Abstract
Nitric oxide (NO) and hydrogen sulfide (H2S) are industrial toxins or pollutants; however, both are produced endogenously and have important biological roles in most mammalian tissues. The recognition that these gasotransmitters have a role in physiological and pathophysiological processes has presented opportunities to harness their intracellular effects either through inhibition of their production; or more commonly, through inducing their levels and or delivering them by various modalities. In this review article, we have focused on an array of NO and H2S donors, their hybrids with other established class of drugs, and the various engineered delivery platforms such a fibers, polymers, nanoparticles, hydrogels, and others. In each case, we have reviewed the rationale for their development.
Keywords: Nitric oxide, hydrogen sulfide, NO-releasing compounds, H2S-releasing hybrids, delivery platforms, fibers, polymers, nanoparticles, hydrogels
1. Introduction
Nitric oxide (NO) is a ubiquitous gaseous free radical and hydrogen sulfide (H2S) a gas that bears the pungent smell of rotten eggs; both are toxic, yet they are recognized as having multiple roles in normal physiology. In 1992, the journal Science referred to NO as the “Molecule of the Year” and in 1998 the Nobel Prize in Physiology and Medicine was awarded to Robert F. Furchgott, Louis J. Ignarro, and Ferid Murad for the major discoveries surrounding it. Thus, for many years NO reined supreme as a signaling wonder molecule. However, in 1996 a new player came on the scene when Abe and Kimura in a landmark study established the physiological role of H2S as a neuromodulator [1]. The elucidation of relevant enzymes and cellular signaling mechanisms led to the induction of H2S into a family of small molecule signaling compounds called gasotransmitters. A term first used by Wang in 2002, gasotransmitter refers to the gaseous nature of these compounds at standard temperature and pressure [2]. There are currently three compounds that qualify as gasotransmitters: carbon monoxide (CO), nitric oxide (NO), and H2S. For a molecule to be considered a gasotransmitter, three specific criteria must be met: 1) Endogenous production; 2) Free permeability through cell membranes; and 3) Well-defined biological targets and functions [2]. Over the years, much attention has focused on creating well-defined chemical tools to probe the NO and H2S physiology in an attempt to determine their signaling roles in biological systems. The acknowledgement of NO and H2S as gasotransmitters has led to an interest in pharmacological application of these gases. To that end, many NO and H2S “releasing” compounds, also termed “donors”, have been developed/designed that have been and are continuing to undergo intensive investigation. However, a limiting factor to the use of NO and H2S as therapeutic agents are their delivery to the target organs, in many cases in a sustained manner. In this review we provide an overview of the various NO and H2S-donating single agents and hybrid compounds together with the various platforms that are used for their delivery. This is an evolving landscape that is at the interface of basic life/physical sciences and complex medical applications.
2. Endogenous production of NO and H2S
NO is synthesized in all cells by nitric oxide synthase (NOS)-dependent (l-arginine- NO pathway) and independent (nitrate-nitrite-NO pathway) pathways (Fig 1). NO is synthesized by three major isoforms of NOS including constitutive neuronal (nNOS/NOS1), endothelial (eNOS/NOS3), and inducible (iNOS/NOS2), reviewed in [3]. The expression levels of these enzymes varies in different tissues; nNOS and eNOS produce low concentrations of NO for short periods of time, whereas iNOS produces relatively higher levels and for longer time periods. NOS-independent NO production from nitrate and nitrite comes from the stomach following protonation of swallowed salivary nitrite (Fig 1) [4]. For details and in-depth presentation of this general area please see Gheibi et al in this special issue [5].
Figure 1.
