Abstract
Ductal carcinoma in situ (DCIS) is a non-obligate precursor to invasive breast cancer. Only a small percentage of DCIS cases are predicted to progress; however, there is no method to determine which DCIS lesions will remain innocuous from those that will become invasive disease. Therefore, DCIS is treated aggressively creating a current state of over diagnosis and over treatment. There is a critical need to identify functional determinants of progression of DCIS to IDC. Interrogating biopsies from five patients with contiguous DCIS and IDC lesions, we have shown that expression of the long noncoding RNA BHLHE40-AS1 increases with disease progression. BHLHE40-AS1 expression supports DCIS cell proliferation, motility, and invasive potential. Mechanistically, BHLHE40-AS1 modulates IL-6 / STAT3 activity and a pro-inflammatory cytokine signature, in part through interaction with ILF3. These data suggest that BHLHE40-AS1 supports early breast cancer progression by engaging STAT3 signaling, creating an immune-permissive microenvironment.
Keywords: Ductal carcinoma in situ (DCIS), long noncoding RNA, BHLHE40-AS1, STAT3, IL-6, ILF3, breast cancer progression
Background:
Increased emphasis on early breast cancer detection, accompanied by better imaging technologies, has led to a dramatic increase in the diagnosis of ductal carcinoma in situ (DCIS). Despite this increased diagnosis and treatment of early stage disease, a complementary decline in late stage diagnosis has not been observed (Gorringe & Fox, 2017). This discrepancy underscores the reality that only a small percentage of early stage lesions progress to invasive disease. In fact, the majority are innocuous (Groen et al., 2017). The molecular mechanisms promoting DCIS progression to invasive disease remain largely unknown (Behbod, Gomes, & Machado, 2018). Current diagnostic strategies focus on assessing the risk of recurrence after DCIS treatment but do not evaluate or define if a DCIS lesion is predicted to progress (Bremer et al., 2018; Solin et al., 2013). Without a clinical method to identify which DCIS will progress, diagnosed women will undergo surgery and post-operative radiotherapy and/or endocrine therapy. This has created a current state of over diagnosis, over treatment, and a significant public health problem where low-risk patients undergo unnecessary intensive treatment without benefit (Groen et al., 2017). Therefore there is urgent need to define clear molecular determinants of progression.
Long noncoding RNAs (lncRNAs) are noncoding transcripts over 200 nucleotides in length that were previously discarded as spurious transcripts with no function within the cell. Now it is appreciated that lncRNAs can function at every level of gene regulation. The majority of lncRNAs with defined functions are located in the nucleus. Nuclear lncRNAs impact gene regulation through recruiting or blocking transcription factors, supporting chromatin looping around enhancer regions, or through interaction with histone modifying complexes to impact global chromatin architecture (Morlando, Ballarino, & Fatica, 2015). LncRNAs are also found to impact mRNA stability, translation and splicing, can function as micro RNA sponges, and as multiple protein complex scaffolds (Gutschner & Diederichs, 2012; Kumar, DeVaux, & Herschkowitz, 2016). It is now appreciated that lncRNAs play critical roles in development, and are often dysregulated in disease progression (Gutschner & Diederichs, 2012; Kumar et al., 2016). In breast cancer, an expression panel of lncRNAs has been shown to perform as well as the Prediction Analysis of Microarray 50 (PAM50) at classifying breast cancer intrinsic subtypes and predicting over-all survival (Su et al., 2014). Furthermore, lncRNAs can function as oncogenes as exemplified by HOTAIR which supports tumor cell invasion, cell proliferation, and metastasis in breast cancer (Avazpour, Hajjari, & Tahmasebi Birgani, 2017). Despite increased evidence that lncRNAs are functional molecules, the analysis of the majority of lncRNAs is preliminary and has not resulted in a clear pattern of expression in models of progression (Kumar et al., 2016).
Given the number of potential mechanisms that impact on cell function, and clear regulation in breast cancer subtypes, we sought to identify lncRNAs that play a mechanistic role in breast cancer progression. Molecular study of DCIS progression has been limited by a lack of both DCIS cell lines and early progression models. In this study we utilized a unique cohort of patient matched biopsies in which a DCIS lesion was identified contiguous with an invasive ductal carcinoma (IDC) lesion, allowing for the direct interrogation of transcriptional changes during progression. To validate the findings in vitro, we used the MCF10A progression cell line series. This series is derived from the normal, spontaneously immortalized MCF10A cells, and includes three additional cell lines that mimic progression in the forms of atypia (MCF10A-AT1 cells), DCIS (MCF10A-DCIS), and invasive (MCF10A-CA1) cells (M. Hu et al., 2008; Imbalzano, Tatarkova, Imbalzano, & Nickerson, 2009; Miller, Santner, Tait, & Dawson, 2000; Soule et al., 1990). We also validated the results in patient derived SUM225 cells, HER2+ cells that mimic DCIS in vivo (Barnabas & Cohen, 2013).
This study identifies 132 lncRNAs whose expression can distinguish between early stage DCIS and patient matched IDC. From this candidate list, we have identified a previously uncharacterized lncRNA, BHLHE40-AS1, which is increased during breast cancer progression and contributes to invasive phenotypes. BHLHE40-AS1 expression modulates IL6 / STAT3 signaling suggesting a potential mechanism by which non-invasive DCIS lesions progress to invasive disease.
RESULTS
BHLHE40-AS1 expression is increased in breast cancer progression
To identify lncRNAs relevant to patient tumor progression, we have taken advantage of a unique model of patient based DCIS progression wherein patients were identified that exhibited a DCIS lesion directly contiguous with an IDC lesion (Elsarraj et al., 2015). As DCIS is a non-obligate precursor lesion, these tandem lesions are a patient derived model of progression from pre-invasive to invasive disease and can be used to interrogate the molecular differences that occur during transition. RNA-sequencing results from five matched DCIS-IDC pairs (GSE66301) were mapped to the human genome build UCSC hg38 and long noncoding RNAs were identified using the GENCODE gene model considering all classes of lncRNAs, and antisense RNAs (Frankish et al., 2015). 132 lncRNAs showed discrimination potential between DCIS and IDC (Figure 1, Panel A). The lncRNA BHLHE40-AS1 is enriched in the patient DCIS-IDC tandem samples and its expression is statistically significant when comparing all DCIS versus all IDC (Figure 1, Panel B). In the MCF10A progression model, BHLHE40-AS1 expression increases with disease progression (Figure 1, Panel C). Compared to the MCF10A controls, the expression of BHLHE40-AS1 increases 3 fold in the MCF10A-DCIS cells and 5 fold in the MCF10A-CA1 cells. These data suggest that BHLHE40-AS1 is a potential biomarker of disease progression.
Figure 1. BHLHE40-AS1 is enriched during early breast cancer progression.
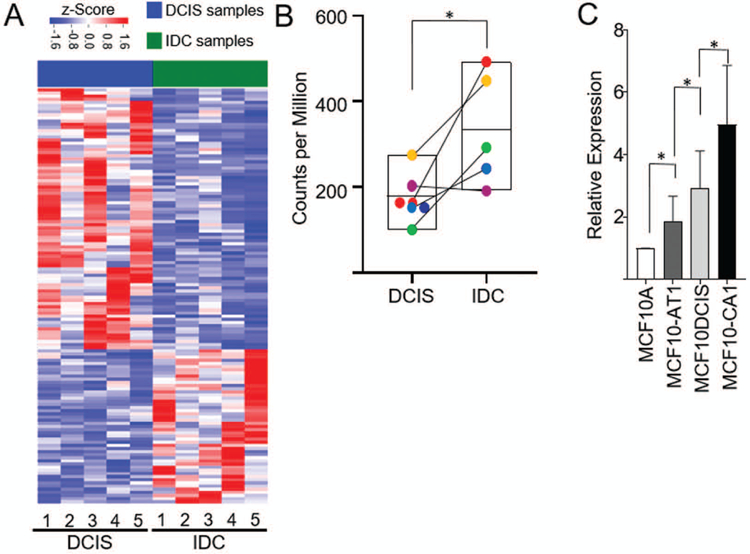
Panel A: Expression of lncRNAs in patient matched DCIS-IDC samples. Represented lncRNAs demonstrate a fold change exceeding 2 and p-value <0.05. RNA expression was z-score transformed. Panel B: Expression of BHLHE40-AS1 in the patient matched tandem lesions. * p<0.05, two-tailed Student’s t test. Panel C: 2.0x105 cells from indicated MCF10A progression series cell lines were seeded into 6-well plates. Total RNA was extracted from each cell line 48 h post-seeding and assessed for BHLHE40-AS1 expression. Bars represent mean ± SD for 6 independent biological replicates. * p<0.05, two-tailed Student’s t test.
