Abstract
BACKGROUND/OBJECTIVES
Brain aging is a major risk factor for severe neurodegenerative diseases. Conversely, L-histidine and L-carnosine are known to exhibit neuroprotective effects. The aim of this study was to examine the potential for L-histidine, L-carnosine, and their combination to mediate anti-brain aging effects in neuronal cells subjected to D-galactose-induced aging.
MATERIALS/METHODS
The neuroprotective potential of L-histidine, L-carnosine, and their combination was examined in a retinoic acid-induced neuronal differentiated SH-SY5Y cell line exposed to D-galactose (200 mM) for 48 h. Neuronal cell proliferation, differentiation, and expression of anti-oxidant enzymes and apoptosis markers were subsequently evaluated.
RESULTS
Treatment with L-histidine (1 mM), L-carnosine (10 mM), or both for 48 h efficiently improved the proliferation, neurogenesis, and senescence of D-galactose-treated SH-SY5Y cells. In addition, protein expression levels of both neuronal markers (β tubulin-III and neurofilament heavy protein) and anti-oxidant enzymes, glutathione peroxidase-1 and superoxide dismutase-1 were up-regulated. Conversely, protein expression levels of amyloid β (1-42) and cleaved caspase-3 were down-regulated. Levels of mRNA for the pro-inflammatory cytokines, interleukin (IL)-8, IL-1β, and tumor necrosis factor-α were also down-regulated.
CONCLUSIONS
To the best of our knowledge, we provide the first evidence that L-histidine, L-carnosine, and their combination mediate anti-aging effects in a neuronal cell line subjected to D-galactose-induced aging. These results suggest the potential benefits of L-histidine and L-carnosine as anti-brain aging agents and they support further research of these amino acid molecules.
Keywords: Histidine, carnosine, brain, aging, neurogenesis
INTRODUCTION
Brain aging is a worldwide concern since it has been identified as the most critical risk factor for severe neurodegenerative diseases, including Alzheimer's disease and Parkinson's disease [1]. As a person ages, cells in the brain experience increased oxidative stress and metabolic impairment which can result in protein aggregation and DNA damage. These changes can subsequently manifest as increased anxiety and impaired cognitive function. In addition, the rate of neurogenesis decreases with age [2]. Both neuronal differentiation/neurite outgrowth and proliferation are important processes for neurogenesis [3,4]. Neurogenesis can be identified by analyzing β-tubulin III, a neuronal cell marker, and neurofilament heavy protein (NEFH), a major component of the neuronal cytoskeleton [5].
Aged brain tissue is characterized by down-regulation of major anti-oxidant enzymes, including superoxide dismutase (SOD) and glutathione peroxidase (GPX) [6]. It has been shown that plaques composed of aggregated amyloid β (Aβ) are leading causes of Alzheimer's disease pathology. Aβ is produced following cleavage of amyloid protein precursor (APP) by pro-apoptotic enzymes, such as caspases [7]. Brain aging has also been directly associated with neurodegenerative diseases characterized by increased chronic neuro-inflammation [8]. Correspondingly, higher levels of interleukin (IL)-8, IL-1β, and tumor necrosis factor (TNF)-α have been detected in aged animal brains [9,10].
D-galactose is a reducing sugar which converts to aldose and hydrogen peroxide when galactose oxidase presents at high concentrations. These by-products increase oxidative stress and accelerate the aging process [11]. A continuous low dose administration of D-galactose in mice has shown to induce changes which resemble accelerated brain aging [12,13]. In particular, aging of various types of brain cells, including astrocytes [14], neuronal cells [15], and neuroblastoma cells [16,17] have been observed in these models.
L-histidine is a common amino acid which is conditionally essential [18] and is a strong scavenger of hydroxyl radicals and singlet oxygen [19,20]. In a previous study, supplementation with 1-10 mM L-histidine extended the lifespan of Caenorhabditis elegans [21]. L-histidine has shown to protect against diseases related to brain aging such as brain infarction [22], cerebral ischemia [23], brain edema [24], sciatic nerve lesions, and neuropathic pain [25]. In rats with acute liver failure, administration of L-histidine increased the anti-oxidant capacities of the brain cerebral cortex [26]. Moreover, L-histidine has shown to significantly attenuate ammonia-induced neurotoxicity by blocking glutamine transport into mitochondria in primary cultures of astrocyte [27].
L-carnosine is a natural dipeptide composed of β-alanine and L-histidine and it is present in human muscle and brain tissues [28]. Studies in male Drosophila melanogaster flies and senescence-accelerated mice have shown that L-carnosine supplementation can efficiently increase lifespan [29,30]. Previous studies have also demonstrated that L-carnosine could exert neuroprotective effects against toxicity induced by N-methyl-D-aspartate, zinc, rotenone, and Aβ [31,32,33,34]. Moreover, L-carnosine attenuated early brain injuries by regulating oxidative stress and apoptosis in rats [35] and it was able to rescue cognitive decline induced by a high-fat diet in AD mice [36].
It has been demonstrated in previous reports that L-carnosine and L-histidine exert neuroprotective effects by activating the carnosine-histidine-histamine pathway [31,33]. Moreover, carnosine is a dipeptide molecule which contains histidine [37]. However, while their close connection in terms of brain function and physiology is well-established, the neuroprotective effects of L-histidine and L-carnosine in combination remain poorly understood.
Despite these insights into the function of L-histidine and L-carnosine, their potential to mediate anti-aging effects in D-galactose-induced brain aging models has not been characterized. Therefore, in the present study, D-galactose-induced aging in retinoic acid (RA)-induced neuronal differentiated SH-SY5Y cells was investigated following exposure to L-histidine, L-carnosine, and a combination of both. In particular, brain neuronal regeneration, Aβ (1-42) production, apoptosis, antioxidation, and inflammation were examined.
MATERIALS AND METHODS
Cell culture and reagents
A human neuroblastoma cell line, SH-SY5Y, was purchased from American Type Culture Collection (ATCC, Manassas, VA, USA). SH-SY5Y cells were maintained in a 1:1 mixture of Minimum Essential Medium and Ham's F-12 (Welgene, Daegu, Korea) supplemented with 10% fetal bovine serum (FBS; Hyclone, Logan, UT, USA) and 1% penicillin (100 U/mL)/streptomycin (100 µg/mL) (Invitrogen, Carlsbad, CA, USA). Cells were incubated in 5% CO2 at 37°C.
All-trans-retinoic acid (RA), D-galactose, L-histidine, and L-carnosine were purchased from Sigma-Aldrich (St. Louis, MO, USA). SH-SY5Y cells were treated with 10 µM RA for 6 d to differentiate them into neuronal cells. Stock preparations and treatments of D-galactose were performed under dim light due to light sensitivity of this sugar.
MTT assay
Neuronal proliferation was evaluated in MTT assays as described previously [38]. Briefly, differentiated SH-SY5Y cells were seeded onto 96-well plates (4 × 104 cells/well). Twenty-four hours later, the cells were treated with 200 mM D-galactose, 1 mM L-histidine, and 10 mM L-carnosine both individually, and in combination in media without FBS. After 48 h, media was removed and then cells were media containing MTT (500 µg/mL). After 4 h in 5% CO2 at 37°C, the supernatant of each well was discarded. Remaining formazan crystals were dissolved in 100 µL DMSO and absorbance values at 560 nm were recorded with a microplate reader (Molecular Devices, Sunnyvale, CA, USA).
