Abstract
Background
Cardiovascular (CV) disease is the leading cause of death for people with serious mental illness (SMI), but clinicians are often slow to address this risk.
Methods/Design
78 Midwestern primary care clinics were randomized to receive or not receive access to a clinical decision support (CDS) tool. Between March 2016 and September 2018, primary care clinicians (PCPs) received CDS alerts during visits with adult patients with SMI who met minimal inclusion criteria and had at least one CV risk factor not at goal. The PCP CDS included a summary of six modifiable CV risk factors and patient-specific treatment recommendations. Psychiatrists received CDS alerts during their next visit with an eligible patient with SMI that alerted them to an elevated body mass index or recent weight gain and the presence of an obesogenic SMI medication. Study outcomes include total modifiable CV risk, six modifiable CV risk factors, and use of obesogenic SMI medications.
Discussion
This cluster-randomized pragmatic trial allowed PCPs and psychiatrists the opportunity to improve CV risk in a timely manner for patients with SMI. Effectiveness will be assessed using an intent-to-treat analysis, and outcomes will be assessed largely through electronic health record data harvested by the CDS tool itself. In total, 10,347 patients with SMI had an index primary care visit in a randomized clinic, and 8937 patients had at least one follow-up visit. Analyses are ongoing, and trial results are expected in mid-2020.
Keywords: Serious mental illness, schizophrenia, bipolar disorder, cardiovascular disease, clinical decision support, primary care
INTRODUCTION
Cardiovascular (CV) disease is the leading cause of death in people with serious mental illness (SMI), including those with bipolar disorder, schizophrenia or schizoaffective disorder. Patients with SMI die at 2.3 times the rate of the general population and 10–20 years earlier than their age- and gender-matched peers. 1,2 In a study of Minnesota health insurance enrollees, women with SMI died at a median age of 63 years (compared to 85 years for female controls), while men with SMI died at a median age of 53 years (compared to 74 years for male controls).3 Of the 387 people who died of CV disease, patients with SMI died at a median age of 56 years, compared with 83 years in controls—an average of 27 years of life lost.3 Other research has found the estimated relative risk (RR) of several CV risk factors to be higher in those with SMI compared to the general population, including obesity (RR=1.5–2.0), smoking (RR=2.0–3.0), diabetes mellitus (RR=2.0), and dyslipidemia (RR=5).4,5
Even though it is fairly well-known that patients with SMI have higher CV risk, primary care providers (PCPs) are often slow to take timely and appropriate clinical actions to address CV risk factors in this population.6–8 Thus, deficits in the quality of preventive medical care may account for much of this excess CV mortality in adults with SMI.9 Although the American Diabetes Association published a consensus guideline for monitoring patients on antipsychotic medications, it is seldom followed.2,10 Moreover, patients with SMI often receive substandard care, such as lower rates of CV procedures when they are indicated.11–13
SMI medications can unfortunately contribute to cardiometabolic risk. Some SMI medications can cause significant weight gain, often associated with changes in insulin resistance and lipid metabolism, while other SMI medications may be less likely to cause weight gain.14–16 Given these differences, moving from a SMI medication that causes weight gain to one that may be more weight neutral can be an effective strategy for helping patients with SMI and high body mass indexes lose or stablize their weight.17,18
Clinical decision support (CDS) has been identified as one strategy to reduce gaps in evidence-based care. Unfortunately, many CDS studies have not demonstrated improvements in clinical outcomes because of design problems related to sample size, bias, or low use rates.19–24 With more recent studies and improved designs, electronic health record (EHR)-linked CDS has been shown to reduce CV risk and improve control of CV risk factors such as blood pressure (BP) and glucose and reduce 10-year CV risk scores.25–27 In this study, we implemented a CDS tool that alerted both the PCP and the psychiatrist for a given patient with SMI about increased CV risk, with the goal of having the PCP consider appropriate management of CV risk and the psychiatrist consider whether a change of SMI medication could be appropriate. This paper describes a primary care clinic cluster-randomized trial of such a CDS intervention, called CV Wizard, to improve CV risk in people with SMI in three large healthcare systems.
STUDY DESIGN
Study Settings
Three healthcare delivery organizations (Essentia Health, HealthPartners and Park Nicollet) with 78 primary care clinics in Minnesota, Wisconsin and North Dakota participated in the study. Study enrollment started in March 2016 at Site A, October 2016 at Site B, and March 2017 at Site C. Study enrollment at all sites ended on 9/19/2017. The intervention period at all sites ended on 9/19/2018 to allow at least one year of follow-up for all participants.
