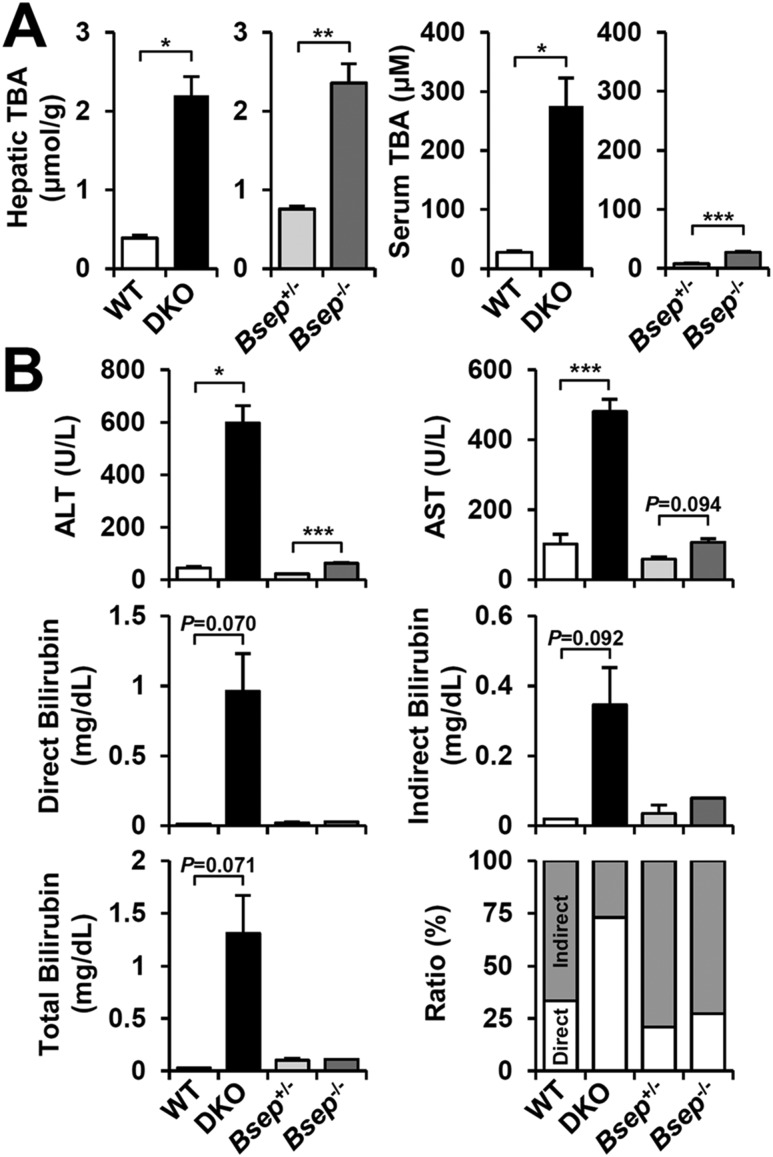
Decreased BA transport due to the loss of BSEP expression causes BA accumulation in liver and blood. Hepatic BAs were highly elevated in both DKO andBsep−/− mice, but only DKO mice showed a robust increase of circulating BAs (Fig. 2A). DKO mice harbored severe liver injury with increased serum ALT, AST (500 to 600 U/L) and conjugated hyperbilirubinemia (1 to 2 mg/dL;Fig. 2B, lower-right panel). In contrast,Bsep−/− mice showed only modest increases in these parameters (Fig. 2B). Overall, despite similar extents of hepatomegaly and hepatic total BA accumulation, DKO showed more severe cholestatic liver injury than didBsep−/−, which is generally consistent with previous reports (8,13).
Figure 2.
Differential BA accumulation and liver injury in DKO andBsep−/−. (A) Hepatic and serum total BA levels in DKO andBsep−/− serum (n = 3 for each group). (B) Serum ALT, AST, and bilirubin (direct, indirect, and total) levels. Ratio (%) indicated the proportions of direct (white) and indirect (gray) bilirubin. Statistics: Studentt test: *P < 0.05, **P < 0.01, ***P < 0.005 compared with each control. In some cases, the actualP value is presented. TBA, total bile acid.
DKO preferentially enriches cholic acid–derived BAs
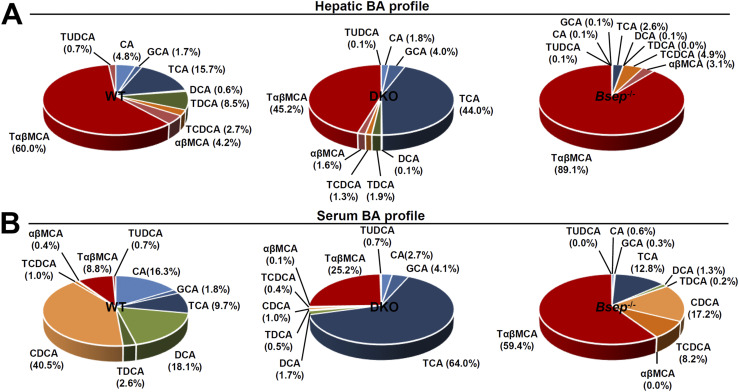
The primary BAs, cholic acid (CA) and chenodeoxycholic acid (CDCA), are further modified into CA-derived BAs (i.e., glyco-CA, tauro-CA) or CDCA-derived BAs [i.e., tauro-CDCA,α/β–muricholic acid (MCA), tauro-α/βMCA] in liver (18). Metabolomic analysis revealed that DKO liver preferentially contains CA-derived BAs (CA, glyco-CA, and tauro-CA), andBsep−/− liver accumulated CDCA-derived BAs (tauro-CDCA,α/βMCA, and tauro-α/βMCA;Fig. 3A;Supplemental Fig. 1A). Similarly, although both types of BAs were mostly elevated (Supplemental Fig. 1B), CA-derived BAs were more proportionally increased in DKO serum, and CDCA-derived BAs were elevated inBsep−/− serum (Fig. 3B). Overall, these results strongly suggest differential regulation of BA synthetic pathways in DKO andBsep−/− livers.
Figure 3.
