Abstract
Background:
Cancer-related fatigue is a significant problem and is associated with poor quality of life. Behavioral interventions include exercise and cognitive-behavioral therapy, which survivors may be unwilling or unable to adopt. Pharmacologic interventions (e.g., selective serotonin reuptake inhibitors) have been disappointing. One potential therapy is the antidepressant bupropion, a norepinephrine-dopamine reuptake inhibitor that targets both inflammation and the hypothalamic-pituitary-adrenal axis. The current study is intended to provide a rigorous test of the efficacy and tolerability of bupropion for cancer-related fatigue.
Methods:
A randomized, double-blind, placebo-controlled trial will examine the effects of bupropion on cancer-related fatigue. The trial will be conducted nationwide through the University of Rochester Medical Center (URMC) National Cancer Institute Community Oncology Research Program (NCORP). Disease-free breast cancer survivors (n=422) who completed chemotherapy and/or radiotherapy 12–60 months previously and report significant fatigue will be randomized 1:1 to receive bupropion (300 mg/day) or placebo. Outcomes will be assessed at baseline and the 12-week follow-up. The primary outcome, fatigue, will be measured with the Functional Assessment of Chronic Illness Therapy – Fatigue (FACIT-F). Secondary outcomes include quality of life, depression, and drug tolerability. Exploratory outcomes include cognition and symptomatology. Potential biological mechanisms and genetic moderators of cancer-related fatigue will also be explored.
Discussion:
This study is the first placebo-controlled trial to our knowledge to evaluate bupropion for cancer-related fatigue. Positive results could revolutionize the treatment of cancer-related fatigue, as bupropion is safe, inexpensive, widely-available, and may be more tolerable and acceptable for many patients than current, limited treatment options.
Keywords: fatigue, bupropion, breast cancer, randomized controlled trial, protocol
1. Background
Fatigue is one of the most prevalent and distressing symptoms experienced by cancer patients.1–4 By recent estimates, moderate to severe fatigue (i.e., ≥4 on a 0–10 scale) occurs in up to 60% of cancer patients during treatment and in nearly 30% following treatment completion.2,5 Similar rates of fatigue have been reported in breast cancer patients,2,6 in whom fatigue has been studied most extensively. Longitudinal studies of breast cancer patients suggest fatigue often persists long after treatment completion; late-onset fatigue can also occur.7–10 Unlike normal fatigue, cancer-related fatigue tends to be more severe, distressing, and unlikely to be relieved by rest.11 Patients have described it as “devastating,” “never-ending,” and “totally consuming.”12,13 Fatigue is associated with worse quality of life and reduced likelihood of returning to work following cancer treatment.14–17 Effective treatment of cancer-related fatigue is a high priority.18,19
Treatment options for fatigued cancer patients are limited. Although exercise has been shown to reduce cancer-related fatigue,20,21 many patients are unwilling or unable to exercise consistently.22,23 Psychosocial interventions demonstrate benefit,24–27 although they tend to be time-intensive, limiting uptake, compliance, maintenance, and disseminability. Pharmacologic interventions such as paroxetine, sertraline, modafinil, and armodafinil have produced no benefit for cancer-related fatigue in randomized trials.28–33 Evidence is mixed regarding the effects of methylphenidate34–37 and uptake has been limited, perhaps because methylphenidate can be habit-forming and is associated with side effects including agitation.38 Additional treatment options for cancer-related fatigue are clearly needed.
One potential therapy is bupropion, which is widely available as a generic and has a long history of safety. Since 1999, there have been repeated calls for investigation of bupropion as a treatment for cancer-related fatigue.19,39,40 Nevertheless, only two small, single-arm, open-label studies have been conducted to date.41,42 These pilot studies have demonstrated promising results. In 2004, Cullum and colleagues41 reported improvements in fatigue in 13 of 15 cancer patients who were experiencing fatigue or depression with marked fatigue. In 2006, Moss and colleagues42 reported significantly reduced fatigue in 21 cancer patients with fatigue with or without depression over four weeks of treatment with bupropion. These data are consistent with trials of bupropion for major depressive disorder (MDD).43 Specifically, an analysis of six randomized, double-blind clinical trials comparing bupropion to an SSRI in individuals with MDD indicated that bupropion was superior to the SSRIs and placebo in reducing fatigue and hypersomnia.43 Moreover, bupropion has an excellent safety profile.44,45
Bupropion has diverse actions that target pathways associated with cancer-related fatigue. One pathway is inflammation. Bupropion suppresses production of tumor necrosis factor (TNF),46,47 a potent mediator of inflammation through activation of nuclear factor-κB (NF-κB), which in turn induces a wide variety of pro-inflammatory cytokines, chemokines, and receptors.48 A large body of literature, much of it in breast cancer, has demonstrated associations between cancer-related fatigue and increased circulating markers of inflammation including TNF, interleukin-1β (IL-1β), interleukin-6 (IL-6), C-reactive protein (CRP), and their receptors.49–57 Cancer-related fatigue is also associated with increased expression of NF-κB. Thus, bupropion may reduce cancer-related fatigue by dampening pro-inflammatory activity.58
A second pathway is the hypothalamic-pituitary-adrenal (HPA) axis. Bupropion increases synaptic availability of norepinephrine (noradrenaline) in the locus coeruleus, an area of the brain that activates the HPA axis.59,60 Norepinephrine is involved in the “fight or flight” response to stress, increasing alertness and arousal.61 Additionally, norepinephrine afferents from the locus coeruleus stimulate release of corticotropin-releasing factor (CRF) from the hypothalamus, inducing adrenocorticotropic hormone (ACTH) release from the anterior pituitary and in turn cortisol release from the adrenal glands. One of cortisol’s many functions is to promote alertness.62 The HPA axis may impact fatigue directly and indirectly through its effects on inflammation. Specifically, in combination with glucocorticoid receptor, cortisol blocks the transcriptional activity of NF-κB,63 suppressing the cascade of pro-inflammatory cytokines described above. Breast cancer patients with fatigue demonstrate dysregulated HPA axis functioning through flattened diurnal cortisol slopes (i.e., low in the morning, high in the evening).64–66 Two small randomized placebo-controlled trials have examined the effects of bupropion on cortisol levels.67,68 One study conducted in healthy male athletes reported significant increases in circulating cortisol six hours after morning bupropion administration,67 around the time peak plasma concentrations of bupropion occur.69 Another study conducted in individuals with depression reported no differences in evening urinary cortisol 16 hours after morning bupropion administration.68 These data suggest that morning administration of bupropion may elevate cortisol (and in turn, alertness) early in the day without affecting evening cortisol. Collectively, these studies provide support for a definitive randomized trial of bupropion for cancer-related fatigue.
The current study will determine the efficacy of bupropion versus placebo in reducing fatigue in a double-blinded, placebo-controlled, randomized clinical trial of breast cancer survivors with fatigue. Secondary aims are to assess the tolerability of bupropion and the efficacy of bupropion versus placebo on quality of life and depression. Exploratory aims are to: 1) assess the efficacy of bupropion versus placebo on symptomatology and cognition, 2) explore the effects of bupropion on putative mechanisms of cancer-related fatigue, and 3) explore associations of CYP2B6 genotype with bupropion metabolism and changes in fatigue.
2. Study Procedures
2.1. Study Population
2.1.1. Recruitment
Inclusion and exclusion criteria are presented in Table 1. Patients (target N = 422) who report moderate to severe cancer-related fatigue in the past week (i.e., score ≥ 4 on the Fatigue Symptom Inventory)70 will be recruited through the University of Rochester Cancer Center (URCC) National Cancer Institute Community Oncology Research Program (NCORP). This program has extensive experience conducting multisite intervention studies,28,32,71,72 provides ready access to up to 20 sites with demographically-diverse breast cancer patients, and offers an important opportunity to evaluate the efficacy of bupropion in a community oncology (“real world”) setting. Eligibility will be confirmed with the treating oncologist to rule out other medical conditions in which fatigue is a prominent symptom (e.g., anemia, autoimmune disease, chronic fatigue syndrome) and contraindications to receiving bupropion.
Table 1.