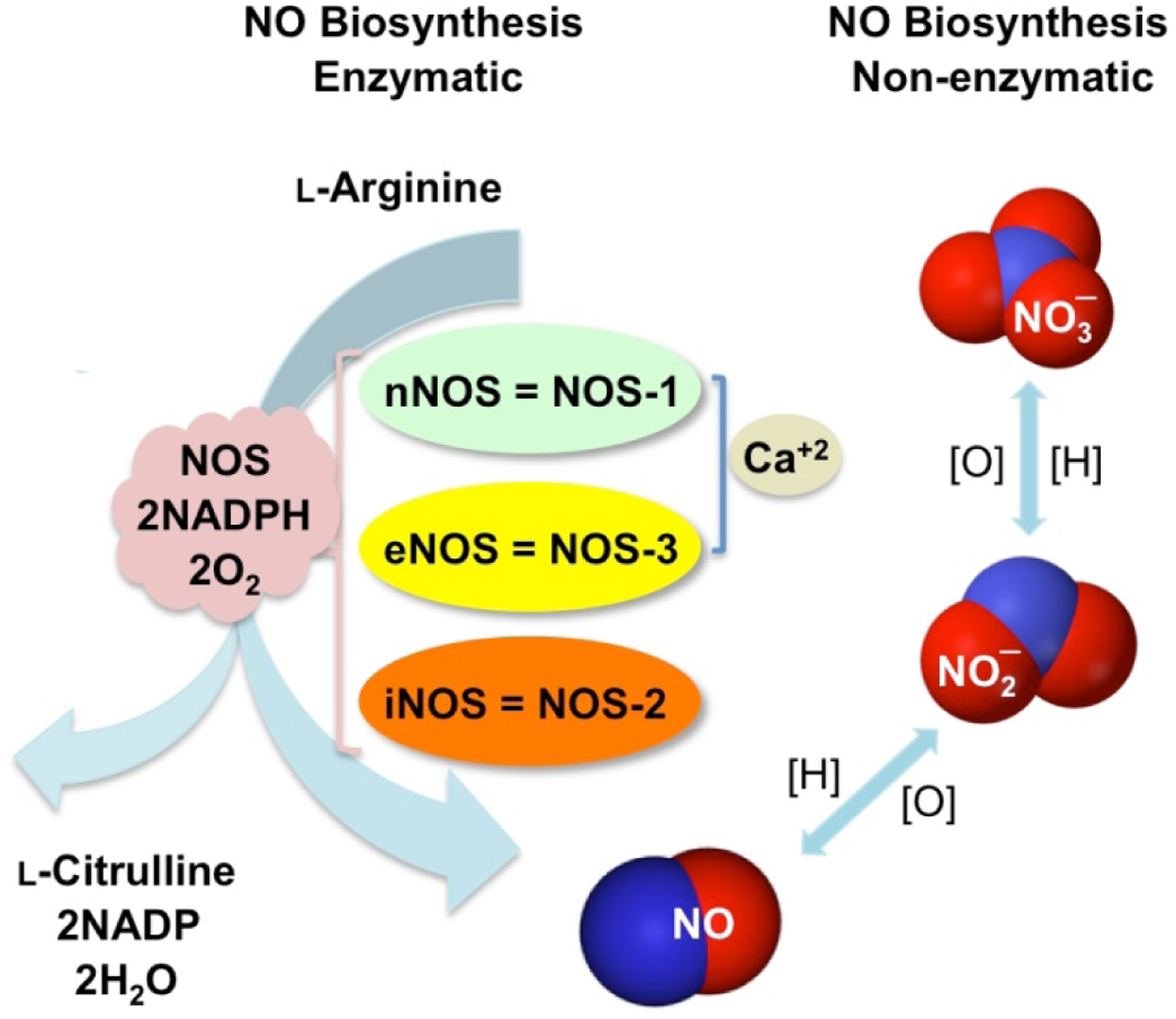
Biosynthesis of nitric oxide. NO is produced by three nitric oxide synthase (NOS) isoforms: neuronal, endothelial, and inducible (nNOS, eNOS, and iNOS) that catalyze the oxidation of l-arginine to l-citrulline, the enzymatic pathway. NO is also produced through reduction of nitrite/nitrate under low oxygen conditions, the non-enzymatic pathway. [O] = oxidation, and [H] = reduction.
