Abstract
A novel coronavirus emerged in human populations and spread rapidly to cause the global coronavirus disease 2019 pandemic. Although the origin of the associated virus (severe acute respiratory syndrome coronavirus 2 [SARS-CoV-2]) remains unclear, genetic evidence suggests that bats are a reservoir host of the virus, and pangolins are a probable intermediate. SARS-CoV-2 has crossed the species barrier to infect humans and other animal species, and infected humans can facilitate reverse-zoonotic transmission to animals. Considering the rapidly changing interconnections among people, animals, and ecosystems, traditional roles of veterinarians should evolve to include transdisciplinary roles.
Keywords: COVID-19, SARS-CoV-2, coronavirus, zoonosis, transmission, one health, animal model, veterinarian
Clusters of pneumonia with unknown etiology were detected in Wuhan, China in December 2019, and shortly after, a novel coronavirus (severe acute respiratory syndrome coronavirus 2 [SARS-CoV-2]) was identified as the causative agent. The virus spread rapidly to other parts of China and many other countries. Despite tremendous efforts to contain the disease, the World Health Organization declared the spread as a global pandemic, referred to as coronavirus disease 2019 (COVID-19). As of May 15, 2020, 216 countries and territories have reported nearly 4.5 million confirmed COVID-19 cases and 300,000 related deaths (https://www.who.int/emergencies/diseases/novel-coronavirus-2019), and those numbers continue to increase. As veterinarian researchers, we have been following the development of COVID-19 in order to identify: 1) zoonotic transmission from animal to human, 2) potential risks to animals, 3) intra- and inter-species dissemination between animals, 4) possible reverse-zoonotic transmission from human to animal, and 5) animal models that are crucial for the development of vaccines and antiviral drugs.
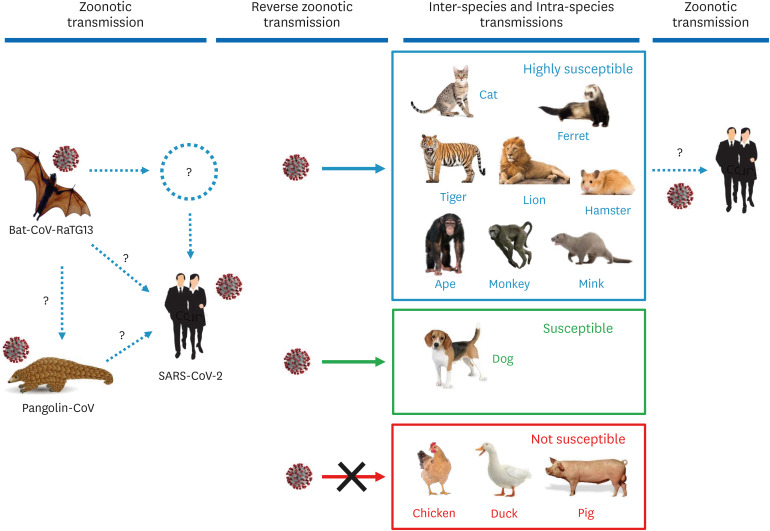
SARS-CoV-2 initiates infection via the binding of its spike (S) protein to a specific cellular receptor. The human receptor for SARS-CoV-2 is angiotensin-converting enzyme-2 (ACE2). A bat coronavirus (bat-CoV), RaTG13, has been isolated from Yunnan, China, and its whole genomic sequence is 96% identical to that of SARS-CoV-2. Another coronavirus was recently isolated from Malayan pangolins, and the whole genomic sequence of the pangolin coronavirus (pangolin-CoV) is 91.02% and 90.55% identical to that of SARS-CoV-2 and bat-CoV-RaTG13, respectively [1]. Although the bat-CoV-RaTG13 sequence is closest to that of SARS-CoV-2 (96% similarity), the receptor-binding domain of the pangolin-CoV S protein is more similar to that of SARS-CoV-2 than that of bat-CoV-RaTG13. Five key amino acids essential for binding to the human receptor are identical between pangolin-CoV and SARS-CoV-2, but four of the five residues are mutated in bat-CoV-RaTG13, indicating that bat-CoV-RaTG13 may not efficiently infect humans [2]. These findings suggest that SARS-CoV-2 may have evolved from pangolin-CoV and adapted to humans via natural selection (Fig. 1). Further studies are needed to substantiate that pangolins are an intermediate host.
