Abstract
Regenerative medicine using stem cells from various sources are emerging treatment modality in several refractory diseases in veterinary medicine. It is well-known that stem cells can differentiate into specific cell types, self-renew, and regenerate. In addition, the unique immunomodulatory effects of stem cells have made stem cell transplantation a promising option for treating a wide range of disease and injuries. Recently, the medical demands for companion animals have been rapidly increasing, and certain disease conditions require alternative treatment options. In this review, we focused on stem cell application research in companion animals including experimental models, case reports and clinical trials in dogs and cats. The clinical studies and therapeutic protocols were categorized, evaluated and summarized according to the organ systems involved. The results indicate that evidence for the effectiveness of cell-based treatment in specific diseases or organ systems is not yet conclusive. Nonetheless, stem cell therapy may be a realistic treatment option in the near future, therefore, considerable efforts are needed to find optimized cell sources, cell numbers and delivery methods in order to standardize treatment methods and evaluation processes.
Keywords: Canine, clinical trials as topic, feline, regenerative medicines, stem cells
INTRODUCTION
Recently, the demand for cell-based therapies for various refractory diseases has been increasing. Stem cells have a wide, sometimes unlimited, differentiation potential in various body organs, and possess the capacity for self-renewal. This makes stem cell transplantation an attractive therapeutic candidate for patients with a wide range of incurable diseases and injuries [1,2,3,4]. According to recent human studies, the number and type of stem cells in clinical trials have expanded [5,6,7,8,9].
Among the various stem cells, mesenchymal stem cells (MSCs) are the most favored and routinely exploited cell type in the clinical trials [1,10]. Basically, they can be easily collected and isolated from bone marrow (bone-marrow-derived MSCs; BMSCs) and adipose tissue (adipose tissue-derived MSCs; AD-MSCs). MSCs have the capability to differentiate into chondrocytes, adipocytes, osteoblasts, myocytes, neural cells and hepatocytes [11,12,13,14,15]. Although MSCs from various sources share many biological features and characteristics, differences have been reported in their immunophenotype, proliferative capacity, differentiation potential, immune modulation and gene expression profiles [1,16,17]. Consequently, the application and effectiveness of each type in veterinary clinical practice may differ [18,19].
Even though stem cell treatment has potential benefits, the true therapeutic efficacy and adverse effects of stem cell therapy are not fully understood [12,13,20]. Several studies have suggested the possibility of adverse reactions during intravenous stem cell transplantation [13,20,21]. Additionally, many veterinary stem-cell treatments studies contain design flaws that limit the reliability of the results. For example, some failed to maintain consistent therapeutic protocols and lacked control groups or blinded evaluation [22,23,24,25,26].
Most recent stem cell reviews in veterinary medicine describe animal models for stem cell research for human disease. These studies mainly focused on various stem cell types and their potencies [27,28,29]. Expanded cell types and treatment protocols have been tested in canine models for clinical application in both humans and animals. Only one literature review describes the clinical use of AD-MSCs for spontaneous animal disease [19]. Before stem cells can be used in companion animal treatment, their safety and efficacy should be proven. The present literature review focuses on the clinical application of cell-based treatment for spontaneous diseases of different organ system in dogs and cats. To determine the status, challenges, and future prospects of stem cell therapy in veterinary medicine, we analyzed some of the most relevant clinical studies, and investigated treatment and evaluation methods.
BASICS OF STEM CELL TRIALS
It is well known that stem cells are unspecialized cells with the ability to self-renewal and differentiation of specialized cell types [1,28]. Regenerative medicine using stem cells was first used to treat hematologic diseases via bone marrow transplantation in late 1900s [30]. By 2000, the utility of stem cells had expanded to include non-hematologic disease such as cardiologic and neurologic diseases [5,10,31,32,33]. Stem cells can be classified under 2 large categories based on their sources: embryonic stem cells (ESCs) and adult stem cells (ASCs) [34]. ESCs have more developmental possibility than ASCs, but these stem cells have ethical and legal issues and safety concerns, including tumorigenicity [35]. ASCs can derived from bone marrow, peripheral blood, umbilical cord blood and tissue, adipose tissue, skin, neuron and muscle [1]. Recently, it has been discovered that pluripotent stem cells can be generated directly from adult somatic cells via genetic reprogramming. There are known as induced pluripotent stem cells (iPSCs) [36].
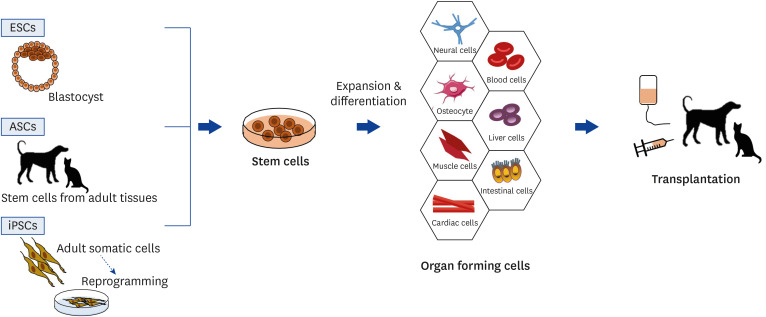
Stem cell treatment involves using stem cells to treat various disease or conditions. Stem cells are collected, transformed into specific types of cells via cell culture, and transplanted into the body. The stem cells and their derivatives then replace and heal damaged tissues. The curative effects of stem cell treatment can be evaluated differently depending on the primary disease, though it is typically accomplished by testing the structural and functional restoration of the target organ (Fig. 1) [34].
Fig. 1. Schemes of basic concept and procedures for stem cell transplantation. Stem cells can be classified into ESCs, ASCs and iPSCs based on their sources. Simply, stem cells are expanded to specific types of cells and transplanted into the body.
ESC, embryonic stem cell; ASC, adult stem cell; iPSC, induced pluripotent stem cell.
STEM CELL TRIALS IN CARDIOVASCULAR DISEASE
Interestingly, stem cell therapy is an emerging potential therapeutic modality for cardiovascular disease [31]. Stem cells are commonly applied in myocardial infarction cases, because treatment is difficult and damaged cardiomyocytes are rarely regenerated [10]. Treatment protocols vary depending on the transplanted cell type, delivery method, time of administration and frequency [33].
Various types of stem cells have been used for cardiac therapy, including skeletal myoblasts, BMSCs, ESCs, endogenous cardiac stem cells (CSCs), AD-MSCs and even iPSCs generated from human hair follicle keratinocytes [37,38,39]. Among these, BMSCs have 2 advantages: lower immunogenicity compared to the other types of stem cells, and the action of paracrine growth factors [39]. It is demonstrated that preclinical studies have shown the ability of MSCs to attenuate cardiac remodeling, restore cardiac function, and regenerate damaged myocytes in acute and chronic canine models [40,41]. iPSCs generated from human hair follicle keratinocytes can differentiate into a cardiogenic lineage and function as a biological pacemaker, allowing for their potential application as a novel therapy for the advanced treatment of atrioventricular block [37]. Theoretically, autologous transplantation of CSCs is also possible. A previous study has demonstrated that attenuation of left ventricle remodeling causes less fibrosis in canine model of myocardial infarction after autologous CSCs intramyocardial injection [33].
The stem cell transplantation method is also important for enhancing therapeutic efficacy and safety. Previously, stem cells were introduced into cardiomyocytes by either direct intramyocardial (subendocardial or subepicardial) injection or intracoronary injection [42,43]. Direct intramyocardial injection has the advantages of providing precise targeting of cell delivery to the ischemic region and higher cell retention compared with intracoronary injection. However, direct injection may induce subsequent inflammation, which reduces the survival of engrafted cells [43,44]. Intracoronary injection presents a high risk of coronary embolism and low stem cell retention. To overcome these disadvantages, other stem cell delivery methods have been developed and utilized. Ultrasound-mediated microbubble destruction induced by micropump in intracoronary injection increases the vascular permeability, which enhances the homing of BMSCs into cardiomyocytes and increases cardiac function [44]. Another study introduced percutaneous retrograde coronary injection in combination with basic fibroblast growth factor (bFGF) [43]. Combination with bFGF enhances the efficacy of BMSCs by increasing the migration and viability of MSCs and promoting their differentiation into the cardiomyocyte phenotype.
Only 2 studies have described cell-based treatment in veterinary clinical cardiac disease (Table 1). One study used allogeneic cardiosphere-derived cells in 5 dilated cardiomyopathy (DCM) dogs [45]. The beneficial effects of stem cell application were not detected in dogs with DCM during a 1-year follow-up. Another study transplanted allogeneic puppy deciduous teeth stem cells for the treatment of degenerative valvular heart disease [46]. The efficacy of treatment was evaluated for 2 months and resulted in improved heart function with alleviated clinical signs of heart failure.
Table 1. Veterinary clinical stem cell trials in cardiovascular disease.
| Disease | Cell therapy | No. of dogs | Control | Evaluation periods/effects | Ref. |
|---|---|---|---|---|---|
| Dilated cardiomyopathy | Allogeneic cardiosphere-derived cells; intra-coronary artery (20 × 106 cells at left main artery and 10 × 106 cells at right coronary artery) | 5 dogs in stem cell group | Yes | At day 1, 1, 2, 6, 12 months; No adverse events and no significant effects occurred during and after cell infusion. | [45] |
| Degenerative valvular heart disease | Allogeneic puppy deciduous teeth stem cells; intravenous; 1 × 106 cells 2 times with 14-day interval | 10 dogs in stem cell group (combination with stem cell therapy and standard treatment); 10 dogs in control group (only standard treatment) | Yes | At 30 and 60 days; left ventricular ejection fraction and quality of life scores were improved in study group. | [46] |
Because myocardial infarction is rare in dogs and cats [47], all of these implications of stem cell therapy for the resolution of cardiomyocyte damage are limited by the experiments due to the use of small and no-randomized samples. Although few pilot studies provide alternative treatment options for cardiovascular disease in veterinary clinics, further research and clinical trials are needed to evaluate the short- and long-term efficacy and complications of stem-cell treatment.
STEM CELL TRIALS IN NEUROLOGIC DISEASE
Spinal cord injury (SCI) causes temporary or permanent neurological defect in humans and companion animals [32]. Because SCI is a devastating condition in humans, several therapeutic experiments using canine SCI models and clinical trials with natural spinal cord-injured dogs have been conducted as preclinical trials [27,32]. Most of the injuries were induced by balloon catheter compression methods between T13 and L3 [48,49,50,51,52,53,54,55]. Few studies have used unilateral spinal cord hemisection between T11 and L2 [56,57]. Stem cells from either canine or human origin were used, most being allogeneic canine MSCs [51,52,53,54,55]. The transplantation time and dose after injury varied, and a small number of dogs (under 10 dogs in each group) were treated. Although these limitations make the efficacy of stem cell therapy inconclusive, several studies have shown promising improvements in functional outcomes and have proven a greater clinical benefit for stem cell therapy in comparison with the traditional treatment.
