Abstract
Background:
Antecedents of chronic pelvic pain are not well characterized, but pelvic organ visceral sensitivity is a hallmark of these disorders. Recent studies have identified that some dysmenorrhea sufferers are much more likely to exhibit comorbid bladder hypersensitivity. Presumably, these otherwise healthy women may be at higher risk of developing full-blown chronic bladder pain later in life. To encourage early identification of patients harboring potential future risk of chronic pain, we describe the clinical profile of women matching this putative pain-risk phenotype.
Objective(s):
Characterize demographic, menstrual, pelvic exam, and psychosocial profiles of young women with comorbid dysmenorrhea and bladder hypersensitivity, defined using a standardized experimental visceral provocation test, contrasted with healthy controls, pure dysmenorrhea sufferers, and women with existing bladder pain syndrome.
Study Design:
This prospective cohort study acquired data on participants with moderate-to-severe dysmenorrhea (n=212), healthy controls (n=44), and bladder pain syndrome (n=27). A subgroup of dysmenorrhea patients was found on screening with noninvasive oral water challenge to report significantly higher bladder pain during experimentally monitored spontaneous bladder filling (>15/100 on visual analogue scale, based on prior validation studies) and separately defined as a group with dysmenorrhea+bladder pain. Medical/menstrual history and pain history were evaluated with questionnaires. Psychosocial profile and impact were measured with validated self-reported health status PROMIS short forms and a Brief Symptom Inventory for somatic sensitivity. Pelvic anatomy and sensory sensitivity were examined via a standardized physical examination and a tampon provocation test.
Results:
In our largely young, single, nulliparous cohort (24 ± 1 yo), roughly a quarter (46/212) of dysmenorrhea sufferers tested positive for the dysmenorrhea+bladder pain phenotype. Dysmenorrhea only sufferers were more likely to be African-American (24%) than healthy controls (5%, post-hoc X2, p=0.007). Pelvic exam findings did not differ in the non-chronic pain groups, except for tampon test sensitivity, which was worse in dysmenorrhea+bladder pain and dysmenorrhea sufferers vs. healthy controls (2.6 ± 0.3 and 1.7 ± 0.2, vs. 0.7 ± 0.2, p <0.05). Consistent with heightened pelvic sensitivity, participants with dysmenorrhea+bladder pain also had more nonmenstrual pain, dysuria, dyschezia and dyspareunia (p’s <0.05). Participants with dysmenorrhea+bladder pain had PROMIS Global Physical T-scores of 47.7±0.9, lower than in women with dysmenorrhea only (52.3±0.5), and healthy controls 56.1±0.7 (p< 0.001). Similarly they had lower PROMIS Global Mental T-score than healthy controls (47.8±1.1 vs. 52.8±1.2, p = 0.017). Similar specific impairments were observed on PROMIS scales for anxiety, depression, and sleep in participants with dysmenorrhea+bladder pain vs. healthy controls.
Conclusion(s):
Women with dysmenorrhea who are unaware they also have bladder sensitivity, exhibit broad somatic sensitivity and elevated psychological distress, suggesting combined preclinical visceral sensitivity may be a precursor to chronic pelvic pain. Defining such precursor states is essential to conceptualize and test preventative interventions for chronic pelvic pain emergence. Dysmenorrhea+bladder pain is also associated with higher self-reported pelvic pain unrelated to menses, suggesting central nervous system changes are present in this potential precursor state.
Condensation:
Dysmenorrheic women with experimental bladder sensitivity exhibit increased psychological distress and sensory sensitivity, suggesting they may have higher future risk for chronic pelvic pain.
INTRODUCTION
Precursors to chronic pelvic pain (CPP) syndromes are largely unknown1,2. CPP can be devastating and highly disruptive, with inadequate treatments in many cases3,4. These become harder to treat late in their course due to overlapping biopsychosocial contributors – central sensitization (heightened awareness of unpleasant sensations), loss of social support, medication side effects, and expanded psychosocial distress5. Dysmenorrhea is commonly associated with CPP (up to 80% of the time), but does not inexorably lead to CPP6. Thus, additional complementary markers of chronic pain vulnerability need to be identified to formulate a practical definition of a precursor state. Known risk factors for CPP include injury, early life trauma, infection, anxiety, and depression7–10.
Hypersensitivity to organ distension is a core feature observed in chronic visceral pain states. Prolonged experience of such visceral pain, even if episodic, may enhance transmission of noxious information from the pelvis via spinal dorsal horn neuronal activity, or unfavorably alter connectivity of brain regions monitoring the state of pelvic organs, core processes thought to underlie emergence of chronic pain11. Our team has previously validated an experimentally controlled, noninvasive bladder hyperalgesia task in CPP patients12,13 and furthermore have found such visceral hyperalgesia in up to a quarter of women with moderate-to-severe dysmenorrhea, but no chronic pain issues14. Definition of clinical features that discriminate women with and without experimental bladder sensitivity is of practical value for potential risk screening, but has not been done to date. Our research question describes findings from the initial four years of a prospective bladder pain risk cohort study, Chronic Pain Risk Associated with Menstrual Pelvic Pain (CRAMPP). We compare the basic demographic, clinical exam features, menstrual cycle characteristics, and psychosocial profile of this dysmenorrhea+bladder pain cohort with a larger group of similarly-aged, isolated moderate-to-severe dysmenorrhea sufferers, healthy controls, and women with bladder pain syndrome (BPS).
METHODS
The study was approved by the NorthShore University HealthSystem Institutional Review Board, and we obtained informed consent for all participants. As previously described, the prospective observational study Chronic pain Risk Associated with Menstrual Pelvic Pain (CRAMPP) was designed to characterize the prevalence of viscero-visceral convergence between uterine pain sensitivity on bladder pain, and possible underlying mechanisms14. Between August 2014 and December 2018, we recruited female participants with community flyers, through contact via the Illinois Women’s Health Registry (http://www.womenshealth.northwestern.edu/programs/illinois-womens-health-registry), and by referral from NorthShore University HealthSystem affiliated gynecology clinics. Candidates were initially phone screened, or completed an online pre-screen. Eligible participants were scheduled for a screening visit at Evanston Hospital.
Inclusion criteria.
