Abstract
Introduction
Total knee arthroplasty (TKA) reduces joint symptoms, but habitual movement compensations persist years after surgery. Preliminary research on movement training interventions have signaled initial efficacy for remediating movement compensations and restoring knee joint loading symmetry during dynamic functional tasks after TKA. The purpose of this clinical trial is to determine if physical rehabilitation that includes movement training restores healthy movement patterns after TKA and reduces the risk of osteoarthritis (OA) progression in the contralateral knee.
Methods/design
150 participants will be enrolled into this randomized controlled trial. Participants will be randomly allocated to one of two dose-equivalent treatment groups: standard rehabilitation plus movement training (MOVE) or standard rehabilitation without movement training (CONTROL). Movement training will promote between-limb symmetry and surgical knee loading during activity-based exercises. Movement training strategies will include real-time biofeedback using in-shoe pressure sensors and verbal, visual, and tactile cues from the physical therapist. The primary outcome will be change in peak knee extension moment in the surgical knee during walking, from before surgery to six months after surgery. Secondary outcomes will include lower extremity movement symmetry during functional tasks, physical function, quadriceps strength, range of motion, satisfaction, adherence, contralateral knee OA progression, and incidence of contralateral TKA.
Discussion
This study will provide insights into the efficacy of movement training after unilateral TKA, along with mechanisms for optimizing long-term physical function and minimizing negative sequelae of compensatory movement patterns.
Keywords: joint replacement, biofeedback, osteoarthritis, rehabilitation, physical therapy
1. Introduction
Total knee arthroplasty (TKA) is one of the most commonly performed surgical procedures in the United States, and its utilization is expected to increase exponentially in the coming decade due to the aging of the population and an increased rate of obesity.[1, 2] Although most patients report substantial improvements in pain and self-reported function after unilateral TKA, movement compensations acquired prior to surgery often persist years after surgery.[3–7] These movement compensations are characterized by disuse of the surgical limb and a reliance on the non-surgical limb during walking and other functional tasks. Such between-limb movement asymmetries after TKA are associated with decreased knee extensor moments and persistent quadriceps weakness in the surgical limb along with poor physical function in both the short and long-term.[5–8] Moreover, these movement compensations are associated with a higher risk of developing osteoarthritis (OA) in the non-surgical limb and requiring a second contralateral TKA.[3, 9] Thus, failure to resolve habitual movement compensations after unilateral TKA may lead to poor recovery of muscle strength and function as well as increased contralateral OA progression.
Traditional rehabilitation protocols after TKA focus primarily on critical impairments such as pain, range of motion, and strength, but often lack any components of movement training.[10, 11] Preliminary research has found that providing patients with biofeedback during weight-bearing activities can improve movement patterns after surgery, improve strength recovery, and increase patient enjoyment. These interventions have been delivered through the use of commercially-available video gaming systems[12] and through biofeedback of weight-bearing symmetry during functional tasks and closed-chain strengthening exercises.[13] While these previous movement interventions allowed therapists to monitor force symmetry and encourage normal movement patterns, they were limited in their scope of use. These interventions required constrained foot positions and did not easily allow patients to receive biofeedback outside of the clinical setting during a range of daily activities. The current study protocol has addressed such limitations by integrating commercially-available in-shoe pressure sensors that provide real-time feedback during any weight-bearing activity at any location. Integration of this technology, along with specific movement training methods and motor learning principles into a comprehensive rehabilitation strategy, are the core elements of the experimental protocol.
As the incidence of TKA continues to increase, it is essential that rehabilitation protocols adequately address all residual physical, functional, and biomechanical impairments after surgery to optimize recovery and long-term health. Therefore, the purpose of this randomized clinical trial is to determine if a novel movement training program (MOVE) remediates pre-operative movement compensation more than standard rehabilitation (CONTROL) without movement training. Secondarily, we will determine if MOVE improves long-term physical function, strength, and lessens contralateral OA progression. Our hypotheses are that MOVE will lead to improved biomechanical outcomes, strength, and function after surgery while reducing the incidence of OA progression in the non-surgical knee.
2. Methods
2.1. Study Design
This will be a two-arm, randomized controlled trial that enrolls 150 participants undergoing unilateral TKA to determine if the addition of MOVE to standard rehabilitation improves movement pattern quality more than standard rehabilitation without movement training (CONTROL). This study has been prospectively registered on clinicaltrials.gov (NCT03325062).