Along with the experimental research, 9 studies evaluated the efficacy of the stem cell treatment in veterinary SCI clinical cases [58,59,60,61,62,63,64,65,66] (Table 2). Acute and chronic clinical spinal cord-injured dogs were treated with various stem cell protocols. Most of the trials used autologous BMSCs, one study used allogeneic AD-MSCs [63], and 2 other studies used olfactory mucosal or glial cells [60,61]. Seven studies concluded that stem cell transplantation was beneficial and improved locomotor function; however, most of the studies involved a small number of dogs, and only 3 studies include control groups. Two studies failed to show the distinct clinical beneficial effects of stem cell treatment [60,64]; however, they also concluded that stem cell treatment was safe, and there may be some beneficial effects of stem cell therapy in companion animals with SCI.
Table 2. Veterinary clinical stem cell trials in neurologic disease.
| Disease | Cell therapy | No. of dogs | Control | Evaluation periods/effects | Ref. |
|---|---|---|---|---|---|
| SCI (T3-L7) | Autologous NIBM-MSCs; intra spinal injection; 5.0 × 106 cells for 2 times with a 21-day interval. | 13 dogs in stem cell group | No | At 2, 5, 7, and 12 months; improvement in gait score in 6 of the cases, and improvement in proprioception and nociception in 2 cases | [58] |
| SCI | Autologous NIBM-MSCs; intra spinal injection; 5.0 × 106 cells 2 times with a 21-day interval. | 7 dogs in stem cell group | No | At 2, 4 and 8 months; some beneficial effect of intraspinal injection of autologous NIBM-MSCs in dogs with paraplegia | [59] |
| SCI (T10-L4) | Autologous olfactory mucosal cells; intraspinal transplant; 6.24 × 106 cells | 23 in stem cell group; 11 in control group (received cell transport medium alone) | Yes | At 1, 3 and 6 months; no evidence for concomitant improvement in long tract function | [60] |
| Severe SCI (T11-L2) | Autologous olfactory glial cells; intraspinal transplant; 5 × 104 cells | 8 dogs in stem cell group | No | From 2 months to 1 year; the transplantation procedure itself is non-injurious and feasible; beneficial effect on locomotion | [61] |
| SCI | Allogenic AD-MSCs; intra spinal injection; 1 × 107 cells | 9 dogs in surgery and stem cell group; 25 dogs in surgery group | Yes | Follow-up more than 6 months; better recovery outcomes compared to decompression surgery alone | [62] |
| Severe acute SCI (T6-L5) | Autologous BM stromal cells; IT into the CSF; 1.0 × 106 cells to 6 × 106 cells (mean, 3 × 106 cells) 3 times at 1-week intervals | 7 dogs in stem cell group | No | Follow-up until 29-62 months after SCI; there were no complications; Only 2 of 7 dogs regained the ability to walk, no changes in sensory function | [63] |
| SCI (T13-L7) | Autologous BMSCs; intraspinal transplant (intraparenchymal); 1 × 106 cells in each 1 cm3 of lesion | 4 dogs in stem cell group | No | At 100 days, 12 months and 18 months; faster clinical recovery and improved movement in 3 of the 4 dogs; no changes in magnetic resonance imaging | [65] |
| Severe SCI (T11-L4) | Autologous BM-MNCs; subarachnodal to the lesioned spinal cord; 4.5 × 106 to 2.3 × 109 cells (mean, 8.88 × 107 cells) | 36 dogs in stem cell group; 46 dogs in control group | Yes | Ambulatory recovery rate was assessed (mean time of ambulatory recovery was 34.84 days); significant increase in the recovery rate was revealed | [66] |
| Chronic SCI | Autologous BMSCs; IT into the cerebrospinal fluid; 0.3 × 105 cells to 3 × 106 cells (median, 1.3 × 106 cells) 3 times at 1-week intervals | 10 dogs in stem cell group; 13 dogs in control group | Yes | At 1, 2, 3, 4, 5, and 6 months, until 6-35 months; there were no complications; improvement of pelvic limb locomotor function | [64] |
| Meningoencephalomyelitis of unknown origin | Autologous BMSCs; IT in the cisterna magna (2.0 × 106 cells), IV (0.5 × 106 cells), and IA in the right carotid artery (4.0 × 106 cells) | 8 dogs in stem cell group (3 in IT + IA, 4 in IT + IV, 1 in IT + IA after IT + IV) | No | For 6 months up to 2-year follow-up; No major short- or long-term adverse effects; early improvement in general and neurological conditions, IT + IA group showed a shorter time of reaction to therapy | [78] |
| Fibrocartilaginous embolic myelopathy | hUCB-MSCs; percutaneous transplantation into parenchyma; 1.0 × 106 cells | 1 dog | No | At 12 weeks; locomotor functions improved following transplantation. | [68] |
SCI, spinal cord injury; NIBM-MSC, neurogenically-induced bone marrow-derived mesenchymal stem cell; IV, intravenous; IA, intra-arterial; IT, intrathecal; AD-MSC, adipose tissue-derived mesenchymal stem cell; BM, bone-marrow; BM-MNC, bone marrow-derived mononuclear cell; BMSC, bone-marrow-derived mesenchymal stem cell; hUCB-MSC, human umbilical cord derived mesenchymal stem cell.
Fibrocartilaginous embolism (FCE), which results from SCI, cause ischemic myelopathy in dogs [67]. One clinical trial utilized human umbilical cord (hUCB)-derived MSCs in an FCE-suspected dog [68]. hUCB-derived MSCs was transplanted 7 days after decompression surgery. As a result, locomotor functions improved following transplantation in this dog.
Along with SCI, stem cell treatment is applied in other neurological disease cases due to its immunomodulatory capacity and the neuroprotective and regenerative effects of paracrine factors induced by stem cells [69,70,71]. Canine meningoencephalomyelitis of unknown origin (MUO) is an intracranial non-infectious inflammatory disease [72,73]. The exact pathogenesis is still unclear, and immunosuppressive drugs are widely used as treatment options [72,74]. Similar to canine MUO, human multiple sclerosis, also known as chronic autoimmune inflammatory disease, is induced by the attack of autoreactive T-cells [75]. The immunomodulatory capacity of MSCs was tested in an experimental rodent model of autoimmune encephalomyelitis, and beneficial effects were seen as a result [76,77]. One study was conducted in which dogs affected by MUO were given MSC treatment [78]. Autologous BMSCs were administered by intrathecal injection in conjunction with intravenous and intra-arterial injection in the right carotid artery in 8 dogs. All dogs showed early improvement in their general and neurological conditions without complications. However, this study divided 8 dogs into 3 treatment groups with different stem cell administration protocols and had no control groups. Thus, the treatment effects of stem cells in canine MUO are still uncertain.
Promising improvements have been made in numerous studies using canine experimental SCI models, and these early results have led to clinical trials of stem cell therapy for various neurologic disease, including SCI cases. Further studies with controlled conditions are needed to verify the efficacy of stem cell therapy in companion animals with neurological disease.
STEM CELL TRIALS IN DERMATOLOGIC DISEASE
Normal skin is constantly being renewed and maintaining homeostasis using a pool of ASCs [79]. The basic process of skin wound-healing can be classified into inflammatory, proliferative, and maturation phases. The proliferation and remodeling phases require the complex processes of re-epithelialization, angiogenesis, stem cell activation, extracellular matrix remodeling, and scar formation [80]. As previously described [81,82,83], MSCs transplantation have demonstrated the therapeutic effects in skin wounds and dermal regeneration [81,82,83]. One study demonstrated the topical injection of BMSCs in an experimentally induced skin wound canine model [83]. The researchers concluded that BMSCs migrated to the region of inflammation, resulting in rapid re-epithelialization, angiogenesis, and increased collagen deposition. Two clinical cases demonstrated application of stem cells in large skin wound (Table 3). Autologous AD-MSCs with platelet-rich plasm was applied in a dog with the large skin defects due to train accident [84]. This case report showed complete closure of the wound 3 months after the stem cell transplantation. Another study showed 2 dogs with chronic chemical burn injuries that were not resolved with conventional treatments (16 and 24-month history) [85]. Both dogs were treated with regenerative therapy using human MSCs with poly (vynil-alcohol) hydrogel membranes, and complete epithelialization was observed after 2 months. These 2 clinical cases only demonstrated individual cases without controls. However, considering the treatment results of these cases, it can be concluded with some certainty that regenerative therapy using stem cells improves the wound healing process. In addition to skin wound repair, application of AD-MSCs in one dog with hepatocutaneous syndrome (HS) has been reported [86]. This dog showed a favorable response to stem cell treatment for a long time. HS induces superficial necrolytic dermatitis associated with livers disease [87], and MSCs were applied for dermal and hepatocyte regeneration [80,83,88].
Table 3. Veterinary clinical stem cell trials in dermatologic disease.
| Disease | Cell therapy | No. of dogs | Control | Evaluation periods/effects | Ref. |
|---|---|---|---|---|---|
| Skin wound (trauma) | Autologous adipose derived MSCs + platelet-rich plasma; spraying the cells over the wound surface (5 applications at day 11, 17, 23, 31, 41) | 1 dog | No | A complete closure of the wound occurred 3 months after the start of the regenerative therapy | [84] |
| Chronic skin wound | Human MSCs + poly (vynil-alcohol) hydrogel membranes; locally infiltrated; 1 × 105 cells/cm2 | 2 dogs | No | A complete epithelialisation was observed after 2 months | [85] |
| Hepatocutaneous syndrome | Allogenic adipose-derived MSCs; IV and intrahepatic injection; 5 × 107 cells for 46 times | 1 dog | No | Follow-up for 32 months; stem cell therapy may extend a patient's survival time. | [86] |
| AD | Autologous adipose-derived MSCs; intravenous route; 1.3 × 106 cells/kg | 5 dogs in stem cell group | No | At 2–3, 6–8, 10–12 weeks; the results were safe but not effective for controlling clinical signs and pruritus induced by AD. | [23] |
AD, atopic dermatitis; MSC, mesenchymal stem cell; IV, intravenous.
MSCs exert their beneficial effects on the treatment of immune-mediated diseases by inhibiting the proliferation of T-cells, B-cells, and dendritic cells [76]. They also alter the maturation of antigen-presenting cells and the cytokine secretion [2]. Because of the immunomodulatory effects of the MSCs, clinical trials using MSCs in atopic dermatitis (AD) have been conducted in dogs [23]. In this study, autologous AD-MSCs were intravenously administrated to 5 dogs with AD. No specific adverse effects were observed, but they failed to improve clinical signs.
Because of the repairing and regenerative capabilities of stem cells, most research has focused on clinical therapeutic applications of stem cells for damaged tissues and skin repair [3]. Recently, the application of stem cells has extended to incurable and recurrent immune-mediated skin diseases that have not responded to the conventional treatment. However, there is a lack of research on appropriate cell sources, mechanisms of action, efficacy, and safety of clinical trials in veterinary medicine.
STEM CELL TRIALS IN GASTROINTESTINAL (GI) DISEASE
The management of GI diseases is often challenging because of the complex pathogenesis, morphology and function of the GI system [89]. Various pathogeneses include infection, inflammation, neoplasm, and functional disturbance. Stem cell therapy may play an important role in human GI disease. Ongoing clinical trials with stem cells have been reported in disease that are difficult to treat, such as cirrhosis and liver failure, inflammatory bowel disease (IBD), and pancreatitis [89,90,91]. Many preclinical studies have shown promising results in human medicine, and these clinical approaches have also been tested in veterinary medicine.