We initially recruited three different cohorts (age 18–45) into CRAMPP – healthy pain-free controls, women with self-reported moderate-to-severe dysmenorrhea, and chronic pain-positive controls. Dysmenorrhea participants had to rank menses pain > 4/10 on a numeric rating scale (NRS: 0: No pain, 10: Worst pain imaginable) and have no concurrent chronic pain diagnoses. This threshold corresponds to a moderate-to-severe subjective pain cutpoint, based on previous studies15. Our prior work has shown that bladder pain sensitivity and other CPP syndromes are uncommon with menstrual pain ≤ 3/10, but are 13-fold higher for pain > 4/1013,14,16. Healthy controls were required to rate their average menstrual pain ≤ 3/10 (NRS) during menses and be free of chronic pain. Status was further confirmed with a prospective, one month menstrual diary to ensure dysmenorrhea patients had ≥ 3/10 menstrual pain (they could be on analgesics during the diary period). The key subgroup that was the focus of this study, dysmenorrhea+bladder pain, was defined in a subsequent assessment visit detailed below.
BPS was diagnosed as >3 months pelvic pain (average intensity ≥ 3/10), pressure, or discomfort related to the bladder accompanied by at least one other urinary symptom such as persistent urgency or frequency17. Other conditions commonly presenting with similar irritative urinary symptoms had to have been ruled out by clinical examination or cystoscopy if necessary. BPS participants could have other chronic pain conditions.
Exclusion criteria.
Participants were excluded for the presence of active pelvic or abdominal malignancies, absence of regular menses, active genitourinary infection in the last four weeks, inability to read or comprehend the informed consent in English, unwillingness to undergo pelvic examination/testing, hypertension, and unwillingness to withdraw from oral contraceptives for two months prior to the study due to other study aims. Study enrollment flow is shown in Supplemental Figure 1.
Screening Visit.
Participants completed questionnaires encompassing medical, surgical, psychological, and gynecological history. Standard demographic questions (age, ethnicity, marital status) were included. Chronic pain states often are accompanied by psychosocial distress, so the validated NIH Patient Reported Outcomes Measurement Information System (PROMIS) short forms were used: anxiety(8a), depression(8b), fatigue(7a), sleep(8b), pain behavior(7a), pain interference(6b), physical functioning(10a), social role satisfaction(7), and the global health-10 (which includes an NRS 0–10 pain score)18. We used 0–100 mm visual analogue scales (VAS) for self-reported pelvic pain (over last week) as well as dyspareunia, dysuria, and dyschezia. We assessed overall somatic symptom sensitivity with the six-item validated Brief Symptom Inventory-somatization subscale (BSI/S)19.
We also conducted standardized examination for pelvic floor dysfunction, frequently comorbid with bladder pain issues; this was done by a fellowship-trained gynecologist (FT or SS), blinded to subject identity (except for 13/20 BPS patients who had seen their division for clinical evaluation)20. During an abdominal and pelvic exam, the midline bladder (corresponding to the trigone) was palpated transvaginally along with levator ani and obturator interni bilaterally, as well as the urethra, uterus, fornices, and uterosacral ligaments. The examiner’s finger palpation pressure was calibrated using a pressure algometer to apply roughly 0.6–0.8 kg/cm2, while vaginal pain was also assessed by having the finger sit at rest inside the canal without movement21. Participants rated palpation-induced pain on a 0–10 NRS. Pelvic floor strength (0–5 Likert scale) and control (none, some, complete ability to relax) were also assessed21. Patients also self-performed a standardized tampon sensitivity test as a marker of vulvar sensitivity, and rated the discomfort on a 0–10 NRS22.
Assessment Visit.
A formal experimental visit was conducted following the case confirmation diaries, generally about a month later, at Evanston Hospital. This also was the point where we formally identified women with the dysmenorrhea+bladder pain phenotype. The primary assessment for bladder sensitivity used our previously published oral water challenge to provoke diuresis and mimic cystometry, with ratings of pain and urgency obtained every fifteen minutes as well as at baseline, first sensation, first urge, and maximum tolerance12,13. This was scheduled for the luteal phase days 17–25 wherever possible, with ovulation kits used for confirmation. Abnormal bladder filling-provoked pain was defined as pain at first urge > 15/100 VAS, a threshold that differentiates participants with and without heightened bladder pain sensitivity on multiple bladder health parameters14. With this cutoff 24% (24/98) of dysmenorrhea sufferers also exhibit this unexpected bladder cross-organ sensitization (labeled as dysmenorrhea+bladder pain hereafter), while only 6% of healthy controls show such sensitivity (2/35).
Statistics.
Analyses were performed in SAS version 9.3. Complete data sets were obtained for all analyzed variables except for the tampon test in 24 participants who either did not feel comfortable with the test or failed to complete it. Clinical assessments were performed up to February 8, 2017, when objectives of the parent study for that phase had been met. All data was entered into REDCap with specified field range restrictions23. Group comparisons were made with ANOVA and Chi-squared test of proportions for parametric data or with a Kruskal-Wallis test (based on Shapiro-Wilks test). Post hoc-tests with a p<0.05 threshold were made to test our primary research questions: does the dysmenorrhea+bladder pain group differ from healthy controls on a) self-report of pelvic pain (bladder, bowel, and pain during intercourse 0–100 VAS), b) pelvic myofascial function (pelvic relaxation and pain) during a structured exam, and c) tampon test (as a measure of clinical pelvic sensitivity). Post-hoc power sensitivity analysis for these two groups (Table 1) confirmed we could detect an effect size d=0.6 with 80% power. Secondary analyses compared a) dysmenorrhea+bladder pain vs. dysmenorrhea only and b) dysmenorrhea only vs. healthy controls, using Tukey’s multiple comparisons adjustment. Finally, exploratory analyses without adjusting for comparisons were also done for findings in healthy controls vs. BPS. P-values to facilitate comparisons are also presented for all assessed demographic, clinical exam, and psychosocial profile variables separately, without any formal research question. Our sample sizes are unequal since CRAMPP primarily focused on recruiting dysmenorrhea patients. To accommodate the potential effects of unequal sample sizes, we confirmed the homogeneity of variance in all analyses.