Endogenous production of H2S is a result of direct enzymatic desulfhydration of cysteine, catalyzed by cystathionine-γ-lyase (CSE) and cystathionine-β-synthase (CBS), and indirect desulfhydration catalyzed by 3-mercapto-sulfurtransferase (3-MST) in the presence of reductants (Fig 2) [6]. CBS is present mostly in the central nervous system and the liver, while CSE is primarily responsible for H2S production in the cardiovascular system. 3-MST is located predominantly in the mitochondria and produces H2S in concert with cysteine aminotransferase (CAT) [7–11]. Non-enzymatic production of H2S (Fig 2) is responsible for a limited amount of H2S in mammalian cells [12] and is mediated through reducing elemental sulfur or organic polysulfides via glucose-supported and thiol-dependent reactions [13–15].
Figure 2.
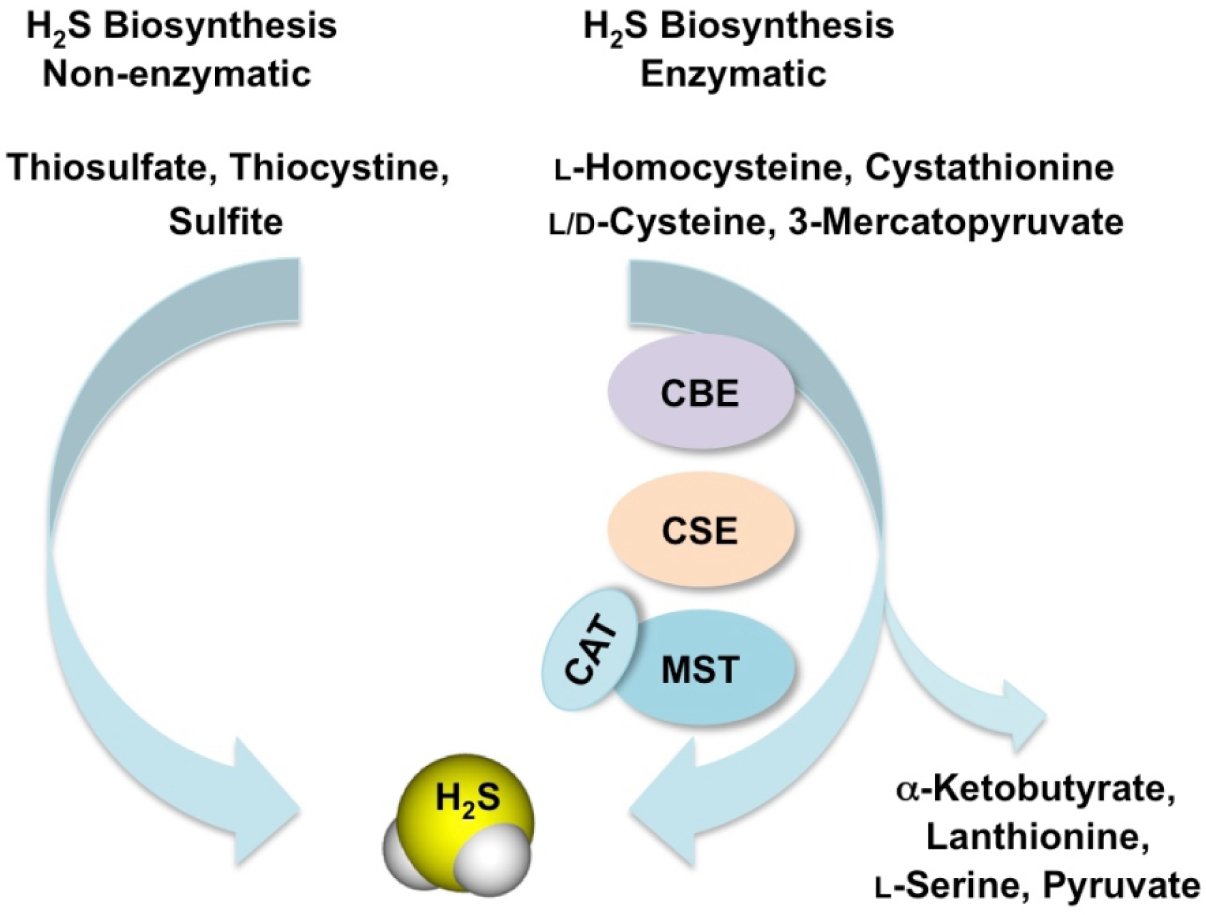
Biosynthesis of hydrogen sulfide. H2S is generated from oxidation of the substrates l-homocysteine, cystathionine, l-cysteine and 3-mercaptopyruvate through the enzymes cystathionine β-synthase (CBS) and cystathionine γ-lyase (CSE) and the tandem enzymes cysteine aminotransferase (CAT) and 3-mercaptopyruvate sulfurtransferase (3-MST). α-Ketobutyrate, lanthionine, L-serine and pyruvate are the secondary products formed. Mammalian enzymes generally metabolize l-amino acids, however, H2S can also be synthesized from d-cysteine by the peroxisomal enzyme d-amino acid oxidase (DAO) to 3-MP, which is a substrate for 3-MST. Alternatively, production of H2S occurs non-enzymatically from various storage forms of sulfur such as thiosulfate, thiocysteine and sulfite.
