Abstract
Background
Long noncoding RNAs (lncRNAs) are known as key regulators in many cancer types, but their biological functions in nasopharyngeal carcinoma (NPC) remain largely unknown. In the present study, we aim to explore the role of the lncRNA ZNRD1-AS1 in NPC tumor development.
Methods
The role of ZNRD1-AS1 in NPC tissues and cells was explored by using quantitative real-time PCR assay. Cellular behavioral experiments were used in testing NPC cell proliferation, invasion, and migration. Luciferase reporter assay, RNA-binding protein immunoprecipitation, and Western blot analysis were used in estimating the associations among ZNRD1-AS1, miR-335, and ROCK1.
Results
ZNRD1-AS1 expression was elevated in the NPC tissues and cells, and ZNRD1-AS1 overexpression was positively correlated with advanced TNM stage and the presence of lymph node metastasis. Our biological experiments indicated that ZNRD1-AS1 knockdown reduces NPC cell invasion and metastasis. Further analyses revealed that ZNRD1-AS1 as a ceRNA promotes the migration and invasion of NPC cells by sponging miR-335. We provided evidence that ZNRD1-AS1 facilitates the invasion and metastasis of NPC cells via the miR-335–ROCK1 axis.
Conclusion
Our data shed light on the oncogenic role of ZNRD1-AS1 in NPC tumor development, and a promising therapeutic target for NPC was identified.
Keywords: nasopharyngeal carcinoma, ZNRD1-AS1, miR-335
Introduction
Nasopharyngeal carcinoma (NPC) is an important malignant tumor of the head and neck and etiologically relevant to the Epstein–Barr virus.1,2 NPC has a specific geographic distribution characterized by high incidence in south China, Southeast Asia, and North Africa.2,3 Although intensity-modulated radiation therapy (IMRT) and comprehensive chemotherapy strategies have been widely used, distant metastasis and recurrence manifest as the dominant pattern of disease relapse in locoregionally advanced NPC.3–5 Therefore, the mechanisms of invasion and migration and efficient therapeutic targets for NPC should be explored.
Mounting evidence demonstrates that the dysregulated expression of long noncoding RNAs (lncRNAs) in human cancer plays important regulatory roles in tumorigenesis, tumor proliferation, and cancer cell metastasis.6 lncRNAs are defined as RNA transcripts that are longer than 200 nucleotides and have no or limited protein-coding capacity.7 LncRNAs can serve as numerous master gene regulators through epigenetic mechanisms, including transcriptional and post-transcriptional modifications, such as (1) interference of transcription, (2) induction of chromatin remodeling and nucleosome modification, (3) regulation of variable splicing mode, (4) generation of endogenous siRNAs, (5) regulation of protein activity, (6) regulation of structural or organizational function, and (7) regulation of signals for proteins and microRNAs.7,8 We recently found a new lncRNA called zinc ribbon domain containing 1 antisense 1 (ZNRD1-AS1, ENST00000431012), also known as the pseudogene of ZNRD1, which is upregulated in NPC cells and tissues.9 It is located in chromosome 6p21.3 and highly expressed in many cancer types.9–11 However, the biological role and molecular mechanisms of ZNRD1-AS1 in NPC carcinogenicity remain ambiguous.
In the present study, we discovered that the expression of ZNRD1-AS1 in NPC tissues is higher than that in corresponding normal nasopharyngeal tissues. In addition, ZNRD1-AS1 facilitated the invasion and migration of NPC cells in vivo and in vitro. We focused on ZNRD1-AS1 and explored its downstream molecular mechanisms during NPC progression. Our results showed that the ZNRD1-AS1–miR-335–Rho‐associated coiled-oil kinase 1 (ROCK1) regulatory axis may be associated with NPC development and serve as a potential therapeutic target for NPC.
Materials and Methods
Cell Culture and Patient Samples
RPMI-1640 (Corning, Manassas, VA, USA) supplemented with 5% fetal bovine serum (Gibco, Grand Island, USA) was used in incubating six human NPC cell lines, namely, 5–8F, 6–10B, CNE-1, CNE-2, SUNE-1, and HNE1. The human immortalized nasopharyngeal epithelial cell line NP69 was cultivated in a serum-free medium covered with bovine pituitary extract (Gibco). All materials were purchased from the American Tissue Culture Collection (ATCC, Manassas, VA, USA) and maintained at 37 °C in a humidified incubator with 5% CO2. Fresh-frozen NPC tissue samples from 40 cases and five normal nasopharyngeal tissue samples were obtained from Jiangsu Cancer Hospital (Nanjing, China) and stored in a laboratory freezer at −80 °C. All the specimens were histologically diagnosed as NPC tumor samples. The research protocols were approved by the Institutional Ethical Review Board of Jiangsu Cancer, and each patient provided written informed consent.
Quantitative Real-Time PCR
Total RNA was extracted from the NPC cell lines or clinical samples with TRIzol reagent (Invitrogen). Thereafter, RNA was reverse-transcribed for the synthesis of complementary DNA (cDNA) with a Prime Script™ RT reagent kit (TAKARA, Shiga, Japan). Quantitative real-time PCR was carried out using the 2−∆∆CT method for the detection of ZNRD1-AS1, miR-335, and ROCK1 expression in the tissues and cells. The expression levels obtained were normalized with those of U6 and β-actin, and the sequences of the primers used were as follows: ZNRD1-AS1:5′-TTTTGTCACTTCCTATCACCCAC-3′ and 5′-GCTGAGTTTGATTATCCCTGAGTG-3′ β-actin: 5′-GGACTTCGAGCAAGAGATGG-3′ and 5′-AGCACTGTGTTGGCGTACAG-3′.
