Abstract
Objective:
The objective of this study was to design, code, and pilot test the feasibility and preliminary efficacy of a self-management digital therapeutic tool for adolescents with migraine.
Background:
Self-management of migraine in adolescents is complex and has important implications for health outcomes. A comprehensive and accessible approach to self-management is needed for youth with migraine, their parents, and clinicians.
Methods:
An iterative co-design process was used to develop and optimize the Migraine Manager digital therapeutic self-management tool. Subsequently, 40 adolescents age 11-18 years were enrolled in an 8-week single arm open label trial (N=36 analyzed). The primary outcome was headache days.
Results:
Usage data for Migraine Manager were similar to other health app usage data, and feedback from participants was uniformly positive, indicating acceptable feasibility. Preliminary efficacy was demonstrated by a reduction in headache days from 17.2 ± 8.5 at baseline to 7.9 ± 7.2 at 8 weeks (95% CI, −13.0 to −7.8; p<.001). There was also statistically significant improvement in patient physical functioning quality of life reported by both patients (baseline = 55.7 ± 20.4; 8 weeks = 69.7 ± 21.9, p = .005) and parents (baseline = 58.5 ± 22.8; 8 weeks = 74.3 ± 18.1, p = .002), and in parent-reported ingestion issues subscale of the adherence barriers scale from baseline to 8 weeks (baseline = 6.0 ± 2.6; 8 weeks = 5.2 ± 3.0, p = .020).
Conclusions:
A self-management digital therapeutic tool for adolescents with migraine can offer care to patients who might not otherwise receive such services. Migraine Manager demonstrated feasibility and preliminary efficacy in this pilot trial, highlighting the potential beneficial impact of this tool. Larger controlled trials with long-term follow-up are needed to definitively determine clinical efficacy of Migraine Manager.
Keywords: Pediatric, Adolescent, Migraine, Headache, Self-Management, Technology
Migraine affects approximately 8% of children and adolescents,1 with estimates ranging up to 28%,2,3 and the majority continue to experience attacks into adulthood.4 The annual economic impact of migraine in the United States is approximately $36 billion including both direct medical costs and lost productivity.5 Additionally, over 130,000 school days are missed by children every two weeks due to migraine.6 The negative effects of migraine on overall quality of life is similar to childhood cancer, heart disease, and rheumatic disease.7 Treatment for migraine focuses on reduction of headache days, duration, and disability. It includes drug and non-drug therapies as well as consistent engagement in healthy lifestyle behaviors, and involves daily patient effort to ensure optimal outcomes8,9. Thus, effective and sustained self-management including behavioral and pharmacological management is critical for patients and their families.
Poor self-management of chronic conditions in children and adolescents is a pervasive problem resulting in poor health outcomes and treatment nonadherence10,11, increased risk of relapse12, and more than a $300 billion increase in annual health care costs in the US13,14 Adolescents have significant difficulty with treatment11,15, with 65-88% of adolescents nonadherent to medication15,16 Our research has documented several barriers to effective self-management, including forgetting, interference with activities, medication unavailability, and oppositional behavior17,18. Similar barriers occur in patients with migraine taking preventive medications. Our clinical trials have demonstrated the efficacy of behavioral interventions targeting self-management barriers19,20 as well as cognitive-behavioral therapy (CBT) in the treatment of migraine21. However, weekly treatment attendance can be a significant barrier for families. Additionally, clinic-based efforts to address self-management are inadequate due to: 1) time constraints (e.g., brief/infrequent clinic visits), 2) exclusive use of educational approaches, which are insufficient for behavior change22-27, and 3) limited number of clinicians trained to provide patients with self-management behavior skills training and/or CBT. Thus, a more comprehensive and accessible approach is needed for youth with migraine, parents, and clinicians so that evidence-based assessment and intervention can be disseminated broadly.
