Abstract
Background
High concentrations of particulate matter less than 2.5 µm in diameter (PM2.5) in poultry houses is an important cause of respiratory disease in animals and humans. Pseudomonas aeruginosa is an opportunistic pathogen that can induce severe respiratory disease in animals under stress or with abnormal immune functions. When excessively high concentrations of PM2.5 in poultry houses damage the respiratory system and impair host immunity, secondary infections with P. aeruginosa can occur and produce a more intense inflammatory response, resulting in more severe lung injury.
Objectives
In this study, we focused on the synergistic induction of inflammatory injury in the respiratory system and the related molecular mechanisms induced by PM2.5 and P. aeruginosa in poultry houses.
Methods
High-throughput 16S rDNA sequence analysis was used for characterizing the bacterial diversity and relative abundance of the PM2.5 samples, and the effects of PM2.5 and P. aeruginosa stimulation on inflammation were detected by in vitro and in vivo.
Results
Sequencing results indicated that the PM2.5 in poultry houses contained a high abundance of potentially pathogenic genera, such as Pseudomonas (2.94%). The lung tissues of mice had more significant pathological damage when co-stimulated by PM2.5 and P. aeruginosa, and it can increase the expression levels of interleukin (IL)-6, IL-8, and tumor necrosis factor-α through nuclear factor (NF)-κB pathway in vivo and in vitro.
Conclusions
The results confirmed that poultry house PM2.5 in combination with P. aeruginosa could aggravate the inflammatory response and cause more severe respiratory system injuries through a process closely related to the activation of the NF-κB pathway.
Keywords: Inflammation, lung injury, poultry house, PM2.5, Pseudomonas
INTRODUCTION
The composition of particulate matter less than 2.5 µm in diameter (PM2.5) in the air is very complex and includes heavy metals, organics, bacteria, fungi, and viruses [1]. PM2.5 can cause great harm to the health of humans and animals, particularly to the respiratory system [2]. Studies have shown that pathogenic microorganisms in the particulate matter in the environment of poultry houses can harm the health of poultry [3]. Moreover, a high concentration of nonpathogenic microorganisms in particulate matter in poultry houses can overload the body's immune system and reduce the body's immunity, thus making livestock and poultry more susceptible to infection [4]. When PM2.5 is inhaled into the human body and deposited in the trachea of the respiratory tract, it can cause or aggravate various respiratory diseases, such as asthma and chronic obstructive pulmonary disease [5,6].
In recent years, the hazards of PM2.5 have received increasing attention. Studies have demonstrated that PM2.5 can interfere with a variety of physiological activities after entering the organism through the respiratory tract, including inducing cellular inflammatory responses, stimulating oxidative stress, and causing calcium homeostasis imbalance, thereby initiating damage to subcellular structures and functions and leading to the onset of diseases [7]. Studies have shown that stimulation with PM2.5 can reduce macrophage viability and induce the secretion of proinflammatory factors (e.g., interleukin [IL]-6, IL-8, and tumor necrosis factor [TNF]-α). The nuclear factor (NF)-κB signaling pathway is involved in the process of this immune response [8,9]. Researchers have experimentally demonstrated that PM2.5 from different sources may induce inflammation. However, studies of the pathogenic mechanisms of PM2.5 in the body are often focused on the inorganic and organic components of PM2.5, while there are relatively few studies on the microbial components of PM2.5 and their pathogenic mechanisms.
Livestock and poultry houses are closed breeding environments with high stocking densities and poor ventilation. Since particulate matter with a complex composition tends to form during poultry respiration, excretion, and feeding processes, the microbial composition of poultry house PM2.5 is more complex [10]. Compared with the open-air environment, PM2.5 in poultry houses may cause more serious injuries to livestock and humans [11]. In a previous study we found that the odds of a respiratory disease outbreak were greater in poultry houses with high PM2.5 concentrations. Moreover, the opportunistic bacterial pathogen Pseudomonas aeruginosa has been consistently detected during compositional analyses of PM2.5 samples from different seasons in poultry houses [12]. Breeders work long hours in poultry houses every day. Microbial aerosols in livestock and poultry houses, especially opportunistic pathogens, can affect poultry and, to a certain extent, affect human health as well [4,13].
P. aeruginosa is the main bacterial pathogen that causes a related infection and is extremely likely to cause secondary infection [14]. Many studies have also shown that Pseudomonas aeruginosa is one of the most common pathogens of human lung infection and can cause patients with low immunity to develop chronic lung infection and cystic fibrosis [15]. At the same time, P. aeruginosa can also cause disease in poultry, leading to septicemia and death in poultry and causing serious losses to the poultry industry [16]. Under normal conditions, opportunistic pathogenic microorganisms do not lead to the occurrence of diseases, but when the environmental conditions of the poultry houses change, the immunity of poultry will be reduced, and opportunistic pathogenic microorganisms will multiply and affect poultry health. It has been documented that high concentrations of PM2.5 can cause secondary infection by P. aeruginosa, leading to cystic fibrosis, but the specific mechanism has not been reported [17].
Macrophages play an important role in the inflammatory response process and are able to participate in the inflammatory response by producing a variety of different cytokines and inflammatory mediators [18]. Therefore, in this study, macrophages were used to establish an in vitro model of inflammation. The mouse pneumonia model is a widely used animal model that well reflects the occurrence and development of diseases in the human body. To a certain extent, mouse models can also simulate the pathological process and pathogenesis of chickens. Many studies have also documented the frequent use of mouse models to study the effects of PM2.5 on the health of humans and animals in livestock and poultry houses, so this study chose mice to establish animal pneumonia models [19,20].
In these experiments, we collected PM2.5 from poultry houses, analyzed the bacterial community composition using 16S rDNA sequencing, and preliminarily evaluated the proinflammatory effects. Synergistic stimulation of mice with PM2.5 and P. aeruginosa was used to establish an animal model in which their body weight change, pathological lung injury, and IL-6, IL-8, and TNF-α protein expression were detected. Furthermore, synergistic stimulation of RAW264.7 macrophages with PM2.5 and P. aeruginosa was used to establish a cellular model in which the expression of IL-6, IL-8, TNF-α, and NF-κB-related proteins were detected. We aim to elucidate the molecular mechanisms by which PM2.5 synergizes with P. aeruginosa to cause damage to the respiratory system and induce immune responses in human and poultry.
MATERIALS AND METHODS
Ethics statement
All animal experimental protocols were approved by the requirements and management guidelines of the Animal Ethical and Experimental Committee of Ludong University (license number LDU-IACUC2018007) based on the National Institutes of Health Guide for the Care and Use of Laboratory Animals. No endangered animals were involved in these experiments, and animal suffering was minimized as much as possible during the procedure.
Sample collection
The sampling location was conducted in a broiler house that was located in Zhaojia Village (37°25′3.78′′ N, 121°40′33.29′′ E) in Muping District, Yantai, Shandong Province. Samples were collected from July 24 to August 24, 2018. An air particulate sampler (ZR-3920, China) was used to collect PM2.5. The flow rate of the sampler was set at 100 L/min, and the sampler was placed in the center of the poultry house at 1 m above the ground for sampling. The sampling time was 72 h/sampling. PM2.5 was collected with a 9 cm × 9 cm waterproof glass filtration membrane, which was sterilized by dry heat before sampling.
Sample handling
The bacterial diversity and relative abundance of the collection of PM2.5 samples were characterized using DNA extraction and 16S rDNA sequence analysis. The remaining filter papers were divided into two groups in which one group was subjected to a heat treatment (PM2.5-) group, while the other group was not heat treated (PM2.5). PM2.5 was dissolved and diluted to the desired concentration with sterile pyrogen-free phosphate-buffered saline (PBS) as needed during the experiment.