2.2. Participants
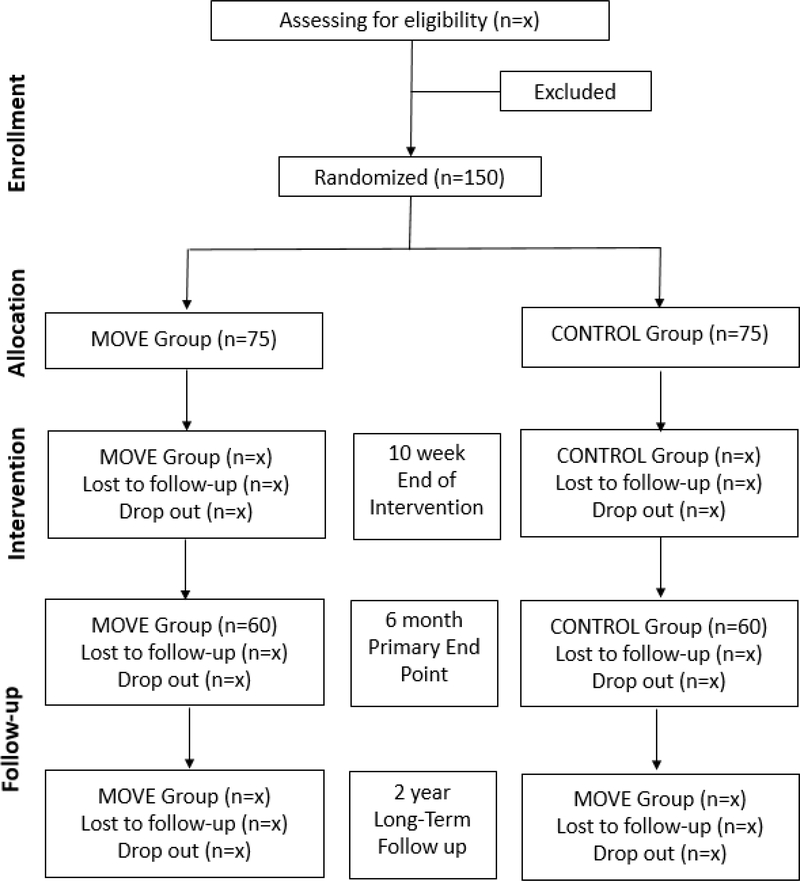
Participants will be recruited from local orthopedic clinics in the Denver metro area. One hundred and fifty participants will be consecutively recruited, with at least 120 expected to complete testing at the six-month primary end-point. Participants can be included in the study if they are between 50–85 years old and are scheduled for a primary unilateral TKA for end-stage OA. Exclusion criteria are: severe contralateral knee OA (>4/10 verbal numeric pain scale or Kellgren-Lawrence grade >3/4), current smoking or drug abuse, comorbid conditions that limit physical function or would interfere with the participant’s ability to successfully complete rehabilitation (e.g. neurologic, vascular, cardiac problems, or ongoing medical treatments), unstable orthopedic conditions that limit function, uncontrolled diabetes (hemoglobin A1c level >8.0), body mass index >40 kg/m2, unable to safely walk 30 m without an assistive device, surgical complication necessitating an altered course of rehabilitation, discharge to a location other than home after surgery, or previous contralateral TKA. Additional exclusion criteria for magnetic resonance imaging (MRI) are ferromagnetic metal implants or pacemakers and other contraindications to MRI. Informed consent will be obtained from all participants prior to engagement in the study protocol. The proposed CONSORT diagram for participants is shown in Figure 1.
Figure 1:
Proposed CONSORT Diagram
2.3. Randomization and Blinding
Following surgery, each participant will be randomized to a treatment arm: MOVE or CONTROL. Randomization will be carried out in a 1:1 ratio using a computer-generated allocation table with stratification on sex and random block sizes of two and four within each stratum. An unblinded clinical coordinator, who is not involved in testing or recruitment, will randomize subjects, contact the appropriate outpatient clinic with randomization assignments, and assign the appropriate therapist to each participant per the randomization assignment. Blinded study personnel will consist of the principal investigator, study coordinators, outcomes assessors and the biostatistician. Unblinded study personnel will consist of the clinical coordinator and physical therapists delivering the intervention. Participants will only be aware that they are participating in one of two potential rehabilitation programs and will be instructed to not discuss any details of their program with any blinded personnel.
2.4. Intervention
All patients will receive a unilateral, tricompartmental TKA. Following hospital discharge, all participants will be seen in outpatient rehabilitation twice a week for weeks 1–4, once a week for weeks 5–8, and once a week 10. Treatment session duration will average 45 minutes for both groups. Participants in both groups will be given an additional booster visit at four months postoperatively to review their discharge exercise programs for a total of 14 visits. Booster visits have been demonstrated to improve long-term pain and physical function in individuals with knee and hip osteoarthritis, as well as booster visits may improve motor learning.[14]
Both groups will participate in a standardized progressive rehabilitation program, which consists of the following: activity-based exercises focusing on improving walking, standing, rising from a chair, rising from the floor, and stair climbing; range of motion and flexibility exercises; progressive resistive strengthening to key musculature of the lower extremities; balance exercises; manual therapy; modalities; and education on pain, swelling, wound management, return to activity progressions, and home exercises (Appendix A). Home exercises will be based on clinic performance and all exercises will be progressed based upon participant tolerance.[15] Home and clinic-based exercises in both groups will be dosed at the same frequency and intensity and will be based on clinic performance of each participant. The key difference between groups is the MOVE group will receive the movement training intervention (detailed below) during the performance of activity-based exercises whereas the CONTROL group will not. Activities in both groups will be progressed throughout the intervention period from simple (e.g., standing) to more complex (e.g., stair climbing) based upon clinical milestones. To decrease the potential for contamination between groups, treatment schedules for each group will not overlap, and primary therapists will be trained and assigned to treat one group. Details on therapist selection and training are provided in Appendix B.
2.4.1. MOVE Treatment Arm
Participants enrolled in the MOVE program will focus on promoting surgical knee use with emphasis on symmetry in functional knee motion and surgical-limb loading without postural compensation. The MOVE program utilizes the Loadsol® insoles (Novel.de, Munich, Germany) and associated iOS app to deliver real-time visual biofeedback during activity performance. Participants in the MOVE group will use the insoles during clinic treatments, at home during their home exercise program, and during the performance of routine daily activities. This will provide a high number of repetitions during task-specific practice (e.g. greater than 2000 repetitions of sit to stand practice over the course of the intervention) to enhance motor learning.[16, 17] Clinicians will also be allowed to use verbal, visual, auditory, and tactile cues during clinic sessions based upon the optimal strategy for each participant and activity.
During each clinic session in the MOVE group, the participant will be assessed for retention of motor learning from the previous session on their current activities to determine if they are ready for intervention progression.[18] Once a participant is able to complete a given activity with less than 5% between-limb loading asymmetry and no movement compensations, the difficulty will be progressed based upon tolerance criteria or to a difficulty level where the participant is unable to correct the task without biofeedback. Then, the frequency of biofeedback will be faded to 50% using an intermittent biofeedback schedule along with random practice of the activities to promote retention of the improved movement pattern.[19] Participants will be instructed to use intermittent biofeedback throughout the day when performing these activities as a part of daily living to encourage the development of an internal representation of the movement pattern. Intermittent biofeedback has been shown to increase motivation and motor learning.[20] The MOVE group home exercise program will be the same as the CONTROL group home exercise program; however, the MOVE group will utilize biofeedback during the performance of their activity-based exercises. The home-based biofeedback and practice schedule will mirror what is performed in clinic.