Measurement of neurite length
Differentiated SH-SY5Y cells were treated with 200 mM D-galactose, 1 mM L-histidine and 10 mM L-carnosine individually and in combination in media without FBS for 48 h. Digital images of randomly chosen fields of cells were obtained with a light microscope (Olympus, Tokyo, Japan). Neurite length was measured with ImageJ software (National Institute of Health, Bethesda, MD, USA).
Assessment of cellular senescence
A commercially available senescence-associated β-galactosidase (SA-β-gal) assay kit was purchased from Cell Signaling Technology (Danvers, MA, USA) to examine cellular senescence. Briefly, differentiated SH-SY5Y cells were plated onto 6-well plates. Twenty-four hours later, cells were treated with 200 mM D-galactose, 1 mM L-histidine, and 10 mM L-carnosine individually, and in combination without FBS. After 48 h, the cells were rinsed with phosphate buffered saline (PBS) and then incubated with a fixative solution for 15 min at room temperature. After the cells were rinsed twice with PBS, they were incubated with β-gal staining solution at 37°C in the absence of CO2 overnight. Cells were imaged at 100x magnification with a light microscope (Olympus). Stained cells were counted in five randomly chosen fields at 100x magnification. The percentage of SA-β-gal-positive cells was calculated as follows: number of blue-stained cells / total number of cells.
Western blotting
Differentiated SH-SY5Y cells were treated with 200 mM D-galactose, 1 mM L-histidine, and 10 mM L-carnosine individually and in combination in the absence of FBS. After 48 h, protein concentrations were measured with a Bio-Rad Protein Assay Kit (Bio-Rad, Hercules, CA, USA). Briefly, protein samples were denatured, subjected to SDS-PAGE electrophoresis, and transferred to polyvinylidene fluoride membranes (Millipore, Billerica, MA, USA). The, membranes were subsequently blocked with 5% bovine serum albumin or non-fat skim milk dissolved in Tris-buffered saline containing Tween 20 (TBS-T) at room temperature. After 1 h, the membranes were incubated with primary antibodies recognizing β-tubulin III (Sigma-Aldrich), NEFH (Sigma-Aldrich), APP (Abcam, Cambridge, UK), Aβ (1-42) (Cell Signaling), GPX-1 (Abfrontier, Seoul, Korea), SOD-1 (Santa Cruz Biotechnology, Dallas, TX, USA), cleaved caspase-3 (Santa Cruz Biotechnology), and β-actin (Abcam) at 4°C overnight. After the membranes were rinsed with TBS-T, they were incubated with secondary antibodies at room temperature for 1 h. To detect bound antibodies, an enhanced chemiluminescence reagent (Animal Genetics Inc., Suwon, Korea) was used and signals were detected on X-ray film.
Reverse transcriptase polymerase chain reaction (RT-PCR)
Differentiated SH-SY5Y cells were treated with 200 mM D-galactose, 1 mM L-histidine and 10 mM L-carnosine individually and in combination in the absence of FBS. After 48 h, RNA was isolated with Trizol reagent (Invitrogen) in accordance with the manufacturer's protocol. Quality confirmed RNA was used to produce cDNA by reverse-transcription with a Revert Aid First Strand cDNA Synthesis Kit (Thermo Fisher Scientific, Waltham, MA, USA). The cDNA generated was amplified with Taq polymerase (TAKARA, Tokyo, Japan) and primers specific for IL-8, IL-1β, TNF-α, and GAPDH as follows :(1) human IL-8: (forward) 5′-TGG CTC TCT TGG CAG CCT TC-3′ and (reverse) 5′-TGC ACC CAG TTT TCC TTG GG-3′ (2) human IL-1β: (forward) 5′-GGA TAT GGA GCA ACA AGT GG-3′ and (reverse) 5′-ATG TAC CAG TTG GGG AAC TG-3′ (3) human TNF-α: (forward) 5′-ACA AGC CTG TAG CCC ATG TT-3′ and (reverse) 5′-AAA GTA GAC CTG CCC AGA CT-3′ (4) human GAPDH (forward) 5′-AG AAG GCT GGG GCT CAT TTG-3′ and (reverse) 5′-AG GGG CCA TCC ACA GTC TTC-3′. PCR products were separated on a 2% agarose gel containing ethidium bromide and visualized under ultra-violet light. GAPDH was detected as an internal control.
Statistical analysis
All results are presented as the mean ± SEM of independent experiments performed at least three times. GraphPad Prism (GraphPad Software, Inc., La Jolla, CA, USA) was used to perform statistical analyses. Statistical significances between groups were determined by using one-way analysis of variance (ANOVA), followed by Newman-Keuls post-hoc test. P-values less than 0.05 were considered statistically significant.
RESULTS
Effects of L-histidine, L-carnosine, and their combination on neuronal cell proliferation
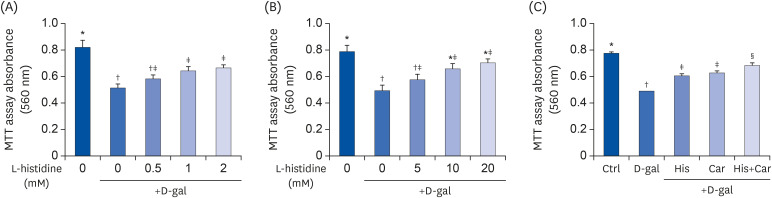
To assess the effects of L-histidine, L-carnosine, and their combination on D-galactose-induced aged and RA-induced neuronal differentiated SH-SY5Y cell proliferation, MTT assays were performed. Cell proliferation was increased when the cells were treated with 1 mM (25.3%, P < 0.05) or 2 mM (29.0%, P < 0.05) L-histidine compared with the D-gal group (Fig. 1A). Similarly, L-carnosine treatment significantly increased cell proliferation at doses of 10 mM and 20 mM compared to the D-galgroup (33.0% and 42.1%, respectively; each P < 0.05) (Fig. 1B).
Fig. 1. Effects of L-histidine, L-carnosine, and their combination on proliferation.
The neuronal proliferation was assessed by MTT assay. (A) L-histidine and (B) L-carnosine were treated with various concentrations for 48 h. (C) His, Car, and His+Car were treated for 48 h and assessed by MTT assay. The values shown are the mean ± standard error of the mean (n = 3–4).
Ctrl, Control; D-gal, 200 mM D-galactose; His, 1 mM L-histidine; Car, 10 mM L-carnosine; His+Car, 1 mM L-histidine + 10 mM L-carnosine.
*,†,‡,§Different superscript marks indicate significant differences between groups (P < 0.05).