Mice
Sixty specific pathogen free (SPF) grade, 5-week-old C57BL/6 wild-type mice were used in the animal experiments. All of the mice were purchased from Jinan Pengyue Experimental Animal Breeding Co., Ltd. (batch number: PY20180812; China). The average body weight of the mice was approximately 20 ± 2 g, and they were kept on a 12-h light/dark cycle in the environment with controlled temperature (23–25°C) and humidity (50 ± 10%). During the feeding period, the animals had free access to water and regular food. The mice were sacrificed with an acetaldehyde pentobarbital sodium injection during the experiment [20].
Bacterial strains and culture conditions
The PAO1 strain ATCC BAA-47 of P. aeruginosa was purchased from the American Type Culture Collection (ATCC, USA). After the PAO1 strain was activated, it was cultured and identified by streaking onto CN agar plates and incubating at 37°C for 24 h. Then, single colonies were picked and inoculated into the LB liquid medium and incubated at 37°C with shaking for the purpose of expanding the culture. In the experiments, sterile and pyrogen-free PBS was used to dilute the bacterial solution.
Cell culture
During the experiments, the murine RAW264.7 cell line (ATCC TIB-71, purchased from ATCC) was cultured for the detection of cellular inflammation. After the cells were revived, they were cultured with high glucose DMEM (HyClone, USA) containing 10% inactivated fetal bovine serum (TransGen, China) at 37°C with 5% CO2. Passages were performed using a cell scraper.
Griess assay
Different concentrations of PM2.5 exhibit different proinflammatory effects [20,21,22]. We selected three different concentrations of PM2.5 to stimulate macrophages to preliminarily evaluate the proinflammatory effects of different concentrations of PM2.5. The Griess method was used to evaluate the effect of PM2.5 on NO production by assaying the RAW264.7 culture supernatant. The cells were cultured to logarithmic phase and then seeded into 96-well cell culture plates (Corning, USA) at a density of 4 × 105 cells/well. Experiments consisted of seven groups: a normal control group, noninactivated PM2.5 groups (PM2.5) (1.00 mg/mL, 0.50 mg/mL, and 0.25 mg/mL), and inactivated PM2.5 groups (PM2.5-) (1.00 mg/mL, 0.50 mg/mL, and 0.25 mg/mL). After 20 μL of the corresponding sample were added to each well of each group, the cells were cultured for 6 h. The cell supernatant was then aspirated, and the Griess method procedure was performed using an NO detection kit (Beyotime, China) according to the manufacturer's instructions. The absorbance was measured at 540 nm. The concentration of NO was calculated according to a standard curve. The experiment was repeated three times.
In vivo: stimulation in mice
Animal model
All of the C57BL/6 mice were divided into six groups using the random number table method. The groups included the normal control group (PBS), non-heat-treated PM2.5 group (PM2.5), heat-treated PM2.5 group (PM2.5-), PAO1 group (PA), non-heat-treated PM2.5 and PAO1 group (PM2.5 + PA), and a heat-treated PM2.5 and PAO1 group (PM2.5- + PA), with 10 mice in each group.
During model establishment, each group of mice was intranasally inoculated twice daily. Then, 10 μL of the PM2.5 suspension (1 mg/mL) or P. aeruginosa (1 × 108 CFU/mL) was dripped into the nostrils of the mice with a micropipette, while 10 μL of only PBS was dripped into the nostrils of the mice in the control group. The experiment was conducted for 14 days, during which time the mice were weighed and their activity status was observed on days 0, 7, and 14.
The mice were dissected at the end of the model establishment period. The blood of each mouse was obtained by taking blood from the eyeball. The blood was allowed to stand for 30 min at 4°C and then centrifuged at 1,500 ×g for 15 min. The supernatant serum was aspirated and stored at −80°C for detecting the expression of proinflammatory cytokines by enzyme-linked immunosorbent assay (ELISA). Lung tissues from the mice were used for histopathological observations, as well as real-time polymerase chain reaction (PCR).
Lung histopathology
The mice were dissected, and the tissues of the left lower lobes of the lungs were harvested. The obtained specimens were immediately fixed in 4% paraformaldehyde solution for 48 h. The fixed tissues were then dehydrated with gradient ethanol solutions and embedded in paraffin, and serial sections with a thickness of 4–5 μm were cut with a rotary microtome and stained with hematoxylin and eosin (HE) and Masson stain. After the sections were mounted with neutral tree gum, photomicrographs were taken using a light microscope (Olympus, Japan) to observe and assess the degree of lung inflammation and collagen deposition. HE-stained sections were independently scored in a blinded manner according to established criteria [23,24].
ELISA
ELISAs were used to detect the expression of IL-6, TNF-α (ExCell Biotech, China) and IL-8 (XiTang Biotech, China) in the preserved mouse serum. The procedure was performed by strictly following the instructions of the ELISA kit.
Real-time PCR
The right lung tissues of the control and experimental groups of mice were homogenized in TRIzol reagent (Ambion, USA) using a tissue homogenizer. Total RNA was extracted, and the concentration and purity of the total RNA were determined. The cDNA was synthesized with an M-MLV reverse transcription kit (Thermo Scientific, USA). Real-time PCR was performed using a real-time fluorescence quantification kit (Vazyme, China). The reaction conditions were as follows: initial denaturation at 95°C for 30 s, followed by a total of 40 cycles of denaturation at 95°C for 10 s and annealing at 60°C for 30 s. The PCR mixture was 20 μL in total, and gene expression analysis was performed using the 2−∆∆Ct method. The primers that were used are listed in Supplementary Table 1.
Western blotting
First, the lung tissues of the mice in each group were rapidly ground in liquid nitrogen, and then the samples were transferred to prechilled 1.5 mL centrifuge tubes and lysed using a RIPA lysis solution (Solarbio, China) containing protease inhibitors and phenylmethylsulfonyl fluoride (PMSF) for 30 min, and the cellular proteins were extracted. After the protein concentration was determined by the BCA method, equal amounts of proteins from each group were loaded for SDS-PAGE and then transferred to a PVDF membrane. The membrane was blocked with 5% fat-free milk for 2 h. The membrane was incubated with a rabbit anti-mouse NF-κB p65 antibody (1:1,000; Cell Signaling Technology, USA), rabbit anti-mouse phospho-NF-κB p65 antibody (1:1,000; Cell Signaling Technology), and rabbit anti-mouse β-actin antibody (1:5,000; Proteintech, USA) for 2 h at room temperature. Then, the membrane was incubated with goat anti-rabbit IgG DyLight 800 antibody (1:30,000; Cell Signaling Technology) for 1 h at room temperature in the dark. Protein expression was detected using a fluorescence imager (LI-COR, USA), and relative gray values were calculated.
In vitro: stimulation in RAW264.7 cells
ELISA
RAW264.7 cells were cultured to log phase for all experiments. The cells were seeded into 96-well cell culture plates at a density of 5 × 104 cells/well and incubated at 37°C with 5% CO2 for 24 h before the subsequent experiments. The experiment was divided into six groups: a normal control group (PBS), a non-inactivated PM2.5 group (PM2.5), an inactivated PM2.5 group (PM2.5-), a PAO1 group (PA), a non-inactivated PM2.5 and PAO1 group (PM2.5 + PA), and an inactivated PM2.5 and PAO1 group (PM2.5- + PA). The PM2.5 suspension (1 mg/mL) or P. aeruginosa (1 × 108 CFU/mL) was added at a volume of 10 μL, or 10 μL of PBS was added to the control group. The corresponding samples were added separately to each group, and the cells were stimulated for 6 h at 37 °C. The cell supernatants were collected and used to measure the expression of IL-6, TNF-α (ExCell Biotech, China) and IL-8 (XiTang Biotech, China) in the serum by ELISA. The procedure was performed by strictly following the instructions of the ELISA kit.