Digital therapeutic tools are ideal for targeting pediatric self-management challenges due to their accessibility and acceptability to adolescents. Unfortunately, existing self-management resources for migraine are limited to behavioral/CBT pain management,28,29 neglecting the impact of lifestyle modification and medication on overall management of migraine. In general, internet resources are limited in scope and effectiveness, lack a guiding theoretical framework, target only one user (e.g., patients but not clinicians), neglect adolescent developmental issues that impact self-management, and do not build transferrable, sustainable skills. Also, they are often developed without the input of non-clinician users, leading to rejection by patients and families30.
To address these shortcomings in migraine self-management research, we developed “Migraine Manager”, the first comprehensive digital therapeutic self-management tool for adolescents with migraine, their parents, and clinicians. Migraine Manager incorporates both migraine-specific and general evidence-based assessment and intervention components that target the barriers we have identified and successfully treated in our prior research and clinical experience. This approach capitalizes on the need to teach users cross-cutting skills (e.g., problem solving) along with condition-specific skills (e.g., monitoring of headache frequency and duration). Once developed, we conducted a pilot test of Migraine Manager with the aims of 1) determining the feasibility of delivering self-management assessment and intervention for migraine via an interactive digital therapeutic resource, and 2) testing the preliminary efficacy of this intervention tool on clinical outcomes, specifically headache frequency. We hypothesized that Migraine Manager would demonstrate adequate feasibility and preliminary efficacy in this pilot trial.
Methods
Development of Migraine Manager
The development of Migraine Manager was an iterative co-design process that occurred via a series of focus group/interview sessions with key stakeholders including patients, parents/caregivers, and headache clinicians. An initial concept of a digital therapeutic self-management tool was presented and feedback was solicited from each of these end user groups. Once an alpha prototype was designed and coded, confirmatory interviews were conducted to determine if initial coding was consistent with recommendations. Additional modifications were made following these interviews and a fully functional digital therapeutic tool was finalized for testing in a clinical trial. This tool consisted of 16 intervention modules. Participants were given login information and completed an assessment battery prior to receiving intervention. An algorithm was used to individually tailor treatment module assignments based on patient self-management needs. Although specific modules were assigned to each participant in a personalized treatment manner, all participants were allowed to access all modules if they chose to do so during their 8-week treatment course. That is, specific modules were highlighted in their treatment plan, but we did not restrict access to modules that were not specifically assigned to a given participant.
Study Design and Participants
This single arm open label trial (ClinicalTrial.gov number: NCT03157739) was approved by the Institutional Review Board at a large Midwestern children’s hospital. All eligible patients were recruited in 2018 from a multidisciplinary Headache Center. All patients received a detailed headache evaluation, using a semi-structured interview process that included a full physical, neurological and headache examination, diagnosis using the International Classification of Headache Disorders, and were provided a detailed headache treatment plan that included acute, preventive and biobehavioral treatment. For the study, the participants met the following inclusion criteria: 1) diagnosis of migraine using the International Classification of Headache Disorders criteria (ICHD-3 beta) for migraine with or without aura, 2) 11-18 years of age, inclusive, 3) headache frequency of 8+ per month, 4) access to the internet whether public (e.g., library) or private (e.g., home, personal), and 4) fluent in English. Exclusion criteria were 1) diagnosis of pervasive developmental disorder or serious mental illness (e.g., schizophrenia) as determined by medical chart review. Written informed consent and assent were obtained by participants and their parents/caregivers.
Forty patients consented to participate in the study, and 6 declined. Four participants withdrew prior to beginning study procedures and 3 withdrew after partially completing procedures; these participants’ data were retained for analyses. Therefore, the final sample was comprised 36 adolescents (75% female) with physician-diagnosed migraine, ages 11-18 (M = 14.5). See Figure 2 for participant flow chart. Thirty-one participants were White (86%), 4 were Black or African American (11%), and 1 was Asian (3%). Participants were provided a modest compensation for their participation.
Figure 2.