Characterization of BHLHE40-AS1
BHLHE40-AS1 has not been functionally characterized. Multiple sequence alignments indicate that BHLHE40-AS1 exhibits a high degree of evolutionary conservation in primates, with the exception of a late insertion of a 6000 bp L1HS long interspersed nuclear element (LINE) into the human intronic region (Supplemental Figure 1, Panel A). The human transcript shares 45% homology with the positionally conserved mouse RIKEN 0610040F04 transcript (Supplemental Figure 1, Panel A). Rapid Amplification of cDNA Ends (RACE) was used to characterize and clone BHLHE40-AS1 from MCF10A-DCIS cells. This study confirms the presence of an 80kb intronic region. BHLHE40-AS1 is an antisense head-to-head transcript with its 5’ end overlapping the coding gene BHLHE40. RACE results identifies a BHLHE40-AS1 transcript with an extended 5’ end increasing the sequence overlap with BHLHE40. GRO-seq data from MCF7 breast cancer cells suggests that the extended transcript is found in additional backgrounds (Supplemental Figure 1, Panel B). Despite this extended overlap, BHLHE40-AS1 does not appear to interact with the BHLHE40 transcript (Supplemental Figure 1, Panels C&D). Although antisense transcripts frequently function to directly regulate the associated overlapping genes (Latge, Poulet, Bours, Josse, & Jerusalem, 2018), depletion of BHLHE40-AS1 does not consistently impact BHLHE40 transcription (Supplemental Figure 1, Panel E), nor is there a consistent change in BHLHE40 protein accumulation in multiple cell lines (data not shown). While a functional impact of BHLHE40-AS1 on BHLHE40 cannot be ruled out, based on these data, it appears that BHLHE40-AS1 functions independently of BHLHE40.
To determine the molecular context in which BHLHE40-AS1 functions, BHLHE40-AS1 expression was evaluated in a panel of breast cancer cell lines representing all molecular subtypes. BHLHE40-AS1 is expressed in all subtypes and enriched in HER2 positive cell lines (Figure 2, Panel A). Further, MCF10A cells stably overexpressing HER2 demonstrate a 3 fold increase of BHLHE40-AS1 expression that can be attenuated by treatment with the HER2 inhibitor lapatinib (Figure 2, Panels B & C). Confocal imaging in MCF10A-CA1 cells and HER2 positive BT474 cells demonstrate the lncRNA is predominantly found in the cytoplasmic compartment (Figure 2, Panels D & E). These data demonstrate that BHLHE40-AS1 is a conserved, cytoplasmic lncRNA, expressed downstream of HER2.
Figure 2. BHLHE40-AS1 is a predominantly cytoplasmic lncRNA regulated by HER2.
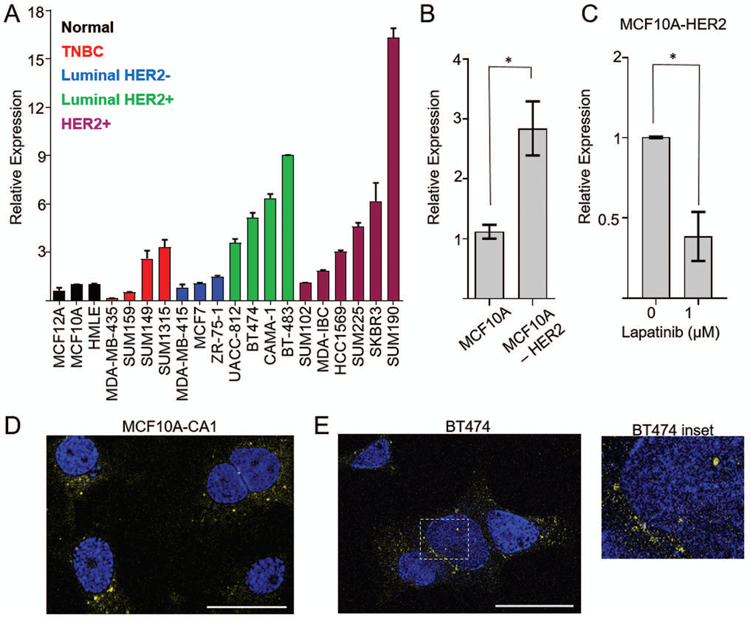
Panel A: Expression of BHLHE40-AS1 was assessed in a panel of breast cancer cell lines representative of all breast cancer subtypes. Bars represent mean ± range of 2 independent biological replicates. Panel B: 2.0x105 MCF10A or MCF10A-HER2 were seeded into a 6-well dish. Total RNA was extracted after 48 h and assessed for BHLHE40-AS1 expression. Bars represent mean ± SD of 4 independent biological replicates. Panel C: 2.0x105 cells were seeded into a 6 well dish. 24 h post-seeding cells were treated with vehicle or lapatinib for an additional 24h. Bars represent mean ± SD for 3 independent biological replicates. * p<0.05, two-tailed Student’s t test. Panel D: Maximum projection of confocal Z-stacks collected from MCF10A-CA1 cells processed by RNA FISH. Bar represents 25 μm. Blue represents DAPI, yellow represents BHLHE40-AS1. Panel E: Confocal imaging of HER2+ BT474 cells. One Z-stack slice representing 0.387μm thickness is shown. Bar represents 25 μm. Blue represents DAPI, yellow represents BHLHE40-AS1. Inset demonstrates nuclear localization of BHLHE40-AS1.
BHLHE40-AS1 supports cell migration, invasion, and proliferation.
To determine if expression functionally impacts invasive phenotypes, BHLHE40-AS1 was overexpressed in the normal, non-transformed, MCF10A cells. Cells overexpressing BHLHE40-AS1 exhibit increased cell spreading relative to pBABE vector control cells (Supplemental Figure 2). To determine if BHLHE40-AS1 impacts cell migration, pBABE control cells and BHLHE40-AS1 overexpressing cells were grown to confluence, scratched, and monitored for migration. Cells overexpressing BHLHE40-AS1 migrate at an average rate of 310 µm2/min compared to the vector control cells at 184 µm2/min (Figure 3, Panel A & B, and supplemental videos). BHLHE40-AS1 overexpressing cells also exhibit increased invasive potential in the Boyden chamber assay (Figure 3, Panel C). BHLHE40-AS1 overexpression did not significantly impact cell growth as evaluated by CellTiter-Glo (CTG) suggesting migration and invasion phenotypes were not affected by changes in cell proliferation (Figure 3, Panel D). To complement these gain of function studies, MCF10A-DCIS cells were transfected with siRNA targeting BHLHE40-AS1 (Figure 4, Panel A). Depletion of the lncRNA for 72 h attenuates cell cycle progression leading to significant accumulation of cells in G0 / G1 from 51.8% ± 8.8 with control siRNA to 71.0% ± 9.9 and 61.5% ± 6.6 with siRNA #1 and #2 respectively (Figure 4, Panels B & C, Table 1). CTG analysis 96 h post depletion of BHLHE40-AS1 shows a significant loss in overall cell number (Figure 4, Panel D).
Figure 3. BHLHE40-AS1 supports an invasive phenotype.
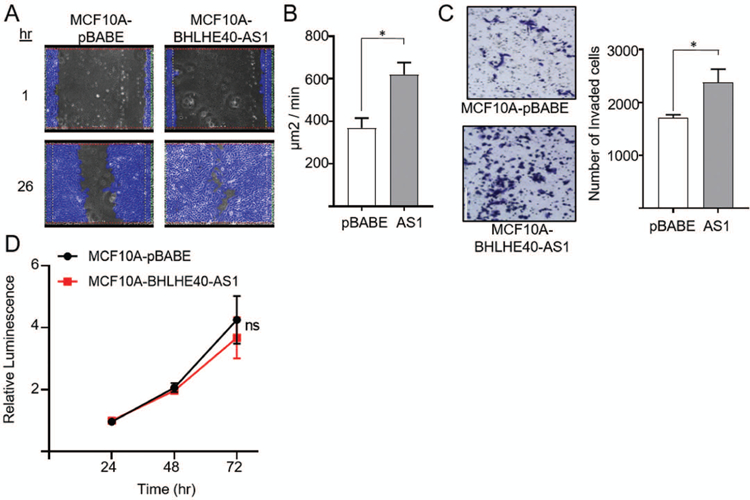
Panel A: 2.0x105 MCF10A-pBABE and MCF10A-BHLHE40-AS1 cells were plated in 6 well dishes. Confluent cells were scratched with a pipette tip 72h after seeding, rinsed with PBS and replenished with fresh medium. Cells were imaged every 30 min to monitor wound closure. Representative examples of the scratch assay are shown. Panel B: Rate of wound closure. Open area was calculated using ibidi MetaViLabs Automated Cellular Analysis System. Bars represent mean ± SD of 3 independent biological replicates * p<0.05, two-tailed Student’s t test. Panel C: 2.0x105 MCF10A-pBABE and MCF10A-BHLHE40-AS1 cells were plated in 6 well dishes. 48h post plating, cells were evaluated for invasive potential by a Boyden chamber assay. 2.5 x 104 cells were added to the upper chamber in serum free media and cultured for 24h. Cells were fixed with methanol, stained with crystal violet and counted using ImageJ. Bars represent mean ± SD of 3 independent biological replicates * p<0.05, two-tailed Student’s t test. Panel D: 4 x 103 cells were seeded into 96 well plates and cell number was measured using the CTG assay at the indicated time points.