Western blotting
The RAW264.7 cells were cultured to log phase and then seeded into six-well plates at a density of 2 × 106 cells/well and incubated at 37°C with 5% CO2 for 24 h before subsequent experiments. First, we add 10 μL of PM2.5 (1.00 mg/mL) to the cells and incubate them at different times (0, 5, 10, 15, 30, or 60 min) to determine the stimulation time. The experimental groupings were the same as for the in vitro ELISA experiments. The corresponding samples were added separately to each group, and the cells were stimulated for 30 min at 37°C. The cells were collected by cell scraping and lysed using a RIPA lysis solution (Solarbio, China) containing protease inhibitors and phenylmethylsulfonyl fluoride (PMSF) for 30 min, and the cellular proteins were extracted. After the protein concentration was determined by the BCA method, carried out the Western Blotting experiments, the method were the same as for the in vivo Western Blotting experiments.
Data analysis
All of the experiments were performed in triplicate, and the data are expressed as the means ± standard error of the mean (SEM). All of the statistical analyses were performed using Prism 7.04 software. Results were analyzed by one-way analysis of variance (ANOVA). The statistical significance of the differences between groups was determined using a two-tailed Student's t-test. A p < 0.05 was considered statistically significant, and p < 0.01 or p < 0.001 was considered extremely statistically significant.
RESULTS
Diversity and relative abundance of bacteria in PM2.5
To more accurately analyze the microbial components of PM2.5 we used 16S rDNA high-throughput sequencing to analyze their diversity and relative abundance. The top 10 most abundant bacterial species in atmospheric PM2.5 are shown in Table 1. Among these bacteria, Acinetobacter (21.21%), Corynebacterium (6.56%), and Pseudomonas (2.94%) are considered potential pathogenic bacteria that are harmful to human and poultry health.
Table 1. The diversity and relative abundance of bacteria in PM2.5.
| No. | Name of bacteria | Relative abundance (%) | G+/G− |
|---|---|---|---|
| 1 | Acinetobacter | 21.21 | G− |
| 2 | Lachnospiraceae | 8.47 | G+ |
| 3 | Brevundimonas | 6.88 | G− |
| 4 | Corynebacterium | 6.56 | G+ |
| 5 | Pseudomonas | 2.94 | G− |
| 6 | Faecalibacterium | 2.91 | G+ |
| 7 | Blautia | 2.20 | G+ |
| 8 | Subdoligranulum | 1.73 | G− |
| 9 | Streptomyces | 1.53 | G+ |
| 10 | Nocardiopsis | 1.25 | G+ |
| 11 | Others | 44.32 | - |
The top 10 bacterial species at the genus level and their relative abundances were detected in PM2.5 using high-throughput sequencing.
G+, gram-positive bacteria; G−, gram-negative bacteria.
PM2.5 raises the release of NO in RAW 264.7 cells
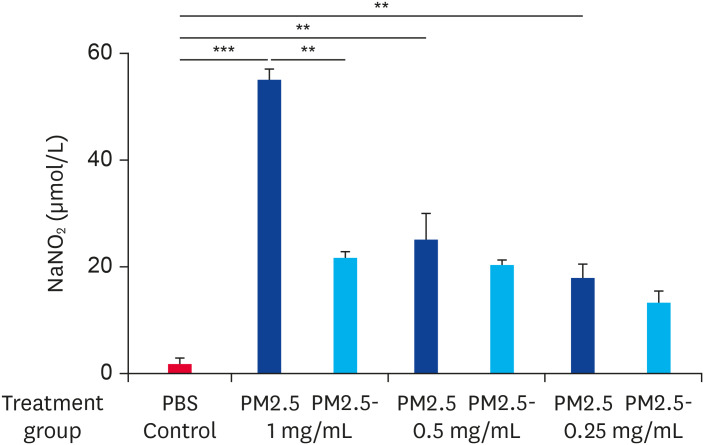
When the concentration of NO produced by RAW264.7 macrophages was measured using the Griess method, we found that the stimulation of cells with PM2.5 at a concentration of 1.00 mg/mL resulted in an approximately 28.03-fold increase in the NO concentration in the supernatant (55.21 ± 1.68 μM) compared to the basal level in normal unstimulated cells (1.97 ± 1.03 μM). When the PM2.5 concentration was lower than 0.50 mg/mL, the NO concentration in the cell supernatant was equivalent to the PM2.5- group (Fig. 1). Thus, we concluded that PM2.5 causes an increase in the expression of NO by cells and the effects of the PM2.5 microbial components are significant.
Fig. 1. PM2.5 stimulated RAW264.7 cells to secrete NO. Cells were stimulated with non-heat-treated PM2.5 or heat-treated PM2.5 at different concentrations (1.00 mg/mL, 0.50 mg/mL, or 0.25 mg/mL) for 6 h. The control group was treated with PBS. Data are presented as the means ± standard error of the mean. Data are representative of three independent experiments.
PBS, phosphate-buffered saline; PM2.5, particulate matter less than 2.5 µm in diameter.
**p < 0.01, ***p < 0.001.
We speculated that there were components of PM2.5 that could induce inflammation in cells and that there was a dose-dependence between the proinflammatory capacity of PM2.5 and its concentration. Interestingly, when equivalent doses of heat-inactivated PM2.5 were used to stimulate cells, its ability to induce NO production was significantly reduced compared to untreated PM2.5. The reason for this may be because when PM2.5 is heat-inactivated, its components, including microorganisms, are unable to function due to a loss of activity.
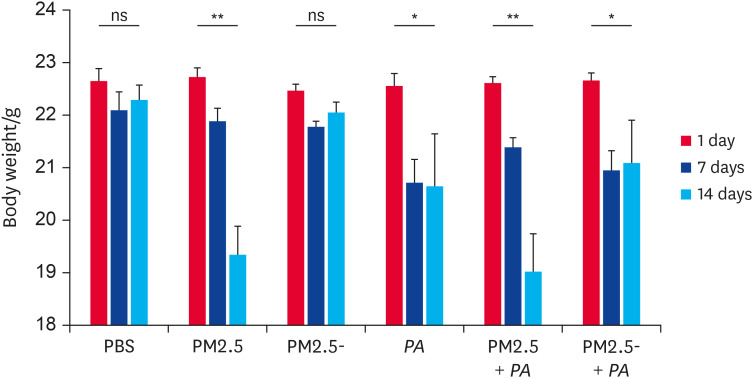
Synergistic stimulation with PM2.5 and P. aeruginosa caused a continuous decrease in mouse body weights
During the experiment, mice in the PM2.5 + PA group experienced the worst health conditions of all of the groups. Mice in this group had slow movement and unkempt hair, their respiratory rates were significantly accelerated, and they were slow to respond to external stimuli (expulsion, light, etc.). The body weights of mice in the experimental groups exposed to PM2.5 were all less than those in the control group. As shown in Fig. 2, mice in the control group showed no significant change in body weight during the experimental period. Mice in the PM2.5 and PM2.5 + PA groups displayed a continuous decrease in body weight during the intranasal inoculation stimulation period. Although the body weights of mice in the PM2.5- group, the PA group, and the PM2.5- + PA group decreased during the early period, their body weights did not continue to decrease in the later period. These results indicated that both PM2.5 and PA had an effect on the health of the mice and in terms of severity and persistence, the effect was more pronounced when they acted together.
Fig. 2. Co-stimulation with PM2.5 and P. aeruginosa resulted in sustained weight loss in mice. C57BL/6 mice were stimulated by an intranasal inoculation twice a day for 14 days. The concentrations of non-heat-treated PM2.5 or heat-treated PM2.5 were 1.00 mg/mL and the concentration of P. aeruginosa was 1 × 108 CFU/mL. The mice were weighed at 0, 7, and 14 days (n = 8–10). Data are presented as the means ± standard error of the mean.
PBS, phosphate-buffered saline; PM2.5, particulate matter less than 2.5 µm in diameter; P. aeruginosa, Pseudomonas aeruginosa; ns, not significant.
*p < 0.05, **p < 0.01.