3. NO and H2S signaling, their interactions and cross talk
NO reacts with the active site of soluble guanylate cyclase (sGC) and produces cyclic GMP (cGMP) (Fig 3). cGMP activates cGMP-dependent Protein Kinase G (PKG), which phosphorylates multiple substrates [16]. In general, an increase in cGMP leads to smooth muscle relaxation, vasorelaxation, and decrease of platelet aggregation [17, 18]. NO can modify proteins by S-nitrosylation of cysteine residues [19–21], which may lead to either progression or inhibition of various diseases [22]. S-nitrosylation of NF-κB and matrix metalloproteinase 9 (MMP9) promotes cell death whereas S-nitrosylation of caspase-3, caspase-9, and c-Jun N-terminal kinase prevents activity and inhibits apoptosis [23]. Hypoxia-inducible factor-1 (HIF-1), estrogen receptor and NF-κB are redox sensitive transcription factors that are regulated by S-nitrosylation [24].
Figure 3.
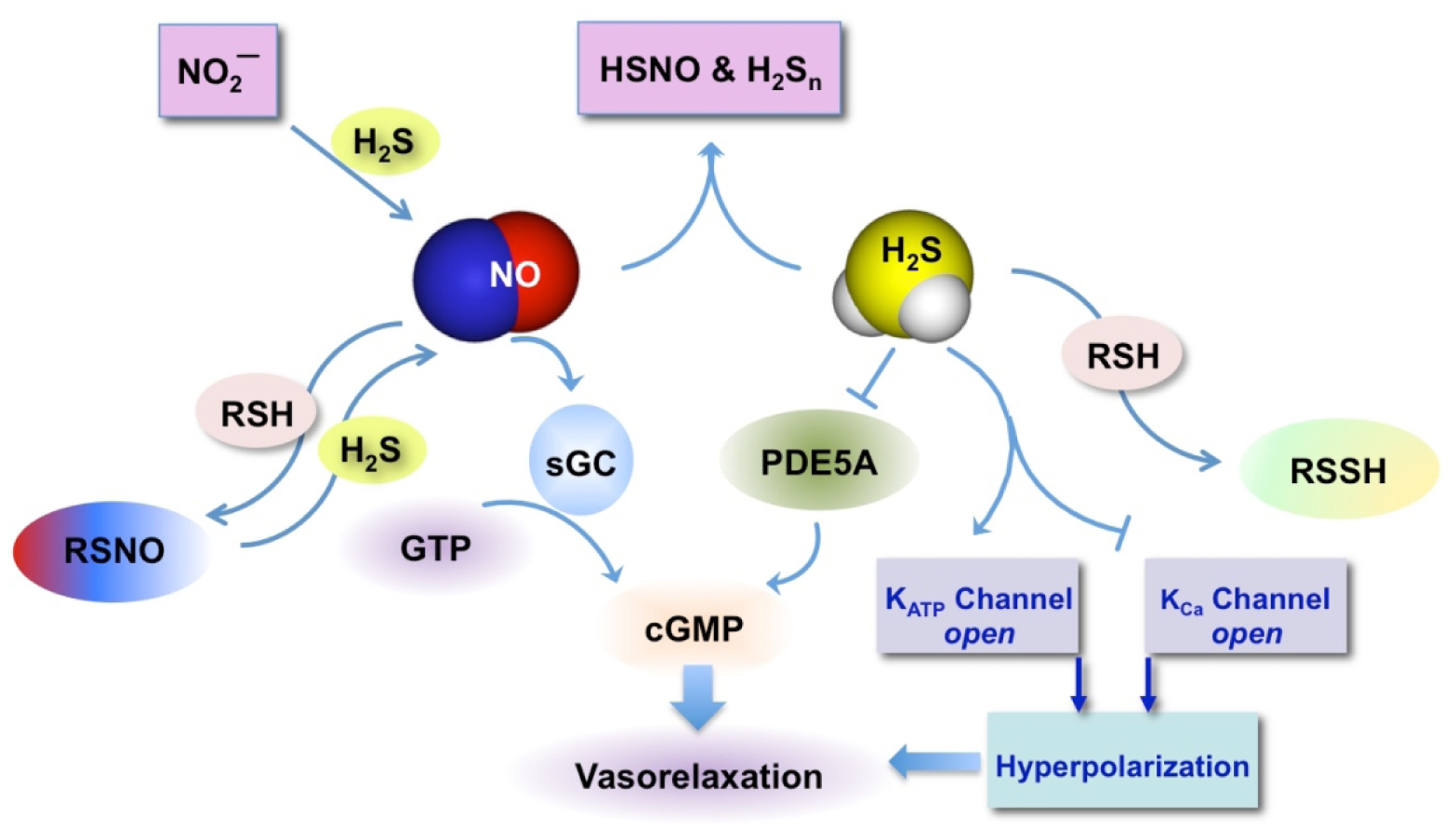
Cellular effects of NO and H2S and their interactions. NO reacts with the active site of soluble guanylate cyclase (sGC) and produces cyclic GMP leading to vasorelaxation. NO can affect cellular proteins by producing peroxynitrite, which in turn can interact with cysteine residues to form S-nitrosothiols (RSNO). The oxidative pathway leads to modification of proteins by S-nitrosylation of cysteine residues. H2S raises cGMP levels