The effects of autologous BMSCs and AD-MSCs were investigated in formocresol-induced oral ulcers in dogs [92,93]. Both studies demonstrated the rapid healing of induced oral ulcer following stem cell therapy compared with other treatments or control groups. This was most likely accomplished through angiogenesis and epithelial/connective tissue proliferation. The gene expression levels of angiogenesis and epithelial/connective tissue markers such as vascular endothelial growth factor (VEGF) and collagen [93], VEGF, collagen, platelets-derived growth factor and epidermal growth factor [92] were significantly higher in the MSC-treated group.
IBD is a multifactorial, idiopathic infiltration of inflammatory cells in the small and large intestines. Lymphocytic-plasmacytic colitis is the most common form in dogs and has several histopathologic and molecular features that resemble human IBD [90,94]. The efficacy of a single intravenous injection of allogeneic AD-MSCs was evaluated in 11 dogs with IBD [95,96]. While the dogs were partially tolerant to conventional therapy, MSCs transplantation improved clinical scores, serum albumin, and serum biomarkers (folate and cobalamin) when compared to baseline [96]. Further evaluation with endoscopic and histological scales showed improved macroscopic changes (endoscopic index), but no improved microscopic histological scores [95]. Although the small sample size and absence of a control group or qualified evaluation methods may obscure the real effects of MSCs, these data provide a short-term safety and therapeutic potentials of allogeneic MSCs in dogs with IBD.
Canine anal furunculosis (CAF) is a chronic, immune-mediated disease in dogs characterized by the occurrence of perianal fistulas that resemble fistulizing Crohn's disease (one type of IBD) in humans [90,97]. Over 80% of CAF is diagnosed in middle-to-old-aged German shepherd dogs, but the pathogenesis of CAF, except for the genetic causes, is not fully understood [98,99]. Long-term immunosuppressive drugs are the most effective therapy, but relapses and refractory cases are common [97]. The efficacy of intralesional injection of human ESC (hESC)-derived MSCs in 6 dogs with refractory CAF was evaluated [100]. The hESC-MSCs were well-tolerated, and all 6 dogs were free of fistulas at 3 months post-injection. However, 2 of the 6 dogs experienced recurrence of fistulas by 6 months, indicating that multiple injection may be required in some cases.
There were only 3 feline stem cell studies in GI disease (Table 4). One feline study was conducted on chronic enteritis (lymphocytic-plasmocytic enteritis) [101]. Allogeneic feline AD-MSCs were administered, and clinical improvements were observed compared with the placebo at the 2-month follow-up. Two other studies administered systemic autologous or allogeneic feline AD-MSCs in refractory feline chronic gingivostomatitis (FCGS) [102,103]. FCGS is a chronic inflammation of the oral mucosa and is associated with a highly reactive immune system [104]. These data support the clinical evidence of immunomodulatory effects of MSCs therapy. To date, compared with autologous MSCs, allogeneic MSCs have shown lower treatment efficacy and delayed clinical response.
Table 4. Veterinary clinical stem cell trials in gastrointestinal disease.
| Disease | Cell therapy | No. of dogs | Control | Evaluation periods/effects | Ref. |
|---|---|---|---|---|---|
| Inflammatory bowel disease | Allogeneic adipose-derived MSCs; IV; 2 × 107 cells/kg | 11 dogs in stem cell group | No | At 6 weeks; the dogs were well tolerated and given clinical benefits. | [95,96] |
| At pre-treatment and between 90 and 120 days post-treatment; endoscopic remission in 4 dogs and histological remission was not achieved | |||||
| Anal furunculosis | hESC-derived MSCs; intra-lesional injection within the dermis and subcutaneous tissue around the perianal fistulas; 2 × 107 cells | 6 dogs in stem cell group | No | At 7, 30, 60, 90, 180 days; the safety and therapeutic potential of hESC-MSCs were revealed. | [100] |
| FCGS | Allogeneic AD-fMSCs; IV; 20 × 106 cells, 2 times, 1 month apart | 7 cats in stem cell group | No | At 1 month, 3 months, and 6 months; clinical improvement and resolution in 4/7 cats; cured ~12–20 months | [102] |
| FCGS | Autologous AD-fMSCs; IV; 20 × 106 cells, 2 times, 1 month apart | 7 cats in stem cell group | No | At 1 month, 3 months, and 6 months; clinical improvement and resolution in 5/7 cats; cured ~3–9 months | [103] |
| Feline chronic enteropathy | Allogeneic AD-fMSCs; IV; 2 × 106 cells/kg, 2 times, 2 weeks apart | 7 cats in stem cell group; 4 cats in control group | Yes | At 2 weeks and 1 to 2 months; significant improvement or complete resolution of clinical signs in 5/7 cats | [101] |
hESC, human embryonic stem cell; FCGS, feline chronic gingivostomatitis; MSC, mesenchymal stem cell; IV, intravenous; AD-fMSC, adipose tissue-derived feline mesenchymal stem cells.
In the treatment of refractory GI disease, stem cell transplantations have been actively studied in both human and veterinary medicine. More specified studies on stem cell sources and treatment protocols for each disease may enable innovative clinical applications of stem cells in refractory chronic GI disease in the near future.
STEM CELL TRIALS IN MUSCULOSKELETAL DISEASE
Musculoskeletal disease includes injuries or pain of the joints and tendons, ligaments, muscles, nerves, and accompanying structures, which affect the ability to move. BMSCs are the progenitors for many mesenchymal tissues, such as bone, cartilage and fat [105]. Stem cell-based bone regeneration was evaluated using canine models. Bone defects were induced by surgery and reconstructed using allogeneic mandibular scaffold-loaded and autologous MSCs [105] or β-tricalcium phosphate and autologous BMSCs via the custom-made stem cell-scaffold device [106]. Both studies demonstrated that the use of MSCs accelerated new bone formation in the mandibular or orbital defects, most likely due to increases in the absorption of bone grafts and osteogenesis.
Stem cells are also used in muscle diseases. Duchenne muscular dystrophy is a devastating genetic disorder that induces severe muscle weakness and atrophy in humans [107]. To evaluate therapeutic stem cell efficacy in this incurable form of muscular dystrophy, golden retriever muscular dystrophy (GRMD) dogs were observed clinically as animal models for humans [108,109,110]. Intra-arterially delivered muscle stem cell [58,95] or mesoangioblasts (vessel-associated stem cells) [110] showed limitations in muscle damage with myofiber regeneration, dystrophin recovery and increased regeneration activity. Thus, these studies concluded that stem cells can provide clinical benefits in GRMD dogs. Few clinical trials related to muscle diseases have been reported [111,112]. Adipose-derived stem cells were injected into 2 dogs with severe skeletal muscle injuries. Clinical improvements and reductions in lesion size were observed after stem cell administration [111]. Semitendinosus myopathy is a rare muscular disorder in certain large breed dogs, and its exact etiology and pathogenesis are still unknown [112]. Because this muscle is responsible for non-weight-bearing positional extension (hip, stifle, and tarsus) and flexion (stifle), lameness and pain are common clinical signs [113]. AD-MSCs treatment of these dogs improved clinical signs without recurrence of lameness, likely due to the prevention of the progression of fibrosis and muscle contracture in semitendinosus myopathy.
Several experimental strategies have provided insight as to whether AD-MSCs therapy can be utilized for the regeneration and maintenance of articular cartilage in osteoarthritis [25,26,114,115]. Administration of autologous AD-MSCs stimulates extracellular matrix synthesis and chondrocyte proliferation and inhibits inflammatory reaction of cartilage [26,114]. In addition, growth factors contained in platelet-rich plasma and hyaluronic acid act as mediators, and potentiators, of the effect of MSCs [26,114]. Significant improvements in limb function, lameness, and force plate gait analysis associated with osteoarthritis observed mostly in the hip joint [22,25,26,114,115], elbow [116], and humeroradial joint [117] (Table 5).
Table 5. Veterinary clinical stem cell trials in musculoskeletal disease.
| Disease | Cell therapy | No. of dogs | Control | Evaluation periods/effects | Ref. |
|---|---|---|---|---|---|
| OA (hip joint) | Autologous AD-MSCs; intraarticular injection; 4.2–5 × 106 cells | 18 dogs divided to stem cell and control group (injection of placebo material) | Yes | At 30, 60, and 90 days; the results showed significantly improved scores for lameness and the compiled scores for lameness, pain, and range of motion. | [22] |
| OA (hip joint) | Autologous AD-MSCs; intraarticular injection; 30 × 106 cells | 9 dogs in stem cell group; 5 healthy dogs in control group | Yes | At 30, 90, 180 days; improvement of limb function in dogs with hip OA was objectively seen. | [25] |
| OA (hip joint) | Autologous AD-MSCs; intraarticular injection; 30 × 106 cells | 8 dogs in stem cell group; 5 healthy dogs in control group | Yes | At 30, 90, 180 days; reduced lameness due to OA was observed after stem cell therapy. | [26] |
| OA (hip joint) | Autologous AD-MSCs; intraarticular injection; 30 × 106 cells | 18 dogs in stem cell group; 17 dogs in PRGF group | Yes | At 1, 3, 6 months; Both groups showed safe and effective outcome and compared to PRGF, cell group showed better results at 6 months. | [114] |
| OA (hip joint) | Autologous AD-MSCs; intraarticular injection; 30 × 106 cells | 10 dogs in stem cell group; 5 healthy dogs in control group | Yes | At 30, 90, 180 days; MSC therapy significantly improved limb function in dogs with hip OA. | [115] |
| OA (elbow joint) | Autologous AD-MSCs; intraarticular injection; 3-5 × 106 cells | 14 dogs in stem cell group | No | At 30, 60, 90, and 180 days; statistically significant improvement in lameness, range of motion, and pain on manipulation over time was shown. | [116] |
| OA (humeroradial joint) | Autologous AD-MSCs; intraarticular injection; 3–5 × 106 cells | 4 dogs in stem cell group | No | At 1 week and 1 month; cellular therapy has a significant potential for clinical use inducing functional improvements. | [117] |
| Skeletal muscle injury | Autologous AD stem cells; case 1, intralesional and IV; 4.7 × 106 cells each, case 2, intralesional 7.5 × 106 cells and IV 3.8 × 106 cells | 2 dogs | No | At 19 weeks (case 1) and 22 weeks (case 2); significant reduction in lesion size and clinical improvements | [111] |
| Semitendinosus myopathy | Autologous AD-MSCs; intralesional and IV | 11 dogs in stem cell group | No | At 6 months and 1 year; stem cell treatment helped prevent progression, of the career-ending fibrosis and muscle contracture. | [112] |
| Gastrocnemius tendon strain | Autologous BMSCs; intralesional; 20 × 106 cells | 1 dog | No | At 30, 60, 90, 180, and 365 days; successful functional outcome; incomplete healing with serial orthopedic and ultrasound examinations | [118] |
| Hip dysplasia | Autologous SVF (2–5 × 106 cells) or allogeneic AD-MSCs (2–8 × 105 cells); acupuncture point injection | 5 dogs in MSC group; 4 dogs in SVF group | No | At 7, 15, and 30 days; clear improvement was observed in both groups. | [24] |
SVF, stromal vascular fraction; PRGF, plasma rich in growth factors; AD-MSC, adipose tissue-derived mesenchymal stem cell; MSC, mesenchymal stem cell; BMSC, bone-marrow-derived mesenchymal stem cell; OA, osteoarthritis.
