Abstract
The recently approved direct-acting antivirals (DAA) agents are effective in terms of sustained virologic response (SVR) rates and are well tolerated in most hepatitis C virus (HCV) patients. This study aimed to analyze the association between serum zinc levels in patients who developed hepatocellular carcinoma (HCC) following HCV eradication after DAA treatment. The retrospective study included 769 HCV-infected patients who achieved SVR after DAA treatment. We calculated the annual incidence rate of HCC and identified risk factors associated with HCC development. We also assessed serum zinc and clinical factors at both baseline and end of treatment (EOT). During follow-up (median duration 35 months), HCC occurred in 18/769 (2.3%) patients. From the multivariate analysis, serum zinc <60 µg/dl [hazard ratio (HR) 5.936] and AFP ≥6.0 ng/dl (HR 5.862) at baseline, baseline-zinc <60 µg/dl (HR 6.283), EOT-serum zinc <63 µg/dl (HR 6.011), baseline-AFP ≥6.0 ng/dl (HR 8.163), and EOT-M2BPGi ≥2.5 (HR 12.194) at baseline and EOT were independently associated with increased HCC risk. In patients who achieved HCV eradication following DAA treatment, serum zinc levels before and at EOT could be a risk factor for developing HCC.
Keywords: zinc, direct-acting antivirals, hepatocellular carcinoma, sustained virologic response, hepatitis C virus
Introduction
Hepatitis C virus (HCV) infections are an important worldwide health problem with chronic consequences, including cirrhosis and hepatocellular carcinoma (HCC).(1–3) The treatment of HCV infections has been revolutionized through the development of direct acting antivirals (DAAs), which are highly effective and well-tolerated.(4–6) Nevertheless, the impact of viral eradication after DAA treatment on disease progression, including the development of HCC, has been questioned.(7–11)
Zinc is well known to be an active center of or coenzyme for >300 types of enzymes that are involved in numerous biological processes, including DNA synthesis, RNA transcription, cell growth and division, among others. As a result, zinc is considered to be an essential trace element for maintaining life.(12–15) In chronic liver disease, a decreased capacity to synthesize albumin and malabsorption of zinc from the intestine cause zinc deficiency.(16–18) Long-term zinc supplementation therapy can improved liver pathology and reduced the incidence of HCC in chronic hepatitis C (CHC) patients.(19,20) Moreover, hypozincemia has been found to be associated with the development of HCC in HCV-related cirrhosis.(21) Therefore, it is important to understand the association between serum zinc and the development of HCC in CHC patients. Here, we examined serum zinc levels at baseline, end of treatment (EOT) and 24 weeks after EOT (p24w) after DAA treatment.
Materials and Methods
Patients and study design
Of the CHC patients who were treated at Sapporo Kosei General Hospital between February 2012 and December 2018, 769 with a sustained virologic response (SVR) were included in this study. Patients with genotype 1 CHC infections were administered 60 mg daclatasvir once daily plus 100 mg asunaprevir twice daily for 24 weeks (n = 258), sofosbuvir/ledipasvir (400/90 mg, combination tablet) once daily for 12 weeks (n = 93), ombitasvir/paritaprevir/ritonavir (25/150/100 mg, combination tablet) once daily for 12 weeks (n = 53), 50 mg elbasvir once daily plus 100 mg grazoprevir once daily for 12 weeks (n = 71), daclatasvir/asunaprevir/beclabuvir (30/200/75 mg, combination tablet) twice daily for 12 weeks (n = 18), and glecaprevir/pibrentasvir (300/120 mg, combination tablet) once daily for 8 or 12 weeks (n = 46). Patients infected with genotype 2 CHC were administered sofosbuvir 400 mg once daily plus ribavirin (weight-based dosing) for 12 weeks (n = 188), ombitasvir/paritaprevir/ritonavir once daily plus ribavirin (weight-based dosing) for 24 weeks (n = 4) and glecaprevir/pibrentasvir (300/120 mg, combination tablet) once daily for 8 or 12 weeks (n = 38). We collected baseline data at the time of DAA treatment initiation including age, gender, HCV genotype, body mass index (BMI), estimated glomerular filtration rate (eGFR), platelets count, aspartate aminotransferase (AST), alanine aminotransferase (ALT), total bilirubin, albumin, Mac-2 binding protein glycosylation isomer (M2BPGi), α-fetoprotein (AFP), and serum zinc. Moreover, we determined the presence of cirrhosis and diabetes. We also examined laboratory tests after EOT such as platelets count, AST, ALT, total bilirubin, albumin, M2BPGi, AFP, and serum zinc.
We defined SVR as a serum HCV RNA viral load below the lower limit of detection performed at least 24 weeks after EOT. Liver cirrhosis was diagnosed based on liver histology, transient elastography (liver stiffness of ≥14.5 kPa measured with Fibroscan (Echosens, Paris, France),(22) or the presence of gastroesophageal varices. We defined diabetes in patients with a HbA1c value ≥6.5% or those undergoing treatment with antidiabetic drugs or insulin. Patients with decompensated cirrhosis, chronic kidney disease (CKD) stage ≥4, concomitant human immunodeficiency virus or hepatitis B virus (HBV) infection, comorbid liver disease associated with autoimmunity, excessive alcohol consumption (daily ethanol consumption was ≥60 g/day), history of HCC, or HCC detected during the DAA treatment and within 24 weeks after EOT were excluded. We defined the observation period, as the time in weeks since the beginning of treatment. All patients were examined for HCC by ultrasonography (US), dynamic computed tomography (CT), and/or magnetic resonance imaging (MRI) at baseline and every 3–6 months after the beginning of treatment. Patients with cirrhosis underwent CT and/or MRI without US in a similar fashion.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional committee (registration no. 506) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This study was a retrospective, single-center, non-randomized, and non-case controlled open trial.