Randomization
Restricted randomization28,29 was implemented at each site in an effort to balance site-specific contextual factors that could impact the effectiveness of the intervention or pose barriers to its full implementation. Although the factors varied by site, the restricted randomization process was implemented similarly across sites in the following manner. Small clinics that posed contamination risk (e.g., shared providers) were combined into a single randomization unit (Site A: n=30, Site B: n=31, Site C: n=15). Clinic characteristics were examined to identify those that warranted stratification and those for which balance would be desirable. Site A clinics were stratified by the proportion of patients insured by Medicaid (proxy for low socioeconomic status (SES)) and the presence of onsite behavioral health services, pilot site status and the number of patients with SMI. At Site B, balance was desired for urban location, proportion of patients who were current smokers (proxy for low SES) and proportion of patients under 30. At Site C, clinics were stratified by the proportion of patients with SMI with balance desired on the total number of patients, proportion of patients receiving optimal vascular care and proportion of patients insured by Medicaid. The study statistician generated five randomization schemes for each site. At Sites A and C, clinics were allocated 1:1 to two groups. At Site B, clinics were allocated 1:1:1 to three groups to accommodate a 3-arm study that would be taking place later in the same clinics. The randomization schemes for each site were assessed, in order, with the first balanced scheme chosen (Site A chose the second scheme; Site B the fifth; Site C the first) for implementation. The study statistician randomly assigned one of the two clinic groups to intervention at Sites A and C, and two of the three groups at Site B. The remaining clinics were assigned to control.
Enrollment and Eligibility
Eligible patients were those with SMI who had an index visit at any randomized primary care clinic and had at least one post-index visit in a randomized primary care clinic during the intervention period. Patients who were residing in a nursing home or receiving hospice care, or who had an active cancer diagnosis, at any time during the enrollment or intervention period were excluded from analyses. Also, patients who requested to be excluded from research studies at their healthcare systems received care consistent with their clinic’s randomized treatment group but were omitted from analyses. Otherwise, data from the index and post-index visits from all patients with an index and at least one post-index visit were included in the analyses.
The automated calculations performed by the CDS tool at each primary care visit in all randomized clinics over the course of the enrollment and intervention periods were used to identify index and post-index visits. This approach assured complete and consistent evaluation of eligibility criteria for all patients seeking care in the randomized clinics.
The index visit was defined as the first visit for a patient at a randomized primary care clinic during the enrollment period that met the following criteria:
Age 18 to 75, inclusive
Not pregnant
Two outpatient diagnostic codes or one inpatient code of SMI documented in the electronic health record (EHR) in the two years prior to index date. SMI was defined as having bipolar disorder (ICD-9 codes of 296.00–296.89, 301.11; ICD-10 codes of F30.1-F31.9), schizophrenia (ICD-9 codes of 295.0–295.6, 295.8–295.9, 297.1, 297.3, 298.8, 298.9, 301.22; ICD-10 codes of F20.0-F24, F28-F29) or schizoaffective disorder (ICD-9 code of 295.6; ICD-10 codes of F25.0-F25.9).
At least one modifiable CV risk factor not at goal Patients were assigned to the clinic-randomized treatment group of the primary care clinic where their index clinic visit took place. The post-index visits were those that took place at a randomized primary care clinic during the intervention period when a patient with an index visit was not pregnant. Eighty-seven percent of patients with an index visit had at least one post-index visit during the intervention period. In this pragmatic trial, the number and timing of post-index visits depended on the decisions made about follow-up between the primary care provider and the patient in the course of usual care.
Leadership and Stakeholder Engagement
During the planning phase of the study, regular meetings with healthcare systems leaders from all care systems representing both primary and mental health specialty care developed consensus regarding content and workflow of the intervention, including:
Inclusion criteria for patients
Primary care clinics to be randomized into the study
Use of the best practice advisory (BPA) alerting clinicians to patients with SMI with at least one modifiable CV risk factor not at goal
Workflow for rooming staff and PCPs
Design of the CDS printout, including agreement that there be no language on the printout indicating that a patient has SMI, given (a) the potential fallibility of EHR diagnoses, and (b) patients’ potential disagreement with a diagnosis of SMI
Training procedures for PCPs and rooming staff regarding the workflow and education about elevated CV morbidity and mortality in people with SMI
Identification, alert to psychiatric prescriber and intervention for patients with SMI who were on an obesogenic medication for SMI and had either a BMI >25 or had gained 7% or more of their body weight in the previous year
Clinician and Staff Training
At HealthPartners and Park Nicollet, training sessions for PCPs and rooming staff occurred via in-person presentations accompanied by breakfast or lunch. At Essentia Health clinics, where primary care clinics are largely rural and geographically dispersed, PCPs and rooming staff attended an online training, reviewed written training material, received training directly from clinic leaders or attended a live presentation.