The DKO andBsep−/− mice showed differential BA profiling. BA composition of (A) liver and (B) serum in WT, DKO, andBsep−/− mice. BAs were quantified as the percentage of total BA analyzed (100%). CA-derived primary BAs shown in blue and dark blue [CA, tauro-CA (TCA), glyco-CA (GCA), deoxycholic acid (DCA), and tauro-DCA (TDCA)]. CDCA-derived primary BAs shown in orange or dark red [CDCA, tauro-CDCA (TCDCA),αβMCA, and tauro-αβMCA (TαβMCA)]. TUDCA, tauro-ursodeoxycholic acid.
Primary BAs are converted into secondary BAs by intestinal bacteria, and then some are absorbed and returned to the liver via enterohepatic circulation. Although a few secondary BAs were detectible in our assay, they were highly increased in DKO serum but not in liver (Supplemental Fig. 1C), indicating that hepatic BA uptake might be blunted in the DKO liver. Circulating levels of secondary BAs were not increased inBsep−/− serum (Supplemental Fig. 1C).
Transcriptomic and proteomic profiling of BA-metabolizing genes
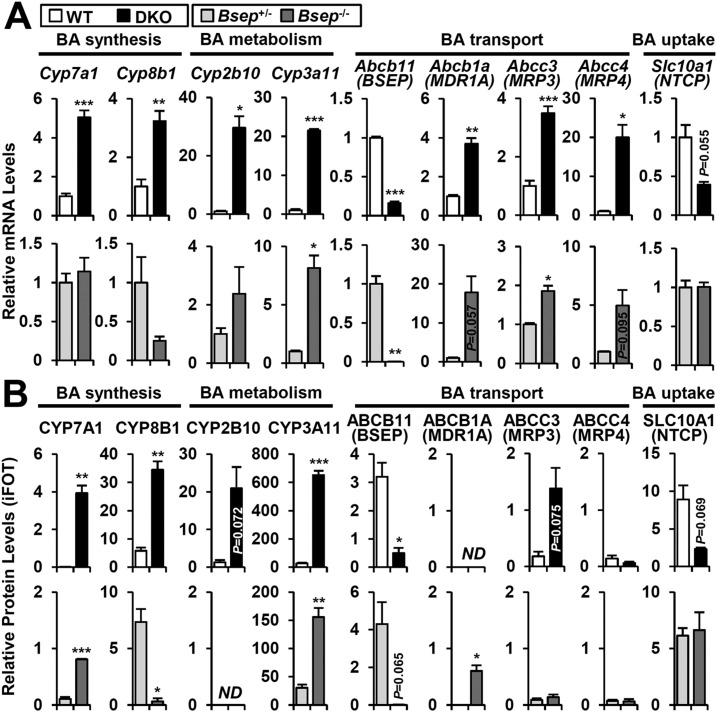
To decipher the molecular basis of differential pathogenesis in DKO andBsep−/− livers, we explored hepatic mRNA and protein expression of a panel of genes involved in BA synthesis, metabolism, and transport (Supplemental Tables 2 and 3). In the DKO liver, mRNA and protein levels of classicde novo BA synthetic enzymes were dramatically induced (CYP7A1 and CYP8B1), and the major canalicular BA transporter BSEP was significantly reduced (Fig. 4A and 4B, upper), explaining the abnormal hepatic BA accumulation in DKO (Fig. 2A). Although the mRNA levels were increased (Fig. 4A and 4B, upper), the protein levels of alternative canalicular BA transporters (ABCB1A and ABCC2) were unchanged, but ABCC3 (MRP3), a basolateral BA transporter, was increased, enabling export of excess BA into the circulation (Fig. 2A) and eventual elimination of toxic BA via the urine. BA influx is mediated by sodium/BA cotransporter (SLC10A1, NTCP), which can be decreased in cholestatic liver injury in response to FXR/SHP independent pathways (19). SLC10A1 expression showed a tendency toward a decrease (Fig. 4A andFig. 5A, upper), potentially restraining excessive uptake of secondary BAs in the DKO liver (Supplemental Fig. 1C).
Figure 4.
Expression profile of representative genes involved in BA homeostasis. The (A) mRNA and (B) protein profile of representative BA synthesis, metabolism, transport, and uptake genes in 3-month-old DKO (upper) andBsep−/− (lower) mice was analyzed using real-time quantitative PCR and proteomics. Relative protein levels are indicated as intensity-based fraction of the total (iFOT). In addition to the official gene symbol, the common gene name is given in parentheses, if necessary. Statistics: Studentt test: *P < 0.05, **P < 0.01, ***P < 0.005 compared with each control. In some cases, the actualP value is presented. ND, not detected.
Figure 5.