Clinical and laboratory evaluations
HCV RNA was determined at a central laboratory using the Roche COBAS TaqMan HCV Auto assay (Roche Diagnostics, Tokyo, Japan; lower limit of quantification, 15 IU/ml). HCV genotype was conducted using a real time polymerase chain reaction assay. Serum zinc levels were measured by atomic absorption spectrometry (Hitachi High-Tech Science Co., Ltd., Tokyo, Japan) until July 2018 and by colorimetry (Jeol Ltd., Tokyo, Japan) from August 2018 onwards.
Statistical analysis
The categorical variables are presented as numbers and percentages and the continuous variables are presented as the median (range). Student’s t test and a Mann-Whitney U test were used to analyze continuous data, as appropriate. We calculated the cumulative HCC incidence using the Kaplan-Meier method. Differences among groups were assessed using the log-rank test. Risk factors associated with the development of HCC were determined by the Cox proportional hazard model. The independent variables included age, gender, HCV genotype, cirrhosis, diabetes, BMI, eGFR, platelet count, albumin, AST, ALT, M2BPGi, AFP, and serum zinc at baseline and EOT. Significant predictive factors that contributed to the development of HCC in the univariate analysis were inputted into the multivariate analysis. A stepwise logistic regression model analysis was used to calculate the adjusted hazards ratio (HR) and 95% confidence intervals (CI) for the various factors. Optimal cut-off values were selected from the receiver operating characteristics curve. A p value <0.05 was considered statistically significant. Statistical analyses were performed with R (http://www.r-project.org/).
Results
Patient flow chart
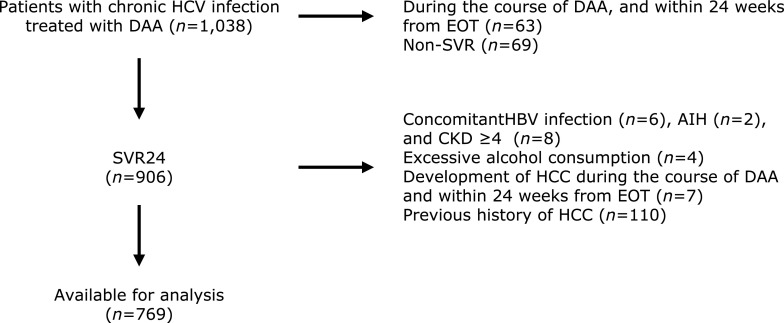
Among the 1,038 patients who initiated their antiviral regimen from between February 2012 and December 2018, 906 (87.2%) achieved SVR. After excluding patients with concomitant HBV infection, CKD stage ≥4, autoimmune hepatitis, excessive alcohol consumption, previous history of HCC, development of HCC during the course of DAA and within 24 weeks from EOT, 769 patients were included in the analysis (Fig. 1).
Fig. 1.
Flowchart for patient selection. AIH, autoimmune hepatitis; HBV, hepatitis B virus; CKD, chronic kidney disease; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; DAA, direct-acting antivirals; EOT, end of treatment; SVR, sustained virologic response.
Characteristics of the study population
The median patient age was 64 years. The patients included 317 men (41.2%) and 539 (70.1%), and 149 (19.4%) patients with genotype 1, and cirrhosis, respectively. Treatment resulted in a significant increase in platelets and albumin, and a significant decrease in AST, ALT, M2BPGi, and AFP (Table 1).
Table 1.
Patients characteristics at baseline and end of treatment
| Characteristics | Baseline | End of treatment | p |
|---|---|---|---|
| Number of patients | 769 | ||
| Age (years) | 64 (18–88) | ||
| Gender, male | 317 (41.2) | ||
| Genotype, 1/2 | 539/230 | ||
| Liver cirrhosis | 149 (19.4) | ||
| Diabetes | 93 (12.1) | ||
| BMI (kg/m2) | 22.9 (14.4–38.8) | ||
| eGFR (ml/min/1.73 m2) | 73.6 (30.4–187.8) | ||
| Treatment | |||
| daclatasvir + asunaprevir | 258 (33.6) | ||
| sofosbuvir/ledipasvir | 93 (12.1) | ||
| ombitasvir/paritaprevir/ritonavir | 53 (6.9) | ||
| elbasvir + grazoprevir | 71 (9.2) | ||
| daclatasvir/asunaprevir/beclabuvir | 18 (2.3) | ||
| glecaprevir/pibrentasvir | 84 (10.9) | ||
| sofosbuvir + ribavirin | 188 (24.4) | ||
| ombitasvir/paritaprevir/ritonavir + ribavirin | 4 (0.5) | ||
| Platelets count (×104/µl) | 15.7 (3.8–22.0) | 16.1 (3.8–43.4) | <0.001 |
| AST (U/L) | 39 (11–272) | 24 (10–99) | <0.001 |
| ALT (U/L) | 39 (7–255) | 17 (4–106) | <0.001 |
| Total bilirubin (mg/dl) | 0.7 (0.2–2.3) | 0.7 (0.1–3.0) | 0.476 |
| Albumin (g/dl) | 4.1 (2.0–5.1) | 4.2 (2.7–5.3) | <0.001 |
| M2BPGi | 2.0 (0.1–24.2) | 1.1 (0.2–11.8) | <0.001 |
| AFP (ng/ml) | 4.2 (1.0–634.9) | 3.1 (0.9–27.1) | <0.001 |
| Serum zinc (µg/dl) | 69 (28–116) | 68 (42–142) | 0.122 |
Categorical variables expressed as number (%) and the continuous variables as median (range). AFP, α-fetoprotein; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; eGFR, estimated glomerular filtration rate; M2BPGi, Mac-2 binding protein glycosylation isomer.