Figure 4. BHLHE40-AS1 supports cell proliferation.
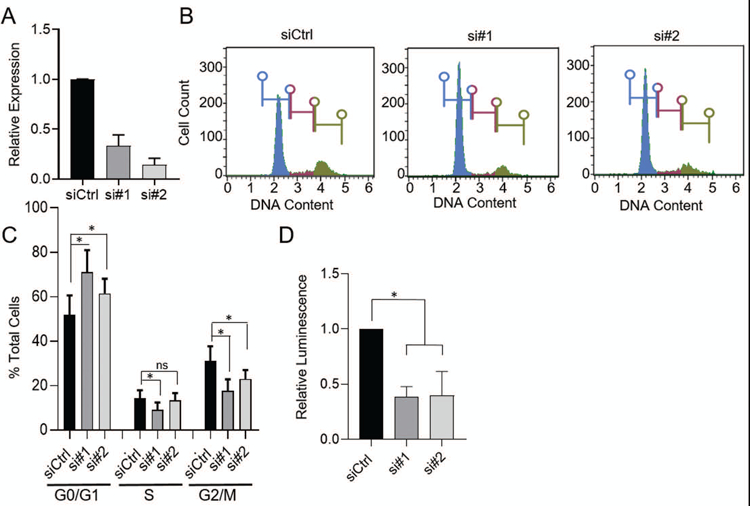
Panel A: 50nM of the indicated siRNA were transfected into 2.0 x105 MCF10A-DCIS cells. Total RNA was extracted 72h later and assessed for BHLHE40-AS1 expression. Panel B: 72h after transfection cells were fixed in cold methanol, stained with propidium iodide and assessed for cell cycle distribution by flow cytometry. One representative histogram is shown. Panel C: Quantitation of cell cycle analysis. Bars represent mean ± SD for 8 independent biological replicates. * p<0.05, two-tailed Student’s t test. Panel D: 4 x 103 MCF10A-DCIS cells were transfected with 50nM siRNA and assessed for relative cell number by the CTG assay 96h post transfection. Bars represent mean ± SD of 3 biological replicates. * p<0.05, two-tailed Student’s t test.
Table 1.
Distribution of cells in the cell cycle (%).
| G0/G1 | S | G2/M | |
|---|---|---|---|
| siCtrl | 51.8 ± 8.8 | 14.3 ± 3.7 | 31.2 ± 6.5 |
| si#1 | 71.0 ± 9.9 | 9.1 ± 3.3 | 17.7 ± 5.2 |
| si#2 | 61.5 ± 6.6 | 13.4 ± 3.2 | 22.9 ± 4.1 |
BHLHE40-AS1 signaling network
To elucidate the molecular impact of BHLHE40-AS1 expression, RNA from pBABE vector control and BHLHE40-AS1 overexpression cells were assessed by the transcriptome profiling Clariom-D array. Filtering the results for expression fold change ±2, p<0.5 and FDR <0.1, 345 genes were identified as differentially regulated (Figure 5, Panel A, Supplemental table 1). Ingenuity Pathway Analysis (IPA), was used to integrate expression data to gain biological insight into BHLHE40-AS1 signaling network (Kramer, Green, Pollard, & Tugendreich, 2014). Upstream regulator analysis predicts the activation state of master regulators by integrating gene expression changes with known biological networks. This generates an activation Z-score which assesses the match of observed and predicted regulation patterns and an associated pathway overlap p-value that measures the enrichment of network-regulated genes in the dataset (Kramer et al., 2014). This analysis, rank ordered by p-value, identifies a strong pro-inflammatory cytokine signature (Figure 5, Panel B). Two interleukin-1 family members (IL-1α and IL-1β), interleukin-6 (IL-6), as well as STAT3 (signal transducer and activator of transcription 3), a transcription factor downstream of all three cytokines (Johnson, O’Keefe, & Grandis, 2018; Liu et al., 2018; Mori et al., 2011), are found in the top seven predicted upstream regulators with activation Z-scores of 4.286, 3.881, 3.667, and 2.367 respectively (Figure 5, Panel B). These data strongly suggest that BHLHE40-AS1 plays an important role in inflammation. Validation of the microarray results using qRT-PCR confirmed a significant increase in expression of IL-1α, IL-1β, and IL-6 in the MCF10A-BHLHE40-AS1 cells (Figure 5, Panel C). Given that STAT3 is functionally downstream of the indicated cytokines, we focused on validating the impact of BHLHE40-AS1 on STAT3 signaling. To determine if BHLHE40-AS1 overexpression leads to the predicted increase in STAT3 pathway activity, vector control and BHLHE40-AS1 overexpressing cells were immunoblotted for phospho-STAT3 (Tyr705) and phospho-STAT3 (Ser727) (Figure 5, Panel D). The data demonstrate a clear increase in the level of p-STAT3 (Tyr705) and p-STAT3 (Ser727) compared to pBABE vector control cells indicating that stable overexpression of BHLHE40-AS1 leads to increased phosphorylation of pSTAT3 and a downstream pro-inflammatory cytokine response. However, these signaling changes may also result from long-term overexpression and may not be indicative of the immediate signaling network of BHLHE40-AS1. Therefore, IL-6 and STAT3 expression and phosphorylation were assessed 48 h post depletion of BHLHE40-AS1 to determine if they rapidly respond to BHLHE40-AS1 signaling. siRNA mediated depletion of BHLHE40-AS1 reduces IL-6 transcripts in the MCF10A-DCIS cells and SUM225 cells by 60% and 50% respectively (Figure 6, Panels A & C). Further, at 48 h there is a loss of total STAT3 protein and pSTAT3 in both cell lines, suggesting that STAT3 activity responds rapidly to BHLHE40-AS1 attenuation (Figure 6, Panels B & D). BHLHE40-AS1 was identified as a primary gene of interest due to its increasing expression both in patient matched DCIS / IDC samples as well as in the MCF10A progression series. Investigating IL-6 in the MCF10A progression series, IL-6 protein expression increases in the progression model (Supplemental Figure 3). Taken together, these data suggest BHLHE40-AS1 supports early breast cancer progression through modulation of IL-6 / STAT3 signaling.
Figure 5. BHLHE40-AS1 expression induces cytokine signature.
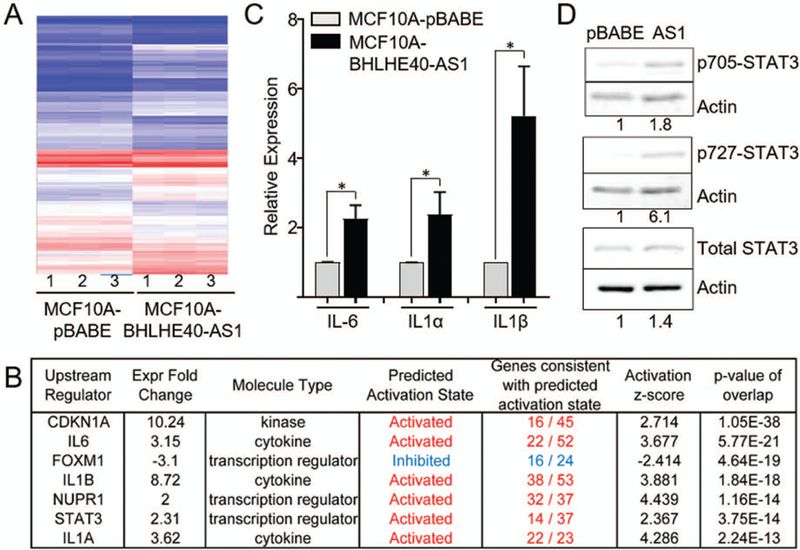
Panel A: 2.0x105 MCF10A-pBABE and MCF10A-BHLHE40-AS1 cells were plated in 6 well dishes. Total RNA was collected 48h after plating. 100ng RNA from three biological replicates were assessed via Clariom™ D Array and analyzed by the Transcriptome Analysis Console (TAC) software. Panel B: Ingenuity Pathway Analysis identified upstream signaling regulators in the MCF10A overexpression cell line. Upstream regulators were filtered for expression fold change of ±2, ordered by p-value of overlap. Panel C: Expression of indicated cytokines was determined by qRT-PCR from 48h total RNA cell extracts. Bars represent mean ± SD from 3 biological replicates. * p<0.05, two-tailed Student’s t test. Panel D: MCF10A-pBABE and MCF10A-BHLHE40-AS1 cells were immunoblotted with indicated antibodies. Densitometry was performed in ImageJ.
