Abstract
The present study examined the acute effect of alcohol and its cues on autonomic and cardiovascular physiology, as indexed by changes in heart rate (HR), in a relatively large sample of healthy young adult men and women. Participants (27–31 years old, final n=145) were administered an alcoholic beverage (88 total, 52 women) or a placebo beverage (57 total, 35 women) in a simulated bar. Target breath alcohol concentration (BrAC) was .08 g%. HR was recorded while participants were seated alone during an initial baseline assessment in a lab room; seated with others during preparation and administration of two beverages in a simulated bar; and seated alone in the lab room at ascending, peak, and descending BrAC. HR increased over time for participants in both beverage groups during beverage preparation. During beverage consumption, HR decreased over time in those who drank placebo whereas HR increased over time in those who drank alcohol, increasing at a faster rate in women compared to men. HR remained elevated at the ascending, peak, and descending limb assessments only in participants who drank alcohol with HR increasing over time at ascending BrAC in the women but not men. Sex differences in HR under alcohol were not explained by sex differences in body mass index, BrAC, recent alcohol use, or subjective stimulation. Our findings suggest that women may be more sensitive to alcohol-induced increases in HR, especially in environments where alcohol cues are abundant. This may have implications for cardiovascular risks associated with alcohol.
Keywords: alcohol, autonomics, biological sex, cardiovascular, cues
Cardiovascular health risks1 associated with chronic alcohol use reported in epidemiological studies (Griswold et al., 2018; Ronksley, Brien, Turner, Mukamal, & Ghali, 2011; Wood et al., 2018) are believed to develop as a function of repeated exposure to the acute effects of alcohol ingestion on cardiovascular physiology. Acute consumption of alcohol at doses that produce peak blood alcohol concentration (BAC) ≥ 0.060 g% can decrease blood pressure, dilate arteries, decrease short- and long-term heart rate variability, increase heart rate (HR), increase cardiac output, and increase peripheral sympathetic nervous system outflow in healthy people tested under highly-controlled laboratory conditions (Bau, Bau, Naujorks, & Rosito, 2005; Bau et al., 2011; Brunelle & Pihl, 2007; Buckman et al., 2015; King, Houle, De Wit, Holdstock, & Schuster, 2002; Mayo & de Wit, 2016; Sher, Bartholow, Peuser, Erickson, & Wood, 2007; Spaak et al., 2008, 2010; Vaschillo et al., 2008). Nevertheless, the acute effects of alcohol on cardiovascular physiology in the natural environment may differ from those observed in the laboratory, at least in part due to the presence of alcohol-associated cues.
Like alcohol, acute exposure to alcohol-associated cues can affect cardiovascular physiology. In fact, discrete cues (e.g., beverage sight, smell, and taste cues) and contextual cues (e.g., place, time, and social cues) can have different acute effects. Specifically, discrete cues can increase HR whereas contextual cues can decrease HR (Dafters & Anderson, 1982; Macfarlane & White, 1989; McCaul, Turkkan, & Stitzer, 1989b, 1989a; Newlin, 1985, 1986; Shapiro & Nathan, 1986; Staiger & White, 1988, 1991). Moreover, discrete and contextual alcohol-associated cues have been shown to moderate the acute effects of alcohol in healthy people tested under highly-controlled laboratory conditions (McKay & Schare, 1999).
Characterizing the acute effects of alcohol and its cues on heart rate is critical for understanding the link between acute and chronic effects of alcohol use on cardiovascular health. Yet, much of the evidence for acute autonomic or cardiovascular effects of alcohol ingestion or alcohol cues is drawn from studies using either small or exclusively male samples. To address these issues, the present study characterized the acute effect of alcohol and its cues on autonomic and cardiovascular physiology, as indexed by changes in HR, in a relatively large sample of healthy, young adult men and women. The study represents primary analysis of HR data collected during a between-subjects, placebo-controlled alcoholic beverage administration experiment. This design is appropriate for the purpose of our study, as the expectation of receiving alcohol (both Told Alcohol/Get Alcohol and Told Alcohol/Get Placebo) is important for explaining individual differences in the acute effects of alcohol (Martin & Sayette, 1993).
In the current study, HR was measured while groups of participants watched beverages being prepared and while they consumed those beverages in a simulated bar room. HR also was measured while each participant was alone in a standard laboratory testing room before and after beverage administration. Thus, HR was monitored before, during, and after acute exposure to discrete and contextual cues with and without acute exposure to alcohol. This allowed each participant to serve as his or her own control for changes in HR under different contextual conditions. In addition, the acute effects of alcohol on HR are positively related to the stimulant-like, positive mood-inducing subjective effects of alcohol (Brunelle, Barrett, & Pihl, 2007; Conrod et al., 2001; King et al., 2002; Ray, McGeary, Marshall, & Hutchison, 2006), which can be amplified by social drinking contexts (for review, see: de Wit & Sayette, 2018) and specific factors such as the number and sex of drinking partners (e.g., Fairbairn et al., 2015; Sayette et al., 2012). Consequently, subjective feelings of stimulation were also assessed to determine whether these might contribute to changes in HR. Finally, given an emerging literature on biological sex differences in the acute effects of alcohol and its cues on other indices of autonomic and cardiovascular physiology (Bates et al., 2011; Chaplin, Hong, Bergquist, & Sinha, 2008; Hartwell & Ray, 2013; Kaplan et al., 1985; Rubonis et al., 1994; Udo et al., 2009), the present study also examined the role of biological sex as a factor in the acute effects of alcohol and its cues on HR.
Method
Participants
We re-recruited participants from a previous 6-year longitudinal study of changes in alcohol use and other behavioral risks among first-time college students (for more information, see: Fromme, Corbin, & Kruse, 2008). These former longitudinal participants were asked to provide salivary samples for DNA analysis in a study of the genetic determinants of drinking patterns and other behavioral risks. Those who provided salivary samples by May 2017 were invited to participate in a laboratory study on the effects of alcohol. Exclusion criteria included scoring > 16 on the Alcohol Use Disorder Identification Test (AUDIT) (Saunders, Aasland, Babor, De La Fuente, & Grant, 1993) to screen out individuals with possible undiagnosed alcohol dependence at the time of the laboratory study, having any medical or other contraindications to alcohol, including certain medications, and for women, pregnancy, nursing, or attempting to become pregnant. Alcohol use over the 30 days prior to the study session was assessed using a modified Time-Line Follow-Back (TLFB) (Sobell & Sobell, 1992).
Of 182 individuals who provided informed consent and participated in the laboratory study, 61 were randomly assigned to the placebo beverage group, and 121 were assigned to the alcohol beverage group. With a primary focus on the pharmacological effects of alcohol, a disproportionate number of participants were assigned to the alcohol beverage group. One individual in the alcohol group became nauseous and was unable to complete the study, reducing the sample size for this group to 120, and the overall laboratory study sample size to 181. Raw HR data from 33 of these participants were lost due to data storage system failures. Of the remaining 148 raw HR data records, 3 were excluded because they did not contain baseline HR data. Thus, the final sample for the present analyses consisted of 145 participants: 88 in the alcohol group (52 women) and 57 in the placebo group (35 women). Table 1 shows their basic biological and sociodemographic characteristics. Table 2 shows their AUDIT scores and recent alcohol use behavior.
Table 1.
Participant Biological and Sociodemographic Characteristics
| Characteristic | Alcohol (n = 88) | Placebo (n = 57) | ||
|---|---|---|---|---|
| Men (n = 36) | Women (n = 52) | Men (n = 22) | Women (n = 35) | |
| M (SD) | M (SD) | M (SD) | M (SD) | |
| Age (yr) | 28.42 (1.27) | 28.31 (0.98) | 27.91 (0.75) | 27.86 (0.69) |
| Height (m) | 1.80 (0.07) | 1.66 (0.06) | 1.76 (0.05) | 1.63 (0.06) |
| Weight (kg) | 81.39 (12.75) | 66.16 (12.84) | 86.17 (19.98) | 64.10 (12.41) |
| BMI (kg/m2) | 25.10 (3.69) | 24.11 (4.58) | 27.95 (6.64) | 24.14 (4.32) |
| n (%) | n (%) | n (%) | n (%) | |
| Ethnicity | ||||
| Hispanic | 5 (14) | 12 (23) | 3 (14) | 9 (26) |
| Race | ||||
| AI/AN | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Asian | 6 (17) | 7 (14) | 4 (18) | 8 (23) |
| Black | 2 (5) | 2 (4) | 0 (0) | 0 (0) |
| White | 22 (61) | 34 (65) | 13 (59) | 18 (51) |
| Multiple | 6 (17) | 9 (17) | 5 (23) | 9 (26) |
| Yearly Income | ||||
| < 60, 000 USD/yr | 23 (64) | 35 (67) | 12 (55) | 21 (60) |
Note. BMI stands for Body Mass Index. AI stands for American Indian. AN stands for Alaska Native. USD stands for U.S. Dollars. There were no beverage group or sex differences in the distributions of ethnicity, race, or income. There were no differences in age, height, weight, or BMI between beverage groups. There were sex differences in height, weight, and BMI, such that values were higher in men, ts ≥ 2.98, ps < .05, ds ≥ .50.
Table 2.