We performed MTT assays to detect neuronal cell proliferation and to select suitable concentrations of L-histidine and L-carnosine for further experiments. In our preliminary experiments, differentiated SH-SY5Y cells were treated with L-histidine and L-carnosine at concentrations ranging from 0.5 mM to 50 mM for 48 h in the presence of D-galactose. Both L-histidine and L-carnosine improved neuronal proliferation in a dose-dependent manner. Statistically, significant effects on proliferation were observed between 1 mM and 10 mM, respectively. Thus, L-histidine exerted a 10-fold higher neuroprotective effect compared to L-carnosine in MTT assays.
Since our goal was to investigate effects of L-histidine and L-carnosine in combination against brain aging, we attempted to identify the minimum concentration exhibiting the similar neuroprotective effects. In addition, we performed MTT assays with various combination doses of L-histidine and L-carnosine. We analyzed neuronal cell proliferations in the presence of 1 mM L-histidine + 10 mM L-carnosine and in the presence of 2 mM L-histidine + 20 mM L-carnosine combinations. Both combination treatments exhibited significant synergistic effects compared with each treatment component alone. In contrast, 0.5 mM L-histidine + 5 mM L-carnosine in MTT assays did not show any additive effects. The combination of 1 mM L-histidine + 10 mM L-carnosine showed similar neuroprotective effects as the combination of 2 mM L-histidine + 20 mM L-carnosine (data not shown). Thus, these concentrations were used for L-histidine and L-carnosine in all subsequent experiments.
As shown in Fig. 1C, neuronal cell proliferation was significantly inhibited by D-galactose treatment compared with the Ctrl group (36.64%, P < 0.001). In contrast, L-histidine and L-carnosine treatments increased cell proliferation by 23.7% (P < 0.001) and 27.2% (P < 0.001), respectively, compared with the D-gal group. Furthermore, treatment with the combination of L-histidine and L-carnosine showed the largest increase in proliferation among the three groups (39.9%, P < 0.001).
Effects of L-histidine, L-carnosine, and their combination on neuronal cell regeneration
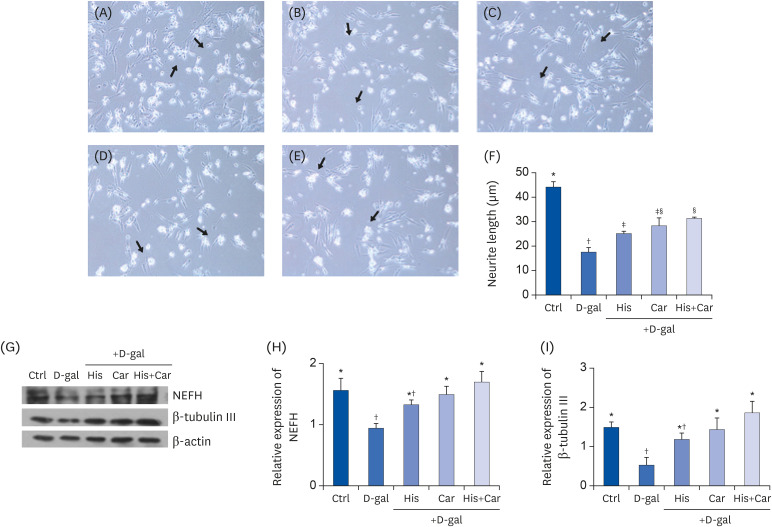
To characterize neuronal regeneration in RA-induced differentiated SH-SY5Y cells, average neurite length was measured. Compared with the Ctrl group, D-galactose treatment significantly decreased average neurite length. In contrast, treatment with L-histidine (40.80%, P < 0.01), L-carnosine (58.9%, P < 0.01), or both treatment group (76.23%, P < 0.001) significantly increased average neurite length compared with the D-gal group (Fig. 2A).
Fig. 2. Effects of L-histidine, L-carnosine, and their combination on neuronal cell regenerations.
Representative pictures for each group (100× magnification). (A) Ctrl, (B) D-gal, (C) His, (D) Car, and (E) His+Car. (F) The average length of the neurites of differentiated SH-SY5Y cells for each group was analyzed. ImageJ software was used to measure the individual neurite length. The protein expressions of an axonal marker, NEFH, and a neuronal marker, β-tubulin III were determined by Western blotting and β-actin was used as a loading control. (G) Representative blots are shown. Quantification of NEFH (H) and β-tubulin III (I) levels to β-actin are shown. The values shown are the mean ± standard error of the mean (n = 3–4).
NEFH, Neurofilament heavy polypeptide; Ctrl, Control; D-gal, 200 mM D-galactose; His, 1 mM L-histidine; Car, 10 mM L-carnosine; His+Car, 1 mM L-histidine + 10 mM L-carnosine.
*,†,‡,§Different superscript marks indicate significant differences between groups (P < 0.05).
NEFH is a major component of axonal cytoskeleton proteins [39] and β-tubulin III is a neuronal marker [40]. Thus, both of these proteins are widely used in neurobiology research. Here, NEFH and β-tubulin III were analyzed to investigate possible effects of L-histidine, L-carnosine, and their combination on neuronal regeneration (Fig. 2B). Expression levels of NEFH and β-tubulin III were 39.6% (P < 0.05) and 64.3% (P < 0.05) lower, respectively, in the D-gal group compared with the Ctrl group (Fig. 2C). In contrast, NEFH and β-tubulin III expression levels were higher following treatment with L-carnosine and following treatment with the L-carnosine and the combination than in the D-gal group. L-histidine also tended to increase the expression of NEFH and β-tubulin III, although not significantly in either case.
Effects of L-histidine, L-carnosine, and their combination on cellular senescence and levels of APP and Aβ (1-42) in neuronal cells
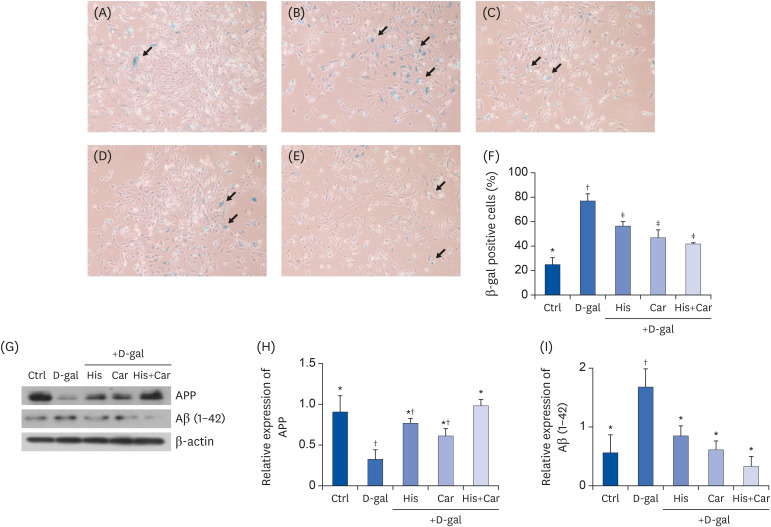
The SA-β-gal assay is a widely used method for verifying the presence of senescent cells [41]. We performed SA-β-gal staining to detect aging cells (Fig. 3A), the D-gal group exhibited 3-fold increase in the number of SA-β-gal positive cells compared to the Ctrl group (P < 0.001). Conversely, the numbers of SA-β-gal positive cells following treatment with L-histidine, L-carnosine, and a combination of both were significantly decreased compared with the D-gal group (His, P < 0.05; Car, P < 0.01; both, P < 0.01).