Figure 6. BHLHE40-AS1 regulates IL-6 / STAT3 activity.
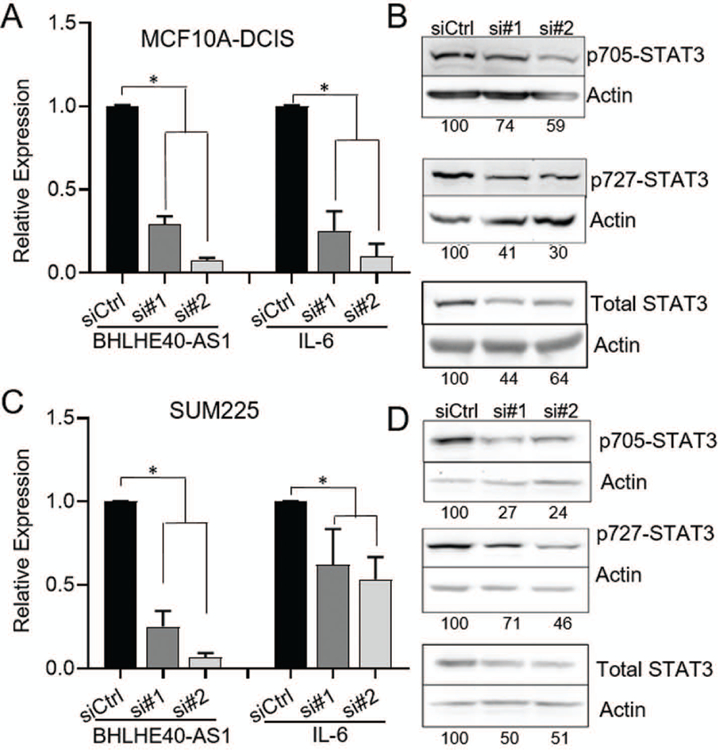
MCF10A-DCIS (Panel A & Panel B) and SUM225 cells (Panel C & Panel D) were transfected with 50nM of siRNA targeting BHLHE40-AS1. Total RNA (A & C) or whole cell lysate (B & D) was collected 48h after transfection and assessed by qRT-PCR or western blot for expression of indicated genes.
BHLHE40-AS1 interacts with ILF3 to mediate IL-6 signaling.
To determine the mechanism by which BHLHE40-AS1 impacts IL-6 signaling, we identified interacting proteins. BHLHE40-AS1, and control antisense transcripts, were in vitro transcribed and biotin labeled. These RNAs were incubated with whole cell lysate from MCF10A-DCIS cells and purified with streptavidin magnetic beads. LncRNA associated proteins were separated by SDS-PAGE and stained with colloidal coomassie for visualization (Supplemental Figure 4). Mass spectrometry analysis identified Interleukin Enhancer Binding Factor 3 (ILF3) as a top candidate interacting protein. To validate ILF3 as an interacting protein, BHLHE40-AS1 pull-down assays were performed in MCF10A-BHLHE40-AS1 cells using biotinylated DNA oligos tiling BHLHE40-AS1, or non-targeting antisense oligos as control. qRT-PCR confirms successful isolation of BHLHE40-AS1 and immunoblotting of associated co-precipitating proteins confirms an association with ILF3 (Figure 8, Panel A). Furthermore, RNA immunoprecipitation (RIP) using an ILF3 antibody was performed to monitor the levels of BHLHE40-AS1 associating with ILF3 relative to control IgG. Co-purifying RNA was isolated and assessed by qRT-PCR for 18s RNA, H19, a cytoplasmic lncRNA, and BHLHE40-AS1. BHLHE40-AS1 is specifically enriched in ILF3 relative to control IgG and control RNAs (Figure 8, Panel B). Taken together these data demonstrate an interaction between BHLHE40-AS1 and ILF3.
ILF3 is a known RNA binding protein that is alternately spliced producing the NF90 and NF110 proteins. These RNA binding proteins interact with several cellular and viral RNAs and participate in diverse cellular functions including miRNA biogenesis, translational regulation, mRNA stabilization, although the physiological importance of some interactions is still to be determined (Castella, Bernard, Corno, Fradin, & Larcher, 2015). ILF3 has previously been identified to interact with IL-6 mRNA in lung fibroblast (WI38 and IDH4) cells (Tominaga-Yamanaka et al., 2012). ILF3 depletion in the MCF10A-BHLHE40-AS1 cells was sufficient to rescue the increased IL-6 expression (Figure 8, Panel C). These data demonstrate that BHLHE40-AS1 modulates IL-6 expression through a direct interaction with ILF3.
Discussion
There are currently no known functional determinants of DCIS progression to an invasive lesion; in fact, DCIS and IDC lesions are very similar transcriptionally and epigenetically (Abba et al., 2015; DeVaux & Herschkowitz, 2018; Nelson, Machado, & Schwertfeger, 2018; Pang et al., 2017). This study profiles global long noncoding RNA expression in a unique patient-based model of breast cancer progression wherein early DCIS lesions are directly contiguous with an IDC lesion. From this unbiased, patient based model we have identified 132 lncRNAs that are differentially expressed with early breast cancer progression. Further, we identify the lncRNA BHLHE40-AS1 as a novel lncRNA that increases with disease progression both in the patient biopsies and in cell culture models of early breast cancer progression.
Phenotypically, BHLHE40-AS1 expression contributes to cell migration, invasion, and potentially cell proliferation. Cell cycle progression is a complex mechanism regulated by many controls and check points. Given that overexpression of BHLHE40-AS1 did not result in a significant change in cell cycle progression, we did not anticipate a function in proliferation. However, BHLHE40-AS1 depletion attenuates normal cell cycle progression and results in an accumulation in G0 / G1. Taken together this suggests that with overexpression of the lncRNA, the G0 / G1 checkpoint remains intact, however loss of BHLHE40-AS1 attenuates normal cell proliferation. It is possible that the lncRNA may be playing an indirect role in cell cycle progression as opposed to directly regulating a checkpoint. Future studies will be needed to elucidate the lncRNAs role in cell cycle progression.
The data presented here demonstrate that BHLHE40-AS1 modulates a pro-inflammatory cytokine signature and is an important mediator of IL-6 / STAT3 signaling. IL-1α and IL-1β are pleiotropic activator cytokines that function as critical signaling mediators of the inflammatory tumor microenvironment (Baker, Houston, & Brint, 2019). IL-1β in particular is found to be upregulated in many cancers including breast cancer, and to support angiogenesis, proliferation, metastasis, and inflammasome activation at both primary and metastatic sites (Baker et al., 2019; Holen et al., 2016; Lewis, Varghese, Xu, & Alexander, 2006). In breast cancer, IL-1β expression induces IL-6 and additional cytokines and growth factors (Oh, Lee, Park, Seo, & Lee, 2016). Further, while less studied in cancer than IL-1β, IL-1α has been found to support inflammation and cancer stem cell expansion downstream of HER2 expression. IL-1α induces IL-6 and STAT3 signaling creating a feed-forward pro-inflammation signaling loop supporting tumor progression (Holen et al., 2016; Liu et al., 2018).
STAT3 is a core signaling integrator of pro-inflammatory cytokines in addition to many oncogenes and growth factors (Dethlefsen, Hojfeldt, & Hojman, 2013). The canonical IL-6 / STAT3 signaling pathway is well known to play a key role in the development of many cancers including breast where it is found constitutively activated in greater than 50% of patient tumors (Dethlefsen et al., 2013; Johnson et al., 2018; Kumari, Dwarakanath, Das, & Bhatt, 2016). In breast cancer cells, STAT3 activation by IL-6 enhances tumor cell migration, invasion and metastasis and is tightly linked to cellular transformation, proliferation and tumor initiation (Ling & Arlinghaus, 2005; Segatto, Baldassarre, & Belletti, 2018; Snyder, Huang, & Zhang, 2011; Yu, Lee, Herrmann, Buettner, & Jove, 2014). BHLHE40-AS1 mediates STAT3 activation in DCIS cell lines. In an inducible PyVmT mammary tumor model, in STAT3 deficient mice, early stage lesions were cleared by immune cell infiltration resulting in delayed tumor development. In contrast, intact STAT3 signaling resulted in pro-tumorigenic inflammation, immune evasion and tumor formation (Jones et al., 2016). The induction of cytokine expression and STAT3 activation by BHLHE40-AS1 implicates the lncRNA as a key mediator of pro-tumorigenic inflammatory response.
Mechanistically, BHLHE40-AS1 may directly target IL-6 transcripts through an interaction with the known RNA binding protein ILF3. ILF3 holds many diverse roles within the cell and has been implicated in promoting invasive potential, proliferation, and migration in breast cancer cells (Q. Hu et al., 2013; Zhang et al., 2017). Future studies will be needed to fully understand the consequences of the interaction between BHLHE40-AS1 and ILF3.