Participant AUDIT Scores and Alcohol Use Over Past 30 Days
| Variable | Alcohol (n = 88) | Placebo (n = 57) | ||
|---|---|---|---|---|
| Men (n = 36) | Women (n = 52) | Men (n = 22) | Women (n = 35) | |
| M (SD) | M (SD) | M (SD) | M (SD) | |
| AUDIT | 6.83 (2.85) | 5.78 (3.10) | 6.04 (4.07) | 4.88 (2.46) |
| Number of days used any alcohol | 13.25 (8.77) | 11.51 (7.07) | 12.77 (8.51) | 11.00 (6.55) |
| Number of standard drinks per drinking day | 3.41 (1.56) | 2.46 (1.01) | 3.20 (1.78) | 2.61 (1.45) |
| Maximum number of standard drinks consumed in one occasion | 7.68 (4.13) | 4.98 (2.85) | 7.23 (4.76) | 5.14 (3.11) |
| Number of binge drinking episodes | 2.92 (3.47) | 2.67 (4.33) | 3.68 (5.32) | 1.94 (2.48) |
Note. Raw scores from the Alcohol Use Disorder Identification Test (AUDIT) are presented (Saunders, Aasland, Babor, De La Fuente, & Grant, 1993). Alcohol use variables were derived from a Time-Line Follow-Back (TLFB) procedure (Sobell & Sobell, 1992). There were no differences between beverage groups. There were sex differences in the number of standard drinks per drinking day and the maximum number of standard drinks consumed in one occasion such that these were higher in men, ts ≥ 2.13, ps < .05, ds ≥ .36
Materials
Breath alcohol concentration (BrAC) measurement.
BrAC readings were taken every 10 min after beverage consumption and absorption using Alco-Sensor IV breathalyzers (Intoximeters, Inc.; St. Louis, Missouri, USA). For the present study, we obtained each participant’s first post-absorption BrAC reading as well as their reading immediately preceding planned assessment of various constructs on the ascending limb, peak, and descending limb of the BrAC timecourse.
HR measurement.
HR was measured in beats per minute (bpm) using Polar RS800CX HR monitors (Polar Electro Inc., Bethpage, NY). Participants were shown how to attach the HR monitor chest strap to their bodies, and then allowed to do so in private. They were also asked to wear the wristwatch that controlled the HR monitor, but the wristwatch was operated by the research assistants (RA) at various points through the study to start and stop HR monitoring.
Each time HR was assessed in the standard lab room, the RA started a slideshow of nature scenes on the computer monitor and started the HR monitor before leaving the room. During this time, participants passively observed the slideshow while seated alone in their testing rooms. RAs returned after 5 min to stop the HR monitor. Data before minute mark 1:00 contained the clear rise and fall of the cardiac orienting response. HR data from minute mark 1:00 until 5:00 (4 min total) in each recording period were aggregated to obtain 4 average HR measurements (1 minute each) within each assessment (baseline, ascending, peak, descending) for each participant.
To assess HR during the dosing phase, RAs started the HR monitors immediately before opening the door to the simulated bar room and stopped the HR monitor 30 min later, upon conclusion of beverage consumption. During the first 10 min of the dosing phase, participants entered the bar, took their seats, listened to the bartender RA deliver instructions, and observed preparation of the first purportedly alcoholic beverages. During the next 10 min, participants consumed their first beverage and observed the second beverage being prepared. Participants consumed the second beverage during the third 10 min. Data before minute mark 2:00 were characterized by the rise and fall of a large, clearly movement-related artifact. HR data from minute mark 2:00 until 30:00 (28 min total) were aggregated to obtain 28 average HR measurements (1 minute each) across the dosing phase in the bar room for each participant.
Placebo manipulation check.
At the beginning of the planned assessment on the ascending limb of the BrAC curve, participants responded to the following item: “Research experiments do not always use the same standard servings as those typically used at bars, restaurants, or parties. Please estimate the number of standard alcoholic drinks you were served during this experiment. One standard drink was defined as 1.5 ounces of liquor in a mixed drink.
Subjective stimulation measurement.
Participants completed the 14-item Subjective Effects of Alcohol Scale (SEAS; Morean, Corbin, & Treat, 2013) at the baseline, ascending, peak, and descending assessments. Since alcohol-induced increases in HR appear to be linked to the positive stimulant-like subjective effects of alcohol (e.g., Conrod, Peterson, & Pihl, 2001), the High Arousal Positive Valence subscale of the SEAS was scored to capture changes in subjective stimulation across the experiment.2 The subscale consists of 4 items (“lively”; “fun”; “funny”; “talkative”). Participants rated the extent to which they were currently experiencing these feelings using a Likert scale ranging from “Not at All” (0) to “Extremely” (10). In their 2013 article, Morean, Corbin & Treat demonstrated high internal reliability (α = .93-.94) for the High Arousal Positive Valence subscale on the ascending and descending limbs of the BrAC timecourse as well as strong measurement invariance for the SEAS across beverage condition (alcohol/placebo) and limbs of the BrAC timecourse. This means that the High Arousal Positive Valence subscale scores can be expected to measure the same underlying factor (i.e., subjective stimulation) when collected repeatedly within an experimental session, to measure the factor equally well over the session, and to measure the factor equally well in alcohol and placebo beverage conditions.
Procedure
Participants were asked to refrain from drinking alcohol for 24 hours and from eating for at least 4 hours before coming to the laboratory. Figure 1 depicts the timeline of laboratory procedures. Upon arrival, participants provided informed consent and were screened for 0.00 g% BrAC. Female participants were also screened for pregnancy using standard urine hCG tests. Next, RAs recorded the participant’s height and weight for calculation of beverage doses. Each participant was then escorted into an individual standard laboratory testing room with a chair and a computer station. Baseline assessments were completed, which included HR and subjective stimulation measurement, and measures not reported here. An individualized bodyweight-adjusted caloric snack of pretzels was provided to control stomach contents prior to beverage administration.
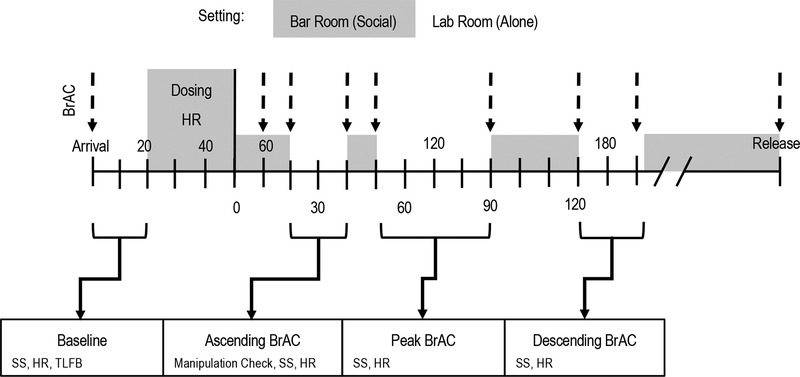
Figure 1.
Timeline of within-session events. “Arrival” indicates completion of certain procedures in the lab hallway lobby area (e.g., consent form, height and weight measurement). Shading indicates times after “arrival” when each participant was in the simulated bar room alongside other participants or confederates. Lack of shading indicates times after “arrival” when each participant was alone in the standard lab room. Breath alcohol concentration (BrAC) was measured approximately every 10 min. BrAC measurements are represented using dashed lines with an arrowhead. Targets for ascending, peak, and descending BrAC assessments were 0.06 g%, 0.08 g%, and 0.06 g%, respectively. Subjective stimulation (SS) was measured using the High Arousal Positive Valence subscale of the Subjective Effects of Alcohol Scale (Morean, Corbin, & Treat, 2013). Heart rate (HR) was measured in beats per minute (bpm). HR in the lab room was measured over 5 min. HR in bar room was measured over 30 min. Recent alcohol use was assessed at baseline using an assisted timeline followback (TLFB) procedure (Sobell & Sobell, 1992). The manipulation check item asked participants to estimate the number of standard drink equivalents they consumed in the simulated bar room. Numbers above the number line indicate time since arrival in minutes. Numbers below the number line indicate time after dosing in minutes.
Each participant attended a single double-blind laboratory session where they were randomly assigned to an alcohol or placebo condition. None of the RAs (bartenders or testers) were aware of the participants’ beverage condition, and a separate RA conducted the breathalyzer tests. As shown in Figure 1, beverage preparation and consumption occurred in a simulated bar room in a small group setting (see Dosing below for details). At different times post-dosing, each participant was escorted into a standard laboratory testing room for individual assessments.
Dosing.
Beverage administration took place in a simulated bar room in variable-sex-composition groups of 3 to 5 participants randomly assigned to the same condition (alcohol or placebo). As needed, RAs served as confederates to meet the desired minimum group size of 3 individuals. Confederates were trained to help maintain a similarly pleasant social milieu across sessions. Confederates always consumed placebo but underwent all procedures.
Participants had 20 min to consume 2 drinks of equal volume containing 1 part vodka (40% ethanol v/v)—or decarbonated tonic water as vodka placebo—and 3 parts mixer. The mixer was 5 parts cranberry juice, 4 parts diet cherry soda, and 0.5 parts lime juice. Participants were instructed to consume each drink at an even pace, such that they finished each drink within 10 minutes. The bartender RA monitored consumption. Alcohol doses were calculated based on the participants’ age, sex, weight, and height using the methods of Curtin and Fairchild (2003) to target a peak BrAC of .08 g%.