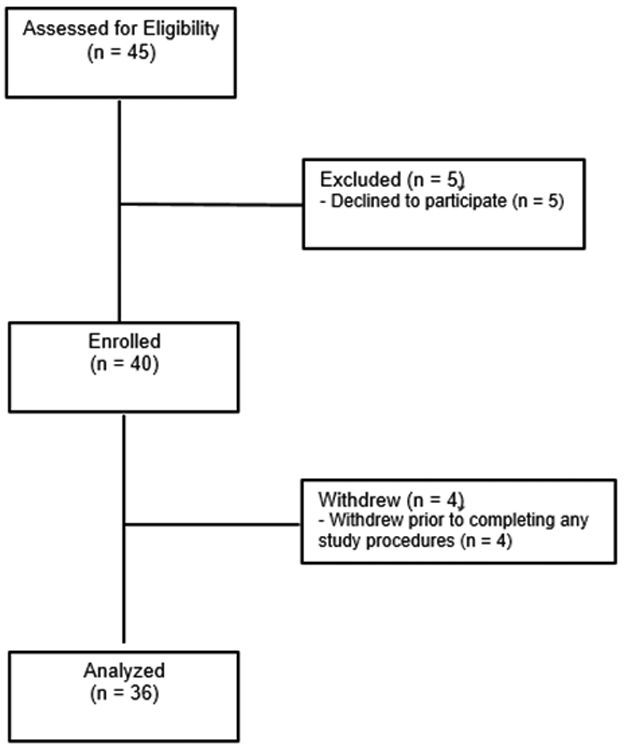
Participant Flow Chart
Measures
A demographic form including parent age, education, marital status, patient ethnicity, and annual household income was completed by caregivers at baseline. Treatment integrity was maintained via automated electronic delivery of the intervention, which precluded human error or variation in intervention delivery.
Daily Diary:
Participants completed a diary each day during the 8-week intervention period. This diary consisted of 16-21 items depending on whether the participant experienced a headache that day. These items assessed headache frequency, pain, duration, approach to treatment, impact on activities, stress, and healthy habits. Headache frequency, the primary clinical endpoint for the trial, was defined as the total number of days with headache reported during the previous month. A headache day was defined as starting and ending at midnight.
Pediatric Quality of Life Inventory (PedsQL 4.0):31
The PedsQL 4.0 is a 23-item self-report measure that evaluates children’s health-related quality of life (HRQOL) across 4 areas of functioning: physical, emotional, social, and school. The PedsQL 4.0 has both patient- and caregiver-report forms and can be used in children ages 2-18 years. Respondents rate how much of a problem each item has been during the past month on an anchored 5-point scale. The PedsQL 4.0 is a well-established reliable and valid measure of HRQOL that has been used extensively in pediatric chronic illness research.
Parent and Adolescent Medication Barriers Scales (PMBS/AMBS):32
The PMBS (16-items) and the AMBS (17-items) are designed to assess perceived barriers to medication taking. Each item is rated on a 5-point Likert-like scale from 1 = ‘strongly disagree’ to 5 = ‘strongly agree.’ The PMBS has strong internal consistency (α = .87) and stability over time33. There are 4 factor-analytically derived subscales: Disease Frustration/Adolescent Issues, Regimen Adaptation/Cognitive, Ingestion Issues, and Parent Reminder.
Data Analyses
Data analyses presented here were the a priori primary analyses of these data. As this single arm trial was a pilot study aimed at determining feasibility and preliminary efficacy, no statistical power calculation was conducted prior to the study. SPSS statistical software was used for descriptive analyses and MPlus statistical software was used to conduct two-tailed pairwise dependent samples t-tests (p < .05) under missing data conditions using specialized commands (i.e., model constraint) that allow data to be missing conditional on other variables in the model. Cohen’s d is reported as an effect size metric for pairwise comparisons. Differences in the following variables were tested in a series of within-subject pairwise comparisons that used the False Discovery Rate34 (FDR) for Type-1 error control: (1) number of headache days from baseline to month 1, month 1 to month 2, and baseline to month 2 of the intervention, (2) PedsQL total scores and subscale scores (physical, emotional, social, and school functioning) at baseline and post-treatment for caregiver and patient forms, and (3) PMBS/AMBS total scores and subscale scores (Disease Frustration/Adolescent Issues, Regimen Adaptation/Cognitive, Ingestion Issues, and Parent Reminder) at baseline and post-treatment. Pairwise missing data ranged between 2.8%-30.3% and was handled via maximum likelihood estimation. To address the possibility of response variable non-normality, all analyses were performed using 5000 bootstrap replications.
