Abstract
Introduction:
Spontaneous third ventriculostomies (STV) have only been reported in relation to obstructive hydrocephalus and increased intracranial pressure (ICP) and is most commonly seen as disruption of the floor of the third ventricle. Hydrocephalus has been reported in patients with Krabbe disease; however, it is clinically difficult to monitor for hydrocephalus in patients with Krabbe disease as symptoms of increased ICP may overlap with symptoms of Krabbe disease. We describe a case series of STV and hydrocephalus, likely in response to increased ICP, in patients with infantile Krabbe disease.
Methods:
Brain magnetic resonance images of patients with infantile Krabbe disease were retrospectively analyzed to assess for ventricular size and presence of STV. A brain atlas was used to standardize calculation of ventricular size. Mid-sagittal, T2 weighted images around the third ventricle were assessed for STV. Developmental outcomes were measured with a series of standardized and validated tests.
Results:
Seventy-five patients with infantile Krabbe disease were evaluated. Twelve cases of STV were identified. Head circumference (SE=8.07; p<0.001) and average ventricular volume were greater (Left:SE=1.47, p<0.001) in patients with STV when compared to patients without STV. Patients with STV also had more delayed development in adaptive (diff=0.2, p<0.01), gross motor (diff =0.0, p<0.01), and fine motor (diff=0.1, p<0.001) function.
Conclusions:
STV, likely in the context of increased ICP, were identified in patients with Krabbe disease. Though difficult to assess, our study highlights the importance of monitoring for increased ICP, which can result in STV, in patients with infantile Krabbe disease.
Keywords: Spontaneous third ventriculostomy, Krabbe disease, Globoid cell leukodystrophy, Intracranial pressure
Introduction
Krabbe disease is a rare progressive, neurodegenerative disorder characterized by central and peripheral demyelination. It is inherited in an autosomal recessive pattern and is due to mutations in the GALC gene leading to a deficiency of galactocerebrosidase, an enzyme that hydrolyzes key sphingolipids – psychosine and galactosylceramide. The resultant accumulation of psychosine is toxic to myelinating Schwann cells and oligodendrocytes, and accumulation of galactosylceramide elicits an inflammatory response causing infiltration of sphingolipid-rich macrophages (Suzuki, 2003). Ultimately, these two processes lead to demyelination and neuronal death in the central and peripheral nervous systems (Castelvetri et al., 2011; Suzuki, 2003). While most patients present before 12 months of age (early infantile onset), later onset forms with more variable presentation - late infantile (12 months to 3 years), juvenile (3–8 years), and adult (8 years and older) - have been well-characterized (Bascou, DeRenzo, Poe, & Escolar, 2018; Beltran-Quintero et al., 2019; Graziano & Cardile, 2015; Liao, Gelinas, & Sirrs, 2014). Common presenting symptoms in early infantile Krabbe disease include regression of developmental milestones, irritability, developmental delays, feeding difficulties, and abnormal muscle tone (Bascou et al., 2018). The median survival rates in early infantile disease range from 2–3 years of age (Escolar, West, Dallavecchia, Poe, & LaPoint, 2016; Liao et al., 2014). However, for patients who present early with minimal symptoms, medical interventions such as hematopoietic stem cell transplantation have shown to prolong median survival age while allowing some patients to live relatively normal lives into their teenage years (Wright, Poe, DeRenzo, Haldal, & Escolar, 2017).
Initial evaluation of a patient with Krabbe disease is based on a comprehensive history and physical examination. Additional ancillary tests such as neuroimaging and nerve conduction studies are important for determining disease severity (Escolar et al., 2016). Brain magnetic resonance imaging (MRI) commonly shows demyelination with increased T2 signaling in the periventricular and subcortical white matter, corticospinal tracts, and cerebellar white tracts (Abdelhalim, Alberico, Barczykowski, & Duffner, 2014; Bascou et al., 2018; Livingston et al., 2012). Optic atrophy (Udow, Bunge, Ryner, Mhanni, & Salman, 2012; Vargiami et al., 2016), enhancement and thickening of cranial nerves and cauda equina nerve roots (Beslow, Schwartz, & Bönnemann, 2008; Hwang et al., 2016; Morana et al., 2009), and hydrocephalus (Breningstall & Patterson, 2008; Caniglia et al., 2002; Laxdal & Hallgrimsson, 1974; Livingston et al., 2012; Richards, Morse, & Howrey, 1999) have also been reported.
Though there is no precise definition, hydrocephalus is the clinical diagnosis of ventricular enlargement due to an imbalance between the production and reabsorption of cerebrospinal fluid (CSF) and it may be associated with increased intracranial pressure (ICP) when it is obstructive or non-communicating in nature (Kahle, Kulkarni, Limbrick Jr, & Warf, 2016). Common symptoms of hydrocephalus are irritability, increasing head circumference, emesis, lethargy, and poor feeding, similar to the symptom profile of patients with Krabbe disease (Bascou et al., 2018). There are two types of hydrocephalus: communicating and non-communicating. Communicating hydrocephalus is characterized by decreased reabsorption of CSF by the arachnoid granulations, whereas non-communicating hydrocephalus is characterized by a mechanical obstruction preventing outflow of CSF (Bullivant, Hader, & Hamilton, 2009). Both communicating and non-communicating hydrocephalus have been reported in Krabbe disease. Cases of communicating hydrocephalus have been reported in patients with Krabbe disease who received hematopoietic stem cell transplantation (Caniglia et al., 2002; Richards et al., 1999). These patients were all treated with ventriculoperitoneal shunts (VPS) with one case reporting improvement of clinical symptoms (Caniglia et al., 2002). A handful of other cases have also described patients with Krabbe disease developing non-communicating or obstructive hydrocephalus. A 3-month-old female was found to have enlargement of her lateral and third ventricles on a pneumoencephalogram. She had increasing head circumference after the procedure leading to concerns that medical intervention possibly led to hydrocephalus, making it unclear if the development of hydrocephalus was directly related to progression of her Krabbe disease (Laxdal & Hallgrimsson, 1974). Breningstall, et al. reported the case of a 21-month-old female who presented with increasing head circumference who was found to have an unexplained parietal skull fracture and tetra-ventriculomegaly. She eventually had a VPS placed for comfort, and her hydrocephalus was thought to be an intermediary condition between communicating and non-communicating hydrocephalus (Breningstall & Patterson, 2008). Neither of these patients went on to receive hematopoietic stem cell transplantation. Regardless, evidence for these findings are limited to a handful of anecdotal case reports as mentioned.