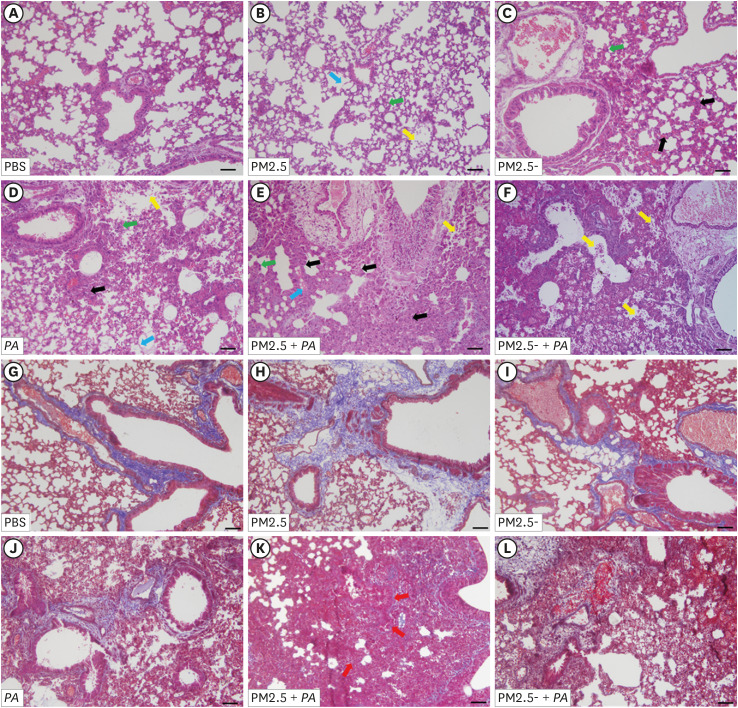
Synergistic stimulation with PM2.5 and P. aeruginosa aggravated lung injury in mice
The pathological sections of mouse lung tissue showed that in the control group, the alveolar wall was thin, the bronchial structure was intact, there was no cell detachment in the airway epithelium, and there was occasional infiltration of alveolar macrophages and a few neutrophils (Fig. 3A). Compared with the control group, the degree of neutrophil exudation in the alveoli was increased in the PM2.5 group (Fig. 3B), while the degree of lung tissue injury was more severe in the PA group. The PA group showed alveolar wall thickening, and this effect was accompanied by the appearance of a large number of neutrophils (Fig. 3D). These results indicated that the degree of lung injury in the PM2.5 + PA group was the most severe (Fig. 3E). In these group, the lung tissues exhibited a phenomenon that was similar to that of pulmonary edema. The alveolar walls already exhibited abnormal morphology, and the degree of thickening of the alveolar walls was the most severe, which was accompanied by the hallmark infiltration of pulmonary macrophages and neutrophils into the alveoli. Moreover, the cilia growth on the inner wall of the bronchi was abnormal, showing airway epithelial cell shedding. In addition, collagen deposition appeared around the blood vessels and airways in the PM2.5 + PA group (Fig. 3K). The above results indicated that PM2.5 could cause lung injury in mice and that when PM2.5 and P. aeruginosa acted synergistically, this inflammatory effect was more significant.
Fig. 3. Co-stimulation with PM2.5 and P. aeruginosa aggravated lung histopathological injury and lung fibrosis in mice. (A-F) Histopathological features of lung tissue by hematoxylin and eosin staining. (G-L) Lung sections from various treatment groups were subjected to Masson trichrome staining. (A, G) The PBS group showed normal lung tissue including thin alveolar walls and few alveolar macrophages. (B-F, H-L) These groups were treated with non-heat-treated PM2.5, heat-treated PM2.5, PAO1, non-heat-treated PM2.5 with PAO1 and heat-treated PM2.5 with PAO1. In these groups, the concentration of PM2.5 and PM2.5- were 1.00 mg/mL, and the concentration of P. aeruginosa was 1 × 108 CFU/mL. Yellow arrows indicate neutrophils in the alveolar space, green arrows indicate neutrophils in the interstitial space, blue arrows indicate hyaline membranes, and black arrows indicate thickening of the alveolar walls. Red arrows indicate the deposition of collagen in the interstitial space. The representative sections are shown at 400× original magnification (scale bars, 50 μm).
PBS, phosphate-buffered saline; PM2.5, particulate matter less than 2.5 µm in diameter; P. aeruginosa, Pseudomonas aeruginosa.
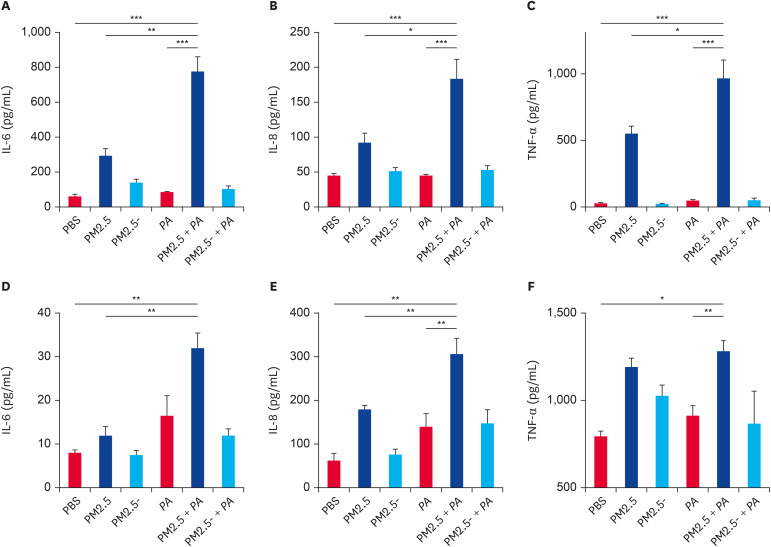
PM2.5 synergized with P. aeruginosa to stimulate the expression of IL-6, IL-8, and TNF-α
When detecting the expression of proinflammatory cytokines in mouse serum, we found that synergistic stimulation by PM2.5 and P. aeruginosa caused a significant increase in the expression levels of IL-6, IL-8, and TNF-α (Fig. 4A-C). Compared with the healthy control group, synergistic stimulation by PM2.5 and PA significantly increased the expression levels of IL-6, IL-8, and TNF-α in serum by approximately 11.77-fold, 4.89-fold, and 30.52-fold, respectively, with stimulated concentrations of 780.40 ± 82.12 pg/mL, 185.3 ± 27.01 pg/mL, and 968.80 ± 135.00 pg/mL, respectively. The above in vivo results showed that the combined action of PM2.5 and P. aeruginosa can significantly increase the expression levels of proinflammatory cytokines in mice and that this effect is more significant than that of the stimulation by PM2.5 or P. aeruginosa alone.
Fig. 4. Co-stimulation with PM2.5 and Pseudomonas aeruginosa increased the expression of IL-6, IL-8, and TNF-α protein in vivo and in vitro. (A-C) Detection of IL-6, IL-8, and TNF-α in serum of mice by ELISA (n = 5). (D-F) The expression levels of IL-6, IL-8, and TNF-α in the cell supernatant were detected by ELISA (n = 3). Data are presented as the means ± standard error of the mean.
IL, interleukin; PBS, phosphate-buffered saline; PM2.5, particulate matter less than 2.5 µm in diameter; P. aeruginosa, Pseudomonas aeruginosa; TNF, tumor necrosis factor; ELISA, enzyme-linked immunosorbent assay.
*p < 0.05, **p < 0.01, ***p < 0.001.
To further determine the proinflammatory capacity of particulate matter, we used PM2.5 and PA together to stimulate RAW264.7 cells and examined the expression of proinflammatory cytokines in the cell supernatants. Compared with the control group, synergistic stimulation by PM2.5 and P. aeruginosa significantly increased the expression levels of IL-6, IL-8, and TNF-α in cell supernatants by approximately 4.01-fold, 4.94-fold, and 1.62-fold, respectively, with concentrations of 32.14 ± 3.269 pg/mL, 308.70 ± 20.27 pg/mL, and 1287.00 ± 56.46 pg/mL, respectively (Fig. 4D-F). The results showed that synergistic stimulation by PM2.5 and P. aeruginosa significantly increased the expression levels of IL-6, IL-8, and TNF-α in cell supernatants. This increase is the same as the results from the in vivo mouse experiments.