Results
Feasibility and Acceptability
The feasibility and acceptability for Migraine Manager was assessed via participant reports of their experiences with the tool as well as usage data obtained from the program. In general, participants viewed Migraine Manager quite favorably; it was seen as a helpful resource that provided valuable information and participants reported using it regularly over the treatment course. Participant usage data were similar to and, in some cases, better than health app usage in general. In a national survey of 934 individuals 79, 65.5% reported using a health app at least once daily and 44.4% reported using their apps for 1-10 minutes. Our usage data revealed that intervention modules focused on headache education, school challenges, and goal setting had completion rates of ≥ 60% by participants. The two parent modules had lower completion rates. Taken together, this suggests that patients were engaged in and completed the intervention modules that teach critical self-management skills (education, goal setting, etc.), whereas parents were not as highly engaged. Given that our primary intervention target was the patient, and our usage data by patients was similar to that which has been published to date, Migraine Manager appears to be a feasible and acceptable intervention program for adolescents with migraine.
Primary Outcome- Headache Days
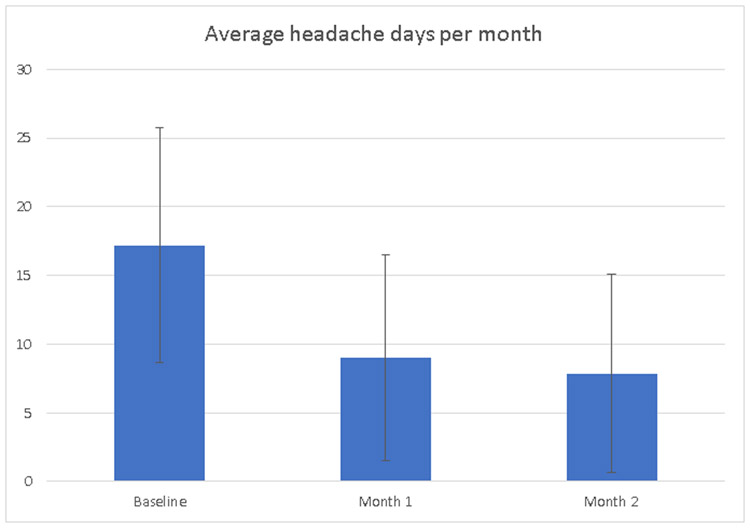
At baseline, participants reported having an average of 17 headache days in the last month. Headache days during the 8-week intervention were reported via daily headache diaries completed online. There was a statistically significant decrease in headache days per month (Figure 1) from baseline (M = 17.2, SD = 8.5) to month 1 (M = 9.0, SD = 7.5; 95% CI, −11.3 to −5.8; p <. 001; d = 1.22), from month 1 to month 2 (M = 7.8, SD = 7.3; 95% CI, −3.3 to −0.4; p = .011; d = 0.37), and from baseline to month 2 (95% CI, −13.0 to −7.8; p < .001; d = 1.23).
Figure 1.
Average number of HA days per month at baseline, month 1, and month 2.
Secondary Outcomes
Health-Related Quality of Life (PedsQL):
Differences in PedsQL average subscale scores (physical, emotional, social, and school functioning) from baseline to post-treatment were also examined. There was statistically significant improvement in patient-reported physical functioning from baseline (M = 55.7, SD = 20.4) to post-treatment (M = 69.7, SD = 21.9; 95% CI, 4.0 to 22.7; p = .005; d = 0.60). Similarly, there was statistically significant improvement in caregiver-reported physical functioning from baseline (M=58.5, SD=22.8) to post-treatment (M = 74.3, SD = 18.1, 95% CI, 5.4 to 23.3; p = .002; d = 0.74). There were no statistically significant differences in social, emotional, or school functioning from baseline to post-treatment.