Inclusion and exclusion criteria
| Inclusion criteria |
| Age at least 18 years |
| Female |
| Diagnosed with stage I-III breast cancer |
| No evidence of current breast cancer |
| Report moderate to severe fatigue in the past week (i.e., a score ≥ 4 on a 0–10 scale) |
| Attribute their fatigue to cancer and/or its treatment |
| Completed surgery, radiation, standard dose chemotherapy, and/or targeted therapy 12–60 months previously |
| Able to read and speak English |
| Women of child-bearing potential must agree to use contraception prior to study entry and for the duration of study participation |
| Be capable of providing written informed consent |
| Exclusion criteria |
| Another medical condition in which fatigue is a prominent symptom (e.g., anemia, autoimmune disease, sleep apnea) |
| Currently taking bupropion, Wellbutrin, Forfivo, Aplenzin, or Zyban (i.e., bupropion prescribed for other indications), an anti-depressant including but not limited to an MAOI inhibitor, anti-psychotic, a systemic anti-TNF agent, linezolid, methylene blue, or a systemic corticosteroid |
| History of renal impairment (i.e., glomerular filtration rate < 45) |
| History of cirrhosis (i.e., Child-Pugh score ≥ 5) |
| History of seizures |
| History of bulimia or anorexia nervosa |
| History of sensitivity to bupropion |
| Allergic to lactose |
| Have psychiatric or neurological disorder(s) that would interfere with study participation (e.g., schizophrenia, alcohol use disorder) |
| Be currently pregnant or breastfeeding or plan to become pregnant in the next four months, due to unknown teratogenicity of bupropion |
Patients who meet all eligibility criteria will be provided with a copy of the IRB-approved informed consent to review. If the patient decides to participate in the study, she will be asked to sign and date the informed consent document. All consented participants will be provided with a photocopy of the signed consent form.
2.1.2. Randomization and Stratification
Randomization will occur at the time of registration. A computer-generated randomization schedule will be used to randomize participants 1:1 to the two intervention conditions (bupropion XL or placebo) stratified by study site, previous receipt of chemotherapy, and current receipt of hormonal therapy with a random (50/50) block of 4 or 8. The schedule will be prepared by a URCC NCORP Research Base biostatistician.
2.2. Baseline Assessment
Eligible patients who provide informed consent will complete a study blood draw and a packet of self-report questionnaires in an outpatient clinic area under the supervision of a research associate. Participants will be randomized as described above. Participants will then be provided with study drug before the end of the baseline study visit. Saliva collection supplies, instructions, and a diary will be provided at the study visit for the participant to complete at home. A postage-paid mailer to return the samples and diary to the study site will also be provided. Alternately, participants can pick up the study drug and/or drop off saliva samples in person at a later date within the same week. Treatment notes will be collected at baseline for all consented participants.
2.3. Baseline Saliva Collection
Starting the day after the baseline study visit, participants will be asked to collect saliva three times per day for three days: upon awakening, between 4:00–6:30 pm, and at bedtime. Participants will be provided with instructions as well as a saliva collection diary/health behavior questionnaire that they will complete daily. Participants will receive text, phone, or email reminders for timely saliva collection. Texting will be the preferred method of reminders for saliva collection as the timing of collection is important. Saliva samples will be used for analysis of salivary cortisol to assess the effects of bupropion on the HPA axis.
2.4. Baseline Blood Collection
Blood will be collected at baseline for analysis of serum cytokines, leukocyte gene expression, and CYP2B6*6 and *18 alleles. Up to 33 ml of blood will be drawn. Participants will be asked to complete a questionnaire asking about exercise, alcohol consumption, caffeine use, medication use (i.e., NSAID, steroid, opioid, beta-blocker) for the 24 hours prior to blood draws.
2.5. Study Agent
The URMC Investigational Drug Service will prepare encapsulated study drug to maintain blinding. Specifically, the active treatment group will receive capsules containing a bupropion XL 150 mg tablet and an inert substance (i.e., lactose); the placebo group will receive identical-looking capsules containing lactose only. Participants will be instructed to take one capsule every morning during week 1 (i.e., 150 mg in the bupropion group) and two capsules every morning during weeks 2–12 (i.e., 300 mg in the bupropion group). Bupropion XL is prescribed in dosages of 150 mg, 300 mg, or 450mg. A target dose of 300 mg was selected to balance tolerability with therapeutic efficacy across CYP2B6 genotypes.73 A duration of twelve weeks was selected based on a pooled analysis of studies of bupropion for MDD documenting improvements in fatigue that were still ongoing at the end of most studies at eight weeks.43 Participants will not take the study drug the morning of their week 12 follow-up study visit. They will take their regularly scheduled study dose after their Week 12 study visit. They will be instructed to take one capsule every morning in week 13 (i.e., 150 mg in the bupropion group) and discontinue study drug in week 14. If they wish to receive bupropion after study completion they will be referred to their oncologist or primary care provider.
2.6. Follow-Up Study Visit
Participants will be scheduled for a follow-up study visit during week 12 in which they will complete a blood draw and self-report questionnaires. One week prior to their scheduled visit, participants will be mailed saliva collection supplies and asked to return the collected samples at the study visit. Saliva collection procedures will occur as described above. Participants will not take the study drug the morning of their week 12 study visit, so that the blood draw can occur at least 24 hours after their most recent dose. They will take their regular study dose after their Week 12 study visit. Up to 33 ml of blood will be drawn at the 12 week follow-up assessment for analysis of serum cytokines, bupropion metabolites, and leukocyte gene expression. Participants will be asked to bring their pill bottle to the follow-up study visit and a pill count will be conducted. The coordinator will leave the participants with enough study capsules to complete the study and will collect the extra capsules. Collection of follow-up data will be attempted from all participants who are randomized, including those who discontinued study medication early. Adverse events will be assessed by phone in weeks 4, 8, and 14. At each participant visit and phone calls, the site study staff will assess adverse events by recording all voluntary reports of the participant.
For participants who wish to withdraw during the course of the study, they will begin tapering the study drug for one week. Participants may not resume bupropion during the study after stopping it. All efforts will be made to collect follow-up data, including the self-report questionnaire, blood, saliva, and remaining study drug.
2.7. Study Measures
2.7.1. Demographic and Clinical Information
Demographic information will be obtained from all participants through the use of a standardized self-report questionnaire. Variables to be assessed include date of questionnaire completion, date of birth, race, ethnicity, marital status, living arrangement, education, current work status, occupation, spouse/partner’s occupation, and patient and household income. Participants will also be asked to list all non-prescription medications, vitamins and supplements, alcohol use, and history of tobacco use. Menopausal status will be assessed by items from the Menopausal Status Questionnaire.74 The following clinical variables will be assessed via medical record review for all participants: date of breast cancer diagnosis, disease stage, hormone receptor status, surgical treatment (dates and type), chemotherapy (agents, doses, start and stop dates, and number of infusions), radiotherapy (start and stop dates, number of treatments, and dose), hormonal therapy (previous/current/none, start and stop dates, agent), height (inches), and weight (pounds). Comorbidities will be abstracted from the medical record using the Charlson Comorbidity Index.75
2.7.2. Primary Outcome
The primary study outcome is fatigue as measured by the Fatigue subscale of the Functional Assessment of Chronic Illness Therapy – Fatigue (FACIT-F).76 The Fatigue subscale consists of 13 items asking about fatigue in the past seven days. Items are scored on a five-point scale (0=not at all, 4=very much). Negatively-worded items are recoded and items are summed to produce a score ranging from 0–52 with lower scores indicating greater fatigue. The FACIT-F has extensive reliability and validity data in cancer patients.77,78 The FACIT-F demonstrates sensitivity to change in previous intervention studies to reduce fatigue in cancer patients.71,79,80 While change in fatigue (continuous variable) is the primary outcome we will also assess the clinically-important difference in fatigue (i.e., difference of 3 points on the Fatigue subscale; dichotomous measure).77
2.7.3. Secondary Outcomes
Secondary outcomes include quality of life, depression, and tolerability. Quality of life will be measured by the Total Score of the Functional Assessment Cancer Therapy – General (FACT-G) scale, which is included in the FACIT-F.81 The FACT-G consists of four subscales: physical well-being (PWB), functional well-being (FWB), emotional well-being (EWB), and social well-being (SWB). Scores on the four subscales are summed to produce a total score ranging from 0 to 108 with higher scores indicating better quality of life. The FACT-G has extensive reliability and validity data and demonstrates sensitivity to change in cancer patients.71,77
Depression during the previous 7 days will be assessed using the eight-item PROMIS Depression Short Form 8a.82 Items are evaluated on a five-point Likert scale (1=never, 5=always) and responses are summed to produce a total score ranging from 8 to 40 with higher scores indicating greater depression.83 T scores standardized to the US general population can also be calculated.83 The PROMIS Depression 8a has previously been used to measure depression in cancer patients and found to be sensitive to change in this population.84–86 The PROMIS Depression 8a was selected because it does not include items overlapping with fatigue, unlike many other measures of depression.87–89
Drug tolerability and adherence will be measured by pill count, incidence of CTCAE grade 2 or above toxicity, early medication discontinuation, and self-reported insomnia. Insomnia will be assessed using the Insomnia Severity Index (ISI)90 which consists of seven items assessing difficulty falling and staying asleep over the past week. Items are evaluated on a five-point Likert scale. Responses are summed to produce a total score ranging from 0–28 with higher scores indicating greater insomnia. A cutoff of 8 is used to indicate clinically-significant insomnia in cancer patients.91 The ISI is recommended for use in cancer patients and found to be sensitive to change.72,92
2.7.4. Exploratory Outcomes
Cognition:
Because cancer-related fatigue is often associated with self-reported cognition,93,94 self-reported cognition will be assessed as an exploratory outcome using the PROMIS Cognitive Functioning 8a and Cognitive Abilities 4a measures.95 Both measures assess cognition over the past seven days. Functioning in mental acuity, concentration, verbal and nonverbal memory, verbal fluency, and perceived changes are assessed. The extent to which cognitive impairment interferes with daily functioning, whether other people observe cognitive impairment, and the impact of cognitive impairment on quality of life are also assessed. The PROMIS Cognitive Functioning 8a is an eight-item measure that assesses perceived deficits with regard to cognitive tasks. Items are evaluated on a five-point Likert scale (1=very often, 5=never) and responses are summed to produce a total score ranging from 8 to 40 with higher scores indicating better functioning. The PROMIS Cognitive Abilities 4a is a four-item measure that assesses perceived functional abilities with regard to cognitive tasks. Items are evaluated on a five-point Likert scale (1=not at all, 5=very much) and responses are summed to produce a total score ranging from 5 to 20 with higher scores indicating better functioning.
