Abstract
Background
Porcine parvovirus (PPV) is a single-stranded DNA virus that causes porcine reproductive failure. It is of critical importance to study PPV pathogenesis for the prevention and control of the disease. NS1, a PPV non-structural protein, is participated in viral DNA replication, transcriptional regulation, and cytotoxicity. Our previous research showed that PPV can activate nuclear factor kappa B (NF-κB) signaling pathway and then up-regulate the expression of interleukin (IL)-6.
Objectives
Herein, the purpose of this study is to determine whether the non-structural protein NS1 of PPV also has the same function.
Methods
Real-time quantitative reverse transcription polymerase chain reaction (RT-qPCR), enzyme-linked immunosorbent assay, western blot, immunofluorescence assay and small interfering RNA (siRNA) were used.
Results
Our findings demonstrated that PPV NS1 protein can up-regulate the expression levels of IL-6 and tumor necrosis factor-alpha in a dose-dependent manner. Moreover, PPV NS1 protein was found to induce the phosphorylation of IκBα, then leading to the phosphorylation and nuclear translocation of NF-κB. In addition, the NS1 protein activated the upstream pathways of NF-κB. Meanwhile, TLR2-siRNA assay showed TLR2 plays an important role in the activation of NF-κB signaling pathway induced by PPV-NS1.
Conclusions
These findings indicated that PPV NS1 protein induced the up-regulated of IL-6 expression through activating the TLR2 and NF-κB signaling pathways. In conclusion, these findings provide a new avenue to study the innate immune mechanism of PPV infection.
Keywords: Porcine parvovirus, IL-6, NF-κB, toll-like receptors
INTRODUCTION
Porcine parvovirus (PPV) is a DNA virus that belongs to the genus Protoparvovirus subfamily Parvoviridae, and family Parvovirinae [1]. PPV was first reported in Germany- in 1968 and was subsequently discovered in several other swine-producing countries, which caused significantly economic losses worldwide [2]. The PPV genome is approximately 5 kb and contains two major open reading frames. The 5' half of the genome encodes the capsid proteins (VP1, VP2, and VP3) and the 3' half of the genome encodes non-structural proteins (NS1, NS2, and NS3) [3,4,5]. NS1 is a highly conserved protein that is produced during the early phase of PPV replication and participated in PPV DNA replication, transcription regulation, and cytotoxicity [6,7,8].
It has been well documented that the first-line of defense for innate immunity induces a cellular reaction via the immediate activation of the nuclear factor kappa B (NF-κB) pathway [9]. NF-κB is part of a family of transcription factors that regulates the innate immune response genes expression, including interleukin (IL)-1, interferon (IFN), IL-6, and tumor necrosis factor-alpha (TNF-α) [10,11]. During homeostasis, NF-κB binds to IκB in the cytoplasm and when exposed to various exogenous stimuli, such as viral and bacterial infections, it entered nucleus from cytoplasm [12]. IκBα is the major protein comprising the IκB complex. In addition, IκBα was the first protein to be described in this family and is also the most extensively studied IκB protein to date [13]. When various signaling pathways are activated, IκBα undergoes phosphorylation and is released from the NF-κB/IκBα dimer. NF-κB is then translocated from the cytoplasm into the nucleus to bind DNA and regulate transcription [14]. Viral nucleic acids and their encoding proteins, pathogen-associated molecular patterns (PAMPs), are important for virus survival and, therefore, ideal targets of host pattern recognition receptors (PRRs) [15]. Among the PRRs, toll-like receptors (TLRs) are type I transmembrane proteins that play a central role in the recognition and response to infection by the innate immune system [16,17]. Following recognition by PAMPs, TLRs selectively recruit adaptor molecules (e.g., myeloid differentiation primary response protein 88 [MyD88]), that initiate downstream signaling events that leads to the activation of IL-1 receptor-associated kinase-4 (IRAK4) [18] tumor necrosis factor receptor associated factor 6 (TRAF6), and NF-κB, which induces the production of inflammatory cytokines [19,20].
The innate immune system is the first line of defense against viral infection, which causes the signaling pathways to activate the expression of protective cellular genes (e.g., IL-6, TNF-α, IFN-γ) [21]. These genes play a critical role in antiviral innate immunity and modulating the adaptive immune response to viral infection [22]. Moreover, Zipris et al. [23] demonstrated that infection with the parvovirus Kilham rat virus, induced the proinflammatory cytokines IL-6 and IL-12p40 by activating TLR9 and NF-κB. The oncolytic parvovirus H-1, significantly enhanced the expression of TLR3 and TLR9 during infection of HEK293 cells, and promoted the translocation of the p65 protein from the cytoplasm to the nucleus, and induced the expression of TNF-α [24]. In addition, cell transfected with human parvovirus B19 (B19) nonstructural protein NS1 could stimulate the level of TLR expression and provide insight into understanding the role of B19-NS1 on innate immunity [25]. However, the mechanism of PPV interaction with host innate immune system remains unclear.
Our previous study indicated that PPV infection could induce IL-6 expression, and this phenomenon was mediated by TLR2, TLR7, and TLR9 through activation of the NF-κB signaling pathway (unpublished data) [26]. In the present study, we further aimed to explore the influence of the PPV NS1 protein on the activation of the NF-κB signaling pathway in vitro. Our results demonstrate that PPV NS1 activates NF-κB and subsequently induces the expression of IL-6 potentially via TLR2. These results have important implications on the understanding of the mechanism of PPV interaction with host cells.