Due to the complex neurologic symptomatology associated with Krabbe disease, the clinical diagnosis of increased ICP or hydrocephalus in these patients is challenging. Given these limitations and the paucity of reported cases of hydrocephalus or increased ICP in this exceedingly rare disease, the purpose of our study was to address the possible etiologies of spontaneous third ventriculostomies (STV) identified in our cohort and the importance of monitoring for increased ICP and hydrocephalus in patients with Krabbe disease as interventions such as VPS may benefit this patient population.
Methods
We conducted a retrospective study of patients with Krabbe disease who were referred to the Program for the Study of Neurodevelopment in Rare Disorders (NDRD) between October 2011 and October 2017 at the University of North Carolina at Chapel Hill and University of Pittsburgh Medical Center - Children’s Hospital of Pittsburgh. All research was conducted with the approval of the institutional review boards of the University of North Carolina (08–0237) and University of Pittsburgh (PRO11050036). All patients in our study had infantile Krabbe disease. Though some patients underwent umbilical cord stem cell transplant (UCBT), all MRI scans included in this study were obtained prior to treatment or from untreated patients. Patients whose MRI scans were severely degraded preventing assessment of midline anatomy and those who were diagnosed with other genetic or neurologic conditions known to alter development and findings on neuroimaging were excluded from this study.
Neuroimaging
MRI images were obtained using a 3 Tesla Siemens Skyra scanner and 20-channel coil. A T1-weighted MPRAGE sagittal image (1×1×1 mm voxel size, TR 2400ms, TE 3.16mm) and a T2-weighted SPACE sagittal image (1×1×1 mm voxel size, TR 3200ms, TE 497ms) were obtained. T1 images were used to calculate volume of the lateral ventricles. T2 images were inspected to detect the presence of a STV at the mid-sagittal slices around the third ventricle. All MRI scans were reviewed by a board certified pediatric neuroradiologist with more than twenty years of experience in the field of pediatric neuroradiology and additional nine years of experience in reviewing MRI scans of patients with Krabbe disease. Affected STV was defined as a flow void at the level of the third ventricular floor on mid-sagittal images and a defect in the floor of the third ventricle anterior to the mammillary bodies confirmed on multiplanar reformatted T2 and T1 weighted images.
To standardize the calculation of ventricular size across a wide range of patient ages, a 2-year-old brain atlas registered to each patient’s T1-weighted MRI was adopted. The brain atlas, labeled with left and right lateral ventricles, was obtained from a brain development study at the University College of London. The registration was performed using rigid, affine, and diffeomorphic transformation to achieve the alignment of the lateral ventricles between atlas and patient images, and all alignments were confirmed by direct visual inspection. In case of difficulty in the registration for young and unmyelinated brains, manual delineation was conducted to calculate the ventricular volumes.
Head Circumference
Head circumference was measured during every NDRD evaluation and within two days of completion of a patient’s MRI. Measurements completed during other medical visits were also used to examine changes in head circumference over time. Head circumference percentiles were calculated using the Center for Disease Control and Prevention norms for children aged birth to three years (Kuczmarski, 2000).
Neurodevelopmental Studies
All patients referred to the NDRD were evaluated following a protocol of standardized testing designed by a multidisciplinary team for longitudinal follow-up. The protocol was designed to minimize fatigue and maximize children’s ability to test successfully (Martin et al., 2008). All evaluations were performed at a single site with a team of neurodevelopmental pediatricians, speech pathologists, audiologists, physical therapists, and psychometricians. The neurodevelopmental tests included an overall physical and neurological exam along with evaluation of signs and symptoms of disease, growth, mobility, adaptive behavior, receptive and expressive language, gross and fine motor, and cognitive behavior. The standardized and validated tools that were used during each visit included Mullen Scales of Early Learning, Peabody Developmental Motor Scales, and Gross Motor Function Measure (Folio & Fewell, 2000; Mullen, 1995; Russell, Rosenbaum, Wright, & Avery, 2002). Parents also completed questionnaires asking about birth history, early signs of disease, development, and behaviors, including a measure of independent adaptive behaviors (Bruininks, 1984) along with the Vineland Adaptive Behavior Scale (Burger-Caplan, Saulnier, & Sparrow, 2018).
Statistics
A series of analyses were performed to understand the relationship between head circumference, ventricular size, developmental outcomes, and the presence or absence of a STV.
To examine the relationship between head circumference and STV, head circumference percentiles were compared between the two groups – patients with STV and patients without STV – while controlling for age and receipt of an UCBT. Analysis was restricted to a subset of children who were less than 3 years of age at the time of their first MRI. To account for repeated measurements for each child, a random intercept mixed model was used to fit the head circumference percentile data. Ventricular size was also compared between the groups, and a mixed regression model was used to test for group differences in the mean ventricle size at 24 months along with the rate of change of ventricular volume over time. Clinical outcomes such as cognitive, adaptive, gross and fine motor, and receptive and expressive language were also compared between the two groups.
Results
Subjects
A total of 75 patients were enrolled in our study. Of the 75 subjects, 39 (52 %) were male and 36 (48%) were female. A vast majority of these patients were white (n=70 (93%), among whom 10 (8%) were Hispanic). Additionally, 2 (3%) patients were black, 2 (3%) were Asian, and 1 (1%) was of unknown race. All children had early or late infantile disease, and eighteen (24%) of these children were ultimately treated with UCBT.
Spontaneous Third Ventriculostomy
STV was identified in 12 (16%) patients at their first MRI, which was obtained at a median age of two years (range: two months to 13 years). Notably, none of the patients who had UCBT showed signs of STV, though several patients with STV did go on to receive UCBT (Table 1). No patients without STV at their first MRI developed STV during their follow-up period.
Head Circumference
To examine the relationship between head circumference and STV, the head circumference percentile was compared between patients with and without STV. Head circumference percentile was greater among patients found to have STV regardless of UCBT status (Table 1a, Figure 2). In our cohort, head circumference was approximately 30 percentile points greater (SE = 8.27; p < 0.001) in patients with STV after controlling for age and UCBT status (Table 1b).
Table 1a.
Head circumference percentile in the presence and absence of STV
| UCBT status | STV | N | Median (%) | Minimum (%) | Maximum (%) |
|---|---|---|---|---|---|
| NH | No STV | 199 | 27.77 | 0.01 | 99.51 |
| STV | 83 | 70.24 | 2.90 | 99.93 | |
| UCBT | No STV | 197 | 18.67 | 0.00 | 80.81 |
| STV | 6 | 44.55 | 27.39 | 97.85 |
NH = natural history, UCBT = Umbilical cord stem cell transplant, STV = Spontaneous third ventriculostomy
Figure 2.