The data presented here demonstrate that BHLHE40-AS1 is a novel lncRNA whose expression is important to the progression of DCIS lesions into invasive IDC. Integrating BHLHE40-AS1 as a marker of increased disease progression and as an activator of STAT3 signaling, we propose BHLHE40-AS1 expression serves as a mediator between primary DCIS lesions and the surrounding cells serving to create a permissive immune microenvironment. Future studies will expand our patient analysis and confirm BHLHE40-AS1 as a biomarker of progression. Incorporating BHLHE40-AS1 and IL-6 expression with STAT3 activation in biopsied DCIS lesions may serve to distinguish those DCIS lesions more likely to progress from those that will remain innocuous.
Materials and Methods:
Cell culture:
The MCF10A progression series was purchased from the Barbara Ann Karmanos Cancer Institute and maintained in a culture of DMEM medium supplemented with 5% horse serum, 20ng/mL EGF, 0.5 mg/mL hydrocortisone, 100 ng/mL cholera toxin, 10μg/mL insulin and 1x antibiotic-antimycotic (Gibco, Grand Island, New York). MCF10A-HER2 cells were also maintained in the same medium. SUM225 cells were obtained from Asterand and cultured in DMEM-F12 supplemented with 5 µg/mL insulin, 1 µg/mL hydrocortisone, 10 mM HEPES, 5% fetal bovine serum (FBS) and 1x antibiotic-antimycotic as previously described (Behbod et al., 2009). 293-Phoenix cells for retrovirus production were maintained in DMEM supplemented with 10% FBS. All cell lines were verified by STR analysis and routinely screened for mycoplasma contamination.
qRT-PCR Primers:
TaqMan® Assays were purchased from ThermoFisher Scientific (Waltham, MA): BHLHE40-AS1 (Hs04274224_ m1), 18s (Hs99999901_s1), Actin (Hs99999903_m1), GAPDH (Hs02758991_g1). Sybr-green assays purchased from Integrated DNA Technologies (Coralville, IA):

Plasmids:
Clonetech SMARTer® RACE kit (Takara BIO USA, Mountain View, CA) was used to PCR amplify BHLHE40-AS1 by PCR from MCF10A-DCIS cells. The PCR product was validated by sequencing and ligated into pCR™4Blunt-TOPO for amplification. BHLHE40-AS1 was subcloned into pBABE-puro for retroviral production between restriction sites BamHI and XhoI. Primer 1: 5’-CAATACGGATCC GCCCTTACGTCTCTTT-3’ Primer 2: 5’-TTGATCTCGAGTAAACGAATTCGCCCTTGTT-3’.
RNA Sequencing Analysis:
RNA sequencing of DCIS-IDC patient sample pairs 1–5 from NCBI GEO dataset GSE66301 were analyzed (Elsarraj et al., 2015). RNA-Seq data was mapped using HISAT2 to the human genome build UCSC hg38 (Pertea, Kim, Pertea, Leek, & Salzberg, 2016). Gene expression quantification was achieved using FEATURECOUNTS (Liao, Smyth, & Shi, 2019) against the GENCODE (Frankish et al., 2015) gene model. Gene expression was further normalized using the R package RUVr (Risso, Ngai, Speed, & Dudoit, 2014). Differential lincRNA expression was evaluated using the R package edgeR (Robinson, McCarthy, & Smyth, 2010). Significance was achieved for a fold change exceeding 2x and p-value<0.05. Graphical representation as heatmaps was generated using the Python visualization library matplotlib.
Gene expression analysis:
48h post-seeding, total RNA was collected using Omega Bio-Tek (Norcross, GA) E.Z.N.A.® Total RNA Kit. RNA was reverse transcribed using Applied Biosystems™ High-Capacity cDNA Reverse Transcription Kit and analyzed by qRT-PCR on the QuantStudio (ThermoFisher Scientific) for indicated genes. The ∆∆CT method was used to determine gene expression fold change.
Florescence In Situ Hybridization:
33 DNA probes tiling BHLHE40-AS1 and labeled with Quasar570 were purchased from LGC Biosearch™Technologies (Novato, CA). Cells plated on glass coverslides were processed for RNA Fluorescence in situ hybridization following the standard Biosearch protocols. Cells were fixed in 3.7% formaldehyde, permeabilized with 0.5% Triton-X 100 and blocked with wash buffer A (LGC Biosearch) with formamide. 250 nM pooled probes in hybridization buffer (LGC Biosearch) were incubated on cells overnight at 37°C followed by DAPI staining. Mounted cells were imaged on the Zeiss LSM780 confocal microscope. Z-stack images were collected with the Plan-Apochromat 63x/1.40 Oil DIC objective and processed in ZEN blue.
Stable cell line generation:
1 µg empty pBABE-puro vector or pBABE-puro-BHLHE40-AS1 vector were transfected into packaging 293 Phoenix cells using lipofectamine X-tremeGENE™HP from Millipore Sigma (Burlington, MA). Viral media was collected 36h post plasmid transfection and incubated with MCF10A cells overnight. Transduced cells were selected with 1 μg puromycin for 2 weeks with medium changes every 48–72 h.
Wound Healing:
2.0x105 MCF10A-pBABE or MCF10A-BHLHE40-AS1 cells were seeded in six well plates and grown to confluency. Cells were scratched with a pipette tip, rinsed with PBS, and incubated in fresh medium in the EVOS FL Auto OnStage Incubator. Cells were imaged every 30 min to monitor wound closing. Images were analyzed by MetaViLabs Automated Cellular Analysis System (Austin, TX) to determine the rate of wound closure. The rate of scratch closure during the linear fit portion of the curve is reported.
Invasion Assay:
2.0x105 MCF10A-pBABE and MCF10A-BHLHE40-AS1 cells were seeded in six well plates. 48h post-seeding cells were trypsinized and counted and 2.5x103 cells were resuspended in serum free medium and loaded into the upper chamber of a growth factor reduced Corning®BioCoat™ Matrigel™ Invasion Chamber (Corning Life Sciences, Tewksbury, MA) previously rehydrated with serum free medium. Chambers were placed into 12 well plates loaded with complete media and maintained for 24h at 37°C. Cells were then fixed in 100% methanol, stained with 0.1% crystal violet and imaged on the EVOS FL Imaging system (Thermofisher Scientific). ImageJ was used to count 3 fields of view per replicate; 3 independent biological replicates were performed.
Cell Cycle Analysis:
50 nM control and targeted siRNA were complexed with RNAiMax (ThermoFisher Scientiic) and transfected into indicated cell lines. 72 h post transfection cells were trypsinized, and fixed in 70% ethanol as a single cell suspension. Cells were rinsed with PBS and stained according to the Muse® Cell Cycle Assay kit standardized instructions. 10,000 events were collected for each experimental replicate.
CellTiter Glo (CTG) Analysis:
Cells were seeded into 96 well plates. At indicated time points, cells were assayed via CellTiter-Glo® (Promega, Madison, WI). Cells were removed from the incubator and allowed to cool to room temperature for 10 min. CTG cell lysis / luminescence reagent was added directly to the well plate and incubated with agitation at room temperature for 10 min. Resulting luminescence (generated via reaction between luciferin and ATP – representing a direct relationship with the number of metabolically active cells) was collected on a VICTOR3V plate reader (PerkinElmer, Waltham, MA).
Microarray:
Total RNA was extracted from replicate MCF10A-pBABE and MCF10A-pBABE-BHLHE40-AS1 cells. RNA quality was assessed by bioanalyzer and 100 ng total RNA with RIN scores of >9.8 were arrayed using Applied Biosystems Human Clariom D Assay by the University at Albany Center for Functional Genomics following standard protocols. Raw CEL files were analyzed with the Transcriptome Analysis Console (TAC). 365 differentially expressed genes were identified with p-value <0.05 and false discovery rate < 0.1. Ingenuity Pathway Analysis (IPA) (Qiagen, Hilden, Germany) was used to identify predicted upstream regulators with an expression fold change with an absolute value of 2 or greater.
Western Blot:
Cells were lysed in cell lysis buffer (20 mM Tris, 150 mM NaCl, 1 mM Na2EDTA, 1mM EGTA, 1% Triton X-100) supplemented with protease and phosphatase inhibitors. Whole cell lysates were resolved by SDS-PAGE and immunoblotted with the following antibodies from Cell Signaling Technology (Danvers, MA): Actin (13E5) 1:1000, GAPDH (14C10) 1:1000, STAT3 (79D7) 1:1000, phospho-Stat3 Tyr705 (D3A7) 1:250, phospho-Stat3 Ser727 (9134) 1:250.
