Abstract
Purpose
To investigate the potential role of the circMTO1/miR-9-5p/NOX4 axis in liver cancer.
Materials and Methods
Human genome-wide circrna microarray V2 was used for analyzing the expression profile of circRNAs in human tissue samples. The TargetScan database was used to predict target genes. Gene overexpression and silencing in hepatoma cell lines were achieved by transfecting the cells with suitable constructs. Quantitative real time PCR and Western blotting were used to analyze gene and protein expression levels. CCK-8 analysis was performed to detect cell proliferation and the transwell assay for analyzing cell migration. Annexin V-FITC/PI staining and immunohistochemistry were respectively used to detect apoptosis and protein expression.
Results
CircMTO1 were down-regulated in the liver cancer tissues and cell lines compared to their respective normal controls. TargetScan database screening and dual luciferase assay revealed that circMTO1 was a molecular sponge of miR-9-5p, and NOX4 was the target gene of miR-9-5p. Overexpression of circMTO1 and NOX4 inhibited proliferation and migration of hepatoma cells, while the overexpression of miR-9-5p had the opposite effects. In contrast, overexpression of circMTO1 and NOX4 promoted apoptosis, while that of miR-9-5p decreased the cell apoptosis rates.
Conclusion
Overexpression of CircMTO1 acts as tumor suppressor in liver cancer by sponging miR-9-5p, which upregulates NOX4.
Keywords: circMTO1, miR-9-5p/NOX4 axis, hepatocellular carcinoma, proliferation, apoptosis
Introduction
Hepatocellular carcinoma (HCC) is one of the most commonly diagnosed human malignancies worldwide, which is associated with high morbidity and mortality. China alone accounts for about 50% of the global liver cancer burden.1,2 An estimated 466,100 patients were diagnosed with HCC in China in 2015, of which 422,100 did not survive.1 Based on the stage of tumor progression, HCC can be managed by surgery, liver transplantation, targeted therapy and palliative care.3 Despite significant progress in the treatment of liver cancer, its incidence and mortality remains high, calling for new potential therapeutic targets.
Circular RNAs (circRNAs) is a class of non-coding RNAs characterized by a covalently closed loop structure. They are expressed in a tissue-specific and developmental stage-specific manner.4 The circRNA regulates gene expression by acting as an microRNAs (miRNAs) sponge, a chelating agent for RNA binding protein or a transcriptional regulator.5,6 Aberrant expression levels of circRNA have been detected in vascular diseases, neurological diseases and cancer, indicating their essential role in both physiological and pathophysiological processes.7,8 CircRNAs involved in HCC progression have been identified, although their effects on HCC cell proliferation and apoptosis are not completely clear.
MiRNAs are short non-coding RNAs that can negatively regulate target gene expression by binding to the 3ʹ untranslated region (UTR) of the mRNAs through complementary base pairing, and inducing decay or transcriptional repression.9 MiRNAs have multiple targets that regulate proliferation, differentiation, apoptosis, protein secretion and viral infection,10,11 and are therefore attractive therapeutic targets. Several miRNAs involved in the development and progression of liver disease have also been identified that can be potential markers for liver cancer diagnosis, prognosis and pharmacogenomics.12
The NADPH oxidase (NOX) family of enzymes are an important source of ROS in signal transduction.13,14 NOX4 plays a crucial role in mediating the action of transforming growth factor-β (TGF-β) in the liver. It activates TGF-β in stellate cells and promotes liver fibrosis,14 and triggers apoptosis of hepatoma cells by upregulating the proapoptotic BIM and BMF.15 Therefore, NOX4 likely plays an important role in the development of liver cancer.
In this study, we performed a global expression analysis of circRNAs, miRNAs and mRNAs in HCC tissues and cell lines, and determined the effect of the circMTO1/miR-9-5p/NOX4 axis on hepatoma cell proliferation and apoptosis. Our findings provide new insights into the molecular mechanisms underlying liver cancer development, and present circMTO1 as a potential diagnostic biomarker and therapeutic target for HCC.
Materials and Methods
Reagents
RPMI-1640 medium, MEM, penicillin, streptomycin, and fetal bovine serum (FBS) were purchased from Gibco, USA. The TRIzol reagent, cDNA reverse transcription kit and SYBR Premix EX TaqTM kit were bought from TaKaRa, Japan. The miR-9-5p mimics, miR-9-5p inhibitor, and the respective scrambled controls were synthesized by Genepharma. The pcDNA-circMTO1 and pcDNA-NOX4 plasmids and Lipofectaminetm 3000 were purchased from Invitrogen, USA. The pmirGLO vector and the Dual Luciferase Activity Assay Kit were purchased from Promega, USA. Annexin V-FITC/PI kit, RIPA lysis buffer and NOX4 antibody were purchased from Abcam, UK. The β-actin antibody and goat anti-rabbit (mouse) IgG secondary antibody were purchased from Cell Signaling Technology Inc., USA. Polyvinylidene fluoride (PVDF) membranes were purchased from Millipore Corporation, USA.
Cell Culture
Six HCC cell lines (Huh7, Hep3B, MHCC-97L, MHCC-97H, SMMC-7721 and HepG2) and normal liver cell lines (L-02) were purchased from Shanghai Institute of Cell Biology, Chinese Academy of Sciences. L-02 cells were cultured in RPMI-1640 medium supplemented with 10% FBS, 100 IU/mL penicillin and 100 IU/mL streptomycin. Huh7 cells, Hep3B cells, MHCC-97L cells, MHCC-97H cells, SMMC-7721 cells and HepG2 cells were cultured in MEM supplemented as above. All cell lines were cultured at 37°C under 5% CO2. The medium was replaced every 2 days and the cells in log phase were used for the experiments.