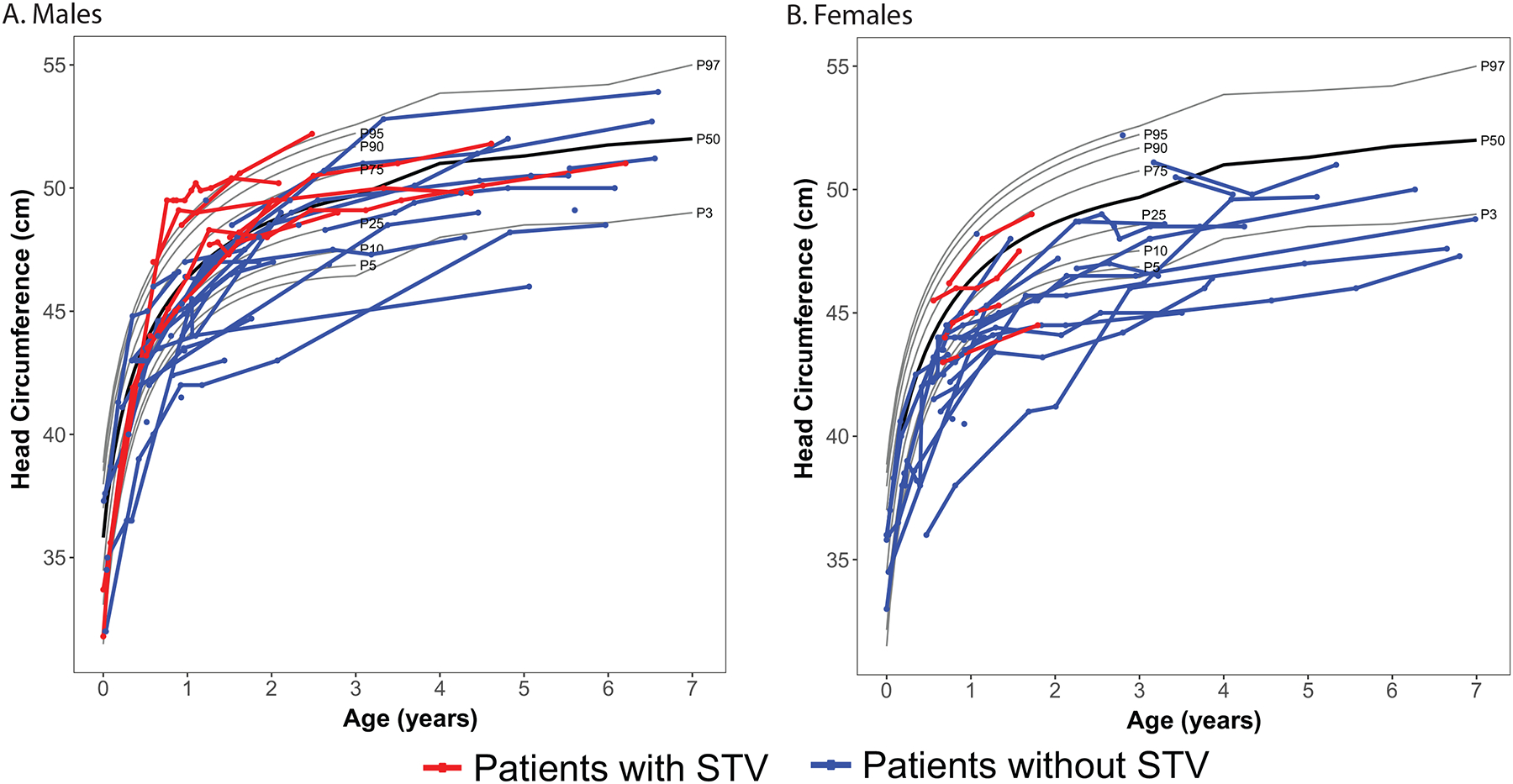
Head circumference over time among male and female patients with Krabbe disease, stratified by the presence and absence of STV. Each point depicts an individual measurement; lines connecting points show multiple measurements for an individual child. The black line represents the mean curve for typical developing children. The red line indicates patients with STV and the blue line patients without STV.
Table 1b:
Mixed effects model of head circumference percentile on STV, controlling for age and UCBT status
| Effect | Estimate | SE | DF | t Value | Pr > |t| |
|---|---|---|---|---|---|
| Intercept | 24.00 | 6.50 | 179.00 | 3.70 | <0.001 |
| STV | 28.90 | 8.27 | 60.00 | 3.50 | <0.001 |
| Age (months) | 0.08 | 0.18 | 210.00 | 0.45 | 0.651 |
| Pre-UCBT | 11.17 | 5.21 | 229.00 | 2.14 | 0.033 |
DF = Degrees of freedom, SE = Standard error, STV = Spontaneous third ventriculostomy, UCBT = Umbilical cord stem cell transplant
Ventricle Size
At least one measurement of ventricular volume was available for 74 of the 75 subjects. Repeated measurements over time were available for 34 subjects, including 9 out of the 12 subjects with STV (Figure 3). No left-right asymmetry was identified, as intra-patient ventricular volume was grossly similar between the left and right ventricles (Table 3). Patients with STV had larger ventricles at 24 months compared to patients without STV (right: diff = 7.81 cm3, SE 1.49, p < 0.001; left: diff = 8.36 cm3, SE = 1.47, p < 0.001). Ventricular size increased over time for both groups; however, the rate of increase was significantly higher in patients with STV at 0.26 cm3/month compared to patients without STV at 0.07 cm3/month. Subjects who had not received UCBT had larger ventricles than those who received UCBT (right: diff = 6.01 cm3, SE = 1.78, p = 0.001; left: diff = 6.97 cm3, SE = 1.86, p < 0.001); however, there was not a significant difference in the rate of ventricular enlargement between the groups (right: p > 0.19; left: p > 0.12), and this interaction was removed from the final model.