Parent and Adolescent Medication Barriers (PMBS/AMBS):
Parent scores on the ingestion issues subscale of the PMBS showed a statistically significant decrease from baseline (M = 6.0, SD = 2.6) to month 2 (M = 5.2, SD = 3.0; 95% CI, −1.4 to −0.1; p = .020, d = 0.42). Patient reported disease frustration/adolescent issues scores increased from baseline (M = 17.3, SD = 6.8) to month 2 (M = 19.5, SD = 6.9; 95% CI, 0.5 – 3.9; p = .010, d = 0.44). There were no statistically significant differences in other subscales on the PMBS or AMBS from baseline to post-treatment.
Discussion
In this study, we sought to design and code a digital therapeutic self-management tool for adolescent patients with migraine and their parents and subsequently test the feasibility and preliminary efficacy of Migraine Manager in a pilot trial. Our iterative co-design process in which we engaged patients, parents, and clinicians, resulted in Migraine Manager being a feasible assessment and intervention tool, with patients demonstrating engagement in and completion of self-management intervention modules. Parent engagement was lower than expected and may represent an area for future development and improvement. Conversely, this may be a reflection of adolescent independence and thus represent a positive outcome. Further inquiry is needed with regard to this issue. Nevertheless, with patients being our primary target for intervention, the feasibility of Migraine Manager was quite good overall.
Regarding clinical outcomes, participants experienced a statistically significant decrease in headache days from 17 at baseline to 8 at 2 months/8 weeks. We also observed an improvement in physical functioning HRQOL per both patient- and caregiver-report. Additionally, there was a decrease in adherence barriers as represented by improved parent scores on the ingestion issues subscale of the PMBS. These clinical findings highlight the potential beneficial clinical impact of this type of digital therapeutic self-management support tool and the need for continued examination.
There are several potential benefits to using digital therapeutics like Migraine Manager in clinical care. Rather than relying solely on patient recall, this type of tool could provide more accurate data for physical history, which might have implications for diagnosis and treatment considerations. The ability of digital therapeutic tools to collect real-time data on headache frequency as well as data on healthy habit engagement, treatment adherence, and other self-management behaviors is highly beneficial. These data can be used to inform ongoing medical and behavioral management of pain and potentially improve outcomes at a faster rate. Additionally, during the time between clinic visits, it is currently unknown how patients are managing their headaches. With Migraine Manager, clinicians can access inter-visit data to provide additional support via telehealth or recommend an additional clinic visit. The ongoing assessment of need for self-management support over the course of treatment is also critically important for developing adolescents who will take on more responsibility for self-management from their parents as they age. Finally, with its assessment-based treatment algorithm, Migraine Manager improves on the current ‘one size fits all’ approach to self-management in chronic conditions by providing a personalized treatment approach to match the specific needs of each patient.
There are important methodological limitations of the current study that have implications for future research in this area. Because this project involved the development and pilot testing of a digital therapeutic tool, the sample size for the trial is modest. Larger, sufficiently powered clinical trials will need to be conducted to definitively determine the efficacy of Migraine Manager. Additionally, the study design was a single arm trial in order to maximize the number of participants providing feasibility data. Future trials should also include an appropriate control arm to determine the efficacy of Migraine Manager compared to usual care. This pilot study also included a sample that was primarily female and White. The predominance of females is to be expected given the gender differential that develops in migraine during adolescence, while the racial distribution is a reflection of the local population demographics. In an appropriately powered and controlled clinical trial, the sample will need to be more diverse to ensure that there are no differential effects in certain demographic groups.
Conclusion
Our goal with this project was to develop a feasible digital therapeutic tool that can be used to promote self-management and potentially improve clinical outcomes in pediatric patients with migraine. Digital therapeutics will continue to be an attractive treatment option for populations in which there is a behavioral health need that is not being currently met by standard practices. Our data suggest that Migraine Manager is feasible with acceptable usage rates in adolescent patients, and that it may have a positive impact on clinical outcomes, particularly headache frequency. Certainly, larger, controlled clinical trials with long-term follow-up are needed to definitively determine clinical efficacy of this tool. The initial positive impact and feedback from participants in this study serve as the starting point for this line of research and the continued development and testing of Migraine Manager.