Patients and Tissue Samples
Twenty pairs of liver tumor tissues and adjacent normal tissues were harvested from patients undergoing liver cancer resection. Written informed consent was obtained from all patients, and all trials were approved and supervised by the Ethics Committee of Jingzhou Central Hospital and compliant with the Declaration of Helsinki. Liver cancer was diagnosed on the basis of histopathological examination. The resected tissue specimens were flash frozen and stored at −80°C.
Bioinformatics Analysis
Total RNA was extracted from the normal and tumor tissues using TRIzol reagent, and the samples were prepared for Human Whole Genome Circrna Microarray V2 as per the protocol of Arraystar Inc. The results were visualized in the form of a heat map. The TargetScan database was then used to identify the putative binding sites between miR-9-5p and the non-coding region of circMTO1 and NOX4 respectively.
Quantitative Real Time RT-PCR (qRT-PCR)
Total RNA was extracted from the tissues or cells using TRIzol reagent, and the concentration was determined using a Nanodrop 2000 instrument. Equal amounts of the total RNA were reverse transcribed to cDNA using the cDNA Reverse Transcription Kit, followed by qRT-PCR using the SYBR Premix EX TaqTM kit in FTC-3000p real-time PCR system. U6 and β-actin were used as the internal references for miRNA, mRNA and circRNA respectively. The relative gene expression levels were analyzed by the 2−ΔΔCt method. The primers are listed in Table 1.
Table 1.
Primer Sequences
| Primer | Sequence (5ʹ-3ʹ) |
|---|---|
| circMTO1 | Forward:5ʹ-TGCATCAGAGGCTTGGAGAA-3’ |
| Reverse:5ʹ-AAGGAAGGGGTGATCTGACG-3’ | |
| miR-9-5p | Forward:5ʹ-TCGCTCTTTGGTTATCTAGCT-3’ |
| Reverse:5ʹ-UCUUUGGUUAUCUAGCUGUAUGA-3’ | |
| NOX4 | Forward:5ʹ-ATTGATGGGCTCCCTCTACCC-3’ |
| Reverse:5ʹ-TGCCTTGACTGGTTCTCGTTCC-3’ | |
| U6 | Forward:5ʹ-GCTTCGGCAGCACATATACTAAAAT-3’ |
| Reverse:5ʹ-CGCTTCACGAATTTGCGTGTCAT-3’ | |
| β-actin | Forward:5ʹ-TGACTTCAACAGCGACACCCA-3’ |
| Reverse:5ʹ-CACCCTGTTGCTGTAGCCAAA-3’ |
Cell Transfection
The HepG2 and Hep3B cells were transfected with various constructs (miR-9-5p mimics, scrambled controls, pcDNA-circMTO1 and pcDNA-NOX4) as appropriate using the LipofectamineTM 3000 Transfection Reagent according to the manufacturer’s instructions. The medium was replaced 6h after transfection, and the cells were harvested 48h later.
Dual Luciferase Reporter Gene Activity Assay
The circMTO1 3ʹUTR wt/mut or NOX4 3ʹUTR wt/mut constructs were cloned into the pmirGLO vector, and the HepG2 and Hep3B cells were co-transfected with pmirGLO-circMTO1-wt/mut or pmirGLO-NOX4-wt/mut along with miR-9-5p mimic or the scrambled control. The luciferase activity of the cells was determined 48h later using a dual luciferase activity assay kit.
CCK-8 Assay
The transfected cells were seeded into 96-well plates and cultured for 24, 48, 72 or 96h. For each time point, 10μL CCK-8 solution was added per well and the cells were incubated at 37°C for 2h. The absorbance of the wells at 450 nm was detected using an automatic microplate reader.
Cell Migration Assay
The transfected cells were seeded into the upper chamber of a transwell insert (24-well plate format) at the density of at 1×105 cells/well in serum-free medium, and the lower chambers were filled with complete medium. After culturing the cells at 37°C under 5% CO2 for 48h, the upper chambers were removed and the unmigrated cells remaining on the membrane surface were swabbed. The migrated cells on the lower side of the membrane were washed thrice with PBS, fixed with 4% paraformaldehyde and stained with 1% crystal violet for 20 min. After washing thrice with PBS, the number of migrating cells was counted in 5 random fields. Each sample was tested in triplicates.
Flow Cytometry
The transfected cells were harvested, centrifuged and re-suspended in binding buffer. The cells were sequentially stained with 10µL Annexin V-FITC and 10µL propidium iodide (PI) for 30 min at room temperature in the dark, and then analyzed using a FACSCaliburTM flow cytometer.
Immunochemistry
The liver tissues were fixed, embedded in paraffin, and cut into 4µm sections. The latter were cleared, dehydrated through an ethanol gradient, and immersed in 3% hydrogen peroxide to quench endogenous peroxidases. After blocking with animal serum for 20 min, the sections were incubated with the primary antibody, washed with PBS, and then incubated with the secondary antibody. The slides were rinsed, and color was developed using the ABC complex and DAB. The stained sections were observed under 400x magnification.