Figure 3:
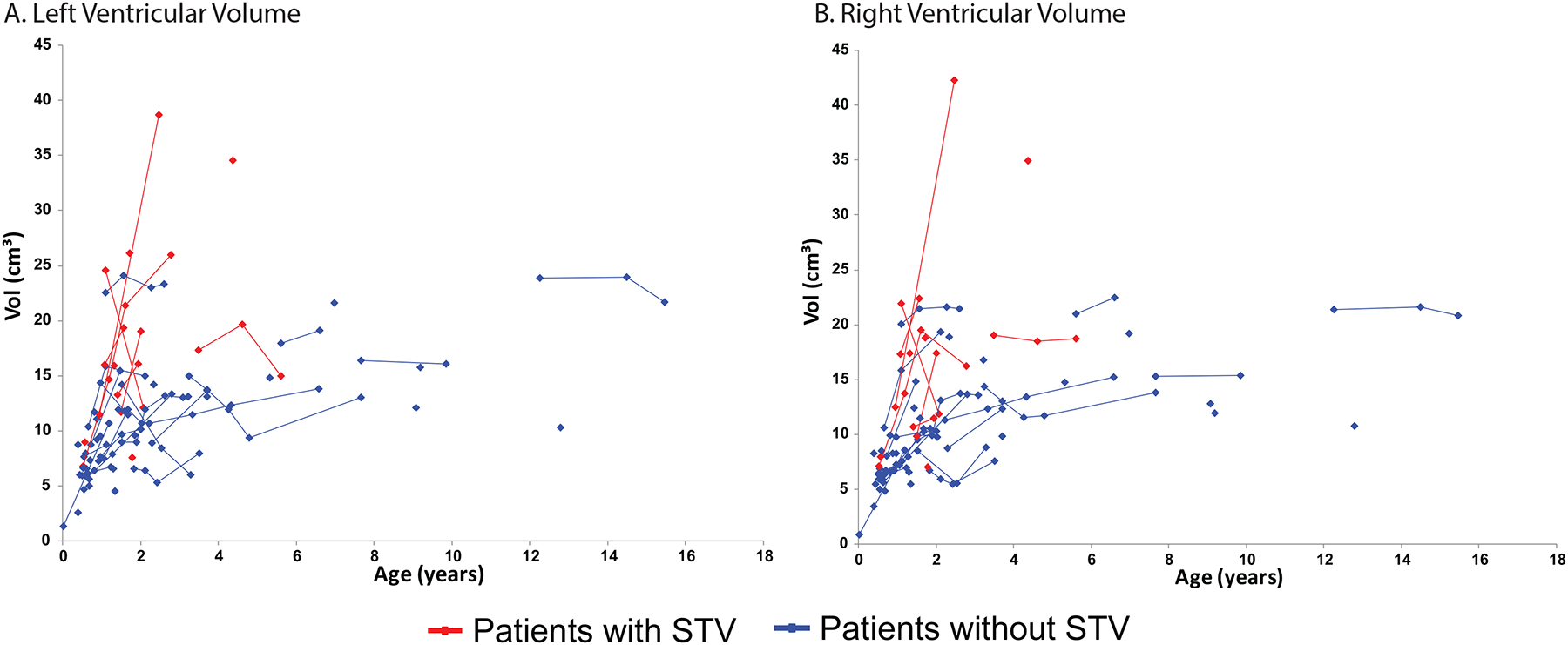
(A) Right ventricular volume over time stratified based on the presence and absence of spontaneous third ventriculostomy (STV). Each point depicts an individual measurement; lines connecting points show multiple measurements for an individual child. The red line indicates patients with STV and the blue line patients without STV. (B) Left ventricular volume over time stratified based on the presence and absence of spontaneous third ventriculostomy (STV). Each point depicts an individual measurement; lines connecting points show multiple measurements for an individual child. The red line indicates patients with STV and the blue line patients without STV.
Table 3.
Multivariate regression model of key predictive variables of right and left ventricular volume.
| Right Ventricle | Left Ventricle | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| B | SE | DF | t Value | p | B | SE | DF | t Value | p | ||
| Intercept | 3.88 | 1.85 | 81.90 | 2.10 | 0.039 | 3.30 | 1.92 | 83.40 | 1.72 | 0.089 | |
| Age (centered at 24 months) | 0.07 | 0.02 | 79.70 | 4.43 | <0.001 | 0.07 | 0.02 | 77.20 | 4.26 | <0.001 | |
| STV | 7.81 | 1.49 | 58.80 | 5.23 | <0.001 | 8.36 | 1.47 | 58.60 | 5.69 | <0.001 | |
| Age × STV | 0.19 | 0.07 | 104.00 | 2.50 | 0.014 | 0.19 | 0.08 | 102.00 | 2.51 | 0.014 | |
| NH | 6.01 | 1.78 | 68.00 | 3.37 | 0.001 | 6.97 | 1.86 | 70.80 | 3.74 | <0.001 | |
B = Beta, DF = Degrees of freedom, NH = Natural history, SE = Standard error, STV = Spontaneous third ventriculostomy
Developmental Skills
Of the 75 patients, 69 patients who had not received UCBT and were less than 5 years of age at the time of their first MRI were included in the analyses of developmental outcomes. Patients with STV were more delayed than patients without STV, with the greatest adverse impact on adaptive development (Diff = 0.20, p=0.005) while the effect sizes on gross and fine motor skills (Diff = 0.06, p=0.003; Diff = 0.10, p = 0.002, respectively) were more modest. While cognitive, receptive language, and expressive language scores were not significantly different between the two groups, the patients with STV had a trend towards worse developmental outcomes in all domains measured (Table 4).
Table 4:
Relationship between STV and developmental scores across six key domains. The mean value of the developmental quotients by STV status along with the mean group difference is shown.
| No STV n = 47 | STV n=12 | ||||||
|---|---|---|---|---|---|---|---|
| Domain | Mean | SD | Mean | SD | Diff | p-value | |
| Cognitive | 0.39 | 0.27 | 0.25 | 0.26 | 0.14 | 0.130 | |
| Adaptive | 0.41 | 0.21 | 0.21 | 0.16 | 0.20 | 0.005 | |
| Gross Motor | 0.13 | 0.09 | 0.07 | 0.05 | 0.06 | 0.003 | |
| Fine Motor | 0.17 | 0.20 | 0.07 | 0.04 | 0.10 | 0.002 | |
| Receptive Language | 0.54 | 0.42 | 0.39 | 0.42 | 0.15 | 0.271 | |
| Expressive Language | 0.38 | 0.34 | 0.23 | 0.31 | 0.15 | 0.169 | |
Discussion
This is the first report of a series of cases in which STV is identified in patients with Krabbe disease. In this retrospective cohort study, we further report on the association between STV and increased head circumference, increased ventricular volume, and delayed development when compared to patients without STV. Our findings imply that there is development of increased ICP with resultant STV in many cases of infantile Krabbe disease that are associated with worse motor and adaptive outcomes.