The real-time PCR results demonstrated that the mRNA expression levels of IL-6, IL-8, and TNF-α in the lungs of mice costimulated with PM2.5 and P. aeruginosa were significantly higher than those in the control group (Fig. 5A-C), which was similar to the ELISA results. The results demonstrated that both PM2.5 and P. aeruginosa can upregulate the expression of inflammatory cytokines to various extents. In particular, when PM2.5 and P. aeruginosa stimulate synergistically, the effect is more significant.
Fig. 5. Co-stimulation with PM2.5 and Pseudomonas aeruginosa increased the expression of IL-6, IL-8, and TNF-α mRNA in the lung of the mice. (A-D) Detection of IL-6, IL-8, and TNF-α in the lung tissue of mice by real-time polymerase chain reaction (n = 5). Data are presented as the mean ± standard error of the mean.
IL, interleukin; PBS, phosphate-buffered saline; PM2.5, particulate matter less than 2.5 µm in diameter; TNF, tumor necrosis factor.
*p < 0.05, **p < 0.01, ***p < 0.001.
PM2.5 synergized with P. aeruginosa to activate the NF-κB pathway
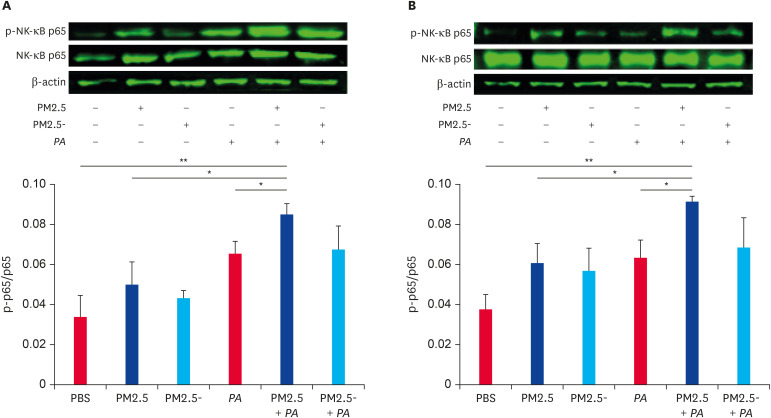
As shown in Fig. 6A, both PM2.5 and P. aeruginosa can increase the expression of the phospho-NF-κB p65 protein in the lung, and protein expression was significantly increased when PM2.5 and P. aeruginosa jointly stimulated the mice. The expression trend of related proteins in the RAW264.7 cells was the same as that in vivo (Fig. 6B). The results indicate that the NF-κB signaling pathway is activated to a greater extent when PM2.5 and P. aeruginosa act synergistically compared with stimulation by PM2.5 or P. aeruginosa alone.
Fig. 6. Co-stimulation of PM2.5 and Pseudomonas aeruginosa activated signaling pathways. (A) Detection of NF-κB p65 and phospho-NF-κB p65 proteins expression levels in lung by western blot and the ratio of phospho-NF-κB p65 protein/NF-κB p65 (n = 3). Data are presented as the means ± SEM. (B) Detection of NF-κB p65 and phospho-NF-κB p65 protein expression levels in RAW264.7 cells by western blot and the ratio of phospho-NF-κB p65 protein/NF-κB p65 (n = 3). Data are presented as the means ± SEM.
PBS, phosphate-buffered saline; PM2.5, particulate matter less than 2.5 µm in diameter; NF, nuclear factor; SEM, standard error of the mean.
*p < 0.05, **p < 0.01.
DISCUSSION
The welfare and healthy farming of poultry under intensive rearing conditions are closely related to the farming environment, and harmful gases such as ammonia and hydrogen sulfide, which are present in breeding houses, can damage the health of livestock and poultry [25,26]. With the development of intensive and large-scale livestock production and the accumulation of farming experience, the importance of microbial aerosols in the transmission of epidemic diseases has garnered much attention. Most infectious diseases in livestock and poultry are transmitted through the air, which causes great harm and loss to the livestock industry and even threatens human health; these issues also hinder the improvement and development of efficient animal husbandry production practices. Our previous results showed that the concentration of airborne particulate matter in poultry houses gradually increased as broilers grew, and the number of bacterial species that were carried by the particulate matter also increased [3,27].
16S rDNA high-throughput sequencing technology is currently the most commonly used analytical method to study the structural diversity of environmental microbial flora. In this study, the 16S rDNA (v4-v5) region of all bacteria in the collected PM samples was sequenced, and the results showed that microbial components were abundant in PM2.5 in poultry houses, and the main dominant bacteria were Acinetobacter, Corynebacterium, and Pseudomonas, among which there may be certain specific opportunistic pathogenic strains [12,28]. For example, Acinetobacter were detected in this study and had a relative abundance as high as 21.21% in PM2.5, and this genus is also often isolated in poultry house environments. Some Acinetobacter strains have been reported in the literature to cause pneumonia [12]. Some Corynebacterium strains can grow and multiply on throat mucosa and secrete exotoxins, which can cause local inflammation and lead to systemic toxic symptoms after entering systemic circulation [29]. In addition, the relative abundance of Pseudomonas was 2.94% in PM2.5 in our study. P. aeruginosa is the most common pathogen in this genus and can induce respiratory infections or septicemia in poultry, while it is also the most common pathogen that is responsible for human lung infections [15,16]. The presence of P. aeruginosa is also frequently detected in many livestock and poultry houses with outbreaks of respiratory diseases [16,30]. P. aeruginosa exhibits lower virulence in healthy hosts; however, when the body's immunity is reduced, P. aeruginosa can cause various secondary infections, including pulmonary infections [31,32]. Therefore, we speculate that the exposure to PM2.5 in the breeding environment can enhance the susceptibility of the respiratory system to P. aeruginosa and that the synergistic effects can lead to more severe lung damage and inflammatory responses.
As a second messenger, NO regulates various cellular functions and participates in physiological and pathological processes in vivo, while macrophages can participate in inflammatory and immune responses by secreting NO [33]. Li et al. [34] experimentally demonstrated that the stimulation of cells using PM2.5 collected from the atmospheric environment could induce the expression of iNOS and secrete large amounts of NO. Our study demonstrated that the levels of NO produced by macrophages stimulated with different concentrations of PM2.5 were different and that the level of NO produced by cells stimulated with PM2.5 without heat inactivation treatment was higher than that with PM2.5 with heat inactivation. In addition, this difference was significant when the sample concentration was 1.00 mg/mL (p < 0.01). This result indicates that the effect of biological components in PM2.5 on inflammation cannot be ignored.
From the pathological results of mouse lung tissue sections, it was found that both PM2.5 and P. aeruginosa could cause different degrees of lung injury, and the degree of lung injury that was caused by untreated PM2.5 was higher than that caused by heat-inactivated PM2.5, indicating that the microbial components played an important role in promoting lung injury in mice, which is consistent with the results of Meng et al. [20]. When PM2.5 and P. aeruginosa were used to stimulate synergistically, the inflammatory damage of the mouse lung tissues was the most severe, and signs of fibrosis began to appear around the pulmonary vessels and airways. This damage may be caused by a strong inflammatory response in the lung tissue [23,35]. The above results showed that when excessively high concentrations of PM2.5 in poultry houses caused respiratory tract injury and impaired host immune function, P. aeruginosa had an opportunity to adhere and colonize on the surface of the respiratory tract, thereby aggravating the inflammatory response and causing more severe lung injury.