Standard procedures were followed to enhance the effectiveness of the placebo manipulation (Rohsenow & Marlatt, 1981). All participants were informed that they would receive alcohol, with doses not to exceed 0.08 g%. All beverages were prepared in full view of the participants by an RA who served as a bartender, with the vodka or decarbonated tonic poured from re-sealed, and therefore ostensibly unopened, vodka bottles. To provide alcohol smell and taste cues, glasses were chilled and rimmed with vodka, and a squirt of 95% alcohol was added to the top of each beverage. Immediately prior to participants entering the barroom for the first time, the bar countertop was wiped with tequila to provide ambient alcohol olfactory cues. The barroom was dimly lit, with neon signs and other typical barroom cues, and contemporary music was played. Sessions were conducted on Friday and Saturday evenings, times during which alcohol is typically consumed by young adults (e.g., Lau-Barraco, Braitman, Linden-Carmichael, & Stamates, 2016; Maggs, Williams, & Lee, 2011). Prior to the first breathalyzer test following consumption, participants rinsed with alcohol-free mouthwash to clear any remaining mouth alcohol. Participants randomized to the alcohol condition were provided accurate BrAC feedback. Participants randomized to placebo were provided bogus BrAC feedback (0.04–0.05 g% for ascending/descending, 0.06–0.08 g% for peak).
Ethics.
All procedures were approved by the University of Texas Institutional Review Board and complied with American Psychological Association ethical standards and guidelines for human alcohol administration provided by the National Institute on Alcohol Abuse and Alcoholism.
Data Analysis Plan
Analyses, data preparation, and visualization were done in R version 3.6.0 (R Core Team, 2018) using packages car (Fox & Weisberg, 2019), emmeans (Lenth, 2019), ggplot2 (Wickham, 2009), and lme4 (Bates, Mächler, Bolker, & Walker, 2015). Placebo manipulation check data were evaluated using two independent samples t-tests. BrAC and subjective stimulation data were evaluated using a repeated measures analysis of variance (ANOVA). HR data were analyzed using linear mixed effect models (LMM), which is synonymous with mixed effects linear regression (MELR), and equivalent to a multilevel model (MLM) or hierarchical linear model (HLM) with repeated measures at level 1 and persons at level 2. We chose LMM analysis for the HR data because we wanted to make inferences about beverage and biological sex-related effects based on data from as many persons as possible, despite incomplete data for some persons in some experiment phases; traditional repeated-measures ANOVA requires listwise deletion of cases with missing data for any phases. Compared to traditional repeated-measures ANOVA, LMM are better able to account for the high degree of dependency among observations nested within persons, decreasing the likelihood of spurious (false positive) statistical findings (Aarts, Verhage, & Veenvliet, 2014). A separate LMM analysis was conducted for each experiment phase because HR was not monitored continuously across phases and we were interested in the effects of beverage group and biological sex (or their interaction) within each phase.
Regardless of experiment phase, HR LMM analyses proceeded the same way. We first fit HR as a function of Time (minute), including linear and quadratic components in the fixed effects part of the model and a random intercept term (capturing between-subject variation in the overall level of HR) in the random effects part of the model. Time was treated as a pseudo-continuous variable, and centered so that the first observation served as the intercept (time 0) for the model. Next, we tested whether more complex random effects would improve model fit: random intercepts plus random slopes on the linear component of Time (capturing between-subject variation in linear trend over time), random intercepts plus random slopes on the quadratic component of Time (capturing between-subject variation in the quadratic trend over time), and random intercepts plus random slopes on both linear and quadratic components of Time. We tried to retain the most complex random effects structure that would allow model convergence and improve model fit, in keeping with standard procedure for balancing statistical power and Type I error in mixed effects regression (Barr, Levy, Scheepers, & Tily, 2013; Matuschek, Kliegl, Vasishth, Baayen, & Bates, 2017; Page-Gould, 2019). We evaluated change in model fit using the X2 likelihood ratio test (LRT).
Having identified the best unconditional model of HR (viz., as a function of only Time-related fixed and random effects), we added all of our fixed effects of interest at once: the main effects of beverage group and biological sex and their interactions, as well as interactions with the linear and quadratic components of Time. The Group factor was represented using a dummy coded variable (alcohol = 1). The Sex factor was also represented using a dummy coded variable (woman = 1). For the dosing, ascending, peak, and descending phase HR analyses, we also entered average HR across the baseline phase as a centered continuous covariate (Baseline Resting HR) as well as its interaction with the linear and quadratic components of Time, in order to control for between-person differences in resting HR and the potential association between resting HR and HR change over time. We then evaluated the contribution of each fixed effect of interest to model fit using X2 LRT, and removed non-contributing predictors until we arrived at the best conditional model of HR. Finally, model-estimated means (and SEs) were obtained for visualization.
Results
Placebo check
The estimated number of standard drinks consumed in the placebo group was significantly greater than zero, M (SD) = 2.19 (1.16), t (56) = 14.32, p < .001, d = 3.83, as was the estimated number in the alcohol group, M (SD) = 2.82 (3.32), t (87) = 22.60, p < .001, d = 4.84.
BrAC
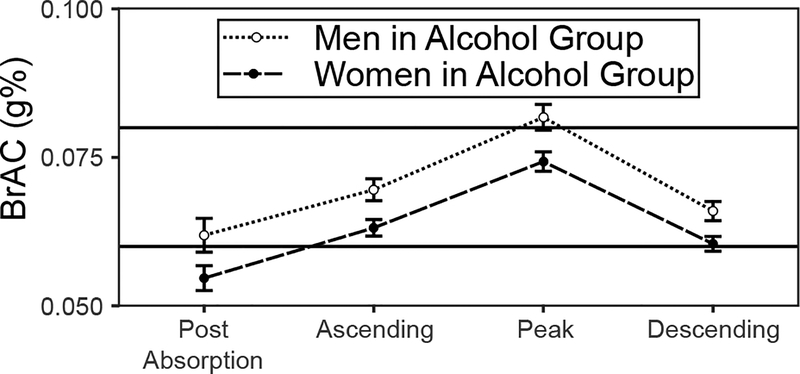
ANOVA detected a significant main effect of sex, F (1, 86) = 8.15, p = .005, ηp2 = .087, and a significant main effect of time, F (3, 258) = 59.96, p < .001, ηp2 = .411, but no Sex × Time interaction, F (3, 258) = 0.377, ηp2 = .005. Controlling for time, BrAC was lower in women compared to men, t (86) = −2.85, p = .005, d = .62. Controlling for sex, BrAC increased from the post-absorption to ascending limb reading, t (86) = 6.27, p < .001, d = 1.35, increased again from the ascending limb to peak reading, t (86) = 11.95, p < .001, d = 2.58, and decreased from the peak to descending limb reading, t (86) = −18.06, p < .001, d = 3.89. Model-estimated means and standard errors are shown in Figure 2.
Figure 2.
Model-estimated BrAC M ± SE across readings. Horizontal lines at .06 g% and .08 g% represent targets for ascending/descending and peak BrAC.
HR
Baseline HR assessed in the standard lab room.
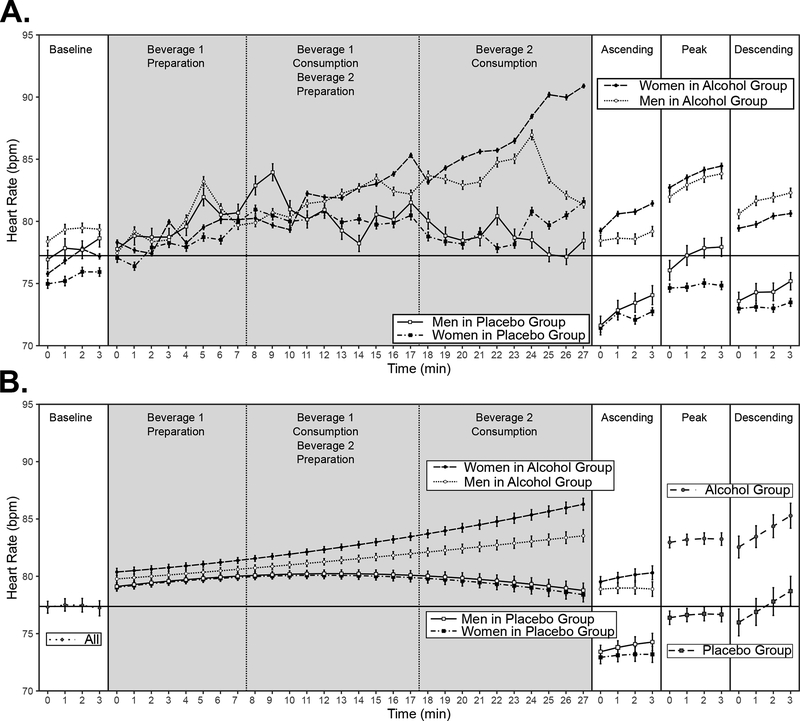
The best unconditional model was one containing the linear and quadratic components of time as well as the random intercept and both random slope terms, Fixed Effects R2 = .00, Total R2 = .98. The best conditional model was ultimately identical to the best unconditional model. All terms involving group and sex were dropped without loss of model fit, LRT X2 (9) = 7.37, p = .60. Only the linear component of time, b ± SE = 1.12 ± .24, t (143) = 4.65, p < .001, and the quadratic component of time, b ± SE = −.24 ± .08, t (143) = −2.98, p < .001, remained in the fixed effects part of the model. Sample means and standard errors are shown in the Baseline phase portion of Figure 3A. Means and standard errors estimated from the best conditional model are shown in the Baseline phase portion of Figure 3B.
Figure 3.