Table 1:
Demographic Information for women with dysmenorrhea and controls.
| HC (n=44) | DYS (n=166) | DYSB (n=46) | BPS (n=27) | Group | DYS vs. HC | DYSB vs. HC | DYS vs. DYSB | |
|---|---|---|---|---|---|---|---|---|
| Age | 23.8 (1.0) | 24.5 (0.5) | 23.8 (0.9) | 29.0 (1.1) | 0.003 | 0.927 | 1.000 | 0.919 |
| Education | ||||||||
| Completed high school | 4 (9.1%) | 18 (10.8%) | 5 (10.9%) | 3 (11.1%) | 0.023 | 0.146 | 0.085 | 0.766 |
| Some college | 22 (50.0%) | 68 (41.0%) | 19 (41.3%) | 8 (29.6%) | ||||
| Associate | 2 (4.6%) | 6 (3.6%) | 0 (0.0%) | 4 (14.8%) | ||||
| Bachelor | 5 (11.4%) | 48 (28.9%) | 15 (32.6%) | 3 (11.1%) | ||||
| Postgraduate | 11 (25.0%) | 26 (15.7%) | 7 (15.2%) | 9 (33.3%) | ||||
| Race | ||||||||
| White | 24 (55.8%) | 84 (50.9%) | 25 (54.4%) | 22 (81.5%) | 0.007 | 0.023 | 0.183 | 0.290 |
| African American | 2 (4.6%) | 39 (23.6%) | 8 (17.4%) | 3 (11.1%) | ||||
| Asian | 12 (27.9%) | 25 (15.1%) | 11 (23.9%) | 0 (0.0%) | ||||
| Other | 5 (11.6%) | 17 (10.3%) | 2 (4.3%) | 2 (7.4%) | ||||
| Marital Status | ||||||||
| Single | 39 (88.6%) | 136 (81.9%) | 36 (78.3%) | 12 (44.4%) | 0.011 | 0.625 | 0.394 | 0.932 |
| Living with partner | 1 (2.3%) | 13 (7.8%) | 4 (8.7%) | 5 (18.5%) | ||||
| Married | 4 (9.1%) | 14 (8.4%) | 5 (10.9%) | 9 (33.3%) | ||||
| Separated | 0 (0.0%) | 1 (0.6%) | 0 (0.0%) | 0 (0.0%) | ||||
| Divorced/widowed | 0 (0.0%) | 2 (1.2%) | 1 (2.2%) | 1 (3.7%) | ||||
| Body mass index | 22.1 (0.4) | 23.6 (0.4) | 23.7 (0.7) | 25.2 (1.2) | 0.053 | 0.214 | 0.385 | 1.000 |
| Do you smoke cigarettes? | ||||||||
| Yes | 2 (4.5%) | 8 (4.8%) | 0 (0.0%) | 2 (7.4%) | 0.046 | 0.936 | 0.341 | 0.277 |
| No | 39 (88.6%) | 144 (86.7%) | 43 (93.5%) | 18 (66.7%) | ||||
| Not currently, but used to | 3 (6.8%) | 14 (8.4%) | 3 (6.5%) | 7 (25.9%) | ||||
| Moderate Exercise (Hours/ Week) | 6.9 (0.6) | 9.0 (0.7) | 7.3 (0.8) | 8.6 (1.6) | 0.293 | 0.371 | 0.996 | 0.522 |
| Vigorous Exercise (Hours/Week) | 3.6 (0.4) | 3.4 (0.3) | 3.4 (0.5) | 1.8 (0.4) | 0.108 | 0.994 | 0.996 | 1.000 |
The mean (SEM - standard error of the mean) or sample size (percentage) for each item for each of the 4 groups is shown. Note bivariate contrasts for BPS are not shown fully in Supplemental Table 1. The right 4 columns indicate the significance for contrasts for aggregate group differences, DYS vs. HC, DYSB vs. HC, DYS vs. DYSB. DYS – dysmenorrhea, DYSB-dysmenorrhea with bladder sensitivity, HC healthy controls, BPS bladder pain syndrome
Results
Demographics
At the time of this analysis, we enrolled a total of 284 participants in CRAMPP, 212 women with moderate-to-severe dysmenorrhea, 44 healthy controls, and 27 BPS patients. One participant was excluded due to inconsistent responses to her pelvic pain profile questions. Forty-six of the dysmenorrhea cohort met our criteria for experimental bladder pain sensitivity and comprise the dysmenorrhea+bladder pain cohort for the subsequent analyses.
As shown in Table 1, the dysmenorrhea and healthy control participants were young, predominantly single, and nulliparous. More African American women were recruited into the dysmenorrhea only group (24%), compared to healthy controls (5%; p = 0.023). Conversely, BPS participants were mostly Caucasian and 5 years older on average. The overall cohort had fairly high educational attainment, with ~90% of all subgroups noting some level of college attainment. The groups did not differ in the amount of self-reported weekly intensity of moderate or vigorous exercise.
Menstrual & Clinical Exam Characteristics
As expected, participants with BPS, dysmenorrhea, and dysmenorrhea+bladder pain had similarly high levels of menstrual pain vs. healthy controls (Table 2, p< 0.001). The majority of all participants (>70%) had comparable menstrual cycle length, with a mean duration of 5.8 – 6.4 days (Table 2). There were no differences across the groups for menstrual cycle characteristics. Previous birth control pill exposure did not differ between dysmenorrhea cohorts and healthy controls (33–40%) but was markedly higher in BPS participants (70%; p = 0.008). Most participants (89% or higher in all cohorts) had never had a prior vaginal delivery. Only one patient (BPS) reported a prior pelvic inflammatory disease diagnosis.