through inhibition of phosphodiesterase 5A (PDE5A) an enzyme that catabolizes it. H2S can also interact with the sulfhydryl group of cysteines and proteins to form persulfides (R-SSH). NO can interact with H2S to form HSNO [32] and H2Sn [45]; H2S can interact with NO2− [34] or with RSNO [268–270] to produce NO. H2S can interact with membrane ion channels and/or voltage-dependent calcium channels leading to vasorelaxation in vascular smooth muscle [26].
KATP = ATP-sensitive potassium, KCa = voltage-dependent calcium channels
H2S can also increase cGMP levels by inhibiting PDE5A, the enzyme that is involved in its catabolism [25]. H2S interacts with ATP-sensitive potassium (KATP) channels leading to vasorelaxation in vascular smooth muscle [26] and enhancing cardiovascular function [27]. Voltage-dependent calcium channels are also important targets of H2S [28]. H2S signaling may be through sulfuration (S-sulfhydration) of target proteins, where a sulfhydryl group (-SH) is transferred to a cysteine residue to form hydropersulfide (-SSH) [29, 30] or persulfide groups [31]. H2S also reacts with S-nitrosothiols to form thionitrous acid (HSNO), the smallest S-nitrosothiol [32]. HSNO can be metabolized to NO+, NO, and NO−, all of which have distinct physiological effects. Thus, HSNO can act a signaling molecule that may play a key role in cellular redox regulation [32].
