Abstract
Background
The poultry red mite, Dermanyssus gallinae, is a serious problem in the laying hen industry worldwide. Currently, the foremost control method for D. gallinae is the implementation of integrated pest management, the effective application of which necessitates a precise monitoring method.
Objectives
The aim of the study was to propose an accurate monitoring method with a reliable protocol for caged-layer poultry farms, and to suggest an objective classification for assessing D. gallinae infestation on caged-layer poultry farms according to the number of mites collected using the developed monitoring method.
Methods
We compared the numbers of mites collected from corrugated cardboard traps, regarding with length of sampling periods, sampling sites on cage, and sampling positions in farm buildings. The study also compared the mean numbers of mites collected by the developed method with the infestation levels using by the conventional monitoring methods in 37 caged-layer farm buildings.
Results
The statistical validation provided the suitable monitoring method that the traps were installed for 2 days on feed boxes at 27 sampling points which included three vertical levels across nine equally divided zones of farms. Using this monitoring method, the D. gallinae infestation level can be assessed objectively on caged-layer poultry farms. Moreover, the method is more sensitive than the conventional method in detecting very small populations of mites.
Conclusions
This method can be used to identify the initial stages of D. gallinae infestation in the caged-layer poultry farms, and therefore, will contribute to establishment of effective control strategies for this mite.
Keywords: Poultry red mite, Dermanyssus gallinae, monitoring method, caged-layer poultry farm, infestation level
INTRODUCTION
Dermanyssus gallinae (De Geer, 1875), also known as the poultry red mite, is a serious problem in the laying hen industry worldwide [1,2,3]. D. gallinae is a small ectoparasite (approximately 1.5 mm in length) that is characterized by five developmental stages: egg, larva, protonymph, deutonymph, and adult [4]. To develop from the protonymph to adult stage, and to lay eggs thereafter, these mites need to feed on blood meals [4]. During these developmental stages, D. gallinae causes several adverse effects in host birds, including anemia in hens, reduced egg quality, and increased feed and water intake [1,5,6]. Moreover, given that the life cycle of this mite can be completed within 1 week, heavy infestations of D. gallinae in layer poultry farms can occur within a short time period (30–70 days) [3,7,8,9]. D. gallinae is also known as a vector of avian pathogens, including paramyxovirus, Escherichia coli, Salmonella Enteritidis, and avian influenza A virus, that can infect animals and humans [2,10,11,12,13]. To overcome the problems caused by this mite, it is necessary to establish control strategies that are appropriate for the layer farm environment.
Currently, the best-known control method for D. gallinae is the implementation of integrated pest management (IPM) [3,14]. Briefly, IPM comprises the following five stages: 1) prevention, 2) monitoring for diagnosis, 3) application of non-chemical control strategies, 4) application of chemical control strategies, and 5) monitoring the effects of control strategy application. Among these steps, monitoring is a key component for the appropriate application of IPM programs for D. gallinae [1]. However, given the particular life cycle characteristics of D. gallinae, notably the fact that it lives off-host and feeds only intermittently during the night, the monitoring of this mite tends to be difficult [15]. For this reason, numerous methods and devices have been introduced for detecting infestations of D. gallinae on layer poultry farms, including the mite monitoring score (MMS), cardboard or tube traps, examination of droppings or dust, and automatic counter devices [16,17,18,19,20]. Although each of these methods has its own strengths, most only can be used to identify the present or proliferative trends of mites [21]. Moreover, the efficacy of these methods has only been validated with respect to European aviary farms, and there are no practical guidelines for monitoring under field conditions on caged-layer farms [22]. In most Asian countries, including Korea, laying hens are raised in cage system houses, and to the best of our knowledge, there have been no detailed studies that have examined the monitoring of D. gallinae at these facilities.
Previously, Working Group 2 of the Control of the poultry red mite project (COST Action FA 1404) has reported that the optimal monitoring tool for this mite should be durable and reliable, have low handling costs, and be readily implemented (https://www.coremi.eu/fileadmin/documents_organicresearch/coremi/On-farm_monitoring_protocol__under_construction_.pdf). Among the several monitoring approaches, the corrugated cardboard trap method is economically viable and simple to implement [18]. In this study, we describe a simple method for objectively assessing the levels of D. gallinae infestation in caged-layer poultry houses by using corrugated cardboard traps. Moreover, we provide a detailed sampling method for monitoring D. gallinae under field conditions on cage system farms, including specifications relating to the sampling period, the location of installed traps, and the number of traps required. In addition, we propose an objective classification of D. gallinae infestation levels for caged-layer poultry farms on the basis of the number of mites collected from traps.
MATERIALS AND METHODS
Appropriate length of sampling period using corrugated cardboard traps
To determine a suitable sampling period duration for monitoring D. gallinae infestation on caged-layer poultry farms, we conducted a study over a 7-day period on a caged-layer poultry farm for egg production in Korea, which had previously experienced recurrent D. gallinae-associated problems. The traps were constructed from rectangular pieces of corrugated cardboard measuring 100 by 70 mm with 2.5 mm thickness. The corrugated cardboard was cut to expose corrugations along the longer side. Four traps were attached by double-sided tape to the exterior of feed boxes at identical cages for monitoring periods of 1, 2, 4, and 7 days. A total of 108 traps (27 traps per sampling period) were used. After collection, the traps were immediately placed separately in self-sealing storage bags for transport to the laboratory, where they were frozen at −20°C for 24 h. Thereafter, we poured trapped frozen mites onto a petri dish and counted the number of all stages of the mite (except eggs) in each trap upon thawing using a stereo microscope (20×) or a magnifying glass (10×).