Table 2:
Menstrual and reproductive history of women with dysmenorrhea and controls
| HC (n=44) | DYS (n=166) | DYSB (n=46) | BPS (n=27) | P-value | DYS vs. HC | DYSB vs. HC | DYS vs. DYSB | |
|---|---|---|---|---|---|---|---|---|
| Menstrual pain without analgesics (VAS) | 15 (2) | 72 (1) | 73 (2) | 72 (5) | <.001 | <.001 | <.001 | 0.985 |
| Menstrual pain with analgesics (VAS) | 10 (3) | 41 (2) | 50 (4) | 56 (6) | <.001 | <.001 | <.001 | 0.092 |
| Average length of menstrual cycle | ||||||||
| 21 days or less | 2 (4.5%) | 11 (6.6%) | 5 (11.1%) | 4 (17.4%) | 0.599 | 0.475 | 0.626 | 0.530 |
| 22–34 days | 36 (81.8%) | 142 (85.5%) | 36 (80.0%) | 17 (73.9%) | ||||
| 35 days or longer | 4 (9.1%) | 6 (3.6%) | 3 (6.7%) | 1 (4.3%) | ||||
| Not sure | 2 (4.5%) | 7 (4.2%) | 1 (2.2%) | 1 (4.3%) | ||||
| Number of days menstrual bleeding | 5.9 (0.5) | 5.8 (0.1) | 5.7 (0.2) | 6.4 (0.5) | 0.428 | 0.944 | 0.955 | 1.000 |
| Prior birth control pill usage | ||||||||
| Yes | 15 (34.1%) | 67 (40.4%) | 15 (32.6%) | 19 (70.4%) | 0.008 | 0.448 | 0.882 | 0.339 |
| I never have had sex with a male partner | ||||||||
| Yes | 11 (25.0%) | 36 (21.7%) | 7 (15.2%) | 0 (0.0%) | 0.036 | 0.639 | 0.246 | 0.334 |
| Children delivered | ||||||||
| None | 43 (97.7%) | 156 (94.0%) | 43 (93.5%) | 24 (88.9%) | 0.502 | 0.321 | 0.328 | 0.901 |
| Vaginal births | ||||||||
| None | 43 (97.7%) | 159 (95.8%) | 44 (95.7%) | 24 (88.9%) | 0.359 | 0.549 | 0.584 | 0.969 |
| Previously diagnosed with PID? | ||||||||
| Yes | 40 (97.6%) | 107 (91.5%) | 29 (100.0%) | 17 (85.0%) | 0.109 | 0.186 | 0.397 | 0.103 |
The mean (SEM - standard error of the mean) or sample size (percentage) for each item for each of the 4 groups is shown. Note bivariate contrasts for BPS are fully shown in Supplemental Table 1. The right 4 columns indicate the significance for contrasts for aggregate group differences, DYS vs. HC, DYSB vs. HC, DYS vs. DYSB. DYS – dysmenorrhea, DYSB-dysmenorrhea with bladder sensitivity, HC healthy controls, BPS bladder pain syndrome, VAS – visual analogue scale (0–100), PID – pelvic inflammatory disease
Next, we sought to establish whether clinical exam characteristics would discriminate women with either dysmenorrhea or bladder sensitivity (Table 3). Notably, this was the first pelvic exam for 30% of participants. Most participants (82–90%) were able to voluntarily relax their pelvic floor. We found no group differences in pelvic floor flexibility, control of pelvic floor contraction, or strength of voluntary activation (p’s > .1).
Table 3:
Comparative pelvic exam characteristics of women with dysmenorrhea vs. controls
| HC (n=41) | DYS (n=117) | DYSB (n=29) | BPS (n=20) | P-value | DYS vs. HC | DYSB vs. HC | DYS vs. DYSB | |
|---|---|---|---|---|---|---|---|---|
| Width of the vaginal opening | ||||||||
| One Finger | 33 (80.5%) | 73 (62.4%) | 19 (65.5%) | 11 (55.0%) | 0.401 | 0.075 | 0.240 | 0.946 |
| Two Fingers | 8 (19.5%) | 39 (33.3%) | 9 (31.0%) | 8 (40.0%) | ||||
| Two Fingers, Can Wiggle | 0 (0.0%) | 5 (4.3%) | 1 (3.5%) | 1 (5.0%) | ||||
| Genital hiatus (cm) | 1.04 (0.07) | 1.04 (0.04) | 1.16 (0.09) | 1.01 (0.08) | 0.568 | 1.000 | 0.655 | 0.569 |
| Perineal body (cm) | 2.01 (0.06) | 2.06 (0.04) | 2.12 (0.08) | 2.00 (0.08) | 0.692 | 0.928 | 0.717 | 0.898 |
| Ability to relax PFM after contraction | ||||||||
| 100% Relaxation | 34 (82.9%) | 104 (88.9%) | 25 (86.2%) | 18 (90.0%) | 0.767 | 0.323 | 0.710 | 0.687 |
| Some Relaxation | 7 (17.1%) | 13 (11.1%) | 4 (13.8%) | 2 (10.0%) | ||||
| General PFM strength | ||||||||
| 0 (unable to contract) | 9 (22.0%) | 9 (7.7%) | 2 (6.9%) | 1 (5.0%) | 0.289 | 0.222 | 0.257 | 0.559 |