Description of the CDS Technology
Hosting the CDS on a secure web service securely linked with the EHR allowed for maximum efficiency and versatility. As in much of healthcare, CV risk factor management is continuously evolving, with updated evidence and guidelines emerging fairly frequently. Maintaining the CDS on a single web service allowed the study team to make necessary updates to risk equations and algorithms relatively easily. It also avoided potentially disruptive changes to the CDS with each EHR upgrade, and allowed the CDS to run without slowing down the EHR production environment, with an average web service run time of less than one second. Most importantly, the web service algorithms not only drove the intervention, but also assured complete collection of necessary data for analysis at both intervention and control clinics. This greatly reduced the burden and increases the accuracy of collecting data needed for Data Safety Monitoring Board monitoring of the study, and for assessment of intervention effectiveness at the end of the study.
Description of CDS Content
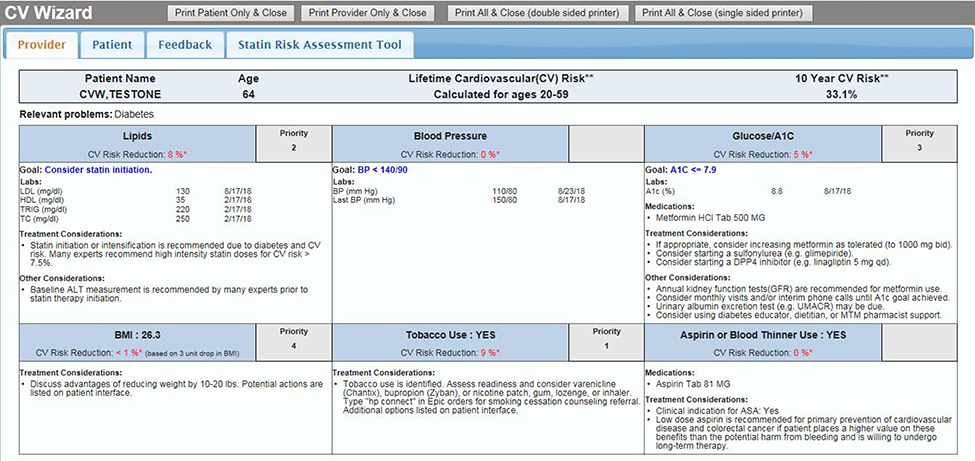
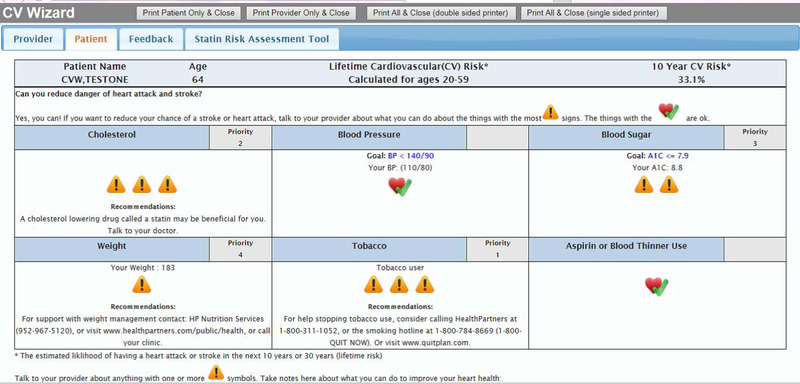
CV Wizard is a set of point-of-care CDS tools that include both a PCP-oriented decision support display as well as a companion patient-oriented display that are designed to be printed and reviewed with patients during their primary care visit. The PCP CDS summarizes six modifiable CV risk factors for each eligible patient: BP, lipids, glucose, smoking status, body mass index (BMI) and aspirin use (Figure 1). It also summarizes the patient’s medications, relevant lab values, allergies and any contraindications to treatment. The PCP CDS estimates the patient’s 10-year and/or 30-year total CV risk (depending on age and availability of patient data) and gives prioritized patient-specific treatment recommendations based on national guidelines for each uncontrolled CV risk factor, including specific medications with dose recommendations. The patient CDS contains similar information about uncontrolled CV risk factors and total CV risk designed for patients at all levels of health literacy and numeracy (Figure 2). The CDS uses evidence-based algorithms and evidence-based CV risk models (such as ACC/AHA30,31 and UKPDS32,33 guidelines) to prioritize the risk factors according to what is likely to be of the most benefit to a specific patient. For each risk factor, CV Wizard calculates a modifiable CV risk (the absolute amount that the 10-year atherosclerotic CV disease (ASCVD) risk could be reversed if the patient were to achieve the goal for that risk factor).
Figure 1.
Example PCP Interface.
Figure 2.
Example Patient Interface.
