Abstract
The immune system is composed of a diverse array of cell types, each with a specialized role in orchestrating the immune response to pathogens or cancer. Even within a single cell ‘type,’ individual cells can access a wide spectrum of differentiation and activation states, which reflect the physiological response of each cell to the tissue environment and immune stimuli. Thus, the cellular diversity of the immune system is inherently quite complex and understanding this complexity has greatly benefited from technologies that measure immune responses at single-cell resolution, in addition to the systems-level response as a whole. In this Commentary, we focus on recent work at the interface of immunology and single-cell genomics and highlight advances in technologies and their application to immune cells. We highlight recent single cell genomic profiling of T cells in particular, as somatic rearrangements in the T cell receptor (TCR) loci enable the tracking of clonal T cell responses through space and time. Finally, we discuss opportunities for future use of these technologies in understanding vaccination and the basis for effective vaccine-induced immunity.
Single-cell genomic technologies to study the immune system
For decades, immunologists have focused on the single cell as a fundamental unit of the immune response, often generating new technologies to advance the possible resolution of cellular analysis, such as flow cytometry and in situ fluorescence imaging1,2. As a result, the field has accumulated a deep understanding of the immune system, including cell type diversity, cell lineage and differentiation pathways, and cell-cell interactions within tissues, to an extent that is largely unrivaled by any other tissue system. However, these studies have primarily relied on the expression of cell surface proteins chosen a priori, since most single-cell methods required the use of monoclonal antibodies recognizing surface proteins. In contrast, genomic measurements including DNA or RNA sequencing have generally been performed in bulk after the isolation of cell populations using previously identified surface markers.
Over the past few years, methods to measure genomic profiles of single cells have revolutionized the study of tissue biology, enabling unbiased analyses of cells without prior knowledge of cell types or surface markers. Among these methods, single-cell RNA-seq (scRNA-seq) stands out as the most rapidly evolving and widely adopted technique. In current iterations, this method can generate genome-wide transcriptome profiles – measuring thousands of unique gene transcripts – from tens of thousands of single cells per experiment. The technological advances in scRNA-seq have recently been reviewed elsewhere3–5, but include improvements in: 1) cell profiling throughput, increasing from tens to thousands of single-cell profiles per experiment, 2) sensitivity and reproducibility, such as the use of unique molecular identifiers for quantification of RNA transcripts, and 3) analytical tools, including tools for unbiased single-cell clustering and visualization6, including lineage trajectory mapping7. As a result, generation of these datasets is now achievable in most laboratories equipped for standard molecular biology, and the analysis of single-cell data is increasingly automated.
A series of studies in mouse dendritic cells provided insights into the potential of this technology for studies of the immune system. Shalek and Satija et al. performed scRNA-seq in stimulated bone marrow-derived dendritic cells (BMDCs), which revealed significant transcriptional heterogeneity in cells that appeared similar by cell surface marker profiles8,9. Among 18 profiled single cells, 3 appeared distinct by transcriptional profile and were classified as a ‘mature’ BMDC differentiation state. The ‘mature’ BMDCs, later verified as a distinct cell type10, expressed high levels of genes critical for stimulating a T cell response, including Ccr7, Ccl22, and Cd83. In contrast, the other cells, classified as ‘maturing’ cells, expressed high levels of inflammatory cytokines, such as Tnf and Il1a. In a second study, the same authors expanded the dataset to profile more than 1,700 BMDCs and showed that even within ‘maturing’ cells, significant intra-cell type heterogeneity could be observed9. Namely, after stimulation with lipopolysaccharide (LPS), a small fraction of these cells exhibited precocious behavior and transcribed anti-viral response genes before the rest of the population. Importantly, this heterogeneity was functional, since the removal of precocious cells or the cytokines secreted by these cells blunted the response of all cells in culture.