Changes in serum zinc and albumin levels before and after DAA treatment
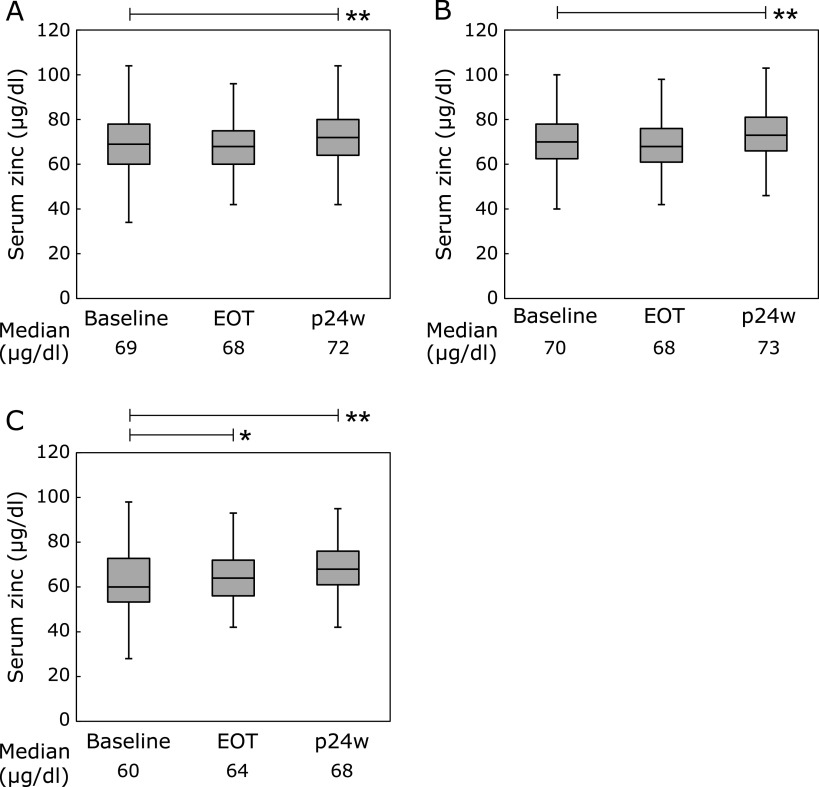
The median serum zinc levels were 69, 68, and 72 µg/dl at baseline, EOT, and p24w, respectively (baseline vs p24w; p<0.001; Fig. 2A). The median serum zinc levels were 70, 68, and 73 µg/dl at baseline, EOT, and p24w in patients without cirrhosis (baseline vs p24w; p<0.001; Fig. 2B). The median serum zinc levels were 60, 64, and 68 µg/dl at baseline, EOT, and p24w in patients with cirrhosis (baseline vs EOT; p<0.01, baseline vs p24w ; p<0.001; Fig. 2C). The median albumin levels were 4.1, 4.2, and 4.3 g/dl, at baseline, EOT, and p24w, respectively (baseline vs EOT, baseline vs p24w; all p<0.001; Supplemental Fig. 1A*). The median albumin levels were 4.2, 4.3, and 4.4 g/dl at baseline, EOT, and p24w in patients without cirrhosis (baseline vs p24w; p<0.001; Supplemental Fig. 1B*). The median albumin levels were 3.7, 4.0, and 4.2 g/dl at baseline, EOT, and p24w in patients with cirrhosis (baseline vs EOT, baseline vs p24w; all p<0.001; Supplemental Fig. 1C*). Serum zinc levels were positively correlated with albumin levels at baseline (r = 0.449. p<0.001; Supplemental Fig. 2A*). The increase in serum zinc levels from baseline to p24w was significantly associated with the increase in albumin levels from baseline to p24w (r = 0.351, p<0.001; Supplemental Fig. 2B*).
Fig. 2.
Changes in the serum zinc levels at baseline, EOT, and p24W. (*p<0.01, **p<0.001). EOT, end of treatment; p24w, 24 weeks after EOT. (A) Overall. (B) Non-cirrhotic patients. (C) Cirrhotic patients.
HCC incidence and predictors
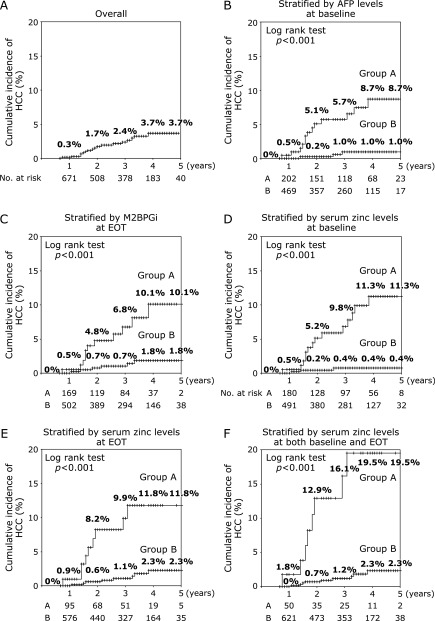
During follow-up (median duration 35 months, range 8–107 months), HCC occurred in 18/769 (2.3%) patients. The 1, 2, 3, 4, and 5 year overall cumulative rates of HCC were 0.3, 1.7, 2.4, 3.7, and 3.7%, respectively (Fig. 3A). Factors associated with developing HCC at baseline were older age, cirrhosis, higher BMI, lower eGFR, lower platelet count, higher AST, lower albumin, higher M2BPGi, higher AFP, and lower serum zinc (Table 2). A multivariate Cox regression analysis showed that serum zinc <60 µg/dl (HR 5.936, p = 0.003) and AFP ≥6.0 ng/dl (HR 5.862, p = 0.007) at baseline were independently associated with a higher risk of developing HCC (Table 3). Factors associated with developing HCC at EOT were lower platelet count, higher AST, lower albumin, higher M2BPGi, higher AFP, and lower serum zinc (Table 4). A multivariate Cox regression analysis was carried out including both baseline and EOT factors; accordingly, baseline-serum zinc <60 µg/dl (HR 6.283, p = 0.007), EOT-serum zinc <63 µg/dl (HR 6.011, p<0.001), baseline AFP ≥6.0 ng/dl (HR 8.163, p = 0.002), and EOT-M2BPGi ≥2.5 (HR 12.194, p<0.001) were identified as independent factors for developing HCC (Table 5).