Fig. 1. Possible origin of SARS-CoV-2 and transboundary transmissions between humans and animals. Bat-CoV-RaTG13 is a coronavirus identified in bats in Yunnan, China. Pangolin-CoV is a coronavirus isolated from Malayan pangolins smuggled to Guangdong, China. The whole-genome sequence of SARS-CoV-2 is 96% identical to bat-CoV-RaTG13. The whole-genome sequence of pangolin-CoV is 91.02% and 90.55% identical to SARS-CoV-2 and bat-CoV-RaTG13, respectively [1]. The receptor-binding domain sequences in the spike (S) protein of SARS-CoV-2 and pangolin-CoV are almost identical, suggesting efficient binding of both viruses to the human receptor angiotensin-converting enzyme 2. This genetic evidence suggests that pangolin-CoV is a possible ancestor of SARS-CoV-2.
SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; bat-CoV, bat coronavirus; pangolin-CoV, pangolin coronavirus.
Cats and dogs are often in close contact with humans, and thus, it is important to determine their susceptibility to SARS-CoV-2. COVID-19 has been reported in two dogs in Hong Kong. Dogs that live with COVID-19 patients have become infected and tested positive. One of the dogs developed specific antibodies against SARS-CoV-2 and seroconverted, indicating an active infection.
Canine cases of COVID-19 were also reported in the Netherlands and the US (https://6abc.com/coronavirus-dogs-dog-update/6134707/). A family in North Carolina experienced mild COVID-19 symptoms, and their pug also showed mild symptoms with inappetence. All three family members tested SARS-CoV-2 positive, and the virus was detected in the dog. The family owned two dogs and a cat, but only one dog tested positive. In the Netherlands, four house pets tested COVID-19 positive. A COVID-19 patient owned a dog and three cats, and the dog was suffering severe breathing problems. This bulldog tested SARS-CoV-2 positive and was euthanized due to the illness. The three cats also developed specific antibodies for SARS-CoV-2. All four animals appeared to have contracted the virus from their COVID-19 owner.
In contrast to dogs, cats appear to be highly susceptible to the virus. In Belgium, a cat living with its COVID-19 owner became clinically ill, exhibiting respiratory problems accompanied by diarrhea and vomiting. The specific viral sequence of SARS-CoV-2 was detected in the feces and gastric vomitus of the cat, and that sequence was identical to that of the cat owner, indicating the occurrence of reverse-zoonotic transmission of SARS-CoV-2 from human to animal. In Wuhan, 102 cats were tested, and 15% of them were seroconverted, indicating SARS-CoV-2 exposure of cats from either people or other cats [3]. An additional case of COVID-19 in cats was reported in Hong Kong. This cat did not show any clinical signs, but oral, nasal, and fecal samples tested positive. In the US, SARS-CoV-2 positive cats have been reported in two separate areas in the state of New York [4]. A veterinarian tested one cat after it exhibited mild respiratory symptoms. The cat was SARS-CoV-2 positive, but no one in the household was positive. This cat may have contracted the coronavirus outside of the household from a person with COVID-19. In another case, a cat with respiratory symptoms and living with a COVID-19 patient tested positive. These data clearly show that cats are susceptible to SARS-CoV-2 and may contract COVID-19 (Fig. 1). A controlled experiment was conducted to support the observations showing that cats are susceptible to COVID-19 [5]. The results of that experiment showed that cats are highly susceptible to SARS-CoV, with the viral sequence detected in (on) the nasal turbinates, soft palate, tonsils, and small intestine. The infections led to massive lesions in their nasal passages, trachea, and lungs, indicating efficient replication of the virus in cats. Interestingly, kittens and adolescent cats were especially vulnerable to COVID-19. Notably, the coronavirus spread from infected cats to uninfected cats via respiratory droplets. Feline cases of COVID-19 have additionally been reported in Spain, France, and Germany (https://www.oie.int/scientific-expertise/specific-information-and-recommendations/questions-and-answers-on-2019novel-coronavirus/).
It is logical to speculate that secondary transmission may occur from COVID-19 animals to humans, despite no direct evidence showing whether transmission from cats to humans can occur (Fig. 1). Owning a pet cat is commonplace, thus, the possible transmission of SARS-CoV between owners and cats or between cats, for example, in a veterinary hospital setting, is of concern. Experimental infections under varying conditions, wide-ranging surveillance using specific diagnostic tests, and collection of epidemiological data will help elucidate the role of companion animals in the spread of COVID-19. Among wildlife, lions and tigers are susceptible to SARS-CoV-2. Four tigers and two lions at the Bronx Zoo in New York City, developed clinical symptoms associated with respiratory illness, and testing confirmed they were SARS-CoV-2 positive [6]. The big cats had been exposed to a zookeeper who was COVID-19 positive and actively shedding the virus, indicating occurrences of reverse-zoonotic transmission (Fig. 1).