Western Blot
Protein expression was evaluated by Western blot analysis. We used a RIPA buffer containing a phosphatase inhibitor and PMSF (Beyotime, Shanghai, China) to extract total proteins from the NPC cell lines and tissues in accordance with the manufacturer’s protocols. Protein concentration was detected with a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific). All proteins were subsequently separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred onto polyvinylidene fluoride membranes (Millipore, Bedford, MA, USA). The membranes were blocked for 2 h with bovine serum albumin and washed three times with TBST (TBS, 1 mL/L Tween-20). The membranes were cultured with the primary antibodies anti-ROCK1 and anti-β-actin (1:1000; Cell Signaling Technology, MA, USA) overnight at 4 °C and then with a secondary antibody after washing. Immunoreactive bands were visualized with an ECL detection reagent (Millipore, MA, USA). Optical density analysis was performed on Image Lab (Bio-Rad, CA, USA).
Cell Transfection
Small interfering RNAs (siRNAs) of ZNRD1-AS1 and miR-335 mimics/inhibitors (RiboBio, Guangzhou, China) were designed and synthesized by the RiboBio Company. Transfection into 5–8F and SUNE1 cells was performed with Lipofectamine RNAiMAX (Invitrogen) according to the manufacturer’s protocols. Transfection efficiency was validated by quantitative real-time PCR (qRT-PCR) analysis.
Colony Formation Assay
After 24–48 h of transfection, 5–8F and SUNE1 cells and their controls were seeded in triplicate into six-well plates (1×103 cells/well). After cultivation at 37 °C in 5% CO2 for 2 weeks, the colonies were washed with PBS, fixed with paraformaldehyde (Sigma), and stained with 0.1% crystal violet solution (Sigma) for 20 min. The colonies were counted under a microscope.
Cell Counting Kit-8 Assay
A total of 3000 cells/well and five replicate wells for each experimental group were prepared on 96-well plates after transfection. Then, 10 μL of Cell Counting Kit-8 (CCK-8) reagent and 100 μL of RPMI-1640 medium were added to each well. Cellular proliferation and viability were detected by measuring the absorbance at 450 nm every 24 h after culturing for 1 h.
Wound Healing Assay
Si-ZNRD1-AS1 or si-NC cells were seeded into six-well plates and cultivated with serum-free RPMI-1640 for 24 h. When the cells had grown to approximately 50–80% confluence, wounds were made on the cell monolayers of each well by using 200 µL RNase-free pipettes. The width of the scratch was photographed at 0 and 24 h under a microscope.
Transwell Assays
Cell migration and invasion abilities were detected by transwell assay. This task was completed by using transwell chambers (8 μm pores; Corning, NY, USA) as previously described.12,13
Dual Luciferase Reporter Assays
DIANA (http://diana.imis.athena-innovation) was used in predicting the interactions between ZNRD1-AS1 and miR-335. Previous studies reported that ROCK1 is the downstream target of miR-335. Wild-type and mutant ZNRD1-AS1 were cotransfected with miR-335 or NC mimic into NPC cells for 2 days. Dual-luciferase reporter assay was then used to determine relative luciferase activity. The relative luciferase activities of ROCK1 and miR-335 were evaluated.
Tumor Xenograft Model
Six-to-eight-week-old immunodeficient male BALB/c nude mice (10 mice) were provided by Yangzhou University Medical Center (Yangzhou, China). Our team established an axillary lymph node metastasis model, and 200 µL of 5–8F cells transfected with si-ZNRD1-AS1 and si-NC (2×106) were subcutaneously injected into the foot pads of the mice.12,13 Four weeks after inoculation, the mice were euthanized, and autopsy was performed on the mice. We observed the presence of axillary lymph node metastasis. The animal experiments were approved by Nanjing Medical University Animal Care Committee and in accordance with the Guide for the Care and Use of Laboratory Animals (eighth edition, 2011, National Institutes of Health, USA).
RNA Immunoprecipitation
RNA immunoprecipitation (RIP) experiments were used in examining the relationship between ZNRD1-AS1 and miR-335 using EZMagna RIP kit (Millipore, Massachusetts, USA) in accordance to the manufacturer’s instructions. The abundance levels of co-precipitated RNAs were tested using qRT-PCR. The antibodies of anti-argonaute-2 (Ago2, Abcam, USA) and anti-IgG antibodies were selected for immunoprecipitation.
Statistical Analysis
Each experiment was performed at least three times. Statistical analyses were carried out using SPSS 20.0 (IBM, USA) and GraphPad Prism 6 (GraphPad, USA). Data were presented as the mean ± SD of three independent experiments and analyzed by using one-way ANOVA and t-test. The correlations between ZNRD1-AS1 expression and clinicopathological features were calculated by using the χ2 test. Outcomes were considered statistically significant at p values of <0.05 and <0.01.
Result
ZNRD1-AS1 Is Overexpressed in NPC Tissues and Cells
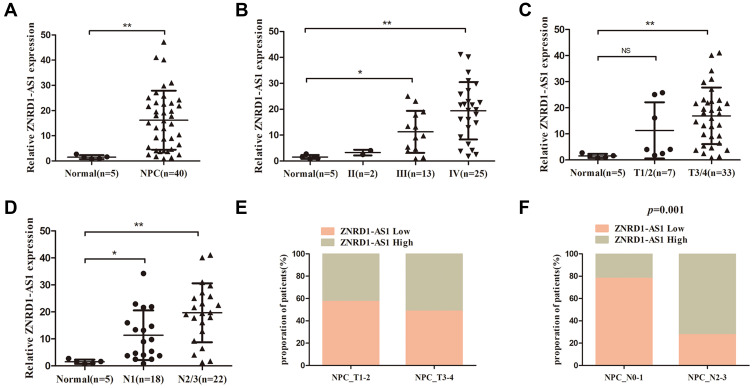
In the present study, we first detected the expression of ZNRD1-AS1 in five normal nasopharyngeal and 40 NPC tissue samples through qRT-PCR. Figure 1A reveals that ZNRD1-AS1 expression is higher in the NPC tissues than in the normal nasopharyngeal tissues. We then explored the association between ZNRD1-AS1 expression and the clinicopathological features of the NPC patients (Table 1). The results indicate that ZNRD1-AS1 overexpression is positively correlated with adverse TNM stage and the presence of lymph node metastasis (Figure 1B, D, and F). No obvious correlation with primary tumor size was observed (Figure 1C and E). Finally, we measured the expression of ZNRD1-AS1 in NP69 and NPC cells. The data reveal that ZNRD1-AS1 is expressed more extensively in the NPC cells than in the NP69 cells (Figure 2A). In summary, the results imply that ZNRD1-AS1 plays an important role in NPC tumorigenesis.