The CDS patient and PCP versions are displayed on the screen and are usually printed by rooming staff to allow them to be used as shared-decision-making tools by patients and PCPs. Treatment recommendations include both pharmacologic and lifestyle recommendations based on sophisticated algorithms that account for a patient’s most recent clinical values, distance from goals, current therapy, and relevant comorbidities, such as abnormal kidney function. The CDS also provides safety and monitoring alerts.
CDS Implementation
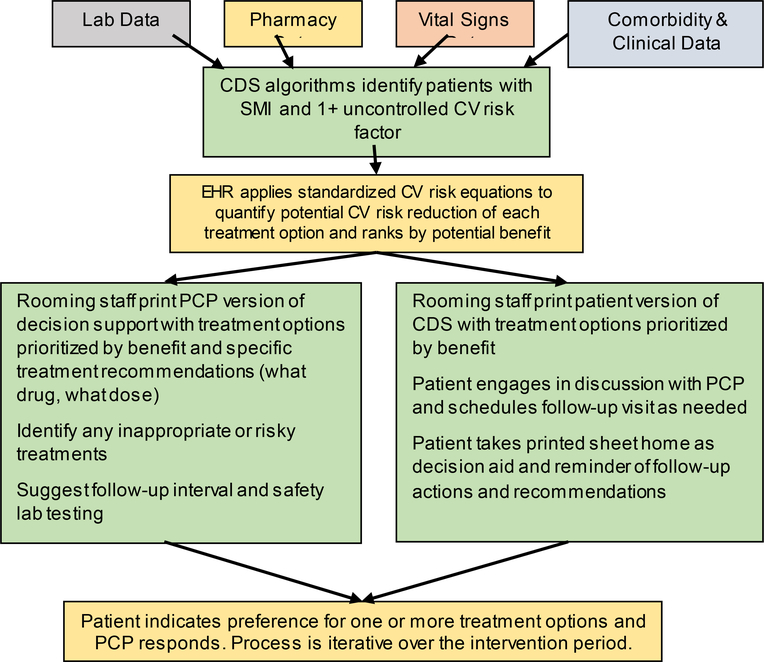
For patients who presented for an appointment at any participating primary care clinic (intervention or control), when a blood pressure was entered into the electronic health record (EHR) and the vitals section closed, selected clinical data were sent from the EHR to the CDS website to assess eligibility for the CV Wizard intervention (Figure 3). For eligible patients at intervention randomized clinics, a BPA was displayed in real time to clinic rooming staff (the staff who typically prepare a patient for a provider visit including obtaining vital signs such as blood pressure). Rooming staff clicked on the BPA to open and print patient and PCP versions of the CDS. Rooming staff handed the patient version to the patient in the exam room and suggested that patients talk with their PCPs about ways they could reduce their CV risk. Rooming staff placed the PCP version of the CDS on the exam room door.
Figure 3.
Schematic Representation of CV Wizard Intervention: How Treatment Options are Identified, Prioritized, and Presented to Patients and Primary Care Providers (PCPs)
In control clinics, the same data flowed from the EHR to the CDS web service, but no BPA or CDS was provided to rooming staff or PCPs. However, for study-eligible SMI patients in both intervention and control clinics, the CDS web service collected and retained relevant data needed for analysis on a secure server, including a randomly-assigned patient identification number, demographic data, vitals, current medications and comorbidities, allergies and laboratory data. CV Wizard was also available to PCPs for any patient by clicking on a button in the navigator bar of the EHR in intervention clinics.
Best Practice Advisory for Psychiatrists
Patients with SMI who met study criteria and had (a) a BMI >25 or a normal BMI with ≥ 7% weight gain in the last year, and (b) were on an obesogenic antipsychotic or antidepressant were identified and tagged in the EHR. Patients who were hospitalized for a psychiatric condition in the last two years or were under court commitment were excluded. In two care systems, at the patient’s next appointment with his or her psychiatric clinician, the clinician received a BPA alerting him or her to the patient’s elevated BMI or recent weight gain and suggested consideration of non-obesogenic alternatives. At the third care system, the patient record was first assessed by a psychiatric nurse, who determined patient eligibility and then contacted the psychiatric clinician to suggest consideration of a medication change. Medications triggering the intervention included amitriptyline, clomipramine, desipramine, doxepin, imipramine, nortriptyline, paroxetine, asenapine, chlorpromazine, clozapine, olanzapine, quetiapine, risperidone, thioridazine, phenelzine, selegiline, tranylcypromine, and mirtazapine. Potentially obesogenic mood stabilizers, such as divalproex and lithium, were not included, as study team members and clinic leaders concluded that were no good non-obesogenic alternatives to these medications. If psychiatric providers wanted to consider transitioning patients to less obesogenic medications, referrals to pharmacists (Sites A and C) or nurse care managers (Site B) were available to support patients and providers during this transition.