Susceptibility to COVID-19 has been evaluated in laboratory animals, companion animals, and farm animals in attempts to identify animal models of SARS-CoV-2 infection. Upon infection, susceptible animals may not show symptoms, or even if they do exhibit clinical signs, their symptoms may not match those of COVID-19 in humans. Thus, the establishment of an appropriate animal model is crucial. Among laboratory animals, ferrets, golden Syrian hamsters, and monkeys have been shown to be susceptible to SARS-CoV-2, and they develop clinical symptoms [7]. Ferrets are a good model for human influenza, particularly as they can sneeze, spreading the virus in the air. SARS-CoV-2 infects ferrets, but fatalities have not been observed [8]. The virus was detected in ferret nasal washes, saliva, urine, and feces, and airborne transmission was observed. However, viral replication in other organs was undetectable and did not lead to symptoms other than an increase in body temperature. A monkey-based study was conducted using four rhesus macaques [9]. These Old-World monkeys were successfully infected, and viral replication occurred in the nose, pharynx, lung, and gut. The infections caused weight loss and a moderate level of interstitial pneumonia with lesions and lymphocyte infiltration occurring in the lung, which were confirmed by H&E staining and immunohistochemical analysis. This monkey study also showed the presence of an antibody response against the virus. Using this monkey model, the efficacy of remdesivir, an antiviral drug, was examined [10]. Recovery in remdesivir-treated animals was significantly better than that in control animals. Only one of six treated animals had mild breathing difficulty, whereas all animals in the control group showed breathing difficulty. In treated monkeys, the viral load in the lungs was significantly lower, and lung damage was significantly milder than those in the control animals. Regardless of the animal model used, a significant challenge in such studies is that the experimental infection must be performed in a biosafety level 3 facility.
The high susceptibility of the Felidae family (lions, tigers, jaguars, leopards, cougars, and cheetahs) and, in particular, monkeys to COVID-19 implies a potential for outbreaks in great apes (chimpanzees, gorillas, orangutans) in zoos, animal-holding facilities, primate research centers, and national wildlife parks. Risk assessments are needed to prepare for the conservation and protection of such animals.
Among farm animals, minks at two breeding farms in the Netherlands showed various symptoms including respiratory illness and were found to have been infected with SARS-CoV-2. The minks were likely to have contracted the virus from farm staff, and the mink farms were subsequently placed under quarantine (https://www.oie.int/scientific-expertise/specific-information-and-recommendations/questions-and-answers-on-2019novel-coronavirus/). A subsequent study suggested that an infection took place from minks to humans, and cats played a role in the spread of the virus between farms, implicating possible occurrence of interspecies transmission (cat to mink) and secondary zoonotic transmission (mink to human) of SARS-CoV-2 (Fig, 1). In experimental infections, pigs, chickens, and ducks remained SARS-CoV-2 negative and did not develop any clinical signs [5]. Viral sequences were not detected in any swabs collected from virus-inoculated animals, and the animals remained seronegative for two weeks post-infection. Therefore, pigs, chickens, and ducks seem not to be susceptible to SARS-CoV-2. Regardless, a wider range of farm animal species needs to be examined to assess the risks of SARS-CoV-2 infection and to identify possible impacts of COVID-19 on the agricultural and food supply industries.
A combination of ecological disturbances, landscape changes, human behaviors, and public health factors contributes to the frequency of contacts between humans and wildlife, and such contacts pose a risk of exposure to transboundary animal viruses [11]. Moreover, viruses can easily mutate, switch hosts, and adapt to a new host. For the control of infectious diseases in animal populations, veterinarians typically apply 1) biosecurity measures to prevent the introduction of viruses to populations, 2) surveillance via diagnostic testing to identify infected animals, 3) removal of infected animals from uninfected populations, and 4) vaccination for long-term control by promoting immunity. These approaches have proven to be effective and successful in many countries when used to contain some transboundary epizootics; foot-and-mouth disease, African swine fever, and Newcastle disease, to name a few. In this period within the COVID-19 pandemic, societies and human behaviors are quickly changing, and to accommodate these changes, the traditional roles of veterinarians should evolve accordingly. Veterinarians should have significant roles in maintaining healthy ecosystems and protecting animals and humans from emerging and transboundary infections. Such roles should be based on the One Health framework, the application of which can reduce economic impacts on the livestock industry and food supply.
Footnotes
Funding: This study was supported by the Cooperative Research Program of the Center for Companion Animal Research (Project No. PJ013985012020), BK21 PLUS and the Research Institute for Veterinary Science, Seoul National University, Republic of Korea, awarded to HY, as well as Agriculture and Food Research Initiative (AFRI) Competitive Grants No. 2018-67015-28287 from the US Department of Agriculture (USDA) National Institute of Food and Agriculture (NIFA) awarded to DY.