Heart rate (HR) across experiment phases. A-B: Horizontal line represents the average Baseline HR across Group and Sex. Solid vertical lines indicate boundaries of each experiment phase. Dashed vertical lines indicate boundaries between events of interest inside the dosing phase. Gray plot area indicates participants were in the bar room (social). White plot area indicates participants were in the lab room (alone). A: Sample mean and standard error for each experiment phase. B: Means and standard errors for each experiment phase estimated from the best statistical model. Best model for baseline phase did not include Group or Sex effects. Best models for dosing and ascending phases included both Group and Sex effects. Best models for peak and descending phases included Group effects, but not Sex effects. Means and standard errors for dosing, ascending, peak, and descending phases are estimated while controlling for between-person differences in Baseline HR.
HR across beverage preparation and consumption in the bar room.
The best unconditional model was one containing the linear and quadratic components of time as well as the random intercept and a random slope term for linear time. The best conditional model contained the following significant interactions: Group × Sex × quadratic Time, Group × quadratic Time, Sex × quadratic Time, Group × linear Time, and Baseline HR × linear Time. The two models are presented in Table 3. Sample means and SEs are shown in the Dosing phase portion of Figure 3A. Means and standard errors estimated from the best conditional model are shown in the Dosing phase portion of Figure 3B.
Table 3.
Regression output from best unconditional and conditional multi-level models of heart rate (HR, beats per minute) across 28-minute Dosing phase in simulated bar room (social)
| Dosing HR unconditional | Dosing HR conditional | |||||||
|---|---|---|---|---|---|---|---|---|
| Fixed Effects | b | SE | t | p | b | SE | t | p |
| (Intercept) | 77.984 | 0.960 | 81.210 | <0.001 | 78.192 | 0.453 | 172.450 | <0.001 |
| linear Time | 0.310 | 0.042 | 7.445 | <0.001 | 0.383 | 0.057 | 6.696 | <0.001 |
| quadratic Time | −0.004 | 0.001 | −2.985 | 0.003 | −0.014 | 0.002 | −6.203 | <0.001 |
| Baseline HR | 0.805 | 0.035 | 23.301 | <0.001 | ||||
| linear Time × Baseline HR | −0.004 | 0.002 | −2.653 | 0.008 | ||||
| linear Time × Group | −0.164 | 0.072 | −2.257 | 0.024 | ||||
| quadratic Time × Group | 0.016 | 0.003 | 5.382 | <0.001 | ||||
| quadratic Time × Sex | −0.001 | 0.002 | −0.335 | 0.738 | ||||
| quadratic Time × Group × Sex | 0.006 | 0.003 | 2.174 | 0.030 | ||||
| Random Effects | ||||||||
| Residual Error Variance | 17.44 | 17.14 | ||||||
| Random Intercept SD | 127.70person | 24.60person | ||||||
| Random Slope SD | 0.08linear Time | 0.05linear Time | ||||||
| Random Intercept-Slope Correlation | −0.28 | −0.19 | ||||||
| Intraclass Correlation | 0.88 | 0.64 | ||||||
| n | 144person | 144person | ||||||
| Observations | 3778 | 3778 | ||||||
| Fixed Effects R2 / Total R2 | 0.019 / 0.878 | 0.665 / 0.879 | ||||||
Note. Time was measured in minutes. Time = 0 represents the first of the twenty-eight minutes. Group = 1 indicates alcohol beverage group. Sex = 1 indicates woman. Likelihood ratio X2 tests indicated that each fixed effect term shown above was a significant contributor to model fit.
The trends illustrated in Figure 3B indicated that HR increased across beverage preparation and consumption in the bar for men and women alike in the alcohol group, but at an apparently faster rate in women compared to men. In the placebo group, HR increased at first (while observing beverage 1 preparation), but then decreased across the remainder of time in the bar and at the same rate for men and women. Simple slopes analysis confirmed these patterns. In the placebo group, the simple slope of linear time was positive (b ± SE = .384 ± .057, asymptotic z = 6.71, p < .001), and the simple slope of quadratic time was negative for both men (b ± SE = −.0141 ± .002, asymptotic z = 6.20, p < .001) and women (b ± SE = −.0148 ± .002, asymptotic z = −7.19, p < .001), and did not differ between them: mean difference ± SE = .001 ± .002, asymptotic z = 0.33, p = .737. In the alcohol group, the simple slope of linear time was positive (b ± SE = .220 ± .049, asymptotic z = 4.47, p < .001), and the simple slope of quadratic time was positive in women (b ± SE = .007 ± .002, asymptotic z = 4.00, p < .001), but not significantly different from zero in men (b ± SE = .002 ± .002, asymptotic z = 1.00, p = .319), such that the simple slope of quadratic time in the alcohol group was significant in women compared to men: mean difference ± SE = .005 ± .002, asymptotic z = 2.93, p = .003.
Moderator analyses.
Since there were differences between the sexes in body mass index (Table 1) and alcohol use (Table 2), we tested the ability of those differences to moderate sex differences in HR. Neither the BMI nor alcohol use moderation terms improved model fit, LRT ps > .189. There were also differences in BrAC between men and women in the alcohol group (Figure 2), which also could explain the sex-related effects on HR. In order to test for moderation of sex-related effects on HR by initial post-dosing BrAC reading, we fit a separate set of models using only bar room HR data from the alcohol group. The best conditional model of these data contained a significant Sex × quadratic Time interaction. Model fit was improved by introducing the moderation effect by BrAC, LRT X2 (2) = 12.82, p = .002. In order to understand the moderating effect of BrAC, we computed the simple slopes of quadratic time for each sex while holding BrAC at its median (.06 g%) or 25% above or below median (.07 and .05 g%, respectively), and tested for a difference between the sexes in the simple slope at each level of BrAC. At 25% below median BrAC, the simple slope of quadratic time was positive in women (b ± SE = .007 ± .002, t (1131) = 3.13, p = .002) and not significantly different from zero in men (b ± SE = −.003 ± .003, t (553) = −1.31, p = .192) such that the simple slope was significantly greater in women compared to men: mean difference ± SE = .010 ± .003, t (167) = 3.85, p < .001. At the median BrAC, the simple slope of quadratic time was positive in women (b ± SE = .008 ± .002, t (1686) = 3.82, p < .001) and not significantly different from zero in men (b ± SE = .000 ± .002, t (1058) = 0.05, p = .963) such that the simple slope was significantly greater in women compared to men: mean difference ± SE = .008 ± .002, t (166) = 3.61, p < .001. At 25% above median BrAC, the simple slope of quadratic time was positive in women (b ± SE = .008±.002, t (756) = 3.56, p < .001) and not significantly different from zero in men (b ± SE = .003 ± .002, t (1041) = 1.49, p = .136), such that the simple slope was significantly greater in women compared to men: mean difference ± SE = .005 ± .002, t (166) = 2.10, p = .038. Thus, at all levels of BrAC, HR accelerated faster in women than men, although the size of this sex difference was slightly diminished at higher BrAC.
Finally, given that the number and sex of drinking partners can influence subjective stimulation when alcohol is consumed (e.g., Fairbairn et al., 2015; Sayette et al., 2012), and that there is a link between alcohol-induced changes in subjective stimulation and HR (e.g., Conrod, Peterson, & Pihl, 2001), we also tested for the ability of the number and sex of drinking partners as well as subjective stimulation change scores (ascending – baseline) to moderate group- and sex-related effects on HR. Moderation terms involving social factors and subjective stimulation alike failed to improve model fit, LRT ps > 0.152. This was also the case when we tested sex difference-moderation effects in the alcohol group alone, LRT ps> .365.
HR at ascending assessment in the standard lab room.
The best unconditional model was one containing the linear and quadratic components of time as well as the random intercept and both random slope terms. The best conditional model contained a significant Group × Sex × linear Time interaction. The two models are presented in Table 4. Sample means and standard errors are shown in the Ascending phase portion of Figure 3A. Means and standard errors estimated from the best conditional model are shown in the Ascending phase portion of Figure 3B.
Table 4.
Regression output from best unconditional and conditional multi-level models of heart rate (HR, beats per minute) across 4-minutes in the standard lab room (alone) at ascending limb assessment
| Ascending HR unconditional | Ascending HR conditional | |||||||
|---|---|---|---|---|---|---|---|---|
| Fixed Effects | b | SE | t | p | b | SE | t | p |
| (Intercept) | 76.080 | 1.127 | 67.509 | <0.001 | 72.344 | 1.139 | 63.526 | <0.001 |
| linear Time | 0.780 | 0.286 | 2.730 | 0.006 | 1.028 | 0.355 | 2.893 | 0.004 |
| quadratic Time | −0.093 | 0.085 | −1.099 | 0.272 | −0.093 | 0.085 | −1.099 | 0.272 |
| Baseline HR | 0.771 | 0.054 | 14.212 | <0.001 | ||||
| Group | 6.156 | 1.458 | 4.224 | <0.001 | ||||
| linear Time × Sex | −0.390 | 0.293 | −1.331 | 0.183 | ||||
| linear Time × Group | −0.558 | 0.296 | −1.888 | 0.059 | ||||
| linear Time × Group × Sex | 0.348 | 0.241 | 1.447 | 0.148 | ||||
| Random Effects | ||||||||
| Residual Error Variance | 2.73 | 2.73 | ||||||
| Random Intercept SD | 181.56person | 72.00person | ||||||
| Random Slope 1 SD | 5.16linear Time | 5.00linear Time | ||||||
| Random Slope 2 SD | 0.36quadratic Time | 0.36quadratic Time | ||||||
| Random Intercept-Slope 1 Correlation | 0.02 | −0.18 | ||||||
| Random Intercept-Slope 2 Correlation | −0.15 | 0.10 | ||||||
| Intraclass Correlation | 0.98 | 0.96 | ||||||
| n | 145person | 145person | ||||||
| Observations | 580 | 580 | ||||||
| Fixed Effects R2 / Total R2 | 0.002 / 0.985 | 0.601 / 0.985 | ||||||
Note. Time was measured in minutes. Time = 0 represents the first of the four minutes. Group = 1 indicates alcohol beverage group. Sex = 1 indicates woman. Likelihood ratio X2 tests indicated that each fixed effect term shown above was a significant contributor to model fit.