MATERIALS AND METHODS
Cell lines and recombinant plasmid
The 293T cell line (ATCC: CRL-11268) was used to transfect the NS1 recombinant plasmid. The 293T cells were cultured in Dulbecco's modified eagle medium (DMEM, Gibco, Beijing, China) supplemented with 10% (v/v) fetal bovine serum (FBS, Gibco, Beijing, China). The NS1 gene was successfully cloned into pEGFP-N1 and the recombinant plasmid, named pEGFP-N1-NS1, was verified by sequencing. The recombinant plasmid was amplified in E. coli DH5α and purified using the high purity plasmid rapid extraction kit (Omega Bio-tek, Inc, USA).
Transfection of the NS1 recombinant plasmid in 293T cells
One-day-old, cell monolayers exhibiting 70% to 80% confluency in six-well plates were used for recombinant plasmid transfection. Briefly, the plasmid and liposome complexes were prepared according to the recommended ratio of the Lipofectamine™ 2000 Reagent kit (Invitrogen, USA) with 1 μg DNA per 2.5 μL of liposomes. Both 4 μg of plasmid DNA and 10 μL of LipofectamineTM 2000 were separately diluted into 100 μL OPTI-MEM medium (Invitrogen) and maintained at room temperature for 5 min, before gently mixing. The plasmid/liposome complexes were incubated at room temperature for 20 min. Then, 200 μL of the plasmid/liposome mixture was diluted in 800 μL of the OPTI-MEM medium, and subsequently injected into the well. The six-well plates were cultured in 5% CO2 at 37°C, and 6 h after transfection, the cultural medium was replaced by DMEM supplemented with 2% FBS.
NF-κB inhibitor assay
The pre-transfected group was treated with NF-κB specific-inhibitor of BAY 11-7082 (10 μM) (Beyotime, China) following by transfection with pEGFP-N1-NS1 (4 μg/mL) for 1 h. The pEGFP-N1-NS1 plus BAY 11-7082 group was treated with BAY 11-7082 (10 μM) and simultaneously transfected with pEGFP-N1-NS1 (4 μg/mL). The pre-inhibitor group was treated with BAY 11-7082 (10 μM) for 1 h before the transfection with pEGFP-N1-NS1 (4 μg/mL). Following by transfection for 6 h, DMEM supplemented with 2% FBS containing fresh BAY 11-7082 inhibitor (10 μM) was added to the culture. At 24 h-post transfection, the cell supernatants were collected, and the cells were washed twice with cold PBS, and collected in 500 μL DMEM.
Immunofluorescence assay (IFA)
The 293T cells grown to 80% confluent monolayers in 24-well plates within 24 h were transfected with 4 μg vector plasmid or recombinant plasmid. After 24 h of transfection, cells were washed five times with cold PBS (5 min each) before being fixed in ethyl alcohol absolute for 30 min. Cells were washed three times with cold PBS. Then cells were permeabilized in 0.05% Triton X-100 for 30 min at room temperature. After-washing three times with cold PBS, the cells were blocked in PBS containing 5% BSA (Sigma, USA) for 2 h at 37°C. The cells were then incubated with a rabbit anti-mouse p65 monoclonal antibody (Proteintech, China; 1:1,000 dilution) overnight at 4°C. The cells were washed with PBST twice (5–10 min each) and incubated with a goat anti-rabbit secondary antibodies (Proteintech, China; 1:5,000 dilution) at room temperature for 1 h. The cells were then incubated with DAPI (Invitrogen) for another 5 min at room temperature and were washed three times (5 min each) with cold PBST. Finally, the 24-well culture plate was observed under a fluorescence microscope (Zeiss, Germany).
Cytoplasmic and nuclear protein extraction and western blot analysis
Six-well plates containing 293T cells were transfected with or without pEGFP-N1-NS1 (4 μg). After the cells were transfected with NS1 for 0, 1, 3, 6, 12, 24, 36, and 48 h, the cytoplasmic and nuclear protein were extracted using a nuclear and cytoplasmic extraction kit (Boster, China) according to the manufacturer's instructions [26]. The protein concentration was measured using BCA Protein Assay Kit (Boster), and the collected protein was stored at −80°C until use.
For the total protein treatment, the cells were collected at 0, 1, 3, 6, 12, 24, 36, or 48 h following plasmid transfection. The protein extracts were prepared from cells by suspension in lysis buffer containing protease inhibitor cocktail for 30 min on ice. Then 20 μg protein from each sample was used for western blot analysis as previously described [27]. The following rabbit polyclonal antibodies were used as primary antibody of the corresponding proteins: p65 with 1:1,000 dilution (Proteintech, China), phospho-p65 with 1:1000 dilution (p-p65 Ser536, Cell Signaling Technology, America), IκBα with 1:1,000 dilution (Proteintech), MyD88 with 1:100 dilution (Boster, China), TLR2 with 1:100 dilution (Boster),β-actin (1:1,000, Proteintech),and TBP (1:1,000, Proteintech). A HRP-conjugated goat anti-rabbit secondary antibody (1:5,000, Proteintech) were used in this study.
Cytokine detection by enzyme-linked immunosorbent assay (ELISA)
The 293T cells were seeded into six-well plates one day prior to plasmid transfection. After 1, 3, 6, 12, 24, 36, and 48 h of plasmid transfection, cell culture supernatants were harvested and used to analyze the production of TNF-α and IL-6 protein using ELISA kits (Boster, China) [26]. The optical density (OD) was read at an absorbance of 450 nm using an ELISA plate reader (Bio-Rad). The data were analyzed using Curve Exert 1.3 software to create a standard curve.