Fig. 3.
The cumulative rate of HCC by Kaplan-Meier analysis. AFP, α-fetoprotein; EOT, end of treatment; HCC, hepatocellular carcinoma; M2BPGi, Mac-2 binding protein glycosylation isomer. (A) Overall cumulative incidence of HCC. (B) Cumulative incidence of HCC stratified by AFP at baseline. Group A (n = 220); baseline-AFP ≥6.0 ng/ml, Group B (n = 549); baseline-AFP <6.0 ng/ml (log-rank test p<0.001). (C) Cumulative incidence of HCC stratified by M2BPGi at EOT. Group A (n = 194); EOT-M2BPGi ≥2.5, Group B (n = 575); EOT-M2BPGi <2.5 (log-rank test p<0.001). (D) Cumulative incidence of HCC stratified by serum zinc levels at baseline. Group A (n = 197); baseline-serum zinc levels <60 µg/dl, Group B (n = 572); baseline-serum zinc levels ≥60 µg/dl (log-rank test p<0.001). (E) Cumulative incidence of HCC stratified by serum zinc at EOT. Group A (n = 107); EOT <63 µg/dl, Group B (n = 662); EOT ≥63 µg/dl (log-rank test p<0.001). (F) Cumulative incidence of HCC stratified by serum zinc at both baseline and EOT. Group A (n = 58); baseline-serum zinc levels <60 µg/dl and EOT-serum zinc levels <63 µg/dl, Group B (n = 711); baseline-serum zinc levels ≥60 µg/dl and/or EOT-serum zinc levels ≥63 µg/dl (log-rank test p<0.001).
Table 2.
Factors associated with developing HCC at baseline
| Characteristics | Developing HCC | Non developing HCC | p |
|---|---|---|---|
| Number | 18 | 751 | |
| Age (years) | 72 (52–82) | 66 (18–88) | 0.033 |
| Gender, male/female | 11/7 | 306/445 | 0.083 |
| Genotype, 1/2 | 16/2 | 523/228 | 0.078 |
| Liver cirrhosis, yes/no | 12/6 | 137/614 | <0.001 |
| Diabetes, yes/no | 1/17 | 92/659 | 0.389 |
| BMI (kg/m2) | 25.2 (19.4–32.0) | 23.0 (14.4–38.8) | 0.010 |
| eGFR (ml/min/1.73 m2) | 64.8 (33.2–105.3) | 74.5 (30.4–187.8) | 0.026 |
| Platelets count (×104/µl) | 12.5 (3.8–22.0) | 16.1 (12.3–20.1) | 0.014 |
| AST (U/L) | 47 (28–125) | 39 (11–272) | 0.039 |
| ALT (U/L) | 42 (15–101) | 39 (7–255) | 0.976 |
| Total bilirubin (mg/dl) | 0.9 (0.4–2.2) | 0.7 (0.2–2.3) | 0.083 |
| Albumin (g/dl) | 3.6 (2.0–4.7) | 4.2 (2.6–5.1) | <0.001 |
| M2BPGi | 7.3 (2.2–22.8) | 1.9 (0.1–24.2) | <0.001 |
| AFP (ng/ml) | 7.3 (1.8–68.5) | 4.1 (1.0–634.9) | 0.001 |
| Serum zinc (µg/dl) | 57 (28–84) | 70 (34–116) | <0.001 |
Categorical variables expressed as number (%) and the continuous variables as median (range). AFP, α-fetoprotein; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; eGFR, estimated glomerular filtration rate; HCC, hepatocellular carcinoma; M2BPGi, Mac-2 binding protein glycosylation isomer.
Table 3.
Factors associated with developing HCC at baseline on multivariate analysis
| Factors | Hazard Ratio | 95%CI | p | |
|---|---|---|---|---|
| Serum zinc <60 µg/dl | 5.936 | 1.865 | 18.886 | 0.003 |
| AFP ≥6.0 ng/dl | 5.862 | 1.625 | 21.14 | 0.007 |
AFP, α-fetoprotein; CI, confidence interval; HCC, hepatocellular carcinoma.
Table 4.
Factors associated with developing HCC at end of treatment
| Characteristics | Developing HCC | Non developing HCC | p |
|---|---|---|---|
| Platelets count (×104/µl) | 13.1 (4.0–20.1) | 16.1 (3.8–43.4) | 0.005 |
| AST (U/L) | 29 (16–81) | 24 (10–99) | 0.009 |
| ALT (U/L) | 23 (8–51) | 17 (4–106) | 0.306 |
| Total bilirubin (mg/dl) | 0.7 (0.4–1.6) | 0.7 (0.1–3.0) | 0.343 |
| Albumin (g/dl) | 3.8 (2.7–4.6) | 4.2 (2.7–5.3) | <0.001 |
| M2BPGi | 2.8 (1.0–9.2) | 1.1 (0.2–11.8) | <0.001 |
| AFP (ng/ml) | 4.6 (2.3–10.7) | 3.1 (0.9–27.1) | 0.008 |
| Serum zinc (µg/dl) | 56 (42–90) | 68 (42–142) | <0.001 |
Continuous variables expressed as median (range). AFP, α-fetoprotein; ALT, alanine aminotransferase; AST, aspartate aminotransferase; HCC, hepatocellular carcinoma; M2BPGi, Mac-2 binding protein glycosylation isomer.