Position of the sampling site on cages
To assess the effect of the trap position within the cage structure for quantitative evaluation of the presence of D. gallinae, we installed a total of 54 traps on metal cages, positioned on the exterior of the feed box (n = 18), on the floor of the cage (n = 18), and under the egg channel (n = 18). The traps on the floor of the cage were positioned at the inter-space of the hen cages where the traps were not pecked or damaged by the hens. Traps were collected after 2 days and the mites in traps were counted as described above.
Optimal sampling position and site in farm buildings
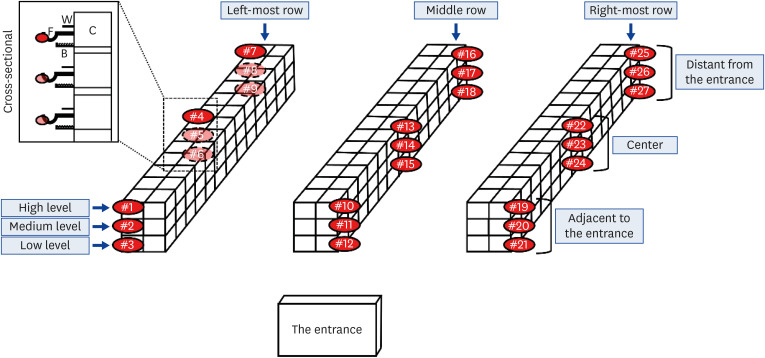
To determine the optimal sampling positions and sites to provide representative sampling of D. gallinae infestation within an entire caged-layer poultry house, we compared the mean numbers of mites collected from six farms in different regional locations. The experiment was performed using six cage farm buildings where low (n = 3) and moderate to heavy (n = 3) D. gallinae infestation had been detected according to the conventional monitoring method (MMS), which provides an indication of farm mite populations at five levels [16,17]: 0 = no mites visible; I = mites visible in cracks and crevices; II = mites visible in unprotected sites; III = clusters of mites (groups of mites larger than 1 cm2) visible in cracks and crevices; and IV = clusters of mites visible in unprotected sites in and on farm equipment. The number of optimal sampling sites (n = 27) was predetermined based on a previous study, which reported that by using 11 to 19 corrugated cardboard traps (100 by 70 mm with 3 mm thickness) to routinely monitor mite populations, a relative variability of ~20% would be obtained [18]. As shown in Fig. 1, the traps were placed on feed boxes located at three horizontal positions (adjacent to the entrance of a farm building zone, the central zone, and distant from the entrance zone) in three selected rows (left-most row, middle row, and right-most row relative to the entrance of a farm building) at three vertical levels (low, medium, and high). The traps (n = 27) installed on each farm (n = 6) were removed after 2 days and mites were counted as previously described. The mean numbers of mites recovered from the three different sampling zones (horizontal positions, vertical levels, and different rows) were compared to evaluate the variation in infestation burdens in a single caged-layer poultry farm building. Moreover, the mean numbers of mites collected from each sampling point were compared with other points to determine variations in the numbers of mites recovered from the different sampling points.
Fig. 1. Schematic diagram of a caged-layer poultry house. Reddish circles represent the locations of traps constructed from rectangular pieces of corrugated cardboard measuring 100 × 70 × 2.5 mm. A schematic cross-sectional side view.
C, cage; W, water pipe; F, feed box; B, egg channel.
Correlation between our developed trap method and the MMS method
To validate our monitoring method and to establish objective criteria for assessing D. gallinae infestation levels in caged-layer poultry houses, we compared the average numbers of mites collected in traps with the infestation levels determined using the MMS method [16,18]. For the purposes of this assessment, D. gallinae was monitored on 37 caged-layer poultry farms in Korea using the two methods. Among the 37 selected poultry houses, 21 (56.76%) had no D. gallinae problem, whereas D. gallinae infestation had been reported in the remaining 16 (43.24%).
Statistical analysis
Statistical analysis was performed using SPSS software, version 25.0 (IBM Corp., Armonk, USA). To analyze the data, we used a one-way ANOVA, with a p value of < 0.05 being considered significant. Duncan's test was also used to determine statistically significant differences. Data from the experiment comparing the numbers of collected mites were analyzed using Pearson's correlation coefficient, which was used to calculate the relationship among the number of mites collected from the exterior of the feedbox, on the floor of the cage, and under the egg channel of the cage.
RESULTS
Appropriate length of sampling period and position of corrugated cardboard traps on cages
The mean (± SEM) number of D. gallinae collected from the corrugated cardboard traps (n = 108) over collection periods of 1, 2, 4, and 7 days was 28.07 ± 10.34, 49.48 ± 23.98, 197.44 ± 115.06, and 1315.46 ± 880.14, respectively. With regard to the developmental stages of the mites, the proportions of D. gallinae's eggs, larvae, nymphs, and adults among the overall collected mites during the sampled period in this study as followed: 1 day (1.99%, 2.51%, 36.42%, and 59.08%, respectively), 2 days (0.92%, 2.15%, 47.15%, 49.79%, respectively), 4 days (24.78%, 0.98%, 32.72%, 41.52%, respectively) and 7 days (31.28%, 2.51%, 40.36%, 25.86%, respectively).