Data Sources
The automated EHR data collection and calculations performed by the CDS tool at each primary care visit in the randomized clinics were also used for data collection for the primary outcomes. The CDS tool harvested vitals, medications, diagnoses and orders documented in the EHR over the two years prior to each web service call. These data elements were used to calculate total modifiable CVR at each index and post-index visit and document all CV risk factors. In addition, EHR data were harvested to obtain information on potential important safety events such as suicidal ideation (documented in the ninth item of the Patient Health Questionnaire (PHQ9)34), suicide attempts, hospitalizations and emergency department visits. The EHR data are assumed to completely capture care delivered to patients; to the extent that care was delivered to patients but not documented, it is assumed that these are extremely rare and random events. PCPs were asked to complete surveys regarding their perception of the usefulness of CV Wizard, their likelihood to recommend the tool to colleagues, and recommendations on how to improve the tool.
Fidelity to the Intervention
Use of CV Wizard was estimated by tracking CDS print rates. Monthly print rate reports were provided to clinic leadership. Reports compared print rates across clinics in each care system, and also provided PCP-level use rates for each individual clinic. At Sites A and C, clinic print rates were listed by clinic in the order of highest to lowest print rate for the current month, and by displaying the print rates of each clinic for the past 12 months in a grid that highlighted print rate levels in green (meeting goal), yellow (close to meeting goal) and red (not close to meeting goal). At Site B, clinic print rates were compared to the goal rate and the average of all clinics. In addition to distribution of monthly print rate information, study staff at all three sites periodically contacted the lead nurse at clinics with low print rates to inquire if assistance in improving rates was desired. Study staff also periodically contacted the lead nurse at clinics with low print rates to inquire if assistance in improving print rates was desired. Use of CV Wizard was incentivized at Sites A and C by providing lunch to clinic staff when a clinic achieved the goal of printing at or above 75% of eligible visits for at least four months. Use of CV Wizard was not incentivized at Site B.
Study Outcomes
The primary study outcomes are the change in total modifiable CV risk and in the six individual modifiable CV risk factors (blood pressure, cholesterol, glycated hemoglobin, tobacco smoking, elevated BMI, and appropriate use of aspirin/clopidogrel); and the post-index use of obesogenic SMI medications. Secondary outcomes include CV risk factor identification, treatment initiation and intensification, outpatient and inpatient utilization, risky prescribing events, and CV events. Total modifiable CV risk will be calculated by the CDS tool at the index and all post-index visits for all enrolled patients. Similarly, all modifiable CV risk components documented in the EHR from the index visit through the end of the intervention period will be collected by the CDS tool. All total modifiable CV risk values and modifiable risk components calculated or collected by the CDS tool between the index visit and the end of the intervention period will be used to calculate rates of change in each outcome among patients in intervention relative to control clinics.
Outcome Definitions
Total (10-year) modifiable CV risk, the primary outcome, was calculated as the sum of modifiable CV risk associated with each of 6 modifiable CV risk factors (BP, cholesterol, glycated hemoglobin, tobacco smoking, elevated BMI, and appropriate use of aspirin/clopidogrel). At each encounter the web service extracted the most recent data elements needed for computing CV risk from the EHR, looking back over a period appropriate for each risk component. A modifiable risk component for each CV risk factor not at optimal goal was calculated as the difference between the total 10-year risk calculated from the patient’s actual values and the goal value using validated risk prediction equations, including those from Framingham35, the ACC/AHA30,31 and the UKPDS32,33. Total modifiable CV risk, the primary outcome measure, was the sum of modifiable CV risk components across CV risk factors not at optimal goal at the time of the encounter. Total modifiable CV risk was calculated for each enrolled patient at the index visit and each post-index encounter on the web service of the CDS through the end of the intervention period, ensuring consistent estimates of modifiable CV risk that were blind to study staff.
Analysis Plan
The primary analyses will use generalized linear mixed models to test for differences in the rates of change in total modifiable CV risk (H1) and modifiable risk factors (H2) among intervention relative to control patients in the 12 months post-index. Total modifiable CV risk and risk factors documented for each patient between the index visit and the end of the intervention period will be predicted from fixed effects for clinic-randomized treatment group (intervention vs. control), time elapsed in years between index and the outcome (time), the treatment by time interaction, patient covariates (index outcome value, age, sex), and a random clinic intercept. Study-wide models will also include fixed covariates for site, balancing covariates used in randomization and site by constrained covariate interaction terms to uniquely relate site-specific balancing covariates to the outcomes. Site-specific models will include fixed effects for the clinic covariates balanced at the site.