STV is a rare finding that has been reported as a paraphysiological response to obstructive hydrocephalus, which in turn leads to increased ICP (Bailey, Pipitone, & Zuccoli, 2010; Pinto VL, 2018). The most common location of STV is the floor of the third ventricle, which is composed of the optic chiasm, infundibulum and tuber cinereum of the hypothalamus, mammillary bodies, posterior perforated substance, and the tegmentum of the midbrain. The thinnest portion is the anterior portion of the floor of the third ventricle extending from the infundibulum to the pre-mamillary sulcus (Mortazavi et al., 2014). In fact, during operative endoscopic third ventriculostomies, thinning of the anterior floor of the third ventricle has been observed (Bullivant et al., 2009). All reported cases of STV have been in context of obstructive hydrocephalus and is thought to be associated with chronic increased ICP (Alonso, Taboada, Alvarez, Paramo, & Vila, 1979; Bailey et al., 2010; Bhatia, Banerji, & Rao, 1977; Bullivant et al., 2009; Deniz, Ece, Çelik, Akalan, & Fırat, 2008; Gallia & Teo, 2008; Kanjilal, 1972; Kim, Feiz-Erfan, Clatterbuck, & Spetzler, 2005; Leslie & Alker Jr, 1964; Luigetti, Pravatà, Bartalena, & Cianfoni, 2013; Öğrenci, Ekşi, & Koban, 2016; Parmar, Aquilina, & Carter, 2009; Rovira et al., 1999; Yuen, Bulluss, Trost, & Murphy, 2008). The associated chronic increase in ICP leads to CSF pulsations pushing against the floor of the third ventricle, causing thinning and eventual rupture of the floor, thus relieving the increased ICP by CSF diversion into the subarachnoid space (Öğrenci et al., 2016; Parmar et al., 2009). Although none of the 75 patients in our cohort demonstrated evidence of CSF flow obstruction, the 12 patients with STV were found to have a statistically significant increase in head circumference and ventricular volume in keeping with the diagnosis of hydrocephalus and increased ICP. As Krabbe patients are known to have brain and brainstem atrophy, we propose that the cases of STV identified in our cohort are likely resulting from increased ICP superimposed on an atrophic ventricular wall leading to the spontaneous perforation of the floor of the third ventricle (Abdelhalim et al., 2014; Beslow et al., 2008; Liao et al., 2014; Orsini JJ, 2000 [Updated 2018 Oct 11]; Zuccoli, Narayanan, Panigrahy, Poe, & Escolar, 2015).
Cases of hydrocephalus in patients with Krabbe disease have been reported in the literature – both with and without transplantation (Breningstall & Patterson, 2008; Caniglia et al., 2002; Laxdal & Hallgrimsson, 1974; Richards et al., 1999). Age at transplant is known to be an important factor in long-term outcomes of patients with Krabbe disease (Wright et al., 2017). In contrast to recent trends to undergo transplantation within weeks of birth, prior reported cases of hydrocephalus were identified in patients who received transplantation at or after four months of age (Caniglia et al., 2002; Richards et al., 1999; Wright et al., 2017). Though our findings are undoubtedly confounded by selection bias, no patient who received transplantation developed a STV. This is despite the fact that most patients who received an UCBT are on immune prophylaxis, most commonly steroids, which are known to be associated with brain atrophy and total reduction in brain tissue volume – both of which can lead to ventriculomegaly (Murphy et al., 2001; Zivadinov, 2005). Therefore, the presence of ventriculomegaly and STV are likely indicative of advanced Krabbe disease. Future studies on UCBT in patients with Krabbe disease should prospectively evaluate the ventricular anatomy among transplanted patients to better characterize the extent to which transplantation may impact the incidence of these prognostically negative imaging findings.
Furthermore, patients with STV had significantly more delayed development in adaptive behavior, gross motor skills, and fine motor skills. The presence of hydrocephalus has been associated with worse developmental outcomes across many diseases including very low birth weight infants, cephaloceles, meningitis, and intraventricular hemorrhages (Campbell et al., 1993; Lo et al., 2008; Perdaens, Koerts, & Nassogne, 2018). Therefore the presence of a STV, which likely indicates increased ICP and hydrocephalus, might also predict poor developmental outcomes in patients with Krabbe disease. Knowing the associations between STV and both developmental outcomes and transplant status in our cohort, the identification of STV may guide our understanding of disease burden and outcomes after transplantation. Additionally, increased ICP and hydrocephalus have also been associated with delayed myelination, damage to the periventricular white matter, and altered neuronal connections which all affect neurodevelopmental outcomes and can compound the effect of demyelination seen in Krabbe disease (Del Bigio, 2004, 2010; Hanlo et al., 1997). Fortunately, it has been noted that these changes can be reversed to some degree with interventions such as VPS, highlighting the importance of monitoring ICP in Krabbe patients (Del Bigio, 2010). To date, hydrocephalus and increased ICP are not typically monitored in patients with Krabbe disease (Bascou et al., 2018; Escolar et al., 2016; Wright et al., 2017). Given the complex neurologic symptomatology exhibited by this patient population, detecting and monitoring increased ICP can be clinically challenging. However, our data highlights the importance of doing so, with the guidance of additional ancillary tests such as neuroimaging and lumbar punctures when CSF protein is measured, as increased ICP may be treatable in a subset of patients. Increasing ICP would be especially important to consider in patients with increasing head circumference and worsening clinical symptoms such as irritability, poor feeding, emesis, and loss of developmental milestones (Breningstall & Patterson, 2008; Caniglia et al., 2002; Del Bigio, 2010; Laxdal & Hallgrimsson, 1974). Especially as newborn screening for Krabbe disease becomes more prevalent, it is important that clinicians should have a high index of suspicion for increased ICP, and neuroimaging or lumbar punctures should be pursued in appropriate cases when evaluating a patient with Krabbe disease (Orsini, Saavedra-Matiz, Gelb, & Caggana, 2016).