Symptom Inventory:
Patient-reported symptoms of cancer and its treatment will be assessed as an exploratory outcome using the M.D. Anderson Symptom Inventory (MDASI).96 This measure consists of a series of 11-point Likert scales in which the severity of each symptom is indicated by filling in the appropriate circle on an 11-point scale, anchored by “0” = “Not Present” and “10” = “As Bad As You Can Imagine.” It will serve as a concurrent self-report measure of symptoms. Four symptom interference items assess the impact of symptoms on activities of daily living, general physical activity, exercise, and quality of life.
Salivary Cortisol and Inflammatory Biomarkers:
Salivary cortisol and circulating markers of inflammation will be evaluated as putative mechanisms of cancer-related fatigue. Assays of circulating markers of inflammation will be conducted on all serum samples; baseline and follow-up samples on a given individual will be assayed together on the same assay plate. Cytokine levels (e.g., IL-1β, IL-6, TNFα) will be determined by a high sensitivity multiplex assay according to the manufacturer’s protocol. Additional cytokines (e.g., CRP) may be determined by a high sensitivity enzyme-linked immunosorbent assay (ELISA). All samples will be assayed in duplicate, and inter- and intra-assay variability will be monitored by inclusion of an internal laboratory quality control sample on all assay plates.
Plasma samples collected at the follow-up assessment from all participants will be analyzed for bupropion and metabolites (i.e., 4-hydroxybupropion, threohydrobupropion, erythro-hydrobupropion). Analyses will be conducted using a novel, sensitive, and precise chiral liquid chromatography–tandem mass spectrometry (LC-MS/MS) method to separate and quantify bupropion enantiomers and diastereomers of 4-hydroxybupropion as well as threo- and erythro-hydrobupropion.97,98 Although our focus in this project will be the major metabolites of bupropion, as they are pharmacologically active, this method will be implemented if quantification of glucuronides are deemed important.
SNP Analysis:
DNA will be extracted from whole blood samples. Only active treatment samples will be analyzed. The CYP2B6*6 allele is characterized by the presence of two nonsynonymous single nucleotide polymorphisms (SNPs), 516G>T in exon 4 (rs3745274) and 785A>G in exon 5 (rs2279343). The CYP2B6*18 is characterized by the 983T>C SNP (rs28399499). DNA will be extracted from whole blood. Extracted DNA will be genotyped by laboratory-developed multiplex PCR-based assays followed by single base primer extension for variant detection by mass spectrometry.
Gene Expression Analysis:
RNA will be extracted from PaxGene tubes collected at baseline and follow up. RNA will be assayed following the manufacturer’s standard protocol, and analyzed using linear model analysis of quintile normalized log2-transformed gene expression values and subsequent Transcription Element Listening System (TELiS) bioinformatics analysis of genes showing > 1.2-fold differential expression.99 Bioinformatics analyses will test the hypothesis that bupropion as measured by group assignment and bupropion metabolites reduce activity of the pro-inflammatory NF-κB signaling pathways.
2.7.5. Potential Covariates
Patient-reported physical activity will be assessed as a potential covariate using the International Physical Activity Questionnaire (IPAQ).100 The IPAQ consists of seven questions asking participants to estimate how much time over the previous seven days they engaged in vigorous activity, moderate activity, walking, and sitting. The IPAQ has been shown to be a valid measure of physical activity in cancer patients.101
3. Statistical Considerations
3.1. Sample Size Justification
The primary outcome will be the FACIT-F Fatigue subscale. Based on sedentary breast cancer survivors, the standard deviation is assumed to be 10.79 The minimal clinically important difference is assumed to be 3 points,77 which corresponds to an effect size of 0.3 (standardized mean difference). We will use ANCOVA and assume a conservative pre-post correlation of 0.5. At 90% power and a 0.05 significance level, we will need 176 subjects per group.102 Including 20% attrition, this gives 211 subjects per group, or 422 subjects total.
3.2. Analytic Plan
3.2.1. Preliminary Analyses
Before conducting the analyses described below, outcome variable distributions will be assessed using histograms and density estimates to determine if variance stabilizing or normalizing transformations should be applied, or the analyses should be done with nonparametric or robust methods. Participants who complete the study will be compared to those who do not to identify predictors of attrition. Study arms will be compared on baseline demographic (e.g., age), clinical (e.g., time since treatment, history of radiation), and behavioral (e.g., physical activity, alcohol consumption, caffeine use) variables.
3.2.2. Primary Aim
This objective is to determine the efficacy of bupropion versus placebo in reducing fatigue as measured by the FACIT-F Fatigue subscale at 12 weeks. We will use ANCOVA with group as the main factor and baseline fatigue as a covariate. Study site will be included as a random effect independent of residual error. This initial linear mixed model (LMM) will be fit using Restricted Maximum Likelihood (REML) estimation. The significance of the variance due to study site will be tested using a 95% profile likelihood confidence interval. If this variance is statistically significant, we will include site in the final model and also investigate the individual study sites by calculating the Empirical Best Linear Unbiased Predictions of each site.103 An F test will be used to test whether the group difference in mean change is significantly different than zero. The mean between arm difference including 95% confidence intervals via marginal means. We will also examine the effect of clinically relevant covariates (e.g., smoking status, current receipt of hormonal therapy, time since treatment, etc.) on the results. Model’s linearity assumption will be evaluated based on normality of the model’s residuals. In case of the major deviation from the linearity, we will also conduct sensitivity analysis using either transformation semiparametric or parametric modeling. In addition to viewing fatigue as a continuous measure, we will examine a dichotomized version of the fatigue based on the MCID of 3 points specified by the measure developer.77 This outcome will be evaluated using a generalized linear mixed model (GLMM) with a logit link function to determine whether rates of clinically meaningful improvement differ as a function of group assignment.
3.2.3. Secondary Aims
Secondary Aim 1.
The objective is to assess the efficacy of bupropion versus placebo in improving depression (measured by PROMIS Depression Short Form) and quality of life (measured by FACT-G) at 12 weeks (Secondary Aim 1a), and to evaluate whether the beneficial effects of bupropion on fatigue will be independent of its effects on depression (Secondary Aim 1b). Secondary Aim 1a will be performed using the same analytic approach described for the Primary Aim. Secondary Aim 1b will be performed using a mediation model (see Figure 1). MPlus104 will be utilized to estimate the indirect (mediation) and direct effects and to obtain bootstrap-based 95% confidence intervals for these effects.105–108
Figure 1.