The time parameter will estimate the annual rate of change in outcomes in the control clinics; and the sum of the time and treatment by time (interaction) parameters will estimate the annual rate of change in the intervention clinics. The interaction parameter will test the significance of the difference in the rates of change in the intervention clinics relative to the control clinics. It is expected that the interaction parameter will reflect less increase in total modifiable CVR or CV risk factors among intervention clinics relative to control clinics. The null hypothesis of no difference in changes in each outcome among intervention relative to control clinics will be rejected if α2<0.05 for the interaction parameter.
Effects of the intervention on individual risk factors will be assessed following this same approach with adaptations to accommodate the measurement frequency and distribution of each factor. Outcomes will be normalized, as needed, using error distributions and link functions (e.g., negative binomial distribution, log link) appropriate to their observed distributions.
Analyses regarding the use of obesogenic medications will predict the presence of an open prescription for an obesogenic medication among patients with 7% or greater weight gain in the previous 12 months or most recent BMI > 25 mg/m2. The likelihood of an obesogenic medication 12 months after index date (binomial error, logit link) will be predicted from clinic-randomized treatment group (intervention vs. control), assessment point (12m vs. 0m) and the treatment by assessment point interaction.
Sample Size
A priori power estimates were based on a plan to recruit and randomly assign 52 primary care clinics 1:1 with an estimated total of 2,250 patients with SMI. It was expected that eligible patients would have a mean of four modifiable CV risk measures per person per year. Preliminary data from patients in a prior clinic-randomized CDS trial investigating CV risk were used to estimate clinic- and patient-level intraclass correlations (ICC) and standard deviations (SD) for CV risk factors. The effective patient sample size (Neffpt) was estimated by reducing the correlated patient sample size, N≈2,250, to that of an equivalent independent sample size by dividing N by the design effect introduced by patients being clustered within clinics (Neffpt = N / [1 + (npt/clin – 1)ρclin]. Assuming npt/clin = 43 and ρclin=.01-.02, the effective patient sample sizes were Neffpt≈1,219 to 1,582. The effective outcome sample size (Neffout) was estimated by reducing Neffpt by the design effect introduced from repeated outcomes within patients (Neffout = Neffot / [1 + (nout/pt – 1)ρpt]. Assuming nout/pt = 4 and ρpt=.35-.90, the effective outcome sample sizes were Neffout≈1,318 to 3,086. Given these assumptions and equal distribution of outcomes between patients in intervention and control clinics, the minimum detectable standardized effect (MDSE, β) for the fixed time by treatment parameter was β = .071-.109 (power=.80, α2=.05), which are very small between-groups differences in changes in CV risk outcomes. As examples, these MDSE correspond to a predicted .071*9.6=0.68 to .109*9.6=1.05 slower rate of increase in total modifiable CV risk, or in SBP (SD=21.4) of 1.5–2.6 mmHg, among patients in intervention relative to control clinics in the 12 months post-index. Therefore, we anticipated the primary analyses would be sufficiently powered to detect small differences (d<.10) in CV risk and CV risk factors among CV Wizard versus control patients 12 months after exposure to the intervention.
Ethical and Regulatory Approval
Study design and procedures were reviewed and approved by Institutional Review Boards at all three health systems.
Informed Consent
As the care recommendations in the CV Wizard intervention were limited to evidence-based care from current national clinical guidelines, the Institutional Review Boards granted waivers of consent for PCPs and patients to use CV Wizard in clinical care. PCPs were consented to complete surveys about their experience with CV Wizard.
Interim Analyses of Benefit or Harm
We did not conduct interim analyses to evaluate benefit of the intervention. This was because early detection of benefit of the intervention was unlikely, and early termination of the intervention was not thought to offer any additional protection to current or future study participants, as early termination would have returned all participants to care as usual. We did conduct interim analyses testing for evidence of significant harm, defined as increased rates of suicidal ideation as reported on item 9 of the PHQ934 collected as part of usual clinical practice, suicide attempts, emergency department visits or hospitalizations in the intervention group compared to the control group. Interim analyses for these safety outcomes were conducted twice per year and reported to the Data and Safety Monitoring Board by the study statistician.
Discussion
This cluster-randomized pragmatic trial conducted in 78 primary care clinics aimed to decrease modifiable CV risk in adults with SMI, an issue that significantly impacts morbidity and mortality in this population. Identifying people with SMI with one or more of six selected major CV risk factors not at goal allowed PCPs the opportunity to identify and intervene to improve CV risk factor control in a timely way. Effectiveness will be assessed using an intent-to-treat analysis, and outcomes will be assessed largely through EHR data harvested by the CDS tool itself and securely stored as a limited data set on the firewall protected CDS web service.
The use of a CDS tool that acted as both a means of intervention delivery and a means of data collection in intervention and control clinics was an important innovation in this study, allowing a comparison of similar data across clinics. Having the tool run silently in the background in control clinics identified targeted patients and assessed their individual CV risk factors and 10- and/or 30-year total modifiable risk, medications and lab values in identical ways for patients in intervention and control clinics.