2.4.2. CONTROL Treatment Arm
Participants enrolled in the CONTROL program will focus on the same exercise protocol as the MOVE program, though the treating physical therapists will not provide any instructed feedback on movement patterns during their treatment beyond minimal cues for safety. Progression within activities in the CONTROL group will be based upon the participant’s tolerance and safety in performing the activity. The CONTROL home exercise program will be the same as the MOVE group but without use of biofeedback. If a therapist is directly questioned on movement patterns by a participant, the therapist will encourage the participant to move “naturally” and “comfortably.”
2.5. Fidelity Oversight
Onsite fidelity assessments and chart reviews will be used to maximize study treatment fidelity (Appendix B). For each clinical site, the clinical coordinator will observe at least 50% of clinic sessions for the first two participants with each therapist to ensure protocol adherence. Such onsite observations will be repeated with at least one clinic session for every participant and a study treatment fidelity checklist will be completed and graded. If treatment fidelity is below 90%, additional training sessions will be scheduled with individual therapists. We have averaged 97% fidelity when using this approach to ensure fidelity of a previous study (n=162 participants) at the same clinical sites.[15] Therefore, we expect treatment fidelity to be greater than 90% for all observations. Should new therapists require training during the course of the trial, the same procedures for initial selection, training, and monitoring will be implemented. Furthermore, monthly email, phone calls and/or in-person meetings will occur involving each of the two intervention group therapists (MOVE and CONTROL) with the clinical coordinator to answer any questions, review current and past participants, and receive feedback. For chart reviews, a daily note fidelity checklist for adherence to the rehabilitation protocol across therapists will be completed for every visit and charts will be reviewed monthly in each treatment group at each clinical site.
2. 6. Outcomes
All outcomes (Table 1) will be assessed 1–2 weeks preoperatively, at the end of intervention (10 weeks), at six months (primary endpoint), and at two years postoperatively at the University of Colorado Denver Anschutz Medical Campus (Aurora, CO, USA).
Table 1.
Timeline of outcome assessments
| Outcome Measures | Baseline | 10 Weeks | 6 Months | 2 Years |
|---|---|---|---|---|
| Motion Analysis: Peak Knee Extension Moment | X | X | X* | X |
| Physical Function: SCT, 30-STS, 6MW, TUG, Physical Activity | X | X | X | X |
| Self-Report Measures: WOMAC, VR-12 | X | X | X | X |
| Quadriceps Muscle Strength: Dynamometry | X | X | X | X |
| Knee Range of Motion: Goniometry | X | X | X | X |
| Satisfaction | X | |||
| Adherence | X | |||
| Knee OA progression: MRI (WORMS score) | X | X | ||
| Incidence of contralateral TKA | X |
Note: SCT, stair climb test; 30-STS, 30 second sit-to-stand test; 6MW, six minute walk test; TUG, timed up and go test; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index survey; VR-12, Veterans Rand 12-item health survey; MRI, magnetic resonance imaging; OA, osteoarthritis; WORMS, Whole-Organ Magnetic Resonance Imaging Score.
indicate primary outcome and time point.
2.6.1. Primary Outcome
The primary outcome for this study is change in peak knee extension moment in the surgical limb from baseline (preoperative) to six months after TKA during walking at a fixed speed of 1.0 m/s. We have selected the primary outcome of peak knee extension moment as a clear indicator of movement quality for the following reasons: 1) speed on functional tasks can improve acutely (within one year) despite persistent movement compensations[2, 4, 7, 8], 2) knee extension (quadriceps) muscle weakness is a chronic impairment following TKA[21–23], 3) small surgical limb knee extension moments are a primary movement deviation following TKA[24–26], and 4) the MOVE intervention specifically targets surgical limb loading and thus assessment of knee extension moment is a direct assessment of intervention efficacy. We selected the 6-month time point as primary based on preliminary data indicating response to movement training becomes more pronounced at this point as opposed to immediately following the intervention.[12]
2.6.2. Secondary Outcomes
Secondary biomechanical outcome measures include peak surgical limb knee extension moments measured: 1) at the participant’s preferred walking speed, 2) during rising from a chair, and 3) ascending/descending a step. Secondary physical performance and self-report measures include the stair climb test (SCT)[27], 30 second sit-to-stand test (30STS)[28], 6-minute walk test (6MW)[29], timed up and go test (TUG)[30], average steps per day, Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC)[31], The Veteran’s RAND 12-item health survey (VR-12)[32], quadriceps strength[21] and knee range of motion[33]. Weekly home exercise program logs, averaged over the course of the intervention, will be used to quantify adherence to the intervention in addition to number of clinical sessions attended. Adherence to the movement training intervention will also be quantified by calculating the number of minutes of Loadsol® insole wear time over the course of the intervention. Satisfaction with each of the rehabilitation programs will be assessed utilizing a 5-point Likert scale. The relationship between between-limb peak knee extension moment asymmetry (six months) and contralateral knee OA progression assessed by magnetic resonance imaging (MRI) at two years following TKA will be evaluated. Knee OA progression will primarily be quantified using the whole-organ MRI scoring method (WORMS) total sum score, with the maximum WORMS cartilage subscore and maximum WORMS medial meniscus subscore serving as additional descriptors of knee OA progression.
2.6.3. Testing Procedures
Biomechanical and clinical testing will take place in one day, at each test point. Biomechanical testing will be completed prior to the clinical testing and the order of assessment items are standardized. Magnetic resonance imaging assessments will be completed within the same week as biomechanical and clinical testing. Questionnaires will be completed electronically via Research Electronic Data Capture software at home or in-person at the testing session based on participant preference. Outcome assessors will review all questionnaires to ensure no incomplete data.