| 1 | 11 (26.8%) | 31 (26.5%) | 7 (24.1%) | 3 (15.0%) | ||||
| 2 | 6 (14.6%) | 26 (22.2%) | 10 (34.5%) | 4 (20.0%) | ||||
| 3 | 8 (19.5%) | 24 (20.5%) | 7 (24.1%) | 7 (35.0%) | ||||
| 4 | 6 (14.6%) | 21 (18.0%) | 3 (10.3%) | 5 (25.0%) | ||||
| 5 (strong contraction) | 1 (2.4%) | 6 (5.1%) | 0 (0.0%) | 0 (0.0%) | ||||
| Uterine mobility | ||||||||
| Yes | 40 (97.6%) | 107 (91.5%) | 29 (100.0%) | 17 (85.0%) | 0.109 | 0.186 | 0.397 | 0.103 |
| Uterine size | ||||||||
| Normal | 40 (97.6%) | 113 (96.6%) | 28 (96.6%) | 20 (100.0%) | 0.856 | 0.758 | 0.803 | 0.994 |
| Enlarged | 1 (2.4%) | 4 (3.4%) | 1 (3.4%) | 0 (0.0%) | ||||
| Vaginal tenderness* | 0.1 (0.1) | 0.1 (0.0) | 0.2 (0.2) | 0.6 (0.3) | 0.027 | 0.991 | 0.758 | 0.809 |
| Abdominal tenderness | 0.0 (0.0) | 0.1 (0.0) | 0.3 (0.2) | 1.5 (0.4) | <.001 | 0.906 | 0.385 | 0.580 |
| Pubic tenderness | 0.9 (0.3) | 0.7 (0.1) | 1.7 (0.4) | 3.1 (0.6) | <.001 | 0.869 | 0.122 | 0.007 |
| SIJ tenderness | 0.0 (0.0) | 0.1 (0.0) | 0.5 (0.2) | 1.0 (0.5) | <.001 | 0.972 | 0.067 | 0.061 |
| Iliococcygeus tenderness | 1.0 (0.3) | 0.6 (0.1) | 1.5 (0.4) | 2.8 (0.6) | <.001 | 0.589 | 0.660 | 0.072 |
| Bladder tenderness | 1.1 (0.3) | 0.8 (0.2) | 1.5 (0.4) | 3.3 (0.9) | <.001 | 0.838 | 0.826 | 0.297 |
| Urethral tenderness | 0.7 (0.3) | 0.2 (0.1) | 0.8 (0.3) | 2.7 (0.7) | <.001 | 0.331 | 0.998 | 0.313 |
| Uterine tenderness | 1.2 (0.4) | 1.1 (0.2) | 2.1 (0.5) | 4.7 (0.8) | <.001 | 0.978 | 0.373 | 0.110 |
| Tampon test | 0.7 (0.2) | 1.7 (0.2) | 2.6 (0.3) | 3.1 (0.5) | <.001 | 0.019 | <.001 | 0.043 |
The mean (SEM - standard error of the mean) or sample size (percentage) for each item for each of the 4 groups is shown. Note bivariate contrasts for BPS are fully shown in Supplemental Table 1. The right 4 columns indicate the significance for contrasts for aggregate group differences, DYS vs. HC, DYSB vs. HC, DYS vs. DYSB. DYS – dysmenorrhea, DYSB-dysmenorrhea with bladder sensitivity, HC healthy controls, BPS bladder pain syndrome, PFM – pelvic floor muscle, SIJ – sacroiliac joint,
all tenderness is on 0–10 numeric rating scale
Clinical exam-evoked tenderness at all sites was significantly different between groups by ANOVA (p < 0.001), but outside of expected worse pain at all sites for BPS vs. healthy controls, significant individual contrasts were only seen at the pubic bone, and only for dysmenorrhea+bladder pain (DYSB) vs. dysmenorrhea (DYS). In a subanalysis of 20 BPS participants (13 unblinded, 7 blinded), clinical exam results were similar whether the patient was known to the examiner (Supplemental Table 1). Tampon test pain ratings (0–10 NRS) were higher in both dysmenorrhea groups (DYS:1.7 ± 0.2, DYSB: 2.6 ± 0.2) and BPS patients (3.1±0.5) compared with healthy controls (0.7 ± 0.2 all p < 0.05), and dysmenorrhea+bladder pain patients reported significantly higher pain than dysmenorrhea only (p = 0.04).
Pain and psychological characteristics
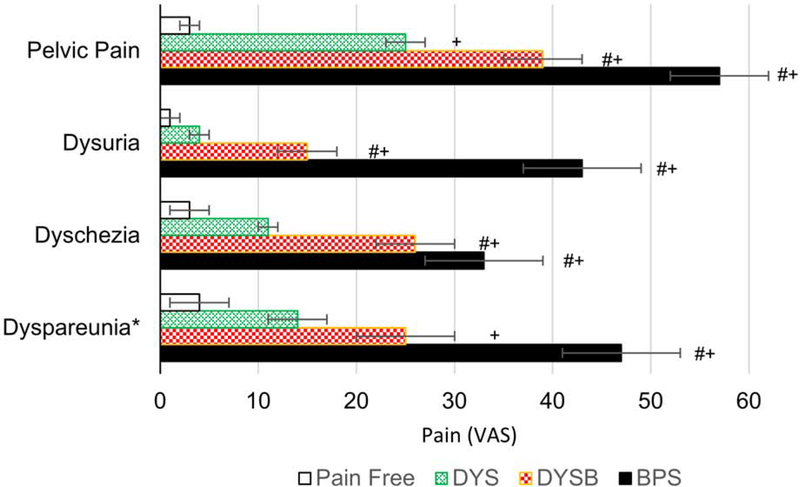
Patients with dysmenorrhea+bladder pain and BPS had more bodily pain (PROMIS global 0–10 NRS, 3.2±0.3 and 5.4±0.4 respectively) compared to women with dysmenorrhea (2.2±0.2) and healthy controls (0.4±0.2, p’s<0.01). While dysmenorrhea sufferers reported significantly higher bodily pain and pelvic pain in the last week than healthy controls (outside menstrual phase), dysmenorrhea+bladder pain sufferers like BPS patients reported higher pelvic pain scores than healthy controls on all pain domains. Dysmenorrhea+bladder pain participants’ pelvic pain subdomain scores were also higher than in dysmenorrhea sufferers, except for dyspareunia (p’s< 0.05).