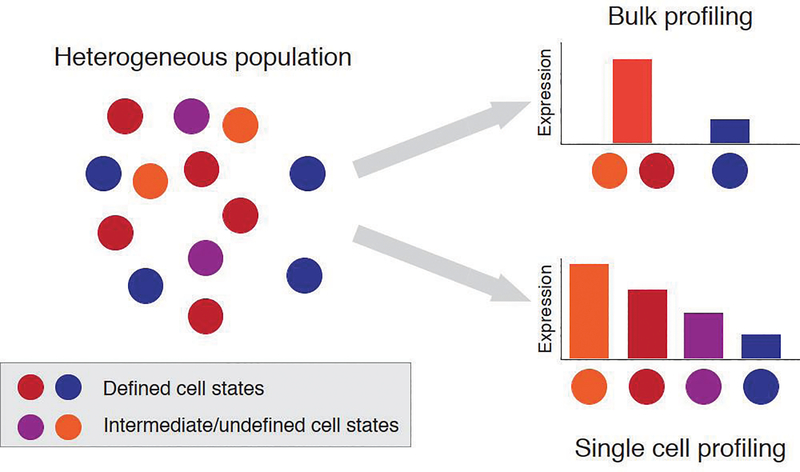
To demonstrate that scRNA-seq could also be used to de-convolute more complex cellular mixtures, Jaitin et al. analyzed 4,000 single mouse splenocytes11. This analysis identified the expected immune lineages in the spleen, such as B and T cells, as well as new cell type distinctions. For example, analysis of splenic dendritic cells (DCs) identified at least 4 subtypes, which were not previously identified using standard cell surface marker demarcations. Furthermore, as observed in BMDCs, activation of splenic DCs with LPS led to substantial heterogeneity in gene expression across the population. For example, while interferon pathway genes were up-regulated uniformly in all CD4+ DCs after stimulation, tumor necrosis factor (TNF) and transforming growth factor-β (TGFβ) pathways were up-regulated only in a subset of CD4+ DCs. In subsequent studies, similar insights were observed in other immune cell types, including in CD4+ and CD8+ T cells, innate lymphoid cells (ILCs), macrophages, and hematopoietic progenitors12–16. In nearly every study, scRNA-seq revealed previously unrecognized features of each cell type, such as heterogeneity within conventional surface marker-defined populations or rare functional subsets. For example, in hematopoietic progenitors, scRNA-seq identified significant heterogeneity in common myeloid progenitors (CMPs), including cells with restricted potential for single myeloid cell fates15. Similarly, in T helper 17 (Th17) cells, scRNA-seq identified subsets of pathogenic and non-pathogenic cells, both in vitro and in vivo, and nominated critical gene pathways governing their development12,13. In summary, these studies demonstrated that scRNA-seq can be used to identify inter- and intra-population variability without prior knowledge of cell types or activation states (Fig. 1).
Figure 1. Interrogation of heterogenous immune populations enabled by single-cell genomics.
A heterogenous population of immune cell contains defined cell states characterized by previously identified surface markers (red and blue), intermediate (purple) and undefined cell states (orange). Bulk genomic profiling relies on isolating cells based on pre-defined surface markers, which can lead to profiling of mixed cell states and the absence of intermediate cell states (top). Single-cell genomic profiling enables unbiased interrogation of heterogenous populations, revealing uncharacterized and intermediate cell states (bottom).
Tracking the T cell response with TCR- and scRNA-seq
One unique feature of scRNA-seq profiles in T and B cells is the presence of RNA transcripts that encode for the T- and B-cell receptors, respectively. In T cells, the predominant TCR type is generated through somatic recombination of TCRα and TCRβ subunit genes, which produces a highly diverse repertoire of nearly 1014 unique heterodimers in each individual17. As a result, the genetic sequence of the TCR is uniquely informative; not only does it determine antigen specificity, but it also serves as a cellular barcode that traces the lineage of each cell. Indeed, initial studies demonstrated the value of pairing cellular phenotype with clonal identity. Han et al. performed targeted sequencing of TCR and RNA transcripts in primary human T cells and demonstrated that T cells arising from the same clonal population could adopt distinct phenotypes18. For example, in a patient with colorectal cancer, a clonally-expanded population of tumor-infiltrating T cells adopted both regulatory T cell (Treg) and T helper 17 (Th17) phenotypes, indicating phenotypic plasticity in cells arising from the same parental clone. Conversely, different expanded T cell clones that were predicted to bind a shared antigen (based on TCR sequence specificity) shared a common phenotype, suggesting that antigen specificity can also direct T cell phenotype.