Western Blotting
The total cellular protein was extracted with RIPA lysis buffer, and quantified by the BCA method. Equal amounts of protein per sample were denatured, resolved by SDS-PAGE and transferred to PVDF membranes. The blots were blocked with 5% skim milk, incubated overnight with the primary antibody at 4°C, and then with the secondary antibody at room temperature for 1h. The immunoblots were developed as per standard procedures, and Image Pro Plus image analysis system was used to quantify the protein bands.
Statistical Analysis
Statistical analysis was performed using SPSS 18.0 software. The data was represented as mean ± standard deviation ( ) of three independent experiments. Multiple groups were compared by one-way ANOVA. P<0.05 was considered statistically significant.
) of three independent experiments. Multiple groups were compared by one-way ANOVA. P<0.05 was considered statistically significant.
Results
CircMTO1 Is Expressed at Low Levels in Liver Cancer Tissues and Cells
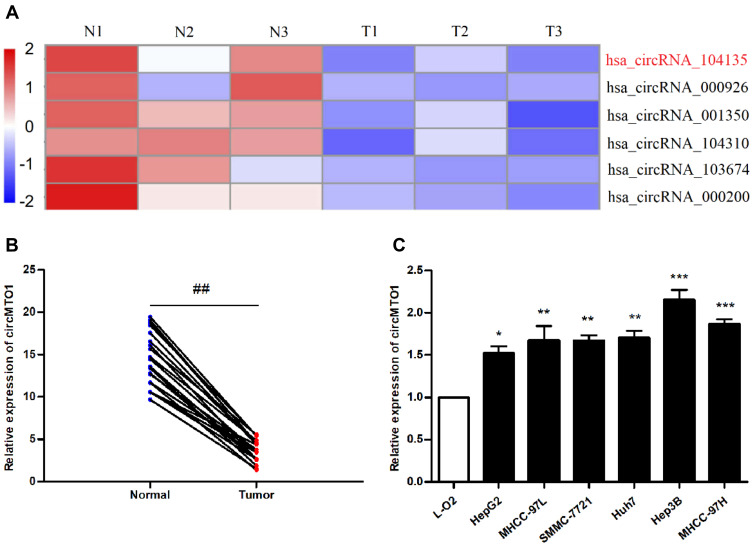
As shown in the heat map in Figure 1A, 6 circRNAs were down-regulated in HCC tissues compared to the normal liver tissues, among which circMTO1 (hsa_circRNA_104135) showed the most significant down-regulation. The lower levels of circMTO1 were verified in both hepatocarcinoma tissues (P<0.01; Figure 1B). Compared with L-02 cells, low expression of circMTO1 was observed in all six HCC cell lines. Among them, circMTO1 expression was the lowest in HepG2 cells (P <0.05), while circMTO1 expression was the highest in Hep3B cells (P<0.001;Figure 1C). For subsequent experiments, these two cell lines were selected.
Figure 1.
Expression of circMTO1 in liver cancer tissues and cells. (A) Heat map of differentially expressed circRNAs in liver cancer tissues. N: normal tissue adjacent to the tumor; T: tumor tissue. Red indicates high expression and blue indicates low expression. (B, C) Results of qRT-PCR analysis showing that circMTO1 is down-regulated in (B) tumor tissues, and in (C) HCC cells. ##P<0.01 compared with adjacent normal tissues, *P<0.05, **P<0.01, ***P<0.001 compared with L-02 cells.
Abbreviation: HCC, hepatocellular carcinoma.
CircMTO1 Affects the Proliferation and Apoptosis of Hepatoma Cells in vitro
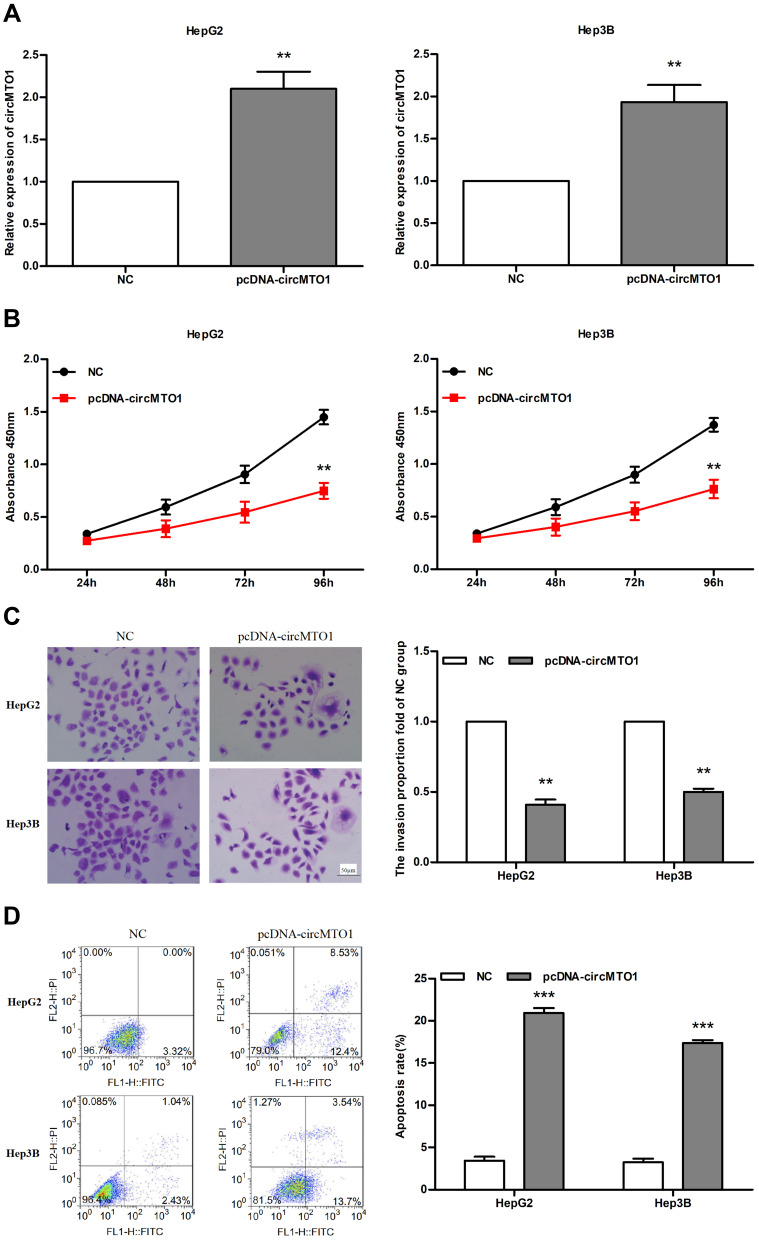