It is visible in Figure 3B that HR was overall higher in the alcohol compared to placebo group controlling for baseline HR, in keeping with the significant group beta estimate in Table 4. The trends illustrated in Figure 3B also indicate, however, that whereas women’s HR in the alcohol group increased over the course of the ascending limb assessment, men in the alcohol group showed no such increase. Placebo group HR increased over the course of the ascending limb assessment, but similarly for men and women. This was confirmed by simple slopes analysis. In the placebo group, the simple slope of linear time was positive in men (b ± SE = 1.03 ± .36, t (245) = 2.88, p = .004) and women (b ± SE = .64 ± .33, t (215) = 1.95, p = .053), and did not differ between them: mean difference ± SE = .39 ± .30, t (141) = 1.31, p = .192. In the alcohol group, the simple slope of linear time was positive in women (b ± SE = .99 ± .31, t (187) = 3.20, p = .002), but not significantly different from zero in men (b ± SE = .47 ± .32, t (212) = 1.44, p = .150), such that the simple slope was significantly greater in women compared to men: mean difference ± SE = .52 ± .24, t (141) = 2.18, p = .031.
Moderator analyses.
As with the bar room HR data, we tested potential moderators of group- and sex effects on HR at the ascending assessment in the standard lab room. Neither the BMI nor alcohol use moderation terms improved fit, LRT ps > .124. Moderation terms involving social factors and/or subjective stimulation also failed to improve fit, LRT ps > .116. We also re-analyzed ascending phase HR data from the alcohol group alone. The best conditional model for these data contained a significant Sex × linear Time interaction, and its fit was not improved by introducing the moderation effects of ascending BrAC, social factors, or subjective stimulation, LRT ps > .309.
HR at peak assessment in the standard lab room.
The best unconditional model was one containing the linear and quadratic components of Time, the random intercept term, and a random slope term for quadratic time, Fixed Effects R2 = .00, Total R2 = .98. The best conditional model, Fixed Effects R2 = .58, Total R2 = .98, was one containing the linear component of time, b ± SE = .91 ± .22, t (287) = 4.15, p < .001, the quadratic component of time, b ± SE = −.14 ± .07, t (374) = −1.97, p = .049, the main effect of group, b ± SE = 6.57 ± 1.49, t (141) = 4.40, p < .001, and the main effect of baseline resting HR, b ± SE = .76 ± .06, t (141) = 13.26, p < .001. Sample means and standard errors are shown in the Peak phase portion of Figure 3A. Means and standard errors estimated from the best conditional model are shown in the Peak phase portion of Figure 3B. It is visible in Figure 3B that HR was overall higher in the alcohol compared to placebo group controlling for baseline HR, in line with the significant main effect of group.
HR at descending assessment in the standard lab room.
The best unconditional model was one containing only the linear component of time, the random intercept term, and a random slope term for linear time, Fixed Effects R2 = .00, Total R2 = .98. The best conditional model, Fixed Effects R2 = .56, Total R2 = .98, was one containing the linear component of time, b ± SE = .39 ± .08, t (142) = 4.91, p < .001, the main effect of group, b ± SE = 6.24 ± 1.47, t (140) = 6.42, p < .001, and the main effect of baseline resting HR, b ± SE = .73 ± .06, t (140) = 12.79, p < .001. Sample means and standard errors are shown in the Descending phase portion of Figure 3A. Means and standard errors estimated from the best conditional model are shown in the Descending phase portion of Figure 3B. It is visible in Figure 3B that HR was overall higher in the alcohol compared to placebo group controlling for baseline HR, in line with the significant main effect of group.
Subjective stimulation
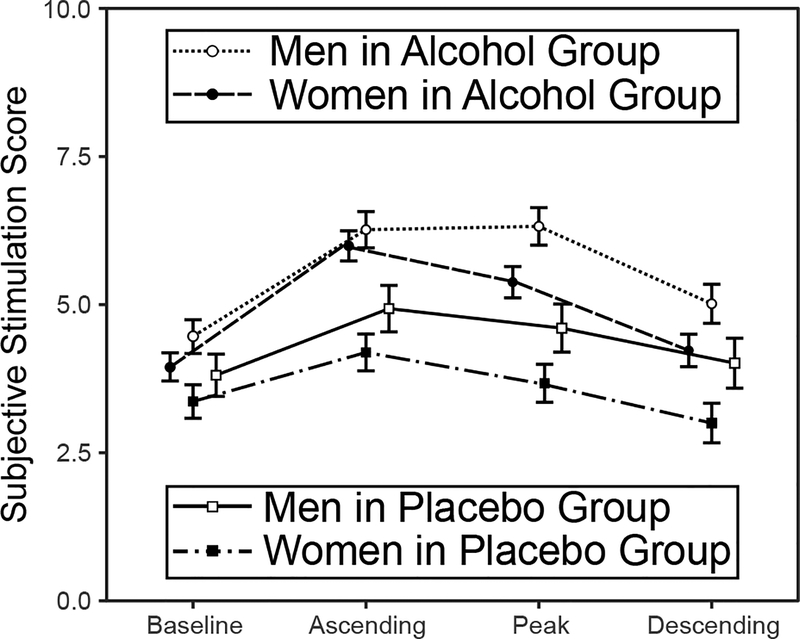
Given that there is a link between alcohol-induced changes in HR and subjective stimulation (e.g., Conrod, Peterson, & Pihl, 2001), one potential explanation for the sex differences in HR under alcohol observed here is that women may experience greater subjective stimulation from drinking alcohol in social contexts compared to men. Recently, Corbin, Scott, Boyd, Menary, & Enders (2015) found that young adult women drinking in mixed-sex groups in a simulated bar room reported higher levels of stimulation than women drinking in a standard lab room, and that across contexts, levels of stimulation reported by women (but not men) drinking alcohol were greater than counterparts drinking placebo. To address this possibility, we examined subjective stimulation levels for an interaction of beverage group and biological sex across the baseline, ascending, peak, and descending assessments in the standard lab room. ANOVA detected significant main effects of group, F (1, 141) = 8.62, p = .004, ηp2 = .058, sex, F (1, 141) = 3.75, p = .054, ηp2 = .026, and time, F (3, 423) = 3.64, p = .013, ηp2 = .025, but no significant interaction effects. Controlling for time and sex, stimulation was greater in the alcohol compared to placebo group, t (141) = 4.86, p < .001, d = 0.82. Controlling for time and group, stimulation was lower in women compared to men, t (141) = 2.74, p = .007, d = 0.46. Controlling for group and sex, stimulation increased from baseline to ascending, t (141) = 8.09, p < .001, d = 1.36, decreased from ascending to peak, t (141) = −2.86, p = .014, d = 0.48, and decreased further from peak to descending, t (141) = −6.91, p < .001, d = 1.16. Model-estimated means and standard errors are shown in Figure 4. It should be noted, however, that the main effect of sex was no longer significant, F (1, 133) = 1.62, p = .205, ηp2 = .012, when controlling for the number of drinking partners during the dosing phase, a social factor that affects subjective stimulation from drinking (e.g., Fairbairn et al., 2015; Sayette et al., 2012). We also tested whether the apparent sex difference in subjective stimulation in the alcohol group specifically might be due to overall lower BrAC among women in the alcohol group (Figure 2). Controlling for BrAC in the alcohol group, there were no significant main effects of sex in subjective stimulation at ascending, peak, or descending, Fs (1, 85) < 2.54, ps > .114, ηp2s < .025.
Figure 4.
Model-estimated subjective stimulation score M ± SE across asessments. Stimulation was measured using the SEAS High Arousal Positive Valence subscale. Lines and points are displaced on the x-axis to show error bars.
Discussion
The present study characterized the acute effect of alcohol and its cues on heart rate (HR) in young adult men and women, and examined potential sex differences in those acute effects. We found that HR increased over time for both men and women alike, independent of alcohol or placebo beverage content, while observing preparation of the first beverage inside the bar room. While consuming the first and second beverage inside the bar room, men and women in the placebo beverage group exhibited HR decrease over time whereas counterparts in the alcohol beverage group exhibited HR increase over time. HR increased at a faster rate in women compared to men in the alcohol group such that HR was higher in women than men at the end of the beverage consumption period, at which time alcohol is being absorbed and distributed throughout the body. At the ascending limb assessment in the standard lab room, HR decreased to a level below baseline (viz., below HR level while at rest in the standard lab room before bar room procedures) in the placebo beverage group whereas in the alcohol group, HR remained elevated (viz., above baseline). For women, but not men, in the alcohol group, HR increased over time at the ascending limb assessment. At the peak and descending limb assessments in the standard lab room, HR remained elevated in the alcohol group whereas HR returned to baseline in the placebo group.