Cytokines play a crucial role in the regulation of immune system function, so to a certain extent, the changes in their expression levels can reflect the status of the body's immune function [26]. As an important inflammatory transmitter, TNF-α plays a very important role in the initiation and maintenance of inflammation. An increase in the secretion of TNF-α can induce the secretion of other proinflammatory cytokines (e.g., IL-1, IL-6, IL-8) and inflammatory chemokines so that the inflammatory signals are amplified to cause a cascade effect [36]. IL-6 and IL-8 are important factors that are involved in inflammation and are able to affect the entire process of the body's inflammatory response by promoting the release of proinflammatory factors from macrophages [37,38]. Mononuclear phagocytes have been shown to be the main source of proinflammatory mediators in respiratory tract infections. Upon infection or external stimulation, proinflammatory factors such as IL-6, IL-8, and TNF-α that are secreted by macrophages are able to stimulate and activate endothelial cells and aggravate the pulmonary inflammatory response [39,40]. Real-time PCR and ELISA results showed that after co-stimulation with PM2.5 and P. aeruginosa, the mRNA and protein expression levels of IL-6, IL-8, and TNF-α were significantly increased. These abnormally secreted inflammatory factors may further cause immune damage to local tissues, alter normal immune function, and promote the occurrence of the inflammatory response. Therefore, PM2.5 and P. aeruginosa in poultry houses enter the bodies of humans and poultry through the respiratory tract to stimulate inflammatory cells such as macrophages and induce the expression and release of a variety of inflammatory factors. These abnormally secreted inflammatory factors may further alter normal immune function and promote the occurrence of inflammation.
Many studies have reported that both PM2.5 and P. aeruginosa could promote the release of inflammatory factors such as IL-6, IL-8, and TNF-α and mediate the pulmonary inflammatory response by activating the NF-κB signaling pathway [41,42]. The NF-κB signaling pathway is one of the ubiquitous signaling cascades mediating inflammatory responses in eukaryotic cells [43,44]. NF-κB is usually bound to its inhibitory protein (inhibitor of kappa B [IκB]) in the form of p50-p65 heterodimers and is in an inactive state. After it is stimulated by inflammatory signals, IκB is phosphorylated and degraded through ubiquitination to release the NF-κB heterodimer, which enters the nucleus to initiate the transcription and translation of various inflammatory mediator genes [45]. It has been shown that the development of inflammation in poultry is also closely related to the activation of the NF-κB pathway [46]. These results showed that under the synergistic stimulation of PM2.5 with P. aeruginosa, the expression of phospho-NF-κB protein was significantly increased in macrophages and mouse lung tissue. This result indicated that when PM2.5 in poultry houses acted synergistically with P. aeruginosa, it could further induce the expression of a large number of inflammatory factors by activating the NF-κB signaling pathway, leading to immune dysfunction and thereby inducing respiratory inflammation and causing severe lung injury.
In summary, excessively high concentrations of PM2.5 in poultry houses will induce the abnormal expression of multiple inflammatory factors, damaging the immune functions of the hosts. When P. aeruginosa adheres, colonizes, and grows in the respiratory epithelium, it will further activate macrophages to secrete various inflammatory factors and produce a more intense inflammatory response, and this process is closely related to the activation of the NF-κB pathway. This study will provide a certain theoretical basis for optimizing the breeding environment of poultry houses and for preventing and treating P. aeruginosa infections. To better evaluate the pathogenesis of avian P. aeruginosa disease in the future, we will use broilers to establish animal infection models to study the relevant mechanisms.
Footnotes
Funding: This research was financially supported by the National Key Research and Development Program of China (Grant No. 2018YFD0501402), the Key Research and Development Plan of Shandong Province (No. 2017NC210009), the Major Agricultural Applied Technological Innovation Projects of Shandong Province, the Key Research and Development Plan of Yantai (No. 2018XSCC045), and the Innovation Team Project for Modern Agricultural Industrious Technology System of Shandong Province (SDAIT-11-10).
Conflict of Interest: The authors declare no conflicts of interest.
- Conceptualization: Zhang J, Zhang X.
- Data curation: Zhu H.
- Formal analysis: Li M, Tang J.
- Funding acquisition: Zhang J, Zhang X.
- Investigation: Li M, Wei X, Tang J.
- Methodology: Chen G.
- Project administration: Zhang J.
- Resources: Zhang X.
- Software: Zhu H.
- Supervision: Zhang X.
- Validation: Li Y, Feng T, Chen G.
- Visualization: Li M.
- Writing - original draft: Li M, Wei X.
- Writing - review & editing: Jiang L, Yu X, Zhu H, Zhang J, Zhang X.
SUPPLEMENTARY MATERIALS
Primer sequences for real-time polymerase chain reaction
High concentration of PM2.5 can inhibit cell proliferation. The antiproliferative activity of PM2.5 was evaluated by WST-1 (Roch, Germany) assay. RAW 264.7 were seeded in 96-well plates (Corning, USA) at a density of 5 × 104 cells/well and cultured for 24 h at 37°C. (A) PM2.5 with different concentration gradients were added, each group was added 10 μL. Experiments consisted of seven groups: a normal control group, non-inactivated PM2.5 groups (PM2.5) (1.00 mg/mL, 0.50 mg/mL, 0.25 mg/mL), and inactivated PM2.5 groups (PM2.5-) (1.00 mg/mL, 0.50 mg/mL, 0.25 mg/mL).
Lung injury scoring about hematoxylin and eosin. Lung injury scores were assessed. Ten fields of view were randomly selected from each section to evaluate the severity of lung injury according to five indicators: the number of neutrophils in the alveolar space, the number of neutrophils in the pulmonary interstitial space, the presence or absence of the formation of hyaline membranes, the presence or absence of protein debris in the alveolar space, and the degree of alveolar wall thickening. Each index was weighted and averaged according to its correlation with disease and the number of fields under the microscope and finally, a lung tissue injury pathological score was obtained for each group. Data are presented as the mean ± standard error of the mean.
Detection of NF-κB p65 proteins expression levels in RAW264.7 cells by western blot.