Because people with SMI die earlier than their peers, our study targeted CV risk in people with SMI at younger ages than is common for general primary care patients. CV risk is traditionally targeted starting around age 40, when 10-year modifiable CV risk equations to estimate risk are available, and CV risk is often not identified and addressed until patients have a 10-year estimated CV risk of at least 10%. In contrast, patients with SMI in this study who were 18–75 years old and had at least one CV risk factor not at goal were targeted, regardless of their total 10- or 30-year risk. These changes in age and CV risk thresholds were made because waiting until patients with SMI were 40 years old or met traditional risk thresholds would missed the opportunity to focus in a timely way on CV risk factor control in this relatively high-risk patient population.
Trial Status
The trial was funded through a cooperative agreement with the National Institute of Mental Health (U19MH092201). Participant enrollment began at Site A in March 2016, Site B in October 2016, and Site C in March 2017. Enrollment continued through 9/19/2017 at all sites, and follow-up occurred through 9/19/2018 at all sites, allowing for at least one year of follow-up for each enrolled patient. Our recruitment sample includes 10,347 patients with an index primary care visit, and 8937 patients with at least one follow-up visit. Analyses are ongoing, and trial results are expected in late 2019.
Acknowledgments
Funding: Supported by a Cooperative Agreement with the National Institute of Mental Health (U19MH092201)
Footnotes
Trial Registration: ClinicalTrials.gov Identifier: NCT02451670
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Osby U, Correia N, Brandt L, Ekbom A, Sparen P. Mortality and causes of death in schizophrenia in Stockholm county, Sweden. Schizophr Res. 2000;45(1–2):21–28. [DOI] [PubMed] [Google Scholar]
- 2.Parks J, Svendsen D, Singer P, Foti M. Morbidity and Mortality in people with serious mental illness. National Association of State Mental Health Program Directors 2006; http://www.nasmhpd.org/general_files/publications/med_directors_pubs/Technical%20Report%20on%20Morbidity%20and%20Mortaility%20-%20Final%2011-06.pdf.
- 3.Trangle M, Gary M, Paul G, Christensen R. Minnesota 10 by 10. Reducing morbidity and mortality in people with serious mental illnesses. Minn Med. 2010;93(6):38–41. [PubMed] [Google Scholar]
- 4.Dixon L, Postrado L, Delahanty J, Fischer PJ, Lehman A. The association of medical comorbidity in schizophrenia with poor physical and mental health. J Nerv Ment Dis. 1999;187(8):496–502. [DOI] [PubMed] [Google Scholar]
- 5.Davidson S, Judd F, Jolley D, Hocking B, Thompson S, Hyland B. Cardiovascular risk factors for people with mental illness. Aust N Z J Psychiatry. 2001;35(2):196–202. [DOI] [PubMed] [Google Scholar]
- 6.Weiner M, Warren L, Fiedorowicz JG. Cardiovascular morbidity and mortality in bipolar disorder. Ann Clin Psychiatry. 2011;23(1):40–47. [PMC free article] [PubMed] [Google Scholar]
- 7.Osborn DP, Levy G, Nazareth I, Petersen I, Islam A, King MB. Relative risk of cardiovascular and cancer mortality in people with severe mental illness from the United Kingdom’s General Practice Rsearch Database. Arch Gen Psychiatry. 2007;64(2):242–249. [DOI] [PubMed] [Google Scholar]
- 8.Hennekens CH, Hennekens AR, Hollar D, Casey DE. Schizophrenia and increased risks of cardiovascular disease. Am Heart J. 2005;150(6):1115–1121. [DOI] [PubMed] [Google Scholar]
- 9.Druss BG, Bradford WD, Rosenheck RA, Radford MJ, Krumholz HM. Quality of medical care and excess mortality in older patients with mental disorders. Arch Gen Psychiatry. 2001;58(6):565–572. [DOI] [PubMed] [Google Scholar]
- 10.Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care. 2004;27(2):596–601. [DOI] [PubMed] [Google Scholar]
- 11.Druss BG, Bradford DW, Rosenheck RA, Radford MJ, Krumholz HM. Mental disorders and use of cardiovascular procedures after myocardial infarction. JAMA. 2000;283(4):506–511. [DOI] [PubMed] [Google Scholar]
- 12.Frayne SM, Halanych JH, Miller DR, et al. Disparities in diabetes care: impact of mental illness. Arch Intern Med. 2005;165(22):2631–2638. [DOI] [PubMed] [Google Scholar]
- 13.Desai MM, Rosenheck RA, Druss BG, Perlin JB. Mental disorders and quality of diabetes care in the veterans health administration. Am J Psychiatry. 2002;159(9):1584–1590. [DOI] [PubMed] [Google Scholar]