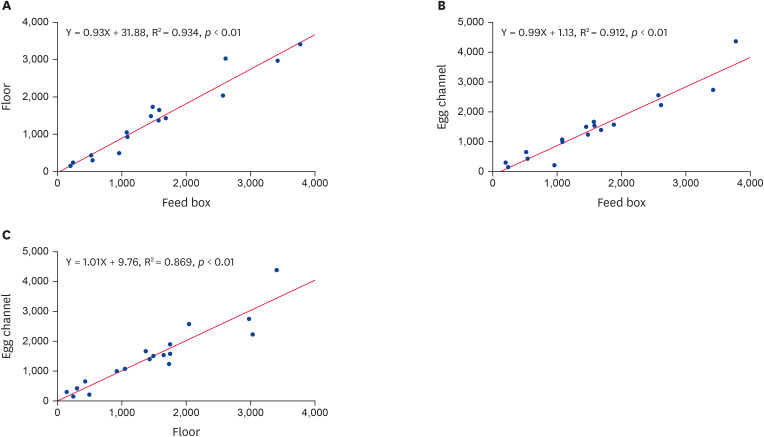
The 18 data points for each of the three trap installation positions within the metal cages and the modeled regression lines obtained from the analysis are shown in Fig. 2. The R2 values for comparisons between traps installed on feed boxes and under egg channels (R2 = 0.934, p < 0.01), between traps on feed boxes and on floors (R2 = 0.912, p < 0.01), and between traps on floors and under egg channels (R2 = 0.869, p < 0.01) all showed highly positive linear correlations.
Fig. 2. Comparison of the average number of Dermanyssus gallinae collected from three different sites on the same cage. Values of R2 and P were obtained from linear regression analysis. (A) Feed box vs. floor traps, (B) feed box vs. egg channel traps, and (C) floor vs. egg channel traps.
Comparison of the average number of mites obtained from different sampling zones
As described in the Materials and Methods section, the average number of mites collected from corrugated cardboard traps was examined in relation to nine trap installation sites (three horizontal positions, three vertical levels, and three selected rows). Among the six monitored caged-layer poultry farms in this study, three were scored as MMS level I, whereas the other three were classified as level II to III (Table 1). With regard to horizontal positions in the same row, on farms with MMS level I, the average number of mites collected from traps sited distant from the entrance zone (109.07 mites) was significantly higher than that obtained from traps sited both adjacent to the entrance (53.48) and in the center of the row (55.85). In contrast, on farms with MMS level II to III, the number of mites collected from traps distant from the entrance (182.15) was significantly lower than that collected from traps sited near the entrance (463.33) and in the row center (445.37). For traps installed at different vertical levels within the same horizontal zone, we detected no significant difference on farms with either low or moderate to heavy infestation. However, with respect to selected rows within a building, the numbers of mites recovered from the right-most row (100.30 and 519.44 for low and moderate to heavy infestation farms, respectively) were significantly higher than those collected from the left-most row (52.74 and 231.48, respectively) in the experimental poultry houses.
Table 1. The average number of Dermanyssus gallinae collected from corrugated cardboard traps located in three different horizontal zones, vertical levels, and rows in caged-layer poultry houses with either low (n = 3) or moderate to heavy (n = 3) infestation.
| D. gallinae infestation | Variable | Sampling zones | Mean ± SEM* | p value |
|---|---|---|---|---|
| Low infestation level using the MMS method (level I) | Horizontal positions | Adjacent to entrance zone | 53.48 ± 12.73a | 0.009 |
| Center zone | 55.85 ± 9.32a | |||
| Distant from entrance zone | 109.07 ± 19.85b | |||
| Vertical levels | High level zone | 77.96 ± 13.52a | 0.190 | |
| Medium level zone | 87.70 ± 19.05a | |||
| Low level zone | 52.74 ± 12.18a | |||
| Selected rows | Left-most row zone | 51.96 ± 8.80a | 0.047 | |
| Middle row zone | 66.15 ± 14.79ab | |||
| Right-most row zone | 100.30 ± 19.38b | |||
| Moderate to heavy infestation level using the MMS method (level II–III) | Horizontal positions | Adjacent to entrance zone | 463.33 ± 114.68a | 0.031 |
| Center zone | 445.37 ± 106.76a | |||
| Distant from entrance zone | 182.15 ± 40.71b | |||
| Vertical levels | High level zone | 202.56 ± 43.58a | 0.060 | |
| Medium level zone | 426.85 ± 123.05a | |||
| Low level zone | 461.44 ± 97.74a | |||
| Selected rows | Left-most row zone | 231.48 ± 47.06a | 0.050 | |
| Middle row zone | 339.93 ± 83.09ab | |||
| Right-most row zone | 519.44 ± 55.19b |
MMS, mite monitoring score.
*Means within a column followed by the same superscript are not significantly different at the 5% level of significance (Duncan test).