Real-time quantitative reverse transcription polymerase chain reaction (RT-qPCR)
RT-qPCR was used to detect the adapter molecules in the TLR signaling pathway. The 293T cells were transfected with the plasmid (4 μg) for 1, 3, 6, 12, 24, 36, and 48 h, total RNA was extracted using TRIzol Reagent (Invitrogen) and then reverse-transcribed into cDNA using reverse transcriptase (TaKaRa, China) to detect the level of TLRs and adapter molecule transcripts. RT-qPCR was performed in triplicate in three separate experiments and the gene expression was monitored through a Power SYBR Premix Ex Taq II (TaKaRa) with corresponding primers (Table 1). All reactions were performed for 45 cycles, and the gene expression was calculated using the comparative method to quantify the relative level of expression after normalizing to levels of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene expression using the 2-∆∆CT method [27]. The fluorescence signals were measured using the Roche LightCycler 480 Real-Time PCR System (Roche, Switzerland). The primers that were used are listed in Table 1.
Table 1. Primer sequences for mRNA analysis by real-time quantitative reverse transcription polymerase chain reaction.
| Genes | Accession number in GenBank | Primer (5′→3′) | Annealing temperature (°C) | Size of amplicon (bp) |
|---|---|---|---|---|
| GAPDH | NC_000012.12 | F: AAGGTCGGAGTCAACGG | 50/55/58 | 220 |
| R: GGAAGATGGTGATGGGATT | ||||
| TLR2 | NC_000004.12 | F: CCCAAGCTTATGACTACAGCTCAGGAAGC | 58 | 221 |
| R: CGCGGATCCATAGCTAGCCATTGTTGCT | ||||
| MyD88 | NC_000003.12 | F: ACAAGGCAATGAAGAAAGAGTT | 58 | 100 |
| R: GCAAGGCGAGTCCAGAA | ||||
| IRAK4 | NC_000012.12 | F: ATGCCACCTGACTCCT | 50 | 160 |
| R: CTCCAAATCCTCCCTC | ||||
| TRAF6 | U78798.1 | F: GGCACGCCACCTACAA | 58 | 362 |
| R: CAGGGCTATGAATCACAACA | ||||
| TAK1 | XM_017007119.1 | F: ATGTAGTGACCTCCCTTGC | 58 | 176 |
| R: GGTGTCTATCCCATCTGC | ||||
| NF-κB | NM_001165412.1 | F: GGACTACCTGGTGCCTCTA | 58 | 280 |
| R: ACGCCTCTGTCATTCG | ||||
| TNF-α | BC028148.1 | F: CGAGTGACAAGCCTGTAGCC | 58 | 171 |
| R: TGAAGAGGACCTGGGAGTAGAT | ||||
| IL-6 | NC_000007.14 | F: GACAGCCACTCACCTCTTC | 55 | 221 |
| R: TTCACCAGGCAAGTCTCA |
Statistical analysis
All data were analyzed using SPSS version 17.0 software for windows (SPSS Inc., USA). A Duncan's multiple range test and χ2 test were used to determine the differences between groups. The value of p < 0.05 was considered statistically significant.
RESULTS
PPV NS1 stimulates the production of IL-6 and TNF-α in a dose-dependent manner
Our previous study indicated that an PPV infection can induce IL-6 expression in vitro [27]. The influence of PPV NS1 protein on the production of pro-inflammatory cytokines IL-6 and TNF-α in 293T cells was further quantified by RT-qPCR and ELISA. The mock and empty vector- transfected cells were used as the controls. The RT-qPCR results showed that the IL-6 mRNA was expressed abundantly at 1 h post-transfection and subsequently decreased gradually. However, the expression of IL-6 mRNA peaked at 24 h post-transfection. It had a significantly higher expression of IL-6 mRNA when compared with the control groups at all times (p < 0.01) (Fig. 1A). The level of TNF-α mRNA expression peaked at 1 h post-transfection and gradually decreased. At 12 h, the level of expression was the lowest but remained higher than that of the control group (p < 0.05) (Fig. 1B).
Fig. 1. PPV NS1 stimulates the production of IL-6 and TNF-α in a dose-dependent manner. The 293T cells were transfected with 4 μg of pEGFP-N1-NS1, mock- and 4 μg pEGFP-N1-transfected 293T cells were used as controls. The level of IL-6 (A) and TNF-α (B) at mRNA expression were detected by real-time reverse transcription polymerase chain reaction. The level of IL-6 (C) and TNF-α (D) protein expression were detected by enzyme-linked immunosorbent assay. (E and F) The 293T cells transfected with 1, 2, 4, and 6 μg of pEGFP-N1-NS1 were harvested after 12 h of transfection. All data are expressed as the mean ± SD of three independent experiments. Compared with the control group at the same time, there was a highly significant difference between groups (**p < 0.01), or significant difference between groups (*p < 0.05, Student's t-test).
PPV, porcine parvovirus; IL, interleukin; TNF-α, tumor necrosis factor-alpha.
The cell culture supernatants were harvested at 12, 24, 36, and 48 h post-transfection, and the concentration of IL-6 and TNF-α was quantified using commercial ELISA kits. We found that the level of IL-6 and TNF-α protein expression was significantly higher compared to the control groups at all times points (p < 0.01) (Fig. 1C and D). These results demonstrated that pEGFP-N1-NS1 induced the quantifiable expression of both IL-6 and TNF-α both in mRNA and protein expression levels in 293T cells.
An RT-qPCR assay was used to further determine whether the transfection dose of the pEGFP-N1-NS1 plasmid could affect the IL-6 and TNF-α expression. Briefly, pEGFP-N1-NS1 was transfected into 293T cells at a dose of 1, 2, 4, and 6 μg, respectively. Cells were harvested after 12 h transfection. The mock-transfected cells were used as controls. As shown in Fig. 1E and F, IL-6 expression was increased by 1.4, 2.5, 5.3, and 2.4-fold in 293T cells in the groups of transfection with pEGFP-N1-NS1 at a dose of 1, 2, 4, and 6 μg, respectively. The level of TNF-α expression increased by 1.8, 2.5, 9.2, and 2.4-fold in 293T cells at a dose of 1, 2, 4, and 6 μg of pEGFP-N1-NS1 transfection, respectively. These results suggest that transfection with 4 μg of pEGFP-N1-NS1 induced peak levels of IL-6 and TNF-α expression. Moreover, these findings indicate that pEGFP-N1-NS1 transfection enhanced IL-6 and TNF-α expression in a dose-dependent manner to the plasmid pEGFP-N1-NS1 concentration.