Table 5.
Factors associated with developing HCC at both baseline and end of treatment on multivariate analysis
| Factors | Hazard Ratio | 95%CI | p | |
|---|---|---|---|---|
| Baseline-serum zinc <60 µg/dl | 6.283 | 1.655 | 23.847 | 0.007 |
| EOT-serum zinc <63 µg/dl | 6.011 | 2.093 | 17.265 | <0.001 |
| Baseline-AFP ≥6.0 ng/dl | 8.163 | 2.188 | 35.852 | 0.002 |
| EOT-M2BPGi ≥2.5 | 12.194 | 4.184 | 58.325 | <0.001 |
AFP, α-fetoprotein; CI, confidence interval; HCC, hepatocellular carcinoma; M2BPGi, Mac-2 binding protein glycosylation isomer.
The analysis of HCC incidence according to AFP levels at baseline is shown in Fig. 3B. Stratified by baseline AFP levels, the cumulative rates of HCC at 1, 3, and 5 years were 0.5%, 5.7%, and 8.7%, respectively, in patients with a baseline AFP ≥6.0 ng/ml (Group A, n = 220) and 0%, 1.0%, and 1.0%, respectively, in patients with a baseline AFP <6.0 ng/ml (Group B, n = 549; p<0.001 by log-rank test). The analysis of HCC incidence according to EOT-M2BPGi levels at EOT is shown in Fig. 3C. Stratified by EOT-M2BPGi levels, the cumulative rates of HCC at 1, 3, and 5 years were 0.5, 6.8, and 10.1%, respectively, in patients with EOT-M2BPGi ≥2.5 (Group A, n = 194) and 0, 0.7, and 1.8%, respectively, in patients with EOT-M2BPGi <2.5 (Group B, n = 575; p<0.001 by log-rank test). The analysis of HCC incidence according to baseline serum zinc levels at baseline is shown in Fig. 3D. Stratified by baseline serum zinc levels, the cumulative rates of HCC at 1, 3, and 5 years were 0.5, 9.8, and 11.3%, respectively, in patients with baseline serum zinc levels <60 µg/dl (Group A, n = 197) and 0, 0.4, and 0.4%, respectively, in patients with baseline serum zinc levels ≥60 µg/dl (Group B, n = 572; p<0.001 by log-rank test). The analysis of HCC incidence according to EOT-serum zinc levels is shown in Fig. 3E. Stratified by EOT-serum zinc levels, the cumulative rates of HCC at 1, 3, and 5 years were 0.9, 9.9, and 11.8%, respectively, in patients with EOT-serum zinc levels <63 µg/dl (Group A, n = 107) and 0, 1.1, and 2.3%, respectively, in patients with EOT-serum zinc levels ≥63 µg/dl (Group B, n = 662; p<0.001 by log-rank test). Stratified by both baseline and EOT-serum zinc levels, the cumulative rates of HCC at 1, 3, and 5 years were 1.8, 16.1, and 19.5%, respectively, in patients with baseline serum zinc levels <60 µg/dl and EOT-serum zinc levels <63 µg/dl (Group A, n = 58) and 0, 1.2, and 2.3%, respectively, in patients with baseline serum zinc levels ≥60 µg/dl and/or EOT-serum zinc levels ≥63 µg/dl (Group B, n = 711; p<0.001 by log-rank test).
Discussion
Mean serum zinc levels are known to increase to the greatest extent during follow-up after interferon treatment from zinc.(23) In two recent studies, serum zinc levels were also found to change in HCV patients before and after DAA treatment.(24,25) However, the mechanism underlying these effects has not yet been clarified. It is known that the production of albumin is inhibited by acute phase cytokines, such as interleukin 6 and tumor necrosis factor α.(26) Furthermore, inflammation increases the catabolic rate, resulting in hypoalbuminemia.(27) Viral eradication of HCV by DAA treatment suppresses hepatic inflammation and acute phase cytokines.(28,29) In the present study, significant increases in serum zinc levels were observed at EOT in cirrhotic patients, and at p24w in all patients. Similarly, significant increases in albumin levels were observed at EOT in all patients, and cirrhotic patients and at p24w in all patients. It has been reported that the baseline albumin levels are lower than the normal limit, and, after achieving SVR, the levels increase, approaching normal levels.(30) The same mechanism is likely involved in the increased albumin levels observed, particularly in cirrhotic patients who achieved SVR following DAA, in the present study. We also observed that serum zinc levels were positively correlated with albumin levels at baseline. Furthermore, the increase in serum zinc levels from baseline to p24w was significantly associated with the increase in albumin levels from baseline to p24w. Therefore, as albumin levels increase following DAA, the increase in serum zinc might be a result of the increase in albumin levels.