Two recent advances have enabled pairing T cell clonotype with phenotype at higher resolution and throughput. First, development of analytic tools including TraCeR and MiXCR have enabled the reconstruction of TCR sequences from scRNA-seq and bulk RNA-seq data19,20. In these methods, bulk or single-cell RNA-seq is performed using standard workflows and reads aligning to the TCR locus are computationally extracted and reassembled into contigs based on overlapping sequences within individual reads. These methods benefit from the fact that they can be used on existing datasets to faithfully reconstruct TCR sequences from a high proportion of single cells (>70% paired TCRα and TCRβ), although these methods were designed for compatibility with full-length scRNA-seq methods such as SmartSeq2. Second, experimental methods that produce paired TCR-and scRNA-seq libraries have been adapted for a number of platforms, including droplet-based methods such as InDrops21 and the 10X Genomics platform22,23. These methods split input RNA and produce two sequencing libraries matched by a unique cellular barcode – one for whole transcriptome and the second for targeted TCR sequencing, taking advantage of the high throughput possible on droplet-based platforms while maintaining a high TCR recovery rate (>70%).
These techniques have now been applied to variety of biological systems, revealing complex clonal dynamics in the T cell response to pathogens and cancer. Initial descriptions of T cell phenotypes in the context of melanoma by Tirosh et al. demonstrated the ability of scRNA-seq to distinguish not only the major phenotypes of tumor-infiltrating T cells (CD4+, CD8+ and Treg) but also a spectrum of activation and exhaustion states defined by co-expression of cytotoxic (GZMA, GZMB, IFNG) and inhibitory (PD1, TIGIT, LAG3, CTLA4) genes, respectively24. Reconstruction of TCR sequence in individual cells identified clonally-expanded subsets of tumor-infiltrating T cells which were enriched for exhaustion and activation signatures, supporting a link between clonal expansion, T cell activation, and exhaustion within the tumor microenvironment. Subsequent studies have shown high concordance in phenotype between T cells sharing a common TCR sequence, even within different activation states in major phenotype classifications such as Tregs21,22.
Despite the high correlation between TCR sequence and phenotype, additional studies have characterized transitions between distinct phenotypic states among clonally related cells. In the context of malaria infection, Lönnberg et al. demonstrated a bifurcation between closely related but functionally distinct populations of T helper 1 (Th1) and T follicular helper (Tfh) T cell subsets25. Inference of the clonal origin of these cells based on TCR sequence demonstrated that this bifurcation can occur within single clones of cells sharing an identical TCR, suggesting that the progeny of a single parental cell can adopt distinct phenotypic states. Within the tumor microenvironment, Li et al. compared TCR sequences shared between different phenotypic states to provide insight into the origin and differentiation lineages that connect distinct T cell phenotypes. TCR sharing between TIL phenotypes suggested that dysfunctional, exhausted CD8+ T cells derive from a differentiation gradient which includes partially exhausted, transitional cells, while cytotoxic CD8+ T cells derive from distinct clonal populations26. Finally, methods to link TCR sequence to epigenetic profile in single cells have enabled interrogation of the cis-regulatory landscapes that distinguish both distinct T cell phenotypes and clonal populations within the same phenotypic state27. In summary, recent studies combining TCR and scRNA-seq have shed light on the diversity of clonal dynamics during T cell response, revealing both convergent phenotypes and transitions between distinct phenotypic states within clonally related cells. Importantly, these studies required single-cell technologies, since it would be difficult, if not impossible, to answer these questions with population measurements.