NS1 induces the expression of IL-6 through the activation of NF-κB
To further evaluate the involvement of NF-κB in mediating the expression of IL-6, the NF-κB inhibitor, BAY 11-7082, was applied to verify whether IL-6 was secreted in response to NF-κB activation in 293T cells during transfection with pEGFP-N1-NS1. The mock-transfected cells were used as the control group.
The levels of IL-6 mRNA and protein expression were significantly reduced in the groups treated with BAY11-7082 at 24 h (Fig. 2A and B). The results showed that the ability of pEGFP-N1-NS1 to enhance the level of IL-6 mRNA and protein expression was significantly reduced after BAY11-7082 treatment when compared with the pEGFP-N1-NS1 transfected group (p < 0.01). Although the expression level of IL-6 mRNA was substantially higher in the pre-inhibitor group, there were no significant differences in the protein expression between the three BAY11-7082-treated groups. The inhibition of IL-6 production by BAY 11-7082 suggested that the classical pathway of NF-κB activation was critical for the subsequent up-regulation of IL-6.
Fig. 2. NS1 induces the expression of IL-6 through NF-κB activation. To further evaluate the involvement of NF-κB in mediating the expression of IL-6, the effect of the NF-κB inhibitor, BAY 11-7082, on IL-6 was examined by real-time quantitative reverse transcription polymerase chain reaction (A) and enzyme-linked immunosorbent assay (B). The mock-transfected cells were used as the control. All experiments were performed in triplicate and repeated three times.
IL, interleukin; NF-κB, nuclear factor kappa B.
PPV NS1 protein induces NF-κB p65 nuclear translocation and p65 phosphorylation
To further test whether the PPV NS1 protein could promote the nuclear translocation of p65, 293T cells were transfected with pEGFP-N1-NS1 for 24 h. The immunofluorescence analyses found that transfection with the empty vector failed to induce NF-κB nuclear translocation, as p65 was located exclusively in the cytoplasm (Fig. 3, top panels). However, p65 rapidly translocated to the nucleus when the cells were transfected with pEGFP-N1-NS1 (Fig. 3, bottom panels). These results demonstrate that NS1 can stimulate the translocation of NF-κB from the cytoplasm into the nucleus.
Fig. 3. PPV NS1 protein induced NF-κB p65 nuclear translocation. An indirect immunofluorescence analysis was used to measure the cellular localization of NF-κB in PPV NS1-transfected 293T cells. The 293T cells were subjected to immunofluorescence staining for the detection of p65 subcellular localization using rabbit anti-p65 and FITC-conjugated secondary antibody (green). The nuclei were stained with DAPI (blue).
PPV, porcine parvovirus; NF-κB, nuclear factor kappa B.
NF-κB activation was also confirmed following transfection with 4 μg pEGFP-N1-NS1 for 1, 3, 6, 12, 24, 36, and 48 h by RT-qPCR and western blotting (Fig. 4). Mock-transfected cells and 4 μg pEGFP-N1 transfected into 293T cells were used as controls. The level of NF-κB mRNA expression was significantly higher than that of the control groups at 1, 6, and 12 h post-transfection (p < 0.05), whereas the level of NF-κB transcription decreased substantially at 24, 36, and 48 h post-transfection (Fig. 4A). The western blot analysis revealed that the level of p65 in cells remained relatively unchanged, whereas the level of p-p65 expression in the nucleus increased significantly at 3, 12, 24, 36, and 48 h, peaking at 48 h. The level of p-p65 expression in the cytoplasm gradually decreased at 1, 3, 6, and 12 h, and subsequently increased at 24 h and 36 h (Fig. 4B).
Fig. 4. PPV NS1 induced the phosphorylation of p65 after transfection. The activation of NF-κB was also confirmed after 293T cells were transfected with 4 μg pEGFP-N1-NS1 for 1, 3, 6, 12, 24, 36, and 48 h by real-time quantitative reverse transcription polymerase chain reaction (A) and western blotting (B). The expression of NS1 in 293T cells was confirmed at the mRNA level (C). To eliminate the effect of the empty vector on p65, we conducted an empty vector control test at the protein level (D). Compared with the control group at the same time, there was a highly significant difference between groups (**p < 0.01, Student's t-test).
PPV, porcine parvovirus; NF-κB, nuclear factor kappa B.
To eliminate the effect of the empty vector on p65, we conducted an empty vector control test at the protein level. To this end, 293T cells were transfected with different doses of pEGFP-N1-NS1 (0, 1, 2, and 4 μg) and the transfected plasmids were supplemented in each sample with up to 4 μg of the empty vector. After 36 h, the cell samples were collected and the total p65 protein, p-p65 cytoplasmic protein and p-p65 nuclear protein were measured by western blot. As shown in Fig. 4C and D, the expression of NS1 in 293T cells after transfection 24 h was confirmed at mRNA level by RT-qPCR (Fig. 4C). The total level of p65 in the cells remained unchanged, and p-p65 expression in both the cytoplasm and nucleus was enhanced with the increase of pEGFP-N1-NS1 concentration. These results suggested that the NF-κB signaling pathway was strongly activated with substantially increase of p65 phosphorylation at Ser536 in response to pEGFP-N1-NS1 but not pEGFP-N1.