- 14.Newcomer JW. Medical risk in patients with bipolar disorder and schizophrenia. J Clin Psychiatry. 2006;67 Suppl 9:25–30; discussion 36–42. [PubMed] [Google Scholar]
- 15.Pylvanen V, Knip M, Pakarinen A, Kotila M, Turkka J, Isojarvi JI. Serum insulin and leptin levels in valproate-associated obesity. Epilepsia. 2002;43(5):514–517. [DOI] [PubMed] [Google Scholar]
- 16.Lieberman JA, Stroup TS, McEvoy JP, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353(12):1209–1223. [DOI] [PubMed] [Google Scholar]
- 17.Dayabandara M, Hanwella R, Ratnatunga S, Seneviratne S, Suraweera C, de Silva VA. Antipsychotic-associated weight gain: management strategies and impact on treatment adherence. Neuropsychiatric disease and treatment. 2017;13:2231–2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mukundan A, Faulkner G, Cohn T, Remington G. Antipsychotic switching for people with schizophrenia who have neuroleptic-induced weight or metabolic problems. Cochrane Database Syst Rev. 2010(12):Cd006629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meigs JB, Cagliero E, Dubey A, et al. A controlled trial of web-based diabetes disease management: the MGH diabetes primary care improvement project. Diabetes Care. 2003;26(3):750–757. [DOI] [PubMed] [Google Scholar]
- 20.Montori VM, Dinneen SF, Gorman CA, et al. The impact of planned care and a diabetes electronic management system on community-based diabetes care: the Mayo Health System Diabetes Translation Project. Diabetes Care. 2002;25(11):1952–1957. [DOI] [PubMed] [Google Scholar]
- 21.Crosson JC, Stroebel C, Scott JG, Stello B, Crabtree BF. Implementing an electronic medical record in a family medicine practice: communication, decision making, and conflict. Ann Fam Med. 2005;3(4):307–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Orzano AJ, Strickland PO, Tallia AF, et al. Improving outcomes for high-risk diabetics using information systems. J Am Board Fam Med. 2007;20(3):245–251. [DOI] [PubMed] [Google Scholar]
- 23.Welch WP, Bazarko D, Ritten K, Burgess Y, Harmon R, Sandy LG. Electronic health records in four community physician practices: impact on quality and cost of care. J Am Med Inform Assoc. 2007;14(3):320–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O’Connor PJ, Crain AL, Rush WA, Sperl-Hillen JM, Gutenkauf JJ, Duncan JE. Impact of an electronic medical record on diabetes quality of care. Ann Fam Med. 2005;3(4):300–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O’Connor PJ, Sperl-Hillen JM, Rush WA, et al. Impact of electronic health record clinical decision support on diabetes care: a randomized trial. Ann Fam Med. 2011;9(1):12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sperl-Hillen JM, Crain AL, Margolis KL, et al. Clinical decision support directed to primary care patients and providers reduces cardiovascular risk: a randomized trial. J Am Med Inform Assoc. 2018;25(9):1137–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kharbanda EO, Asche SE, Sinaiko AR, et al. Clinical Decision Support for Recognition and Management of Hypertension: A Randomized Trial. Pediatrics. 2018;141(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moulton LH. Covariate-based constrained randomization of group-randomized trials. Clin Trials. 2004;1(3):297–305. [DOI] [PubMed] [Google Scholar]
- 29.Imbens G Experimental design for unit and cluster randomized trials. 2011; http://cyrussamii.com/wp-content/uploads/2011/06/Imbens_June_8_paper.pdf. Accessed 10 June 2015.
- 30.Goff DC Jr., Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25 Pt B):2935–2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goff DC Jr., Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 Suppl 2):S49–73. [DOI] [PubMed] [Google Scholar]
- 32.Hayes AJ, Leal J, Gray AM, Holman RR, Clarke PM. UKPDS outcomes model 2: a new version of a model to simulate lifetime health outcomes of patients with type 2 diabetes mellitus using data from the 30 year United Kingdom Prospective Diabetes Study: UKPDS 82. Diabetologia. 2013;56(9):1925–1933. [DOI] [PubMed] [Google Scholar]
- 33.Stevens RJ, Kothari V, Adler AI, Stratton IM. The UKPDS risk engine: a model for the risk of coronary heart disease in Type II diabetes (UKPDS 56). Clin Sci (Lond). 2001;101(6):671–679. [PubMed] [Google Scholar]
- 34.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lloyd-Jones DM, Leip EP, Larson MG, et al. Prediction of lifetime risk for cardiovascular disease by risk factor burden at 50 years of age. Circulation. 2006;113(6):791–798. [DOI] [PubMed] [Google Scholar]