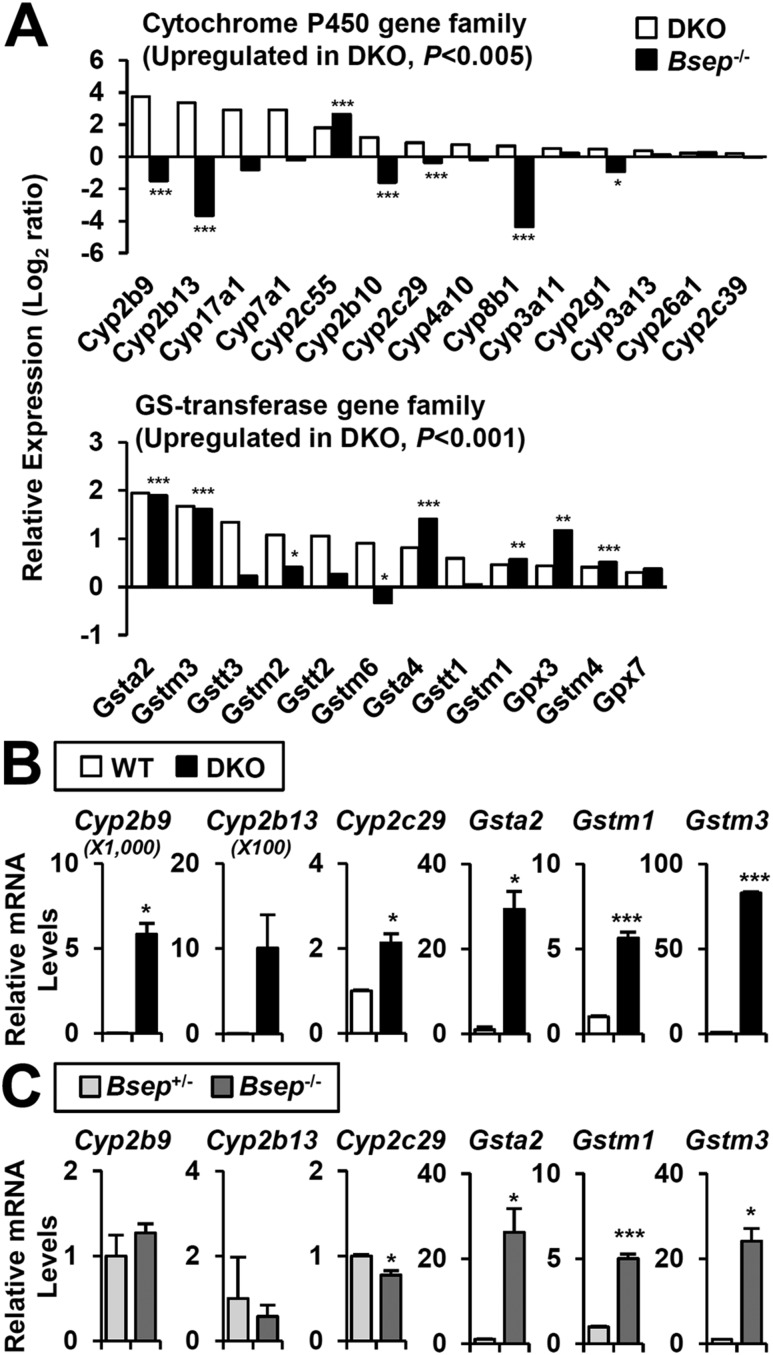
Specific activation of CYP2B and CYP2C in DKO. (A) Upregulated genes of CYP and GST (GS transferase) gene family (P < 0.005 andP < 0.001 in DKO, respectively).P values in DKO separately indicated by asterisks (*, **, ***). Representative CYP and GST gene expression in (B) DKO and (C)Bsep−/− were confirmed by real-time quantitative PCR. Statistics: Studentt test: *P < 0.05, **P < 0.01, ***P < 0.005 compared with each control. In some cases, the actualP value is presented.
TheBsep−/− liver showed quite distinct mRNA and protein profiles compared with the DKO liver. Despite unchanged mRNA levels, CYP7A1 protein was significantly increased but to a lesser extent than in DKO liver (Fig. 4A and 4B, lower). CYP8B1 expression, which mediates conversion of 7α-hydroxycholesterol to CA, was totally abolished, favoring CDCA production over CA synthesis (Fig. 3A andFig. 4A and 4B, lower) (20). For the BA metabolism genes, CYP3A11 was significantly induced but other enzymes such as CYP2B10, UGT1A1, and SULT2A1 were unchanged (Fig. 4A and 4B, lower;Supplemental Tables 3 and 4). CYP3A11 upregulation is thought to be responsible for the polyhydroxylation of BAs inBsep−/− (21). The BSEP level was strongly diminished, but other BA transporters (ABCB1A and ABCC2) were highly induced, compensating for blunted BSEP function through alternative canalicular export of conjugated BAs. In contrast to DKO, basolateral BA transporter proteins were unchanged inBsep−/− (ABCC3 and ABCC4;Fig. 4A and 4B, lower), restraining BA efflux into the blood circulation (Fig. 2A). Overall, these results collectively identify underlying molecular mechanisms for the distinct BA phenotypes of DKO andBsep−/− mice (Figs. 1 and2).
DKO specifically activates CYP gene family
To further define the specific molecular responses in two different models, we compared available DKO andBsep−/− microarrays (GSE20599 and GSE70179). Among 12,959 common gene symbols, DKO andBsep−/− microarrays identified 2210 (17.1% of total) and 2069 (16.0% of total) of differentially regulated genes (P < 0.01), respectively. The GO-BP and KEGG analyses using DAVID tools indicated overlapping and nonoverlapping groups in DKO andBsep−/− microarrays (Supplemental Fig. 2B;Supplemental Tables 4 and 5).
A total of 662 genes were altered in both microarrays, and they overlapped very significantly (hypergeometric test,P = 7.41*10−76;Supplemental Fig. 2A and 2C). GO-BP and KEGG analysis of the 252 increased genes identified groups of genes in chemical carcinogenesis [glutathioneS-transferases (GSTs):Gsta2,Gstm1,etc.], immune responses (Cd14,Tlr2,Cxcl10,Il17,etc.), and cellular proliferation (Jun,Jund,etc.), which correlates with liver injury and hepatomegaly phenotype. In contrast, genes in the peroxisome and PPAR signaling pathway were largely downregulated (Ppara,Acox1,Acsl1,etc.). Interestingly, 256 genes regulated in the opposite manner (increased in DKO and decreased inBsep−/−) were also grouped into chemical carcinogenesis according to the CYP gene responses (i.e.,Cyp2b9,Cyp2b10,Cyp2b13;Supplemental Fig. 2D).
The CYP genes and GSTs are responsible for clearance of xenobiotics and toxic cellular components such as BA and bilirubin. Many CYP genes and GSTs were highly increased in DKO (Fig. 5A). In contrast,Bsep−/− had only increased GSTs and showed a mixed pattern of CYP genes (Fig. 5A;Supplemental Fig. 3A and 3B). In particular, bothCyp2b andCyp2c genes were clearly induced in DKO (Fig. 5B) but were completely blunted inBsep−/− (Fig. 5C). The protein levels showed similar patterns (Supplemental Fig. 4A and 4B). Expression of GSTs and glutathione peroxidases was commonly upregulated in both DKO andBsep−/− livers (Fig. 5B and 5C, right). These results strongly indicate differential transcriptional control accounting for DKO-specific induction of CYP genes.