PPV induces the degradation of IκBα gene expression
Since the degradation of IκBα will release the NF-κB, the level of IκBα expression was also confirmed following transfection with 4 μg of pEGFP-N1-NS1. Cells were collected at 1, 3, 6, 12, 24, 36, and 48 h after transfection and IFTα expression were detected by RT-qPCR and western blotting. Mock - transfected and 4 μg of pEGFP-N1-transfected 293T cells were used as the controls. The expression of IκBα in mRNA was significantly higher than that of the control groups at 1, 3, and 12 h post-transfection (p < 0.05) (Fig. 5A). The cytoplasmic proteins were subsequently prepared, and the western blot analysis showed that IκBα expression in the cytoplasm was significantly decreased at 3, 6, 36, and 48 h post-transfection (Fig. 5B). The empty vector control test indicated that the expression of IκBα in the cytoplasm decreased as the NS1 plasmid concentration increased (Fig. 5C). This finding suggested that the PPV NS1 protein can promote IκBα degradation and subsequently induce p65 phosphorylation.
Fig. 5. PPV NS1 induced IκBα degradation following transfection. The level of IκBα expression was confirmed following transfection with 4 μg of pEGFP-N1-NS1 at 1, 3, 6, 12, 24, 36, and 48 h by real-time quantitative reverse transcription polymerase chain reaction (A) and western blotting (B). The empty vector control test at the protein level was used to detect the expression of IκBα degradation (C). Compared with the control group at the same time, there was a highly significant difference between groups (**p < 0.01, Student's t-test).
PPV, porcine parvovirus.
Detection of NF-κB signaling pathway-related genes during pEGFP-N1-NS1 transfection
The expression of the key factors in the NF-κB signaling pathway (MyD88, IRAK4, TRAF6, and TAK1) was detected at 1, 3, 6, 12, 24, 36, and 48 h post-transfection by RT-qPCR or western blot. Mock- and 4 μg pEGFP-N1-transfected 293T cells were used as controls. As shown in Fig. 6, the trends for the level of MyD88, IRAK4, and TRAF6 mRNA expression were similar when compared with the control groups (Fig. 6A, D, E, and F). Briefly, the expression of MyD88, IRAK4, and TRAF6 in mRNA level peaked at 1 h post-transfection, and then gradually decreased to a minimum levels at 12 h post-transfection, while there was still a higher level of MyD88 and TRAF6 mRNA expression when compared that of the control groups (p < 0.05). The expression of TAK1 was significantly higher compared to that of the control groups during the initial stage of infection (within 24 h post-transfection) (Fig. 6F). The western blot analysis revealed that the total level of MyD88 protein expression in the cells increased significantly from 1 h to 48 h (Fig. 6B). The empty vector control test indicated that the expression of MyD88 in the cells increased with the NS1 plasmid concentration (Fig. 6C).
Fig. 6. The detection of NF-κB signaling pathway-related genes during pEGFP-N1-NS1 transfection. The expression of MyD88 (A), IRAK4 (D), TRAF6 (E), and TAK1 (F) mRNA in the 293T cells were tested after pEGFP-N1-NS1 transfection at different time points. The mock- and 4 μg pEGFP-N1-transfected 293T cells were used as the controls. The level of MyD88 protein expression was confirmed after transfection with 4 μg pEGFP-N1-NS1 by western blot (B) and the empty vector control test at the protein level were used to detect the expression of MyD88 (C). Compared with the control group at the same time, there was a highly significant difference between groups (**p < 0.01), or significant difference between groups (*p < 0.05, Student's t-test).
NF-κB, nuclear factor kappa B.
In summary, pEGFP-N1-NS1 induced the expression of MyD88, IRAK4, TRAF6, and TAK1 in mRNA level, and also increased the MyD88 protein expression. These results indicated that the upstream molecules in the NF-κB signaling pathway were activated by pEGFP-N1-NS1.
The PPV NS1 protein induces TLR2 expression through activating the NF-κB signaling pathway
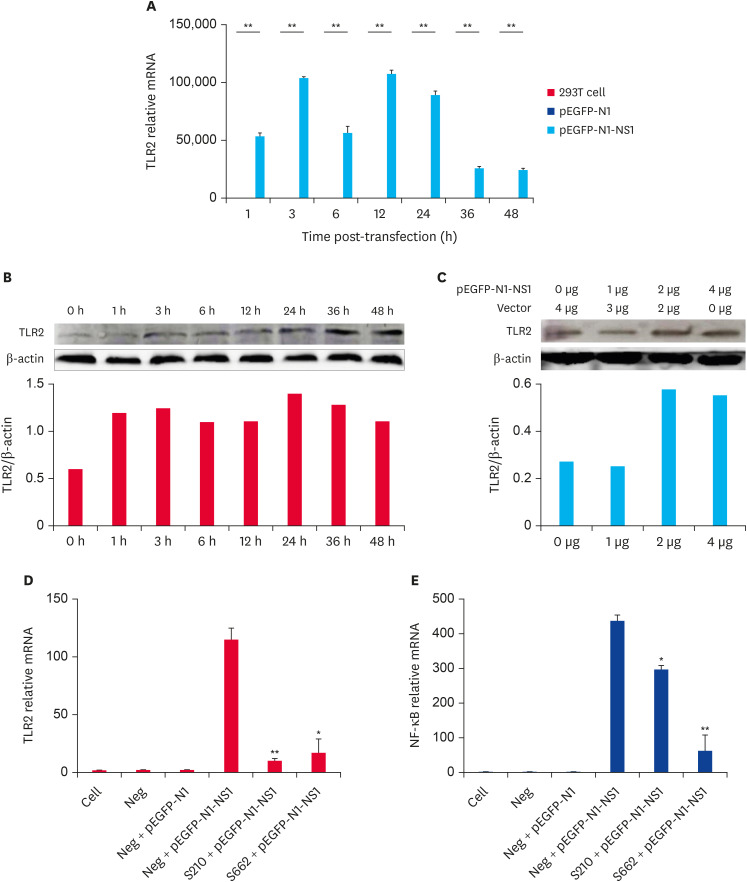
Our previous studies revealed that PPV infection can induce TLR2, TLR7, and TLR9 expression (unpublished date). To investigate the transcription pattern of TLRs following the transfection of pEGFP-N1-NS1, 4 μg of pEGFP-N1-NS1 plasmid was transfected into 293T cells. The cell precipitates were harvested at 1, 3, 6, 12, 24, 36, and 48 h post-transfection, and was detected by RT-qPCR and western blot. The mock- and 4 μg pEGFP-N1-transfected 293T cells were used as the controls. Our results showed that the level of TLR2, TLR7, and TLR9 gene expression were significantly higher than that of the control groups at all of the tested time points following the transfection (p < 0.05) (Fig. 7A, some data not shown). However, the level of TLR2 expression was the lowest at 48 h, which was 25 000 times than that of the control groups. The western blot analysis showed that the total amount of TLR2 protein in the cells increased significantly at all indicated time (Fig. 7B). The empty vector control test indicated that the level of TLR2 expression in the cells increased with the NS1 plasmid concentration (Fig. 7C).