Figure 1.
ZNRD1-AS1 is overexpressed in NPC tissues. (A) The expression of ZNRD1-AS1 in 5 normal nasopharyngeal and 40 NPC tissue samples was detected by qRT-PCR. (B) The expression of ZNRD1-AS1 was tightly associated with TNM stage. (C) The expression of ZNRD1-AS1 was correlated with the T stage of NPC patients. (D) The expression of ZNRD1-AS1 was correlated with lymph node metastasis. (E) Relationship between ZNRD1-AS1 expression and T stage of NPC patients. (F) ZNRD1-AS1 expression was positively correlated with lymph node metastasis in NPC patients. *p < 0.05, **p < 0.01.
Table 1.
Relationship Between ZNRD1-AS1 Expression with Clinical Characteristics of NPC Patients
| Variables | No. of Patients (n=40) | ZNRD1-AS1 Low Group | ZNRD1-AS1 High Group | P-value |
|---|---|---|---|---|
| Gender | ||||
| Male | 28 | 15 | 13 | 0.490 |
| Female | 12 | 5 | 7 | |
| Age | ||||
| <50 | 20 | 8 | 12 | 0.206 |
| ≤50 | 20 | 12 | 8 | |
| TNM stage | ||||
| II–III | 15 | 11 | 4 | 0.022 |
| IV | 25 | 9 | 16 | |
| T stage | ||||
| T1–T2 | 7 | 4 | 3 | 1.00 |
| T3–T4 | 33 | 16 | 17 | |
| N stage | ||||
| N0–N1 | 18 | 14 | 4 | 0.001 |
| N2–N3 | 22 | 6 | 16 |
Note: P-value obtained from Pearson χ-test or Fisher’s exact test.
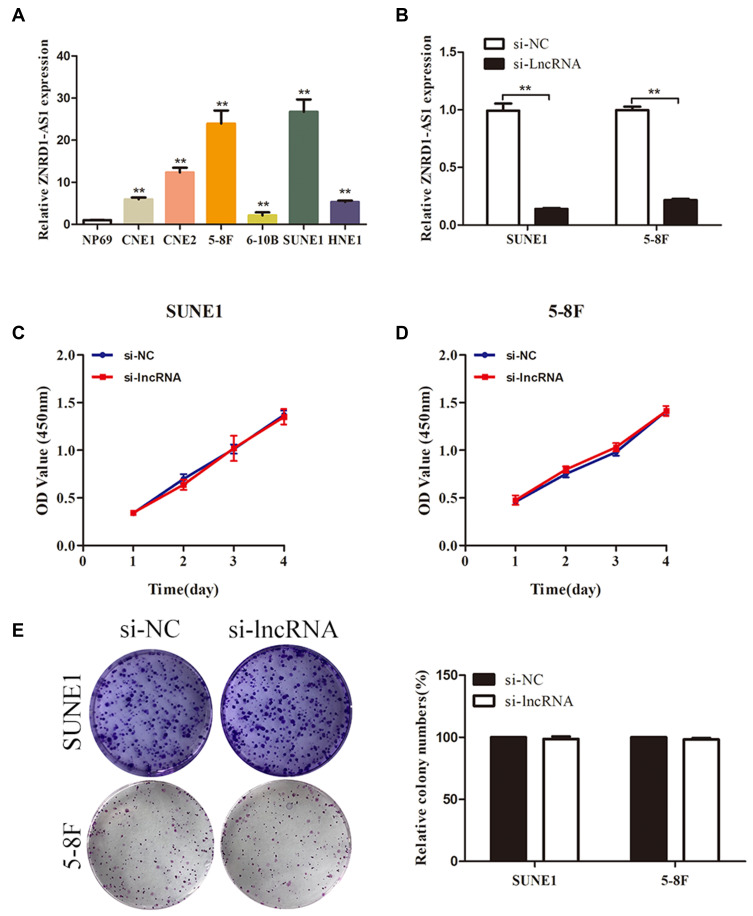
Figure 2.
ZNRD1-AS1 knockdown showed no obvious effects on cell proliferation. (A) The expression of ZNRD1-AS1 in NPC and NP69 cells was detected by qRT-PCR. (B) The expression of ZNRD1-AS1 in NPC cells was tested by qRT-PCR after transfection with si-lncRNA and si-NC. (C and D) CCK8 assays showed that ZNRD1-AS1 knockdown has no obvious effects on cell proliferation. (E) Colony formation analyses indicated that ZNRD1-AS1 knockdown has no obvious effects on cell viability. **p < 0.01.