Numerous studies have demonstrated that interferon-based antiviral treatment reduces the incidence of HCC in patients with HCV-induced SVR.(31–33) Similarly, several recent retrospective studies and one recent prospective study have suggested that the risk of HCC decreased after DAA treatment.(34–39) Cox regression analyses have highlighted cirrhosis,(7,8) low platelet count,(7,9) alcohol abuse,(8,36) low albumin levels,(9) higher AFP levels,(10) and older age,(11,38) as independent predictors for developing HCC. Therefore, various factors were selected, due to review period, back patient characteristics, and selected independent factors. Moreover, we excluded patients with a history of HCC and those who reported a high rate of HCC recurrence in advance.(7,40–44)
In the present study, a multivariate analysis showed lower serum zinc levels and higher AFP levels at baseline. As several laboratory parameters changed in patients who achieved SVR following DAA, including reductions in AFP and M2BPGi levels, increases in platelet count, albumin, and serum zinc levels,(28,29,45–47) we carried out the multivariate analysis at both baseline and EOT. The results showed lower serum zinc levels at baseline and EOT, higher AFP at baseline, and higher M2BPGi at EOT. AFP has previously been identified as a candidate risk factor for the development of HCC.(10) Although M2BPGi has been shown to be a noninvasive biomarker for fibrosis, post treatment levels of M2BPGi have been associated with the risk of developing HCC among patients with SVR.(46) Serum zinc levels were also lower in patients who developed HCC in the univariate analysis at baseline and EOT. Based on the multivariate analysis, serum zinc was selected as the only common factor associated with HCC development at both baseline and EOT.
Although the long-term administration of zinc to CHC patients with zinc deficiency has been reported to reduce hepatic fibrosis and the risk of developing HCC,(48) no consistent views have been reached on whether zinc deficiency increase the risk of developing HCC or not. Many patients with concomitant HCC presumably have advanced hepatic fibrosis. Currently, it is not known whether hypozincemia results from advanced hepatic fibrosis or whether a zinc-deficient state initiates or promotes HCC. Although zinc is an essential nutrient for numerous biological activities, such as suppressing oxidative stress and maintaining the immune system, mitochondrial destruction by HCV severely disrupts zinc homeostasis.(49) A recent paper also revealed that hypozincemia is associated with the development of HCC in HCV-related cirrhosis. This study concluded that HCV induces hypozincemia due to a reduction in copper-zinc superoxide dismutase and antioxidative activity, which results in the development HCC.(21) However, it did not been analyze serum zinc levels in patients who developed HCC following HCV eradication by DAA. Despite HCV eradication, improvements in serum zinc levels were still dependent on the liver fibrosis states.(21) Accordingly, we postulate that serum zinc levels increase after DAA treatment and that patients whose serum zinc levels remain unchanged are likely to develop HCC. The association between serum zinc levels and the development of HCC should be further investigated.
This study had several limitations. First, this was a retrospective single-center study. Second, the serum zinc levels show circadian variations, being high in the early morning and decreasing toward the afternoon.(50,51) Therefore, blood sample collection should preferably have been done in the early morning when patients were fasting. However, because of the difficulty in collecting samples at the same time from all patients in a clinical setting, this study did not define a fixed sampling time. Lastly, as a major limitation, due to the small number of patients who developed HCC in the present study, the factors, such as serum zinc, identified in the multivariate analysis as contributing to HCC incidence might differ in a larger study. Consequently, larger multi-center studies will be needed to confirm these results.
In summary, the most novel finding of this study was that serum zinc levels were strongly associated with the development of HCC in CHC patients who exhibited eradication of HCV following DAA treatment. Therefore, it is important to pay attention to the serum zinc levels between baseline and EOT following DAA treatment.
Abbreviations
- AFP
α-fetoprotein
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- BMI
body mass index
- CHC
chronic hepatitis C
- CI
confidence intervals
- CKD
chronic kidney disease
- CT
computed tomography
- DAAs
direct acting antivirals
- eGFR
estimated glomerular filtration rate
- EOT
end of treatment
- HCC
hepatocellular carcinoma
- HBV
hepatitis B virus
- HCV
hepatitis C virus
- HR
hazards ratio
- M2BPGi
Mac-2 binding protein glycosylation isomer
- MRI
magnetic resonance imaging
- p24w
24 weeks after EOT
- SVR
sustained virologic response
- US
ultrasonography
Conflict of Interest
No potential conflicts of interest were disclosed.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional committee (registration no. 506) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Supplementary Material
References
- 1.Lavanchy D. The global burden of hepatitis C. Liver Int 2009; 29 Suppl 1: 74–81. [DOI] [PubMed] [Google Scholar]