Activated CAR/PXR increases CYP gene transcription to improve DKO cholestasis
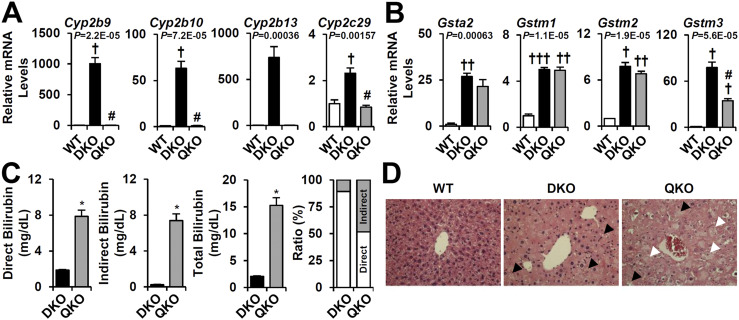
Expression of CYP genes is regulated by multiple transcription factors, including, not only the xenobiotic receptors CAR and PXR, but also PPARα, aryl hydrocarbon receptor (AHR), and nuclear transcription factor E2-related factor 2 (NRF2) (22). In addition to a variety of exogenous activators, the two xenobiotic nuclear receptors are known to be activated by a variety of endogenous ligands and stress signals, including hydrophobic BAs and bilirubin (23). Initial studies withFxr:Shp:Car andFxr:Shp:Pxr triple mutant mice did not show a major impact on P450 gene responses, suggesting functional redundancy. Thus, to genetically test the transcriptional function of CAR and PXR in the DKO liver, we generatedFxr:Shp:Car:Pxr QKO mice (Supplemental Fig. 5). The strong DKO induction ofCyp2b andCyp2c was totally blunted in QKO livers (Fig. 6A). In contrast, the GST genes, which are also upregulated inBsep−/− livers (Fig. 1C), were partly decreased or unaffected (Fig. 6B). These results are consistent with the well-recognized regulation ofCyp2b andCyp2c genes by CAR and PXR (24,25).
Figure 6.
Deletion of CAR/PXR exacerbates BA-induced hepatotoxicity. The mRNA expression of representative (A) CYP and (B) GSTs was evaluated by real-time quantitative PCR (n = 3 for each group). (C) Serum bilirubin levels in DKO and QKO. Ratio (%) indicated the proportions of direct (white) or indirect (gray) bilirubin compared with total bilirubin. (D) Hematoxylin and eosin images of WT, DKO, and QKO liver. Black arrowheads indicate hepatic steatosis; white arrowheads indicate cytoplasmic clearing and severe hepatocyte swelling. Statistics: (A and B)P values of one-way ANOVA test indicated.Post hoc t test with Bonferroni correction:†,#P < 0.0167,††,##P < 0.00333,†††,###P < 0.00167;†compared with WT;#compared with DKO. (D) Studentt test: *P < 0.05, ***P < 0.005 compared with DKO.
Both CAR and PXR have been strongly linked to BA detoxification in response to cholestatic liver injury (26,27). However, both the somewhat unexpected viability of the QKO mice and their retention of the GST gene responses raised the question of whether CAR and PXR activation in the DKO livers is protective. Consistent with the predicted beneficial effect, loss of CAR/PXR in the cholestatic DKO liver not only affectedCyp2b/2c gene transcription but also exacerbated BA-induced liver injury. The QKO mice experienced substantial growth retardation, in particular from 3 to 6 months of age, and an increase in external signs of poor health relative to the DKO mice. Although the serum AST and ALT levels did not differ from those of the DKO mice (data not shown), the total serum bilirubin levels were strongly upregulated (sevenfold) in QKO relative to DKO. This was stronger for unconjugated indirect bilirubin (32-fold) compared with glucuronidated direct bilirubin (4-fold;Fig. 6C). Histological analysis also showed accelerated liver injury in QKO, as indicated by more severe cytoplasmic clearing, giant cell transformation, and hepatocyte swelling (Fig. 6D, white arrowhead). These results suggest that activation of CAR/PXR signaling in DKO protects against the liver injury due to excess bilirubin accumulation and defective bilirubin conjugation.
Upregulated CYP2B protein expression in DKO and PFIC5 liver
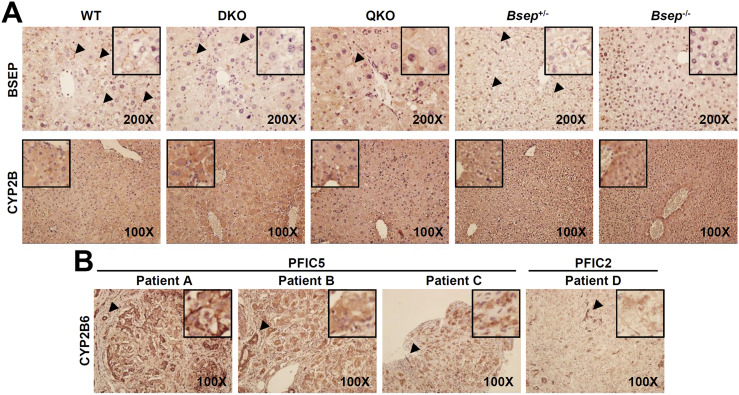
To directly analyze the induction of CYP2B expression in the DKO liver, we stained liver sections with CYP2B-specific antibody. As expected, canalicular BSEP expression was specifically detectible in WT andBsep+/− livers, which was markedly reduced in DKO and QKO and, as expected, totally absent inBsep−/− (Fig. 7A, arrowhead in upper panel). Basal CYP2B expression was weakly present around the central veins in the WT and was highly increased in the DKO liver. This induction was mostly blunted by CAR/PXR deletion (QKO). As expected from the mRNA and protein expression (Fig. 4), no change was found in CYP2B protein expression in theBsep−/− liver (Fig. 7A, lower panel).
Figure 7.
Upregulation of CYP2B expression in DKO and PFIC5 livers. (A) Mouse liver sections from WT, DKO, QKO,Bsep+/−, andBsep−/− mice were stained with anti-BSEP (upper panel) and anti-CYP2B6 (lower panel) antibodies. Next, nuclei were counterstained with hematoxylin. Arrowheads indicate canalicular BSEP expression. (B) Cirrhotic liver sections from end-stage disease of patients with PFIC5 and PFIC2 were stained with anti-CYP2B6 antibody. (Inset) Parenchymal CYP2B6 staining. Arrowhead indicates positive CYP2B6 staining in a biliary tract.