Acknowledgments
Source Funding: This study was supported by a grant (R21 NS094476) from the National Institutes of Health/National Institute of Neurological Disorders and Stroke (NIH/NINDS).
Abbreviations:
- CBT
Cognitive-behavioral therapy
- HRQOL
Health-related quality of life
- PMBS/AMBS
Parent and Adolescent Medication Barriers Scales
- FDR
False Discovery Rate
Footnotes
Conflict of Interest: The authors have no conflict of interest to report.
References
- 1.Abu-Arafeh I, Razak S, Sivaraman B, Graham C. Prevalence of headache and migraine in children and adolescents: a systematic review of population-based studies. Dev Med Child Neurol. 2010;52(12):1088–1097. [DOI] [PubMed] [Google Scholar]
- 2.Abu-Arefeh I, Russell G. Prevalence of headache and migraine in schoolchildren. BMJ. 1994;309(6957):765–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Split W, Neuman W. Epidemiology of migraine among students from randomly selected secondary schools in Lodz. Headache. 1999;39:494–501. [DOI] [PubMed] [Google Scholar]
- 4.Bille BA 40-year follow-up of school children with migraine. Cephalalgia. 1997;17(4):488–491; discussion 487. [DOI] [PubMed] [Google Scholar]
- 5.Stewart WF, Ricci JA, Chee E, Morganstein D, Lipton R. Lost productive time and cost due to common pain conditions in the US workforce. JAMA. 2003;290(18):2443–2454. [DOI] [PubMed] [Google Scholar]
- 6.Stang PE, Osterhaus JT. Impact of migraine in the United States: data from the National Health Interview Survey. Headache. 1993;33(1):29–35. [DOI] [PubMed] [Google Scholar]
- 7.Powers SW, Patton SR, Hommel KA, Hershey AD. Quality of life in childhood migraines: clinical impact and comparison to other chronic illnesses. Pediatrics. 2003;112(1 Pt 1):e1–5. [DOI] [PubMed] [Google Scholar]
- 8.Hershey AD, Powers SW, Winner P, Kabbouche M. Pediatric Headaches in Clinical Practice. West Sussex, UK: John WIley & Sons, Ltd; 2009. [Google Scholar]
- 9.Hershey AD. Current approaches to the diagnosis and management of paediatric migraine. Lancet Neurol. 2010;9(2):190–204. [DOI] [PubMed] [Google Scholar]
- 10.World-Health-Organization. Adherence to long-term therapies: Evidence for action. Geneva, Switzerland: World Health Organization;2003. [Google Scholar]
- 11.Rapoff MA. Adherence to Pediatric Medical Regimens. 2nd ed. New York: Springer; 2010. [Google Scholar]
- 12.Kane S, Huo D, Aikens J, Hanauer S. Medication nonadherence and the outcomes of patients with quiescent ulcerative colitis. The American Journal of Medicine. 2003;114(1):39–43. [DOI] [PubMed] [Google Scholar]
- 13.DiMatteo MR. The role of effective communication with children and their families in fostering adherence to pediatric regimens. Patient Educ Couns. 2004;55(3):339–344. [DOI] [PubMed] [Google Scholar]
- 14.Berg JS, Dischler J, Wagner DJ, Raia JJ, Palmer-Shevlin N. Medication compliance: a healthcare problem. The Annals of pharmacotherapy. 1993;27(9 Suppl):S1–24. [PubMed] [Google Scholar]
- 15.Logan DE, Zelikovsky N, Labay L, Spergel J. The Illness Management Survey: Identifying adolescents' perceptions of barriers to adherence. J Pediatr Psychol. 2003;28(6):383–392. [DOI] [PubMed] [Google Scholar]