References
- 1.Lippmann M. Toxicological and epidemiological studies of cardiovascular effects of ambient air fine particulate matter (PM2.5) and its chemical components: coherence and public health implications. Crit Rev Toxicol. 2014;44(4):299–347. doi: 10.3109/10408444.2013.861796. [DOI] [PubMed] [Google Scholar]
- 2.Xing YF, Xu YH, Shi MH, Lian YX. The impact of PM2.5 on the human respiratory system. J Thorac Dis. 2016;8(1):E69–E74. doi: 10.3978/j.issn.2072-1439.2016.01.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang J, Wei X, Jiang L, Li Y, Li M, Zhu H, Yu X, Tang J, Chen G, Zhang X. Bacterial Community Diversity in Particulate Matter (PM2.5 and PM10) Within Broiler Houses in Different Broiler Growth Stages Under Intensive Rearing Conditions in Summer. J Appl Poult Res. 2019;28(2):479–489. [Google Scholar]
- 4.Fiegel J, Clarke R, Edwards DA. Airborne infectious disease and the suppression of pulmonary bioaerosols. Drug Discov Today. 2006;11(1-2):51–57. doi: 10.1016/S1359-6446(05)03687-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Isiugo K, Jandarov R, Cox J, Ryan P, Newman N, Grinshpun SA, Indugula R, Vesper S, Reponen T. Indoor particulate matter and lung function in children. Sci Total Environ. 2019;663:408–417. doi: 10.1016/j.scitotenv.2019.01.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang Q, Hu D, Wang X, Chen Y, Wu Y, Pan L, Li H, Zhang J, Deng F, Guo X, Shen H. The modification of indoor PM2.5 exposure to chronic obstructive pulmonary disease in Chinese elderly people: A meet-in-metabolite analysis. Environ Int. 2018;121(Pt 2):1243–1252. doi: 10.1016/j.envint.2018.10.046. [DOI] [PubMed] [Google Scholar]
- 7.Chenxu G, Minxuan X, Yuting Q, Tingting G, Jinxiao L, Mingxing W, Sujun W, Yongjie M, Deshuai L, Qiang L, Linfeng H, Jun T. iRhom2 loss alleviates renal injury in long-term PM2.5-exposed mice by suppression of inflammation and oxidative stress. Redox Biol. 2018;19:147–157. doi: 10.1016/j.redox.2018.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hiraiwa K, van Eeden SF. Contribution of lung macrophages to the inflammatory responses induced by exposure to air pollutants. Mediators Inflamm. 2013;2013:619523. doi: 10.1155/2013/619523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tang Q, Huang K, Liu J, Wu S, Shen D, Dai P, Li C. Fine particulate matter from pig house induced immune response by activating TLR4/MAPK/NF-κB pathway and NLRP3 inflammasome in alveolar macrophages. Chemosphere. 2019;236:124373. doi: 10.1016/j.chemosphere.2019.124373. [DOI] [PubMed] [Google Scholar]
- 10.Just N, Kirychuk S, Gilbert Y, Létourneau V, Veillette M, Singh B, Duchaine C. Bacterial diversity characterization of bioaerosols from cage-housed and floor-housed poultry operations. Environ Res. 2011;111(4):492–498. doi: 10.1016/j.envres.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 11.Kearney GD, Shaw R, Prentice M, Tutor-Marcom R. Evaluation of respiratory symptoms and respiratory protection behavior among poultry workers in small farming operations. J Agromed. 2014;19(2):162–170. doi: 10.1080/1059924X.2014.886536. [DOI] [PubMed] [Google Scholar]
- 12.Jiang L, Zhang J, Tang J, Li M, Zhao X, Zhu H, Yu X, Li Y, Feng T, Zhang X. Analyses of aerosol concentrations and bacterial community structures for closed cage broiler houses at different broiler growth stages in winter. J Food Prot. 2018;81(9):1557–1564. doi: 10.4315/0362-028X.JFP-17-524. [DOI] [PubMed] [Google Scholar]
- 13.Arteaga V, Mitchell D, Armitage T, Tancredi D, Schenker M, Mitloehner F. Cage versus noncage laying-hen housings: respiratory exposures. J Agromed. 2015;20(3):245–255. doi: 10.1080/1059924X.2015.1044681. [DOI] [PubMed] [Google Scholar]
- 14.Musavi L, Lopez J, Cho R, Siegel N, Seal S, Dorafshar AH, Steinberg JP. Infectious complications after open cranial vault remodeling for craniosynostosis. J Craniofac Surg. 2019 doi: 10.1097/SCS.0000000000005695. [DOI] [PubMed] [Google Scholar]
- 15.Rybtke M, Hultqvist LD, Givskov M, Tolker-Nielsen T. Pseudomonas aeruginosa biofilm infections: community structure, antimicrobial tolerance and immune response. J Mol Biol. 2015;427(23):3628–3645. doi: 10.1016/j.jmb.2015.08.016. [DOI] [PubMed] [Google Scholar]
- 16.Walker SE, Sander JE, Cline JL, Helton JS. Characterization of Pseudomonas aeruginosa isolates associated with mortality in broiler chicks. Avian Dis. 2002;46(4):1045–1050. doi: 10.1637/0005-2086(2002)046[1045:COPAIA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 17.Psoter KJ, De Roos AJ, Mayer JD, Kaufman JD, Wakefield J, Rosenfeld M. Fine particulate matter exposure and initial Pseudomonas aeruginosa acquisition in cystic fibrosis. Ann Am Thorac Soc. 2015;12(3):385–391. doi: 10.1513/AnnalsATS.201408-400OC. [DOI] [PubMed] [Google Scholar]
- 18.Tamura N, Hazeki K, Okazaki N, Kametani Y, Murakami H, Takaba Y, Ishikawa Y, Nigorikawa K, Hazeki O. Specific role of phosphoinositide 3-kinase p110alpha in the regulation of phagocytosis and pinocytosis in macrophages. Biochem J. 2009;423(1):99–108. doi: 10.1042/BJ20090687. [DOI] [PubMed] [Google Scholar]
- 19.Franzi LM, Linderholm AL, Rabowsky M, Last JA. Lung toxicity in mice of airborne particulate matter from a modern layer hen facility containing Proposition 2-compliant animal caging. Toxicol Ind Health. 2017;33(3):211–221. doi: 10.1177/0748233716630490. [DOI] [PubMed] [Google Scholar]
- 20.Meng K, Wu B, Gao J, Cai Y, Yao M, Wei L, Chai T. Immunity-related protein expression and pathological lung damage in mice poststimulation with ambient particulate matter from live bird markets. Front Immunol. 2016;7:252. doi: 10.3389/fimmu.2016.00252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ma QY, Huang DY, Zhang HJ, Wang S, Chen XF. Exposure to particulate matter 2.5 (PM2.5) induced macrophage-dependent inflammation, characterized by increased Th1/Th17 cytokine secretion and cytotoxicity. Int Immunopharmacol. 2017;50:139–145. doi: 10.1016/j.intimp.2017.06.019. [DOI] [PubMed] [Google Scholar]
- 22.He M, Ichinose T, Yoshida S, Ito T, He C, Yoshida Y, Arashidani K, Takano H, Sun G, Shibamoto T. PM2.5-induced lung inflammation in mice: differences of inflammatory response in macrophages and type II alveolar cells. J Appl Toxicol. 2017;37(10):1203–1218. doi: 10.1002/jat.3482. [DOI] [PubMed] [Google Scholar]
- 23.Zhou Y, Li P, Goodwin AJ, Cook JA, Halushka PV, Chang E, Zingarelli B, Fan H. Exosomes from endothelial progenitor cells improve outcomes of the lipopolysaccharide-induced acute lung injury. Crit Care. 2019;23(1):44. doi: 10.1186/s13054-019-2339-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matute-Bello G, Downey G, Moore BB, Groshong SD, Matthay MA, Slutsky AS, Kuebler WM Acute Lung Injury in Animals Study Group. An official American Thoracic Society workshop report: features and measurements of experimental acute lung injury in animals. Am J Respir Cell Mol Biol. 2011;44(5):725–738. doi: 10.1165/rcmb.2009-0210ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang S, Chi Q, Hu X, Cong Y, Li S. Hydrogen sulfide-induced oxidative stress leads to excessive mitochondrial fission to activate apoptosis in broiler myocardia. Ecotoxicol Environ Saf. 2019;183:109578–109578. doi: 10.1016/j.ecoenv.2019.109578. [DOI] [PubMed] [Google Scholar]
- 26.Shi Q, Wang W, Chen M, Zhang H, Xu S. Ammonia induces Treg/Th1 imbalance with triggered NF-κB pathway leading to chicken respiratory inflammation response. Sci Total Environ. 2019;659:354–362. doi: 10.1016/j.scitotenv.2018.12.375. [DOI] [PubMed] [Google Scholar]