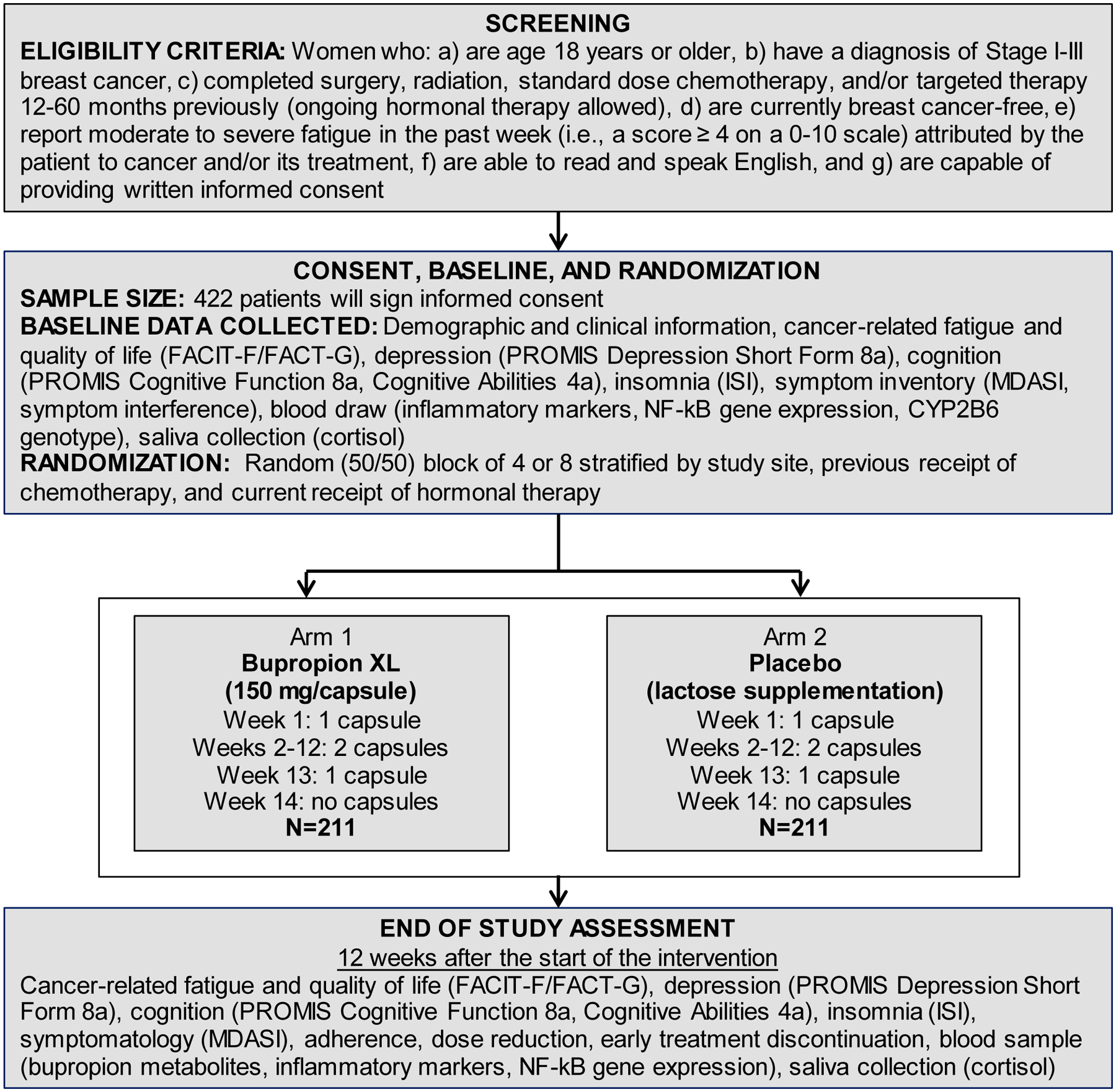
Study flow diagram
Secondary Aim 2.
This objective is to assess the tolerability of bupropion in breast cancer survivors with fatigue. We hypothesize that adherence as measured by pill count, incidence of CTCAE grade 2 or above toxicity, self-reported insomnia, and early discontinuation will be similar between the bupropion and placebo groups. These variables will be evaluated as separate analyses using the same analytic approach as the primary analysis. For insomnia, the model will assume a Gaussian response. The same analysis will be used for pill count, but a log transformation may be required. For toxicity, we will assume a Bernoulli distribution and a logit link. LMMs will be used for insomnia and pill count. GLMMs will be used for toxicity and early discontinuation.
3.2.4. Exploratory Aims
Exploratory Aim 1:
The objective is to assess the efficacy of bupropion versus placebo in improving cognition (measured by PROMIS Cognitive Functioning 8a and Cognitive Abilities 4a) and symptomatology (measured by the MDASI) at 12 weeks (Exploratory Aim 1a). The analysis for Exploratory Aim 1a will be performed using the same analytic approach described for the Primary Aim. Because this aim is exploratory, results of the analysis will be considered hypothesis generating, subject to verification in independent studies.
Exploratory Aim 2:
The objective is to explore the effects of bupropion on putative mechanisms of cancer-related fatigue. It is hypothesized that the relationship between group assignment and reductions in fatigue will be mediated by cytokines (e.g., IL-1B, IL-6, TNF-A, CRP) and cortisol slope (Exploratory Aim 2a). It is further hypothesized that the relationship between bupropion metabolites (e.g., bupropion, 4-hydroxybupropion, threo-hydrobupropion, erythro-hydrobupropion) and fatigue within the bupropion arm will be mediated by cytokines and cortisol slope (Exploratory Aim 2b). To assess these aims, we will perform mediation analyses following the conceptual framework as shown in Figure 2. We will use same estimation approach as in Secondary Aim 1b. Because of the number of hypotheses tested for this aim, we will adjust for multiplicity using the false discovery rate (FDR) procedure of Benjamini and Hochberg with target rate of 0.10.109 Because this aim is exploratory, results of the analysis will be considered hypothesis generating, subject to verification in independent studies.
Figure 2.
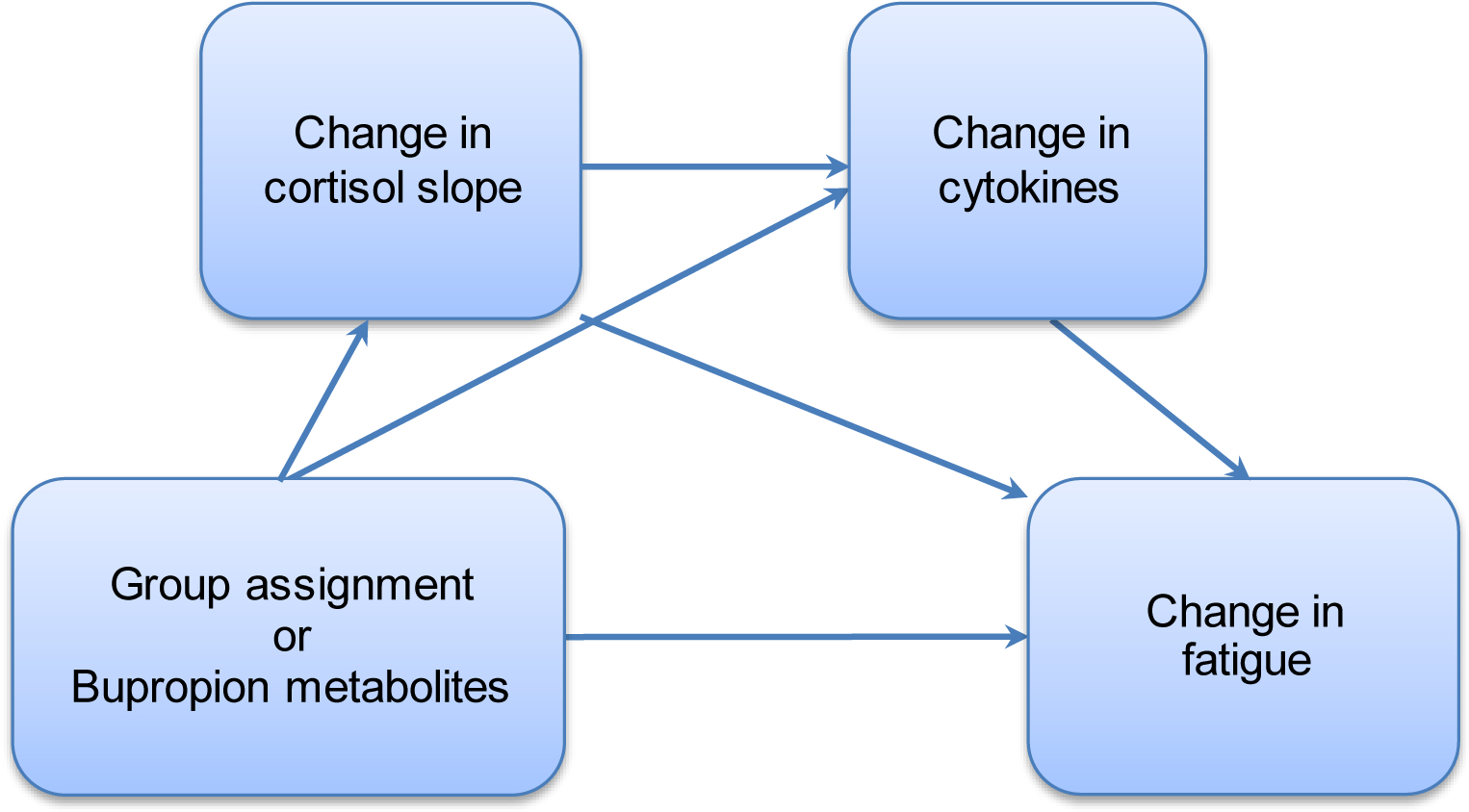
Model examining Exploratory Aims 2 and 3: cortisol-inflammation pathways mediating the relationship between bupropion exposure and change in fatigue.