In tendon injuries, autologous BMSCs combination with custom orthosis have great potential for modulating inflammation and stimulating tendon regeneration [118]. After autologous MSCs transplantation, lameness resolved and peak vertical and propulsive forces of contralateral pelvic limb increased. However, incomplete healing was observed via serial orthopedic and ultrasound examinations, so further research is required for clinical application of stem cells in tendon injuries.
Hip dysplasia (HD) is an inherited orthopedic disease that affects dogs of all breeds. Common treatments applied in HD dogs include an energy-restricted diet, exercise-restriction, medical management with analgesics and/or chondroprotective agents, or surgical correction [119]. One study used autologous or allogeneic adipose-derived stem cells in 9 HD dogs [24]. Acupoint was suggested as a stem cell injection site, and stem cell administration resulted in functional improvement and marked decrease in pain on manipulation in 8 dogs with HD. Only one dog showed no remarkable improvements or pathological alterations.
The utility of stem cells in musculoskeletal disease has been actively studied in humans, and preclinical study results using animal models provide useful information for the clinical applications of stem cells in animal disease. The clinical relevance of some disease has been identified, but the pathophysiology, disease process, and treatment responses could vary between species. In addition, preclinical animal study results were overstated due to inappropriate controls and different evaluation methods. Thus, human study results cannot be applied directly to animals, and further research is required.
STEM CELL TRIALS IN NEOPLASIA
According to a previous report [120], cytotoxic chemotherapy has been exploited for a variety of tumor treatments in small animal medicine. These chemotherapy regimens often have limitations due to their dose-dependent toxicities and drug resistance. Thus, over the past decades, veterinary oncologists have searched for novel therapies to achieve a more efficient tumor treatment.
Among them, MSCs transplantation is considered a highly valuable and promising approach for a variety of neoplasia [4]. The positive aspects of MSCs (easily isolated, extensive proliferation, and the differentiation capacity into various cell types) have been previously described in clinical application [16,121]. In addition, genetically modified MSCs can accumulate at the site of cancer and be utilized for cancer gene therapy, which is effective for the cellular delivery of anticancer agents and/or molecules including cytokines, interferons, or pro-drugs that inhibit tumor growth and angiogenesis [122,123,124]. Gene therapy using genetically modified stem cells has been demonstrated in several human cancer studies. However, few studies have been performed in veterinary medicine.
Canine splenic hemangiosarcoma (HSA) is a highly metastatic malignant tumor, and either splenectomy alone or adjuvant chemotherapy have been considered as the treatments of choice. In one clinical case, human neural stem cells (hNSCs) were used with 5-fluorocytosine in a dog with HSA lung metastasis [125]. Significant reduction in the size and number of metastatic lung nodules with fewer side effect was observed 2 weeks after the hNSCs were treated with 5-fluorocytosine. The injected hNSCs had been engineered to express the cytosine deaminase gene, which can converts the prodrug 5-fluorocytosine into the active form. This led to a significant size reduction of the metastatic lung nodules [126]. In addition, hNSCs delivered an anticancer agent to the neoplasm, including the metastatic lesions. Another study demonstrated the use of MSCs combined with recombinant human bone morphogenetic protein 2 (rhBMP-2) and chemotherapeutic agents in a canine osteosarcoma model [127]. The results showed that the MSCs/rhBMP-2/chemotherapeutic agent's injected groups experienced more effective tumor size reduction and neoplastic cell infiltration than the conventional treatment group. This preliminary test result indicates the potential for a promising new modality in cancer treatment, but further studies and clinical trials are necessary.
In the aspects of hematological tumor, leukemia and lymphoma have been considered a refractory malignant neoplasia, even though several chemotherapy protocols have been known [128]. Suter et al. [129] reported the first clinical case of suspected acute lymphocytic leukemia treatment with allogeneic hematopoietic cell transplantation (HCT) with dog leukocyte antigen-matched CD34+ cells (from related siblings). Because acute leukemias are rare aggressive neoplasms of immature lymphopoietic or hematopoietic progenitor cells, the prognosis is poor and treatment outcomes are limited. The dog in this study presented favorable clinical outcomes within the 2-year follow-up period, and this pilot case report provides valuable clinical possibilities for the use of allogeneic HCT in dogs. Lymphoma is a well-recognized lymphoid origin neoplasm in dogs. Canine T-cell lymphoma has a poorer prognosis than that of B-cell lymphoma, and the median survival time is 6–9 months and 8–16 months with multiagent chemotherapy, respectively [130]. Two clinical trials used apheresis and peripheral blood HCT (PBHCT) in canine B- and T-cell lymphoma [131,132]. Both studies used CD34+ cells after total body irradiation as a treatment for lymphoma and recommended PBHCT in B- and T-cell lymphoma as a valuable alternative treatment (Table 6).
Table 6. Veterinary clinical stem cell trials in neoplasia.
| Disease | Cell therapy | No. of dogs | Control | Evaluation periods/effects | Ref. |
|---|---|---|---|---|---|
| Hemangiosarcoma with pulmonary metastasis | hNSCs; IV; 1 × 107 cells | 1 dog (stem cell injection with 5-fluorocytosine therapy about 30 days after surgery) | No | Follow-up until the patient died (105 days); hNSCs/5-FC therapy can improve the quality of dog's life with therapeutic effects and lower side effects | [125] |
| Acute large granular lymphocytic leukemia | Allogeneic PBHCT; IV; 5 × 106 CD 34+ cells/kg | 1 dog | No | Follow-up for 2 years; considerable clinical benefit over chemotherapy alone. | [129] |
| T-cell lymphoma | Autologous PBHCT; IV; more than 2 × 106 CD 34+ cells/kg | 15 dogs in stem cell group | No | Follow-up for overall survival of median 239.5 days (range, 4–738 days); PBHCT may be considered as a treatment option for dogs with T-cell lymphoma. | [131] |
| B-cell lymphoma | Autologous PBHCT; IV; more than 2 × 106 CD 34+ cells/kg | 24 dogs in stem cell group | No | Follow-up for assessment of disease-free interval (median 271 days) and overall survival (median 463 days); PBHCT may be considered as a treatment option for dogs with B-cell lymphoma. | [132] |
hNSC, human neural stem cell; IV, intravenous; PBHCT, peripheral blood hematopoietic cell transplantation.
Because of the natural occurrence and biological similarities of cancer in animals and humans, the field of comparative oncology would benefit from a focus on companion animals for the development of new cancer therapy. Few therapeutic approaches for spontaneous cancer in dogs have revealed promising effects of treatment with stem cells. However, the utility of stem cells for targeted cancer therapy is still vague in veterinary medicine due to the low number of clinical studies and the unclear mechanisms of action.
FUTURE DIRECTIONS FOR VETERINARY CLINICAL STEM CELL TRIALS
Regenerative medicine is an emerging field in both human and veterinary medicine. Dogs have been usually used in experimental models and preclinical studies for stem cell treatments in humans. The need for stem cell treatment has expanded in veterinary medicine, and clinical applications are being continuously attempted.
This study reviewed 6 clinical categories of stem cell application in clinically ill dog and cats. Among them, major progress in stem cell application has been made in the field of neurology and musculoskeletal disease. The most promising results were found by studies conducted on spinal cord injuries and osteoarthritis using autologous MSCs from bone marrow- or adipose tissue. Some of the studies provided controls for more efficient evaluations, but treatment frequency, interval, and stem cell dosages varied, making it difficult to generalize the application. Other clinical trials looking at tissue and organ repair/regeneration effects of stem cells were conducted for the treatment of skin or muscle wounds. The results of these studies were also favorable, but they failed to demonstrate objective indicators with only a few clinical cases. Because of the immunomodulatory capacity and paracrine role of some types of stem cells, clinical trials were performed on immune-mediated inflammatory disease such as meningoencephalitis, AD, IBD, anal furunculosis, and FCGS. However, understanding the exact mechanisms of cell therapy for each disease requires further study. In addition, more cases that have clinical, scientific controls and long-term follow-up are needed. Stem cells for the treatment of cancer is an emerging modality in humans, but there is a lack of clinical application in veterinary medicine for evaluation.
Because standardized treatment and evaluation methods (including case selection, optimal cell type, delivery route, time of administration, cell dose, evaluation methods, and periods) are not optimized for each trial, treatment efficacy is uncertain and there are always questions that remained unanswered. To use stem cells in clinical therapy, ‘safety’ and ‘efficacy’ are the 2 main issues to consider. In human medicine, there are regulations and guidelines for using stem cell-based products for clinical and commercial use [133].
The basic principles for using cell-based products in veterinary clinical trial should include:
1) Donor selection criteria
- The donor should be screened for infectious diseases and other risk factors to prevent the transmission of disease agents.
2) Quality-controlled cells
- The origin of the cells, conditions for storage, composition of the products should be adequately defined and labeled.
- Demonstration that cellular function and integrity have been preserved.
- Prove free of contamination from viruses, bacteria, fungi, mycoplasma and endotoxins.
3) Efficacy and safety
- The efficacy and safety of the cells after delivery have been demonstrated in target animals.
- Long-term safety evaluation is highly recommended.
- Adverse events after stem cell intervention should be reported.
- Consider risk factors such as toxicity, tumorigenicity, and immune reactions.
Recently, the United States Food and Drug Administration (FDA) and the European Medicine Agency's (EMA) approved guidance for cell-based products for veterinary use [133,134,135]. In 2018, the Animal and Plant Quarantine Agency (APQA) of Korea also documented ‘Guideline on safety assessment of cell-based products for animal use’ [136]. If researchers and clinicians follow the guidelines of FDA, EMA, and APQA of Korea on the above 3 principles, the problems of standardized treatment protocols and evaluation methods of stem cell therapy will be rapidly improved.
Based on the previous pilot and preliminary studies, clinicians' and researchers' efforts to standardize stem cell treatments are needed. In addition, further preclinical and clinical stem cell studies are necessary for the progress of this treatment modality in both human and veterinary medicine.
ACKNOWLEDGMENTS
The authors thanks to the members of our internal medicine laboratory for their support during drafting of this manuscript.
Footnotes
Funding: This research was supported by the Bio & Medical Technology Development Program of the National Research Foundation (NRF) funded by the Korean government (MSIT) (2016M3A9B6903437).
Conflict of Interest: The authors declare no conflicts of interest.
- Conceptualization: Kang MH, Park HM.
- Data curation: Kang MH.
- Investigation: Kang MH, Park HM.
- Supervision: Park HM.
- Writing - original draft: Kang MH, Park HM.
- Writing - review & editing: Kang MH, Park HM.