Our finding that HR increased over time in the alcohol and placebo beverage groups alike while watching beverage preparation in the bar room (i.e., during initial exposure to discrete and contextual alcohol cues) is in line with drug cue conditioning theories proposing that drug-related cues acquire excitatory properties as a function of classical conditioning to the stimulant-like effects of drugs of abuse (Eikelboom & Stewart, 1982; Robinson & Berridge, 1993; Stewart, de Wit, & Eikelboom, 1984). Our finding that HR was decreased in the alcohol group at the ascending limb assessment in the lab room compared to at the end of beverage consumption in the bar room (i.e., upon initial offset of discrete and contextual alcohol cues) could be interpreted as reflecting the offset of excitatory cues, but such an account has trouble explaining why HR would decrease to a level below baseline in the placebo group at the same assessment or why HR in the placebo group decelerated during beverage consumption in the bar room. These findings may be better interpreted as evidence that contextual alcohol cues elicited an alcohol effect-opposing physiological reaction that was masked by the co-occurring excitatory effects of discrete alcohol cues. Such an interpretation would be in line with drug cue conditioning theories proposing drug-related cues acquire the ability to elicit physiological reactions that compensate and oppose the anticipated physiological effects of the drug as a function of classical conditioning (Siegel & Ramos, 2002).
Our finding of a sex difference in HR under alcohol on the ascending limb, especially while individuals were still in the bar room, adds to an emerging literature on sex differences in the acute effects of alcohol and its cues on autonomic and cardiovascular physiology (Bates et al., 2011; Chaplin et al., 2008; Hartwell & Ray, 2013; Kaplan et al., 1985; Rubonis et al., 1994; Udo et al., 2009). This finding was robust to several alternative explanations. First, the difference persisted in analyses controlling for sex differences in BMI. Second, women in our study reached a slightly lower average BrAC than men (Δ ≈ .005 g%) due to a slight overcorrection for sex differences in alcohol pharmacokinetics by the dosing algorithm. The existing literature indicates a positive relationship between BrAC and HR (King et al., 2002; Spaak et al., 2008, 2010), whereby women in our study would be expected to respond to alcohol with lower increases in HR but they did not. Although between-person differences in BrAC might account for what appears to be a sex difference in HR under alcohol, our moderator analyses continued to find sex differences in the alcohol group HR data even when controlling for between-person differences in BrAC. Third, observed sex differences in HR under alcohol might be a function of sex differences in acquired tolerance due to differences in alcohol use over time. In our moderator analyses, however, we observed sex differences in the alcohol group HR data even when controlling for between-person differences in recent alcohol use.
Beyond beverage and bar room cues, the presence of social drinking cues represents one of the key differences between the present study and previous studies of acute alcohol-induced increases in HR. In previous studies, participants drank alone (e.g., Sayette, Smith, Breiner, & Wilson, 1992; Spaak et al., 2008, 2010) whereas in the present study and others like it (e.g., Doty & de Wit, 1995; Kirkpatrick & De Wit, 2013; Sayette et al., 2012), participants drank in groups. A fourth potential explanation for our observed sex difference in HR under alcohol could be that compared to men, women in the present study may have been more stimulated by drinking in the social context of the bar room, as suggested by the results of the study by Corbin and colleagues (2015). Nevertheless, in the present study, women reported marginally lower levels of subjective stimulation than men (regardless of beverage condition). Although, it should be noted that this apparent sex difference in overall subjective stimulation was not robust: it disappeared from the alcohol group when controlling for lower BrAC among women in the alcohol group, and it disappeared from both groups when controlling for differences in specific social factors between sessions such as the number of drinking partners during dosing in the bar. Critically, however, the observed sex difference in HR under alcohol persisted in moderator analyses controlling for between-person differences in subjective stimulation as well as in moderator analyses controlling for between-session differences in specific social factors such as the number and sex of drinking partners. In sum, our observed sex difference in HR under alcohol on the ascending limb, especially while individuals were still in the bar room, was robust to explanation by potential sex differences in BMI, BrAC, recent alcohol use, sensitivity to the stimulant-like subjective effects of alcohol, and sensitivity to social drinking context factors that can amplify the stimulant-like effects of alcohol. Thus, our finding appears to reflect biological sex as a factor determining the magnitude of an acute pharmacological effect of alcohol in a simulated naturalistic setting: social drinking in a bar room.
Limitations
Despite the robust nature of current findings, our conclusions are tempered by several caveats. First, we used a single session between-subject, single-dose design. Although manipulating beverage contents within participants across multiple sessions would have some advantages, such a design was not feasible here as we were re-recruiting prior longitudinal participants who came in to the lab from across the U.S. The current design does, however, allow each participant to serve as their own within-person control from baseline through ascending, peak, and descending assessments. Moreover, within-person manipulation of beverage contents adds other complications related to beverage order effects, which were not an issue in the current design. Another possible perceived limitation is that everyone expected to receive alcohol; hence, observed effects of alcohol and its cues are not independent of the effects of expectancies. Yet, as noted by Martin and Sayette (1993) expectancies are important determinants of individual differences in alcohol responses, thus were necessary to include in our study. Whereas random assignment to condition generated groups that were well-matched on a number of sociodemographic and biological characteristics important to the study (e.g., baseline resting heart rate), we cannot rule out the possibility that the observed effects of alcohol and placebo are a function of unmeasured person/group differences.
Consistent with previous research (e.g., Quinn & Fromme, 2016), we collected the placebo manipulation check at the beginning of the ascending limb assessment. This timing is thought to capture the strongest placebo effects, but we cannot be certain the placebo effect was active across subsequent assessments (i.e., peak and descending). Thus, we are cautious about interpreting the decrease in raw HR observed in the placebo group on the descending limb, at which point it had been 2 hours since beverage consumption. Second, participants had to stand up from their seat in the social setting of the barroom and walk to their individual lab testing rooms for the ascending, peak, and descending limb assessments. This may have weakened the placebo effect as an intact sense of balance might have raised suspicion about whether they received alcohol. It is highly unlikely, however, that these movements created artifacts that contribute to our HR effects because at least 10 min elapsed from the time each participant sat down inside their testing room to the time of HR recording. Additionally, we inspected and excluded the first minute of each of these HR recordings from analysis.
Lastly, we used HR as our index of the acute autonomic and cardiovascular effects of alcohol. Although relatively crude, this measure was appropriate for answering the basic questions posed in our study. In the absence of HR variability indices, however, we cannot attribute the observed effects to any specific central or peripheral neuro-cardiac regulatory mechanisms. In addition, without saliva or blood samples, we cannot rule out the possibility that the observed effects are due to some central or peripheral neuro-endocrine mechanism.
Implications for alcohol use-associated cardiovascular health risks
Our study has implications for the link between acute and chronic effects of alcohol use on the cardiovascular system. Although recent studies (Griswold et al., 2018; Wood et al., 2018) report similar magnitudes of association between chronic alcohol use and most cardiovascular system-related health risks among men and women, earlier studies indicated that cardiovascular system-related health risks were of greater concern among women (Chou & Dawson, 1994; WHO, 2014). These earlier findings could be explained by a failure to account for pharmacokinetic differences between men and women that lead to higher BrACs in women after acute alcohol ingestion and, thus, greater toxicity per unit consumed. However, it could also be explained by a sex difference in the acute effects of alcohol on autonomic, cardiovascular, and/or endocrine physiology. Our study supports the latter. In our study, women exhibited higher HR than men under alcohol on the ascending limb, especially in the simulated naturalistic drinking context, despite controlling for recent alcohol use, BrAC, and BMI. Although our study cannot discriminate among autonomic, cardiovascular, and endocrine explanations for the sex difference under alcohol, there is an emerging literature on adaptive and resting HR variability that suggests the autonomic nervous system regulates the cardiovascular system differently in men and women, and these differences may translate into differential effects of alcohol on HR (Bates et al., 2011; Koenig & Thayer, 2016; Udo et al., 2009). Given the population-level associations between alcohol use and cardiovascular health risks are believed to develop over the course of repeated exposure to the acute effects of alcohol in the natural environment, more research is warranted on biological sex as a factor determining the acute effects of alcohol and its cues on autonomic, cardiovascular, and/or endocrine physiology.
Public significance statement:
This study suggests that compared to men, women may experience greater increases in heart rate while drinking alcoholic beverages.
Disclosures and acknowledgements
Funding for the project was provided by NIH R01-AA-013967 and R01-AA-020637 (KF). BDB’s contributions were supported by NIH R01-AA-025451. RUC’s contributions were enabled by a pre-doctoral traineeship from the University of Texas Alcohol Training Grant (NIH T32-AA-007471), a post-doctoral fellowship from the University of Missouri College of Arts and Science Mission Enhancement Fund, and affiliation with the University of Missouri Alcohol & Addiction Training Grant (NIH T32-AA-013526). Funding sources had no role other than financial support.
We thank former University of Texas at Austin Studies on Alcohol, Health, and Risky Behaviors (SAHARA) lab managers, graduate students (Dr. Emily Wilhite, Dr. Elise Marino, and Travis Mallard), and undergraduate research assistants for their assistance with alcohol administration sessions. We thank current SAHARA lab graduate student Valeria Tretyak for her help with data organization. We also thank current University of Missouri Social Cognitive and Addiction Neuroscience (SCAN) lab graduate students Hannah Volpert-Esmond and Jorge Martins for their suggestion to consider social factors in moderator analyses. We also thank Dr. Kenneth J. Sher for his feedback on alcohol versus cue and context-related effects.
Footnotes
The ideas and data appearing in this manuscript were presented in poster format at the 59th annual meeting of the Society for Psychophysiological Research.
The authors have no conflicts of interest to declare.
The potential health benefits of chronic light to moderate drinking relative to abstention detected in epidemiological studies represent a highly controversial and hotly debated finding. There is good reason to believe that these potential health benefits are either artifactual or overestimated (Fillmore, Kerr, Stockwell, Chikritzhs, & Bostrom, 2006; Stockwell et al., 2016).