Fig. 3. Effects of L-histidine, L-carnosine, and their combination on cellular senescence and levels of APP and Aβ (1-42) in neuronal cells.
SA-β-gal assay was performed to investigate cellular senescence. Representative images for each group at 100× magnification were shown. (A) Ctrl, (B) D-gal, (C) His, (D) Car (E) His+Car. (F) % of SA-β-gal positive cells in total counted cells. The protein expressions of APP and Aβ (1-42) were determined by Western blotting and β-actin was used as a loading control. (G) Representative blots are represented. Quantification of APP (H) and Aβ (1-42) (I) levels to β-actin are shown. The values shown are the mean ± standard error of the mean (n = 3–4).
APP, Amyloid β precursor protein; Aβ (1-42), Amyloid β (1-42); SA-β-gal, Senescence-associated β-galactosidase; Ctrl, Control; D-gal, 200 mM D-galactose; His, 1 mM L-histidine; Car, 10 mM L-carnosine; His+Car, 1 mM L-histidine + 10 mM L-carnosine.
*,†,‡Different superscript marks indicate significant differences between groups (P < 0.05).
Western blotting was also performed to determine whether treatment with L-histidine, L-carnosine, or their combination affected protein levels of APP and Aβ (1-42) in RA-differentiated SH-SY5Y cells (Fig. 3B). D-galactose treatment significantly down-regulated levels of APP (P < 0.05), whereas treatment with L-histidine and L-carnosine inhibited this decrease (P < 0.05). In addition, D-galactose treatment increased protein expression of Aβ (1-42) by 50% compared to the Ctrl group (P < 0.05). When cells were treated with L-histidine, L-carnosine, or their combination significant down-regulation of Aβ (1-42) expression by 50%, 64%, and 81%, respectively, was observed (His and Car, P < 0.05; His+Car, P < 0.01). Taken together, these results indicate that L-histidine and L-carnosine, when administered individually or in combination, they suppress D-galactose-induced aging-related Aβ formation.
Effects of L-histidine, L-carnosine, and their combination on expression of anti-oxidant enzymes and caspase-3 in neuronal cells
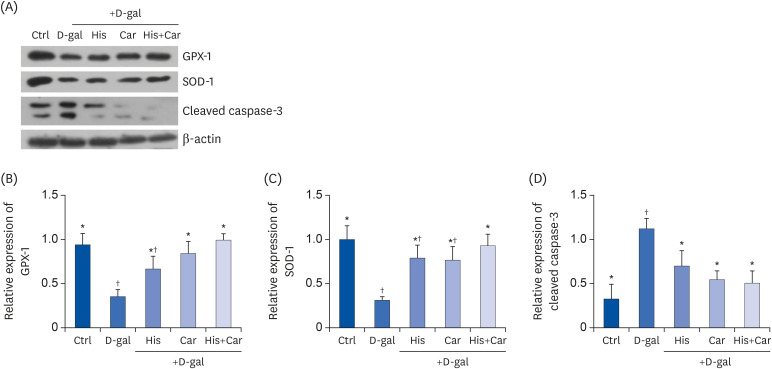
As a person ages, their brain experiences increased oxidative stress, metabolic impairment, and DNA damage [42]. In the present study, treatment with D-galactose decreased levels of the antioxidant enzymes, GPX-1 (P < 0.05) and SOD-1 (P < 0.05), and increased levels of the apoptosis marker, cleaved caspase-3 (P < 0.01) compared with the Ctrl group (Fig. 4A). In contrast, treatment with L-histidine, L-carnosine, or their combination recovered levels of expression of these proteins from D-galactose-induced decreases. For example, both L-carnosine and the combination of L-histidine and L-carnosine protected the level of GPX-1 which was down-regulated by D-galactose (P < 0.05 for both, respectively). Meanwhile, the level of cleaved caspase-3 was significantly down-regulated following treatment with L-histidine (37.2%, P < 0.05), L-carnosine (51.2%, P < 0.05), and their combination (54.6%, P < 0.05) compared to the D-gal group. Overall, these results suggest that L-histidine, L-carnosine, and their combination enhance anti-oxidation and inhibit apoptosis against D-galactose-induced aging in differentiated SH-SY5Y cells.
Fig. 4. Effects of L-histidine, L-carnosine, and their combination on anti-oxidant enzymes and caspase-3.
The protein expressions of GPX-1, SOD-1, and cleaved caspase-3 were determined by Western blotting and β-actin was used as a loading control. (A) Representative blots are represented (left panels). Quantification of (B) GPX-1, (C) SOD-1, and (D) Cleaved caspase-3 levels to β-actin are shown. The values shown are the mean ± standard error of the mean (n = 3–4).
GPX-1, glutathione peroxidase-1; SOD-1, superoxide dismutase-1; Ctrl, Control; D-gal, 200 mM D-galactose; His, 1 mM L-histidine; Car, 10 mM L-carnosine; His+Car, 1 mM L-hisitidne + 10 mM L-carnosine.
*,†Different superscript marks indicate significant differences between groups (P < 0.05).
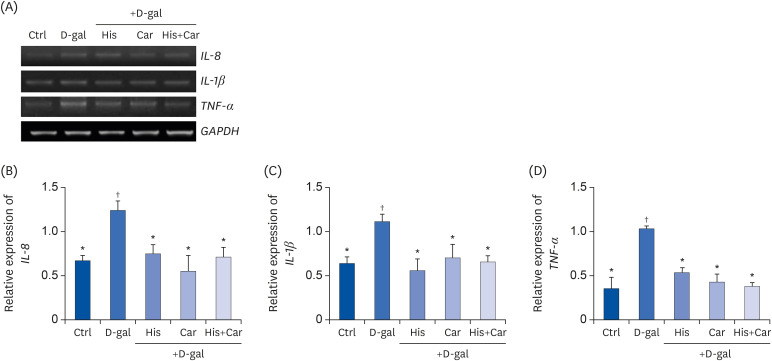
Effects of L-histidine, L-carnosine, and their combination on pro-inflammatory cytokines in neuronal cells
High-levels of chronic inflammation strongly contribute to the cognitive decline associated with brain aging. In particular, IL-8, IL-1β, and TNF-α are major inflammatory cytokines which are involved in the initiation and orchestration of neuro-inflammatory responses [43,44]. To investigate the anti-inflammatory effects of L-histidine and L-carnosine both individually and in combination on D-galactose-induced inflammation, mRNA levels of IL-8, IL-1β, and TNF-α were analyzed in RA-differentiated SH-SY5Y cells (Fig. 5A). Following D-galactose treatment, mRNA levels of all three cytokines were up-regulated (IL-8 and IL-1β, P < 0.05; TNF-α, P < 0.001). In contrast, following treatment with L-histidine, L-carnosine, or their combination levels of IL-8, IL-1β, and TNF-α were restored to the levels observed in the Ctrl group.
Fig. 5. Effects of L-histidine, L-carnosine, and their combination on pro-inflammatory cytokines in neuronal cells.