References
- 1.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 2.Götherström C. Immunomodulation by multipotent mesenchymal stromal cells. Transplantation. 2007;84(1 Suppl):S35–S37. doi: 10.1097/01.tp.0000269200.67707.c8. [DOI] [PubMed] [Google Scholar]
- 3.Mizuno H. Adipose-derived stem cells for tissue repair and regeneration: ten years of research and a literature review. J Nippon Med Sch. 2009;76(2):56–66. doi: 10.1272/jnms.76.56. [DOI] [PubMed] [Google Scholar]
- 4.Ozawa K, Sato K, Oh I, Ozaki K, Uchibori R, Obara Y, Kikuchi Y, Ito T, Okada T, Urabe M, Mizukami H, Kume A. Cell and gene therapy using mesenchymal stem cells (MSCs) J Autoimmun. 2008;30(3):121–127. doi: 10.1016/j.jaut.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 5.Barker RA. Developing stem cell therapies for Parkinson's disease: waiting until the time is right. Cell Stem Cell. 2014;15(5):539–542. doi: 10.1016/j.stem.2014.09.016. [DOI] [PubMed] [Google Scholar]
- 6.Li C, Dong N, Wu H, Dong F, Xu Y, Du H, He H, Liu Z, Li W. A novel method for preservation of human corneal limbal tissue. Invest Ophthalmol Vis Sci. 2013;54(6):4041–4047. doi: 10.1167/iovs.13-11648. [DOI] [PubMed] [Google Scholar]
- 7.Mitchell A, Fujisawa T, Newby D, Mills N, Cruden NL. Vascular injury and repair: a potential target for cell therapies. Future Cardiol. 2015;11(1):45–60. doi: 10.2217/fca.14.77. [DOI] [PubMed] [Google Scholar]
- 8.Murphy SV, Kidyoor A, Reid T, Atala A, Wallace EM, Lim R. Isolation, cryopreservation and culture of human amnion epithelial cells for clinical applications. J Vis Exp. 2014;94(94):52085. doi: 10.3791/52085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schwartz SD, Hubschman JP, Heilwell G, Franco-Cardenas V, Pan CK, Ostrick RM, Mickunas E, Gay R, Klimanskaya I, Lanza R. Embryonic stem cell trials for macular degeneration: a preliminary report. Lancet. 2012;379(9817):713–720. doi: 10.1016/S0140-6736(12)60028-2. [DOI] [PubMed] [Google Scholar]
- 10.Strioga M, Viswanathan S, Darinskas A, Slaby O, Michalek J. Same or not the same? Comparison of adipose tissue-derived versus bone marrow-derived mesenchymal stem and stromal cells. Stem Cells Dev. 2012;21(14):2724–2752. doi: 10.1089/scd.2011.0722. [DOI] [PubMed] [Google Scholar]
- 11.Bang OY, Lee JS, Lee PH, Lee G. Autologous mesenchymal stem cell transplantation in stroke patients. Ann Neurol. 2005;57(6):874–882. doi: 10.1002/ana.20501. [DOI] [PubMed] [Google Scholar]
- 12.Chen J, Li Y, Wang L, Zhang Z, Lu D, Lu M, Chopp M. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke. 2001;32(4):1005–1011. doi: 10.1161/01.str.32.4.1005. [DOI] [PubMed] [Google Scholar]
- 13.Furlani D, Ugurlucan M, Ong L, Bieback K, Pittermann E, Westien I, Wang W, Yerebakan C, Li W, Gaebel R, Li RK, Vollmar B, Steinhoff G, Ma N. Is the intravascular administration of mesenchymal stem cells safe? Mesenchymal stem cells and intravital microscopy. Microvasc Res. 2009;77(3):370–376. doi: 10.1016/j.mvr.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 14.Kang WJ, Kang HJ, Kim HS, Chung JK, Lee MC, Lee DS. Tissue distribution of 18F-FDG-labeled peripheral hematopoietic stem cells after intracoronary administration in patients with myocardial infarction. J Nucl Med. 2006;47(8):1295–1301. [PubMed] [Google Scholar]
- 15.Kursova LV, Konoplyannikov AG, Pasov VV, Ivanova IN, Poluektova MV, Konoplyannikova OA. Possibilities for the use of autologous mesenchymal stem cells in the therapy of radiation-induced lung injuries. Bull Exp Biol Med. 2009;147(4):542–546. doi: 10.1007/s10517-009-0538-7. [DOI] [PubMed] [Google Scholar]
- 16.Kern S, Eichler H, Stoeve J, Klüter H, Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24(5):1294–1301. doi: 10.1634/stemcells.2005-0342. [DOI] [PubMed] [Google Scholar]
- 17.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 18.Alves EG, Serakides R, Boeloni JN, Rosado IR, Ocarino NM, Oliveira HP, Góes AM, Rezende CM. Comparison of the osteogenic potential of mesenchymal stem cells from the bone marrow and adipose tissue of young dogs. BMC Vet Res. 2014;10(1):190. doi: 10.1186/s12917-014-0190-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marx C, Silveira MD, Beyer Nardi N. Adipose-derived stem cells in veterinary medicine: characterization and therapeutic applications. Stem Cells Dev. 2015;24(7):803–813. doi: 10.1089/scd.2014.0407. [DOI] [PubMed] [Google Scholar]
- 20.Vulliet PR, Greeley M, Halloran SM, MacDonald KA, Kittleson MD. Intra-coronary arterial injection of mesenchymal stromal cells and microinfarction in dogs. Lancet. 2004;363(9411):783–784. doi: 10.1016/S0140-6736(04)15695-X. [DOI] [PubMed] [Google Scholar]
- 21.Kang MH, Park HM. Evaluation of adverse reactions in dogs following intravenous mesenchymal stem cell transplantation. Acta Vet Scand. 2014;56(1):16. doi: 10.1186/1751-0147-56-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Black LL, Gaynor J, Gahring D, Adams C, Aron D, Harman S, Gingerich DA, Harman R. Effect of adipose-derived mesenchymal stem and regenerative cells on lameness in dogs with chronic osteoarthritis of the coxofemoral joints: a randomized, double-blinded, multicenter, controlled trial. Vet Ther. 2007;8(4):272–284. [PubMed] [Google Scholar]
- 23.Hall MN, Rosenkrantz WS, Hong JH, Griffin CE, Mendelsohn CM. Evaluation of the potential use of adipose-derived mesenchymal stromal cells in the treatment of canine atopic dermatitis: a pilot study. Vet Ther. 2010;11(2):E1–E14. [PubMed] [Google Scholar]
- 24.Marx C, Silveira MD, Selbach I, da Silva AS, Braga LM, Camassola M, Nardi NB. Acupoint injection of autologous stromal vascular fraction and allogeneic adipose-derived stem cells to treat hip dysplasia in dogs. Stem Cells Int. 2014;2014:391274. doi: 10.1155/2014/391274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vilar JM, Batista M, Morales M, Santana A, Cuervo B, Rubio M, Cugat R, Sopena J, Carrillo JM. Assessment of the effect of intraarticular injection of autologous adipose-derived mesenchymal stem cells in osteoarthritic dogs using a double blinded force platform analysis. BMC Vet Res. 2014;10(1):143. doi: 10.1186/1746-6148-10-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vilar JM, Morales M, Santana A, Spinella G, Rubio M, Cuervo B, Cugat R, Carrillo JM. Controlled, blinded force platform analysis of the effect of intraarticular injection of autologous adipose-derived mesenchymal stem cells associated to PRGF-Endoret in osteoarthritic dogs. BMC Vet Res. 2013;9(1):131. doi: 10.1186/1746-6148-9-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gabel BC, Curtis EI, Marsala M, Ciacci JD. A review of stem cell therapy for spinal cord injury: large animal models and the frontier in humans. World Neurosurg. 2017;98:438–443. doi: 10.1016/j.wneu.2016.11.053. [DOI] [PubMed] [Google Scholar]
- 28.Fortier LA, Travis AJ. Stem cells in veterinary medicine. Stem Cell Res Ther. 2011;2(1):9. doi: 10.1186/scrt50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Markoski MM. Advances in the use of stem cells in veterinary medicine: from basic research to clinical practice. Scientifica (Cairo) 2016;2016:4516920. doi: 10.1155/2016/4516920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thomas ED, Lochte HL, Jr, Lu WC, Ferrebee JW. Intravenous infusion of bone marrow in patients receiving radiation and chemotherapy. N Engl J Med. 1957;257(11):491–496. doi: 10.1056/NEJM195709122571102. [DOI] [PubMed] [Google Scholar]
- 31.Behfar A, Crespo-Diaz R, Terzic A, Gersh BJ. Cell therapy for cardiac repair--lessons from clinical trials. Nat Rev Cardiol. 2014;11(4):232–246. doi: 10.1038/nrcardio.2014.9. [DOI] [PubMed] [Google Scholar]
- 32.McMahill BG, Borjesson DL, Sieber-Blum M, Nolta JA, Sturges BK. Stem cells in canine spinal cord injury--promise for regenerative therapy in a large animal model of human disease. Stem Cell Rev Rep. 2015;11(1):180–193. doi: 10.1007/s12015-014-9553-9. [DOI] [PubMed] [Google Scholar]
- 33.Welt FG, Gallegos R, Connell J, Kajstura J, D'Amario D, Kwong RY, Coelho-Filho O, Shah R, Mitchell R, Leri A, Foley L, Anversa P, Pfeffer MA. Effect of cardiac stem cells on left-ventricular remodeling in a canine model of chronic myocardial infarction. Circ Heart Fail. 2013;6(1):99–106. doi: 10.1161/CIRCHEARTFAILURE.112.972273. [DOI] [PubMed] [Google Scholar]
- 34.Mahla RS. Stem cells applications in regenerative medicine and disease therapeutics. Int J Cell Biol. 2016;2016:6940283. doi: 10.1155/2016/6940283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282(5391):1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 36.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 37.Chauveau S, Anyukhovsky EP, Ben-Ari M, Naor S, Jiang YP, Danilo P, Jr, Rahim T, Burke S, Qiu X, Potapova IA, Doronin SV, Brink PR, Binah O, Cohen IS, Rosen MR. Induced pluripotent stem cell-derived cardiomyocytes provide in vivo biological pacemaker function. Circ Arrhythm Electrophysiol. 2017;10(5):e004508. doi: 10.1161/CIRCEP.116.004508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Novak A, Shtrichman R, Germanguz I, Segev H, Zeevi-Levin N, Fishman B, Mandel YE, Barad L, Domev H, Kotton D, Mostoslavsky G, Binah O, Itskovitz-Eldor J. Enhanced reprogramming and cardiac differentiation of human keratinocytes derived from plucked hair follicles, using a single excisable lentivirus. Cell Reprogram. 2010;12(6):665–678. doi: 10.1089/cell.2010.0027. [DOI] [PubMed] [Google Scholar]
- 39.Segers VF, Lee RT. Stem-cell therapy for cardiac disease. Nature. 2008;451(7181):937–942. doi: 10.1038/nature06800. [DOI] [PubMed] [Google Scholar]