Biomechanical testing will utilize an 8-camera motion capture system (Vicon Motion Systems, Oxford, United Kingdom) and two force plates (Bertec Corporation, Columbus, OH) embedded in an over ground walkway (12 m). Reflective markers will be placed on anatomical landmarks of the lower limbs, trunk, and upper extremities according to a modified Helen-Hayes marker set.[34] Force and marker position data will be sampled at 2000 Hz and 100 Hz and filtered using a fourth order low-pass Butterworth filter with cut-off frequencies of 20 Hz and 6 Hz, respectively. For gait testing, subjects will be asked to walk at their self-selected speed. Following this, subjects will be asked to walk at a set speed of 1.0 meters/second (±5%). Five acceptable trials at each speed will be collected. Subjects will then be asked to ascend and descend a single step. The wooden box (width 40.26 cm, length 59.69 cm, height 19.05 cm) will be mounted to one of the embedded force plates. Subjects will also perform a sit-to-stand task during the motion capture. The chair will be placed so that the knees are in 90 degrees of flexion when sitting and both feet will be placed symmetrically on adjacent force plates. Subjects will be asked to rise from the chair, come to a complete standing position, and return to a seated position without using their arms. For all activities, joint angles and moments will be calculated using Visual 3D (C-Motion Inc., Germantown, MD) using Euler sequences. Tasks will be time-normalized based on kinetically-derived gait events for the walking and stepping tasks or movement of the pelvis for the sit-to-stand task.
Clinical testing will include a battery of performance-based tests, questionnaires, and clinical measures. Performance-based tests will include the SCT, 30STS, 6MW, and TUG. For the SCT, subjects will be asked to ascend and descend a flight of 12 stairs (17.1 cm step height). A handrail will be available if needed for safety. During the 30STS, the number of times a subject can rise from a seated position without the use of a hands will be recorded. A repetition will only count if the individual has completed standing from the 40 cm chair. In the event the individual cannot perform the test without the use of the hands, he or she will receive a score of 0, but a modified score with the use of hands will be recorded, but not used in the analysis. The 6MW will test how far a subject can walk in six-minutes in a 30.5 m hallway. Subjects cannot run and rest breaks are allowed, although time will continue. The TUG will measure the amount of time it takes for an individual to rise from a chair with a seat height of 40 cm, walk 3 meters, turn-around, walk back to the chair, and return to a seated position. For all tests, subjects will be asked to complete them as fast as possible while still being safe. Time will start on the investigators command of “Go”. These are common, valid, reliable, and responsive measures to measure functional ability before and after TKA.[27–30]
The isometric strength of the knee extensors will be collected using an electromechanical dynamometer (HUMAC Norm, Computer Sports Medicine Inc., Stoughton, MA). Subjects will be positioned in a seated position with hips flexed to 80 degrees and knee flexed to 60 degrees. The ankle will be secured to the dynamometer using Velcro strap, which will be placed on the anterior shank 2 cm proximal to the lateral malleolus. The axis of the dynamometer arm will be aligned with the lateral femoral condyle. Subjects will complete three submaximal warm-up contractions (50%, 75%, 100%), followed by up to three maximal efforts lasting 3 seconds each until two trials are within 5% peak torque of each other. One minute of rest will be given between each of the trials. Strength of the knee extensors will be defined as the peak maximal voluntary isometric contraction. This will be quantified as the maximal torque production during any of the trials, normalized to the subject’s body mass (Nm/kg). Knee flexion and extension range of motion will be measured using a standard long-arm goniometer. Subjects will be positioned supine and asked to draw their heel toward their buttocks as far as possible for knee flexion, and straighten the knee as much as possible for knee extension. All goniometric measurements will be active and no overpressure will be applied by the tester during range of motion measurement.
Physical activity will be quantified objectively using accelerometers (ActiGraph wGT3X-BT, ActiGraph Corp., Pensacola, FL, USA). Subjects will be issued the activity monitor and instructed to place it on their hip during all waking hours for 10 days. The number of steps per day will be extracted and averaged across all valid wear days. Valid wear days will be considered days in which the subject wore the monitor for at least 10 hours.
Magnetic resonance images will be collected at the 10-week and two-year time points. Immediately prior to the MRI, the subject will complete an MRI safety and contraindication form. Images of the non-operated knee will be collected on a Siemens MAGNETOM Skyra 3.0 Tesla whole-body scanner (Siemens Healthcare, Germany). The patient will be positioned in supine with the foot vertical with the leg relaxed. The bottom of the patella will be aligned with the center of the multi-purpose, flexible coil.
The knee MRI acquisition will begin with a three-plane localizer. The following three sequences will be used for the morphological analysis of the knee joint: 1) a coronal intermediate-weighted 2D turbo spin-echo sequence (TR/TE: 3550/22 ms; FOV: 140 mm2; Matrix: 384 × 307; Slice thickness: 3 mm; Acquisition time: 3:45 min); 2) a sagittal 3D dual-echo in steady state sequence (TR/TE: 16.30/5 ms; FOV: 150 mm2; Matrix: 384 × 307; Slice thickness: 0.7 mm; Acquisition time: 11:33 min) followed by two multi-planar reformations to reconstruct coronal and axial plane images; and 3) a sagittal intermediate-weighted 2D turbo spin-echo sequence with fat suppression (TR/TE: 3550/30 ms; FOV: 150 mm2; Matrix: 448 × 314; Slice thickness: 3 mm; Acquisition time: 6:51 min). Further MRI sequence details can be found in the OAI protocol.[35]
De-identified images will be reviewed on a picture-archiving communication system workstation (Agfa Healthcare, USA) by a radiologist with over 10 years of experience and scored by a blinded assessor according to the WORMS scoring method.[36] WORMS separately evaluates menisci, cartilage, bone marrow lesions, ligaments, and other structural findings, with higher scores corresponding to increasing severity. Meniscal lesions will be assessed in six regions (anterior/body/posterior regions of the medial and lateral menisci) and graded from 0 to 4. Cartilage lesions will be assessed in six regions (patella, trochlea, medial femoral condyle, lateral femoral condyle, medial tibia, and lateral tibia) and graded from 0 to 6. Bone marrow edema pattern lesions will be assessed in the subchondral zone of the same six regions as described for cartilage, and graded from 0 to 3. Ligamentous abnormalities of the anterior cruciate ligament, posterior cruciate ligament, medial collateral ligament, lateral collateral ligament, patellar tendon, and popliteal tendon, as well as other findings (e.g., subchondral cysts, effusion, loose bodies, and popliteal cysts) will be graded according to WORMS as previously described.[36]
The WORMS total sum score will primarily define the progression of OA, calculated as the sum of grades for all the knee features. Secondary descriptors of knee OA progression will include the maximum WORMS cartilage subscore and the maximum WORMS medial meniscus subscore. Additionally, incidence of contralateral TKA at the 2-year time point will also be collected.