ELISA:
2.0x105 cells of each of the MCF10A progression series cell lines were plated in a 6 well dish. 48h post-seeding, conditioned media was collected and centrifuged at 1000xg for 10 min and assayed for IL-6 concentration using Invitrogen™ eBioscience™ Human IL-6 ELISA Ready-SET-Go! Kit (ThermoFisher Scientific). The remaining cells were trypsinized and counted to determine cell number for normalization.
BHLHE40-AS1 pull down with biotinylated oligos.
RNA purification was performed as previously described (Chu, Quinn, & Chang, 2012; Chu et al., 2015). Briefly, MCF10A-DCIS or MCF10A-BHLHE40-AS1 cells were grown to 90% confluence, crosslinked in 3% formaldehyde for 30 min followed by quenching with 0.125M glycine for 5 min. Cells were lysed in lysis buffer (50 mM Tris pH 7.0, 10 mM EDTA, 1% SDS, protease inhibitors, and 100U/mL RNAse Out) and sonicated until clear. Lysate was diluted in hybridization buffer (750 mM NaCl, 1% SDS, 50 mM Tris pH 7.0, 1 mM EDTA, 15% formamide, protease inhibitors, and 100U/mL RNAse Out) and incubated with biotinylated probes tiling BHLHE40-AS1 overnight. MyOne Streptavidin C1 Dynabeads were incubated with the lysate for 2 h, beads were washed in wash buffer (2x NaCl and sodium citrate (SSC), 0.5% SDS, protease inhibitors, and 100U/mL RNAse Out), resuspended in RNA proteinase K buffer (100 mM NaCl, 10mM Tris pH 7.0, 1mM EDTA, 0.5% SDS, Proteinase K) at 50C before boiling for 10 min 95C. Reverse crosslinked samples were then incubated with TRIzol and RNA was purified via chloroform extraction.
ILF3 RNA immunoprecipitation (RIP).
ILF3 RIP was performed following standard methods (Feng et al., 2014). 4.5x107 MCF10A-DCIS cells were lysed in RIP buffer (150mM KCl, 25mM Tris pH 7.4, 5mM EDTA, 0.5% NP40, protease and RNAse inhibitors), precleared with streptavidin beads, and incubated overnight with 10ug either control IgG or anti-ILF3 (BD Biosciences cat #612154). Streptavidin beads were added for an additional 2hr incubation. Beads were washed with RIP buffer, incubated in TRIzol, and RNA was purified via chloroform extraction.
Supplementary Material
Supplemental Figure 1. Characterization of BHLHE40-AS1. Panel A: Schematic representation of BHLHE40-AS1 with associated UCSC Genome Browser tracks depicting mammalian conservation, vertebrate conservation and repeat elements. Panel B: The transcript cloned from RACE was aligned to the genome using UCSC BLAT tool demonstrating an elongated 5’ end. To determine if this transcript is found in additional backgrounds, GRO-seq (Global Run-On Sequencing) data from MCF7 cells (SRR816998, SRR816995, SRR604586) was analyzed. Peaks on the negative strand indicate this extended transcript is present in other genetic backgrounds. Panel C: Biotinylated probes tiling BHLHE40 were incubated with crosslinked MCF10A-DCIS whole cell lysate. Streptavidin beads were used to isolate target RNA. RNA was isolated and assessed for indicated genes by qRT-PCR. Three independent biological replicates were performed, one representative purification is shown. Panel D: RNA purification was performed as in panel C with crosslinked whole cell lysate from MCF10A-BHLHE40-AS1 cells. 4 independent biological replicates were performed, one representative experiment is shown. Panel E: 50nM of indicated siRNA were transfected into MCF10A-DCIS (left) or SUM225 (right) cells. Total RNA was collected 72h after transfection and assessed for expression of indicated genes. Bars represent mean ± SD of 3 independent biological replicates. * p<0.05, two-tailed Student’s t test.
Supplemental Figure 2. Characterization of BHLHE40-AS1. A) 2.0x105 MCF10A-pBABE and MCF10A-BHLHE40-AS1 cells were plated and imaged 24h post-seeding. Bar represents 200 μm.
Supplemental Figure 3. IL-6 expression in the MCF10A series. Panel A: Conditioned media from indicated cell lines was collected 48h post-seeding and IL-6 protein concentration was assessed by ELISA. Signal was normalized to total cell count. Bars indicate mean ± SD for 3 biological replicates. * p<0.05, two-tailed Student’s t test.
Supplemental Figure 4. Protein interaction partner identification. 10pmol synthesized biotinylated BHLHE40-AS1 or antisense control were incubated with 150 ug MCF10A-DCIS whole cell lysate and purified as described in methods. Associated proteins were separated by SDS-PAGE and visualized by colloidal coomassie staining.
Supplemental Videos. MCF10A-pBABE cells (Video 1) or MCF10A-BHLHE40-AS1 cells (Video 2) were grown to confluence, scratched with a pipette tip, and maintained in the EVOS Onstage Incubator for imaging every 30 min over 48 h. Videos are 1 image per hour for 48 images and stitched together at 5 frames per second.
Figure 7. BHLHE40-AS1 interacts with ILF3 to mediate IL-6 production.
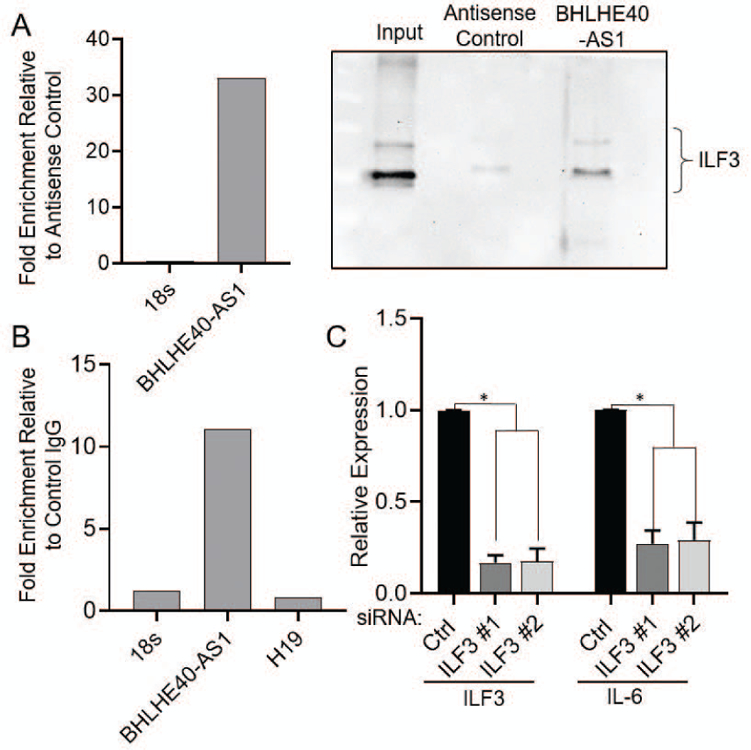
Panel A: 4.5 x107 MCF10A-BHLHE40-AS1 cells were used to pull-down BHLHE40-AS1 RNA with associated proteins with biotinylated DNA oligos as described in methods. qRT-PCR validates successful lncRNA pulldown (left). Associated proteins were separated by SDS-PAGE and immunoblotted for ILF3 (right). Panel B: RNA immunoprecipitation of ILF3 from MCF10A-DCIS cells was performed as described in methods. Associated RNAs were evaluated by qRT-PCR for indicated RNAs. Panel C: 50nM of siRNA targeting ILF3 were transfected into 2.0 x105 MCF10A-BHLHE40-AS1 cells. Total RNA was collected 48 hr later and assessed for indicated gene expression. Bars indicate mean ± SD of 3 biological replicates. * p<0.05, two-tailed Student’s t test.
Acknowledgments:
We would like to thank M. Kuentzel and S. Chittur of the Center for Functional Genomics at the University at Albany for their guidance, discussions, and services. We would like to acknowledge the University of Kansas Cancer Center Biospecimen Repository Core Facility staff for helping obtain human specimens. We thank the University of Kansas Medical Center-Genomics Core for generating the array and sequencing data sets and the Cornell University Biotechnology Resource Center (BRC) for mass spectrometry analysis. We would like to thank Dr. Sun in Dr. Conklin’s lab for the kind gift of the MCF10A-HER2 overexpressing cells. RSD was supported by the DOD-CDMRP award W81XWH-15-1-10495, RSD and JIH were partially supported by the 2015 Breast Cancer Alliance Young Investigator Award, and Susan G. Komen CCR17481765. CC and SLG were partially supported by CPRIT Core Facility grant RP170005, NIH P30 shared resource grant CA125123, and NIEHS P30 Center grant 1P30ES030285-01. FB was supported in part by grants from 2014 Breast Cancer Research Foundation-AACR and NIH/NCI 1R21CA185460-01 and The University of Kansas Cancer Center P30 CA168524.