There are several limitations to the present study, not the least of which is the modest sample size. However, given the exceedingly low incidence of Krabbe disease and the rarity of STV, our data represent a relatively sizeable cohort. Additionally, we did not measure brain atrophy, which is a known phenomenon in patients with Krabbe disease and those treated with transplantation (Liao et al., 2014). The exclusion of brain atrophy from our data can possibly complicate our findings especially in regards to ventricular size as hydrocephalus ex-vacuo is also seen in patients with brain atrophy. However, hydrocephalus ex-vacuo is not associated with increased pressure; therefore, STV would not be expected in patients with hydrocephalus ex-vacuo (Alvin, 2016). Additionally, as it is not standard of care to measure opening pressure in patients with Krabbe disease, objective measurements of ICP were not obtained. Future studies should prospectively assess ICP and ventricular anatomy of patients with Krabbe disease to better understand risk for STV, association with disease severity, and changes after UCBT. Finally, CSF phase contrast imaging analysis of the region of the STV is warranted to confirm our preliminary data.
Conclusion
This series is the first neuroimaging-based description of STV in patients with Krabbe disease, a rare but severe neurodevelopmental condition. Hydrocephalus and increased ICP are challenging to monitor clinically in patients with Krabbe disease. However, our study highlights the importance of obtaining objective measurements of ICP as there are interventions such as VPS placement to relieve symptoms related to increased ICP and possibly alter disease prognosis. The presence of increased ICP and resultant formation of a STV may aid in additional prognostic information to help counsel families about disease burden when considering UCBT.
Figure 1.
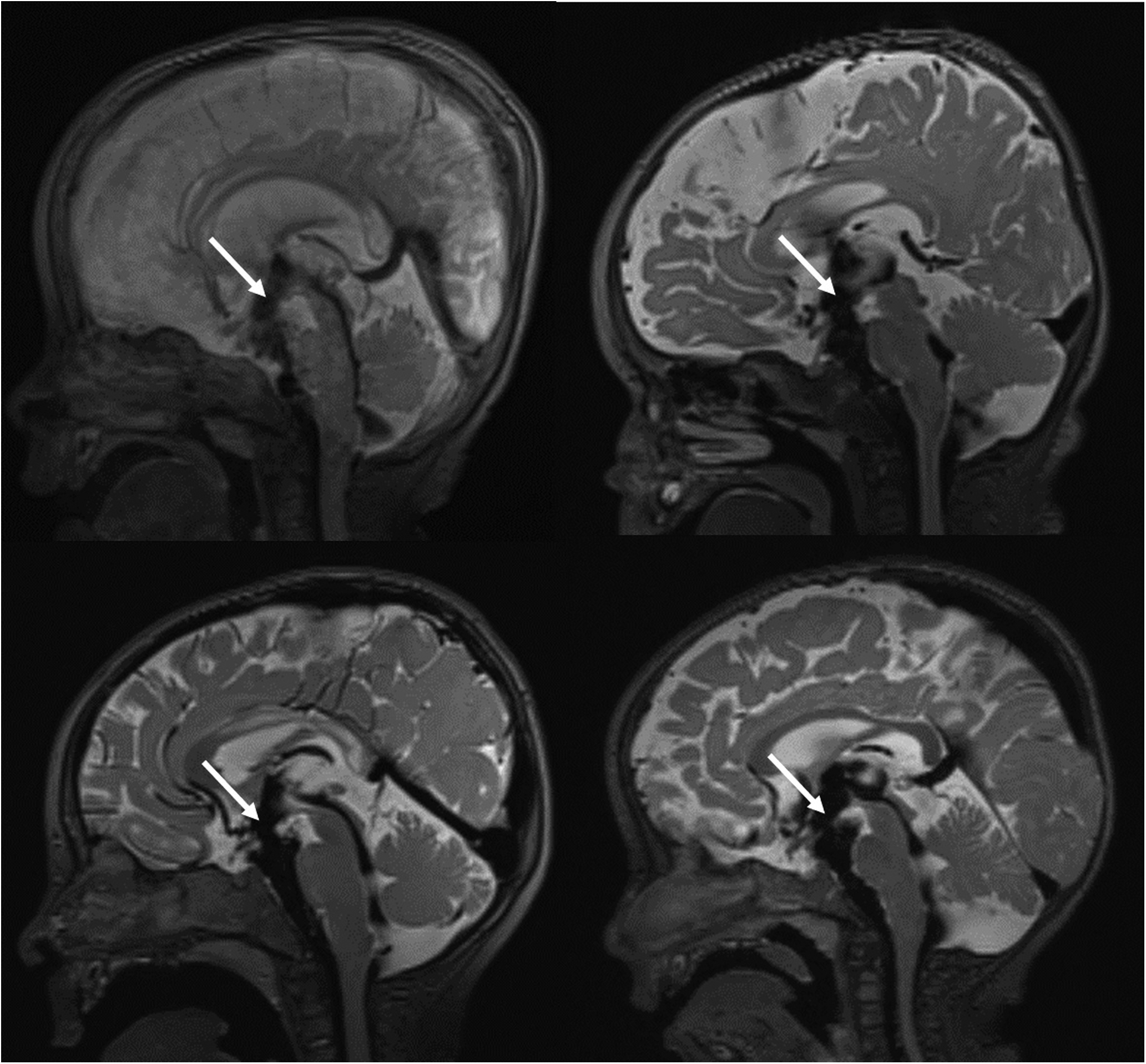
Representative sagittal images demonstrating a large flow void and focal defect in the floor of the third ventricle consistent with STV in four patients with Krabbe disease. The arrowhead indicates the location of STV.