The mRNA expressions of IL-8, IL-1β, and TNF-α were determined by RT-PCR and GAPDH was used as a loading control. (A) Representative blots are shown. Quantification of (B) IL-8, (C) IL-1β, and (D) TNF-α levels to GAPDH are shown. The values shown are the mean ± SE of the mean (n = 3–4).
Ctrl, Control; D-gal, 200 mM D-galactose; His, 1 mM L-histidine; Car, 10 mM L-carnosine; His+Car, 1 mM L-hisitidne + 10 mM L-carnosine.
*,†Different superscript marks indicate significant differences between groups (P < 0.05).
DISCUSSION
In the present study, L-histidine, L-carnosine, and their combination exhibited anti-brain aging effects against D-galactose-induced aging in RA-differentiated SH-SY5Y cells. For example, these three treatments inhibited cellular senescence by restoring aging-related impairments. In addition, these treatments increased neurogenesis, neuronal proliferation, and expression levels of anti-oxidant enzyme, while also blocking Aβ formation, apoptosis, and proinflammatory mediators.
Both L-histidine and L-carnosine have been implicated as potent neuroprotective amino acids. Their ability to cross the blood brain barrier further supports this role [45]. L-histidine can penetrate blood brain barrier through a Na+-independent neutral amino acid transporter (system L) [46], whereas carnosine crosses blood brain barrier by passive diffusion. That's because its permeabilities are very low and similar to those of mannitol which has similar molecular weight with carnosine [47,48]. Since L-histidine and L-carnosine do not compete each other when crossing the blood brain barrier, the combination treatments of L-histidine and L-carnosine would still enable themselves to be transported from blood to brain intact.
Daily consumption of histidine has shown to significantly improve working memory task capacity, clear thinking, and attentiveness [49]. Conversely, insufficient intake of L-histidine leads to diminished histamine levels in the brain. In mice, the latter is associated with anxiety-like behaviors [50]. Anti-brain aging effects of carnosine in a D-galactose-induced mouse model have also been reported [51]. For example, L-carnosine alleviated aging-induced increases in brain mitochondrial monoamine oxidase-A activity [52]. In addition, L-carnosine exerts neuroprotective effects via modulation of the HO-1/Hsp72 system and by reducing neuronal damage caused by oxidative stress [53].
The anti-brain aging properties of L-histidine and L-carnosine are closely related. This is consistent with L-carnosine being a component of L-histidine and β-alanine, thereby potentially providing similar physiological properties between L-histidine and L-carnosine [54]. Decker et al. [55] previously observed that carnosine was able to scavenge peroxyl radicals to a similar level as achieved by L-histidine. However, β-alanine did not exhibit the same effect. These results suggest that the radical scavenging capacity of carnosine is mainly dependent on L-histidine residue, especially its imidazole ring structure [55,56]. Furthermore, considering that administration of L-carnosine increases the availability of L-histidine, neuroprotective activities of L-carnosine appear to be mediated by a carnosine-histidine-histamine signaling pathway in differentiated PC12 cells [31] and via rotenone-induced oxidative stress in brain microvascular endothelial cells [33]. L-histidine may be further converted into histamine, which has exhibited neuroprotective effects in both a neurodegenerative-induced hamster model [57] and a brain infarction model in rats [22]. Hiraga et al. [58] has suggested that L-histidine exerts its protective effects on ischemic events by facilitating the central histaminergic pathway which consequently inhibits recruitment of inflammatory cell by the histamine H2 receptor. However, conflicting reports have described that the neuroprotective activity of L-carnosine is independent of the histidine-histamine pathway, despite a partial role observed for the histamine H1 receptor [59,60].
In the present study, D-galactose exposure significantly increased expression of Aβ (1-42) in differentiated SH-SY5Y cells. This effect was inhibited by treatment with L-histidine, L-carnosine, and their combination. L-carnosine disrupts hydrogen bond networks in the central hydrophobic cluster of Aβ (1-42). As a result, L-carnosine is able to inhibit aggregation of Aβ (1-42) [61]. In the present study, treatment with D-galactose increased Aβ formation. APP is a precursor of Aβ, and its APP cleavage can occur via amyloidogenic or non-amyloidogenic pathways. The former generates neurotoxic Aβ, whereas the latter blocks Aβ production and generates sAPPα, a molecule with neuroprotective functions [62,63]. Based on these insights, we hypothesize that D-galactose up-regulates β-secretase and γ-secretase activities to generate Aβ from APP. Furthermore, these effects appear to be inhibited by L-histidine, L-carnosine, and their combination. Thus, additional studies are needed to measure and analyze levels of α, β, and γ secretases, as well as sAPPα, in order to confirm this hypothesis.
Consistent with the present results, previous in vivo studies have demonstrated that D-galactose induces down-regulation of anti-oxidant enzymes such as SOD and GPX [64], and up-regulation of the apoptosis marker, cleaved caspase-3 [65] in brain. L-histidine, L-carnosine, and their combination were found to efficiently restore expression of SOD and GPX, and to down-regulate the level of cleaved caspase-3 in the present study. L-histidine has exhibited anti-oxidant potential in brain and other tissues types by increasing the total anti-oxidant capacity of these tissues [66]. In addition, L-histidine has been found to increase the activity and mRNA level of GPX in the brain cerebral cortex in rat models of acute liver failure [26]. L-histidine has also been found to increase levels of mitochondrial glutathione (mGSH), a critical cell defense factor against oxidative stress, and GSH content in the prefrontal cortex of rats with acute hepatic encephalopathy [67]. It should be noted that the present results are also consistent with those of Aydin et al. [51]. The latter demonstrated that carnosine supplementation (250 mg/kg) significantly alleviates D-galactose-induced brain oxidative stress by regulating SOD and GPX activities, as well as the levels of cleaved caspase-3. In another study, carnosine (40 mg/kg) supplementation was found to improve GPX levels and oxygen radical anti-oxidant capacity in BALB/c mice receiving D-galactose [68].
A combination treatment of L-histidine and L-carnosine did not produce an additive effect, except in neuronal proliferation and neurite length analyses where the combination treatment induced a significantly better effect than L-histidine or L-carnosine alone. L-histidine and β-alanine which are precursors of carnosine, are able to cross the blood-brain barrier (BBB) [69] and undergo synthesis into carnosine in the forebrain, particularly in the olfactory bulbs [70,71]. Despite the ability of carnosine to cross the BBB, most of the carnosine present in the brain originates from de novo synthesis rather than carnosine passing through the BBB [72]. Therefore, the combination treatment of L-histidine and L-carnosine may not produce a synergistic effect because their concentrations in the brain are tightly regulated through break down and synthesis reactions. Thus, their potentially additive effects may be inhibited. However, further studies are needed to investigate this hypothesis.
In conclusion, L-histidine and L-carnosine, both individually and combination, efficiently exerted protective effects in D-galactose-induced senescent neuronal cells in the present study. Specifically, these treatments improved neuronal proliferation, differentiation, anti-oxidation, apoptosis, and antiinflammation against D-galactose-induced neuronal impairment. These results support further studies of these amino acids as candidates for the treatment and prevention of age-related brain damage. In particular, the additional in vivo animal studies and clinical trials are necessary to confirm the neuroprotective effects of L-histidine, L-carnosine, and their combination in aged brains.