Footnotes
The authors have no conflicts of interest.
Data Availability:
The data that supports the findings of this study can be found under GSE136579.
Supplemental method: Interacting Protein Identification
BHLHE40-AS1 pull-down was performed following standard methods (Feng et al., 2014). BHLHE40-AS1 was cloned into pCR™4Blunt-TOPO® vector (ThermoFisher Scientific) such that it was flanked by T3 and T7 in vitro transcription priming sites. MEGAscript™ T3 and T7 Transcription kits (ThermoFisher Scientific) supplemented with Biotin RNA labelling nucleotide mixture (Millipore Sigma cat# 11685597910) were used to synthesize BHLHE40-AS1 and the antisense transcript as control. MCF10A-DCIS cells were lysed in RIP buffer (150mM KCl, 25mM Tris pH 7.4, 5mM EDTA, 0.5% NP40, protease and RNAse inhibitors) and quantified by BCA. 10 pmol synthesized RNA were diluted into structure buffer (10mM Tris pH 7.0, 0.1M KCl, 10 mM MgCl2), incubated at 90C for 2 min, on ice for 2 min, then at room temperature for 20 min to allow proper formation of RNA secondary structure. Structured RNA was incubated with 150 ug pre-cleared whole cell lysate at 4C for 2h prior to streptavidin bead addition for 1h. Beads were washed with RIP buffer, associated proteins were collected in Laemmli buffer, separated by SDS-PAGE, and stained with colloidal coomassie for visualization. Unique bands were excised and processed for mass spectrometry by the Cornell University Biotechnology Resource Center.
References:
- Abba MC, Gong T, Lu Y, Lee J, Zhong Y, Lacunza E, … Aldaz CM (2015). A Molecular Portrait of High-Grade Ductal Carcinoma In Situ. Cancer Res, 75(18), 3980–3990. doi: 10.1158/0008-5472.CAN-15-0506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avazpour N, Hajjari M, & Tahmasebi Birgani M (2017). HOTAIR: A Promising Long Non-coding RNA with Potential Role in Breast Invasive Carcinoma. Front Genet, 8, 170. doi: 10.3389/fgene.2017.00170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker KJ, Houston A, & Brint E (2019). IL-1 Family Members in Cancer; Two Sides to Every Story. Front Immunol, 10, 1197. doi: 10.3389/fimmu.2019.01197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnabas N, & Cohen D (2013). Phenotypic and Molecular Characterization of MCF10DCIS and SUM Breast Cancer Cell Lines. Int J Breast Cancer, 2013, 872743. doi: 10.1155/2013/872743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behbod F, Gomes AM, & Machado HL (2018). Modeling Human Ductal Carcinoma In Situ in the Mouse. J Mammary Gland Biol Neoplasia, 23(4), 269–278. doi: 10.1007/s10911-018-9408-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behbod F, Kittrell FS, LaMarca H, Edwards D, Kerbawy S, Heestand JC, … Medina D (2009). An intraductal human-in-mouse transplantation model mimics the subtypes of ductal carcinoma in situ. Breast Cancer Res, 11(5), R66. doi: 10.1186/bcr2358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremer T, Whitworth PW, Patel R, Savala J, Barry T, Lyle S, … Warnberg F (2018). A Biological Signature for Breast Ductal Carcinoma In Situ to Predict Radiotherapy Benefit and Assess Recurrence Risk. Clin Cancer Res, 24(23), 5895–5901. doi: 10.1158/1078-0432.CCR-18-0842 [DOI] [PubMed] [Google Scholar]
- Castella S, Bernard R, Corno M, Fradin A, & Larcher JC (2015). Ilf3 and NF90 functions in RNA biology. Wiley Interdiscip Rev RNA, 6(2), 243–256. doi: 10.1002/wrna.1270 [DOI] [PubMed] [Google Scholar]
- Chu C, Quinn J, & Chang HY (2012). Chromatin isolation by RNA purification (ChIRP). J Vis Exp (61). doi: 10.3791/3912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu C, Zhang QC, da Rocha ST, Flynn RA, Bharadwaj M, Calabrese JM, … Chang HY (2015). Systematic discovery of Xist RNA binding proteins. Cell, 161(2), 404–416. doi: 10.1016/j.cell.2015.03.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dethlefsen C, Hojfeldt G, & Hojman P (2013). The role of intratumoral and systemic IL-6 in breast cancer. Breast Cancer Res Treat, 138(3), 657–664. doi: 10.1007/s10549-013-2488-z [DOI] [PubMed] [Google Scholar]
- DeVaux RS, & Herschkowitz JI (2018). Beyond DNA: the Role of Epigenetics in the Premalignant Progression of Breast Cancer. J Mammary Gland Biol Neoplasia, 23(4), 223–235. doi: 10.1007/s10911-018-9414-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsarraj HS, Hong Y, Valdez KE, Michaels W, Hook M, Smith WP, … Behbod F (2015). Expression profiling of in vivo ductal carcinoma in situ progression models identified B cell lymphoma-9 as a molecular driver of breast cancer invasion. Breast Cancer Res, 17, 128. doi: 10.1186/s13058-015-0630-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y, Hu X, Zhang Y, Zhang D, Li C, & Zhang L (2014). Methods for the study of long noncoding RNA in cancer cell signaling. Methods Mol Biol, 1165, 115–143. doi: 10.1007/978-1-4939-0856-1_10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankish A, Uszczynska B, Ritchie GR, Gonzalez JM, Pervouchine D, Petryszak R, … Harrow J (2015). Comparison of GENCODE and RefSeq gene annotation and the impact of reference geneset on variant effect prediction. BMC Genomics, 16 Suppl 8, S2. doi: 10.1186/1471-2164-16-S8-S2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorringe KL, & Fox SB (2017). Ductal Carcinoma In Situ Biology, Biomarkers, and Diagnosis. Front Oncol, 7, 248. doi: 10.3389/fonc.2017.00248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groen EJ, Elshof LE, Visser LL, Rutgers EJT, Winter-Warnars HAO, Lips EH, & Wesseling J (2017). Finding the balance between over- and under-treatment of ductal carcinoma in situ (DCIS). Breast, 31, 274–283. doi: 10.1016/j.breast.2016.09.001 [DOI] [PubMed] [Google Scholar]
- Gutschner T, & Diederichs S (2012). The hallmarks of cancer: a long non-coding RNA point of view. RNA Biol, 9(6), 703–719. doi: 10.4161/rna.20481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holen I, Lefley DV, Francis SE, Rennicks S, Bradbury S, Coleman RE, & Ottewell P (2016). IL-1 drives breast cancer growth and bone metastasis in vivo. Oncotarget, 7(46), 75571–75584. doi: 10.18632/oncotarget.12289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu M, Yao J, Carroll DK, Weremowicz S, Chen H, Carrasco D, … Polyak K (2008). Regulation of in situ to invasive breast carcinoma transition. Cancer Cell, 13(5), 394–406. doi: 10.1016/j.ccr.2008.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Q, Lu YY, Noh H, Hong S, Dong Z, Ding HF, … Huang S (2013). Interleukin enhancer-binding factor 3 promotes breast tumor progression by regulating sustained urokinase-type plasminogen activator expression. Oncogene, 32(34), 3933–3943. doi: 10.1038/onc.2012.414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imbalzano KM, Tatarkova I, Imbalzano AN, & Nickerson JA (2009). Increasingly transformed MCF-10A cells have a progressively tumor-like phenotype in three-dimensional basement membrane culture. Cancer Cell Int, 9, 7. doi: 10.1186/1475-2867-9-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DE, O’Keefe RA, & Grandis JR (2018). Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat Rev Clin Oncol, 15(4), 234–248. doi: 10.1038/nrclinonc.2018.8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones LM, Broz ML, Ranger JJ, Ozcelik J, Ahn R, Zuo D, … Muller WJ (2016). STAT3 Establishes an Immunosuppressive Microenvironment during the Early Stages of Breast Carcinogenesis to Promote Tumor Growth and Metastasis. Cancer Res, 76(6), 1416–1428. doi: 10.1158/0008-5472.CAN-15-2770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer A, Green J, Pollard J Jr., & Tugendreich S (2014). Causal analysis approaches in Ingenuity Pathway Analysis. Bioinformatics, 30(4), 523–530. doi: 10.1093/bioinformatics/btt703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar M, DeVaux RS, & Herschkowitz JI (2016). Molecular and Cellular Changes in Breast Cancer and New Roles of lncRNAs in Breast Cancer Initiation and Progression. Prog Mol Biol Transl Sci, 144, 563–586. doi: 10.1016/bs.pmbts.2016.09.011 [DOI] [PubMed] [Google Scholar]
- Kumari N, Dwarakanath BS, Das A, & Bhatt AN (2016). Role of interleukin-6 in cancer progression and therapeutic resistance. Tumour Biol, 37(9), 11553–11572. doi: 10.1007/s13277-016-5098-7 [DOI] [PubMed] [Google Scholar]