To determine the functional role of circMTO1 in liver cancer, HepG2 and Hep3B cells were transfected with the pcDNA-circMTO1 expression vector. As shown in Figure 2A, the relative expression of circMTO1 was significantly higher in pcDNA-circMTO1 group compared to the NC group (all P<0.01). Overexpression of circMTO1 significantly inhibited the proliferation of the hepatoma cells (all P<0.01; Figure 2B), as well as their in vitro migration (all P<0.01; Figure 2C). Furthermore, the high levels of circMTO1 significantly increased apoptosis in the hepatoma cells (both P<0.001; Figure 2D). Taken together, overexpression of circMTO1 likely acts a tumor suppressor in liver cancer by inhibiting the proliferation of hepatoma cells and promoting apoptosis.
Figure 2.
CircMTO1 regulates cell proliferation and apoptosis in hepatoma cells. (A) Overexpression of circMTO1 in HepG2 and Hep3B cells transfected with pcDNA-circMTO1. (B) CCK-8 assay results showing proliferation rates of pcDNA-circMTO1 and NC cells. (C) Transwell assay showing the percentage of migrating cells in the pcDNA-circMTO1 and NC groups. (D) Flow cytometry plots showing percentage of AnnexinV+/PI+ apoptotic cells in both groups. **P<0.01, ***P<0.001 compared with NC group.
Abbreviations: NC, negative control; FITC, fluorescein isothiocyanate; PI, propidium iodide; FL1-H, fluorescent channel 1; FL2-H, fluorescent channel 2.
CircMTO1 Is a Molecular Sponge of miR-9-5p
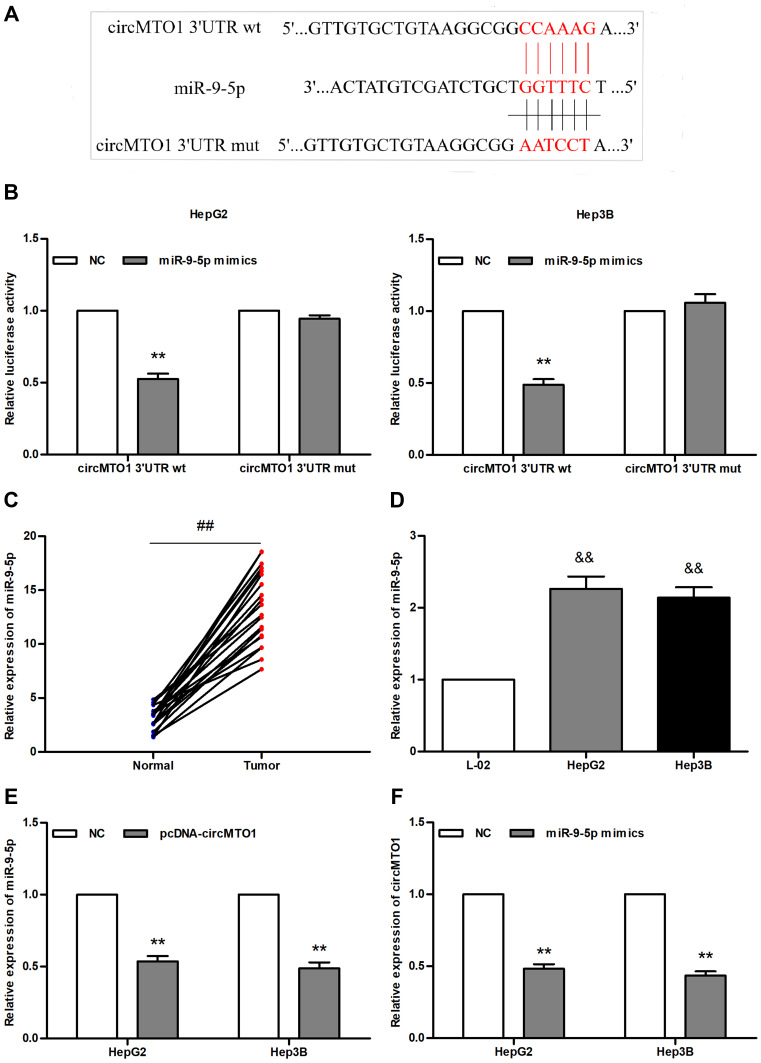
To determine whether circMTO1 targeted miR-9-5p, we first predicted the putative circMTO1-specific binding sites on miR-9-5p using TargetScan (Figure 3A). Cells co-transfected with luciferase reporter plasmid carrying circMTO1 3ʹUTR wt and the miR-9-5p mimic showed significantly reduced fluorescein levels compared to the cells transfected with the circMTO1 3ʹUTR mut (all P<0.01; Figure 3B). As shown in Figure 3C and D, miR-9-5p levels were significantly higher in the hepatocarcinoma tissues and cells compared to that in normal liver tissues and cells respectively (P<0.01 for all). Furthermore, overexpression of circMTO1 significantly inhibited the expression of miR-9-5p (Figure 3E), while the miR-9-5p mimic caused a sharp decrease in circMTO1 levels (Figure 3F). Taken together, circMTO1 and miR-9-5p mutually inhibited each other in hepatoma cells.