ZNRD1-AS1 Knockdown Reduces the Invasion and Metastasis of NPC Cells in vitro and in vivo
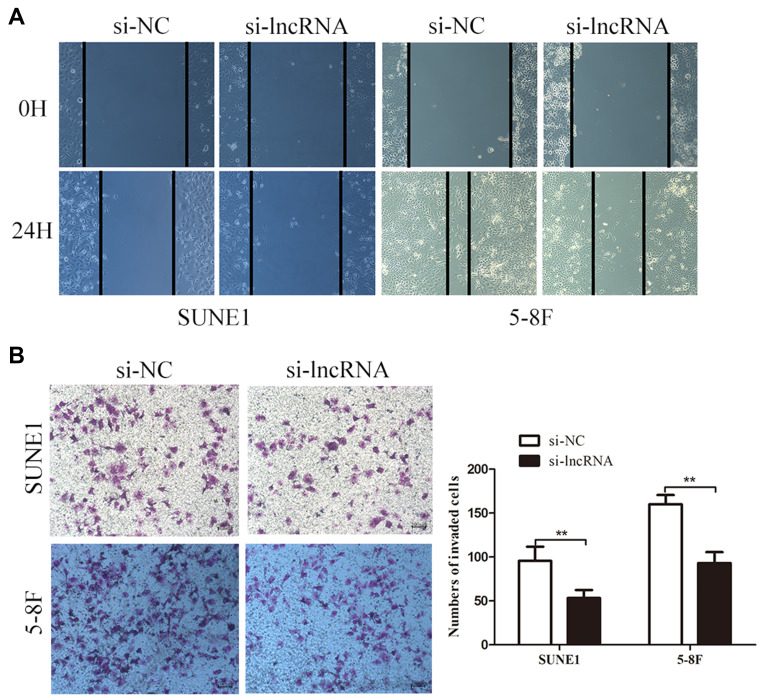
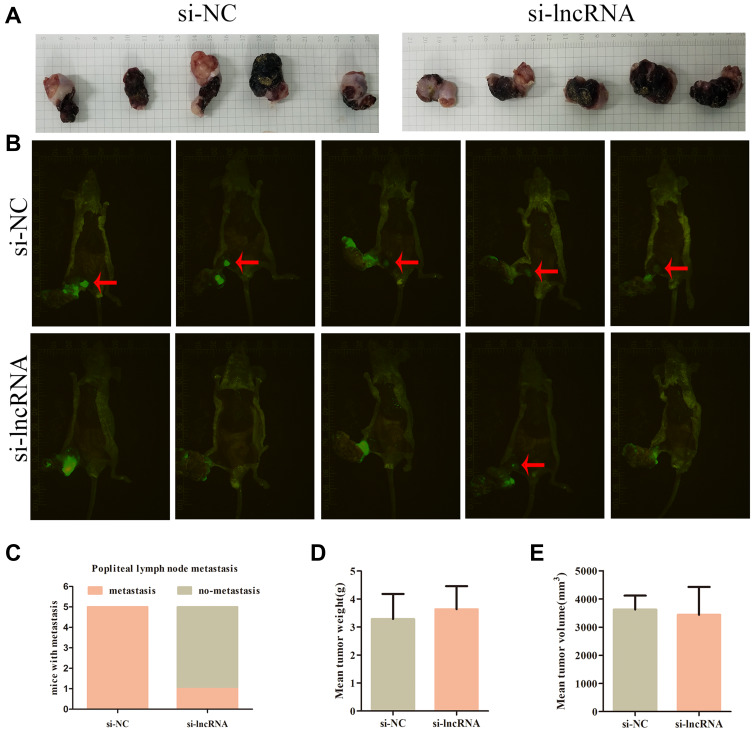
We evaluated the effects of specific siRNAs against ZNRD1-AS1 in 5–8F and SUNE1 cells, which show high endogenous ZNRD1-AS1 levels, to comprehensively evaluate the biological role of ZNRD1-AS1 in NPC progression. Our results indicate that siRNA obviously decreases ZNRD1-AS1 expression in the NPC cells (Figure 2B). CCK8 and colony formation assay were used in exploring the effects of ZNRD1-AS1 knockdown on cell growth. The results indicate that ZNRD1-AS1 knockdown has no obvious effects on cell proliferation compared with the control (Figure 2C–E). The wound healing assay result demonstrates that ZNRD1-AS1 knockdown significantly represses the migration of NPC cells (Figure 3A). The transwell invasion assay results show that ZNRD1-AS1 downregulation reduces the invasion of NPC cells (Figure 3B). According to the established spontaneous lymph node metastasis model, ZNRD1-AS1 knockdown inhibits tumor axillary lymph node metastasis in vivo (Figure 4A–E). In summary, our data reveal that ZNRD1-AS1 knockdown reduces the invasion and metastasis of NPC cells.
Figure 3.
ZNRD1-AS1 knockdown reduced cell invasion and metastasis in vitro. (A) Wound healing assays showed that ZNRD1-AS1 knockdown inhibits the migration of NPC cells. (B) Transwell analyses indicated that ZNRD1-AS1 knockdown suppresses the invasion of NPC cells. **p < 0.01.
Figure 4.
ZNRD1-AS1 knockdown reduced cell invasion and metastasis in vivo. (A) The pictures of nude mice primary tumor sizes in si-NC or si-lncRNA control groups. (B) Image of xenograft tumors in si-NC or si-lncRNA control groups of nude mice. Red arrows indicated popliteal lymph node metastasis. (C) The incidences of popliteal lymph node metastasis of each group were counted. (D) The weights of tumors were detected. (E) The volumes of tumors were detected.