Exploratory Aim 3:
It is hypothesized that major metabolites of bupropion (i.e., 4-hydroxybupropion, threohydrobupropion, erythro-hydrobupropion) and improvements in fatigue will be greater in patients with no copies of CYP2B6*6 and *18 (i.e., normal metabolizers) compared to patients with one or two copies of *6 and/or *18 (i.e., reduced metabolizers). Analyses will focus on the bupropion arm only. The SNPs that compose CYP2B6*6, rs3745274 and rs2279343, and *18 (rs28399499) will be tested for Hardy-Weinberg equilibrium. We will use ANCOVA (with the same structure as the Primary Aim) with normal vs. reduced metabolizers as the group and baseline fatigue as the covariate. Differences in bupropion metabolites and toxicity between normal vs. reduced metabolizers will be evaluated. Relatively few drugs inhibit or induce CYP2B6,110 but we will collect data regarding other medications taken by participants and include them as control variables if they are CYP2B6 inhibitors or inducers (i.e., clopidogrel, carbamazepine, rifampin, voriconazole). Statistical significance will be assessed after applying a FDR adjustment.
3.2.5. Missing Data
Differential attrition by group will be analyzed. Missingness in the data will be addressed as follows. First, trained research personnel who are aware of the difficulties posed by missing data will oversee data collection. Second, assuming that missing values are missing at random (MAR) (i.e., the probability of missing data is not affected by unobserved variables) multiple imputation will be used to obtain parameter estimates and standard errors.111,112 These will be compared with the estimates obtained by omitting subjects that had missing data. If the results are very similar, we will report the latter for simplicity. The possibility that data are missing not at random (MNAR) would reflect a situation in which the probability of missing data is affected by unobserved variables (e.g., participants are too fatigued to participate post-intervention). There has been no evidence of MNAR in previous URCC NCORP trials and it is not expected to occur in the current study. However, if MNAR is suspected after examining the reasons for dropout, etc., sensitivity analyses will be conducted with pattern-mixture models to assess its impact. For the mediation models, Full Information Maximum Likelihood Estimation (FIML) will be used. FIML is robust to missing data under the MAR assumption. In the event of MNAR, sensitivity analyses will again be performed with pattern-mixture models.111,113
4. Discussion
This study is the first rigorous trial of bupropion for cancer-related fatigue. Positive results from the current study of breast cancer survivors would form the basis for future trials to determine the efficacy of bupropion for fatigue in other cancer populations and in patients on active treatment. American Society of Clinical Oncology (ASCO) and National Comprehensive Cancer Network (NCCN) guidelines recommend routine screening for fatigue in cancer patients,18,114 but current treatment options for fatigued patients are suboptimal. Thus, randomized controlled trials of pharmacologic interventions for cancer-related fatigue have been identified as a high priority for research,19 including within the NCORP.115 If found to be efficacious, bupropion would be a safe, low-cost, widely-available treatment that could revolutionize management of cancer-related fatigue.
The study will also be among the first randomized, placebo-controlled pharmacologic trials to assess biological mechanisms underlying changes in cancer-related fatigue. Measurement of biological data enhances the scientific rigor and transparency of the study. It also represents a significant advance beyond the association studies that form the basis of current knowledge. The interventional nature of the study, combined with a rich dataset of biological parameters, will allow for detailed exploration of mechanistic pathways involved in cancer-related fatigue. Findings that bupropion exerts a beneficial effect on fatigue through inflammation and/or HPA axis pathways would spur examination of additional therapies targeting these pathways. Findings of no relationship between the efficacy of bupropion and inflammation and HPA axis pathways would suggest a role for other mechanisms, spurring future research to uncover these mechanisms. Thus, exploration of inflammation and HPA axis pathways will be a significant scientific contribution regardless of whether bupropion is found to be efficacious. Study limitations should also be noted. For example, the sample will be relatively heterogeneous in terms of time since treatment and treatment received, although this design decision will increase the generalizability of results. In addition, to reduce participant burden, we will assess fatigue only at baseline and follow-up. Therefore, we may miss circadian changes in fatigue (e.g., morning fatigue, evening fatigue. Nevertheless, successful completion of the current research is expected to yield important new knowledge regarding a potential treatment for cancer-related fatigue as well as exciting new avenues for future research.
Exploration of CYP2B6 as a moderator of bupropion efficacy further enhances the current project. Significant results would suggest that tailored dosing of bupropion should be considered. Genotype-guided drug dosing is becoming increasingly common in oncology and in some cases is recommended by the FDA (e.g., mercaptopurine).116 With the falling cost of genotyping and greater understanding of the human genome, it is likely that genetic information will become part of routine clinical care and increasingly important in clinical decisions. Thus, the current project anticipates precision medicine in supportive cancer care.
Funding:
This work is supported by the National Cancer Institute grants R01 CA214647 and UG1 CA189961. This work is also been supported in part by the Participant Research, Interventions, and Measurement Core Facility at the H. Lee Moffitt Cancer Center & Research Institute, an NCI designated Comprehensive Cancer Center (P30-CA076292). The authors are grateful to Howard McLeod, PharmD, for his contributions to the project.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hollen PJ, Msaouel P, Gralla RJ. Determining issues of importance for the evaluation of quality of life and patient-reported outcomes in breast cancer: results of a survey of 1072 patients. Breast Cancer Res Treat. 2015;151(3):679–686. [DOI] [PubMed] [Google Scholar]
- 2.Wang XS, Zhao F, Fisch MJ, et al. Prevalence and characteristics of moderate to severe fatigue: a multicenter study in cancer patients and survivors. Cancer. 2014;120(3):425–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ness S, Kokal J, Fee-Schroeder K, Novotny P, Satele D, Barton D. Concerns across the survivorship trajectory: results from a survey of cancer survivors. Oncol Nurs Forum. 2013;40(1):35–42. [DOI] [PubMed] [Google Scholar]
- 4.Butt Z, Rosenbloom SK, Abernethy AP, et al. Fatigue is the most important symptom for advanced cancer patients who have had chemotherapy. J Natl Compr Canc Netw. 2008;6(5):448–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reilly CM, Bruner DW, Mitchell SA, et al. A literature synthesis of symptom prevalence and severity in persons receiving active cancer treatment. Support Care Cancer. 2013;21(6):1525–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abrahams HJ, Gielissen MF, Schmits IC, Verhagen CA, Rovers MM, Knoop H. Risk factors, prevalence, and course of severe fatigue after breast cancer treatment: A meta-analysis involving 12,327 breast cancer survivors. Ann Oncol. 2016. [DOI] [PubMed] [Google Scholar]
- 7.Goedendorp MM, Andrykowski MA, Donovan KA, et al. Prolonged impact of chemotherapy on fatigue in breast cancer survivors: a longitudinal comparison with radiotherapy-treated breast cancer survivors and noncancer controls. Cancer. 2012;118(15):3833–3841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ancoli-Israel S, Liu L, Rissling M, et al. Sleep, fatigue, depression, and circadian activity rhythms in women with breast cancer before and after treatment: a 1-year longitudinal study. Support Care Cancer. 2014;22(9):2535–2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Donovan KA, Jacobsen PB, Andrykowski MA, et al. Course of fatigue in women receiving chemotherapy and/or radiotherapy for early stage breast cancer. J Pain Symptom Manage. 2004;28(4):373–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bower JE, Ganz PA, Desmond KA, et al. Fatigue in long-term breast carcinoma survivors: A longitudinal investigation. Cancer. 2006;106(4):751–758. [DOI] [PubMed] [Google Scholar]