Comparison of the average number of mites obtained from 27 predetermined sampling points
The average numbers of D. gallinae collected in the traps at different predetermined sampling points (n = 27) in the six monitored caged-layer poultry houses are shown in Table 2. The results from the 27 sampling points showed significant differences among farms with MMS level I (p = 0.049) and level II to III (p = 0.009) infestation. In the three farms with low infestation levels, the average numbers of mites recovered from sampling point #26 (231.67 mites) were most significantly higher than those at other points, followed by those collected from sampling points #27 (176.67) and #16 (170.67). In the case of farms with moderate to heavy infestation, the mean numbers of mites collected from sampling point #20 (1,677 mites) were significantly higher than those at other sampling points, followed by those collected from sampling point #15 (1,157.67).
Table 2. The average number of Dermanyssus gallinae collected in corrugated cardboard traps installed at 27 sampling points in caged-layer poultry houses.
| Sampling point | Numbers of collected mites | |
|---|---|---|
| Farms (n = 3) with low infestation | Farms (n = 3) with moderate to heavy infestation | |
| Mean ± SEM* | Mean ± SEM* | |
| Point–#1 | 56.33 ± 32.04abc | 81 ± 22.03a |
| Point–#2 | 27 ± 12.66ab | 211 ± 33.83a |
| Point–#3 | 18 ± 12.58a | 473.33 ± 89.84ab |
| Point–#4 | 99 ± 43.66abc | 316.67 ± 251.15a |
| Point–#5 | 56.33 ± 14.31abc | 211.67 ± 132.75a |
| Point–#6 | 20.33 ± 5.55a | 191.67 ± 103.24a |
| Point–#7 | 74.67 ± 32.05abc | 337 ± 308.65a |
| Point–#8 | 79 ± 35.68abc | 136 ± 48.18a |
| Point–#9 | 37 ± 4.04abc | 125 ± 61.26a |
| Point–#10 | 41.33 ± 34.57abc | 272.33 ± 50.02a |
| Point–#11 | 13.67 ± 10.37a | 327.67 ± 228.02a |
| Point–#12 | 26.67 ± 25.67ab | 315.67 ± 161.60a |
| Point–#13 | 59.67 ± 32.77abc | 203.33 ± 56.63a |
| Point–#14 | 84.33 ± 39.50abc | 338.33 ± 101.62a |
| Point–#15 | 44 ± 27.23abc | 1,157.67 ± 539.84bc |
| Point–#16 | 170.67 ± 78.77cd | 152.33 ± 112.88a |
| Point–#17 | 84.33 ± 47.80abc | 140 ± 69.60a |
| Point–#18 | 70.67 ± 56.90abc | 152 ± 112.88a |
| Point–#19 | 77.67 ± 25.22abc | 182.33 ± 63.18a |
| Point–#20 | 165.33 ± 70.07bcd | 1,677 ± 670.29c |
| Point–#21 | 55.33 ± 36.79abc | 629.67 ± 257.99ab |
| Point–#22 | 65.33 ± 31.74abc | 149.67 ± 32.54a |
| Point–#23 | 47.67 ± 28.00abc | 704 ± 463.54ab |
| Point–#24 | 26 ± 15.72ab | 735.33 ± 495.56ab |
| Point–#25 | 57 ± 37.61abc | 128.33 ± 94.18a |
| Point–#26 | 231.67 ± 108.83d | 96 ± 76.00a |
| Point–#27 | 176.67 ± 32.92cd | 372.67 ± 132.42a |
| p value | 0.049 | 0.009 |
*Means followed by the same superscript are not significantly different at the 5% level of significance (Duncan test).
Correlation between the numbers of D. gallinae collected in corrugated cardboard traps and D. gallinae infestation level estimated using the MMS method
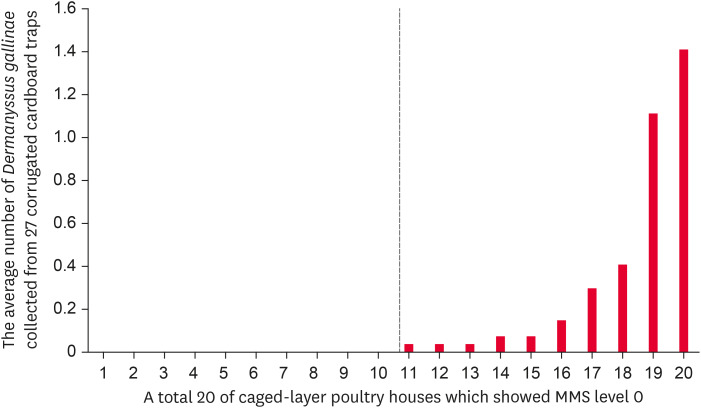
Among the 37 caged-layer poultry farms monitored in the present study, the MMS levels were as follows: 0 (n = 20 farms), I (n = 5), II (n = 4), III (n = 4), and IV (n = 4). The average numbers of mites collected from the 27 traps installed in poultry houses with MMS levels 0, I, II, III, and IV were 0.18, 68.64, 293.62, 415.23, and 923.94, respectively (Table 3). With the exception of MMS levels 0 and I, we found that there were significant differences among the average numbers of mites collected using our developed monitoring method based on the infestation level of poultry houses estimated using the MMS method (p < 0.001). The lowest numbers (mean ± SEM) of mites collected among farms with MMS levels 0, I, II, III, and IV were 0, 2.22 ± 0.55, 205.96 ± 51.47, 379.85 ± 119.60, and 503.93 ± 66.54, respectively, whereas the highest numbers were 1.41 ± 0.50, 122.56 ± 30.83, 370.63 ± 132.36, 451.44 ± 82.75, and 1909.96 ± 392.12, respectively. Notably, when using our developed monitoring method, we succeeded in detecting D. gallinae in the range of 0.37 to 1.41 mites on 10 (50%) of the 20 poultry farms assessed as level 0 using the MMS method (Fig. 3).