Figure 3.
MiR-9-5p and circMTO1 mutually inhibit each other in hepatocarcinoma cells. (A) Putative binding sites between miR-9-5p and circMTO1. (B) Fluorescein activity in cells co-transfected with pmir-GLO-CircMTO1 3ʹUTR wt/mut and miR-9-5p mimic. (C, D) Results of qRT-PCR analysis showing up-regulation of miR-9-5p in (C) liver tumors and (D) HepG2 and Hep3B cells. (E) MiR-9-5p levels in hepatoma cells overexpressing circMTO1. (F) CircMTO1 levels in cells overexpressing miR-9-5p. **P<0.01 compared with NC group, ##P<0.01 compared with the adjacent normal tissues, &&P<0.01 compared with L-02 cells.
Abbreviations: wt, wild type; mut, mutant type; NC, negative control.
MiR-9-5p Targets NOX4 in Liver Cancer Tissues and Cells
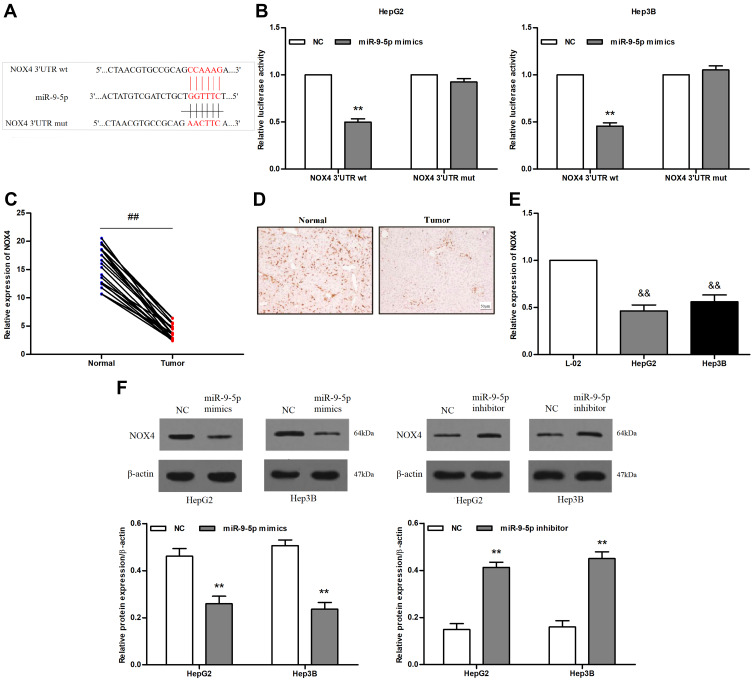
To determine whether miR-9-5p targeted NOX4, we predicted the putative binding sites between miR-9-5p and NOX4 (Figure 4A). The dual luciferase reporter assay further showed that miR-9-5p inhibited fluorescein activity in HepG2 and Hep3B cells transfected with a reporter plasmid carrying NOX4 3ʹUTR wt. However, no significant inhibition was observed when the reporter plasmid carried NOX4 3ʹUTR mut (both P<0.01; Figure 4B). Next, we found NOX4 mRNA levels were significantly down-regulated in hepatocarcinoma tissues compared to the adjacent normal tissues (P<0.01; Figure 4C). Consistent with this, the in situ NOX4 protein expression was significantly less in the tumors compared to the adjacent normal liver tissues (Figure 4D). NOX4 mRNA levels were significantly down-regulated in the hepatoma cell lines compared to L-02 cells (all P<0.01; Figure 4E). Furthermore, miR-9-5p overexpression significantly down-regulated NOX4 whereas the miR-9-5p inhibitor up-regulated NOX4 in HepG2 cells and Hep3B cells (Figure 4F). Thus, miR-9-5p binds to and transcriptionally represses NOX4 in liver cancer cells.
Figure 4.
MiR-9-5p targets NOX4 in hepatocarcinoma cells. (A) Binding sites between miR-9-5p and NOX4. (B) Luciferase activity in cells co-transfected with pmitr-GLO-NOX4 3ʹUTR wt/mut and miR-9-5p mimic. (C) NOX4 mRNA levels in the tumor and normal liver tissues. (D) In situ NOX4 expression in the tumor and normal liver tissues. (E) NOX4 mRNA levels in HepG2, Hep3B and L-02 cells. (F) NOX4 mRNA levels in cells transfected with miR-9-5p mimic or inhibitor. **P<0.01 compared with NC, ##P<0.01 compared with adjacent normal tissues, &&P<0.01 compared with L-02.
Abbreviations: wt, wild type; mut, mutant type; NC, negative control.