We then examined whether differential CYP2B regulation is conserved in human PFIC5 and PFIC2 livers. We analyzed CYP2B6 expression in liver sections from patients with previously diagnosed PFIC5 and PFIC2 showing end-stage liver cirrhosis. CYP2B6 expression was detected in PFIC5 hepatocytes, but PFIC2 did not have CYP2B6-positive hepatocytes (Fig. 7B, inset). Biliary epithelial cells generally expressed high levels of CYP2B6 proteins regardless of the diagnosis (Fig. 7B, arrowhead), consistent with the previous observation in a normal donor [Protein Atlas database (28);Supplemental Fig. 6]. These human data strongly support our findings of activated CAR/PXR-CYP2B signaling in DKO mice but not inBsep−/− mice.
Discussion
The DKO andBsep−/− models of cholestasis showed similar primary responses, in particular, comparable levels of hepatomegaly and total BA elevations. However, both the molecular mechanisms that account for the increased BA levels and the secondary consequences of these elevations are quite different.
Loss of the FXR/SHP axis in DKO livers dramatically stimulates BA synthesis via strong induction of CYP7A1 and CYP8B1 and inhibits canalicular BA transport via suppression of ABCB11/BSEP, leading to BA overload (Supplemental Fig. 7). Elevated hepatic BAs are forced into the circulation by increased ABCC3/MRP3 expression (Fig. 4), resulting in dramatic elevation of serum BA levels (Fig. 2A;Supplemental Fig. 1B). The increased BA pool activates CAR/PXR signaling to enhance BA and bilirubin metabolism and conjugation through the induction of CYP and GST genes. Although the initial results indicated that the P450 and GST responses were not ablated in either theFxr:Shp:Car or theFxr:Shp:Pxr triple knockout models, the functional role of the CAR/PXR pathway was strongly confirmed by the observation that deletion of both (QKO) completely abolishedCyp2b andCyp2c induction (Fig. 6A). This suggests that both CAR and PXR contribute toCyp2b andCyp2c transactivation, which is consistent with numerous previous studies (24,25). The more severe phenotype of the QKO relative to the DKO confirmed the expected protective effects of the CAR/PXR axis, especially in the context of bilirubin clearance. However, both the somewhat unexpected viability of the QKO mice and their continued induction of GST enzymes suggest that important protective pathways are active in the QKO liver and, likely, also in the DKO liver. The specific targets and functions of CAR and PXR in DKO pathogenesis and the identity of such additional pathways need further investigation.
In contrast to DKO, theBsep−/− livers highly induced alternative canalicular BA transporters (MDR1 and MRP2) without changes in basolateral transporter expression (MRP3 and MRP4), relieving excess hepatic BA overload through the biliary system (Fig. 2A andFig. 4;Supplemental Fig. 7). This alternative pathway preferentially exports hydroxylated and, subsequently, conjugated BAs into the bile duct, which partially rescues the total BA output inBsep−/− (29). Instead of CAR/PXR activation as in DKO, FXR is likely activated inBsep−/− livers by the increased BA levels. In accord with this, representative positive target genes of FXR are highly upregulated inBsep−/− but are mostly blunted or slightly increased in DKO (Supplemental Fig. 8). FXR activation is likely also responsible for the opposite regulation of its negative targetCyp8b1 (30), which was strongly decreased inBsep−/− but was increased in bothFxr single knockout and DKO (Fig. 4A and GSE20599 data set; data not shown). AdditionalBsep−/−-specific genes such asAbcb1a (MDR1A),Slc51b (OSTβ),Abcb4 (MDR2), and GSTs arebona fide FXR targets (Fig. 4) that regulate BA homeostasis (31–34). Therefore, despite an intrahepatic cholestasis phenotype that initially appeared similar in DKO andBsep−/− livers, it is apparent that the specific molecular defects in BA homeostasis are quite different. This might be crucial to understanding the pathology of intrahepatic cholestasis in animal models and in human patients.
Our results suggest several functionally interrelated mechanisms that might contribute to the differential pathology. The first is the substantial difference in the composition of the BA pools, because FXR activation inBsep−/− livers nearly eliminates the 12-α-hydroxylation function of CYP8B1. This could be expected to make the BA pool more hydrophobic and toxic; however, it has previously been suggested that substantial secondary hydroxylation of thisBsep−/− BA pool via CYP3A11 decreases toxicity (21). This secondary effect is definitely consistent with the more benignBsep−/− phenotype but raises two important questions that do not yet have clear answers. The first question is because the CAR/PXR axis is apparently not activated in theBsep−/− livers (Fig. 4), what is the protective pathway or pathways that differentially induce CYP3A11 and GSTs? Candidates include the other transcription factors AHR and NRF2 (35–38), because recent evidence has suggested that crosstalk between the AHR and NRF2 pathways might protect cells from oxidative stress and cholestatic liver injury (39–41). The second question is why is the even larger induction ofCyp3a11 and other CYP in DKO livers relative toBsep−/− livers associated with a worse, rather than a better, outcome?
One simple interpretation is that the differential DKO andBsep−/− phenotypes are simply secondary to some other aspect of the BA pool. In this scenario, the somehow more benignBsep−/− BA pool would result in milder cholestasis, less liver injury, and no CAR/PXR activation (13,21,42). However, previous observations with youngFxr single knockout mice (3 weeks old) showed substantial CYP gene induction in circumstances that are largely free of cholestatic liver injury (8) (GSE20599 data set; data not shown), suggesting that that loss of FXR is sufficient to primarily activate CAR/PXR. Previous results also showed that CAR and PXR can be activated by dietary CA, regardless of theFxr genotype (43). CAR and PXR are indirectly and directly activated by bilirubin and certain hydrophobic BAs to control BA/bilirubin detoxification and clearance processes (44–48). Thus, loss of FXR/SHP might preferentially activate CAR/PXR axis through CA-derived BAs and/or bilirubin. In contrast, theBsep−/− livers showed much more modest changes in CA-derived BAs, instead favoring CDCA synthesis to activate FXR (Fig. 3A). Also, FXR can reportedly inhibit CAR/PXR activation in certain gene promoters (49). These results indicate that loss of FXR might be a specific requirement for direct and indirect CAR/PXR activation.