Funding
This research was partially funded by the National Institutes of Health Grant 1R01NS061965-01 to MLE. Funding was also provided by The Legacy of Angels Foundation and the DANA Foundation, MLE and the New York State Department of Health to CASM-M.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdelhalim AN, Alberico RA, Barczykowski AL, & Duffner PK (2014). Patterns of magnetic resonance imaging abnormalities in symptomatic patients with Krabbe disease correspond to phenotype. Pediatric neurology, 50(2), 127–134. [DOI] [PubMed] [Google Scholar]
- Alonso A, Taboada D, Alvarez JA, Paramo C, & Vila M (1979). Spontaneous ventriculostomy and ventricular diverticulum. Radiology, 133(3), 651–654. [DOI] [PubMed] [Google Scholar]
- Alvin M, Miller PE (2016). Compensated hydrocephalus. Lancet, 387(10036), 2422. [DOI] [PubMed] [Google Scholar]
- Bailey A, Pipitone N, & Zuccoli G (2010). Phase-contrast cine MRI revealing en valve mechanism in spontaneous third ventriculostomy: report of a case and literature review. Clinical neurology and neurosurgery, 112(9), 817–820. [DOI] [PubMed] [Google Scholar]
- Bascou N, DeRenzo A, Poe MD, & Escolar ML (2018). A prospective natural history study of Krabbe disease in a patient cohort with onset between 6 months and 3 years of life. Orphanet journal of rare diseases, 13(1), 126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltran-Quintero ML, Bascou NA, Poe MD, Wenger DA, Saavedra-Matiz CA, Nichols MJ, & Escolar ML (2019). Early progression of Krabbe disease in patients with symptom onset between 0 and 5 months. Orphanet journal of rare diseases, 14(1), 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beslow LA, Schwartz ES, & Bönnemann CG (2008). Thickening and enhancement of multiple cranial nerves in conjunction with cystic white matter lesions in early infantile Krabbe disease. Pediatric Radiology, 38(6), 694–696. [DOI] [PubMed] [Google Scholar]
- Bhatia R, Banerji AK, & Rao M (1977). Spontaneous rupture of the third ventricle in obstructive hydrocephalus: a radiographic diagnosis. Acta neurochirurgica, 39(3–4), 159–165. [DOI] [PubMed] [Google Scholar]
- Breningstall GN, & Patterson RJ (2008). Acquired obstructive hydrocephalus in globoid-cell leukodystrophy. Pediatric neurology, 39(4), 279–280. [DOI] [PubMed] [Google Scholar]
- Bruininks RH (1984). Scales of Independent Behavior: Psycho-educational Battery: DLM. [Google Scholar]
- Bullivant KJ, Hader W, & Hamilton M (2009). A pediatric experience with endoscopic third ventriculostomy for hydrocephalus. Can J Neurosci Nurs, 31(2), 16–19. [PubMed] [Google Scholar]
- Burger-Caplan R, Saulnier CA, & Sparrow SS (2018). Vineland Adaptive Behavior Scales Encyclopedia of Clinical Neuropsychology (pp. 1–5): Springer. [Google Scholar]
- Campbell MK, Halinda E, Carlyle MJ, Fox AM, Turner LA, & Chance GW (1993). Factors predictive of follow-up clinic attendance and developmental outcome in a regional cohort of very low birth weight infants. American journal of epidemiology, 138(9), 704–713. [DOI] [PubMed] [Google Scholar]
- Caniglia M, Rana I, Pinto RM, Fariello G, Caruso R, Angioni A, … De Rossi G (2002). Allogeneic bone marrow transplantation for infantile globoid-cell leukodystrophy (Krabbe’s disease). Pediatric Transplantation, 6(5), 427–431. doi: doi: 10.1034/j.1399-3046.2002.02026.x [DOI] [PubMed] [Google Scholar]
- Castelvetri LC, Givogri MI, Zhu H, Smith B, Lopez-Rosas A, Qiu X, … Bongarzone ER (2011). Axonopathy is a compounding factor in the pathogenesis of Krabbe disease. Acta neuropathologica, 122(1), 35–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Bigio MR (2004). Cellular damage and prevention in childhood hydrocephalus. Brain Pathology, 14(3), 317–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Bigio MR (2010). Neuropathology and structural changes in hydrocephalus. Developmental disabilities research reviews, 16(1), 16–22. [DOI] [PubMed] [Google Scholar]
- Deniz FE, Ece K, Çelik Ö, Akalan N, & Fırat MM (2008). Spontaneous third ventriculostomy in chronic obstructive hydrocephalus. Child’s Nervous System, 24(5), 633–634. [DOI] [PubMed] [Google Scholar]
- Escolar ML, West T, Dallavecchia A, Poe MD, & LaPoint K (2016). Clinical management of Krabbe disease. Journal of Neuroscience Research, 94(11), 1118–1125. doi: doi: 10.1002/jnr.23891 [DOI] [PubMed] [Google Scholar]
- Folio MR, & Fewell RR (2000). Peabody developmental motor scales: Examiner’s manual: Pro-ed. [Google Scholar]
- Gallia GL, & Teo C (2008). Spontaneous third ventriculocisternostomy in an infant with obstructive hydrocephalus. Journal of neurosurgery. Pediatrics, 1(6), 477–480. doi: 10.3171/ped/2008/1/6/477 [DOI] [PubMed] [Google Scholar]
- Graziano ACE, & Cardile V (2015). History, genetic, and recent advances on Krabbe disease. Gene, 555(1), 2–13. [DOI] [PubMed] [Google Scholar]
- Hanlo PW, Gooskens RM, Van Schooneveld M, Tulleken CF, van der Knaap MS, Faber JJ, & Willemse J (1997). The effect of intracranial pressure on myelination and the relationship with neurodevelopment in infantile hydrocephalus. Developmental Medicine & Child Neurology, 39(5), 286–291. [DOI] [PubMed] [Google Scholar]
- Hwang M, Zuccoli G, Panigrahy A, Rodriguez D, Poe MD, & Escolar ML (2016). Thickening of the cauda equina roots: a common finding in Krabbe disease. European radiology, 26(10), 3377–3382. [DOI] [PubMed] [Google Scholar]
- Kahle KT, Kulkarni AV, Limbrick DD Jr, & Warf BC (2016). Hydrocephalus in children. The lancet, 387(10020), 788–799. [DOI] [PubMed] [Google Scholar]
- Kanjilal GC (1972). Spontaneous cerebral ventriculostium: two cases. Journal of Neurology, Neurosurgery & Psychiatry, 35(5), 676–681. doi: 10.1136/jnnp.35.5.676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim LJ, Feiz-Erfan I, Clatterbuck RE, & Spetzler RF (2005). Spontaneous ventriculostomy in a patient with obstructive hydrocephalus. Acta neurochirurgica, 147(2), 219–220. [DOI] [PubMed] [Google Scholar]