ZNRD1-AS1 Is the Direct Target of miR-335
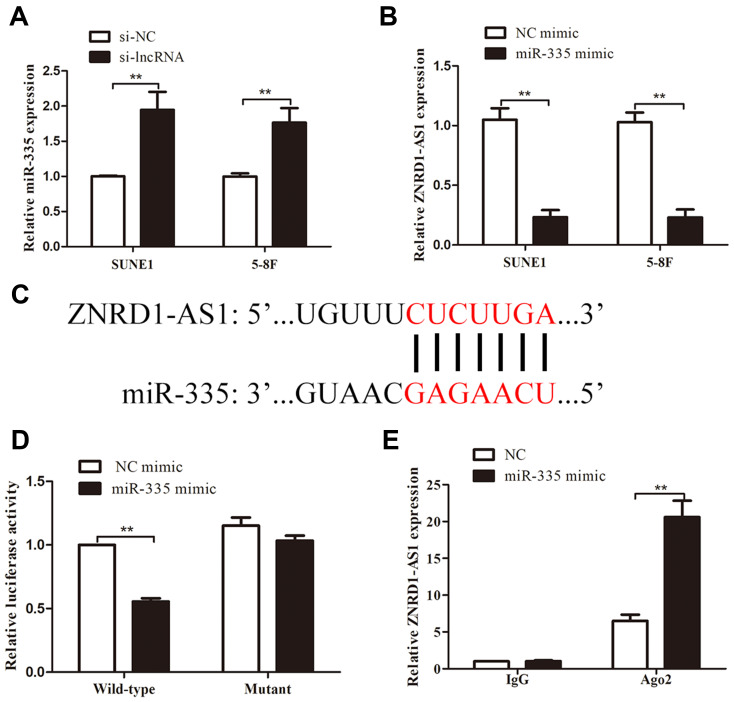
lncRNAs can exert “sponge-like” effects on various miRNAs and block their regulatory functions on target mRNAs.14–16 Thus, we supposed that ZNRD1-AS1 exerts its function by interacting with some miRNAs. The online bioinformatics database DIANA (http://diana.imis.athena-innovation) predicts that miR-335 contains the putative binding sites of ZNRD1-AS1. We quantified miR-335 expression in NPC cells treated with siRNA against ZNRD1-AS1. As shown in Figure 5A and B, ZNRD1-AS1 knockdown promotes the expression of miR-335 in NPC cells, whereas miR-335 overexpression represses ZNRD1-AS1 expression. The dual-luciferase reporter assay results reveal that miR-335 overexpression represses the luciferase activity of wild-type ZNRD1-AS1 3ʹUTR compared with that of mutant ZNRD1-AS1 (Figure 5C and D). This result indicates that miR-335 directly targets ZNRD1-AS1. The RIP assay results confirm that ZNRD1-AS1 and miR-335 are enriched in the beads containing the anti-Ago2 antibody compared with the beads containing anti-IgG immunoprecipitates (Figure 5E). These data imply that ZNRD1-AS1 as ceRNA may sponge miR-335.
Figure 5.
ZNRD1-AS1 was the direct target of miR-335. (A) ZNRD1-AS1 knockdown significantly increased miR-335 expression. (B) MiR-335 overexpression repressed ZNRD1-AS1 expression. (C) Sequence alignment of miR-335 binding sites within the 3′-UTR of ZNRD1-AS1. (D) Luciferase reporter assays revealed that miR-335 overexpression represses the luciferase activity of the wild-type ZNRD1-AS1 3ʹUTR compared with that of the mutant. (E) RIP assays indicated that ZNRD1-AS1 and miR-335 are similarly enriched in beads containing anti-Ago2 antibody. **p < 0.01.
ZNRD1-AS1 as a ceRNA Promotes the Migration and Invasion of NPC Cells by Sponging miR-335
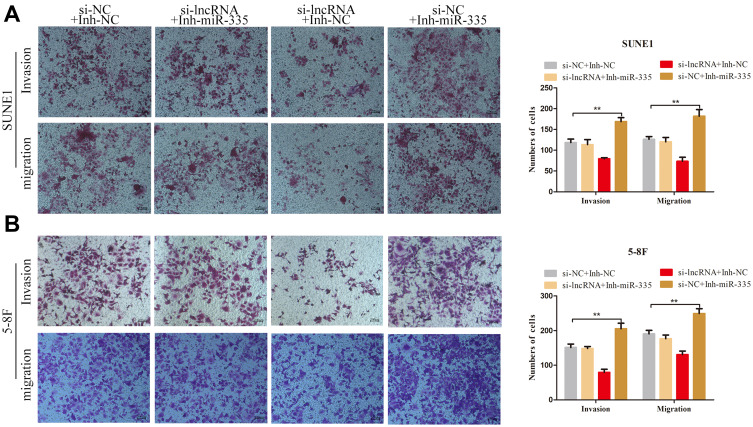
We transfected the miR-335 inhibitor into ZNRD1-AS1 knockdown cells to determine whether the tumor-promoting effects of the latter on cell invasion and migration are influenced by the former. The results of transwell migration assays demonstrate that miR-335 downregulation partly counteracts the suppressive effect of ZNRD1-AS1 knockdown on cell migration (Figure 6A and B). Similar results were observed during transwell invasion analysis. Figure 6A and B show that the simultaneous depletion of miR-335 weakens the ZNRD1-AS1 knockdown-mediated inhibition of NPC cell invasion. These results confirm that ZNRD1-AS1 as a ceRNA promotes the migration and invasion of NPC cells by sponging miR-335.
Figure 6.
ZNRD1-AS1 promoted the migration and invasion of NPC cells via sponging miR-335 as a ceRNA. (A and B) Transwell invasion and migration assays revealed that ZNRD1-AS1 promotes the migration and invasion of NPC cells by inhibiting miR-335 expression. **p < 0.01.