In accordance with this, we found that activation of CAR/PXR signaling is unique for DKO and PFIC5 compared with other cholestasis models, in addition toBsep−/−. Bile duct-ligated mice, a model of extrahepatic cholestasis, do not showCyp2b andCyp2c induction (21) (Supplemental Fig. 9A). In human patients with biliary atresia, the most detrimental type of pediatric cholestasis,CYP2B andCYP2C genes are mostly decreased or unchanged (Supplemental Fig. 9B). Thus, activation of the CAR/PXR-CYP2B/2C axis is specific for PFIC5 and DKO pathogenesis (Fig. 7B).
FXR activation can clearly improve cholestatic liver injury by direct and indirect inhibition of BA synthesis (1). Thus, treatment with the FXR agonist GW4064, an FXR, protects againstα-naphthylisothiocyanate– or bile duct ligation–induced hepatotoxicity in animal models (30). However, decreased FXR activity has also been associated with beneficial effects in the bile duct ligation model by induction of BA conjugation and clearance processes (50,51). The induction of protective CAR/PXR signaling on loss of FXR function might explain this apparent paradox.
In conclusion, collectively, we found that animal models of intrahepatic cholestasis can have very distinct molecular pathogenesis. Although they have overlapping phenotypes and gene expression, the CAR/PXR pathway is differentially activated in DKO, and likely PFIC5, livers to enhance BA and bilirubin detoxification processes. In contrast, inBsep−/− livers, intact FXR signaling has the expected protective effects. These distinct molecular phenotypes might provide insights to understand the unique pathogenesis of the distinct human PFIC syndromes, which might require very different treatment modalities.
Supplementary Material
Acknowledgments
We thank Natalia Gomez-Ospina (Stanford University, Stanford, CA) and Hector Melin-Aldana (Lurie Children’s Hospital, Chicago, IL) for providing PFIC5 liver sections and Jae Man Lee (Kyungpook National University, Daegu, Korea) for their helpful discussions.
Financial Support : This work was supported by the R.P. Doherty, Jr.–Welch Chair in Science (Grant Q-0022).
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- ABC
avidin-biotin complex
- AHR
aryl hydrocarbon receptor
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- BA
bile acid
- BSEP
bile salt export pump
- CA
cholic acid
- CAR
constitutive androstane receptor
- CDCA
chenodeoxycholic acid
- CYP
cytochrome P450
- DKO
Fxr andShp double knockout
- FXR
farnesoid X receptor
- GGT
γ-glutamyl transferase
- GO-BP
Gene Ontology-Biological Pathways
- GST
glutathioneS-transferase
- KEGG
Kyoto Encyclopedia of Genes and Genomes Pathway
- LC-MS/MS
liquid chromatography–tandem mass spectrometry
- MCA
muricholic acid
- MDR
multidrug resistance
- NRF2
nuclear transcription factor E2-related factor 2
- PFIC
progressive familial intrahepatic cholestasis
- PPAR
peroxisome proliferator–activated receptor
- PXR
pregnane X receptor
- QKO
Fxr:Shp:Car:Pxr quadruple knockout
- SHP
small heterodimer partner
- WT
wild-type
References
- 1. Beuers U,Trauner M,Jansen P,Poupon R. New paradigms in the treatment of hepatic cholestasis: from UDCA to FXR, PXR and beyond.J Hepatol.2015;62(1,Suppl):S25–S37. [DOI] [PubMed] [Google Scholar]
- 2. Mazokopakis EE,Papadakis JA,Kofteridis DP. Unusual causes of intrahepatic cholestatic liver disease.World J Gastroenterol.2007;13(12):1879–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schady DA,Finegold MJ. Contemporary evaluation of the pediatric liver biopsy.Gastroenterol Clin North Am.2017;46(2):233–252. [DOI] [PubMed] [Google Scholar]
- 4. Amer S,Hajira A. A comprehensive review of progressive familial intrahepatic cholestasis (PFIC): genetic disorders of hepatocanalicular transporters.Gastroenterol Res.2014;7(2):39–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gomez-Ospina N,Potter CJ,Xiao R,Manickam K,Kim MS,Kim KH,Shneider BL,Picarsic JL,Jacobson TA,Zhang J,He W,Liu P,Knisely AS,Finegold MJ,Muzny DM,Boerwinkle E,Lupski JR,Plon SE,Gibbs RA,Eng CM,Yang Y,Washington GC,Porteus MH,Berquist WE,Kambham N,Singh RJ,Xia F,Enns GM,Moore DD. Mutations in the nuclear bile acid receptor FXR cause progressive familial intrahepatic cholestasis.Nat Commun.2016;7:10713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Modica S,Gadaleta RM,Moschetta A. Deciphering the nuclear bile acid receptor FXR paradigm.Nucl Recept Signal.2010;8:e005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sinal CJ,Tohkin M,Miyata M,Ward JM,Lambert G,Gonzalez FJ. Targeted disruption of the nuclear receptor FXR/BAR impairs bile acid and lipid homeostasis.Cell.2000;102(6):731–744. [DOI] [PubMed] [Google Scholar]