Footnotes
Conflict of Interest: The authors declare no potential conflicts of interests.
- Conceptualization: Kim Y2.
- Formal analysis: Kim Y1.
- Funding acquisition: Kim Y2.
- Investigation: Kim Y2.
- Supervision: Kim Y2.
- Writing - original draft: Kim Y1.
- Writing - review & editing: Kim Y2.
1Kim Y, Yerin Kim; 2Kim Y, Yuri Kim.
References
- 1.Gandhi S, Abramov AY. Mechanism of oxidative stress in neurodegeneration. Oxid Med Cell Longev. 2012;2012:428010. doi: 10.1155/2012/428010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mattson MP, Magnus T. Ageing and neuronal vulnerability. Nat Rev Neurosci. 2006;7:278–294. doi: 10.1038/nrn1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ohnuma S, Harris WA. Neurogenesis and the cell cycle. Neuron. 2003;40:199–208. doi: 10.1016/s0896-6273(03)00632-9. [DOI] [PubMed] [Google Scholar]
- 4.Khodosevich K, Monyer H. Signaling involved in neurite outgrowth of postnatally born subventricular zone neurons in vitro. BMC Neurosci. 2010;11:18. doi: 10.1186/1471-2202-11-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park MH, Son DJ, Nam KT, Kim SY, Oh SY, Song MJ, Chun HO, Lee TH, Hong JT. 2016 [Google Scholar]
- 6.Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 7.Zhang YW, Thompson R, Zhang H, Xu H. APP processing in Alzheimer's disease. Mol Brain. 2011;4:3. doi: 10.1186/1756-6606-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yin F, Sancheti H, Patil I, Cadenas E. Energy metabolism and inflammation in brain aging and Alzheimer's disease. Free Radic Biol Med. 2016;100:108–122. doi: 10.1016/j.freeradbiomed.2016.04.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tha KK, Okuma Y, Miyazaki H, Murayama T, Uehara T, Hatakeyama R, Hayashi Y, Nomura Y. Changes in expressions of proinflammatory cytokines IL-1beta, TNF-alpha and IL-6 in the brain of senescence accelerated mouse (SAM) P8. Brain Res. 2000;885:25–31. doi: 10.1016/s0006-8993(00)02883-3. [DOI] [PubMed] [Google Scholar]
- 10.McLarnon JG. Chemokine interleukin-8 (IL-8) in Alzheimer's and other neurodegenerative diseases. J Alzheimers Dis Parkinsonism. 2016;6:273. [Google Scholar]
- 11.Song X, Bao M, Li D, Li YM. Advanced glycation in D-galactose induced mouse aging model. Mech Ageing Dev. 1999;108:239–251. doi: 10.1016/s0047-6374(99)00022-6. [DOI] [PubMed] [Google Scholar]
- 12.Lu J, Zheng YL, Luo L, Wu DM, Sun DX, Feng YJ. Quercetin reverses D-galactose induced neurotoxicity in mouse brain. Behav Brain Res. 2006;171:251–260. doi: 10.1016/j.bbr.2006.03.043. [DOI] [PubMed] [Google Scholar]
- 13.Shwe T, Pratchayasakul W, Chattipakorn N, Chattipakorn SC. Role of D-galactose-induced brain aging and its potential used for therapeutic interventions. Exp Gerontol. 2018;101:13–36. doi: 10.1016/j.exger.2017.10.029. [DOI] [PubMed] [Google Scholar]
- 14.Shen Y, Gao H, Shi X, Wang N, Ai D, Li J, Ouyang L, Yang J, Tian Y, Lu J. Glutamine synthetase plays a role in D-galactose-induced astrocyte aging in vitro and in vivo. Exp Gerontol. 2014;58:166–173. doi: 10.1016/j.exger.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 15.Hsieh HM, Wu WM, Hu ML. Genistein attenuates D-galactose-induced oxidative damage through decreased reactive oxygen species and NF-κB binding activity in neuronal PC12 cells. Life Sci. 2011;88:82–88. doi: 10.1016/j.lfs.2010.10.021. [DOI] [PubMed] [Google Scholar]
- 16.Liu YY, Nagpure BV, Wong PT, Bian JS. Hydrogen sulfide protects SH-SY5Y neuronal cells against d-galactose induced cell injury by suppression of advanced glycation end products formation and oxidative stress. Neurochem Int. 2013;62:603–609. doi: 10.1016/j.neuint.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 17.Heidari S, Mehri S, Shariaty V, Hosseinzadeh H. Preventive effects of crocin on neuronal damages induced by D-galactose through AGEs and oxidative stress in human neuroblastoma cells (SH-SY5Y) J Pharmacopuncture. 2018;21:18–25. doi: 10.3831/KPI.2018.21.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rassin DK. Essential and non-essential amino acids in neonatal nutrition. Nestle Nutr Workshop Ser. 1994;33:183–195. [Google Scholar]
- 19.Cai Q, Takemura G, Ashraf M. Antioxidative properties of histidine and its effect on myocardial injury during ischemia/reperfusion in isolated rat heart. J Cardiovasc Pharmacol. 1995;25:147–155. doi: 10.1097/00005344-199501000-00023. [DOI] [PubMed] [Google Scholar]
- 20.Matheson IB, Lee J. Chemical reaction rates of amino acids with singlet oxygen. Photochem Photobiol. 1979;29:879–881. [Google Scholar]
- 21.Edwards C, Canfield J, Copes N, Brito A, Rehan M, Lipps D, Brunquell J, Westerheide SD, Bradshaw PC. Mechanisms of amino acid-mediated lifespan extension in Caenorhabditis elegans. BMC Genet. 2015;16:8. doi: 10.1186/s12863-015-0167-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adachi N, Liu K, Arai T. Prevention of brain infarction by postischemic administration of histidine in rats. Brain Res. 2005;1039:220–223. doi: 10.1016/j.brainres.2005.01.061. [DOI] [PubMed] [Google Scholar]
- 23.Liao RJ, Jiang L, Wang RR, Zhao HW, Chen Y, Li Y, Wang L, Jie LY, Zhou YD, Zhang XN, Chen Z, Hu WW. Histidine provides long-term neuroprotection after cerebral ischemia through promoting astrocyte migration. Sci Rep. 2015;5:15356. doi: 10.1038/srep15356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rama Rao KV, Reddy PV, Tong X, Norenberg MD. Brain edema in acute liver failure: inhibition by L-histidine. Am J Pathol. 2010;176:1400–1408. doi: 10.2353/ajpath.2010.090756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Farshid AA, Tamaddonfard E, Najafi S. Effects of histidine and n-acetylcysteine on experimental lesions induced by doxorubicin in sciatic nerve of rats. Drug Chem Toxicol. 2015;38:436–441. doi: 10.3109/01480545.2014.981753. [DOI] [PubMed] [Google Scholar]