Fig. 7. PPV NS1 protein induced the expression of TLR2 during infection. The expression of TLR2 mRNA (A) in 293T cells were tested following pEGFP-N1-NS1 transfection at different time points. Mock- and 4 μg pEGFP-N1-transfected 293T cells were used as the controls. The level of TLR2 protein expression was confirmed following transfection with 4 μg pEGFP-N1-NS1 by western blot (B) and an empty vector control test at the protein level was used to detect the level of TLR2 expression (C). The expression of TLR2 (D) and NF-κB (E) were both inhibited following TLR2 interference. Compared with the control group at the same time, there was a highly significant difference between groups (**p < 0.01), or significant difference between groups (*p < 0.05, Student's t-test).
PPV, porcine parvovirus; TLR, toll-like receptor; NF-κB, nuclear factor kappa B.
To further prove that PPV NS1 can regulate the inflammatory response through TLR2, two TLR2 siRNAs were designed (siRNA210 and siRNA662, synthesized by Shanghai GenePharma Co., Ltd. the primers that were used are listed in Table 2), and transfected into 293T cells to detect the level of NF-κB expression. The results showed that there were no significant changes in the level of TLR2 and NF-κB expression in the negative interference and empty vector group, which indicates that the empty vector and negative interference had no influence on the test results and ensured the accuracy of the test results. The level of TLR2 and NF-κB expression in the TLR2-siRNA and pEGFP-N1-NS1 co-transfected groups were decreased compared to the TLR2-siNeg and pEGFP-N1-NS1 co-transfected group. The results showed that the expression of TLR2 and NF-κB were both inhibited following the interference of TLR2. TLR2 was involved in the PPV NS1-mediated NF-κB signaling pathway activation.
Table 2. Primer sequences for TLR2-siRNA analysis.
| Genes | Primer (5′→3′) |
|---|---|
| TLR2-siRNA 210 | F: CCAGAUCUUUGAGCUCCAUTT |
| R: AUGGAGCUCAAAGAUCUGGTT | |
| TLR2-siRNA 662 | F: CCAAAGAGUCUGAGGUCAATT |
| R: UUGACCUCAGACUCUUUGGTT | |
| NC-siRNA | F: UGACCUCAACUACAUGGUUTT |
| R: AACCAUGUAGUUGAGGUCATT |
DISCUSSION
In response to viral entry and replication, the innate immune system first initiates the host innate response to limit viral spread and reduce the damage to host cells. The innate immune response involves the secretion of cytokines and other mediators, which lead to the induction of an inflammatory response that has antimicrobial effects. It has been well-documented that the first-line innate immunity defense induces a cellular reaction that immediately activates NF-κB signaling [9]. Although PPV was discovered several years ago, and there has been several studies regarding the pathogenesis of PPV, the essential role of NS1 on the pathogenesis of PPV remains unclear. In the present study, the influence of the PPV NS1 protein on the NF-κB signaling pathway was explored in vitro.
IL-6 and TNF-α are critical pro-inflammatory cytokines associated with the host innate response to eliminate pathogenic microorganisms. Our previous research has shown that PPV infection can induce the expression of the inflammatory cytokines IL-6. Therefore, to determine whether NS1 also could stimulate pro-inflammatory cytokines, we quantified the levels of IL-6 and TNF-α mRNA and protein expression by RT-qRCR and western blot. Our results suggested that pEGFP-N1-NS1-transfection enhanced the IL-6 and TNF-α expression in a dose-dependent manner regarding the pEGFP-N1-NS1 plasmid concentration. To further evaluate the involvement of NF-κB in mediating the expression of IL-6, the NF-κB inhibitor, BAY11-7082, was used in this study. The inhibition of IL-6 induction by BAY 11-7082 suggests that activation of the NF-κB pathway was critical for the subsequent up-regulation of IL-6.
NF-κB activation rapidly occurs within minutes after stimulation. The process requires no protein synthesis and can influence critical steps in the host cell life, which makes the NF-κB pathway an attractive target for invading viruses [16]. When activated, NF-κB is released from the NF-κB/IκB complex via the degradation of the IκB proteins in the proteasome and is subsequently transported into the nucleus. Previous studies have demonstrated that infection with PPV activated the NF-κB signaling pathway to induce the transcription of the downstream inflammatory cytokine IL-6 and that the potential mechanisms of PPV-induced NF-κB activation are related to IκBα degradation, while other studies have shown that the TLR signaling pathway was involved in NF-κB activation [26,28]. Moreover, it has been found that B19 nonstructural protein NS1-transfected cells could stimulate TLRs and the innate immunity response [25]. The PPV nonstructural protein NS1 is associated with viral DNA replication, transcription regulation, and cytotoxicity. Given that the nonstructural protein NS1 is crucial in PPV infection, we focused on the mechanism of nonstructural protein NS1 in our present study. The western blot analysis showed that the PPV NS1 protein can promote IκBα degradation, and subsequently cause p65 nuclear translocation and phosphorylation.