Moreover, combined scRNA and TCR sequencing has enabled not only tracking of productive immune responses, but also novel insights into the origin of responsive T cell populations. For example, profiling of phenotypic and clonal dynamics in the context of checkpoint blockade immunotherapy in site-matched biopsies revealed that clonal T cell expansion following therapy preferentially derives from novel clones not previously observed in the tumor rather than reactivation of pre-existing clones28. Additional insights regarding the origins of therapy-responsive T cells enabled by single-cell profiling will have important implications for the rational design of effective therapeutic interventions.
Technologies for the future and opportunities for vaccinology
In summary, the advent of single-cell genomic technologies has revolutionized studies of the immune system, enabling the de-convolution of complex immunological responses without a priori knowledge of cell types or gene pathways. These tools have been particularly useful for the analysis of lymphocytes, since linking each cell’s unique antigen receptor sequence with its molecular phenotype provides an unprecedented view of the relationships between antigen specificity, clonal lineage history, and gene regulation. We primarily focused on describing these principles in the context of transcriptome profiling with scRNA-seq. However, future studies should also take advantage of other emerging single-cell modalities, including methods to profile the epigenome in single cells, such as single-cell assay for transposase-accessible chromatin (scATAC-seq)29, multi-modal genomic methods, such as those that obtain protein, RNA, and/or epigenomic measurements in the same cell27,30–32, and methods that link transcriptome profiling with spatial information33–36. Furthermore, emerging methods to pair TCR sequences with cognate peptide-major histocompatibility complexes (MHC), and ultimately with transcriptome profiles, will facilitate functional profiling of antigen specificity encoded via TCR sequence37–41. Finally, the simultaneous measurement of genomic perturbations (with CRISPR/Cas9) and molecular phenotypes in single cells will enable the functional dissection of gene networks that drive cell types and states42–46.
In patients, scRNA-seq of patient samples has primarily been performed in the settings of infection and cancer, and although more extensive studies are needed, we envision that scRNA-seq could eventually bring value to clinical decision-making. For example, the analysis of the T cell response to checkpoint blockade, as read out by the clonal measurement of T cell expansion in the blood48, could inform whether a therapeutic dose was achieved in each patient, or to optimize combination therapies with other agents. Similarly, tracking the presence, phenotype, and persistence of CAR-T cells post-infusion may also be useful clinically. We also envision significant research and clinical applications for scRNA-seq in studying the response to vaccines. For example, serial analysis of blood cells after vaccination could identify responding immune cell phenotypes and clonotypes, gene expression pathways specifically active in clonally-expanded cells, and the differences between patients who either respond or are resistant to vaccination. Comparisons between vaccine preparations could also identify distinct and shared gene regulatory pathways induced by different adjuvants, and whether certain patient characteristics (such as age or gender) impinge upon these pathways. Finally, similar technological advances in the analysis of B cell responses could be used to understand mechanisms underlying the persistence of the vaccine-induced antibody response48. Altogether, these technologies should provide new insights into the regulation of vaccine-induced immunity, and ultimately provide a molecular basis for improved vaccine design in the future.
Acknowledgments
This work was supported by the Parker Institute for Cancer Immunotherapy (A.T.S. and H.Y.C.), the National Institutes of Health (NIH) P50HG007735 (H.Y.C.) and K08CA230188 (A.T.S.). A.T.S. was supported by a Young Investigator Award from the Human Vaccines Project and Michelson Medical Research Foundation, a Bridge Scholar Award from the Parker Institute for Cancer Immunotherapy, and a Career Award for Medical Scientists from the Burroughs Wellcome Fund. K.E.Y. was supported by the National Science Foundation Graduate Research Fellowship Program (NSF DGE-1656518) and a Stanford Graduate Fellowship. H.Y.C. is an investigator of the Howard Hughes Medical Institute.
Footnotes
Conflict of Interest
A.T.S. is an advisor to Immunai. H.Y.C. is a co-founder of Accent Therapeutics and Epinomics and is an adviser to 10x Genomics and Spring Discovery.