ZNRD1-AS1 Knockdown Suppresses ROCK1 Expression Through miR-335
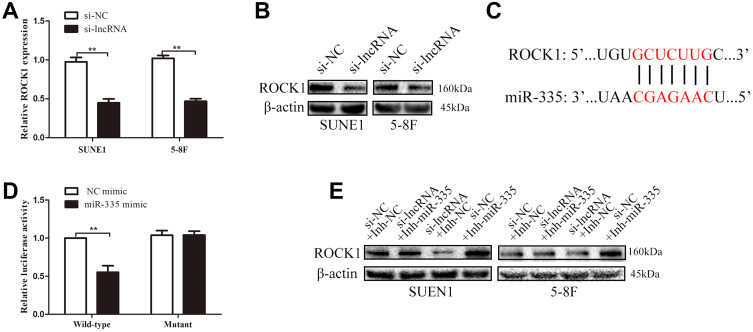
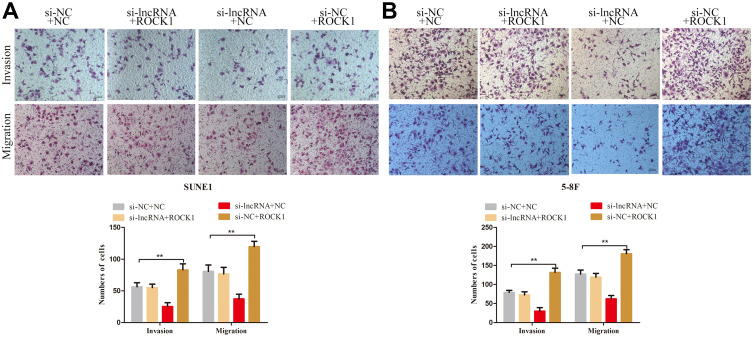
Previous studies reported that ROCK1, which is known as a metastasis-related protein in many types of cancers, is the downstream target of miR-335.17–19 In the present study, we employed PCR and Western blot assays to explore the relationships among ZNRD1-AS1, miR-335, and ROCK1. As shown in Figure 7A and B, ZNRD1-AS1 downregulation reduced ROCK1 expression in the NPC cells. Dual-luciferase reporter assay reveals that miR-335 directly targets ROCK1 in NPC cells (Figure 7C and D). We detected ROCK1 expression in the NPC cells cotransfected with ZNRD1-AS1 siRNA and the miR-335 inhibitor to determine whether ZNRD1-AS1 affects ROCK1 expression by sponging miR-335. The results of the rescue experiments revealed that miR-335 knockdown weakens the negative effects of ZNRD1-AS1 siRNA on ROCK1 expression (Figure 7E). Cellular behavioral experiments indicate that ROCK1 restoration induces the promotion of NPC cell invasion and that migration can be antagonized by ZNRD1-AS1 siRNA (Figure 8A and B). These data confirm that ZNRD1-AS1 induces NPC cell migration and invasion via the miR-335–ROCK1 axis.
Figure 7.
ZNRD1-AS1 knockdown suppressed ROCK1 expression via miR-335. (A) qRT-PCR results revealed that ZNRD1-AS1 knockdown represses ROCK1 expression. (B) Western blot assays showed that ZNRD1-AS1 knockdown suppresses ROCK1 expression. (C) Sequence alignment of miR-335 binding sites within the 3′-UTR of ROCK1. (D) Luciferase reporter assays revealed that ROCK1 is the target of miR-335. (E) Western blot assays showed that miR-335 knockdown weakens the negative effects of ZNRD1-AS1 siRNA on ROCK1 expression. **p < 0.01.
Figure 8.
ZNRD1-AS1 induced NPC cell migration and invasion via the miR-335–ROCK1 axis. (A and B) Transwell invasion and migration analyses indicated that ROCK1 restoration induces the promotion of NPC cell invasion and migration, which could be antagonized by ZNRD1-AS1 siRNA. **p < 0.01.
Discussion
NPC has been managed through IMRT, combined radiotherapy, and chemotherapy, which improve the 5-year overall survival of patients with NPC.1–5 Distant metastasis remains the main reason behind treatment failure.5 Therefore, exploring the molecular mechanisms related to the invasion and metastasis of NPC and finding new targets for intervention are the major challenges that must be overcome.
Researches on the molecular mechanisms of invasion and transfer have expanded from studies on coding genes to those on noncoding RNAs with important regulatory functions.6 lncRNAs have higher tissue- and cell-specific expression than protein-coding genes.7,8 We found that ZNRD1-AS1 is significantly increased in NPC tissues and correlated with clinical outcomes. Human ZNRD1 is a zinc finger-related protein consisting of two zinc ribbon domains located on chromosome 6p21.3 and known as a transcription-associated gene.9 This gene is relevant to the transcription regulation of human leukocyte antigens.20–22 In esophageal squamous cell carcinoma (ESCC), ZNRD1 is upregulated in ESCC tissues, and high ZNRD1 expression is positively associated with adverse clinicopathological characteristics.23 However, no references about the expressions and roles of ZNRD1 and ZNRD1-AS1 in NPC development are available. ZNRD1-AS1, which occurs in the upper region of ZNRD1, can downregulate ZNRD1 expression.9 The aberrant expression of ZNRD1-AS1 has been observed in a number of cancer types, including hepatocellular carcinoma, cervical cancer, and lung cancer, in which it may act as an oncogene.9–11 Nevertheless, the exact molecular mechanisms and global genes mediated by ZNRD1-AS1 in NPC remain incompletely understood.
Our biological experiments showed that the downregulation of ZNRD1-AS1 exerts a negative effect on cell migration and invasion. Bioinformatics analysis and our experiments reveal that ZNRD1-AS1 is the direct target of miR-335. CeRNA is one of the mechanisms linking some ncRNAs, including lncRNAs, mRNA, and pseudogenes, with gene-encoding proteins in cancer.14 The so-called ceRNA hypothesis states that lncRNA transcripts can “talk” with miRNAs if they include the same miRNA binding site.14 miR-335 functions as an important metastasis suppressor in many malignant tumors.17 Recent research on NPC has indicated that miR-335 is downregulated in NPC tissues.24 Zhou et al,25 showed that decrease in miR-335 level inhibits the proliferation and metastasis of NPC cells through TRIM29. Our experiments demonstrate that the miR-335 inhibitor partly counteracts the suppressive effect of si-ZNRD1-AS1 on cell migration and invasion. Thus, we believe that ZNRD1-AS1 interacts with miR-335 through the ceRNA mechanism.