- Kuczmarski RJ (2000). CDC growth charts; United States. [Google Scholar]
- Laxdal T, & Hallgrimsson J (1974). Krabbe’s globoid cell leucodystrophy with hydrocephalus. Archives of Disease in Childhood, 49(3), 232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie EV, & Alker GJ Jr (1964). Spontaneous ventriculostomy. Radiology, 83(4), 683–686. [DOI] [PubMed] [Google Scholar]
- Liao P, Gelinas J, & Sirrs S (2014). Phenotypic variability of Krabbe disease across the lifespan. Canadian Journal of Neurological Sciences, 41(1), 5–12. [DOI] [PubMed] [Google Scholar]
- Livingston JH, Graziano C, Pysden K, Crow YJ, Mordekar SR, Moroni I, & Uziel G (2012). Intracranial calcification in early infantile Krabbe disease: nothing new under the sun. Developmental Medicine & Child Neurology, 54(4), 376–379. [DOI] [PubMed] [Google Scholar]
- Lo BWY, Kulkarni AV, Rutka JT, Jea A, Drake JM, Lamberti-Pasculli M, … Thabane L (2008). Clinical predictors of developmental outcome in patients with cephaloceles. Journal of Neurosurgery: Pediatrics, 2(4), 254–257. [DOI] [PubMed] [Google Scholar]
- Luigetti M, Pravatà E, Bartalena T, & Cianfoni A (2013). An Uncommon Cause of Headache Resolution: Spontaneous Ventriculostomy in Obstructive Hydrocephalus. Headache: The Journal of Head and Face Pain, 53(8), 1356–1357. [DOI] [PubMed] [Google Scholar]
- Martin HR, Poe MD, Reinhartsen D, Pretzel RE, Roush J, Rosenberg A, … Escolar ML (2008). Methods for assessing neurodevelopment in lysosomal storage diseases and related disorders: a multidisciplinary perspective. Acta Paediatrica, 97, 69–75. [DOI] [PubMed] [Google Scholar]
- Morana G, Biancheri R, Dirocco M, Filocamo M, Marazzi MG, Pessagno A, & Rossi A (2009). Enhancing cranial nerves and cauda equina: an emerging magnetic resonance imaging pattern in metachromatic leukodystrophy and krabbe disease. Neuropediatrics, 40(06), 291–294. [DOI] [PubMed] [Google Scholar]
- Mortazavi MM, Adeeb N, Griessenauer CJ, Sheikh H, Shahidi S, Tubbs RI, & Tubbs RS (2014). The ventricular system of the brain: a comprehensive review of its history, anatomy, histology, embryology, and surgical considerations. Child’s Nervous System, 30(1), 19–35. [DOI] [PubMed] [Google Scholar]
- Mullen EM (1995). Mullen scales of early learning: AGS Circle Pines, MN. [Google Scholar]
- Murphy BP, Inder TE, Huppi PS, Warfield S, Zientara GP, Kikinis R, … Volpe JJ (2001). Impaired cerebral cortical gray matter growth after treatment with dexamethasone for neonatal chronic lung disease. Pediatrics, 107(2), 217–221. [DOI] [PubMed] [Google Scholar]
- Öğrenci A, Ekşi MŞ, & Koban O (2016). Spontaneous third ventriculostomy 8 years after diagnosis of obstructive hydrocephalus. Child’s Nervous System, 32(9), 1727–1730. [DOI] [PubMed] [Google Scholar]
- Orsini JJ, E. M, Wasserstein MP, Caggana M et al. (2000. [Updated 2018 Oct 11]). Krabbe disease. GeneReviews [Internet]. [Google Scholar]
- Orsini JJ, Saavedra Matiz CA, Gelb MH, & Caggana M (2016). Newborn screening for K rabbe’s disease. Journal of Neuroscience Research, 94(11), 1063–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmar A, Aquilina K, & Carter MR (2009). Spontaneous third ventriculostomy: definition by endoscopy and cerebrospinal fluid dynamics: Case report. Journal of neurosurgery, 111(3), 628–631. [DOI] [PubMed] [Google Scholar]
- Perdaens O, Koerts G, & Nassogne M-C (2018). Hydrocephalus in children under the age of five from diagnosis to short-/medium-/long-term progression: a retrospective review of 142 children. Acta Neurologica Belgica, 118(1), 97–103. [DOI] [PubMed] [Google Scholar]
- Pinto VL, A. A I. I P. U O. I S. I T. I F. S P. J.-A. f. (2018, [Updated 2018 Oct 27]). Increased Intracranial Pressure. from https://www.ncbi.nlm.nih.gov/books/NBK482119/
- Richards KC, Morse RP, & Howrey R (1999). 393 Stem cell transplantation in Krabbe disease: Outcome in nine cases. European Journal of Paediatric Neurology, 3(6), A102–A103. [Google Scholar]
- Rovira A, Capellades J, Grivé E, Poca MA, Pedraza S, Sahuquillo J, & Rodríguez-Baeza A (1999). Spontaneous ventriculostomy: report of three cases revealed by flow-sensitive phase-contrast cine MR imaging. American journal of neuroradiology, 20(9), 1647–1652. [PMC free article] [PubMed] [Google Scholar]
- Russell DJ, Rosenbaum PL, Wright M, & Avery LM (2002). Gross motor function measure (GMFM-66 & GMFM-88) user’s manual (Vol. 159): Mac keith press London. [Google Scholar]
- Suzuki K (2003). Globoid Cell Leukodystrophy (Krabbe’s Disease): Update. Journal of Child Neurology, 18(9), 595–603. doi: 10.1177/08830738030180090201 [DOI] [PubMed] [Google Scholar]
- Udow S, Bunge M, Ryner L, Mhanni AA, & Salman MS (2012). Prolonged survival and serial magnetic resonance imaging/magnetic resonance spectroscopy changes in infantile Krabbe disease. Pediatric neurology, 47(4), 299–302. [DOI] [PubMed] [Google Scholar]
- Vargiami E, Papathanasiou E, Batzios S, Kyriazi M, Dimitriou E, Anastasiou A, … Zafeiriou DI (2016). Neuroradiological, neurophysiological and molecular findings in infantile Krabbe disease: two case reports. Balkan Journal of Medical Genetics, 19(1), 85–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright MD, Poe MD, DeRenzo A, Haldal S, & Escolar ML (2017). Developmental outcomes of cord blood transplantation for Krabbe disease A 15-year study. Neurology, 10.1212/WNL.0000000000004418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen A, Bulluss KJ, Trost N, & Murphy MA (2008). Spontaneous third ventriculostomy. Journal of Clinical Neuroscience, 15(5), 587–590. [DOI] [PubMed] [Google Scholar]
- Zivadinov R (2005). Steroids and brain atrophy in multiple sclerosis. Journal of the neurological sciences, 233(1–2), 73–81. [DOI] [PubMed] [Google Scholar]
- Zuccoli G, Narayanan S, Panigrahy A, Poe MD, & Escolar ML (2015). Midbrain morphology reflects extent of brain damage in Krabbe disease. Neuroradiology, 57(7), 739–745. [DOI] [PubMed] [Google Scholar]