Table 3. Correlation between MMS levels and the mean numbers of mites collected from 27 corrugated cardboard traps in 37 caged-layer poultry houses.
| MMS level | Number of farms | The average numbers of mites collected using 27 corrugated cardboard traps in caged-layer poultry house | ||
|---|---|---|---|---|
| Mean ± SEM* | Minimum | Maximum | ||
| 0 | 20 | 0.18 ± 0.04a | 0 | 1.41 ± 0.50 |
| I | 5 | 68.64 ± 8.71a | 2.22 ± 0.55 | 122.56 ± 30.83 |
| II | 4 | 293.62 ± 39.10b | 205.96 ± 51.47 | 370.63 ± 132.36 |
| III | 4 | 415.23 ± 49.58c | 379.85 ± 119.60 | 451.44 ± 82.75 |
| IV | 4 | 923.94 ± 121.15d | 503.93 ± 66.54 | 1,909.96 ± 392.12 |
MMS, mite monitoring score.
*Means followed by the same superscript are not significantly different at the 5% level of significance (Duncan test).
Fig. 3. Among the 20 caged-layer poultry houses classified as having a MMS of level 0, Dermanyssus gallinae was detected in 10 poultry houses (50%) using the monitoring method developed in this study. Among these 10 houses, the average number of mites collected from 27 corrugated cardboard traps ranged from 0.37 to 1.41, indicating that the houses were infected with very small populations of D. gallinae.
MMS, mite monitoring score.
DISCUSSION
According to a previous report, most layer farms in Asian countries still use a cage system for egg production, including India (100%), Malaysia (99%), Japan (95%), and China (90%) [23]. The aim of the present study was to develop and demonstrate the utility of a simple monitoring method with a detail protocol that could be employed to precisely establish the presence and extent of D. gallinae infestation on caged-layer poultry farms. Furthermore, we sought to establish objective standards for this method when used to assess D. gallinae infestation levels on these farms.
D. gallinae is a nocturnal feeder that spends daylight hours hidden in myriad cracks and crevices [22]. Given that caged-layer poultry houses provide D. gallinae with numerous available shelters to digest blood meals, mate, and lay eggs, it is difficult to monitor D. gallinae infestation on cage system farms [24]. In this study, we used traps constructed from corrugated cardboard with 2.5 mm thickness as monitoring devices for D. gallinae in caged-layer poultry houses. With regard to the length of the sampling period, we found that the numbers of mites collected increased with an increase in the sampling period. Given that we observed numerous D. gallinae eggs in traps installed for 4 days (24.78% of the overall collected mites) and 7 days (31.28% of the overall collected mites), we determined that sampling periods of 1 and 2 days would provide more accurate monitoring results for determining the level of D. gallinae infestation in caged-layer poultry houses. Although Thomas et al. [25] have indicated that removing traps 24 h after placement would allow adequate time for monitoring in aviary-type poultry houses, we found that the total number of D. gallinae captured in traps installed in caged-layer poultry houses for 1 day provided too small population to obtain reliable monitoring results. Our findings indicate that fast and accurate monitoring of D. gallinae in caged-layer poultry houses can be achieved when the traps are installed for 2 days.
Given that D. gallinae spends the day hidden in cracks and crevices, the required number of traps and the location of sampling points are important considerations for obtaining reliable monitoring data [22]. In this regard, we compared the number of mites recovered from traps installed on the feed box, floor, and egg channel of the same cage to determine the optimal positioning of traps within the cage unit. The results revealed statistically strong correlations among the three different sampling sites on the same cage. In contrast, the findings of a previous study on a cage system farm indicated that traps installed at egg channels contained significantly more mites than those installed on cage floors [26]. We suspect that these differences could be attributable to differences in the length of the sampling period and the design of monitoring devices, as the study conducted by Odaka et al. [26] used two 1 mm thick cedar board traps measuring 45 by 85 mm with a 24 h placement. In the present study, although we detected no significant difference in the numbers of mites collected according to sampling site (on the feed box, on the cage floor, or under the egg channel) within the same metal cage structure, traps attached to feed boxes were simpler to install for farm workers of cage system farms than those deployed at other sites in the cage.