- 40.Perin EC, Silva GV, Assad JA, Vela D, Buja LM, Sousa AL, Litovsky S, Lin J, Vaughn WK, Coulter S, Fernandes MR, Willerson JT. Comparison of intracoronary and transendocardial delivery of allogeneic mesenchymal cells in a canine model of acute myocardial infarction. J Mol Cell Cardiol. 2008;44(3):486–495. doi: 10.1016/j.yjmcc.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 41.Silva GV, Litovsky S, Assad JA, Sousa AL, Martin BJ, Vela D, Coulter SC, Lin J, Ober J, Vaughn WK, Branco RV, Oliveira EM, He R, Geng YJ, Willerson JT, Perin EC. Mesenchymal stem cells differentiate into an endothelial phenotype, enhance vascular density, and improve heart function in a canine chronic ischemia model. Circulation. 2005;111(2):150–156. doi: 10.1161/01.CIR.0000151812.86142.45. [DOI] [PubMed] [Google Scholar]
- 42.Mitchell AJ, Sabondjian E, Sykes J, Deans L, Zhu W, Lu X, Feng Q, Prato FS, Wisenberg G. Comparison of initial cell retention and clearance kinetics after subendocardial or subepicardial injections of endothelial progenitor cells in a canine myocardial infarction model. J Nucl Med. 2010;51(3):413–417. doi: 10.2967/jnumed.109.069732. [DOI] [PubMed] [Google Scholar]
- 43.Wang X, Zhen L, Miao H, Sun Q, Yang Y, Que B, Lopes Lao EP, Wu X, Ren H, Shi S, Lau WB, Ma X, Ma C, Nie S. Concomitant retrograde coronary venous infusion of basic fibroblast growth factor enhances engraftment and differentiation of bone marrow mesenchymal stem cells for cardiac repair after myocardial infarction. Theranostics. 2015;5(9):995–1006. doi: 10.7150/thno.11607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chang X, Liu J, Liao X, Liu G. Ultrasound-mediated microbubble destruction enhances the therapeutic effect of intracoronary transplantation of bone marrow stem cells on myocardial infarction. Int J Clin Exp Pathol. 2015;8(2):2221–2234. [PMC free article] [PubMed] [Google Scholar]
- 45.Hensley MT, Tang J, Woodruff K, Defrancesco T, Tou S, Williams CM, Breen M, Meurs K, Keene B, Cheng K. Intracoronary allogeneic cardiosphere-derived stem cells are safe for use in dogs with dilated cardiomyopathy. J Cell Mol Med. 2017;21(8):1503–1512. doi: 10.1111/jcmm.13077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Petchdee S, Sompeewong S. Intravenous administration of puppy deciduous teeth stem cells in degenerative valve disease. Vet World. 2016;9(12):1429–1434. doi: 10.14202/vetworld.2016.1429-1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Driehuys S, Van Winkle TJ, Sammarco CD, Drobatz KJ. Myocardial infarction in dogs and cats: 37 cases (1985–1994) J Am Vet Med Assoc. 1998;213(10):1444–1448. [PubMed] [Google Scholar]
- 48.Jung DI, Ha J, Kang BT, Kim JW, Quan FS, Lee JH, Woo EJ, Park HM. A comparison of autologous and allogenic bone marrow-derived mesenchymal stem cell transplantation in canine spinal cord injury. J Neurol Sci. 2009;285(1-2):67–77. doi: 10.1016/j.jns.2009.05.027. [DOI] [PubMed] [Google Scholar]
- 49.Lee JH, Chang HS, Kang EH, Chung DJ, Choi CB, Lee JH, Hwang SH, Han H, Kim HY. Percutaneous transplantation of human umbilical cord blood-derived multipotent stem cells in a canine model of spinal cord injury. J Neurosurg Spine. 2009;11(6):749–757. doi: 10.3171/2009.6.SPINE08710. [DOI] [PubMed] [Google Scholar]
- 50.Lee JH, Chung WH, Kang EH, Chung DJ, Choi CB, Chang HS, Lee JH, Hwang SH, Han H, Choe BY, Kim HY. Schwann cell-like remyelination following transplantation of human umbilical cord blood (hUCB)-derived mesenchymal stem cells in dogs with acute spinal cord injury. J Neurol Sci. 2011;300(1-2):86–96. doi: 10.1016/j.jns.2010.09.025. [DOI] [PubMed] [Google Scholar]
- 51.Lim JH, Byeon YE, Ryu HH, Jeong YH, Lee YW, Kim WH, Kang KS, Kweon OK. Transplantation of canine umbilical cord blood-derived mesenchymal stem cells in experimentally induced spinal cord injured dogs. J Vet Sci. 2007;8(3):275–282. doi: 10.4142/jvs.2007.8.3.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Park SS, Byeon YE, Ryu HH, Kang BJ, Kim Y, Kim WH, Kang KS, Han HJ, Kweon OK. Comparison of canine umbilical cord blood-derived mesenchymal stem cell transplantation times: involvement of astrogliosis, inflammation, intracellular actin cytoskeleton pathways, and neurotrophin-3. Cell Transplant. 2011;20(11-12):1867–1880. doi: 10.3727/096368911X566163. [DOI] [PubMed] [Google Scholar]
- 53.Park SS, Lee YJ, Lee SH, Lee D, Choi K, Kim WH, Kweon OK, Han HJ. Functional recovery after spinal cord injury in dogs treated with a combination of matrigel and neural-induced adipose-derived mesenchymal stem cells. Cytotherapy. 2012;14(5):584–597. doi: 10.3109/14653249.2012.658913. [DOI] [PubMed] [Google Scholar]
- 54.Ryu HH, Kang BJ, Park SS, Kim Y, Sung GJ, Woo HM, Kim WH, Kweon OK. Comparison of mesenchymal stem cells derived from fat, bone marrow, Wharton's jelly, and umbilical cord blood for treating spinal cord injuries in dogs. J Vet Med Sci. 2012;74(12):1617–1630. doi: 10.1292/jvms.12-0065. [DOI] [PubMed] [Google Scholar]
- 55.Ryu HH, Lim JH, Byeon YE, Park JR, Seo MS, Lee YW, Kim WH, Kang KS, Kweon OK. Functional recovery and neural differentiation after transplantation of allogenic adipose-derived stem cells in a canine model of acute spinal cord injury. J Vet Sci. 2009;10(4):273–284. doi: 10.4142/jvs.2009.10.4.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim BG, Kang YM, Phi JH, Kim YH, Hwang DH, Choi JY, Ryu S, Elastal AE, Paek SH, Wang KC, Lee SH, Kim SU, Yoon BW. Implantation of polymer scaffolds seeded with neural stem cells in a canine spinal cord injury model. Cytotherapy. 2010;12(6):841–845. doi: 10.3109/14653249.2010.501784. [DOI] [PubMed] [Google Scholar]
- 57.Lee SH, Chung YN, Kim YH, Kim YJ, Park JP, Kwon DK, Kwon OS, Heo JH, Kim YH, Ryu S, Kang HJ, Paek SH, Wang KC, Kim SU, Yoon BW. Effects of human neural stem cell transplantation in canine spinal cord hemisection. Neurol Res. 2009;31(9):996–1002. doi: 10.1179/174313209X385626. [DOI] [PubMed] [Google Scholar]
- 58.Besalti O, Aktas Z, Can P, Akpinar E, Elcin AE, Elcin YM. The use of autologous neurogenically-induced bone marrow-derived mesenchymal stem cells for the treatment of paraplegic dogs without nociception due to spinal trauma. J Vet Med Sci. 2016;78(9):1465–1473. doi: 10.1292/jvms.15-0571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Besalti O, Can P, Akpinar E, Aktas Z, Elcin AE, Elcin YM. Intraspinal transplantation of autologous neurogenically-induced bone marrow-derived mesenchymal stem cells in the treatment of paraplegic dogs without deep pain perception secondary to intervertebral disk disease. Turk Neurosurg. 2015;25(4):625–632. doi: 10.5137/1019-5149.JTN.14502-15.2. [DOI] [PubMed] [Google Scholar]
- 60.Granger N, Blamires H, Franklin RJ, Jeffery ND. Autologous olfactory mucosal cell transplants in clinical spinal cord injury: a randomized double-blinded trial in a canine translational model. Brain. 2012;135(Pt 11):3227–3237. doi: 10.1093/brain/aws268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jeffery ND, Lakatos A, Franklin RJ. Autologous olfactory glial cell transplantation is reliable and safe in naturally occurring canine spinal cord injury. J Neurotrauma. 2005;22(11):1282–1293. doi: 10.1089/neu.2005.22.1282. [DOI] [PubMed] [Google Scholar]
- 62.Kim Y, Lee SH, Kim WH, Kweon OK. Transplantation of adipose derived mesenchymal stem cells for acute thoracolumbar disc disease with no deep pain perception in dogs. J Vet Sci. 2016;17(1):123–126. doi: 10.4142/jvs.2016.17.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nishida H, Nakayama M, Tanaka H, Kitamura M, Hatoya S, Sugiura K, Suzuki Y, Ide C, Inaba T. Evaluation of transplantation of autologous bone marrow stromal cells into the cerebrospinal fluid for treatment of chronic spinal cord injury in dogs. Am J Vet Res. 2011;72(8):1118–1123. doi: 10.2460/ajvr.72.8.1118. [DOI] [PubMed] [Google Scholar]
- 64.Nishida H, Nakayama M, Tanaka H, Kitamura M, Hatoya S, Sugiura K, Harada Y, Suzuki Y, Ide C, Inaba T. Safety of autologous bone marrow stromal cell transplantation in dogs with acute spinal cord injury. Vet Surg. 2012;41(4):437–442. doi: 10.1111/j.1532-950X.2011.00959.x. [DOI] [PubMed] [Google Scholar]
- 65.Penha EM, Meira CS, Guimarães ET, Mendonça MV, Gravely FA, Pinheiro CM, Pinheiro TM, Barrouin-Melo SM, Ribeiro-Dos-Santos R, Soares MB. Use of autologous mesenchymal stem cells derived from bone marrow for the treatment of naturally injured spinal cord in dogs. Stem Cells Int. 2014;2014:437521. doi: 10.1155/2014/437521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tamura K, Harada Y, Nagashima N, Itoi T, Ishino H, Yogo T, Nezu Y, Hara Y, Suzuki Y, Ide C, Tagawa M. Autotransplanting of bone marrow-derived mononuclear cells for complete cases of canine paraplegia and loss of pain perception, secondary to intervertebral disc herniation. Exp Clin Transplant. 2012;10(3):263–272. doi: 10.6002/ect.2011.0151. [DOI] [PubMed] [Google Scholar]
- 67.Cauzinille L, Kornegay JN. Fibrocartilaginous embolism of the spinal cord in dogs: review of 36 histologically confirmed cases and retrospective study of 26 suspected cases. J Vet Intern Med. 1996;10(4):241–245. doi: 10.1111/j.1939-1676.1996.tb02056.x. [DOI] [PubMed] [Google Scholar]