It is well known that TLRs play a key role in the host innate immune response to viral infection. Recognition of viral components by TLRs initiate signal transduction pathways, including triggering the expression of NF-κB-associated genes. In general, TLR signaling is often divided into either MyD88-dependent or TRIF-dependent pathways, of which MyD88 signaling pre-dominantly leads to the activation of NF-κB [18]. To determine whether activation of the TLR signaling pathways is related to NS1-induced NF-κB activation, the mRNA of TLRs and TLR signaling pathway-related genes (MyD88, IRAK4, TRAF6, TAK1, and NF-κB) were detected by RT-qPCR during NS1 transfection. The results showed that PPV-NS1 could up-regulate the expression of these genes, which are key adaptor proteins involved in the NF-κB signaling cascade [29]. Moreover, the western blot analysis indicated that the level of TLR2 expression in the cells increased with the NS1 plasmid concentration. The level of TLR2 and NF-κB expression were both inhibited following TLR2 interference, suggesting that TLR2 is the receptor that identifies PPV and is involved in the activation of the NF-κB signaling pathway. Thus, our data clearly suggest that the TLR signaling pathway can activate NF-κB in response to NS1 transfection.
The PPV NS1 transfected cells began to stimulate the activation of the NF-κB signaling pathway at the early stage, and induced the inflammatory cytokines IL-6 and TNF-α secretion. The extracellular inflammatory cytokines further stimulate the activation of NF-κB, upregulation of the expression levels of signaling pathway related factors and inflammatory cytokines. In addition, after pEGFP-N1-NS1 transfected 293T cells for 24 h, the level of NS1 expression began to increase with the proliferation of cells, which further activate the NF-κB signaling pathway to stimulated the production of inflammatory cytokines.
In conclusion, we demonstrated that PPV nonstructural protein NS1 could induce IL-6 expression through activating the NF-κB signaling pathway which was mediated by TLR2. Thus, our study reveals a potential mechanism by which PPV regulates the innate immune response, providing a basis for elucidating PPV pathogenesis. Further studies are required to determine the functional region in the mechanism by which PPV NS1 is recognized by TLRs, especially TLR2, and promote the innate immune response of host cells.
ACKNOWLEDGEMENTS
We thank Professor Hui Hu from Henan Agricultural University for revising the manuscript.
Footnotes
Funding: This work was supported by the grants from the National Natural Science Foundation of China (31772722) and the Program for Key Scientific Research at the Universities of Henan Province (18A230001).
Conflict of Interest: The authors declare no conflicts of interest.
- Conceptualization: Zhang G.
- Data curation: Jin X.
- Formal analysis: Wei Z.
- Funding acquisition: Wei Z.
- Investigation: Zhan C.
- Methodology: Zhou Y.
- Project administration: Wei Z.
- Resources: Zhang G.
- Software: Yuan Y.
- Supervision: Zhang G.
- Validation: Wei Z.
- Visualization: Song Y.
- Writing - original draft: Jin X.
- Writing - review & editing: Wei Z.
References
- 1.Tamošiūnas PL, Petraitytė-Burneikienė R, Lasickienė R, Akatov A, Kundrotas G, Sereika V, Lelešius R, Žvirblienė A, Sasnauskas K. Generation of recombinant porcine parvovirus virus-like particles in Saccharomyces cerevisiae and development of virus-specific monoclonal antibodies. J Immunol Res. 2014;2014(1):573531. doi: 10.1155/2014/573531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mayr A, Bachmann PA, Siegl G, Mahnel H, Sheffy BE. Characterization of a small porcine DNA virus. Arch Gesamte Virusforsch. 1968;25(1):38–51. doi: 10.1007/BF01243088. [DOI] [PubMed] [Google Scholar]
- 3.Wolf VH, Menossi M, Mourão GB, Gatti MS, Gmr MR. Molecular basis for porcine parvovirus detection in dead fetuses. Genet Mol Res. 2008;7(2):509–517. doi: 10.4238/vol7-2gmr440. [DOI] [PubMed] [Google Scholar]
- 4.Soares RM, Durigon EL, Bersano JG, Richtzenhain LJ. Detection of porcine parvovirus DNA by the polymerase chain reaction assay using primers to the highly conserved nonstructural protein gene, NS-1. J Virol Methods. 1999;78(1-2):191–198. doi: 10.1016/s0166-0934(98)00177-3. [DOI] [PubMed] [Google Scholar]
- 5.Xu YG, Cui LC, Wang HW, Huo GC, Li SL. Characterization of the capsid protein VP2 gene of a virulent strain NE/09 of porcine parvovirus isolated in China. Res Vet Sci. 2013;94(2):219–224. doi: 10.1016/j.rvsc.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 6.Hanson ND, Rhode SL., 3rd Parvovirus NS1 stimulates P4 expression by interaction with the terminal repeats and through DNA amplification. J Virol. 1991;65(8):4325–4333. doi: 10.1128/jvi.65.8.4325-4333.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anouja F, Wattiez R, Mousset S, Caillet-Fauquet P. The cytotoxicity of the parvovirus minute virus of mice nonstructural protein NS1 is related to changes in the synthesis and phosphorylation of cell proteins. J Virol. 1997;71(6):4671–4678. doi: 10.1128/jvi.71.6.4671-4678.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rhode SL., 3rd Both excision and replication of cloned autonomous parvovirus DNA require the NS1 (rep) protein. J Virol. 1989;63(10):4249–4256. doi: 10.1128/jvi.63.10.4249-4256.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hatada EN, Krappmann D, Scheidereit C. NF-kappaB and the innate immune response. Curr Opin Immunol. 2000;12(1):52–58. doi: 10.1016/s0952-7915(99)00050-3. [DOI] [PubMed] [Google Scholar]