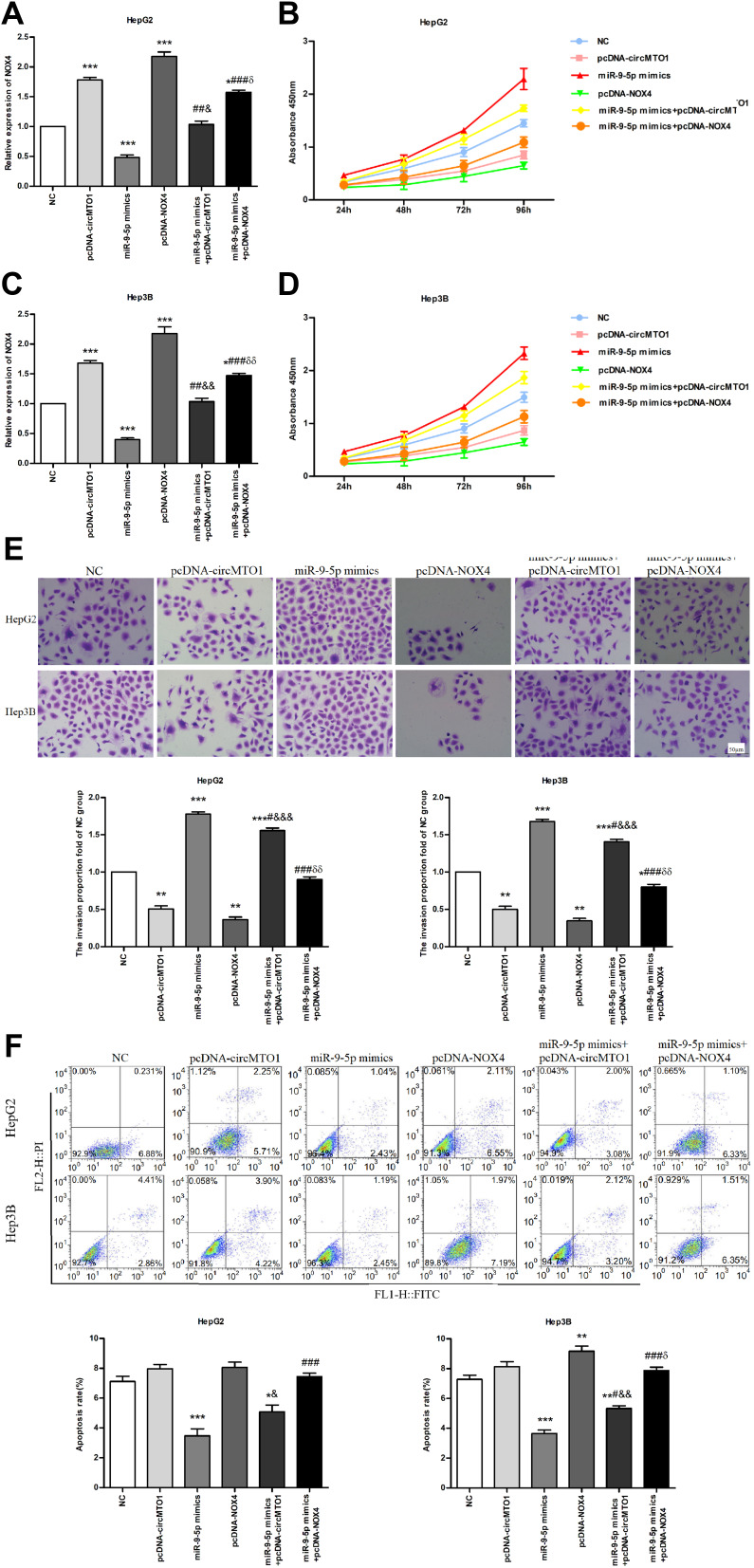
The circMTO1/miR-9-5p/NOX4 Axis Regulates Liver Cancer Progression
The regulatory network of circMTO1, miR-9-5p and NOX4 in the hepatoma cells was further analyzed by co-transfecting the cells with multiple constructs. Overexpression of circMTO1 significantly downregulated miR-9-5p, which in turn increased the expression levels of NOX4 in the HepG2 and Hep3B cells. However, presence of miR-9-5p mimic counteracted the effects of circMTO1 (both P<0.05; Figure 5A and C). Furthermore, miR-9-5p overexpression increased the proliferation and migration of HepG2 and Hep3B cells, which was abrogated by the ectopic expression of circMTO1 or NOX4 (all P<0.05; Figure 5B, D and E). Consistent with this, miR-9-5p overexpression also lowered the apoptosis rate in hepatoma cells, while ectopic expression of circMTO1 and NOX4 had the opposite effect (P<0.05; Figure 5F).
Figure 5.
The circMTO1/miR-9-5p/NOX4 axis regulated the proliferation and apoptosis in hepatoma cells. (A, C) NOX4 mRNA levels in HepG2 and Hep3B cells transfected with pcDNA-circMTO1, pcDNA-NOX4 and miR-9-5p mimic. (B, D) Proliferation rate of cells transfected with the different constructs. (E) In vitro migration rate of cells transfected with the different constructs. (F) Apoptosis rate in the cells transfected with different constructs. * P<0.05, **P<0.01, ***P<0.001 compared with NC, #P<0.05, ##p<0.01, ###p<0.001 compared with miR-9-5p mimic, &P<0.05, &&P<0.01, &&&P<0.001 compared with pcDNA-circMTO1, δP<0.05, δδP<0.01 compared with pcDNA-NOX4.