2.6.4. Participant Safety
Adverse events (injuries, falls) and serious adverse events (pulmonary embolism, manipulation under anesthesia, TKA revision, etc.) will be monitored regularly throughout the duration of the study. The safety officer will meet with the principal investigator on a bi-annual basis to review study progress and adverse events/serious adverse events. Routine monitoring of adverse events will be conducted throughout the intervention (first 10 weeks postoperatively) and continual monitoring will be conducted at each testing time point (10 weeks, six months, two years). Adverse events and serious adverse events will be recorded at each of the intervention visits within both groups (MOVE and CONTROL).
2.7. Sample Size
Statistical power was estimated using effect sizes based on a previous clinical trial.[12] The observed mean change (± SD) from baseline to six months for surgical limb knee extension moment during fixed-speed walking was 0.05 ± 0.24 Nm/kg in the experimental group vs −0.16 ± 0.30 Nm/kg in the control group. A sample size of 120 participants (60 per group) would have over 95% power to detect this observed difference in peak knee extension moments at fixed-speed using a 2-sided, independent samples t-test with an alpha level of 0.05. We will recruit 150 participants (75/group) to allow for as much as a 20% dropout rate.
2.8. Statistical Analyses
The primary analysis will be an intent-to-treat comparison of differences between treatment groups in surgical limb knee extension moment change from baseline to six months after TKA, during fixed-speed walking. No interim analyses are planned. Statistical inference regarding the difference between treatment groups will be based on the estimated coefficient for a treatment group indicator variable in an analysis of covariance model with the change from baseline in peak knee extensor moment at six months as the response variable, and additional covariates that include sex (stratification variable) and the baseline value of peak knee extensor moment to improve the precision of the estimated treatment differences. The conclusion about between-group differences will be determined by this single statistical test to protect against an elevated risk of false positive conclusions.
The secondary outcomes at six months will be analyzed as described above and be evaluated for consistency with the primary outcome. We anticipate secondary outcomes will be correlated with the primary, so that similar effects on secondary outcomes will reinforce significant differences in the primary outcome. Failure to observe consistency between primary and secondary outcomes will be taken as evidence that the effects of movement training are not clear, and that further study is necessary to resolve inconsistencies of the effect of movement pattern training on secondary outcomes. This approach reduces the risk of false-positive conclusions resulting from multiple statistical tests. Measures at other time points will be evaluated using a mixed-effects model to account for the correlation between repeated observations on a subject. Predictors will include group, time and a group by time interaction and the model will additionally control for sex. Linear contrasts will be used to estimate within and between group differences in change over time.
Differences in satisfaction with rehabilitation programs between groups will be evaluated using a Cochran-Mantel-Haenszel test, controlling for sex. Differences in adverse event rates during the intervention between groups will be evaluated using a Chi-square test and differences in adherence will be evaluated using an independent samples t-test. Sensitivity to non-compliance with home exercise program and attendance at PT sessions will also be evaluated in secondary analyses; that is, a per-protocol analysis will be compared to the intention-to-treat results. Loadsol® insole wear time will be used to evaluate how intervention dose impacts intervention efficacy.
We will evaluate the association between two-year progression of knee OA in the non-surgical limb (imaging-based and symptomatic) and asymmetry in peak knee extension moment measured at the primary endpoint (6 months), using Pearson correlations and multiple linear regression with OA progression as the dependent variable. The multiple linear regression model will control for baseline WORMS score, pain, radiographic alignment, body mass index, age, and sex. The independent variable of interest in the model will be relative loading of the contralateral knee at 6 months, measured as the ratio of contralateral to surgical limb peak sagittal plane moments during walking, sit-stand transitions, and step-down tasks. Because relative loading on the contralateral knee is expected to differ by intervention group, we will evaluate the effect of group assignment on the relative loading/OA progression relationship. Variance inflation will be calculated for all variables to detect issues of collinearity. Finally, we will explore rates of contralateral TKA between groups using a Chi-square test.
3. Discussion
Conventional postoperative rehabilitation focuses on pain management, range of motion, strength training and functional activity restoration.[10, 11] However, there is a notable lack of guidelines to address persistent movement compensations after TKA. A National Institutes of Health consensus statement on TKA stated that “the use of rehabilitation services is perhaps the most understudied aspect of the perioperative management of TKA patients.”[37] Furthermore, “there is no evidence supporting the generalized use of any specific preoperative or postoperative rehabilitation intervention.” While evidence has begun to emerge [38], there is still substantial room for improving rehabilitation strategies as movement compensations and functional deficits persist following TKA, leaving patients vulnerable to future disability.[21, 39–42] This study advances current physical rehabilitation by implementing motor control and motor learning techniques, proven successful for other populations[43], to target movement training after TKA.