- 10.Hayden MS, Ghosh S. NF-κB in immunobiology. Cell Res. 2011;21(2):223–244. doi: 10.1038/cr.2011.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haddad JJ, Abdel-Karim NE. NF-κB cellular and molecular regulatory mechanisms and pathways: therapeutic pattern or pseudoregulation? Cell Immunol. 2011;271(1):5–14. doi: 10.1016/j.cellimm.2011.06.021. [DOI] [PubMed] [Google Scholar]
- 12.DeDiego ML, Nieto-Torres JL, Regla-Nava JA, Jimenez-Guardeño JM, Fernandez-Delgado R, Fett C, Castaño-Rodriguez C, Perlman S, Enjuanes L. Inhibition of NF-κB-mediated inflammation in severe acute respiratory syndrome coronavirus-infected mice increases survival. J Virol. 2014;88(2):913–924. doi: 10.1128/JVI.02576-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gagliardo R, Chanez P, Profita M, Bonanno A, Albano GD, Montalbano AM, Pompeo F, Gagliardo C, Merendino AM, Gjomarkaj M. IκB kinase-driven nuclear factor-κB activation in patients with asthma and chronic obstructive pulmonary disease. J Allergy Clin Immunol. 2011;128(3):635–645.e1. doi: 10.1016/j.jaci.2011.03.045. [DOI] [PubMed] [Google Scholar]
- 14.Jiang G, Dandekar S. Targeting NF-κB signaling with protein kinase C agonists as an emerging strategy for combating HIV latency. AIDS Res Hum Retroviruses. 2015;31(1):4–12. doi: 10.1089/aid.2014.0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iwasaki A. A virological view of innate immune recognition. Annu Rev Microbiol. 2012;66(66):177–196. doi: 10.1146/annurev-micro-092611-150203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Santoro MG, Rossi A, Amici C. NF-kappaB and virus infection: who controls whom. EMBO J. 2003;22(11):2552–2560. doi: 10.1093/emboj/cdg267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jenkins KA, Mansell A. TIR-containing adaptors in toll-like receptor signalling. Cytokine. 2010;49(3):237–244. doi: 10.1016/j.cyto.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 18.O'Neill LA, Golenbock D, Bowie AG. The history of toll-like receptors - redefining innate immunity. Nat Rev Immunol. 2013;13(6):453–460. doi: 10.1038/nri3446. [DOI] [PubMed] [Google Scholar]
- 19.Wang Y, Zhang P, Liu Y, Cheng G. TRAF-mediated regulation of immune and inflammatory responses. Sci China Life Sci. 2010;53(2):159–168. doi: 10.1007/s11427-010-0050-3. [DOI] [PubMed] [Google Scholar]
- 20.Tak PP, Firestein GS. NF-kappaB: a key role in inflammatory diseases. J Clin Invest. 2001;107(1):7–11. doi: 10.1172/JCI11830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Randall RE, Goodbourn S. Interferons and viruses: an interplay between induction, signalling, antiviral responses and virus countermeasures. J Gen Virol. 2008;89(Pt 1):1–47. doi: 10.1099/vir.0.83391-0. [DOI] [PubMed] [Google Scholar]
- 22.Chehadeh W, Alkhabbaz M. Differential TLR7-mediated expression of proinflammatory and antiviral cytokines in response to laboratory and clinical enterovirus strains. Virus Res. 2013;174(1-2):88–94. doi: 10.1016/j.virusres.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 23.Zipris D, Lien E, Nair A, Xie JX, Greiner DL, Mordes JP, Rossini AA. TLR9-signaling pathways are involved in Kilham rat virus-induced autoimmune diabetes in the biobreeding diabetes-resistant rat. J Immunol. 2007;178(2):693–701. doi: 10.4049/jimmunol.178.2.693. [DOI] [PubMed] [Google Scholar]
- 24.Sieben M, Schäfer P, Dinsart C, Galle PR, Moehler M. Activation of the human immune system via toll-like receptors by the oncolytic parvovirus H-1. Int J Cancer. 2013;132(11):2548–2556. doi: 10.1002/ijc.27938. [DOI] [PubMed] [Google Scholar]
- 25.Hsu GJ, Tzang BS, Tsai CC, Chiu CC, Huang CY, Hsu TC. Effects of human parvovirus B19 on expression of defensins and toll-like receptors. Chin J Physiol. 2011;54(5):367–376. [PubMed] [Google Scholar]
- 26.Zhou Y, Jin XH, Jing YX, Song Y, He XX, Zheng LL, Wang YB, Wei ZY, Zhang GP. Porcine parvovirus infection activates inflammatory cytokine production through toll-like receptor 9 and NF-κB signaling pathways in porcine kidney cells. Vet Microbiol. 2017;207:56–62. doi: 10.1016/j.vetmic.2017.05.030. [DOI] [PubMed] [Google Scholar]
- 27.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 28.Cao L, Chen J, Wei Y, Shi H, Zhang X, Yuan J, Shi D, Liu J, Zhu X, Wang X, Cui S, Feng L. Porcine parvovirus induces activation of NF-κB signaling pathways in PK-15 cells mediated by toll-like receptors. Mol Immunol. 2017;85:248–255. doi: 10.1016/j.molimm.2016.12.002. [DOI] [PubMed] [Google Scholar]
- 29.Kawai T, Akira S. Signaling to NF-kappaB by Toll-like receptors. Trends Mol Med. 2007;13(11):460–469. doi: 10.1016/j.molmed.2007.09.002. [DOI] [PubMed] [Google Scholar]