For completeness, we also scored and inspected the 3-item High Arousal Negative Valence subscale of the SEAS (“demanding”; “rude”; “aggressive”), which captures negative stimulant-like subjective effects of alcohol. However, scores on this subscale were at floor level (near 0 on a 10-point scale) across the baseline, ascending, peak, and descending assessments. For men and women in the alcohol and placebo groups, respectively, High Arousal Negative Valence subscale score Ms (SDs) were: 0.47 (0.93), 0.39 (0.93), 0.29 (0.51), 0.28 (0.71) at baseline; 0.65 (0.77), 0.49 (0.80), 0.24 (0.51), 0.23 (0.67) at ascending; 0.72 (1.0), 0.46 (0.96), 0.10 (0.20), 0.12 (0.50) at peak; and 0.56 (1.1), 0.47 (1.3), 0.06 (0.22), 0.14 (0.49) at descending. Consequently, neither further analysis nor consideration as a potential moderator of alcohol-induced changes in HR in the present study was warranted.
References
- Aarts E, Verhage M, & Veenvliet J (2014). A solution to dependency: using multilevel analysis to accommodate nested data. Nature Neuroscience, 17(4), 491–496. 10.1038/nn.3648 [DOI] [PubMed] [Google Scholar]
- Barr DJ, Levy R, Scheepers C, & Tily HJ (2013). Random effects structure for confirmatory hypothesis testing: Keep it maximal. Journal of Memory and Language, 68(3), 255–278. 10.1016/j.jml.2012.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates D, Mächler M, Bolker BM, & Walker S (2015). Fitting Linear Mixed-Effects Models Using {lme4}. Journal of Statistical Software, 67(1), 1–48. 10.18637/jss.v067.i01 [DOI] [Google Scholar]
- Bates ME, Buckman JF, Vaschillo EG, Fonoberov VA, Fonoberova M, Vaschillo B, … Mezi I (2011). The redistribution of power: Neurocardiac signaling, alcohol and gender. PLoS ONE, 6(12). 10.1371/journal.pone.0028281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bau PFD, Bau CHD, Naujorks AA, & Rosito GA (2005). Early and late effects of alcohol ingestion on blood pressure and endothelial function. Alcohol, 37(1), 53–58. [DOI] [PubMed] [Google Scholar]
- Bau PFD, Moraes RS, Bau CHD, Ferlin EL, Rosito GA, & Fuchs FD (2011). Acute ingestion of alcohol and cardiac autonomic modulation in healthy volunteers. Alcohol, 45(2), 123–129. 10.1016/j.alcohol.2010.08.011 [DOI] [PubMed] [Google Scholar]
- Brunelle C, Barrett SP, & Pihl RO (2007). Relationship between the cardiac response to acute intoxication and alcohol-induced subjective effects throughout the blood alcohol concentration curve. Human Psychopharmacology: Clinical and Experimental, 22(7), 437–443. 10.1002/hup.866 [DOI] [PubMed] [Google Scholar]
- Brunelle C, & Pihl RO (2007). Effects of conditioned reward and nonreward cues on the heart rate response to alcohol intoxication in male social drinkers. Alcoholism: Clinical and Experimental Research, 31(3), 383–389. 10.1111/j.1530-0277.2006.00318.x [DOI] [PubMed] [Google Scholar]
- Buckman JF, Eddie D, Vaschillo EG, Vaschillo B, Garcia A, & Bates ME (2015). Immediate and Complex Cardiovascular Adaptation to an Acute Alcohol Dose. Alcoholism: Clinical and Experimental Research, 39(12), 2334–2344. 10.1111/acer.12912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaplin TM, Hong KIA, Bergquist KL, & Sinha R (2008). Gender differences in response to emotional stress: An assessment across subjective, behavioral, and physiological domains and relations to alcohol craving. Alcoholism: Clinical and Experimental Research, 32(7), 1242–1250. 10.1111/j.1530-0277.2008.00679.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou PS, & Dawson DA (1994). A study of the gender differences in morbidity among individuals diagnosed with alcohol abuse and/or dependence. Journal of Substance Abuse, 6(4), 381–392. 10.1016/S0899-3289(94)90306-9 [DOI] [PubMed] [Google Scholar]
- Conrod PJ, Peterson JB, & Pihl RO (2001). Reliability and validity of alcohol-induced heart rate increase as a measure of sensitivity to the stimulant properties of alcohol. Psychopharmacology, 157(1), 20–30. 10.1007/s002130100741 [DOI] [PubMed] [Google Scholar]
- Corbin WR, Scott C, Boyd SJ, Menary KR, & Enders CK (2015). Contextual influences on subjective and behavioral responses to alcohol. Experimental and Clinical Psychopharmacology, 23(1), 59–70. 10.1037/a0038760 [DOI] [PubMed] [Google Scholar]
- Curtin JJ, & Fairchild BA (2003). Alcohol and cognitive control: Implications for regulation of behavior during response conflict. Journal of Abnormal Psychology, 112(3), 424–436. 10.1037/0021-843X.112.3.424 [DOI] [PubMed] [Google Scholar]
- Dafters R, & Anderson G (1982). Conditioned tolerance to the tachycardia effect of ethanol in humans. Psychopharmacology, 78(4), 365–367. 10.1007/BF00433743 [DOI] [PubMed] [Google Scholar]
- de Wit H, & Sayette M (2018). Considering the context: social factors in responses to drugs in humans. Psychopharmacology, 235(4), 935–945. 10.1007/s00213-018-4854-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doty P, & de Wit H (1995). Effect of setting on the reinforcing and subjective effects of ethanol in social drinkers. Psychopharmacology, 118(1), 19–27. 10.1007/BF02245245 [DOI] [PubMed] [Google Scholar]
- Eikelboom R, & Stewart J (1982). Conditioning of drug-induced physiological responses. Psychological Review, 89(5), 507–528. 10.1037/0033-295X.89.5.507 [DOI] [PubMed] [Google Scholar]
- Fairbairn CE, Sayette MA, Amole MC, Dimoff JD, Cohn JF, & Girard JM (2015). Speech volume indexes sex differences in the social-emotional effects of alcohol. Experimental and Clinical Psychopharmacology, 23(4), 255–264. 10.1037/pha0000021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillmore KM, Kerr WC, Stockwell T, Chikritzhs T, & Bostrom A (2006). Moderate alcohol use and reduced mortality risk: Systematic error in prospective studies. In Addiction Research and Theory (Vol. 14). 10.1080/16066350500497983 [DOI] [PubMed] [Google Scholar]
- Fox J, & Weisberg S (2019). An {R} Companion to Applied Regression (Third). Retrieved from https://socialsciences.mcmaster.ca/jfox/Books/Companion/ [Google Scholar]
- Fromme K, Corbin WR, & Kruse MI (2008). Behavioral risks during the transition from high school to college. Developmental Psychology, 44(5), 1497–1504. 10.1037/a0012614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griswold MG, Fullman N, Hawley C, Arian N, Zimsen SRM, Tymeson HD, … Gakidou E (2018). Alcohol use and burden for 195 countries and territories, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. The Lancet, 392(10152), 1015–1035. 10.1016/S0140-6736(18)31310-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwell EE, & Ray LA (2013). Sex moderates stress reactivity in heavy drinkers. Addictive Behaviors, 38(11), 2643–2646. 10.1016/j.addbeh.2013.06.016 [DOI] [PubMed] [Google Scholar]
- Kaplan RF, Cooney NL, Baker LH, Gillespie RA, Meyer RE, & Pomerleau OF (1985). Reactivity to Alcohol-Related Cues: Physiological and Subjective Responses in Alcoholics and Nonproblem Drinkers. Journal of Studies on Alcohol, 46(4), 267–272. Retrieved from http://www.jsad.com/doi/abs/10.15288/jsa.1985.46.267 [DOI] [PubMed] [Google Scholar]
- King AC, Houle T, De Wit H, Holdstock L, & Schuster A (2002). Biphasic alcohol response differs in heavy versus light drinkers. Alcoholism: Clinical and Experimental Research, 26(6), 827–835. 10.1097/00000374-200206000-00012 [DOI] [PubMed] [Google Scholar]
- Kirkpatrick MG, & De Wit H (2013). In the company of others: Social factors alter acute alcohol effects. Psychopharmacology, 230(2), 215–226. 10.1007/s00213-013-3147-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig J, & Thayer JF (2016). Sex differences in healthy human heart rate variability: A meta-analysis. Neuroscience and Biobehavioral Reviews, 64(2016), 288–310. 10.1016/j.neubiorev.2016.03.007 [DOI] [PubMed] [Google Scholar]
- Lau-Barraco C, Braitman AL, Linden-Carmichael AN, & Stamates AL (2016). Differences in weekday versus weekend drinking among nonstudent emerging adults. Experimental and Clinical Psychopharmacology, 24(2), 100–109. 10.1037/pha0000068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenth R (2019). emmeans: Estimated Marginal Means, aka Least-Squares Means. Retrieved from https://cran.r-project.org/package=emmeans
- Macfarlane SJ, & White JM (1989). Acquisition and extinction of an alcohol-opposite conditioned response in humans. Psychopharmacology, 97(3), 355–357. 10.1007/BF00439450 [DOI] [PubMed] [Google Scholar]