- 8. Anakk S,Watanabe M,Ochsner SA,McKenna NJ,Finegold MJ,Moore DD. Combined deletion of Fxr and Shp in mice induces Cyp17a1 and results in juvenile onset cholestasis.J Clin Invest.2011;121(1):86–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lee YK,Dell H,Dowhan DH,Hadzopoulou-Cladaras M,Moore DD. The orphan nuclear receptor SHP inhibits hepatocyte nuclear factor 4 and retinoid X receptor transactivation: two mechanisms for repression.Mol Cell Biol.2000;20(1):187–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Park YJ,Kim SC,Kim J,Anakk S,Lee JM,Tseng HT,Yechoor V,Park J,Choi JS,Jang HC,Lee KU,Novak CM,Moore DD,Lee YK. Dissociation of diabetes and obesity in mice lacking orphan nuclear receptor small heterodimer partner.J Lipid Res.2011;52(12):2234–2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chanda D,Xie YB,Choi HS. Transcriptional corepressor SHP recruits SIRT1 histone deacetylase to inhibit LRH-1 transactivation.Nucleic Acids Res.2010;38(14):4607–4619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhang Y,Hagedorn CH,Wang L. Role of nuclear receptor SHP in metabolism and cancer.Biochim Biophys Acta.2011;1812:893–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhang Y,Li F,Patterson AD,Wang Y,Krausz KW,Neale G,Thomas S,Nachagari D,Vogel P,Vore M,Gonzalez FJ,Schuetz JD. Abcb11 deficiency induces cholestasis coupled to impaired β-fatty acid oxidation in mice.J Biol Chem.2012;287(29):24784–24794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhang Y,Li F,Wang Y,Pitre A,Fang ZZ,Frank MW,Calabrese C,Krausz KW,Neale G,Frase S,Vogel P,Rock CO,Gonzalez FJ,Schuetz JD. Maternal bile acid transporter deficiency promotes neonatal demise.Nat Commun.2015;6(1):8186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wei P,Zhang J,Egan-Hafley M,Liang S,Moore DD. The nuclear receptor CAR mediates specific xenobiotic induction of drug metabolism.Nature.2000;407(6806):920–923. [DOI] [PubMed] [Google Scholar]
- 16. Xie W,Barwick JL,Downes M,Blumberg B,Simon CM,Nelson MC,Neuschwander-Tetri BA,Brunt EM,Guzelian PS,Evans RM. Humanized xenobiotic response in mice expressing nuclear receptor SXR.Nature.2000;406(6794):435–439. [DOI] [PubMed] [Google Scholar]
- 17. Jung SY,Choi JM,Rousseaux MW,Malovannaya A,Kim JJ,Kutzera J,Wang Y,Huang Y,Zhu W,Maity S,Zoghbi HY,Qin J. An anatomically resolved mouse brain proteome reveals Parkinson disease-relevant pathways.Mol Cell Proteomics.2017;16(4):581–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Qi Y,Jiang C,Cheng J,Krausz KW,Li T,Ferrell JM,Gonzalez FJ,Chiang JY. Bile acid signaling in lipid metabolism: metabolomic and lipidomic analysis of lipid and bile acid markers linked to anti-obesity and anti-diabetes in mice.Biochim Biophys Acta.2015;1851:19–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zollner G,Wagner M,Fickert P,Geier A,Fuchsbichler A,Silbert D,Gumhold J,Zatloukal K,Kaser A,Tilg H,Denk H,Trauner M. Role of nuclear receptors and hepatocyte-enriched transcription factors for NTCP repression in biliary obstruction in mouse liver.Am J Physiol Gastrointest Liver Physiol.2005;289(5):G798–G805. [DOI] [PubMed] [Google Scholar]
- 20. Li-Hawkins J,Gåfvels M,Olin M,Lund EG,Andersson U,Schuster G,Björkhem I,Russell DW,Eggertsen G. Cholic acid mediates negative feedback regulation of bile acid synthesis in mice.J Clin Invest.2002;110(8):1191–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fuchs CD,Paumgartner G,Wahlström A,Schwabl P,Reiberger T,Leditznig N,Stojakovic T,Rohr-Udilova N,Chiba P,Marschall HU,Trauner M. Metabolic preconditioning protects BSEP/ABCB11−/− mice against cholestatic liver injury.J Hepatol.2017;66(1):95–101. [DOI] [PubMed] [Google Scholar]
- 22. Aleksunes LM,Klaassen CD. Coordinated regulation of hepatic phase I and II drug-metabolizing genes and transporters using AhR-, CAR-, PXR-, PPARα-, and Nrf2-null mice.Drug Metab Dispos.2012;40(7):1366–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kretschmer XC,Baldwin WS. CAR and PXR: xenosensors of endocrine disrupters?Chem Biol Interact.2005;155(3):111–128. [DOI] [PubMed] [Google Scholar]
- 24. Xie W,Barwick JL,Simon CM,Pierce AM,Safe S,Blumberg B,Guzelian PS,Evans RM. Reciprocal activation of xenobiotic response genes by nuclear receptors SXR/PXR and CAR.Genes Dev.2000;14(23):3014–3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ferguson SS,Chen Y,LeCluyse EL,Negishi M,Goldstein JA. Human CYP2C8 is transcriptionally regulated by the nuclear receptors constitutive androstane receptor, pregnane X receptor, glucocorticoid receptor, and hepatic nuclear factor 4alpha.Mol Pharmacol.2005;68(3):747–757. [DOI] [PubMed] [Google Scholar]
- 26. Wagner M,Halilbasic E,Marschall HU,Zollner G,Fickert P,Langner C,Zatloukal K,Denk H,Trauner M. CAR and PXR agonists stimulate hepatic bile acid and bilirubin detoxification and elimination pathways in mice.Hepatology.2005;42(2):420–430. [DOI] [PubMed] [Google Scholar]
- 27. Stedman CA,Liddle C,Coulter SA,Sonoda J,Alvarez JG,Moore DD,Evans RM,Downes M. Nuclear receptors constitutive androstane receptor and pregnane X receptor ameliorate cholestatic liver injury.Proc Natl Acad Sci USA.2005;102(6):2063–2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Uhlén M,Fagerberg L,Hallström BM,Lindskog C,Oksvold P,Mardinoglu A,Sivertsson Å,Kampf C,Sjöstedt E,Asplund A,Olsson I,Edlund K,Lundberg E,Navani S,Szigyarto CA,Odeberg J,Djureinovic D,Takanen JO,Hober S,Alm T,Edqvist PH,Berling H,Tegel H,Mulder J,Rockberg J,Nilsson P,Schwenk JM,Hamsten M,von Feilitzen K,Forsberg M,Persson L,Johansson F,Zwahlen M,von Heijne G,Nielsen J,Pontén F. Proteomics. Tissue-based map of the human proteome.Science.2015;347(6220):1260419. [DOI] [PubMed] [Google Scholar]
- 29. Wang R,Salem M,Yousef IM,Tuchweber B,Lam P,Childs SJ,Helgason CD,Ackerley C,Phillips MJ,Ling V. Targeted inactivation of sister of P-glycoprotein gene (SPGP) in mice results in nonprogressive but persistent intrahepatic cholestasis.Proc Natl Acad Sci USA.2001;98(4):2011–2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Liu Y,Binz J,Numerick MJ,Dennis S,Luo G,Desai B,MacKenzie KI,Mansfield TA,Kliewer SA,Goodwin B,Jones SA. Hepatoprotection by the farnesoid X receptor agonist GW4064 in rat models of intra- and extrahepatic cholestasis.J Clin Invest.2003;112(11):1678–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Halilbasic E,Fuchs C,Traussnigg S,Trauner M. Farnesoid X receptor agonists and other bile acid signaling strategies for treatment of liver disease.Dig Dis.2016;34(5):580–588. [DOI] [PubMed] [Google Scholar]