Luciferase reporter assays confirm that miR-335 can directly bind to the 3′-UTR of ROCK1. ROCK1 is a serine/threonine protein kinase that belongs to the AGC kinase family, which includes angiogenesis inhibitor targets, such as Akt, and a set of the downstream effectors of Rho-GTPase signaling.26,27 Moreover, the expression of ROCK1 is closely related to tumorigenesis and development, participates in the progression of many tumors, and promotes tumor metastasis.28–30 For instance, the knockout of the ROCK1 gene can significantly inhibit breast cancer cell invasion and metastasis.31 ROCK1 inhibitors are currently being studied as a putative drug target in clinical trials on progressive solid tumor patients (NCT01585701). In this work, we found that ROCK1 is involved in the ZNRD1-AS1–miR-335 axis and promotes NPC cell invasion and migration. Moreover, ROCK1 restoration antagonizes the repression of NPC cell invasion and migration induced by ZNRD1-AS1–siRNA.
We found that ZNRD1-AS1 is upregulated in NPC tissues and cells and worked as a ceRNA to decoy miR-335 and promote ROCK1 expression. However, this work has some limitations. The signaling pathway that ZNRD1-AS1-miR-335-ROCK1 axis promotes the invasion and metastasis of NPC cells has not been investigated. Similar to AKT, ROCK1, known as a metastasis-related protein in many types of cancers, activates the downstream effectors of Rho-GTPase signaling.26,27 Shi et al,32 found that lncRNA AFAP1-AS1 exerts its oncogenic functions in osteosarcoma by activating RhoC/ROCK1/p38MAPK/Twist1 signaling transduction, and another study demonstrated that DANCR-miR-27a-3p-ROCK1 axis promotes hepatocellular carcinoma tumorigenesis and EMT progression through the LIMK1/COFILIN1 pathway.33 Herein, further investigations for determining the signaling pathway induced by ZNRD1-AS1-miR-335-ROCK1 axis in NPC tumor development are urgently needed.
Conclusion
We report the functional characterization of ZNRD1-AS1 expressed in NPC tissues and provide the evidence that the gene facilitates the invasion and metastasis of NPC cells via the miR-335–ROCK1 axis. Our study sheds light on the oncogenic role of ZNRD1-AS1 in NPC tumor development and offers a promising therapeutic target for NPC.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Razak AR, Siu LL, Liu FF, et al. Nasopharyngeal carcinoma: the next challenges. Eur J Cancer. 2010;46(11):1967–1978. doi: 10.1016/j.ejca.2010.04.004 [DOI] [PubMed] [Google Scholar]
- 2.Ho CS. Beating ‘Guangdong cancer’: a review and update on nasopharyngeal cancer. Hong Kong Med J. 2017;23(5):497–502. doi: 10.12809/hkmj176834 [DOI] [PubMed] [Google Scholar]
- 3.Lee AW, Ng WT, Chan YH, et al. The battle against nasopharyngeal cancer. Radiother Oncol. 2012;104(3):272–278. doi: 10.1016/j.radonc.2012.08.001 [DOI] [PubMed] [Google Scholar]
- 4.Sun X, Su S, Chen C, et al. Long-term outcomes of intensity-modulated radiotherapy for 868 patients with nasopharyngeal carcinoma: an analysis of survival and treatment toxicities. Radiother Oncol J Eur Soc Ther Radiol Oncol. 2014;110(3):398–403. doi: 10.1016/j.radonc.2013.10.020 [DOI] [PubMed] [Google Scholar]
- 5.Hong RL, Ting LL, Ko JY, et al. Induction chemotherapy with mitomycin, epirubicin, cisplatin, fluorouracil, and leucovorin followed by radiotherapy in the treatment of locoregionally advanced nasopharyngeal carcinoma. J Clin Oncol. 2001;19(23):4305–4313. doi: 10.1200/JCO.2001.19.23.4305 [DOI] [PubMed] [Google Scholar]
- 6.Prabhu KS, Raza A, Karedath T, et al. Non-coding RNAs as regulators and markers for targeting of breast cancer and cancer stem cells. Cancers. 2020;12(2):E351. doi: 10.3390/cancers12020351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iyer MK, Niknafs YS, Malik R, et al. The landscape of long noncoding RNAs in the human transcriptome. Nat Genet. 2015;47(3):199–208. doi: 10.1038/ng.3192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang G, Lu X, Yuan L. LncRNA: a link between RNA and cancer. Biochim Biophys Acta. 2014;1839(11):1097–1109. doi: 10.1016/j.bbagrm.2014.08.012 [DOI] [PubMed] [Google Scholar]
- 9.Guo L, Wen J, Han J, et al. Expression quantitative trait loci in long non-coding RNA ZNRD1-AS1 influence cervical cancer development. Am J Cancer Res. 2015;5:2301–2307. [PMC free article] [PubMed] [Google Scholar]
- 10.Li D, Song L, Wen Z, et al. Strong evidence for LncRNA ZNRD1-AS1, and its functional Cis-eQTL locus contributing more to the susceptibility of lung cancer. Oncotarget. 2016;7(24):35813–35817. doi: 10.18632/oncotarget.8411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wen J, Liu Y, Liu J, et al. Expression quantitative trait loci in long non-coding RNA ZNRD1-AS1 influence both HBV infection and hepatocellular carcinoma development. Mol Carcinog. 2015;54:1275–1282. doi: 10.1002/mc.22200 [DOI] [PubMed] [Google Scholar]
- 12.Zhang W, Du M, Wang T, et al. Long non-coding RNA LINC01133 mediates nasopharyngeal carcinoma tumorigenesis by binding to YBX1. Am J Cancer Res. 2019;9(4):779–790. [PMC free article] [PubMed] [Google Scholar]