Movement compensations acquired prior to TKA can persist years after TKA despite rehabilitation[3–7] and are related to persistent quadriceps weakness and chronic functional limitation.[4–8] Knee pain resolution is common after TKA, however the continual presence of movement compensations remain, suggesting that movement patterns are a learned behavior, possibly as a compensation for chronic unilateral knee OA pain prior to surgery.[8, 44] Although movement compensations are related to pain prior to surgery, there is little to no relation between pain and movement pattern after surgery.[8, 44] Moreover, movement patterns that result in excessive contralateral limb loading may be related to contralateral OA progression and the high rate of contralateral TKA after index TKA.[3]
The proposed study will build upon results of preliminary research by specifically targeting remediation of movement compensations using focused movement training.[12, 13] The MOVE intervention includes novel technology and intervention methods for promoting motor learning during functional training and exercises. While motor learning through the use of biofeedback and practice schedules is not a novel rehabilitative tool in neurological populations,[43] use of the methods for training movement is innovative for TKA rehabilitation. Preliminary data suggests that this approach not only improves biomechanical outcomes but also improves quadriceps strength and function after TKA.[12] As quadriceps strength is reduced chronically after TKA and is highly related to long-term functional performance,[21, 22] it is important to determine which strategies are most effective at improving long-term strength and function. Compensatory movement patterns may persist due to learned disuse of the involved quadriceps muscle and movement training may be a mechanism to address this impairment.[44]
As the TKA patient population becomes progressively younger, there is a need to maintain integrity of the replaced knee, as well as the other lower extremity joints.[45] This study will specifically examine the relationship between compensatory movement patterns and contralateral knee OA progression following unilateral TKA. Following unilateral TKA, the contralateral knee is the next most likely joint to be replaced, followed by the contralateral hip.[9] Up to 50% of individuals undergoing primary unilateral TKA will need their contralateral knee replaced in the subsequent 6 years.[46] Thus, this patient population represents a unique model in which to study accelerated OA progression. The observation of rapid contralateral knee OA progression is consistent with the movement compensations observed before and after TKA, which result in relatively greater loading of the contralateral knee, compensating for reduced loading of the surgical knee. This study will be the first to examine how compensatory movement patterns are related to symptomatic and structural knee OA progression by using a comprehensive approach of imaging analyses over time coupled with biomechanical analyses. Most studies that assess long-term outcomes after TKA are concerned only with the status of the surgical limb. While we believe normalizing movement patterns will increase joint moments and quadriceps function of the surgical limb, we will directly evaluate the non-surgical limb up to two years after surgery using MRI techniques, with proven sensitivity capable of detecting early stages of whole-joint pathology within relatively short time periods of 1–2 years.[35, 47] Results from this innovative approach will provide insight into the biomechanical mechanisms that underlie the rapid progression of contralateral knee OA after unilateral TKA.
In summary, traditional rehabilitation protocols may have acute functional benefit, but rehabilitation must also address remediation of movement compensations to prevent cumulative musculoskeletal stresses that negatively impact long-term function and OA progression. Remediation of movement compensations may reverse muscle weakness and functional limitations, while also reducing contralateral joint loading and its detrimental consequences. Preserving long-term functional ability and reducing future impairments is especially important as the incidence of TKA increases.[2]
Supplementary Material
Acknowledgments
Funding
This trial is funded by the National Institute on Aging (NIH R01 AG056585). Additional support was provided by the Eastern Colorado Geriatric Research Education and Clinical Center.
List of abbreviations
- TKA
total knee arthroplasty
- OA
osteoarthritis
- MRI
magnetic resonance imaging
- SCT
stair climb test
- 30STS
30 second sit-to-stand test
- 6MW
6-minute walk test
- TUG
timed up and go test
- WOMAC
Western Ontario and McMaster Universities Osteoarthritis Index
- VR12
Veteran’s RAND 12-item health survey
- WORMS
whole-organ MRI scoring method
Footnotes
Author Disclosures
Conflict of interest: None of the authors have any conflicts of interest to report. This material is based upon work supported in part by facilities of the VA Eastern Colorado Healthcare System. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States Government.
Trial Status
Active recruitment began 01/09/2018 and 2-year follow-up data collection is estimated to be completed by 12/31/2023.
Ethics approved and consent to participate
Ethics approval to conduct this study was obtained from the University of Colorado Denver Multiple Institutional Review Board (COMIRB No. 17-1701).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Most Frequent Operating Room Procedures Performed in U.S. Hospitals, 2003–2012 2012. https://www.hcup-us.ahrq.gov/reports/statbriefs/sb186-Operating-Room-Procedures-United-States-2012.jsp.