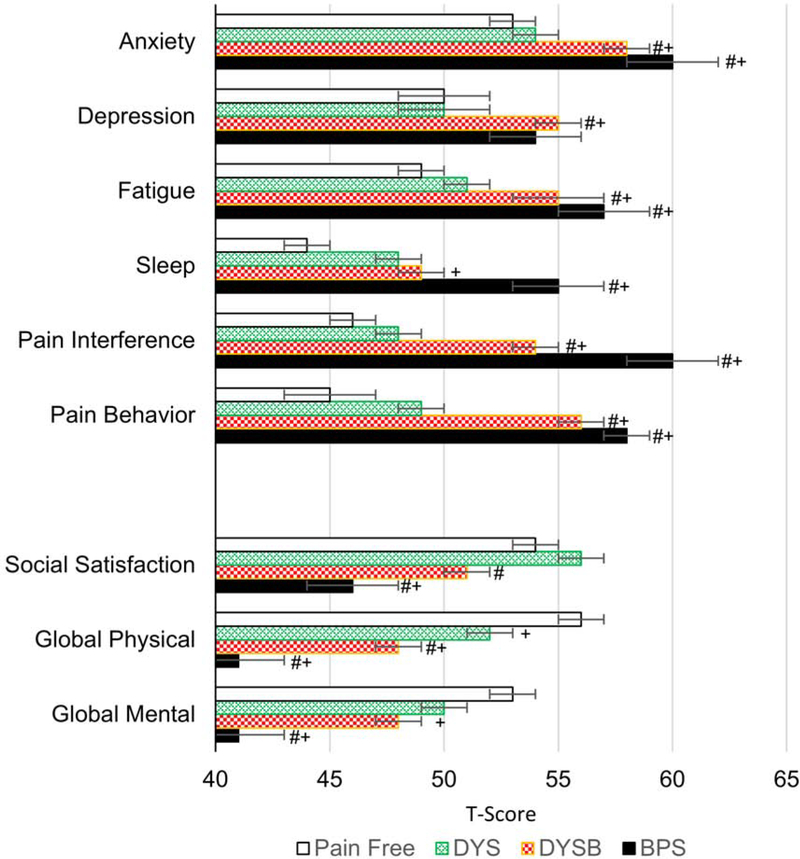
We also confirmed significantly worse mood and anxiety profiles in the dysmenorrhea+bladder pain group vs. both dysmenorrhea only and healthy controls [Fig 2, ex. PROMIS anxiety 58.3±1.2 (DYSB) vs. 54.3±0.8 (DYS) vs. 52.6±1.2 (HC) all p’s < 0.05]. Interestingly PROMIS fatigue, pain interference, pain behavior, physical function, and Brief Symptom Inventory – somatization subscale were all significantly worse for dysmenorrhea+bladder pain vs. dysmenorrhea only and healthy controls, with their ratings intermediate between pure dysmenorrhea and BPS [ex. BSI/S 3.8±0.5 (DYSB) vs. 2.2±0.2 (DYS) vs. 1.6±0.4 (HC), p’s < 0.05]. PROMIS sleep disturbance and social role satisfaction were also rated as worse by dysmenorrhea+bladder pain participants compared to healthy controls (p <0.05).
Figure 2. Comparative patient reported outcomes in dysmenorrhea and controls.
Bars indicate mean PROMIS (Patient-Reported Outcomes Measurement Information System) and SEM in each of the four groups. DYS – dysmenorrhea, DYSB – dysmenorrhea with bladder pain sensitivity, BPS – bladder pain syndrome. + designates p<0.05 vs. healthy controls, # p<0.05 vs. DYS. T-scores for more adverse outcome are positive in the top six categories, and negative in the bottom three.
These group differences in psychosocial domains in dysmenorrhea+bladder pain participants parallel significant reductions in reported quality of life (also seen in BPS patients). Participants with dysmenorrhea+bladder pain had PROMIS Global Physical T-scores of 47.7, lower than in dysmenorrhea only (52.3), and healthy controls 56.1 (p< 0.001). Similarly, they have lower PROMIS Global Mental T-score than healthy controls (47.8 vs. 52.8, p = 0.017).
Comments
Principal Findings
In this young, predominantly nulliparous cohort, we demonstrate that those dysmenorrheic women who also demonstrate experimental bladder sensitivity, have heightened clinical exam sensitivity, self-reported pelvic pain, and psychological distress compared to both healthy controls and women with dysmenorrhea only. There were no significant differences in dysmenorrhea+bladder pain vs. healthy controls for exam-provoked pain, however, except for the tampon test, suggesting clinical exam findings are less likely to be useful in identifying at-risk pelvic pain patients. Women with dysmenorrhea+bladder pain were intermediate in symptom intensity compared to women with formal BPS, despite the latter being only five years older.
Results within context of existing literature
Within the bladder pain literature, other groups have proposed screening strategies for at-risk groups for BPS24–26. Parsons and colleagues have described both a questionnaire (Pelvic Pain and Urgency/Frequency -PUF) surveying irritative and painful bladder symptoms, and direct intravesical infusion of potentially irritating potassium chloride to unmask unrecognized bladder hypersensitivity. Warren and colleagues’ epidemiological studies found that BPS participants more commonly report distant, prodromal symptoms of urinary urgency, frequency or bladder pain persisting for at least 4 weeks, at some point preceding the index presentation of BPS (57% vs. 18%). Although the PUF questionnaire has proven to be nonspecific for identifying full blown BPS, longitudinal studies have not yet been performed to see if it might predict general pelvic pain sensitivity, as suggested by Warren and colleagues. Our strategy moves beyond their thinking, by emphasizing assessment in enriched subgroups with one visceral pain condition that presents early in the pain risk trajectory. Natural bladder filling may also have advantages over both questionnaires and an invasive catheter based method, in being more naturalistic, and permitting instantaneous, real-time assessment of visceral discomfort. Given the tampon test alone discriminated dysmenorrhea+bladder pain from healthy controls, a composite of these two tests might also warrant study.
We also can cast our findings against some known risk factors for dysmenorrhea identified in a previous systematic review conducted by Latthe and colleagues27. We did not observe an association with key potential factors such as low BMI, smoking, longer cycles or heavier cycles, prior pelvic inflammatory disease, exercise involvement, recent hormonal contraceptive use, or nulliparity (although the study was not formally powered to test for many of these factors). An important finding we observed for psychological profiles that extends their work (nonsignificant trends for anxiety and depression) is that for virtually all measures, significant group differences are present in the dysmenorrhea+bladder pain subset, and not dysmenorrhea only sufferers. Given our findings, future studies of psychosocial function with dysmenorrhea should ideally account for comorbid visceral dysfunction even if subclinical, or other features of hypersensitivity, as we have pointed out in a recent paper28.
Clinical implications
Could consistent early abolition of menstrual pain reduce the effects of peripheral inflammation on the central nervous system, and thus reduce the risk of developing central sensitization? Our data only informs half of this question, suggesting that a group of women with dysmenorrhea already harbor worrisome features of sensory dysregulation. A major challenge with dysmenorrhea treatment is that many women do not achieve pain relief with NSAIDs29,30, or are intolerant of hormonal suppression. Clinicians might consider pursuing earlier, and even multimodal treatments for dysmenorrhea to see if chronic pelvic pain can be prevented, although the full causal pathway must be confirmed. Separately, the unexpected finding of heightened pelvic pain (outside of the menses) by dysmenorrhea only sufferers may be important to assess for future pelvic pain drug study approvals, as this imposes significant floor effects on any therapeutic interventions applied to CPP patients.