Data Statement
As this is a Commentary, there is no data in the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lanier LL et al. Correlation of functional properties of human lymphoid cell subsets and surface marker phenotypes using multiparameter analysis and flow cytometry. Immunol. Rev 74, 143–160 (1983). [DOI] [PubMed] [Google Scholar]
- 2.Miller MJ, Wei SH, Parker I & Cahalan MD Two-Photon Imaging of Lymphocyte Motility and Antigen Response in Intact Lymph Node. Science 296, 1869–1873 (2002). [DOI] [PubMed] [Google Scholar]
- 3.Stegle O, Teichmann SA & Marioni JC Computational and analytical challenges in single-cell transcriptomics. Nat. Rev. Genet 16, 133–145 (2015). [DOI] [PubMed] [Google Scholar]
- 4.Papalexi E & Satija R Single-cell RNA sequencing to explore immune cell heterogeneity. Nat. Rev. Immunol 18, 35–45 (2018). [DOI] [PubMed] [Google Scholar]
- 5.Giladi A & Amit I Single-Cell Genomics: A Stepping Stone for Future Immunology Discoveries. Cell 172, 14–21 (2018). [DOI] [PubMed] [Google Scholar]
- 6.Stuart T et al. Comprehensive Integration of Single-Cell Data. Cell 177, 1888–1902.e21 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Trapnell C et al. The dynamics and regulators of cell fate decisions are revealed by pseudotemporal ordering of single cells. Nat. Biotechnol 32, 381–386 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shalek AK et al. Single-cell transcriptomics reveals bimodality in expression and splicing in immune cells. Nature 498, 236–240 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shalek AK et al. Single-cell RNA-seq reveals dynamic paracrine control of cellular variation. Nature 510, 363–369 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Helft J et al. GM-CSF Mouse Bone Marrow Cultures Comprise a Heterogeneous Population of CD11c(+)MHCII(+) Macrophages and Dendritic Cells. Immunity 42, 1197–1211 (2015). [DOI] [PubMed] [Google Scholar]
- 11.Jaitin DA et al. Massively Parallel Single-Cell RNA-Seq for Marker-Free Decomposition of Tissues into Cell Types. Science 343, 776–779 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gaublomme JT et al. Single-Cell Genomics Unveils Critical Regulators of Th17 Cell Pathogenicity. Cell 163, 1400–1412 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang C et al. CD5L/AIM Regulates Lipid Biosynthesis and Restrains Th17 Cell Pathogenicity. Cell 163, 1413–1427 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Avraham R et al. Pathogen Cell-to-Cell Variability Drives Heterogeneity in Host Immune Responses. Cell 162, 1309–1321 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paul F et al. Transcriptional Heterogeneity and Lineage Commitment in Myeloid Progenitors. Cell 163, 1663–1677 (2015). [DOI] [PubMed] [Google Scholar]
- 16.Singer M et al. A Distinct Gene Module for Dysfunction Uncoupled from Activation in Tumor-Infiltrating T Cells. Cell 166, 1500–1511.e9 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davis MM & Bjorkman PJ T-cell antigen receptor genes and T-cell recognition. Nature 334, 395–402 (1988). [DOI] [PubMed] [Google Scholar]
- 18.Han A, Glanville J, Hansmann L & Davis MM Linking T-cell receptor sequence to functional phenotype at the single-cell level. Nat. Biotechnol 32, 684–692 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stubbington MJT et al. T cell fate and clonality inference from single-cell transcriptomes. Nat. Methods 13, 329–332 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bolotin DA et al. Antigen receptor repertoire profiling from RNA-seq data. Nat. Biotechnol 35, 908–911 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zemmour D et al. Single-cell gene expression reveals a landscape of regulatory T cell phenotypes shaped by the TCR. Nat. Immunol 19, 291–301 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Azizi E et al. Single-Cell Map of Diverse Immune Phenotypes in the Breast Tumor Microenvironment. Cell 174, 1293–1308.e36 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Neal JT et al. Organoid Modeling of the Tumor Immune Microenvironment. Cell 175, 1972–1988.e16 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tirosh I et al. Dissecting the multicellular ecosystem of metastatic melanoma by single-cell RNA-seq. Science 352, 189–196 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lönnberg T et