- 11.Berger AM, Mooney K, Alvarez-Perez A, et al. Cancer-Related Fatigue, Version 2.2015. J Natl Compr Canc Netw. 2015;13(8):1012–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Borneman T, Piper BF, Koczywas M, et al. A qualitative analysis of cancer-related fatigue in ambulatory oncology. Clin J Oncol Nurs. 2012;16(1):E26–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poulson MJ. Not just tired. J Clin Oncol. 2001;19(21):4180–4181. [DOI] [PubMed] [Google Scholar]
- 14.Chen ML, Liu LN, Miaskowski C, Chen SC, Lin YC, Wang JS. Presurgical symptom profiles predict quality of life 2 years after surgery in women with breast cancer. Support Care Cancer. 2016;24(1):243–251. [DOI] [PubMed] [Google Scholar]
- 15.Maude SL, Frey N, Shaw PA, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371(16):1507–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lindbohm ML, Kuosma E, Taskila T, et al. Early retirement and non-employment after breast cancer. Psychooncology. 2014;23(6):634–641. [DOI] [PubMed] [Google Scholar]
- 17.Minton O, Stone PC. A comparison of cognitive function, sleep and activity levels in disease-free breast cancer patients with or without cancer-related fatigue syndrome. BMJ Support Palliat Care. 2012;2(3):231–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bower JE, Bak K, Berger A, et al. Screening, assessment, and management of fatigue in adult survivors of cancer: An American Society of Clinical Oncology clinical practice guideline adaptation. J Clin Oncol. 2014;32(17):1840–1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barsevick AM, Irwin MR, Hinds P, et al. Recommendations for high-priority research on cancer-related fatigue in children and adults. J Natl Cancer Inst. 2013;105(19):1432–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meneses-Echavez JF, Gonzalez-Jimenez E, Ramirez-Velez R. Effects of supervised exercise on cancer-related fatigue in breast cancer survivors: a systematic review and meta-analysis. BMC Cancer. 2015;15:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cramp F, Byron-Daniel J. Exercise for the management of cancer-related fatigue in adults. Cochrane Database Syst Rev. 2012;11:CD006145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Waart H, van Harten WH, Buffart LM, Sonke GS, Stuiver MM, Aaronson NK. Why do patients choose (not) to participate in an exercise trial during adjuvant chemotherapy for breast cancer? Psychooncology. 2015. [DOI] [PubMed] [Google Scholar]
- 23.Blaney JM, Lowe-Strong A, Rankin-Watt J, Campbell A, Gracey JH. Cancer survivors’ exercise barriers, facilitators and preferences in the context of fatigue, quality of life and physical activity participation: a questionnaire-survey. Psychooncology. 2013;22(1):186–194. [DOI] [PubMed] [Google Scholar]
- 24.Goedendorp MM, Gielissen MF, Verhagen CA, Bleijenberg G. Psychosocial interventions for reducing fatigue during cancer treatment in adults. Cochrane Database Syst Rev. 2009(1):CD006953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duijts SF, Faber MM, Oldenburg HS, van Beurden M, Aaronson NK. Effectiveness of behavioral techniques and physical exercise on psychosocial functioning and health-related quality of life in breast cancer patients and survivors--a meta-analysis. Psychooncology. 2011;20(2):115–126. [DOI] [PubMed] [Google Scholar]
- 26.Jacobsen PB, Donovan KA, Vadaparampil ST, Small BJ. Systematic review and meta-analysis of psychological and activity-based interventions for cancer-related fatigue. Health Psychol. 2007;26(6):660–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kangas M, Bovbjerg DH, Montgomery GH. Cancer-related fatigue: a systematic and meta-analytic review of non-pharmacological therapies for cancer patients. Psychol Bull. 2008;134(5):700–741. [DOI] [PubMed] [Google Scholar]
- 28.Morrow GR, Hickok JT, Roscoe JA, et al. Differential effects of paroxetine on fatigue and depression: A randomized, double-blind trial from the University of Rochester Cancer Center Community Clinical Oncology Program. J Clin Oncol. 2003;21(24):4635–4641. [DOI] [PubMed] [Google Scholar]
- 29.Stockler MR, O’Connell R, Nowak AK, et al. Effect of sertraline on symptoms and survival in patients with advanced cancer, but without major depression: A placebo-controlled double-blind randomised trial. Lancet Oncol. 2007;8(7):603–612. [DOI] [PubMed] [Google Scholar]
- 30.Roscoe JA, Morrow GR, Hickok JT, et al. Effect of paroxetine hydrochloride (Paxil) on fatigue and depression in breast cancer patients receiving chemotherapy. Breast Cancer Res Treat. 2005;89(3):243–249. [DOI] [PubMed] [Google Scholar]
- 31.Berenson JR, Yellin O, Shamasunder HK, et al. A phase 3 trial of armodafinil for the treatment of cancer-related fatigue for patients with multiple myeloma. Support Care Cancer. 2015;23(6):1503–1512. [DOI] [PubMed] [Google Scholar]
- 32.Jean-Pierre P, Morrow GR, Roscoe JA, et al. A phase 3 randomized, placebo-controlled, double-blind, clinical trial of the effect of modafinil on cancer-related fatigue among 631 patients receiving chemotherapy: a University of Rochester Cancer Center Community Clinical Oncology Program Research base study. Cancer. 2010;116(14):3513–3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spathis A, Fife K, Blackhall F, et al. Modafinil for the treatment of fatigue in lung cancer: results of a placebo-controlled, double-blind, randomized trial. J Clin Oncol. 2014;32(18):1882–1888. [DOI] [PubMed] [Google Scholar]
- 34.Qu D, Zhang Z, Yu X, Zhao J, Qiu F, Huang J. Psychotropic drugs for the management of cancer-related fatigue: a systematic review and meta-analysis. Eur J Cancer Care (Engl). 2015. [DOI] [PubMed] [Google Scholar]
- 35.Gong S, Sheng P, Jin H, et al. Effect of methylphenidate in patients with cancer-related fatigue: a systematic review and meta-analysis. PLoS One. 2014;9(1):e84391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bruera E, Yennurajalingam S, Palmer JL, et al. Methylphenidate and/or a nursing telephone intervention for fatigue in patients with advanced cancer: a randomized, placebo-controlled, phase II trial. J Clin Oncol. 2013;31(19):2421–2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moraska AR, Sood A, Dakhil SR, et al. Phase III, randomized, double-blind, placebo-controlled study of long-acting methylphenidate for cancer-related fatigue: North Central Cancer Treatment Group NCCTG-N05C7 trial. J Clin Oncol. 2010;28(23):3673–3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Breitbart W, Alici Y. Psychostimulants for cancer-related fatigue. J Natl Compr Canc Netw. 2010;8(8):933–942. [DOI] [PubMed] [Google Scholar]
- 39.Portenoy RK, Itri LM. Cancer-related fatigue: guidelines for evaluation and management. Oncologist. 1999;4(1):1–10. [PubMed] [Google Scholar]
- 40.Breitbart W, Alici-Evcimen Y. Update on psychotropic medications for cancer-related fatigue. J Natl Compr Canc Netw. 2007;5(10):1081–1091. [DOI] [PubMed] [Google Scholar]
- 41.Cullum JL, Wojciechowski AE, Pelletier G, Simpson JS. Bupropion sustained release treatment reduces fatigue in cancer patients. Can J Psychiatry. 2004;49(2):139–144. [DOI] [PubMed] [Google Scholar]
- 42.Moss EL, Simpson JS, Pelletier G, Forsyth P. An open-label study of the effects of bupropion SR on fatigue, depression and quality of life of mixed-site cancer patients and their partners. Psychooncology. 2006;15(3):259–267. [DOI] [PubMed] [Google Scholar]
- 43.Papakostas GI, Nutt DJ, Hallett LA, Tucker VL, Krishen A, Fava M. Resolution of sleepiness and fatigue in major depressive disorder: A comparison of bupropion and the selective serotonin reuptake inhibitors. Biol Psychiatry. 2006;60(12):1350–1355. [DOI] [PubMed] [Google Scholar]
- 44.Anthenelli RM, Benowitz NL, West R, et al. Neuropsychiatric safety and efficacy of varenicline, bupropion, and nicotine patch in smokers with and without psychiatric disorders (EAGLES): a double-blind, randomised, placebo-controlled clinical trial. Lancet. 2016;387(10037):2507–2520. [DOI] [PubMed] [Google Scholar]
- 45.Benowitz NL, Pipe A, West R, et al. Cardiovascular Safety of Varenicline, Bupropion, and Nicotine Patch in Smokers: A Randomized Clinical Trial. JAMA Intern Med. 2018;178(5):622–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brustolim D, Ribeiro-dos-Santos R, Kast RE, Altschuler EL, Soares MB. A new chapter opens in anti-inflammatory treatments: the antidepressant bupropion lowers production of tumor necrosis factor-alpha and interferon-gamma in mice. Int Immunopharmacol. 2006;6(6):903–907. [DOI] [PubMed] [Google Scholar]