- Latge G, Poulet C, Bours V, Josse C, & Jerusalem G (2018). Natural Antisense Transcripts: Molecular Mechanisms and Implications in Breast Cancers. Int J Mol Sci, 19(1). doi: 10.3390/ijms19010123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis AM, Varghese S, Xu H, & Alexander HR (2006). Interleukin-1 and cancer progression: the emerging role of interleukin-1 receptor antagonist as a novel therapeutic agent in cancer treatment. J Transl Med, 4, 48. doi: 10.1186/1479-5876-4-48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Y, Smyth GK, & Shi W (2019). The R package Rsubread is easier, faster, cheaper and better for alignment and quantification of RNA sequencing reads. Nucleic Acids Res. doi: 10.1093/nar/gkz114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling X, & Arlinghaus RB (2005). Knockdown of STAT3 expression by RNA interference inhibits the induction of breast tumors in immunocompetent mice. Cancer Res, 65(7), 2532–2536. doi: 10.1158/0008-5472.CAN-04-2425 [DOI] [PubMed] [Google Scholar]
- Liu S, Lee JS, Jie C, Park MH, Iwakura Y, Patel Y, … Chen H (2018). HER2 Overexpression Triggers an IL1alpha Proinflammatory Circuit to Drive Tumorigenesis and Promote Chemotherapy Resistance. Cancer Res, 78(8), 2040–2051. doi: 10.1158/0008-5472.CAN-17-2761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller FR, Santner SJ, Tait L, & Dawson PJ (2000). MCF10DCIS.com xenograft model of human comedo ductal carcinoma in situ. J Natl Cancer Inst, 92(14), 1185–1186. [DOI] [PubMed] [Google Scholar]
- Mori T, Miyamoto T, Yoshida H, Asakawa M, Kawasumi M, Kobayashi T, … Yoshimura A (2011). IL-1beta and TNFalpha-initiated IL-6-STAT3 pathway is critical in mediating inflammatory cytokines and RANKL expression in inflammatory arthritis. Int Immunol, 23(11), 701–712. doi: 10.1093/intimm/dxr077 [DOI] [PubMed] [Google Scholar]
- Morlando M, Ballarino M, & Fatica A (2015). Long Non-Coding RNAs: New Players in Hematopoiesis and Leukemia. Front Med (Lausanne), 2, 23. doi: 10.3389/fmed.2015.00023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson AC, Machado HL, & Schwertfeger KL (2018). Breaking through to the Other Side: Microenvironment Contributions to DCIS Initiation and Progression. J Mammary Gland Biol Neoplasia, 23(4), 207–221. doi: 10.1007/s10911-018-9409-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh K, Lee OY, Park Y, Seo MW, & Lee DS (2016). IL-1beta induces IL-6 production and increases invasiveness and estrogen-independent growth in a TG2-dependent manner in human breast cancer cells. BMC Cancer, 16(1), 724. doi: 10.1186/s12885-016-2746-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang JB, Savas P, Fellowes AP, Mir Arnau G, Kader T, Vedururu R, … Fox SB (2017). Breast ductal carcinoma in situ carry mutational driver events representative of invasive breast cancer. Mod Pathol, 30(7), 952–963. doi: 10.1038/modpathol.2017.21 [DOI] [PubMed] [Google Scholar]
- Pertea M, Kim D, Pertea GM, Leek JT, & Salzberg SL (2016). Transcript-level expression analysis of RNA-seq experiments with HISAT, StringTie and Ballgown. Nat Protoc, 11(9), 1650–1667. doi: 10.1038/nprot.2016.095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risso D, Ngai J, Speed TP, & Dudoit S (2014). Normalization of RNA-seq data using factor analysis of control genes or samples. Nat Biotechnol, 32(9), 896–902. doi: 10.1038/nbt.2931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MD, McCarthy DJ, & Smyth GK (2010). edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics, 26(1), 139–140. doi: 10.1093/bioinformatics/btp616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segatto I, Baldassarre G, & Belletti B (2018). STAT3 in Breast Cancer Onset and Progression: A Matter of Time and Context. Int J Mol Sci, 19(9). doi: 10.3390/ijms19092818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder M, Huang XY, & Zhang JJ (2011). Signal transducers and activators of transcription 3 (STAT3) directly regulates cytokine-induced fascin expression and is required for breast cancer cell migration. J Biol Chem, 286(45), 38886–38893. doi: 10.1074/jbc.M111.286245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solin LJ, Gray R, Baehner FL, Butler SM, Hughes LL, Yoshizawa C, … Badve S (2013). A multigene expression assay to predict local recurrence risk for ductal carcinoma in situ of the breast. J Natl Cancer Inst, 105(10), 701–710. doi: 10.1093/jnci/djt067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soule HD, Maloney TM, Wolman SR, Peterson WD Jr., Brenz R, McGrath CM, … Brooks SC (1990). Isolation and characterization of a spontaneously immortalized human breast epithelial cell line, MCF-10. Cancer Res, 50(18), 6075–6086. [PubMed] [Google Scholar]
- Su X, Malouf GG, Chen Y, Zhang J, Yao H, Valero V, … Esteva FJ (2014). Comprehensive analysis of long non-coding RNAs in human breast cancer clinical subtypes. Oncotarget, 5(20), 9864–9876. doi: 10.18632/oncotarget.2454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tominaga-Yamanaka K, Abdelmohsen K, Martindale JL, Yang X, Taub DD, & Gorospe M (2012). NF90 coordinately represses the senescence-associated secretory phenotype. Aging (Albany NY), 4(10), 695–708. doi: 10.18632/aging.100497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Lee H, Herrmann A, Buettner R, & Jove R (2014). Revisiting STAT3 signalling in cancer: new and unexpected biological functions. Nat Rev Cancer, 14(11), 736–746. doi: 10.1038/nrc3818 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Yang C, Zhang M, Liu H, Gong C, Zhang J, … Li Y (2017). Interleukin enhancer-binding factor 3 and HOXC8 co-activate cadherin 11 transcription to promote breast cancer cells proliferation and migration. Oncotarget, 8(64), 107477–107491. doi: 10.18632/oncotarget.22491 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Characterization of BHLHE40-AS1. Panel A: Schematic representation of BHLHE40-AS1 with associated UCSC Genome Browser tracks depicting mammalian conservation, vertebrate conservation and repeat elements. Panel B: The transcript cloned from RACE was aligned to the genome using UCSC BLAT tool demonstrating an elongated 5’ end. To determine if this transcript is found in additional backgrounds, GRO-seq (Global Run-On Sequencing) data from MCF7 cells (SRR816998, SRR816995, SRR604586) was analyzed. Peaks on the negative strand indicate this extended transcript is present in other genetic backgrounds. Panel C: Biotinylated probes tiling BHLHE40 were incubated with crosslinked MCF10A-DCIS whole cell lysate. Streptavidin beads were used to isolate target RNA. RNA was isolated and assessed for indicated genes by qRT-PCR. Three independent biological replicates were performed, one representative purification is shown. Panel D: RNA purification was performed as in panel C with crosslinked whole cell lysate from MCF10A-BHLHE40-AS1 cells. 4 independent biological replicates were performed, one representative experiment is shown. Panel E: 50nM of indicated siRNA were transfected into MCF10A-DCIS (left) or SUM225 (right) cells. Total RNA was collected 72h after transfection and assessed for expression of indicated genes. Bars represent mean ± SD of 3 independent biological replicates. * p<0.05, two-tailed Student’s t test.
Supplemental Figure 2. Characterization of BHLHE40-AS1. A) 2.0x105 MCF10A-pBABE and MCF10A-BHLHE40-AS1 cells were plated and imaged 24h post-seeding. Bar represents 200 μm.
Supplemental Figure 3. IL-6 expression in the MCF10A series. Panel A: Conditioned media from indicated cell lines was collected 48h post-seeding and IL-6 protein concentration was assessed by ELISA. Signal was normalized to total cell count. Bars indicate mean ± SD for 3 biological replicates. * p<0.05, two-tailed Student’s t test.
Supplemental Figure 4. Protein interaction partner identification. 10pmol synthesized biotinylated BHLHE40-AS1 or antisense control were incubated with 150 ug MCF10A-DCIS whole cell lysate and purified as described in methods. Associated proteins were separated by SDS-PAGE and visualized by colloidal coomassie staining.
Supplemental Videos. MCF10A-pBABE cells (Video 1) or MCF10A-BHLHE40-AS1 cells (Video 2) were grown to confluence, scratched with a pipette tip, and maintained in the EVOS Onstage Incubator for imaging every 30 min over 48 h. Videos are 1 image per hour for 48 images and stitched together at 5 frames per second.