Research implications
Our data suggests that sensory and emotional dysregulation is present for many of these dysmenorrhea+bladder pain patients, although of lower magnitude than that observed in BPS. These higher levels of psychosomatic symptoms are likely prognostic. Nicholl and colleagues found in a large prospective, community-based study that 80% of all new cases of IBS developing over 15 month follow-up exhibited at least two of the following factors – heightened somatic sensitivity, illness behavior, sleep problems, or anxiety31. Longitudinal studies are needed to estimate the specific risk for our dysmenorrhea+bladder pain cohort and identify modifiers. In addition, we observed that our dysmenorrhea only participants have higher average overall pelvic pain symptoms and menstrual pain, as well as an exaggerated objective tampon test response, which suggests we should also look further in these seemingly isolated cases for other permutations of combined organ dysfunction, such as dysmenorrhea plus colonic hyperalgesia or vulvar/pelvic floor hyperalgesia.
Strengths and limitations
We enrolled an ethnically diverse, non-clinical sample of dysmenorrheic women who are in the earlier phase of their life course, before multiple risk factors for chronic pain become entrenched. Our dysmenorrhea+bladder pain phenotype is stable over approximately a month13,14, and our preliminary studies show similar stability at six months (unpublished data). We used validated questionnaires for psychosocial profiling and used standardized assessments for pelvic sensitivity. This is one of the largest studies to employ clinical phenotyping, alongside such assessments, by an experienced gynecological surgeon.
The data we have presented here replicate the dysmenorrhea+bladder pain phenotype we first described in a pilot study13, but still should be externally validated. As BPS was not a primary contrast targeted in this study, we should be cautious interpreting those findings. While we did not see differences in findings between blinded or unblinded exams of these BPS participants, we cannot definitively state which differences meaningfully discriminate the at risk phenotype patients from full blown BPS without a larger chronic pain sample (which this study was not designed to formally answer). The generalizability of our findings is unknown in older reproductive age women, other ethnic groups, or women experiencing early parity. It would also be valuable to explore if there is a male analog involving a non-uterine source of recurrent pain.
Conclusions
Young women with both dysmenorrhea and experimental bladder sensitivity unexpectedly exhibit elevated self-reported and objective evidence of pelvic sensitivity as well as broad mood dysfunction, two constructs identified in prospective studies as key features of full blown chronic pain syndromes. Further study to refine this novel risk phenotype should ensure it has clearly reproducible characteristics, and begin to measure its prospective predictive power for future CPP emergence.
Supplementary Material
Figure 1. Comparative pain symptoms of women with dysmenorrhea and controls.
Bars indicate mean self-reported pain and symptoms scores and SEM in each of the four groups. VAS – visual analogue score (0–100), DYS – dysmenorrhea, DYSB – dysmenorrhea with bladder pain sensitivity, BPS – bladder pain syndrome. + designates p<0.05 vs. healthy controls, # p<0.05 vs. DYS, * dyspareunia contrasts only among women indicating currently sexually active.
3). AJOG at a glance
- Why was this study conducted?
- To describe clinical characteristics of women demonstrating a postulated chronic pelvic pain risk phenotype, presently enrolled in a prospective cohort study of dysmenorrhea, CRAMPP.
- What are the key findings?
- Despite being free of chronic pain, women who have comorbid dysmenorrhea and experimental bladder sensitivity exhibit heightened psychological distress and increased sensory sensitivity.
- What does this study add to what is already known?
- Provides evidence that broad pelvic sensory sensitivity may be a precursor to chronic pelvic pain symptoms, which should encourage formulation of a valid, stable, at-risk phenotypic profile that could be targeted with preventative efforts.
Funding:
This research was supported by NICHD HD081709, NIDDK DK100368, and NorthShore University Health System.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Previously presented at: International Pelvic Pain Society, Toronto, ON, October 18–19. 2019
Conflicts of interest: F.F.T. is a consultant for AbbVie and UroShape, is on speaker’s bureau for AbbVie, and receives royalties from UpToDate. SS is a consultant for Olympus, Emmi, and Allergan, and an owner of Klaas, LLC. D.C. reports grants and personal fees from Aptinyx and Pfizer and personal fees from Daiichi Sankyo, Intec Pharma, Eli Lilly and Company, Samumed, Theravance, Tonix, Williams & Connolly LLP, and Zynerba.
The remaining authors report no conflict of interest.