- 27.Zhang J, Li Y, Xu E, Jiang L, Tang J, Li M, Zhao X, Chen G, Zhu H, Yu X, Zhang X. Bacterial communities in PM2.5 and PM10 in broiler houses at different broiler growth stages in spring. Pol J Vet Sci. 2019;22(3):495–504. doi: 10.24425/pjvs.2019.129957. [DOI] [PubMed] [Google Scholar]
- 28.Jiang L, Li M, Tang J, Zhao X, Zhang J, Zhu H, Yu X, Li Y, Feng T, Zhang X. Effect of different disinfectants on bacterial aerosol diversity in poultry houses. Front Microbiol. 2018;9:2113. doi: 10.3389/fmicb.2018.02113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Los-Arcos I, Len O, Martín-Gómez MT, Baroja A, Berastegui C, Deu M, Sacanell J, Román A, Gavaldà J. Clinical characteristics and outcome of lung transplant recipients with respiratory isolation of Corynebacterium spp. J Clin Microbiol. 2018;56(8):e00142–e00118. doi: 10.1128/JCM.00142-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gong Q, Ruan MD, Niu MF, Qin CL, Hou Y, Guo JZ. Immune efficacy of DNA vaccines based on oprL and oprF genes of Pseudomonas aeruginosa in chickens. Poult Sci. 2018;97(12):4219–4227. doi: 10.3382/ps/pey307. [DOI] [PubMed] [Google Scholar]
- 31.Azam MW, Khan AU. Updates on the pathogenicity status of Pseudomonas aeruginosa. Drug Discov Today. 2019;24(1):350–359. doi: 10.1016/j.drudis.2018.07.003. [DOI] [PubMed] [Google Scholar]
- 32.Garau J, Gomez L. Pseudomonas aeruginosa pneumonia. Curr Opin Infect Dis. 2003;16(2):135–143. doi: 10.1097/00001432-200304000-00010. [DOI] [PubMed] [Google Scholar]
- 33.Do H, Pyo S, Sohn EH. Suppression of iNOS expression by fucoidan is mediated by regulation of p38 MAPK, JAK/STAT, AP-1 and IRF-1, and depends on up-regulation of scavenger receptor B1 expression in TNF-alpha- and IFN-gamma-stimulated C6 glioma cells. J Nutr Biochem. 2010;21(8):671–679. doi: 10.1016/j.jnutbio.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 34.Li W, Cai ZN, Mehmood S, Liang LL, Liu Y, Zhang HY, Chen Y, Lu YM. Anti-inflammatory effects of Morchella esculenta polysaccharide and its derivatives in fine particulate matter-treated NR8383 cells. Int J Biol Macromol. 2019;129:904–915. doi: 10.1016/j.ijbiomac.2019.02.088. [DOI] [PubMed] [Google Scholar]
- 35.Xu Z, Li Z, Liao Z, Gao S, Hua L, Ye X, Wang Y, Jiang S, Wang N, Zhou D, Deng X. PM2.5 induced pulmonary fibrosis in vivo and in vitro. Ecotoxicol Environ Saf. 2019;171:112–121. doi: 10.1016/j.ecoenv.2018.12.061. [DOI] [PubMed] [Google Scholar]
- 36.Li X, Huang Q, Ong CN, Yang XF, Shen HM. Chrysin sensitizes tumor necrosis factor-alpha-induced apoptosis in human tumor cells via suppression of nuclear factor-kappaB. Cancer Lett. 2010;293(1):109–116. doi: 10.1016/j.canlet.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 37.Al Hanai AH, Antkiewicz DS, Hemming JD, Shafer MM, Lai AM, Arhami M, Hosseini V, Schauer JJ. Seasonal variations in the oxidative stress and inflammatory potential of PM2.5 in Tehran using an alveolar macrophage model; the role of chemical composition and sources. Environ Int. 2019;123:417–427. doi: 10.1016/j.envint.2018.12.023. [DOI] [PubMed] [Google Scholar]
- 38.Ayaub EA, Dubey A, Imani J, Botelho F, Kolb MR, Richards CD, Ask K. Overexpression of OSM and IL-6 impacts the polarization of pro-fibrotic macrophages and the development of bleomycin-induced lung fibrosis. Sci Rep. 2017;7(1):13281–13281. doi: 10.1038/s41598-017-13511-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tian G, Wang J, Lu Z, Wang H, Zhang W, Ding W, Zhang F. Indirect effect of PM1 on endothelial cells via inducing the release of respiratory inflammatory cytokines. Toxicol In Vitro. 2019;57:203–210. doi: 10.1016/j.tiv.2019.03.013. [DOI] [PubMed] [Google Scholar]
- 40.Coates BM, Staricha KL, Koch CM, Cheng Y, Shumaker DK, Budinger GR, Perlman H, Misharin AV, Ridge KM. Inflammatory monocytes drive influenza a virus-mediated lung injury in juvenile mice. J Immunol. 2018;200(7):2391–2404. doi: 10.4049/jimmunol.1701543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stravinskas Durigon T, MacKenzie B, Carneiro Oliveira-Junior M, Santos-Dias A, De Angelis K, Malfitano C, Kelly da Palma R, Moreno Guerra J, Damaceno-Rodrigues NR, Garcia Caldini E, de Almeida FM, Aquino-Santos HC, Rigonato-Oliveira NC, Leal de Oliveira DB, Aimbire F, Ligeiro de Oliveira AP, Franco de Oliveira LV, Durigon EL, Hiemstra PS, Vieira RP. Aerobic Exercise Protects from Pseudomonas aeruginosa-Induced Pneumonia in Elderly Mice. J Innate Immun. 2018;10(4):279–290. doi: 10.1159/000488953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dou C, Zhang J, Qi C. Cooking oil fume-derived PM2.5 induces apoptosis in A549 cells and MAPK/NF-кB/STAT1 pathway activation. Environ Sci Pollut Res Int. 2018;25(10):9940–9948. doi: 10.1007/s11356-018-1262-5. [DOI] [PubMed] [Google Scholar]
- 43.Yoon YK, Woo HJ, Kim Y. Orostachys japonicus inhibits expression of the TLR4, NOD2, iNOS, and COX-2 genes in LPS-stimulated human PMA-differentiated THP-1 cells by inhibiting NF-κB and MAPK activation. Evid Based Complement Alternat Med. 2015;2015:682019. doi: 10.1155/2015/682019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Valacchi G, Pagnin E, Phung A, Nardini M, Schock BC, Cross CE, van der Vliet A. Inhibition of NFkappaB activation and IL-8 expression in human bronchial epithelial cells by acrolein. Antioxid Redox Signal. 2005;7(1-2):25–31. doi: 10.1089/ars.2005.7.25. [DOI] [PubMed] [Google Scholar]
- 45.Wang J, Wang H, Zhang H, Liu Z, Ma C, Kang W. Immunomodulation of ADPs-1a and ADPs-3a on RAW264.7 cells through NF-κB/MAPK signaling pathway. Int J Biol Macromol. 2019;132:1024–1030. doi: 10.1016/j.ijbiomac.2019.04.031. [DOI] [PubMed] [Google Scholar]
- 46.Hu X, Chi Q, Liu Q, Wang D, Zhang Y, Li S. Atmospheric H2S triggers immune damage by activating the TLR-7/MyD88/NF-κB pathway and NLRP3 inflammasome in broiler thymus. Chemosphere. 2019;237:124427–124427. doi: 10.1016/j.chemosphere.2019.124427. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Primer sequences for real-time polymerase chain reaction
High concentration of PM2.5 can inhibit cell proliferation. The antiproliferative activity of PM2.5 was evaluated by WST-1 (Roch, Germany) assay. RAW 264.7 were seeded in 96-well plates (Corning, USA) at a density of 5 × 104 cells/well and cultured for 24 h at 37°C. (A) PM2.5 with different concentration gradients were added, each group was added 10 μL. Experiments consisted of seven groups: a normal control group, non-inactivated PM2.5 groups (PM2.5) (1.00 mg/mL, 0.50 mg/mL, 0.25 mg/mL), and inactivated PM2.5 groups (PM2.5-) (1.00 mg/mL, 0.50 mg/mL, 0.25 mg/mL).
Lung injury scoring about hematoxylin and eosin. Lung injury scores were assessed. Ten fields of view were randomly selected from each section to evaluate the severity of lung injury according to five indicators: the number of neutrophils in the alveolar space, the number of neutrophils in the pulmonary interstitial space, the presence or absence of the formation of hyaline membranes, the presence or absence of protein debris in the alveolar space, and the degree of alveolar wall thickening. Each index was weighted and averaged according to its correlation with disease and the number of fields under the microscope and finally, a lung tissue injury pathological score was obtained for each group. Data are presented as the mean ± standard error of the mean.
Detection of NF-κB p65 proteins expression levels in RAW264.7 cells by western blot.