Conflict of Interest: The authors declare no conflicts of interest.
- Conceptualization: Yoo HS, Yoo D.
- Data curation: Yoo HS, Yoo D.
- Formal analysis: Yoo HS, Yoo D.
- Funding acquisition: Yoo HS, Yoo D.
- Investigation: Yoo HS, Yoo D.
- Methodology: Yoo HS, Yoo D.
- Project administration: Yoo HS, Yoo D.
- Resources: Yoo HS, Yoo D.
- Supervision: Yoo D.
- Validation: Yoo HS, Yoo D.
- Visualization: Yoo HS, Yoo D.
- Writing - original draft: Yoo D.
- Writing - review & editing: Yoo HS, Yoo D.
References
- 1.Zhang T, Wu Q, Zhang Z. Probable pangolin origin of SARS-CoV-2 associated with the COVID-19 outbreak. Curr Biol. 2020;30(7):1346–1351.e2. doi: 10.1016/j.cub.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andersen KG, Rambaut A, Lipkin WI, Holmes EC, Garry RF. The proximal origin of SARS-CoV-2. Nat Med. 2020;26(4):450–452. doi: 10.1038/s41591-020-0820-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang Q, Zhang H, Huang K, Yang Y, Hui X, Gao J, He X, Li C, Gong W, Zhang Y, Peng C, Gao X, Chen H, Zou Z, Shi Z, Jin M. SARS-CoV-2 Neutralizing Serum Antibodies in Cats: a Serological Investigation [Internet] Cold Spring Harbor: bioRxiv; [Updated 2020]. https://www.biorxiv.org/content/10.1101/2020.04.01.021196v1. [Google Scholar]
- 4.United States Department of Agriculture. USDA Statement on the Conformation of COVID-19 in Two Pet Cats in New York [Internet] Washington, D.C.: United States Department of Agriculture; [Updated 2020]. https://www.aphis.usda.gov/aphis/newsroom/news/sa_by_date/sa-2020/sars-cov-2-animals. [Google Scholar]
- 5.Shi J, Wen Z, Zhong G, Yang H, Wang C, Huang B, Liu R, He X, Shuai L, Sun Z, Zhao Y, Liu P, Liang L, Cui P, Wang J, Zhang X, Guan Y, Tan W, Wu G, Chen H, Bu Z. Susceptibility of ferrets, cats, dogs, and other domesticated animals to SARS-coronavirus 2. Science. doi: 10.1126/science.abb7015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.United States Department of Agriculture. USDA Statement on the Confirmation of COVID-19 in a Tiger in New York [Internet] Washington, D.C.: United States Department of Agriculture; [Updated 2020]. https://www.aphis.usda.gov/aphis/newsroom/news/sa_by_date/sa-2020/ny-zoo-covid-19. [Google Scholar]
- 7.Cohen J. From mice to monkeys, animals studied for coronavirus answers. Science. 2020;368(6488):221–222. doi: 10.1126/science.368.6488.221. [DOI] [PubMed] [Google Scholar]
- 8.Kim YI, Kim SG, Kim SM, Kim EH, Park SJ, Yu KM, Chang JH, Kim EJ, Lee S, Casel MA, Um J, Song MS, Jeong HW, Lai VD, Kim Y, Chin BS, Park JS, Chung KH, Foo SS, Poo H, Mo IP, Lee OJ, Webby RJ, Jung JU, Choi YK. Infection and rapid transmission of SARS-CoV-2 in ferrets. Cell Host Microbe. 2020;27(5):704–709.e2. doi: 10.1016/j.chom.2020.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Munster VJ, Feldmann F, Williamson BN, van Doremalen N, Pérez-Pérez L, Schulz J, Meade-White K, Okumura A, Callison J, Brumbaugh B, Avanzato VA, Rosenke R, Hanley PW, Saturday G, Scott D, Fischer ER, de Wit E. Respiratory disease in rhesus macaques inoculated with SARS-CoV-2. Nature. doi: 10.1038/s41586-020-2324-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Williamson BN, Feldmann F, Schwarz B, et al. Clinical Benefit of Remdesivir in Rhesus Macaques Infected with SARS-CoV-2 [Internet] Cold Spring Harbor: bioRxiv; [Updated 2020]. https://www.biorxiv.org/content/10.1101/2020.04.15.043166v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leroy EM, Ar Gouilh M, Brugère-Picoux J. The risk of SARS-CoV-2 transmission to pets and other wild and domestic animals strongly mandates a one-health strategy to control the COVID-19 pandemic. One Health. 2020;100133:100133. doi: 10.1016/j.onehlt.2020.100133. [DOI] [PMC free article] [PubMed] [Google Scholar]