- 26.Ruszkiewicz J, Albrecht J. Changes of the thioredoxin system, glutathione peroxidase activity and total antioxidant capacity in rat brain cortex during acute liver failure: modulation by L-histidine. Neurochem Res. 2015;40:293–300. doi: 10.1007/s11064-014-1417-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pichili VB, Rao KV, Jayakumar AR, Norenberg MD. Inhibition of glutamine transport into mitochondria protects astrocytes from ammonia toxicity. Glia. 2007;55:801–809. doi: 10.1002/glia.20499. [DOI] [PubMed] [Google Scholar]
- 28.Kohen R, Yamamoto Y, Cundy KC, Ames BN. Antioxidant activity of carnosine, homocarnosine, and anserine present in muscle and brain. Proc Natl Acad Sci U S A. 1988;85:3175–3179. doi: 10.1073/pnas.85.9.3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yuneva AO, Kramarenko GG, Vetreshchak TV, Gallant S, Boldyrev AA. Effect of carnosine on Drosophila melanogaster lifespan. Bull Exp Biol Med. 2002;133:559–561. doi: 10.1023/a:1020273506970. [DOI] [PubMed] [Google Scholar]
- 30.Gallant S, Semyonova M, Yuneva M. Carnosine as a potential anti-senescence drug. Biochemistry (Mosc) 2000;65:866–868. [PubMed] [Google Scholar]
- 31.Shen Y, Hu WW, Fan YY, Dai HB, Fu QL, Wei EQ, Luo JH, Chen Z. Carnosine protects against NMDA-induced neurotoxicity in differentiated rat PC12 cells through carnosine-histidine-histamine pathway and H(1)/H(3) receptors. Biochem Pharmacol. 2007;73:709–717. doi: 10.1016/j.bcp.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 32.Mizuno D, Konoha-Mizuno K, Mori M, Sadakane Y, Koyama H, Ohkawara S, Kawahara M. Protective activity of carnosine and anserine against zinc-induced neurotoxicity: a possible treatment for vascular dementia. Metallomics. 2015;7:1233–1239. doi: 10.1039/c5mt00049a. [DOI] [PubMed] [Google Scholar]
- 33.Zhang L, Yao K, Fan Y, He P, Wang X, Hu W, Chen Z. Carnosine protects brain microvascular endothelial cells against rotenone-induced oxidative stress injury through histamine H₁ and H₂ receptors in vitro. Clin Exp Pharmacol Physiol. 2012;39:1019–1025. doi: 10.1111/1440-1681.12019. [DOI] [PubMed] [Google Scholar]
- 34.Preston JE, Hipkiss AR, Himsworth DT, Romero IA, Abbott JN. Toxic effects of beta-amyloid(25-35) on immortalised rat brain endothelial cell: protection by carnosine, homocarnosine and beta-alanine. Neurosci Lett. 1998;242:105–108. doi: 10.1016/s0304-3940(98)00058-5. [DOI] [PubMed] [Google Scholar]
- 35.Zhang ZY, Sun BL, Yang MF, Li DW, Fang J, Zhang S. Carnosine attenuates early brain injury through its antioxidative and anti-apoptotic effects in a rat experimental subarachnoid hemorrhage model. Cell Mol Neurobiol. 2015;35:147–157. doi: 10.1007/s10571-014-0106-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Herculano B, Tamura M, Ohba A, Shimatani M, Kutsuna N, Hisatsune T. β-alanyl-L-histidine rescues cognitive deficits caused by feeding a high fat diet in a transgenic mouse model of Alzheimer's disease. J Alzheimers Dis. 2013;33:983–997. doi: 10.3233/JAD-2012-121324. [DOI] [PubMed] [Google Scholar]
- 37.Flancbaum L, Fitzpatrick JC, Brotman DN, Marcoux AM, Kasziba E, Fisher H. The presence and significance of carnosine in histamine-containing tissues of several mammalian species. Agents Actions. 1990;31:190–196. doi: 10.1007/BF01997607. [DOI] [PubMed] [Google Scholar]
- 38.Park S, Kim J, Kim Y. Mulberry leaf extract inhibits cancer cell stemness in neuroblastoma. Nutr Cancer. 2012;64:889–898. doi: 10.1080/01635581.2012.707280. [DOI] [PubMed] [Google Scholar]
- 39.Teunissen CE, Khalil M. Neurofilaments as biomarkers in multiple sclerosis. Mult Scler. 2012;18:552–556. doi: 10.1177/1352458512443092. [DOI] [PubMed] [Google Scholar]
- 40.Dráberová E, Del Valle L, Gordon J, Marková V, Smejkalová B, Bertrand L, de Chadarévian JP, Agamanolis DP, Legido A, Khalili K, Dráber P, Katsetos CD. Class III β-tubulin is constitutively coexpressed with glial fibrillary acidic protein and nestin in midgestational human fetal astrocytes: implications for phenotypic identity. J Neuropathol Exp Neurol. 2008;67:341–354. doi: 10.1097/NEN.0b013e31816a686d. [DOI] [PubMed] [Google Scholar]
- 41.Cho S, Hwang ES. Fluorescence-based detection and quantification of features of cellular senescence. Methods Cell Biol. 2011;103:149–188. doi: 10.1016/B978-0-12-385493-3.00007-3. [DOI] [PubMed] [Google Scholar]
- 42.Mattson MP, Arumugam TV. Hallmarks of brain aging: adaptive and pathological modification by metabolic states. Cell Metab. 2018;27:1176–1199. doi: 10.1016/j.cmet.2018.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Godbout JP, Johnson RW. Age and neuroinflammation: a lifetime of psychoneuroimmune consequences. Immunol Allergy Clin North Am. 2009;29:321–337. doi: 10.1016/j.iac.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 44.Rea IM, Gibson DS, McGilligan V, McNerlan SE, Alexander HD, Ross OA. Age and age-related diseases: role of inflammation triggers and cytokines. Front Immunol. 2018;9:586. doi: 10.3389/fimmu.2018.00586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pardridge WM. Blood-brain barrier transport of nutrients. Nutr Rev. 1986;44(Suppl):15–25. doi: 10.1111/j.1753-4887.1986.tb07674.x. [DOI] [PubMed] [Google Scholar]
- 46.Ohtsuki S, Terasaki T. Contribution of carrier-mediated transport systems to the blood-brain barrier as a supporting and protecting interface for the brain; importance for CNS drug discovery and development. Pharm Res. 2007;24:1745–1758. doi: 10.1007/s11095-007-9374-5. [DOI] [PubMed] [Google Scholar]
- 47.Keep RF, Smith DE. Chapter 198-oligopeptide transport at the blood-brain and blood-CSF barriers. In: Kastin AJ, editor. Handbook of Biologically Active Peptides. Amsterdam: Academic Press; 2006. pp. 1423–1428. [Google Scholar]
- 48.Zlokovic BV. Cerebrovascular permeability to peptides: manipulations of transport systems at the blood-brain barrier. Pharm Res. 1995;12:1395–1406. doi: 10.1023/a:1016254514167. [DOI] [PubMed] [Google Scholar]