References
- 1.Apkarian AV, Baliki MN, Farmer MA. Predicting transition to chronic pain. Curr Opin Neurol. 2013;26(4):360–367. doi: 10.1097/WCO.0b013e32836336ad [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heinricher MM. Pain Modulation and the Transition from Acute to Chronic Pain. Adv Exp Med Biol. 2016;904:105–115. doi: 10.1007/978-94-017-7537-3_8 [DOI] [PubMed] [Google Scholar]
- 3.Fayaz A, Croft P, Langford RM, Donaldson LJ, Jones GT. Prevalence of chronic pain in the UK: a systematic review and meta-analysis of population studies. BMJ Open. 2016;6(6):e010364. doi: 10.1136/bmjopen-2015-010364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johannes CB, Le TK, Zhou X, Johnston JA, Dworkin RH. The prevalence of chronic pain in United States adults: results of an Internet-based survey. J Pain. 2010;11(11):1230–1239. doi: 10.1016/j.jpain.2010.07.002 [DOI] [PubMed] [Google Scholar]
- 5.Dragioti E, Evangelou E, Larsson B, Gerdle B. Effectiveness of multidisciplinary programmes for clinical pain conditions: An umbrella review. J Rehabil Med. 2018;50(9):779–791. doi: 10.2340/16501977-2377 [DOI] [PubMed] [Google Scholar]
- 6.Zondervan KT, Yudkin PL, Vessey MP, et al. Chronic pelvic pain in the community--symptoms, investigations, and diagnoses. Am J Obstet Gynecol. 2001;184(6):1149–1155. [DOI] [PubMed] [Google Scholar]
- 7.Chitkara DK, van Tilburg MAL, Blois-Martin N, Whitehead WE. Early life risk factors that contribute to irritable bowel syndrome in adults: a systematic review. Am J Gastroenterol. 2008;103(3):765–774; quiz 775. doi: 10.1111/j.1572-0241.2007.01722.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lowman BC, Drossman DA, Cramer EM, McKee DC. Recollection of childhood events in adults with irritable bowel syndrome. J Clin Gastroenterol. 1987;9(3):324–330. [DOI] [PubMed] [Google Scholar]
- 9.van Tilburg MAL, Levy RL, Walker LS, et al. Psychosocial mechanisms for the transmission of somatic symptoms from parents to children. World J Gastroenterol. 2015;21(18):5532–5541. doi: 10.3748/wjg.v21.i18.5532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Warren JW, Howard FM, Cross RK, et al. Antecedent nonbladder syndromes in case-control study of interstitial cystitis/painful bladder syndrome. Urology. 2009;73(1):52–57. doi: 10.1016/j.urology.2008.06.031 [DOI] [PubMed] [Google Scholar]
- 11.Apkarian AV, Bushnell MC, Treede R-D, Zubieta J-K. Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain. 2005;9(4):463–484. doi: 10.1016/j.ejpain.2004.11.001 [DOI] [PubMed] [Google Scholar]
- 12.Tu FF, Kane JN, Hellman KM. Noninvasive experimental bladder pain assessment in painful bladder syndrome. BJOG. 2017;124(2):283–291. doi: 10.1111/1471-0528.14433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tu FF, Epstein AE, Pozolo KE, Sexton DL, Melnyk AI, Hellman KM. A noninvasive bladder sensory test supports a role for dysmenorrhea increasing bladder noxious mechanosensitivity. Clin J Pain. 2013;29(10):883–890. doi: 10.1097/AJP.0b013e31827a71a3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hellman KM, Datta A, Steiner ND, et al. Identification of experimental bladder sensitivity among dysmenorrhea sufferers. Am J Obstet Gynecol. April 2018; 219(1):84e1–84.e8 doi: 10.1016/j.ajog.2018.04.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hirschfeld G, Zernikow B. Variability of “optimal” cut points for mild, moderate, and severe pain: neglected problems when comparing groups. Pain. 2013;154(1):154–159. doi: 10.1016/j.pain.2012.10.008 [DOI] [PubMed] [Google Scholar]
- 16.Westling AM, Tu FF, Griffith JW, Hellman KM. The association of dysmenorrhea with noncyclic pelvic pain accounting for psychological factors. Am J Obstet Gynecol. 2013;209(5):422e.1–422.e10. doi: 10.1016/j.ajog.2013.08.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hanno PM, Burks DA, Clemens JQ, et al. AUA guideline for the diagnosis and treatment of interstitial cystitis/bladder pain syndrome. J Urol. 2011;185(6):2162–2170. doi: 10.1016/j.juro.2011.03.064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cella D, Yount S, Rothrock N, et al. The Patient-Reported Outcomes Measurement Information System (PROMIS): progress of an NIH Roadmap cooperative group during its first two years. Med Care. 2007;45(5 Suppl 1):S3–S11. doi: 10.1097/01.mlr.0000258615.42478.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Derogatis LR, Melisaratos N. The Brief Symptom Inventory: an introductory report. Psychol Med. 1983;13(3):595–605. [PubMed] [Google Scholar]
- 20.Fitzgerald CM, Neville CE, Mallinson T, Badillo SA, Hynes CK, Tu FF. Pelvic floor muscle examination in female chronic pelvic pain. J Reprod Med. 2011;56(3–4):117–122. [PubMed] [Google Scholar]
- 21.Hellman KM, Patanwala IY, Pozolo KE, Tu FF. Multimodal nociceptive mechanisms underlying chronic pelvic pain. Am J Obstet Gynecol. 2015;213(6):827.e1–9. doi: 10.1016/j.ajog.2015.08.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Foster DC, Kotok MB, Huang L-S, et al. The tampon test for vulvodynia treatment outcomes research: reliability, construct validity, and responsiveness. Obstet Gynecol. 2009;113(4):825–832. doi: 10.1097/AOG.0b013e31819bda7c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. Journal of biomedical informatics. 2009;42(2):377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parsons CL, Dell J, Stanford EJ, et al. Increased prevalence of interstitial cystitis: previously unrecognized urologic and gynecologic cases identified using a new symptom questionnaire and intravesical potassium sensitivity. Urology. 2002;60(4):573–578. [DOI] [PubMed] [Google Scholar]
- 25.Parsons CL, Tatsis V. Prevalence of interstitial cystitis in young women. Urology. 2004;64(5):866–870. doi: 10.1016/j.urology.2004.06.044 [DOI] [PubMed] [Google Scholar]
- 26.Warren JW, Wesselmann U, Greenberg P, Clauw DJ. Urinary symptoms as a prodrome of bladder pain syndrome/interstitial cystitis. Urology. 2014;83(5):1035–1040. doi: 10.1016/j.urology.2014.01.012 [DOI] [PubMed] [Google Scholar]
- 27.Latthe P, Mignini L, Gray R, Hills R, Khan K. Factors predisposing women to chronic pelvic pain: systematic review. BMJ. 2006;332(7544):749–755. doi: 10.1136/bmj.38748.697465.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zuckerman RM, Silton RL, Tu FF, Eng JS, Hellman KM. Somatic symptoms in women with dysmenorrhea and noncyclic pelvic pain. Arch Womens Ment Health. 2018; October;21(5):533–541, doi: 10.1007/s00737-018-0823-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oladosu FA, Tu FF, Hellman KM. Nonsteroidal antiinflammatory drug resistance in dysmenorrhea: epidemiology, causes, and treatment. Am J Obstet Gynecol. 2018. April;218(4):390–400. doi: 10.1016/j.ajog.2017.08.108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Owen PR. Prostaglandin synthetase inhibitors in the treatment of primary dysmenorrhea. Outcome trials reviewed. Am J Obstet Gynecol. 1984;148(1):96–103. [DOI] [PubMed] [Google Scholar]
- 31.Nicholl BI, Halder SL, Macfarlane GJ, et al. Psychosocial risk markers for new onset irritable bowel syndrome--results of a large prospective population-based study. Pain. 2008;137(1):147–155. doi: 10.1016/j.pain.2007.08.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.