- [2].Kurtz S, Projections of Primary and Revision Hip and Knee Arthroplasty in the United States from 2005 to 2030, The Journal of Bone and Joint Surgery (American) 89(4) (2007) 780. [DOI] [PubMed] [Google Scholar]
- [3].Zeni JA Jr., Flowers P, Bade M, Cheuy V, Stevens-Lapsley J, Snyder-Mackler L, Stiff knee gait may increase risk of second total knee arthroplasty, J. Orthop. Res. (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Farquhar SJ, Reisman DS, Snyder-Mackler L, Persistence of Altered Movement Patterns During a Sit-to-Stand Task 1 Year Following Unilateral Total Knee Arthroplasty, Phys. Ther. 88(5) (2008) 567–579. [DOI] [PubMed] [Google Scholar]
- [5].Naili JE, Iversen MD, Esbjörnsson A-C, Hedström M, Schwartz MH, Häger CK, Broström EW, Deficits in functional performance and gait one year after total knee arthroplasty despite improved self-reported function, Knee Surg. Sports Traumatol. Arthrosc. 25(11) (2017) 3378–3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Yoshida Y, Mizner RL, Ramsey DK, Snyder-Mackler L, Examing outcomes from total knee arthroplasty and the relationship between quadriceps strength and knee function over time, Clinical Biomechanics 23(3) (2008) 320–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Yoshida Y, Zeni J, Snyder-Mackler L, Do Patients Achieve Normal Gait Patterns 3 Years After Total Knee Arthroplasty?, J. Orthop. Sports Phys. Ther. 42(12) (2012) 1039–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Christiansen CL, Bade MJ, Judd DL, Stevens-Lapsley JE, Weight-bearing asymmetry during sit-stand transitions related to impairment and functional mobility after total knee arthroplasty, Arch. Phys. Med. Rehabil. 92(10) (2011) 1624–1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Shakoor N, Block JA, Shott S, Case JP, Nonrandom evolution of end-stage osteoarthritis of the lower limbs, Arthritis Rheum. 46(12) (2002) 3185–9. [DOI] [PubMed] [Google Scholar]
- [10].Meier W, Mizner R, Marcus R, Dibble L, Peters C, Lastayo PC, Total Knee Arthroplasty: Muscle Impairments, Functional Limitations, and Recommended Rehabilitation Approaches, J. Orthop. Sports Phys. Ther. 38(5) (2008) 246–256. [DOI] [PubMed] [Google Scholar]
- [11].Westby MD, Brittain A, Backman CL, Expert Consensus on Best Practices for Post-Acute Rehabilitation After Total Hip and Knee Arthroplasty: A Canada and United States Delphi Study: Rehabilitation Best Practices After Total Joint Arthroplasty, Arthritis Care Res. (Hoboken) 66(3) (2014) 411–423. [DOI] [PubMed] [Google Scholar]
- [12].Christiansen CL, Bade MJ, Davidson BS, Dayton MR, Stevens-Lapsley JE, Effects of Weight-Bearing Biofeedback Training on Functional Movement Patterns Following Total Knee Arthroplasty: A Randomized Controlled Trial, J. Orthop. Sports Phys. Ther. 45(9) (2015) 647–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Zeni J, Abujaber S, Flowers P, Pozzi F, Snyder-Mackler L, Biofeedback to Promote Movement Symmetry After Total Knee Arthroplasty: A Feasibility Study, J. Orthop. Sports Phys. Ther. 43(10) (2013) 715–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Pisters MF, Veenhof C, van Meeteren NL, Ostelo RW, de Bakker DH, Schellevis FG, Dekker J, Long-term effectiveness of exercise therapy in patients with osteoarthritis of the hip or knee: a systematic review, Arthritis Rheum. 57(7) (2007) 1245–53. [DOI] [PubMed] [Google Scholar]
- [15].Bade MJ, Struessel T, Dayton M, Foran J, Kim RH, Miner T, Wolfe P, Kohrt WM, Dennis D, Stevens-Lapsley JE, Early High-Intensity Versus Low-Intensity Rehabilitation After Total Knee Arthroplasty: A Randomized Controlled Trial, Arthritis Care Res. (Hoboken) 69(9) (2017) 1360–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kleim JA, Jones TA, Principles of experience-dependent neural plasticity: implications for rehabilitation after brain damage, J. Speech. Lang. Hear. Res. 51(1) (2008) S225–39. [DOI] [PubMed] [Google Scholar]
- [17].Yang JF, Musselman KE, Livingstone D, Brunton K, Hendricks G, Hill D, Gorassini M, Repetitive mass practice or focused precise practice for retraining walking after incomplete spinal cord injury? A pilot randomized clinical trial, Neurorehabil. Neural Repair 28(4) (2014) 314–24. [DOI] [PubMed] [Google Scholar]
- [18].Lin C-H, Winstein C, Fisher B, Wu A, Neural Correlates of the Contextual Interference Effect in Motor Learning: A Transcranial Magnetic Stimulation Investigation, Journal of Motor Behavior 42(4) (2010) 223–232. [DOI] [PubMed] [Google Scholar]
- [19].Shumway-Cook A, Woollacott MH, Motor control: translating research into clinical practice, Wolters Kluwer Health/Lippincott Williams & Wilkins, Philadelphia, 2012. [Google Scholar]
- [20].Janelle CM, Kim J, Singer RN, Subject-controlled performance feedback and learning of a closed motor skill, Percept. Mot. Skills 81(2) (1995) 627–34. [DOI] [PubMed] [Google Scholar]
- [21].Bade MJ, Kohrt WM, Stevens-Lapsley JE, Outcomes Before and After Total Knee Arthroplasty Compared to Healthy Adults, J. Orthop. Sports Phys. Ther. 40(9) (2010) 559–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Mizner RL, Petterson SC, Snyder-Mackler L, Quadriceps strength and the time course of functional recovery after total knee arthroplasty, J. Orthop. Sports Phys. Ther. 35(7) (2005) 424–436. [DOI] [PubMed] [Google Scholar]
- [23].Meier WA, Marcus RL, Dibble LE, Foreman KB, Peters CL, Mizner RL, LaStayo PC, The long-term contribution of muscle activation and muscle size to quadriceps weakness following total knee arthroplasty, J. Geriatr. Phys. Ther. 32(2) (2009) 79–82. [PubMed] [Google Scholar]
- [24].Hatfield GL, Hubley-Kozey CL, Astephen Wilson JL, Dunbar MJ, The effect of total knee arthroplasty on knee joint kinematics and kinetics during gait, J. Arthroplasty 26(2) (2011) 309–18. [DOI] [PubMed] [Google Scholar]