- 47.Kast RE. Anti- and pro-inflammatory considerations in antidepressant use during medical illness: bupropion lowers and mirtazapine increases circulating tumor necrosis factor-alpha levels. Gen Hosp Psychiatry. 2003;25(6):495–496. [DOI] [PubMed] [Google Scholar]
- 48.Sedger LM, McDermott MF. TNF and TNF-receptors: From mediators of cell death and inflammation to therapeutic giants - past, present and future. Cytokine Growth Factor Rev. 2014;25(4):453–472. [DOI] [PubMed] [Google Scholar]
- 49.Meyers CA, Albitar M, Estey E. Cognitive impairment, fatigue, and cytokine levels in patients with acute myelogenous leukemia or myelodysplastic syndrome. Cancer. 2005;104(4):788–793. [DOI] [PubMed] [Google Scholar]
- 50.Schrepf A, Clevenger L, Christensen D, et al. Cortisol and inflammatory processes in ovarian cancer patients following primary treatment: relationships with depression, fatigue, and disability. Brain Behav Immun. 2013;30 Suppl:S126–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pertl MM, Hevey D, Boyle NT, et al. C-reactive protein predicts fatigue independently of depression in breast cancer patients prior to chemotherapy. Brain Behav Immun. 2013;34:108–119. [DOI] [PubMed] [Google Scholar]
- 52.Wratten C, Kilmurray J, Nash S, et al. Fatigue during breast radiotherapy and its relationship to biological factors. Int J Radiat Oncol Biol Phys. 2004;59(1):160–167. [DOI] [PubMed] [Google Scholar]
- 53.Wang XS, Williams LA, Krishnan S, et al. Serum sTNF-R1, IL-6, and the development of fatigue in patients with gastrointestinal cancer undergoing chemoradiation therapy. Brain Behav Immun. 2012;26(5):699–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bower JE, Ganz PA, Tao ML, et al. Inflammatory biomarkers and fatigue during radiation therapy for breast and prostate cancer. Clin Cancer Res. 2009;15(17):5534–5540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Greenberg DB, Gray JL, Mannix CM, Eisenthal S, Carey M. Treatment-related fatigue and serum interleukin-1 levels in patients during external beam irradiation for prostate cancer. J Pain Symptom Manage. 1993;8(4):196–200. [DOI] [PubMed] [Google Scholar]
- 56.Liu L, Mills PJ, Rissling M, et al. Fatigue and sleep quality are associated with changes in inflammatory markers in breast cancer patients undergoing chemotherapy. Brain Behav Immun. 2012;26(5):706–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bower JE, Ganz PA, Aziz N, Fahey JL. Fatigue and proinflammatory cytokine activity in breast cancer survivors. Psychosom Med. 2002;64(4):604–611. [DOI] [PubMed] [Google Scholar]
- 58.Bower JE, Ganz PA, Irwin MR, Arevalo JM, Cole SW. Fatigue and gene expression in human leukocytes: increased NF-kappaB and decreased glucocorticoid signaling in breast cancer survivors with persistent fatigue. Brain Behav Immun. 2011;25(1):147–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.El Mansari M, Ghanbari R, Janssen S, Blier P. Sustained administration of bupropion alters the neuronal activity of serotonin, norepinephrine but not dopamine neurons in the rat brain. Neuropharmacology. 2008;55(7):1191–1198. [DOI] [PubMed] [Google Scholar]
- 60.Dong J, Blier P. Modification of norepinephrine and serotonin, but not dopamine, neuron firing by sustained bupropion treatment. Psychopharmacology (Berl). 2001;155(1):52–57. [DOI] [PubMed] [Google Scholar]
- 61.Hale MW, Lowry CA. Brain monoaminergic systems in stress neuroendocrinology In: Russell JA, Shipston MJ, eds. Neuroendocrinology of Stress. 1 ed Hoboken, NJ: Wiley-Blackwell; 2015. [Google Scholar]
- 62.Chapotot F, Gronfier C, Jouny C, Muzet A, Brandenberger G. Cortisol secretion is related to electroencephalographic alertness in human subjects during daytime wakefulness. J Clin Endocrinol Metab. 1998;83(12):4263–4268. [DOI] [PubMed] [Google Scholar]
- 63.Rhen T, Cidlowski JA. Antiinflammatory action of glucocorticoids--new mechanisms for old drugs. N Engl J Med. 2005;353(16):1711–1723. [DOI] [PubMed] [Google Scholar]
- 64.Schmidt ME, Semik J, Habermann N, Wiskemann J, Ulrich CM, Steindorf K. Cancer-related fatigue shows a stable association with diurnal cortisol dysregulation in breast cancer patients. Brain Behav Immun. 2016;52:98–105. [DOI] [PubMed] [Google Scholar]
- 65.Bower JE, Ganz PA, Dickerson SS, Petersen L, Aziz N, Fahey JL. Diurnal cortisol rhythm and fatigue in breast cancer survivors. Psychoneuroendocrinology. 2005;30(1):92–100. [DOI] [PubMed] [Google Scholar]
- 66.Bower JE, Ganz PA, Aziz N, Olmstead R, Irwin MR, Cole SW. Inflammatory responses to psychological stress in fatigued breast cancer survivors: relationship to glucocorticoids. Brain Behav Immun. 2007;21(3):251–258. [DOI] [PubMed] [Google Scholar]
- 67.Piacentini MF, Meeusen R, Buyse L, De Schutter G, De Meirleir K. Hormonal responses during prolonged exercise are influenced by a selective DA/NA reuptake inhibitor. Br J Sports Med. 2004;38(2):129–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rao U, Ott GE, Lin KM, Gertsik L, Poland RE. Effect of bupropion on nocturnal urinary free cortisol and its association with antidepressant response. J Psychiatr Res. 2005;39(2):183–190. [DOI] [PubMed] [Google Scholar]
- 69.Fava M, Rush AJ, Thase ME, et al. 15 years of clinical experience with bupropion HCl: from bupropion to bupropion SR to bupropion XL. Prim Care Companion J Clin Psychiatry. 2005;7(3):106–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hann DM, Jacobsen PB, Azzarello LM, et al. Measurement of fatigue in cancer patients: development and validation of the Fatigue Symptom Inventory. Qual Life Res. 1998;7(4):301–310. [DOI] [PubMed] [Google Scholar]
- 71.Heckler CE, Garland SN, Peoples AR, et al. Cognitive behavioral therapy for insomnia, but not armodafinil, improves fatigue in cancer survivors with insomnia: A randomized placebo-controlled trial. Support Care Cancer. 2016;24(5):2059–2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Roscoe JA, Garland SN, Heckler CE, et al. Randomized placebo-controlled trial of cognitive behavioral therapy and armodafinil for insomnia after cancer treatment. J Clin Oncol. 2015;33(2):165–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhu AZ, Cox LS, Nollen N, et al. CYP2B6 and bupropion’s smoking-cessation pharmacology: The role of hydroxybupropion. Clin Pharmacol Ther. 2012;92(6):771–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Carpenter JS, Andrykowski MA. Menopausal symptoms in breast cancer survivors. Oncol Nurs Forum. 1999;26(8):1311–1317. [PubMed] [Google Scholar]
- 75.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. [DOI] [PubMed] [Google Scholar]
- 76.Yellen SB, Cella DF, Webster K, Blendowski C, Kaplan E. Measuring fatigue and other anemia-related symptoms with the Functional Assessment of Cancer Therapy (FACT) measurement system. J Pain Symptom Manage. 1997;13(2):63–74. [DOI] [PubMed] [Google Scholar]
- 77.Cella D, Eton DT, Lai JS, Peterman AH, Merkel DE. Combining anchor and distribution-based methods to derive minimal clinically important differences on the Functional Assessment of Cancer Therapy (FACT) anemia and fatigue scales. J Pain Symptom Manage. 2002;24(6):547–561. [DOI] [PubMed] [Google Scholar]
- 78.Acaster S, Dickerhoof R, DeBusk K, Bernard K, Strauss W, Allen LF. Qualitative and quantitative validation of the FACIT-Fatigue scale in iron deficiency anemia. Health Qual Life Outcomes. 2015;13:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hagstrom AD, Marshall PW, Lonsdale C, Cheema BS, Fiatarone Singh MA, Green S. Resistance training improves fatigue and quality of life in previously sedentary breast cancer survivors: A randomised controlled trial. Eur J Cancer Care (Engl). 2015. [DOI] [PubMed] [Google Scholar]
- 80.Yennurajalingam S, Frisbee-Hume S, Palmer JL, et al. Reduction of cancer-related fatigue with dexamethasone: A double-blind, randomized, placebo-controlled trial in patients with advanced cancer. J Clin Oncol. 2013;31(25):3076–3082. [DOI] [PubMed] [Google Scholar]
- 81.Cella DF, Tulsky DS, Gray G, et al. The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1993;11(3):570–579. [DOI] [PubMed] [Google Scholar]
- 82.Cella D, Riley W, Stone A, et al. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. J Clin Epidemiol. 2010;63(11):1179–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.HealthMeasures. PROMIS Depression Scoring Manual. http://www.healthmeasures.net/administrator/components/com_instruments/uploads/PROMIS%20Depression%20Scoring%20Manual.pdf5. Published Sept. 16, 2016. Accessed Feb. 9, 2017.