- Maggs JL, Williams LR, & Lee CM (2011). Ups and downs of alcohol use among first-year college students: Number of drinks, heavy drinking, and stumble and pass out drinking days. Addictive Behaviors, 36(3), 197–202. 10.1016/j.addbeh.2010.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin CS, & Sayette MA (1993). Experimental design in alcohol administration research: limitations and alternatives in the manipulation of dosage-set. Journal of Studies on Alcohol, 54, 750–761. 10.15288/jsa.1993.54.750 [DOI] [PubMed] [Google Scholar]
- Matuschek H, Kliegl R, Vasishth S, Baayen H, & Bates D (2017). Balancing Type I error and power in linear mixed models. Journal of Memory and Language, 94, 305–315. 10.1016/j.jml.2017.01.001 [DOI] [Google Scholar]
- Mayo LM, & de Wit H (2016). Acquisition of Conditioned Responses to a Novel Alcohol-Paired Cue in Social Drinkers. Journal of Studies on Alcohol and Drugs, 77(2), 317–326. 10.15288/jsad.2016.77.317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaul ME, Turkkan JS, & Stitzer ML (1989a). Conditioned Opponent Responses: Effects of Placebo Challenge in Alcoholic Subjects. Alcoholism: Clinical and Experimental Research, 13(5), 631–635. 10.1111/j.1530-0277.1989.tb00395.x [DOI] [PubMed] [Google Scholar]
- McCaul ME, Turkkan JS, & Stitzer ML (1989b). Psychophysiological Effects of Alcohol‐related Stimuli: I. The Role of Stimulus Intensity. Alcoholism: Clinical and Experimental Research, 13(3), 386–391. 10.1111/j.1530-0277.1989.tb00340.x [DOI] [PubMed] [Google Scholar]
- McKay D, & Schare ML (1999). The effects of alcohol and alcohol expectancies on subjective reports and physiological reactivity: A meta-analysis. Addictive Behaviors, 24(5), 633–647. 10.1016/S0306-4603(99)00021-0 [DOI] [PubMed] [Google Scholar]
- Morean ME, Corbin WR, & Treat TA (2013). The subjective effects of alcohol scale: Development and psychometric evaluation of a novel assessment tool for measuring subjective response to alcohol. Psychological Assessment, 25(3), 780–795. 10.1037/a0032542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newlin DB (1985). The Antagonistic Placebo Response to Alcohol Cues. Alcoholism: Clinical and Experimental Research, 9(5), 411–416. 10.1111/j.1530-0277.1985.tb05573.x [DOI] [PubMed] [Google Scholar]
- Newlin DB (1986). Conditioned compensatory response to alcohol placebo in humans. Psychopharmacology, 88(2), 247–251. 10.1016/0741-8329(85)90124-7 [DOI] [PubMed] [Google Scholar]
- Page-Gould E (2019). Multilevel Modeling In Cacioppo JT, Tassinary LG, & Berntson GG (Eds.), Handbook of Psychophysiology (4th ed.). Cambridge University Press. [Google Scholar]
- Quinn PD, & Fromme K (2016). Individual Differences in Subjective Alcohol Responses and Alcohol-Related Disinhibition. Experimental and Clinical Psychopharmacology, 24(2), 90–99. 10.1037/pha0000065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team. (2018). R: A Language and Environment for Statistical Computing. Retrieved from https://www.r-project.org/
- Ray LA, McGeary JE, Marshall E, & Hutchison KE (2006). Risk factors for alcohol misuse: Examining heart rate reactivity to alcohol, alcohol sensitivity, and personality constructs. Addictive Behaviors, 31(11), 1959–1973. 10.1016/j.addbeh.2006.01.010 [DOI] [PubMed] [Google Scholar]
- Robinson TE, & Berridge KC (1993). The neural basis of drug craving : an incentive-sensitization theory of addiction. Brain Research Reviews, 8, 247–291. [DOI] [PubMed] [Google Scholar]
- Rohsenow DJ, & Marlatt GA (1981). The balanced placebo design: Methodological considerations. Addictive Behaviors, 6(2), 107–122. 10.1016/0306-4603(81)90003-4 [DOI] [PubMed] [Google Scholar]
- Ronksley PE, Brien SE, Turner BJ, Mukamal KJ, & Ghali WA (2011). Association of alcohol consumption with selected cardiovascular disease outcomes: A systematic review and meta-analysis. Bmj, 342(7795), 479 10.1136/bmj.d671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubonis AV, Colby SM, Monti PM, Rohsenow DJ, Gulliver SB, & Sirota AD (1994). Alcohol cue reactivity and mood induction in male and female alcoholics. Journal of Studies on Alcohol, 55(4), 487–494. [DOI] [PubMed] [Google Scholar]
- Saunders JB, Aasland OG, Babor TF, De La Fuente JR, & Grant M (1993). Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption-II. Addiction, 88(6), 791–804. 10.1111/j.1360-0443.1993.tb02093.x [DOI] [PubMed] [Google Scholar]
- Sayette MA, Creswell KG, Dimoff JD, Fairbairn CE, Cohn JF, Heckman BW, … Moreland RL (2012). Alcohol and Group Formation: A Multimodal Investigation of the Effects of Alcohol on Emotion and Social Bonding. Psychological Science, 23(8), 869–878. 10.1177/0956797611435134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayette MA, Smith DW, Breiner MJ, & Wilson GT (1992). The effect of alcohol on emotional response to a social stressor. Journal of Studies on Alcohol, 53(6), 541–545. 10.15288/jsa.1992.53.541 [DOI] [PubMed] [Google Scholar]
- Shapiro AP, & Nathan PE (1986). Human tolerance to alcohol: the role of Pavlovian conditioning processes. Psychopharmacology, 88(1), 90–95. 10.1007/BF00310519 [DOI] [PubMed] [Google Scholar]
- Sher KJ, Bartholow BD, Peuser K, Erickson DJ, & Wood MD (2007). Stress-response-dampening effects of alcohol: Attention as a mediator and moderator. Journal of Abnormal Psychology, 116(2), 362–377. 10.1037/0021-843X.116.2.362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel S, & Ramos BMC (2002). Drug Anticipation and the Treatment of Drug Addiction. Experimental and Clinical Psychopharmacology, 10(3), 162–183. 10.1037//1064-1297.10.3.162 [DOI] [PubMed] [Google Scholar]
- Sobell LC, & Sobell MB (1992). Timeline follow-back: A technique for assessing self-reported alcohol consumption. In Measuring alcohol consumption: Psychosocial and biochemical methods. (pp. 41–72). 10.1007/978-1-4612-0357-5_3 [DOI] [Google Scholar]
- Spaak J, Merlocco AC, Soleas GJ, Tomlinson G, Morris BL, Picton P, … Floras JS (2008). Dose-related effects of red wine and alcohol on hemodynamics, sympathetic nerve activity, and arterial diameter. American Journal of Physiology-Heart and Circulatory Physiology, 294(2), H605–H612. 10.1152/ajpheart.01162.2007 [DOI] [PubMed] [Google Scholar]
- Spaak J, Tomlinson G, McGowan CL, Soleas GJ, Morris BL, Picton P, … Floras JS (2010). Dose-related effects of red wine and alcohol on heart rate variability. AJP: Heart and Circulatory Physiology, 298(6), H2226–H2231. 10.1152/ajpheart.00700.2009 [DOI] [PubMed] [Google Scholar]
- Staiger PK, & White JM (1988). Conditioned alcohol-like and alcohol-opposite responses in humans. Psychopharmacology, 95(1), 87–91. 10.1007/BF00212773 [DOI] [PubMed] [Google Scholar]
- Staiger PK, & White JM (1991). Cue reactivity in alcohol abusers: Stimulus specificity and extinction of the responses. Addictive Behaviors, 16(5), 211–221. 10.1016/0306-4603(91)90014-9 [DOI] [PubMed] [Google Scholar]
- Stewart J, de Wit H, & Eikelboom R (1984). Role of unconditioned and conditioned drug effects in the self-administration of opiates and stimulants. Psychological Review, 91(2), 251–268. 10.1037/0033-295X.91.2.251 [DOI] [PubMed] [Google Scholar]
- Stockwell T, Zhao J, Panwar S, Roemer A, Naimi TS, & Chikritzhs T (2016). Do “ Moderate “ Drinkers Have Reduced Mortality Risk? A Systematic Review and Meta-Analysis of Alcohol Consumption and All-Cause Mortality. Alcohol Drugs, 77, 185–198. 10.15288/jsad.2016.77.185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udo T, Bates ME, Mun E-Y, Vaschillo EG, Vaschillo B, Lehrer P, & Ray S (2009). Gender differences in acute alcohol effects on self-regulation of arousal in response to emotional and alcohol-related picture cues. Psychology of Addictive Behaviors, 23(2), 196–204. 10.1037/a0015015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaschillo EG, Bates ME, Vaschillo B, Lehrer P, Udo T, Mun E-Y, & Ray S (2008). Heart rate variability response to alcohol, placebo, and emotional picture cue challenges: Effects of 0.1-Hz stimulation. Psychophysiology, 45(5), 847–858. 10.1111/j.1469-8986.2008.00673.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. (2014). Global Status Report on Alcohol and Health. Retrieved from https://books.google.com/books?id=HbQXDAAAQBAJ
- Wickham H (2009). ggplot2: elegant graphics for data analysis. Springer. [Google Scholar]
- Wood AM, Kaptoge S, Butterworth AS, Willeit P, Warnakula S, Bolton T, … Davey Smith G (2018). Risk thresholds for alcohol consumption: combined analysis of individual-participant data for 599 912 current drinkers in 83 prospective studies. The Lancet, 391(10129), 1513–1523. 10.1016/S0140-6736(18)30134-X [DOI] [PMC free article] [PubMed] [Google Scholar]