- 2.EI-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology 2012; 142: 1264–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Webster DP, Klenerman P, Dusheiko GM. Hepatitis C. Lancet 2015; 385: 1124–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maan R, van Tilborg M, Deterding K, et al. Safety and effectiveness of direct-acting antiviral agents for treatment of patients with chronic hepatitis C virus infection and cirrhosis. Clin Gastroenterol Hepatol 2016; 14: 1821–1830. [DOI] [PubMed] [Google Scholar]
- 5.Majumdar A, Kitson MT, Roberts SK. Systematic review: current concepts and challenges for the direct-acting antiviral era in hepatitis C cirrhosis. Aliment Pharmacol Ther 2016; 43: 1276–1292. [DOI] [PubMed] [Google Scholar]
- 6.Falade-Nwulia O, Suarez-Cuervo C, Nelson DR, Fried MW, Segal JB, Sulkowski MS. Oral direct-acting agent therapy for hepatitis C virus infection: a systematic review. Ann Intern Med 2017; 166: 637–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Conti F, Buonfiglioli F, Scuteri A, et al. Early occurrence and recurrence of hepatocellular carcinoma in HCV-related cirrhosis treated with direct-acting antivirals. J Hepatol 2016; 65: 727–733. [DOI] [PubMed] [Google Scholar]
- 8.Kanwal F, Kramer J, Asch SM, Chayanupatkul M, Cao Y, El-Serag HB. Risk of hepatocellular cancer in HCV patients treated with direct-acting antiviral agents. Gastroenterology 2017; 153: 996–1005. [DOI] [PubMed] [Google Scholar]
- 9.Calvaruso V, Cabibbo G, Cacciola I, et al. Incidence of hepatocellular carcinoma in patients with HCV-associated cirrhosis treated with direct-acting antiviral agents. Gastroenterology 2018; 155: 411–421. [DOI] [PubMed] [Google Scholar]
- 10.Mettke F, Schlevogt B, Deterding K, et al. Interferon-free therapy of chronic hepatitis C with direct-acting antivirals does not change the short-term risk for de novo hepatocellular carcinoma in patients with liver cirrhosis. Aliment Pharmacol Ther 2018; 47: 516–525. [DOI] [PubMed] [Google Scholar]
- 11.Alavi M, Janjua NZ, Chong M, et al. Trends in hepatocellular carcinoma incidence and survival among people with hepatitis C: an international study. J Viral Hepat 2018; 25: 473–481. [DOI] [PubMed] [Google Scholar]
- 12.Prasad AS. Zinc: an overview. Nutrition 1995; 11 (1 Suppl): 93–99. [PubMed] [Google Scholar]
- 13.Pabo CO, Peisach E, Grant RA. Design and selection of novel Cys2His2 zinc finger proteins. Annu Rev Biochem 2001; 70: 313–340. [DOI] [PubMed] [Google Scholar]
- 14.Joazeiro CA, Weissman AM. RING finger proteins: mediators of ubiquitin ligase activity. Cell 2000; 102: 549–552. [DOI] [PubMed] [Google Scholar]
- 15.Kadrmas JL, Beckerle MC. The LIM domain: from the cytoskeleton to the nucleus. Nat Rev Mol Cell Biol 2000; 5: 920–931. [DOI] [PubMed] [Google Scholar]
- 16.Stamoulis I, Kouraklis G, Theocharis S. Zinc and the liver: an active interaction. Dig Dis Sci 2007; 52: 1595–1612. [DOI] [PubMed] [Google Scholar]
- 17.Grüngreiff K, Reinhold D, Wedemeyer H. The role of zinc in liver cirrhosis. Ann Hepatol 2016; 15: 7–16. [DOI] [PubMed] [Google Scholar]
- 18.Himoto T, Masaki T. Associations between zinc deficiency and metabolic abnormalities in patients with chronic liver disease. Nutrients 2018; 10. pii: E88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matsuoka S, Matsumura H, Nakamura H, et al. Zinc supplementation improves the outcome of chronic hepatitis C and liver cirrhosis. J Clin Biochem Nutr 2009; 45: 292–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matsumura H, Nirei K, Nakamura H, et al. Zinc supplementation therapy improves the outcome of patients with chronic hepatitis C. J Clin Biochem Nutr 2012; 51: 178–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shigefuku R, Iwasa M, Katayama K, et al. Hypozincemia is associated with human hepatocarcinogenesis in hepatitis C virus-related liver cirrhosis. Hepatol Res 2019; 49: 1127–1135. [DOI] [PubMed] [Google Scholar]
- 22.Ziol M, Handra-Luca A, Kettaneh A, et al. Noninvasive assessment of liver fibrosis by measurement of stiffness in patients with chronic hepatitis C. Hepatology 2005; 41: 48–54. [DOI] [PubMed] [Google Scholar]
- 23.Grüngreiff K, Reinhold D, Ansorge S. Serum concentrations of sIL-2R, IL-6, TGF-beta1, neopterin, and zinc in chronic hepatitis C patients treated with interferon-alpha. Cytokine 1999; 11: 1076–1080. [DOI] [PubMed] [Google Scholar]
- 24.Suda T, Okawa O, Shirahashi R, Tokutomi N, Tamano M. Changes in serum zinc levels in hepatitis C patients before and after treatment with direct-acting antiviral agents. Hepatol Res 2019; 49: 1353–1356. [DOI] [PubMed] [Google Scholar]
- 25.Ko YL, Morihara D, Shibata K, et al. Factors attenuating zinc deficiency improvement in direct-acting antiviral agent-treated chronic hepatitis C virus infection. Nutrients 2018; 10. pii: E1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Quinlan GJ, Martin GS, Evans TW. Albumin: biochemical properties and therapeutic potential. Hepatology 2005; 41: 1211–1219. [DOI] [PubMed] [Google Scholar]
- 27.Don BR, Kaysen G. Serum albumin: relationship to inflammation and nutrition. Semin Dial 2004; 17: 432–437. [DOI] [PubMed] [Google Scholar]
- 28.Kan T, Hashimoto S, Kawabe N, Nakano T, Nakaoka K, Yoshioka K. Increase in albumin by daclatasvir/asunaprevir therapy is correlated with decrease in aspartate transaminase. J Transl Int Med 2017; 5: 148–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miyaki E, Imamura M, Hiraga N, et al. Daclatasvir and asunaprevir treatment improves liver function parameters and reduces liver fibrosis markers in chronic hepatitis C patients. Hepatol Res 2016; 46: 758–764. [DOI] [PubMed] [Google Scholar]