- [25].Levinger P, Webster KE, Feller J, Asymmetric knee loading at heel contact during walking in patients with unilateral knee replacement, The Knee 15(6) (2008) 456–60. [DOI] [PubMed] [Google Scholar]
- [26].Levinger P, Menz HB, Morrow AD, Perrott MA, Bartlett JR, Feller JA, Bergman NB, Knee biomechanics early after knee replacement surgery predict abnormal gait patterns 12 months postoperatively, J. Orthop. Res. 30(3) (2012) 371–6. [DOI] [PubMed] [Google Scholar]
- [27].Mizner RL, Petterson SC, Clements KE, Zeni JA, Irrgang J, Snyder-Mackler L, Measuring Functional Improvement after Total Knee Arthroplasty Requires both Performance-Based and Patient-Report Assessments: A Longitudinal Analysis of Outcomes, The Journal of arthroplasty 26(5) (2011) 728–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Gill SD, de Morton NA, Mc Burney H, An investigation of the validity of six measures of physical function in people awaiting joint replacement surgery of the hip or knee, Clin. Rehabil. 26(10) (2012) 945–951. [DOI] [PubMed] [Google Scholar]
- [29].Steffen TM, Hacker TA, Mollinger L, Age- and Gender-Related Test Performance in Community-Dwelling Elderly People: Six-Minute Walk Test, Berg Balance Scale, Timed Up & Go Test, and Gait Speeds, Phys. Ther. 82(2) (2002) 128–137. [DOI] [PubMed] [Google Scholar]
- [30].Podsiadlo D, Richardson S, The timed “Up & Go”: a test of basic functional mobility for frail elderly persons, J. Am. Geriatr. Soc. 39(2) (1991) 142–148. [DOI] [PubMed] [Google Scholar]
- [31].Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW, Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee, The Journal of Rheumatology 15(12) (1988) 1833–1840. [PubMed] [Google Scholar]
- [32].Jones D, Kazis L, Lee A, Rogers W, Skinner K, Cassar L, Wilson N, Hendricks A, Health status assessments using the Veterans SF-12 and SF-36: methods for evaluating otucomes in the Veterans Health Administration, J. Ambul. Care Manage. 24(3) (2001) 68–86. [DOI] [PubMed] [Google Scholar]
- [33].Norkin CC, White DJ, Measurement of Joint Motion : A Guide to Goniometry, 4th Edition, 4 edition ed., F.A. Davis Company, Philadelphia, 2009. [Google Scholar]
- [34].Murray AM, Gaffney BM, Davidson BS, Christiansen CL, Biomechanical compensations of the trunk and lower extremities during stepping tasks after unilateral transtibial amputation, Clin. Biomech. (Bristol, Avon) 49 (2017) 64–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Peterfy CG, Schneider E, Nevitt M, The osteoarthritis initiative: report on the design rationale for the magnetic resonance imaging protocol for the knee, Osteoarthritis Cartilage 16(12) (2008) 1433–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Peterfy CG, Guermazi A, Zaim S, Tirman PF, Miaux Y, White D, Kothari M, Lu Y, Fye K, Zhao S, Genant HK, Whole-Organ Magnetic Resonance Imaging Score (WORMS) of the knee in osteoarthritis, Osteoarthritis Cartilage 12(3) (2004) 177–90. [DOI] [PubMed] [Google Scholar]
- [37].Panel NC, NIH Consensus Statement on total knee replacement: December 8–10, 2003, J Bone Joint Surg [Am] 86(A(6)) (2004) 1328–1335. [DOI] [PubMed] [Google Scholar]
- [38].Artz N, Elvers KT, Lowe CM, Sackley C, Jepson P, Beswick AD, Effectiveness of physiotherapy exercise following total knee replacement: systematic review and meta-analysis, BMC Musculoskelet. Disord. 16 (2015) 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Noble PC, Gordon MJ, Weiss JM, Reddix RN, Conditt MA, Mathis KB, Does total knee replacement restore normal knee function?, Clin. Orthop. Relat. Res. (431) (2005) 157–165. [DOI] [PubMed] [Google Scholar]
- [40].March LM, Cross MJ, Lapsley H, Tribe KL, Courtenay BG, Brooks PM, Outcomes after hip or knee replacement surgery for osteoarthritis, Med. J. Aust. 171(5) (1999). [PubMed] [Google Scholar]
- [41].Walsh M, Woodhouse LJ, Thomas SG, Finch E, Physical Impairments and Functional Limitations: A Comparison of Individuals 1 Year After Total Knee Arthroplasty With Control Subjects, Phys. Ther. 78(3) (1998) 248–258. [DOI] [PubMed] [Google Scholar]
- [42].Ritter MA, Thong AE, Davis KE, Berend ME, Meding JB, Faris PM, Long-term deterioration of joint evaluation scores, J. Bone Joint Surg. Br. 86(3) (2004) 438–42. [DOI] [PubMed] [Google Scholar]
- [43].Tate JJ, Milner CE, Real-Time Kinematic, Temporospatial, and Kinetic Biofeedback During Gait Retraining in Patients: A Systematic Review, Phys. Ther. 90(8) (2010) 1123–1134. [DOI] [PubMed] [Google Scholar]
- [44].Christiansen CL, Bade MJ, Weitzenkamp DA, Stevens-Lapsley JE, Factors predicting weight-bearing asymmetry 1month after unilateral total knee arthroplasty: A cross-sectional study, Gait Posture 37(3) (2013) 363–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Ravi B, Croxford R, Reichmann WM, Losina E, Katz JN, Hawker GA, The changing demographics of total joint arthroplasty recipients in the United States and Ontario from 2001 to 2007, Best Pract. Res. Clin. Rheumatol. 26(5) (2012) 637–47. [DOI] [PubMed] [Google Scholar]
- [46].Shao Y, Zhang C, Charron KD, Macdonald SJ, McCalden RW, Bourne RB, The fate of the remaining knee(s) or hip(s) in osteoarthritic patients undergoing a primary TKA or THA, J. Arthroplasty 28(10) (2013) 1842–5. [DOI] [PubMed] [Google Scholar]
- [47].Laberge MA, Baum T, Virayavanich W, Nardo L, Nevitt MC, Lynch J, McCulloch CE, Link TM, Obesity increases the prevalence and severity of focal knee abnormalities diagnosed using 3T MRI in middle-aged subjects--data from the Osteoarthritis Initiative, Skeletal Radiol. 41(6) (2012) 633–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.