- 84.Jensen RE, Moinpour CM, Potosky AL, et al. Responsiveness of 8 Patient-Reported Outcomes Measurement Information System (PROMIS) measures in a large, community-based cancer study cohort. Cancer. 2017;123(2):327–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Quach CW, Langer MM, Chen RC, et al. Reliability and validity of PROMIS measures administered by telephone interview in a longitudinal localized prostate cancer study. Qual Life Res. 2016;25(11):2811–2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schalet BD, Pilkonis PA, Yu L, et al. Clinical validity of PROMIS Depression, Anxiety, and Anger across diverse clinical samples. J Clin Epidemiol. 2016;73:119–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Radloff L The CES-D Scale: A Self-Report Depression Scale for Research in the General Population. Applied Psychological Measurement. 1977;1(3):385–401. [Google Scholar]
- 88.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta psychiatrica Scandinavica. 1983;67(6):361–370. [DOI] [PubMed] [Google Scholar]
- 89.Kroenke K, Strine TW, Spitzer RL, Williams JB, Berry JT, Mokdad AH. The PHQ-8 as a measure of current depression in the general population. J Affect Disord. 2009;114(1–3):163–173. [DOI] [PubMed] [Google Scholar]
- 90.Morin CM. Insomnia: Psychological Assessment and Management. New York: The Guilford Press; 1993. [Google Scholar]
- 91.Savard MH, Savard J, Simard S, Ivers H. Empirical validation of the Insomnia Severity Index in cancer patients. Psychooncology. 2005;14(6):429–441. [DOI] [PubMed] [Google Scholar]
- 92.Berger AM. Update on the state of the science: Sleep-wake disturbances in adult patients with cancer. Oncol Nurs Forum. 2009;36(4):E165–177. [DOI] [PubMed] [Google Scholar]
- 93.Abrahams HJG, Gielissen MFM, Verhagen C, Knoop H. The relationship of fatigue in breast cancer survivors with quality of life and factors to address in psychological interventions: A systematic review. Clin Psychol Rev. 2018;63:1–11. [DOI] [PubMed] [Google Scholar]
- 94.Abrahams HJG, Smits L, Lugt M, et al. Severe fatigue after treatment of ductal carcinoma in situ: A comparison with age-matched breast cancer survivors and healthy controls. Breast. 2017;31:76–81. [DOI] [PubMed] [Google Scholar]
- 95.Fieo R, Ocepek-Welikson K, Kleinman M, et al. Measurement Equivalence of the Patient Reported Outcomes Measurement Information System((R)) (PROMIS((R))) Applied Cognition - General Concerns, Short Forms in Ethnically Diverse Groups. Psychol Test Assess Model. 2016;58(2):255–307. [PMC free article] [PubMed] [Google Scholar]
- 96.Cleeland CS, Mendoza TR, Wang XS, et al. Assessing symptom distress in cancer patients: the M.D. Anderson Symptom Inventory. Cancer. 2000;89(7):1634–1646. [DOI] [PubMed] [Google Scholar]
- 97.Masters AR, McCoy M, Jones DR, Desta Z. Stereoselective method to quantify bupropion and its three major metabolites, hydroxybupropion, erythro-dihydrobupropion, and threo-dihydrobupropion using HPLC-MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci. 2016;1015–1016:201–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gufford BT, Lu JB, Metzger IF, Jones DR, Desta Z. Stereoselective glucuronidation of bupropion metabolites in vitro and in vivo. Drug Metab Dispos. 2016;44(4):544–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cole SW, Yan W, Galic Z, Arevalo J, Zack JA. Expression-based monitoring of transcription factor activity: The TELiS database. Bioinformatics. 2005;21(6):803–810. [DOI] [PubMed] [Google Scholar]
- 100.Booth M Assessment of physical activity: an international perspective. Res Q Exerc Sport. 2000;71(2 Suppl):S114–120. [PubMed] [Google Scholar]
- 101.Johnson-Kozlow M, Sallis JF, Gilpin EA, Rock CL, Pierce JP. Comparative validation of the IPAQ and the 7-Day PAR among women diagnosed with breast cancer. Int J Behav Nutr Phys Act. 2006;3:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Borm GF, Fransen J, Lemmens WA. A simple sample size formula for analysis of covariance in randomized clinical trials. J Clin Epidemiol. 2007;60(12):1234–1238. [DOI] [PubMed] [Google Scholar]
- 103.Brown H, Prescott R. Applied Mixed Models in Medicine, Second Edition. Edinburgh, UK: John Wiley and Sons, Ltd.; 2006. [Google Scholar]
- 104.Muthén LK, Muthén BO. Mplus User’s Guide. Seventh Edition. Los Angeles CA: Muthén & Muthén; 1998–2015. [Google Scholar]
- 105.MacKinnon DP, Lockwood CM, Hoffman JM, West SG, Sheets V. A comparison of methods to test mediation and other intervening variable effects. Psychol Methods. 2002;7(1):83–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.MacKinnon DP. Introduction to Statistical Mediation Analysis. New York: Lawrence Erlbaum Associates; 2008. [Google Scholar]
- 107.Kraemer HC, Wilson GT, Fairburn CG, Agras WS. Mediators and moderators of treatment effects in randomized clinical trials. Arch Gen Psychiatry. 2002;59(10):877–883. [DOI] [PubMed] [Google Scholar]
- 108.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51(6):1173–1182. [DOI] [PubMed] [Google Scholar]
- 109.Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J R Stat Soc Series B. 1995;57(1):289–300. [Google Scholar]
- 110.Turpeinen M, Raunio H, Pelkonen O. The functional role of CYP2B6 in human drug metabolism: Substrates and inhibitors in vitro, in vivo and in silico. Curr Drug Metab. 2006;7(7):705–714. [DOI] [PubMed] [Google Scholar]
- 111.Molenberghs G, Kenward MG. Missing Data in Clinical Studies. West Sussex, UK: John Wiley and Sons; 2007. [Google Scholar]
- 112.van Buuren S Flexible Imputation of Missing Data. Boca Raton, FL: CRC Press; 2012. [Google Scholar]
- 113.Little RJA, Rubin DB. Statistical Analysis with Missing Data. 2nd ed Hoboken, NJ: Wiley; 2002. [Google Scholar]
- 114.National Comprehensive Cancer Network. Cancer-Related Fatigue (Version 1.2016). http://www.nccn.org/professionals/physician_gls/pdf/fatigue.pdf. Accessed April 22, 2016.
- 115.National Cancer Institute Community Oncology Research Program (NCORP) Symptom Management & Quality of Life Steering Committee (SxQoL SC). Strategic Priorities. https://www.cancer.gov/about-nci/organization/ccct/steering-committees/2015-sxqolsc-strategicpriorities. Published 2015. Accessed October 29, 2019.
- 116.Weng L, Zhang L, Peng Y, Huang RS. Pharmacogenetics and pharmacogenomics: a bridge to individualized cancer therapy. Pharmacogenomics. 2013;14(3):315–324. [DOI] [PMC free article] [PubMed] [Google Scholar]