Abbreviations: NC, negative control; FITC, fluorescein isothiocyanate; PI, propidium iodide; FL1-H, fluorescent channel 1; FL2-H, fluorescent channel 2.
Discussion
HCC is the fourth leading cause of cancer-related deaths worldwide, and the third most lethal cancer in China.16 Despite significant progress in the diagnosis and treatment of liver cancer in recent years, the prognosis is still poor due to its high recurrence and metastasis rates.17,18 A more in-depth understanding of the molecular mechanisms driving metastasis of liver cancer cells can potentially translate to more effective treatment strategies and improved patient prognosis.
CircRNAs, as the name suggests, have a closed circular structure that is impervious to RNA exonuclease and therefore more stable.19 They are derived from exons, introns or intergenic regions, and the exon-derived circRNAs are the final product of mRNA splicing and usually cytoplasmic. Recent studies show that circRNAs molecules have numerous miRNA binding sites and act as miRNA sponges.20 Several circRNA involved in tumor progression have been identified. For example, circ-MTO1 inhibits the proliferation of lung adenocarcinoma cells by regulating the miR-17/QKI-5 axis.21 In this study, we found that circMTO1 was downregulated in human HCC tissues, and the ectopic expression of circMTO1 in hepatoma cell lines inhibited their proliferation. Therefore, circMTO1 likely acts a tumor suppressor in HCC and is a potential biomarker/therapeutic target.
MiRNAs are short single-stranded RNAs that post-transcriptionally regulate gene expression by binding to complementary sequences in the 3ʹ UTR of the target mRNAs, resulting in translational inhibition and gene silencing.22 They play critical roles in physiological processes, such as tissue development and differentiation, cell proliferation and tissue repair.23 MiR-9-5p is up-regulated in liver cancer tissues, and consistent with this, miR-9-5p is highly expressed in HCC and the miR-9a-5p mimic significantly increased the proliferation of hepatoma cells and inhibited apoptosis.24 Therefore, miRNAs are also promising biomarkers for the clinical diagnosis of HCC.24
NOX is a multimeric transmembrane enzyme complex that produces superoxide anions and hydrogen peroxide from molecular oxygen in response to multiple stimuli. The mammalian NOX family includes seven subtypes: NOX1, NOX2, NOX3, NOX4, NOX5, double oxidase 1 and double oxidase 2.25 The NOX-mediated generation of ROS is the pathogenic basis of liver diseases like chronic hepatitis, liver fibrosis and HCC.26–28 The study found that NOX4 plays a role in regulating hepatocyte proliferation under physiological conditions or during tumorigenesis. NOX4 silencing increases the tumorigenic potential of human hepatoma cells in mouse xenografts, leading to earlier tumor formation.29 In addition, NOX4 is significantly down-regulated in liver cancer tissues, as observed in our study as well.30
Based on our findings, we concluded that miR-9-5p targets circMTO1 and NOX4, and that circMTO1 competes with miR-9-5p to regulate the expression of NOX4 in hepatocarcinoma cells. The network comprising of circMTO1, miR-9-5p and NOX4 regulates the progression of liver cancer, and is a promising therapeutic target. For example, overexpression of circMTO1 could arrest liver cancer cells via the miR-9-5p/NOX4 axis. Nevertheless, the molecular mechanisms driving HCC need further investigation.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. doi: 10.3322/caac.21338 [DOI] [PubMed] [Google Scholar]
- 2.Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. doi: 10.3322/caac.21262 [DOI] [PubMed] [Google Scholar]
- 3.Kassahun WT. Contemporary management of fibrolamellar hepatocellular carcinoma: diagnosis, treatment, outcome, prognostic factors, and recent developments. World J Surg Oncol. 2016;14(1):151. doi: 10.1186/s12957-016-0903-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhao W, Dong M, Pan J, et al. Circular RNAs: a novel target among noncoding RNAs with potential roles in malignant tumors. Mol Med Rep. 2019;20(4):3463–3474. doi: 10.3892/mmr.2019.10637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen -L-L. The biogenesis and emerging roles of circular RNAs. Nat Rev Mol Cell Biol. 2016;17(4):205–211. doi: 10.1038/nrm.2015.32 [DOI] [PubMed] [Google Scholar]
- 6.You X, Vlatkovic I, Babic A, et al. Neural circular RNAs are derived from synaptic genes and regulated by development and plasticity. Nat Neurosci. 2015;18(4):603–610. doi: 10.1038/nn.3975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holdt LM, Stahringer A, Sass K, et al. Circular non-coding RNA ANRIL modulates ribosomal RNA maturation and atherosclerosis in humans. Nat Commun. 2016;7(3):12429. doi: 10.1038/ncomms12429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yuan W, Peng S, Wang J, et al. Identification and characterization of circRNAs as competing endogenous RNAs for miRNA-mRNA in colorectal cancer. Peer J. 2019;7:e7602. doi: 10.7717/peerj.7602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Peer G, Mets E, Claeys S, et al. A high-throughput 3ʹ UTR reporter screening identifies microRNA interactomes of cancer genes. PLoS One. 2018;13(3):e0194017. doi: 10.1371/journal.pone.0194017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fan N, Zhang J, Cheng C, et al. MicroRNA-384 represses the growth and invasion of non-small-cell lung cancer by targeting astrocyte elevated gene-1/Wnt signaling. Biomed Pharmacother. 2017;95:1331–1337. doi: 10.1016/j.biopha.2017.08.143 [DOI] [PubMed] [Google Scholar]