- 49.Sasahara I, Fujimura N, Nozawa Y, Furuhata Y, Sato H. The effect of histidine on mental fatigue and cognitive performance in subjects with high fatigue and sleep disruption scores. Physiol Behav. 2015;147:238–244. doi: 10.1016/j.physbeh.2015.04.042. [DOI] [PubMed] [Google Scholar]
- 50.Yoshikawa T, Nakamura T, Shibakusa T, Sugita M, Naganuma F, Iida T, Miura Y, Mohsen A, Harada R, Yanai K. Insufficient intake of L-histidine reduces brain histamine and causes anxiety-like behaviors in male mice. J Nutr. 2014;144:1637–1641. doi: 10.3945/jn.114.196105. [DOI] [PubMed] [Google Scholar]
- 51.Aydın AF, Çoban J, Doğan-Ekici I, Betül-Kalaz E, Doğru-Abbasoğlu S, Uysal M. Carnosine and taurine treatments diminished brain oxidative stress and apoptosis in D-galactose aging model. Metab Brain Dis. 2016;31:337–345. doi: 10.1007/s11011-015-9755-0. [DOI] [PubMed] [Google Scholar]
- 52.Banerjee S, Poddar MK. Carnosine: effect on aging-induced increase in brain regional monoamine oxidase-A activity. Neurosci Res. 2015;92:62–70. doi: 10.1016/j.neures.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 53.Davinelli S, Di Marco R, Bracale R, Quattrone A, Zella D, Scapagnini G. Synergistic effect of L-Carnosine and EGCG in the prevention of physiological brain aging. Curr Pharm Des. 2013;19:2722–2727. doi: 10.2174/1381612811319150007. [DOI] [PubMed] [Google Scholar]
- 54.Boldyrev AA, Aldini G, Derave W. Physiology and pathophysiology of carnosine. Physiol Rev. 2013;93:1803–1845. doi: 10.1152/physrev.00039.2012. [DOI] [PubMed] [Google Scholar]
- 55.Decker EA, Livisay SA, Zhou S. A re-evaluation of the antioxidant activity of purified carnosine. Biochemistry (Mosc) 2000;65:766–770. [PubMed] [Google Scholar]
- 56.Babizhayev MA, Seguin MC, Gueyne J, Evstigneeva RP, Ageyeva EA, Zheltukhina GA. L-carnosine (beta-alanyl-L-histidine) and carcinine (beta-alanylhistamine) act as natural antioxidants with hydroxyl-radical-scavenging and lipid-peroxidase activities. Biochem J. 1994;304:509–516. doi: 10.1042/bj3040509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Canonaco M, Madeo M, Alò R, Giusi G, Granata T, Carelli A, Canonaco A, Facciolo RM. The histaminergic signaling system exerts a neuroprotective role against neurodegenerative-induced processes in the hamster. J Pharmacol Exp Ther. 2005;315:188–195. doi: 10.1124/jpet.105.088153. [DOI] [PubMed] [Google Scholar]
- 58.Hiraga N, Adachi N, Liu K, Nagaro T, Arai T. Suppression of inflammatory cell recruitment by histamine receptor stimulation in ischemic rat brains. Eur J Pharmacol. 2007;557:236–244. doi: 10.1016/j.ejphar.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 59.Fu Q, Dai H, Hu W, Fan Y, Shen Y, Zhang W, Chen Z. Carnosine protects against Abeta42-induced neurotoxicity in differentiated rat PC12 cells. Cell Mol Neurobiol. 2008;28:307–316. doi: 10.1007/s10571-007-9235-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bae ON, Majid A. Role of histidine/histamine in carnosine-induced neuroprotection during ischemic brain damage. Brain Res. 2013;1527:246–254. doi: 10.1016/j.brainres.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 61.Attanasio F, Convertino M, Magno A, Caflisch A, Corazza A, Haridas H, Esposito G, Cataldo S, Pignataro B, Milardi D, Rizzarelli E. Carnosine inhibits Aβ(42) aggregation by perturbing the H-bond network in and around the central hydrophobic cluster. ChemBioChem. 2013;14:583–592. doi: 10.1002/cbic.201200704. [DOI] [PubMed] [Google Scholar]
- 62.Thinakaran G, Koo EH. Amyloid precursor protein trafficking, processing, and function. J Biol Chem. 2008;283:29615–29619. doi: 10.1074/jbc.R800019200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gralle M, Botelho MG, Wouters FS. Neuroprotective secreted amyloid precursor protein acts by disrupting amyloid precursor protein dimers. J Biol Chem. 2009;284:15016–15025. doi: 10.1074/jbc.M808755200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yang WN, Han H, Hu XD, Feng GF, Qian YH. The effects of perindopril on cognitive impairment induced by d-galactose and aluminum trichloride via inhibition of acetylcholinesterase activity and oxidative stress. Pharmacol Biochem Behav. 2013;114-115:31–36. doi: 10.1016/j.pbb.2013.10.027. [DOI] [PubMed] [Google Scholar]
- 65.Li JJ, Zhu Q, Lu YP, Zhao P, Feng ZB, Qian ZM, Zhu L. Ligustilide prevents cognitive impairment and attenuates neurotoxicity in D-galactose induced aging mice brain. Brain Res. 2015;1595:19–28. doi: 10.1016/j.brainres.2014.10.012. [DOI] [PubMed] [Google Scholar]
- 66.Wade AM, Tucker HN. Antioxidant characteristics of L-histidine. J Nutr Biochem. 1998;9:308–315. [Google Scholar]
- 67.Ruszkiewicz J, Fręśko I, Hilgier W, Albrecht J. Decrease of glutathione content in the prefrontal cortical mitochondria of rats with acute hepatic encephalopathy: prevention by histidine. Metab Brain Dis. 2013;28:11–14. doi: 10.1007/s11011-012-9342-6. [DOI] [PubMed] [Google Scholar]
- 68.Han CH, Lin YS, Lee TL, Liang HJ, Hou WC. Asn-Trp dipeptides improve the oxidative stress and learning dysfunctions in D-galactose-induced BALB/c mice. Food Funct. 2014;5:2228–2236. doi: 10.1039/c4fo00205a. [DOI] [PubMed] [Google Scholar]
- 69.Hawkins RA, O'Kane RL, Simpson IA, Viña JR. Structure of the blood-brain barrier and its role in the transport of amino acids. J Nutr. 2006;136:218S–226S. doi: 10.1093/jn/136.1.218S. [DOI] [PubMed] [Google Scholar]
- 70.Margolis FL. Carnosine in the primary olfactory pathway. Science. 1974;184:909–911. doi: 10.1126/science.184.4139.909. [DOI] [PubMed] [Google Scholar]
- 71.Margolis FL, Grillo M. Axoplasmic transport of carnosine (β-alanyl-L-histidine) in the mouse olfactory pathway. Neurochem Res. 1977;2:507–519. doi: 10.1007/BF00966011. [DOI] [PubMed] [Google Scholar]
- 72.Berezhnoy DS, Stvolinsky SL, Lopachev AV, Devyatov AA, Lopacheva OM, Kulikova OI, Abaimov DA, Fedorova TN. Carnosine as an effective neuroprotector in brain pathology and potential neuromodulator in normal conditions. Amino Acids. 2019;51:139–150. doi: 10.1007/s00726-018-2667-7. [DOI] [PubMed] [Google Scholar]