- 68.Chung WH, Park SA, Lee JH, Chung DJ, Yang WJ, Kang EH, Choi CB, Chang HS, Kim DH, Hwang SH, Han H, Kim HY. Percutaneous transplantation of human umbilical cord-derived mesenchymal stem cells in a dog suspected to have fibrocartilaginous embolic myelopathy. J Vet Sci. 2013;14(4):495–497. doi: 10.4142/jvs.2013.14.4.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chao YX, He BP, Tay SS. Mesenchymal stem cell transplantation attenuates blood brain barrier damage and neuroinflammation and protects dopaminergic neurons against MPTP toxicity in the substantia nigra in a model of Parkinson's disease. J Neuroimmunol. 2009;216(1-2):39–50. doi: 10.1016/j.jneuroim.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 70.Parr AM, Tator CH, Keating A. Bone marrow-derived mesenchymal stromal cells for the repair of central nervous system injury. Bone Marrow Transplant. 2007;40(7):609–619. doi: 10.1038/sj.bmt.1705757. [DOI] [PubMed] [Google Scholar]
- 71.Siniscalco D, Giordano C, Galderisi U, Luongo L, Alessio N, Di Bernardo G, de Novellis V, Rossi F, Maione S. Intra-brain microinjection of human mesenchymal stem cells decreases allodynia in neuropathic mice. Cell Mol Life Sci. 2010;67(4):655–669. doi: 10.1007/s00018-009-0202-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Talarico LR, Schatzberg SJ. Idiopathic granulomatous and necrotising inflammatory disorders of the canine central nervous system: a review and future perspectives. J Small Anim Pract. 2010;51(3):138–149. doi: 10.1111/j.1748-5827.2009.00823.x. [DOI] [PubMed] [Google Scholar]
- 73.Uchida K, Park E, Tsuboi M, Chambers JK, Nakayama H. Pathological and immunological features of canine necrotising meningoencephalitis and granulomatous meningoencephalitis. Vet J. 2016;213:72–77. doi: 10.1016/j.tvjl.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 74.Coates JR, Jeffery ND. Perspectives on meningoencephalomyelitis of unknown origin. Vet Clin North Am Small Anim Pract. 2014;44(6):1157–1185. doi: 10.1016/j.cvsm.2014.07.009. [DOI] [PubMed] [Google Scholar]
- 75.Genc B, Bozan HR, Genc S, Genc K. Stem cell therapy for multiple sclerosis. Adv Exp Med Biol. 2019;1084:145–174. doi: 10.1007/5584_2018_247. [DOI] [PubMed] [Google Scholar]
- 76.Gerdoni E, Gallo B, Casazza S, Musio S, Bonanni I, Pedemonte E, Mantegazza R, Frassoni F, Mancardi G, Pedotti R, Uccelli A. Mesenchymal stem cells effectively modulate pathogenic immune response in experimental autoimmune encephalomyelitis. Ann Neurol. 2007;61(3):219–227. doi: 10.1002/ana.21076. [DOI] [PubMed] [Google Scholar]
- 77.Nasri F, Mohtasebi MS, Hashemi E, Zarrabi M, Gholijani N, Sarvestani EK. Therapeutic efficacy of mesenchymal stem cells and mesenchymal stem cells-derived neural progenitors in experimental autoimmune encephalomyelitis. Int J Stem Cells. 2018;11(1):68–77. doi: 10.15283/ijsc17052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zeira O, Asiag N, Aralla M, Ghezzi E, Pettinari L, Martinelli L, Zahirpour D, Dumas MP, Lupi D, Scaccia S, Konar M, Cantile C. Adult autologous mesenchymal stem cells for the treatment of suspected non-infectious inflammatory diseases of the canine central nervous system: safety, feasibility and preliminary clinical findings. J Neuroinflammation. 2015;12(1):181. doi: 10.1186/s12974-015-0402-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Blanpain C, Fuchs E. Epidermal homeostasis: a balancing act of stem cells in the skin. Nat Rev Mol Cell Biol. 2009;10(3):207–217. doi: 10.1038/nrm2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Branski LK, Gauglitz GG, Herndon DN, Jeschke MG. A review of gene and stem cell therapy in cutaneous wound healing. Burns. 2009;35(2):171–180. doi: 10.1016/j.burns.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fathke C, Wilson L, Hutter J, Kapoor V, Smith A, Hocking A, Isik F. Contribution of bone marrow-derived cells to skin: collagen deposition and wound repair. Stem Cells. 2004;22(5):812–822. doi: 10.1634/stemcells.22-5-812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Herdrich BJ, Lind RC, Liechty KW. Multipotent adult progenitor cells: their role in wound healing and the treatment of dermal wounds. Cytotherapy. 2008;10(6):543–550. doi: 10.1080/14653240802345820. [DOI] [PubMed] [Google Scholar]
- 83.Kim JW, Lee JH, Lyoo YS, Jung DI, Park HM. The effects of topical mesenchymal stem cell transplantation in canine experimental cutaneous wounds. Vet Dermatol. 2013;24(2):242–e53. doi: 10.1111/vde.12011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zubin E, Conti V, Leonardi F, Zanichelli S, Ramoni R, Grolli S. Regenerative therapy for the management of a large skin wound in a dog. Clin Case Rep. 2015;3(7):598–603. doi: 10.1002/ccr3.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ribeiro J, Pereira T, Amorim I, Caseiro AR, Lopes MA, Lima J, Gartner A, Santos JD, Bártolo PJ, Rodrigues JM, Mauricio AC, Luís AL. Cell therapy with human MSCs isolated from the umbilical cord Wharton jelly associated to a PVA membrane in the treatment of chronic skin wounds. Int J Med Sci. 2014;11(10):979–987. doi: 10.7150/ijms.9139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nam A, Han SM, Go DM, Kim DY, Seo KW, Youn HY. Long-term management with adipose tissue-derived mesenchymal stem cells and conventional treatment in a dog with hepatocutaneous syndrome. J Vet Intern Med. 2017;31(5):1514–1519. doi: 10.1111/jvim.14798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hill PB, Auxilia ST, Munro E, Genovese L, Silkstone MA, Kirby B. Resolution of skin lesions and long-term survival in a dog with superficial necrolytic dermatitis and liver cirrhosis. J Small Anim Pract. 2000;41(11):519–523. doi: 10.1111/j.1748-5827.2000.tb03976.x. [DOI] [PubMed] [Google Scholar]
- 88.Banas A, Teratani T, Yamamoto Y, Tokuhara M, Takeshita F, Quinn G, Okochi H, Ochiya T. Adipose tissue-derived mesenchymal stem cells as a source of human hepatocytes. Hepatology. 2007;46(1):219–228. doi: 10.1002/hep.21704. [DOI] [PubMed] [Google Scholar]
- 89.Trebol Lopez J, Georgiev Hristov T, García-Arranz M, García-Olmo D. Stem cell therapy for digestive tract diseases: current state and future perspectives. Stem Cells Dev. 2011;20(7):1113–1129. doi: 10.1089/scd.2010.0277. [DOI] [PubMed] [Google Scholar]
- 90.Ibraheim H, Giacomini C, Kassam Z, Dazzi F, Powell N. Advances in mesenchymal stromal cell therapy in the management of Crohn's disease. Expert Rev Gastroenterol Hepatol. 2018;12(2):141–153. doi: 10.1080/17474124.2018.1393332. [DOI] [PubMed] [Google Scholar]
- 91.Schneider G, Saur D. Mesenchymal stem cells: therapeutic potential for acute pancreatitis. Gastroenterology. 2011;140(3):779–782. doi: 10.1053/j.gastro.2011.01.026. [DOI] [PubMed] [Google Scholar]
- 92.Alamoudi NM, El Ashiry EA, Farsi NM, El Derwi DA, Atta HM. Treatment of oral ulcers in dogs using adipose tissue-derived mesenchymal stem cells. J Clin Pediatr Dent. 2014;38(3):215–222. doi: 10.17796/jcpd.38.3.193115427jg6vl60. [DOI] [PubMed] [Google Scholar]
- 93.El-Menoufy H, Aly LA, Aziz MT, Atta HM, Roshdy NK, Rashed LA, Sabry D. The role of bone marrow-derived mesenchymal stem cells in treating formocresol induced oral ulcers in dogs. J Oral Pathol Med. 2010;39(4):281–289. doi: 10.1111/j.1600-0714.2009.00819.x. [DOI] [PubMed] [Google Scholar]
- 94.Jergens AE, Simpson KW. Inflammatory bowel disease in veterinary medicine. Front Biosci (Elite Ed) 2012;4(4):1404–1419. doi: 10.2741/e470. [DOI] [PubMed] [Google Scholar]
- 95.Pérez-Merino EM, Usón-Casaús JM, Duque-Carrasco J, Zaragoza-Bayle C, Mariñas-Pardo L, Hermida-Prieto M, Vilafranca-Compte M, Barrera-Chacón R, Gualtieri M. Safety and efficacy of allogeneic adipose tissue-derived mesenchymal stem cells for treatment of dogs with inflammatory bowel disease: endoscopic and histological outcomes. Vet J. 2015;206(3):391–397. doi: 10.1016/j.tvjl.2015.07.023. [DOI] [PubMed] [Google Scholar]
- 96.Pérez-Merino EM, Usón-Casaús JM, Zaragoza-Bayle C, Duque-Carrasco J, Mariñas-Pardo L, Hermida-Prieto M, Barrera-Chacón R, Gualtieri M. Safety and efficacy of allogeneic adipose tissue-derived mesenchymal stem cells for treatment of dogs with inflammatory bowel disease: clinical and laboratory outcomes. Vet J. 2015;206(3):385–390. doi: 10.1016/j.tvjl.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 97.Hardie RJ, Gregory SP, Tomlin J, Sturgeon C, Lipscomb V, Ladlow J. Cyclosporine treatment of anal furunculosis in 26 dogs. J Small Anim Pract. 2005;46(1):3–9. doi: 10.1111/j.1748-5827.2005.tb00267.x. [DOI] [PubMed] [Google Scholar]
- 98.Day MJ, Weaver BM. Pathology of surgically resected tissue from 305 cases of anal furunculosis in the dog. J Small Anim Pract. 1992;33(12):583–589. [Google Scholar]
- 99.Massey J, Short AD, Catchpole B, House A, Day MJ, Lohi H, Ollier WE, Kennedy LJ. Genetics of canine anal furunculosis in the German shepherd dog. Immunogenetics. 2014;66(5):311–324. doi: 10.1007/s00251-014-0766-5. [DOI] [PubMed] [Google Scholar]
- 100.Ferrer L, Kimbrel EA, Lam A, Falk EB, Zewe C, Juopperi T, Lanza R, Hoffman A. Treatment of perianal fistulas with human embryonic stem cell-derived mesenchymal stem cells: a canine model of human fistulizing Crohn's disease. Regen Med. 2016;11(1):33–43. doi: 10.2217/rme.15.69. [DOI] [PubMed] [Google Scholar]