- 16.Hommel KA, Davis CM, Baldassano RN. Objective versus subjective assessment of oral medication adherence in pediatric inflammatory bowel disease. Inflamm Bowel Dis. 2009;15(4):589–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hommel KA, Baldassano RN. Brief report: Barriers to treatment adherence in pediatric inflammatory bowel disease. Journal of Pediatric Psychology. 2010;35(9):1005–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ingerski LM, Baldassano RN, Denson LA, Hommel KA. Barriers to oral medication adherence for adolescents with inflammatory bowel disease. Journal of Pediatric Psychology. 2010;35(6):683–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hommel KA EH, Odell S, et al. Evaluation of a group-based behavioral intervention to promote adherence in adolescents with Inflammatory Bowel Disease. Eur J Gastroenterol Hepatol. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ingerski LM, Hente EA, Modi AC, Hommel KA. Electronic measurement of medication adherence in pediatric chronic illness: A review of measures. J Pediatr. 2011;159(4):528–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Powers SW, Kashikar-Zuck SM, Allen JR, et al. Cognitive Behavioral Therapy Plus Amitriptyline for Chronic Migraine in Children and Adolescents A Randomized Clinical Trial. Jama-J Am Med Assoc. 2013;310(24):2622–2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sluijs EMFv McMinn AM, Griffin SJ. Effectiveness of interventions to promote physical activity in children and adolescents: systematic review of controlled trials. BMJ (Clinical research ed). 2007;335(7622):703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cooper H, Booth K, Fear S, Gill G. Chronic disease patient education: lessons from meta-analyses. Patient Education and Counseling. 2001;44(2):107–117. [DOI] [PubMed] [Google Scholar]
- 24.Mazzuca SA. Does patient education in chronic disease have therapeutic value? J Chron Dis. 1982;35(7):521–529. [DOI] [PubMed] [Google Scholar]
- 25.Bodenheimer T, Lorig K, Holman H, Grumbach K. Patient self-management of chronic disease in primary care. JAMA. 2002;288(19):2469–2475. [DOI] [PubMed] [Google Scholar]
- 26.Partridge M, Hill S. Enhancing care for people with asthma: the role of communication, education, training and self-management. 1998 World Asthma Meeting Education and Delivery of Care Working Group. European Respiratory Journal. 2000;16(2):333–348. [DOI] [PubMed] [Google Scholar]
- 27.Gibson PG, Powell H, WIlson A, et al. Limited (information only) patient education programs for adults with asthma. Cochrane Database Syst Rev. 2001(1). [DOI] [PubMed] [Google Scholar]
- 28.Connelly M, Rapoff MA, Thompson N, Connelly W. Headstrong: a pilot study of a CD-ROM intervention for recurrent pediatric headache. J Pediatr Psychol. 2006;31(7):737–747. [DOI] [PubMed] [Google Scholar]
- 29.Rapoff MA, Connelly M, Bickel JL, et al. Headstrong intervention for pediatric migraine headache: a randomized clinical trial. J Headache Pain. 2014;15(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Evers KE, Prochaska JM, Prochaska JO, Driskell M-M, Cummins CO, Velicer WF. Strengths and Weaknesses of Health Behavior Change Programs on the Internet. Journal of Health Psychology. 2003;8(1):63–70. [DOI] [PubMed] [Google Scholar]
- 31.Varni JW, Seid M, Rode CA. The PedsQL: Measurement model for the pediatric quality of life inventory. Medical care. 1999;37(2):126–139. PMID: 10024117. [DOI] [PubMed] [Google Scholar]
- 32.Simons LE, Blount RL. Identifying barriers to medication adherence in adolescent transplant recipients. J Pediatr Psychol. 2007;32(7):831–844. [DOI] [PubMed] [Google Scholar]
- 33.Simons LE, McCormick ML, Devine K, Blount RL. Medication barriers predict adolescent transplant recipients' adherence and clinical outcomes at 18-month follow-up. Journal of Pediatric Psychology. 2010;35(9):1038–1048. [DOI] [PubMed] [Google Scholar]
- 34.Benjamin Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society. 1995;57(289–300). [Google Scholar]