- 11.Wang Y, Xue J, Kuang H, et al. MicroRNA-1297 inhibits the growth and metastasis of colorectal cancer by suppressing cyclin D2 expression. DNA Cell Biol. 2017;36(11):991–999. doi: 10.1089/dna.2017.3829 [DOI] [PubMed] [Google Scholar]
- 12.Lakner MA. microRNAs: fad or future of liver disease. World J Gastroenterol. 2011;17(20):2536–2542. doi: 10.3748/wjg.v17.i20.2536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown DI, Griendling KK. Nox proteins in signal transduction. Free Radic Biol Med. 2009;47(9):1239–1253. doi: 10.1016/j.freeradbiomed.2009.07.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crosas-Molist E, Fabregat I. Role of NADPH oxidases in the redox biology of liver fibrosis. Redox Biol. 2015;6:106–111. doi: 10.1016/j.redox.2015.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carmona-Cuenca I, Roncero C, Sancho P, et al. Upregulation of the NADPH oxidase NOX4 by TGF-beta in hepatocytes is required for its pro-apoptotic activity. J Hepatol. 2008;49(6):965–976. doi: 10.1016/j.jhep.2008.07.021 [DOI] [PubMed] [Google Scholar]
- 16.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 17.Bruix J, Reig M, Sherman M. Evidence-based diagnosis, staging, and treatment of patients with hepatocellular carcinoma. Gastroenterology. 2016;150(4):835–853. doi: 10.1053/j.gastro.2015.12.041 [DOI] [PubMed] [Google Scholar]
- 18.Fong ZV, Tanabe KK. The clinical management of hepatocellular carcinoma in the United States, Europe, and Asia: a comprehensive and evidence-based comparison and review. Cancer. 2014;120(18):2824–2838. doi: 10.1002/cncr.28730 [DOI] [PubMed] [Google Scholar]
- 19.Chen J, Li Y, Zheng Q, et al. Circular RNA profile identifies circPVT1 as a proliferative factor and prognostic marker in gastric cancer. Cancer Lett. 2017;388:208–219. doi: 10.1016/j.canlet.2016.12.006 [DOI] [PubMed] [Google Scholar]
- 20.Li Z, Huang C, Bao C, et al. Exon-intron circular RNAs regulate transcription in the nucleus. Nat Struct Mol Biol. 2015;22(3):256–264. doi: 10.1038/nsmb.2959 [DOI] [PubMed] [Google Scholar]
- 21.Zhang B, Chen M, Jiang N, et al. A regulatory circuit of circ-MTO1/miR-17/QKI-5 inhibits the proliferation of lung adenocarcinoma. Cancer Biol Ther. 2019;20(8):1127–1135. doi: 10.1080/15384047.2019.1598762 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 22.Gan M, Zheng T, Shen L, et al. Genistein reverses isoproterenol-induced cardiac hypertrophy by regulating miR-451/TIMP2. Biomed Pharmacother. 2019;112:108618. doi: 10.1016/j.biopha.2019.108618 [DOI] [PubMed] [Google Scholar]
- 23.Suttamanatwong S. MicroRNAs in bone development and their diagnostic and therapeutic potentials in osteoporosis. Connect Tissue Res. 2017;58(1):90–102. doi: 10.3109/03008207.2016.1139580 [DOI] [PubMed] [Google Scholar]
- 24.Liu Z, Chen JY, Zhong Y, et al. lncRNA MEG3 inhibits the growth of hepatocellular carcinoma cells by sponging miR-9-5p to upregulate SOX11. Braz J Med Biol Res. 2019;52:10. doi: 10.1590/1414-431x20198631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bedard K, Krause K-H. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87(1):245–313. [DOI] [PubMed] [Google Scholar]
- 26.De Mochel NSR, Seronello S, Wang SH, et al. Hepatocyte NAD (P) H oxidases as an endogenous source of reactive oxygen species during hepatitis C virus infection. Hepatology. 2010;52(1):47–59. doi: 10.1002/hep.23671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiang JX, Chen X, Serizawa N, et al. Liver fibrosis and hepatocyte apoptosis are attenuated by GKT137831, a novel NOX4/NOX1 inhibitor in vivo. Free Radic Biol Med. 2012;53(2):289–296. doi: 10.1016/j.freeradbiomed.2012.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu CL, Qiu JL, Huang PZ, et al. NADPH oxidase DUOX1 and DUOX2 but not NOX4 are independent predictors in hepatocellular carcinoma after hepatectomy. Tumor Biol. 2011;32(6):1173–1182. doi: 10.1007/s13277-011-0220-3 [DOI] [PubMed] [Google Scholar]
- 29.Crosas-Molist E, Bertran E, Sancho P, et al. The NADPH oxidase NOX4 inhibits hepatocyte proliferation and liver cancer progression. Free Radic Biol Med. 2014;69:338–347. doi: 10.1016/j.freeradbiomed.2014.01.040 [DOI] [PubMed] [Google Scholar]
- 30.Sang YH, Paik YH, Yang JW, et al. NADPH oxidase 1 and NADPH oxidase 4 have opposite prognostic effects for patients with hepatocellular carcinoma after hepatectomy. Gut Liver. 2016;10(5):826–835. doi: 10.5009/gnl15543 [DOI] [PMC free article] [PubMed] [Google Scholar]