- 101.Webb TL, Webb CB. Stem cell therapy in cats with chronic enteropathy: a proof-of-concept study. J Feline Med Surg. 2015;17(10):901–908. doi: 10.1177/1098612X14561105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Arzi B, Clark KC, Sundaram A, Spriet M, Verstraete FJ, Walker NJ, Loscar MR, Fazel N, Murphy WJ, Vapniarsky N, Borjesson DL. Therapeutic efficacy of fresh, allogeneic mesenchymal stem cells for severe refractory feline chronic gingivostomatitis. Stem Cells Transl Med. 2017;6(8):1710–1722. doi: 10.1002/sctm.17-0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Arzi B, Mills-Ko E, Verstraete FJ, Kol A, Walker NJ, Badgley MR, Fazel N, Murphy WJ, Vapniarsky N, Borjesson DL. Therapeutic efficacy of fresh, autologous mesenchymal stem cells for severe refractory gingivostomatitis in cats. Stem Cells Transl Med. 2016;5(1):75–86. doi: 10.5966/sctm.2015-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lyon KF. Gingivostomatitis. Vet Clin North Am Small Anim Pract. 2005;35(4):891–911. doi: 10.1016/j.cvsm.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 105.Liu C, Tan X, Luo J, Liu H, Hu M, Yue W. Reconstruction of beagle hemi-mandibular defects with allogenic mandibular scaffolds and autologous mesenchymal stem cells. PLoS One. 2014;9(8):e105733. doi: 10.1371/journal.pone.0105733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wang Y, Bi X, Zhou H, Deng Y, Sun J, Xiao C, Gu P, Fan X. Repair of orbital bone defects in canines using grafts of enriched autologous bone marrow stromal cells. J Transl Med. 2014;12(1):123. doi: 10.1186/1479-5876-12-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Moat SJ, Bradley DM, Salmon R, Clarke A, Hartley L. Newborn bloodspot screening for Duchenne muscular dystrophy: 21 years experience in Wales (UK) Eur J Hum Genet. 2013;21(10):1049–1053. doi: 10.1038/ejhg.2012.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lardenois A, Jagot S, Lagarrigue M, Guével B, Ledevin M, Larcher T, Dubreil L, Pineau C, Rouger K, Guével L. Quantitative proteome profiling of dystrophic dog skeletal muscle reveals a stabilized muscular architecture and protection against oxidative stress after systemic delivery of MuStem cells. Proteomics. 2016;16(14):2028–2042. doi: 10.1002/pmic.201600002. [DOI] [PubMed] [Google Scholar]
- 109.Rouger K, Larcher T, Dubreil L, Deschamps JY, Le Guiner C, Jouvion G, Delorme B, Lieubeau B, Carlus M, Fornasari B, Theret M, Orlando P, Ledevin M, Zuber C, Leroux I, Deleau S, Guigand L, Testault I, Le Rumeur E, Fiszman M, Chérel Y. Systemic delivery of allogenic muscle stem cells induces long-term muscle repair and clinical efficacy in duchenne muscular dystrophy dogs. Am J Pathol. 2011;179(5):2501–2518. doi: 10.1016/j.ajpath.2011.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sampaolesi M, Blot S, D'Antona G, Granger N, Tonlorenzi R, Innocenzi A, Mognol P, Thibaud JL, Galvez BG, Barthélémy I, Perani L, Mantero S, Guttinger M, Pansarasa O, Rinaldi C, Cusella De Angelis MG, Torrente Y, Bordignon C, Bottinelli R, Cossu G. Mesoangioblast stem cells ameliorate muscle function in dystrophic dogs. Nature. 2006;444(7119):574–579. doi: 10.1038/nature05282. [DOI] [PubMed] [Google Scholar]
- 111.Brown SG, Harman RJ, Black LL. Adipose-derived stem cell therapy for severe muscle tears in working German shepherds: two case reports. Stem Cell Discovery. 2012;2:41–44. [Google Scholar]
- 112.Gibson MA, Brown SG, Brown NO. Semitendinosus myopathy and treatment with adipose-derived stem cells in working German shepherd police dogs. Can Vet J. 2017;58(3):241–246. [PMC free article] [PubMed] [Google Scholar]
- 113.Lewis DD, Shelton GD, Piras A, Dee JF, Robins GM, Herron AJ, Fries C, Ginn PE, Hulse DA, Simpson DL, Allen DA. Gracilis or semitendinosus myopathy in 18 dogs. J Am Anim Hosp Assoc. 1997;33(2):177–188. doi: 10.5326/15473317-33-2-177. [DOI] [PubMed] [Google Scholar]
- 114.Cuervo B, Rubio M, Sopena J, Dominguez JM, Vilar J, Morales M, Cugat R, Carrillo JM. Hip osteoarthritis in dogs: a randomized study using mesenchymal stem cells from adipose tissue and plasma rich in growth factors. Int J Mol Sci. 2014;15(8):13437–13460. doi: 10.3390/ijms150813437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Vilar JM, Cuervo B, Rubio M, Sopena J, Domínguez JM, Santana A, Carrillo JM. Effect of intraarticular inoculation of mesenchymal stem cells in dogs with hip osteoarthritis by means of objective force platform gait analysis: concordance with numeric subjective scoring scales. BMC Vet Res. 2016;12(1):223. doi: 10.1186/s12917-016-0852-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Black LL, Gaynor J, Adams C, Dhupa S, Sams AE, Taylor R, Harman S, Gingerich DA, Harman R. Effect of intraarticular injection of autologous adipose-derived mesenchymal stem and regenerative cells on clinical signs of chronic osteoarthritis of the elbow joint in dogs. Vet Ther. 2008;9(3):192–200. [PubMed] [Google Scholar]
- 117.Guercio A, Di Marco P, Casella S, Cannella V, Russotto L, Purpari G, Di Bella S, Piccione G. Production of canine mesenchymal stem cells from adipose tissue and their application in dogs with chronic osteoarthritis of the humeroradial joints. Cell Biol Int. 2012;36(2):189–194. doi: 10.1042/CBI20110304. [DOI] [PubMed] [Google Scholar]
- 118.Case JB, Palmer R, Valdes-Martinez A, Egger EL, Haussler KK. Gastrocnemius tendon strain in a dog treated with autologous mesenchymal stem cells and a custom orthosis. Vet Surg. 2013;42(4):355–360. doi: 10.1111/j.1532-950X.2013.12007.x. [DOI] [PubMed] [Google Scholar]
- 119.Pascual-Garrido C, Guilak F, Rai MF, Harris MD, Lopez MJ, Todhunter RJ, Clohisy JC. Canine hip dysplasia: a natural animal model for human developmental dysplasia of the hip. J Orthop Res. 2018;36(7):1807–1817. doi: 10.1002/jor.23828. [DOI] [PubMed] [Google Scholar]
- 120.Biller B. Metronomic chemotherapy in veterinary patients with cancer: rethinking the targets and strategies of chemotherapy. Vet Clin North Am Small Anim Pract. 2014;44(5):817–829. doi: 10.1016/j.cvsm.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 121.Wagner W, Wein F, Seckinger A, Frankhauser M, Wirkner U, Krause U, Blake J, Schwager C, Eckstein V, Ansorge W, Ho AD. Comparative characteristics of mesenchymal stem cells from human bone marrow, adipose tissue, and umbilical cord blood. Exp Hematol. 2005;33(11):1402–1416. doi: 10.1016/j.exphem.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 122.Fritz V, Jorgensen C. Mesenchymal stem cells: an emerging tool for cancer targeting and therapy. Curr Stem Cell Res Ther. 2008;3(1):32–42. doi: 10.2174/157488808783489462. [DOI] [PubMed] [Google Scholar]
- 123.Kim SK, Kim SU, Park IH, Bang JH, Aboody KS, Wang KC, Cho BK, Kim M, Menon LG, Black PM, Carroll RS. Human neural stem cells target experimental intracranial medulloblastoma and deliver a therapeutic gene leading to tumor regression. Clin Cancer Res. 2006;12(18):5550–5556. doi: 10.1158/1078-0432.CCR-05-2508. [DOI] [PubMed] [Google Scholar]
- 124.Studeny M, Marini FC, Champlin RE, Zompetta C, Fidler IJ, Andreeff M. Bone marrow-derived mesenchymal stem cells as vehicles for interferon-beta delivery into tumors. Cancer Res. 2002;62(13):3603–3608. [PubMed] [Google Scholar]
- 125.Hwang Y, Kim D, Chang D, Ahn B, Kim YB, Kim G. Effects of neural stem cells and 5-fluorocytosine in canine metastatic lung tumor. J Vet Sci. 2017;18(2):257–260. doi: 10.4142/jvs.2017.18.2.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yi BR, Hwang KA, Kim YB, Kim SU, Choi KC. Effects of genetically engineered stem cells expressing cytosine deaminase and interferon-beta or carboxyl esterase on the growth of LNCaP rrostate cancer cells. Int J Mol Sci. 2012;13(10):12519–12532. doi: 10.3390/ijms131012519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Rici RE, Will SE, Luna AC, Melo LF, Santos AC, Rodrigues RF, Leandro RM, Maria DA. Combination therapy of canine osteosarcoma with canine bone marrow stem cells, bone morphogenetic protein and carboplatin in an in vivo model. Vet Comp Oncol. 2018;16(4):478–488. doi: 10.1111/vco.12404. [DOI] [PubMed] [Google Scholar]
- 128.Rout ED, Avery PR. Lymphoid neoplasia: correlations between morphology and flow cytometry. Vet Clin North Am Small Anim Pract. 2017;47(1):53–70. doi: 10.1016/j.cvsm.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 129.Suter SE, Hamilton MJ, Sullivan EW, Venkataraman GM. Allogeneic hematopoietic cell transplantation in a dog with acute large granular lymphocytic leukemia. J Am Vet Med Assoc. 2015;246(9):994–997. doi: 10.2460/javma.246.9.994. [DOI] [PubMed] [Google Scholar]
- 130.Vail DM. Hematopoietic tumors. In: Ettinger SJ, Feldman EC, editors. Textbook of Veterinary Internal Medicine. 7th ed. Philadelphia: WB saunders; 2010. pp. 2148–2163. [Google Scholar]
- 131.Warry EE, Willcox JL, Suter SE. Autologous peripheral blood hematopoietic cell transplantation in dogs with T-cell lymphoma. J Vet Intern Med. 2014;28(2):529–537. doi: 10.1111/jvim.12302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Willcox JL, Pruitt A, Suter SE. Autologous peripheral blood hematopoietic cell transplantation in dogs with B-cell lymphoma. J Vet Intern Med. 2012;26(5):1155–1163. doi: 10.1111/j.1939-1676.2012.00980.x. [DOI] [PubMed] [Google Scholar]
- 133.Questions and Answers on Allogenic Stem Cell-Based Products for Veterinary Use: Specific Questions on Sterility [Internet] London: European Medicines Agency; [Updated 2017]. [Accessed 2018 Sep 12]. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2017/06/WC500229927.pdf. [Google Scholar]
- 134.Cell-Based Products for Animal Use [Internet] Rockville: Food and Drug Administration; [Updated 2015]. [Accessed 2018 Sep 12]. https://www.fda.gov/downloads/AnimalVeterinary/GuidanceComplianceEnforcement/GuidanceforIndustry/UCM405679.pdf. [Google Scholar]
- 135.Stem Cell-Based Products for Veterinary Use: Specific Questions on Target Animal Safety to be Addressed by ADVENT [Internet] London: European Medicines Agency; [Updated 2016]. [Accessed 2018 Sep 12]. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2016/07/WC500210915.pdf. [Google Scholar]
- 136.Guideline on Safety Assessment of Cell-Based Products for Animal Use [Internet] Gimcheon: Animal and Plant Quarantine Agency; [Updated 2018]. [Accessed 2018 Sep 12]. http://www.qia.go.kr/viewwebQiaCom.do?id=44816&type=2_14dwyp. [Google Scholar]