al. Single-cell RNA-seq and computational analysis using temporal mixture modeling resolves TH1/TFH fate bifurcation in malaria. Sci. Immunol 2, eaal2192 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li H et al. Dysfunctional CD8 T Cells Form a Proliferative, Dynamically Regulated Compartment within Human Melanoma. Cell 176, 775–789.e18 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Satpathy AT et al. Transcript-indexed ATAC-seq for precision immune profiling. Nat. Med 24, 580 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yost KE et al. Clonal replacement of tumor-specific T cells following PD-1 blockade. Nat. Med 25, 1251–1259 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buenrostro JD et al. Single-cell chromatin accessibility reveals principles of regulatory variation. Nature 523, 486–490 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shahi P, Kim SC, Haliburton JR, Gartner ZJ & Abate AR Abseq: Ultrahigh-throughput single cell protein profiling with droplet microfluidic barcoding. Sci. Rep 7, 44447 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stoeckius M et al. Simultaneous epitope and transcriptome measurement in single cells. Nat. Methods 14, 865–868 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cao J et al. Joint profiling of chromatin accessibility and gene expression in thousands of single cells. Science 361, 1380–1385 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Satija R, Farrell JA, Gennert D, Schier AF & Regev A Spatial reconstruction of single-cell gene expression data. Nat. Biotechnol 33, 495–502 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ståhl PL et al. Visualization and analysis of gene expression in tissue sections by spatial transcriptomics. Science 353, 78–82 (2016). [DOI] [PubMed] [Google Scholar]
- 35.Cohen M et al. Lung Single-Cell Signaling Interaction Map Reveals Basophil Role in Macrophage Imprinting. Cell 175, 1031–1044.e18 (2018). [DOI] [PubMed] [Google Scholar]
- 36.Vickovic S et al. High-definition spatial transcriptomics for in situ tissue profiling. Nat. Methods 1–4 (2019) doi: 10.1038/s41592-019-0548-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bentzen AK et al. Large-scale detection of antigen-specific T cells using peptide-MHC-I multimers labeled with DNA barcodes. Nat. Biotechnol 34, 1037–1045 (2016). [DOI] [PubMed] [Google Scholar]
- 38.Gee MH et al. Antigen Identification for Orphan T Cell Receptors Expressed on Tumor-Infiltrating Lymphocytes. Cell 172, 549–563.e16 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li G et al. T cell antigen discovery via trogocytosis. Nat. Methods 16, 183–190 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Joglekar AV et al. T cell antigen discovery via signaling and antigen-presenting bifunctional receptors. Nat. Methods 16, 191–198 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kisielow J, Obermair F-J & Kopf M Deciphering CD4 + T cell specificity using novel MHC–TCR chimeric receptors. Nat. Immunol 20, 652–662 (2019). [DOI] [PubMed] [Google Scholar]
- 42.Dixit A et al. Perturb-Seq: Dissecting Molecular Circuits with Scalable Single-Cell RNA Profiling of Pooled Genetic Screens. Cell 167, 1853–1866.e17 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Adamson B et al. A Multiplexed Single-Cell CRISPR Screening Platform Enables Systematic Dissection of the Unfolded Protein Response. Cell 167, 1867–1882.e21 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jaitin DA et al. Dissecting Immune Circuits by Linking CRISPR-Pooled Screens with Single-Cell RNA-Seq. Cell 167, 1883–1896.e15 (2016). [DOI] [PubMed] [Google Scholar]
- 45.Datlinger P et al. Pooled CRISPR screening with single-cell transcriptome readout. Nat. Methods 14, 297–301 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rubin AJ et al. Coupled Single-Cell CRISPR Screening and Epigenomic Profiling Reveals Causal Gene Regulatory Networks. Cell 176, 361–376.e17 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang AC et al. A single dose of neoadjuvant PD-1 blockade predicts clinical outcomes in resectable melanoma. Nat. Med 25, 454 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lindeman I et al. BraCeR: B-cell-receptor reconstruction and clonality inference from single-cell RNA-seq. Nat. Methods 15, 563–565 (2018). [DOI] [PubMed] [Google Scholar]