- 30.Deterding K, Höner Zu Siederdissen C, Port K, et al. Improvement of liver function parameters in advanced HCV-associated liver cirrhosis by IFN-free antiviral therapies. Aliment Pharmacol Ther 2015; 42: 889–901. [DOI] [PubMed] [Google Scholar]
- 31.Bruno S, Stroffolini T, Colombo M, et al. Sustained virological response to interferon-alpha is associated with improved outcome in HCV-related cirrhosis: a retrospective study. Hepatology 2007; 45: 579–587. [DOI] [PubMed] [Google Scholar]
- 32.Asahina Y, Tsuchiya K, Tamaki N, et al. Effect of aging on risk for hepatocellular carcinoma in chronic hepatitis C virus infection. Hepatology 2010; 52: 518–527. [DOI] [PubMed] [Google Scholar]
- 33.Morgan RL, Baack B, Smith BD, Yartel A, Pitasi M, Falck-Ytter Y. Eradication of hepatitis C virus infection and the development of hepatocellular carcinoma: a meta-analysis of observational studies. Ann Intern Med 2013; 158 (5 Pt 1): 329–337. [DOI] [PubMed] [Google Scholar]
- 34.Ioannou GN, Green PK, Berry K. HCV eradication induced by direct-acting antiviral agents reduces the risk of hepatocellular carcinoma. J Hepatol 2018; 68: 25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guarino M, Sessa A, Cossiga V, et al. Direct-acting antivirals and hepatocellular carcinoma in chronic hepatitis C: a few lights and many shadows. World J Gastroenterol 2018; 24: 2582–2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kanwal F, Kramer JR, Asch SM, Cao Y, Li L, El-Serag HB. Long-term risk of hepatocellular carcinoma in HCV patients treated with direct acting antiviral agents. Hepatology 2019. DOI: 10.1002/hep.30823. [DOI] [PubMed] [Google Scholar]
- 37.Singal AG, Lim JK, Kanwal F. AGA clinical practice update on interaction between oral direct-acting antivirals for chronic hepatitis C infection and hepatocellular carcinoma: expert review. Gastroenterology 2019; 156: 2149–2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lleo A, Aglitti A, Aghemo A, et al. Predictors of hepatocellular carcinoma in HCV cirrhotic patients treated with direct acting antivirals. Dig Liver Dis 2019; 51: 310–317. [DOI] [PubMed] [Google Scholar]
- 39.Carrat F, Fontaine H, Dorival C, et al. Clinical outcomes in patients with chronic hepatitis C after direct-acting antiviral treatment: a prospective cohort study. Lancet 2019; 393: 1453–1464. [DOI] [PubMed] [Google Scholar]
- 40.Calleja JL, Crespo J, Rincón D, et al. Effectiveness, safety and clinical outcomes of direct-acting antiviral therapy in HCV genotype 1 infection: results from a Spanish real-world cohort. J Hepatol 2017; 66: 1138–1148. [DOI] [PubMed] [Google Scholar]
- 41.Kolly P, Waidmann O, Vermehren J, et al. Hepatocellular carcinoma recurrence after direct antiviral agent treatment: a European multicentre study. J Hepatol 2017; 67: 876–878. [DOI] [PubMed] [Google Scholar]
- 42.El Kassas M, Funk AL, Salaheldin M, et al. Increased recurrence rates of hepatocellular carcinoma after DAA therapy in a hepatitis C-infected Egyptian cohort: a comparative analysis. J Viral Hepat 2018; 25: 623–630. [DOI] [PubMed] [Google Scholar]
- 43.Ikeda K, Kawamura Y, Kobayashi M, et al. Direct-acting antivirals decreased tumor recurrence after initial treatment of hepatitis C virus-related hepatocellular carcinoma. Dig Dis Sci 2017; 62: 2932–2942. [DOI] [PubMed] [Google Scholar]
- 44.Ogawa E, Furusyo N, Nomura H, et al. Short-term risk of hepatocellular carcinoma after hepatitis C virus eradication following direct-acting anti-viral treatment. Aliment Pharmacol Ther 2018; 47: 104–113. [DOI] [PubMed] [Google Scholar]
- 45.Hu KQ, Kyulo NL, Lim N, Elhazin B, Hillebrand DJ, Bock T. Clinical significance of elevated alpha-fetoprotein (AFP) in patients with chronic hepatitis C, but not hepatocellular carcinoma. Am J Gastroenterol 2004; 99: 860–865. [DOI] [PubMed] [Google Scholar]
- 46.Sasaki R, Yamasaki K, Abiru S, et al. Serum Wisteria floribunda agglutinin-positive mac-2 binding protein values predict the development of hepatocellular carcinoma among patients with chronic hepatitis C after sustained virological response. PLoS One 2015; 10: e0129053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nagata H, Nakagawa M, Nishimura-Sakurai Y, et al. Serial measurement of Wisteria floribunda agglutinin positive Mac-2-binding protein is useful for predicting liver fibrosis and the development of hepatocellular carcinoma in chronic hepatitis C patients treated with IFN-based and IFN-free therapy. Hepatol Int 2016; 10: 956–964. [DOI] [PubMed] [Google Scholar]
- 48.Takahashi M, Saito H, Higashimoto M, Hibi T. Possible inhibitory effect of oral zinc supplementation on hepatic fibrosis through downregulation of TIMP-1: a pilot study. Hepatol Res 2007; 37: 405–409. [DOI] [PubMed] [Google Scholar]
- 49.Grüngreiff K, Reinhold D. Zinc: a complementary factor in the treatment of chronic hepatitis C? (Review). Mol Med Rep 2010; 3: 371–375. [DOI] [PubMed] [Google Scholar]
- 50.Hambidge KM, Goodall MJ, Stall C, Pritts J. Post-prandial and daily changes in plasma zinc. J Trace Elem Electrolytes Health Dis 1989; 3: 55–57. [PubMed] [Google Scholar]
- 51.Kanabrocki EL, Sothern RB, Ryan MD, et al. Circadian characteristics of serum calcium, magnesium and eight trace elements and of their metallo-moieties in urine of healthy middle-aged men. Clin Ter 2008; 159: 329–346. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.