For the effective estimation of the D. gallinae infestation level on poultry farms, it is important to optimize the number and positions of traps. In this regard, a previous study showed that 11 to 19 corrugated cardboard traps (100 by 70 mm with 3 mm thickness) would be sufficiently effective for monitoring D. gallinae populations, with a relative variability of ~20% [18]. On the basis of this result, we determined 27 trap positions per caged-layer poultry house (Fig. 1). To assess the efficacy of traps installed at these 27 predetermined positions, we examined variations in the number of D. gallinae recovered from each sampling zone on three farms with low D. gallinae infestation and three with moderate to heavy infestation. We compared the number of mites collected from cages uniformly divided into three equal zones, according to horizontal position in the same row, vertical level, and row position in the caged-layer poultry farm building relative to the entrance. Interestingly, on farms with low D. gallinae infestation, the number of mites collected from the horizontal zone most distant from the entrance was significantly higher than that collected from either the zone near the entrance or the center of buildings, whereas on farms with moderate to heavy D. gallinae infestation, significantly fewer mites were collected from the zone most distant from the entrance compared with those collected from the other zones. Accordingly, these results could indicate that D. gallinae proliferation initially commences in a zone distant from the entrance of a farm building, with mites moving closer to the door concomitant with an increase in their numbers. Therefore, the results imply that monitoring of different horizontal zones within the same row is essential for the detection of D. gallinae on farms with variable levels of infestation. Furthermore, the results also indicated the possibility of a simpler and more reasonable method for the early stage of mite infection, which would involve the installation of a small number of traps in zones distant from the entrance. In contrast, on farms with low levels of D. gallinae infestation, we detected no significant difference with respect to the vertical distribution of monitoring devices. However, on farms with moderate to heavy infestation, we observed that the number of mites collected from the lower two levels was two-fold higher than that captured by traps installed at the upper-most level, implying that the vertical distribution should also be monitored to precisely evaluate D. gallinae infestation in caged-layer poultry houses. These findings are consistent with those obtained in a previous study conducted on an aviary system farm, which indicated that the type of hybrid hens used and their preferential vertical mobility may promote differences in vertical mite distribution [27]. We also selected the left-most, middle, and right-most rows in poultry houses relative to a farm building entrance to compare the numbers of D. gallinae collected. Contrary to expectation, a significantly higher number of mites were collected from the right-most rows than were collected from the left-most rows on farms with both low (p = 0.047) and moderate to heavy (p = 0.05) infestations. We suspect that this discrepancy could be attributable to a difference in temperature. Among the farms examined in this study, three farms (two with low and one with heavy infestation) had windows on the right-hand side of the farm building. Accordingly, the temperature may have been slightly higher in the right-most row than in the other two rows, and this may have acted as an attractant for D. gallinae, as has been described in previous studies [28,29]. Our results indicate that selection of the two end rows and the middle row could yield reliable monitoring results. In agreement with previous studies conducted on aviary and free-range layer farms, the overall results demonstrated that the distribution of D. gallinae in caged-layer poultry houses shows spatial variation, depending upon environmental conditions and the infestation level of D. gallinae on individual farms [20,27]. Furthermore, with regard to the 27 sampling points assessed in the present study, we found that the differences in the number of collected mites from each sampling point were significantly different at both low and moderate to heavy D. gallinae infestation farms, which also supports our assertion that the pre-determined sampling points are suitable for monitoring D. gallinae in caged-layer poultry houses.
Therefore, we believe that the sampling method we propose for monitoring mites on caged-layer poultry farms will enable those monitoring these farms to obtain objective and consistent results in various situations. However, further studies will be needed to adjust our established monitoring method for different structural types of caged-layer poultry farm buildings. This monitoring method has a limitation in that farm workers must count the D. gallinae collection individually. If mites were abundant in traps, the numbers of D. gallinae present could be calculated by weight according to the method proposed by Meyer-Kühling et al. [30].
In the present study, we monitored D. gallinae on 37 caged-layer poultry farms using both our established method and the MMS method. We accordingly found that among 20 hen houses with designated MMS level 0, we were able to detect D. gallinae in 10 hen houses (50%), thereby indicating that our monitoring method is very sensitive when used on caged-layer poultry farms. We therefore believe that this method represents an effective monitoring tool that can be used to enhance IPM for the control of D. gallinae on cage-system farms based on the initial confirmation of mite infection. Comparison of our established method and the MMS method indicated that, with the exception of MMS levels 0 and I, the average numbers of mites collected showed a significant difference according to the MMS levels of farms. We believe that the monitoring method described in this study can be used to provide an objective assessment of D. gallinae infestation levels on caged-layer poultry farms and could be used as an alternative to the conventional MMS method for identifying D. gallinae infestation on farms. Further, we established detailed criteria based on the minimum and maximum number of mites (mean ± SEM) collected on farms corresponding to each MMS level. Corresponding to MMS levels 0, I, II, and III, and IV, the infestation levels determined using our method were based on the average number of D. gallinae covering four levels of mite infestation: clean = 0 mites, low infestation = 1 to 150 mites, heavy infestation = 151 to 500 mites, and severe infestation = more than 501 mites. Although the definition of a threshold level obtained in this study did not correspond with whole numbers of D. gallinae populations on farms, the overall results can be considered valuable from a monitoring perspective for determining the level of D. gallinae infestation and the timing of control treatment initiation for the effective control of this mite in caged-layer hen houses.
In this study, we found that installation of 27 corrugated cardboard monitoring traps with dimensions of 100 × 70 × 2.5 mm on feed boxes for 2 days was an effective approach for monitoring D. gallinae within the hen houses of caged-layer poultry farms. This study also established four reliable levels of D. gallinae infestation that correspond to the categories of the MMS method. The method introduced in this study is a simple and relevant method for monitoring the D. gallinae infestation level on caged-layer poultry farms, for which we also established a detailed protocol for its application in the field. Moreover, the method is particularly useful with respect to identifying the initial stages of D. gallinae infestation, and therefore will be extremely beneficial for establishing preventative strategies and assessing the effectiveness of such prevention methods.