- 32. Baghdasaryan A,Chiba P,Trauner M. Clinical application of transcriptional activators of bile salt transporters.Mol Aspects Med.2014;37:57–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Geier A,Wagner M,Dietrich CG,Trauner M. Principles of hepatic organic anion transporter regulation during cholestasis, inflammation and liver regeneration.Biochim Biophys Acta.2007;1773:283–308. [DOI] [PubMed] [Google Scholar]
- 34. Jiang Y,Jin J,Iakova P,Hernandez JC,Jawanmardi N,Sullivan E,Guo GL,Timchenko NA,Darlington GJ. Farnesoid X receptor directly regulates xenobiotic detoxification genes in the long-lived Little mice.Mech Ageing Dev.2013;134(9):407–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shen G,Kong AN. NRF2 plays an important role in coordinated regulation of phase II drug metabolism enzymes and phase III drug transporters.Biopharm Drug Dispos.2009;30(7):345–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Boverhof DR,Burgoon LD,Tashiro C,Chittim B,Harkema JR,Jump DB,Zacharewski TR. Temporal and dose-dependent hepatic gene expression patterns in mice provide new insights into TCDD-mediated hepatotoxicity.Toxicol Sci.2005;85(2):1048–1063. [DOI] [PubMed] [Google Scholar]
- 37. Yeager RL,Reisman SA,Aleksunes LM,Klaassen CD. Introducing the “TCDD-inducible AhR-Nrf2 gene battery.” Toxicol Sci.2009;111(2):238–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cheng X,Maher J,Dieter MZ,Klaassen CD. Regulation of mouse organic anion-transporting polypeptides (Oatps) in liver by prototypical microsomal enzyme inducers that activate distinct transcription factor pathways.Drug Metab Dispos.2005;33(9):1276–1282. [DOI] [PubMed] [Google Scholar]
- 39. Xu S,Weerachayaphorn J,Cai SY,Soroka CJ,Boyer JL. Aryl hydrocarbon receptor and NF-E2-related factor 2 are key regulators of human MRP4 expression.Am J Physiol Gastrointest Liver Physiol.2010;299(1):G126–G135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kalthoff S,Ehmer U,Freiberg N,Manns MP,Strassburg CP. Interaction between oxidative stress sensor Nrf2 and xenobiotic-activated aryl hydrocarbon receptor in the regulation of the human phase II detoxifying UDP-glucuronosyltransferase 1A10.J Biol Chem.2010;285(9):5993–6002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zollner G,Wagner M,Trauner M. Nuclear receptors as drug targets in cholestasis and drug-induced hepatotoxicity.Pharmacol Ther.2010;126(3):228–243. [DOI] [PubMed] [Google Scholar]
- 42. Wang R,Chen HL,Liu L,Sheps JA,Phillips MJ,Ling V. Compensatory role of P-glycoproteins in knockout mice lacking the bile salt export pump.Hepatology.2009;50(3):948–956. [DOI] [PubMed] [Google Scholar]
- 43. Zollner G,Wagner M,Moustafa T,Fickert P,Silbert D,Gumhold J,Fuchsbichler A,Halilbasic E,Denk H,Marschall HU,Trauner M. Coordinated induction of bile acid detoxification and alternative elimination in mice: role of FXR-regulated organic solute transporter-alpha/beta in the adaptive response to bile acids.Am J Physiol Gastrointest Liver Physiol.2006;290(5):G923–G932. [DOI] [PubMed] [Google Scholar]
- 44. Staudinger JL,Goodwin B,Jones SA,Hawkins-Brown D,MacKenzie KI,LaTour A,Liu Y,Klaassen CD,Brown KK,Reinhard J,Willson TM,Koller BH,Kliewer SA. The nuclear receptor PXR is a lithocholic acid sensor that protects against liver toxicity.Proc Natl Acad Sci USA.2001;98(6):3369–3374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Xie W,Radominska-Pandya A,Shi Y,Simon CM,Nelson MC,Ong ES,Waxman DJ,Evans RM. An essential role for nuclear receptors SXR/PXR in detoxification of cholestatic bile acids.Proc Natl Acad Sci USA.2001;98(6):3375–3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zhang J,Huang W,Qatanani M,Evans RM,Moore DD. The constitutive androstane receptor and pregnane X receptor function coordinately to prevent bile acid-induced hepatotoxicity.J Biol Chem.2004;279(47):49517–49522. [DOI] [PubMed] [Google Scholar]
- 47. Huang W,Zhang J,Chua SS,Qatanani M,Han Y,Granata R,Moore DD. Induction of bilirubin clearance by the constitutive androstane receptor (CAR).Proc Natl Acad Sci USA.2003;100(7):4156–4161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Uppal H,Toma D,Saini SP,Ren S,Jones TJ,Xie W. Combined loss of orphan receptors PXR and CAR heightens sensitivity to toxic bile acids in mice.Hepatology.2005;41(1):168–176. [DOI] [PubMed] [Google Scholar]
- 49. Renga B,Migliorati M,Mencarelli A,Cipriani S,D'Amore C,Distrutti E,Fiorucci S.. Farnesoid X receptor suppresses constitutive androstane receptor activity at the multidrug resistance protein-4 promoter.Biochim Biophys Acta.2011;1809:157–165. [DOI] [PubMed] [Google Scholar]
- 50. Stedman C,Liddle C,Coulter S,Sonoda J,Alvarez JG,Evans RM,Downes M. Benefit of farnesoid X receptor inhibition in obstructive cholestasis.Proc Natl Acad Sci USA.2006;103(30):11323–11328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wagner M,Fickert P,Zollner G,Fuchsbichler A,Silbert D,Tsybrovskyy O,Zatloukal K,Guo GL,Schuetz JD,Gonzalez FJ,Marschall HU,Denk H,Trauner M. Role of farnesoid X receptor in determining hepatic ABC transporter expression and liver injury in bile duct-ligated mice.Gastroenterology.2003;125(3):825–838. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.