- 13.Chen W, Du M, Hu X, et al. Long noncoding RNA cytoskeleton regulator RNA promotes cell invasion and metastasis by titrating miR-613 to regulate ANXA2 in nasopharyngeal carcinoma. Cancer Med. 2020;9(3):1209–1219. doi: 10.1002/cam4.2778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Salmena L, Poliseno L, Tay Y, et al. A ceRNA hypothesis: the rosetta stone of a hidden RNA language? Cell. 2011;146(3):353–358. doi: 10.1016/j.cell.2011.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu XH, Sun M, Nie FQ, et al. Lnc RNA HOTAIR functions as a competing endogenous RNA to regulate HER2 expression by sponging miR-331-3p in gastric cancer. Mol Cancer. 2014;13:92. doi: 10.1186/1476-4598-13-92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yuan JH, Yang F, Wang F, et al. A long noncoding RNA activated by TGF-β promotes the invasion-metastasis cascade in hepatocellular carcinoma. Cancer Cell. 2014;25(5):666–681. doi: 10.1016/j.ccr.2014.03.010 [DOI] [PubMed] [Google Scholar]
- 17.Tang H, Du W, Jiang Y, et al. Upregulated expression of ROCK1 promotes cell proliferation by functioning as a target of miR-335-5p in non-small cell lung cancer. J Cell Physiol. 2019. doi: 10.1002/jcp.28886 [DOI] [PubMed] [Google Scholar]
- 18.Wang Y, Yang T, Zhang Z, et al. Long non-coding RNA TUG1 promotes migration and invasion by acting as a ceRNA of miR-335-5p in osteosarcoma cells. Cancer Sci. 2017;108(5):859–867. doi: 10.1111/cas.13201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liang H, Zhang C, Guan H, et al. LncRNA DANCR promotes cervical cancer progression by upregulating ROCK1 via sponging miR-335-5p. J Cell Physiol. 2019;234(5):7266–7278. doi: 10.1002/jcp.27484 [DOI] [PubMed] [Google Scholar]
- 20.Fan W, Wang Z, Kyzysztof F, et al. A new zinc ribbon gene (ZNRD1) is cloned from the human MHC class I region. Genomics. 2000;63:139–141. doi: 10.1006/geno.1999.6040 [DOI] [PubMed] [Google Scholar]
- 21.Qian X, Jeon C, Yoon H, et al. Structure of a new nucleic-acid-binding motif in eukaryotic transcriptional elongation factor TFIIS. Nature. 1993;365:277–279. doi: 10.1038/365277a0 [DOI] [PubMed] [Google Scholar]
- 22.Chu TW, Capossela A, Coleman R, et al. Cloning of a new “finger” protein gene (ZNF173) within the class I region of the human MHC. Genomics. 1995;29:229–239. doi: 10.1006/geno.1995.1236 [DOI] [PubMed] [Google Scholar]
- 23.Yunping Z, Liu H. Wang Ruwen et al. Expression and prognostic value of ZNRD1 in esophageal squamous cell carcinoma. Dig Dis Sci. 2009;54:586–592. doi: 10.1007/s10620-008-0380-1 [DOI] [PubMed] [Google Scholar]
- 24.Zhu D, Shao M, Yang J, et al. Curcumin enhances radiosensitization of nasopharyngeal carcinoma via mediating regulation of tumor stem-like cells by a CircRNA network. J Cancer. 2020;11(8):2360–2370. doi: 10.7150/jca.39511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xiao-Min Z, Rui S, Dong-Hua L, et al. Upregulated TRIM29 promotes proliferation and metastasis of nasopharyngeal carcinoma via PTEN/AKT/mTOR signal pathway. Oncotarget. 2016;7:13634–13650. doi: 10.18632/oncotarget.7215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laura DB, Gagliardi Paolo A, Alberto P, et al. Serine/Threonine kinase 3-phosphoinositide-dependent protein kinase-1 (PDK1) as a key regulator of cell migration and cancer dissemination. Cancers. 2017;9(3):cancers9030025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Olivier D, Sandro B. Rho kinase inhibitors: a patent review (2014-2016). Expert Opin Ther Pat. 2017. [DOI] [PubMed] [Google Scholar]
- 28.Jinyang W, Lei Z, Rongmei Q, et al. Rho A regulates epidermal growth factor-induced human osteosarcoma MG63 cell migration. Int J Mol Sci. 2018;19(5):1437. doi: 10.3390/ijms19051437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gao-Xiang H, Yan W, Jie S, et al. Up-regulation of Rho-associated kinase 1/2 by glucocorticoids promotes migration, invasion and metastasis of melanoma. Cancer Lett. 2017;1–11. [DOI] [PubMed] [Google Scholar]
- 30.Zheng W, Tian-En L, Chen M, et al. miR-106b-5p contributes to the lung metastasis of breast cancer via targeting CNN1 and regulating Rho/ROCK1 pathway. Aging. 2020;12:1867–1887. doi: 10.18632/aging.102719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lei W, Michelle S, Stephanie S, et al. Novel insights into the roles of Rho Kinase in Cancer. Arch Immunol Ther Exp. 2016;64(4):259–278. doi: 10.1007/s00005-015-0382-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shi D, Wu F, Mu S, et al. LncRNA AFAP1-AS1 promotes tumorigenesis and epithelial-mesenchymal transition of osteosarcoma through RhoC/ROCK1/p38MAPK/Twist1 signaling pathway. J Exp Clin Cancer Res. 2019;38(1):375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guo D, Li Y, Chen Y, et al. DANCR promotes HCC progression and regulates EMT by sponging miR-27a-3p via ROCK1/LIMK1/COFILIN1 pathway. Cell Prolif. 2019;52(4):e12628. doi: 10.1111/cpr.12628 [DOI] [PMC free article] [PubMed] [Google Scholar]