ACKNOWLEDGMENTS
The authors appreciate Guntai Noh for assistance in sampling. We also thank the Korea Corrugated Packaging Case Industry Association for assistance in manufacturing the traps used in this study.
Footnotes
Funding: This work was carried out with the support of “Cooperative Research Program for Agriculture Science and Technology Development (Project title: Development of monitoring techniques for poultry red mite (Dermanyssus gallinae), Project No.: PJ01345502)”, Rural Development Administration, Republic of Korea.
Conflict of interest: The authors have no conflicts of interest to declare.
- Conceptualization: Oh SI, Yoo JG.
- Data curation: Oh SI, Park KT, Yoo JG.
- Formal analysis: Oh SI.
- Funding acquisition: Yoo JG.
- Investigation: Oh SI, Jung Y, Do YJ, Choe C, Cho A.
- Methodology: Oh SI, Park KT, Yoo JG.
- Project administration: Yoo JG, Do YJ.
- Resources: Oh SI, Park KT.
- Software: Oh SI, Kim S.
- Supervision: Yoo JG.
- Validation: Kim S, Yoo JG.
- Visualization: Oh SI.
- Writing - original draft: Oh SI.
- Writing - review & editing: Yoo JG.
References
- 1.Sigognault Flochlay A, Thomas E, Sparagano O. Poultry red mite (Dermanyssus gallinae) infestation: a broad impact parasitological disease that still remains a significant challenge for the egg-laying industry in Europe. Parasit Vectors. 2017;10(1):357. doi: 10.1186/s13071-017-2292-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.George DR, Finn RD, Graham KM, Mul MF, Maurer V, Moro CV, Sparagano OA. Should the poultry red mite Dermanyssus gallinae be of wider concern for veterinary and medical science? Parasit Vectors. 2015;8(1):178. doi: 10.1186/s13071-015-0768-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sparagano OA, George DR, Harrington DW, Giangaspero A. Significance and control of the poultry red mite, Dermanyssus gallinae . Annu Rev Entomol. 2014;59(1):447–466. doi: 10.1146/annurev-ento-011613-162101. [DOI] [PubMed] [Google Scholar]
- 4.Axtell RC, Arends JJ. Ecology and management of arthropod pests of poultry. Annu Rev Entomol. 1990;35(1):101–126. doi: 10.1146/annurev.en.35.010190.000533. [DOI] [PubMed] [Google Scholar]
- 5.Kilpinen O, Roepstorff A, Permin A, Nørgaard-Nielsen G, Lawson LG, Simonsen HB. Influence of Dermanyssus gallinae and Ascaridia galli infections on behaviour and health of laying hens (Gallus gallus domesticus) Br Poult Sci. 2005;46(1):26–34. doi: 10.1080/00071660400023839. [DOI] [PubMed] [Google Scholar]
- 6.Mul M, van Niekerk T, Chirico J, Maurer V, Kilpinen O, Sparagano O, Thind B, Zoons J, Moore D, Bell B, Gjevre AG, Chauve C. Control methods for Dermanyssus gallinae in systems for laying hens: results of an international seminar. Worlds Poult Sci J. 2009;65(4):589–600. [Google Scholar]
- 7.Brännström S, Morrison DA, Mattsson JG, Chirico J. Genetic differences in internal transcribed spacer 1 between Dermanyssus gallinae from wild birds and domestic chickens. Med Vet Entomol. 2008;22(2):152–155. doi: 10.1111/j.1365-2915.2008.00722.x. [DOI] [PubMed] [Google Scholar]
- 8.Liebisch A, Liebisch G. Biologie, Schäden und Bekämpfung beim Befall durch die Rote Vogelmilbe (Dermanyssus gallinae) Lomhmann Inf. 2003;4:1. [Google Scholar]
- 9.Maurer V, Baumgärtner J. A population model for Dermanyssus gallinae (Acari: Dermanyssidae) Exp Appl Acarol. 1994;18(7):409–422. doi: 10.1007/BF00051523. [DOI] [PubMed] [Google Scholar]
- 10.De Luna CJ, Arkle S, Harrington D, George DR, Guy JH, Sparagano OA. The poultry red mite Dermanyssus gallinae as a potential carrier of vector-borne diseases. Ann N Y Acad Sci. 2008;1149(1):255–258. doi: 10.1196/annals.1428.085. [DOI] [PubMed] [Google Scholar]
- 11.Valiente Moro C, Chauve C, Zenner L. Experimental infection of Salmonella Enteritidis by the poultry red mite, Dermanyssus gallinae . Vet Parasitol. 2007;146(3-4):329–336. doi: 10.1016/j.vetpar.2007.02.024. [DOI] [PubMed] [Google Scholar]
- 12.Valiente Moro C, De Luna CJ, Tod A, Guy JH, Sparagano OA, Zenner L. The poultry red mite (Dermanyssus gallinae): a potential vector of pathogenic agents. Exp Appl Acarol. 2009;48(1-2):93–104. doi: 10.1007/s10493-009-9248-0. [DOI] [PubMed] [Google Scholar]
- 13.Sommer D, Heffels-Redmann U, Köhler K, Lierz M, Kaleta EF. Role of the poultry red mite (Dermanyssus gallinae) in the transmission of avian influenza A virus. Tierarztl Prax Ausg G Grosstiere Nutztiere. 2016;44(1):26–33. doi: 10.15653/TPG-150413. [DOI] [PubMed] [Google Scholar]
- 14.Mul MF, van Riel JW, Roy L, Zoons J, André G, George DR, Meerburg BG, Dicke M, van Mourik S, Groot Koerkamp PW. Development of a model forecasting Dermanyssus gallinae’s population dynamics for advancing Integrated Pest Management in laying hen facilities. Vet Parasitol. 2017;245:128–140. doi: 10.1016/j.vetpar.2017.07.027. [DOI] [PubMed] [Google Scholar]
- 15.Mul MF, Ploegaert JP, George DR, Meerburg BG, Dicke M, Koerkamp PW. Structured design of an automated monitoring tool for pest species. Biosyst Eng. 2016;151:126–140. [Google Scholar]
- 16.Cox M, De Baere K, Vervaet E, Zoons J, Fiks-Van Niekerk T. Book of 8th European Symposium on Poultry Welfare, Cervia, Italy. Beekbergen: World's Poultry Science Association; 2009. Red mites: monitoring method and treatment; p. 83. [Google Scholar]
- 17.Mul MF, van Riel JW, Meerburg BG, Dicke M, George DR, Groot Koerkamp PW. Validation of an automated mite counter for Dermanyssus gallinae in experimental laying hen cages. Exp Appl Acarol. 2015;66(4):589–603. doi: 10.1007/s10493-015-9923-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nordenfors H, Chirico J. Evaluation of a sampling trap for Dermanyssus gallinae (Acari: Dermanyssidae) J Econ Entomol. 2001;94(6):1617–1621. doi: 10.1603/0022-0493-94.6.1617. [DOI] [PubMed] [Google Scholar]
- 19.Pavličević A, Pavlović I, Stajković N. Method for early detection of poultry red mite Dermanyssus gallinae (De Geer, 1778) Biotechnol Anim Husb. 2007;23(3-4):119–127. [Google Scholar]
- 20.Zenner L, Bon G, Chauve C, Nemoz C, Lubac S. Monitoring of Dermanyssus gallinae in free-range poultry farms. Exp Appl Acarol. 2009;48(1-2):157–166. doi: 10.1007/s10493-009-9253-3. [DOI] [PubMed] [Google Scholar]
- 21.Lammers GA, Bronneberg RG, Vernooij JC, Stegeman JA. Experimental validation of the AVIVET trap, a tool to quantitatively monitor the dynamics of Dermanyssus gallinae populations in laying hens. Poult Sci. 2017;96(6):1563–1572. doi: 10.3382/ps/pew428. [DOI] [PubMed] [Google Scholar]
- 22.Hinkle N, Hickle L. External parasites and poultry pests. In: Saif YM, Fadly AM, Glisson JR, McDougald LB, Nolan LK, Swayne DE, editors. Diseases of Poultry. Ames: Iowa State University Press; 2008. pp. 1011–1024. [Google Scholar]
- 23.International Egg Commission. Egg Industry Review 2015. London: International Egg Commission; 2015. pp. 20–32. [Google Scholar]
- 24.Maurer V, Bieri M, Fölsch D. Das suchverhalten von Dermanyssus gallinae in Hühnerställen. Arch Geflugelkd. 1988;52:209–215. [Google Scholar]
- 25.Thomas E, Chiquet M, Sander B, Zschiesche E, Flochlay AS. Field efficacy and safety of fluralaner solution for administration in drinking water for the treatment of poultry red mite (Dermanyssus gallinae) infestations in commercial flocks in Europe. Parasit Vectors. 2017;10(1):457. doi: 10.1186/s13071-017-2390-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Odaka M, Ogino K, Shikada M, Asada K, Kasa S, Inoue T, Maeda K. Correlation between the proportion of stained eggs and the number of mites (Dermanyssus gallinae) monitored using a ‘non-parallel board trap’. Anim Sci J. 2017;88(12):2077–2083. doi: 10.1111/asj.12860. [DOI] [PubMed] [Google Scholar]
- 27.Nordenfors H, Höglund J. Long term dynamics of dermanyssus gallinae in relation to mite control measures in aviary systems for layers. Br Poult Sci. 2000;41(5):533–540. doi: 10.1080/713654991. [DOI] [PubMed] [Google Scholar]
- 28.Kilpinen O. Activation of the poultry red mite, Dermanyssus gallinae (Acari: Dermanyssidae), by increasing temperatures. Exp Appl Acarol. 2001;25(10-11):859–867. doi: 10.1023/a:1020409221348. [DOI] [PubMed] [Google Scholar]
- 29.Koenraadt CJ, Dicke M. The role of volatiles in aggregation and host-seeking of the haematophagous poultry red mite Dermanyssus gallinae (Acari: Dermanyssidae) Exp Appl Acarol. 2010;50(3):191–199. doi: 10.1007/s10493-009-9305-8. [DOI] [PubMed] [Google Scholar]
- 30.Meyer-Kühling B, Pfister K, Müller-Lindloff J, Heine J. Field efficacy of phoxim 50% (ByeMite) against the poultry red mite Dermanyssus gallinae in battery cages stocked with laying hens. Vet Parasitol. 2007;147(3-4):289–296. doi: 10.1016/j.vetpar.2007.04.012. [DOI] [PubMed] [Google Scholar]