NO and H2S bind avidly to hemoglobin [2] leading to the formation of nitrosyl hemoglobin and sulfhemoglobin, respectively [33]. This competition for the common hemoglobin sink can potentiate the biological activity of the other. NO and H2S can interact with each other, affecting each other’s bioavailability and reactivity [34–36]. For example, NO inhibits CBS activity by binding to the heme group of the enzyme [37] and NaSH inhibits a recombinant form of bovine eNOS by an interaction between co-factors such as NADPH or tetrahydrobiopterin [38, 39]. NO can increase H2S biosynthesis through increases in expression of CBS and CSE enzymes in vascular smooth cells [26, 40]. H2S increases NO levels by increasing IL-1β-induced iNOS expression in vascular smooth cells [41] through NF-κB activation by a mechanism involving the ERK1/2 signaling cascade. In bovine arterial endothelial cells, H2S has been shown to increase eNOS activation either indirectly by inducing its phosphorylation through an Akt-dependent mechanism [42] or directly by inducing Ca2+ release from the intracellular storage in the endoplasmic reticulum [43]. In human umbilical vein endothelial cells L-cysteine supplementation stimulated NO production, while inhibition of CSE blocked it [44]. Emerging data show that the interaction between H2S and NO can also generate polysulfides (H2Sn; H2S2 and H2S3) [45], reviewed in [3]. Some of the main features of NO and H2S signaling and their interactions are depicted in Fig 3.
4. Synthetic H2S donors and H2S-NSAID Conjugates
The need for chemical tools to study H2S biology grew out of an interest in determining the physiological roles of H2S. These tools include probes for the detection of H2S, which change their spectroscopic properties in response to H2S, CBS and CSE inhibitors, which reduce endogenous production of H2S, and H2S donors, which are compounds designed to release H2S under specific conditions. To study H2S in a physiologically relevant manner, donors with variable release rates and triggers are needed. We do not attempt to provide a comprehensive description of all available H2S donors here; rather, we critically examine recent developments in H2S donor chemistry, highlight specific examples of donors, and discuss important considerations in designing and choosing donors for biological studies and translation to H2S-releasing therapeutics.
4.1. Small molecule H2S donors
4.1.1. Sulfide salts
The most commonly used H2S donors employed in biological studies are sodium hydrosulfide (NaSH) and sodium sulfide (Na2S). Although routinely referred to as H2S donors, sulfide salts are simply solid analogs of H2S gas, providing instantaneous access to the biologically relevant forms of sulfide (H2S and HS–) in aqueous media. Sulfide salts have been extremely important in the establishment of H2S as a gasotransmitter, and these salts have been applied to evaluate the biological roles and therapeutic potential of exogenous H2S delivery.
One of the first studies on exogenous H2S delivery by Wang used aqueous NaSH solutions in the in vitro evaluation of rat aortic rings [46]. Exogenously delivered NaSH led to a 60% greater relaxation versus controls, showcasing the properties of H2S as a vasorelaxant. In a separate study by Du, exogenous delivery of NaSH via IV injection in rats with oleic acid-induced acute lung injury (ALI) alleviated symptoms by diminishing IL-6 and IL-8 levels, while simultaneously increasing IL-10 levels in the lung tissues [47]. This landmark study also provided verification of the hypothesis that down-regulation of endogenous H2S production in the cardiovascular system is involved in ALI pathogenesis and disease progression.
Despite these successes in early work, Na2S and NaHS are not ideal H2S donors for several reasons. First, they are instantaneously converted into H2S/HS−, which is starkly different from biological H2S signaling, which is tightly regulated by CSE and CBS. Second, commercial sulfide salts are impure, contaminated with polysulfides and other potential sulfur signaling species. Finally, without special precautions to prevent oxidation and volatilization, solutions of H2S quickly become depleted, creating inconsistencies in the amount of H2S added across a treatment group in an experiment because all animals or cell groups cannot be treated simultaneously.
4.1.2. Hydrolysis-Triggered H2S Donors
Several types of H2S donors have been developed that begin releasing H2S as they dissolve in water. Release rates vary, and some have pH-dependent release profiles. A few well studied classes of hydrolysis-triggered H2S donors are shown in Figure 4.
Figure 4.
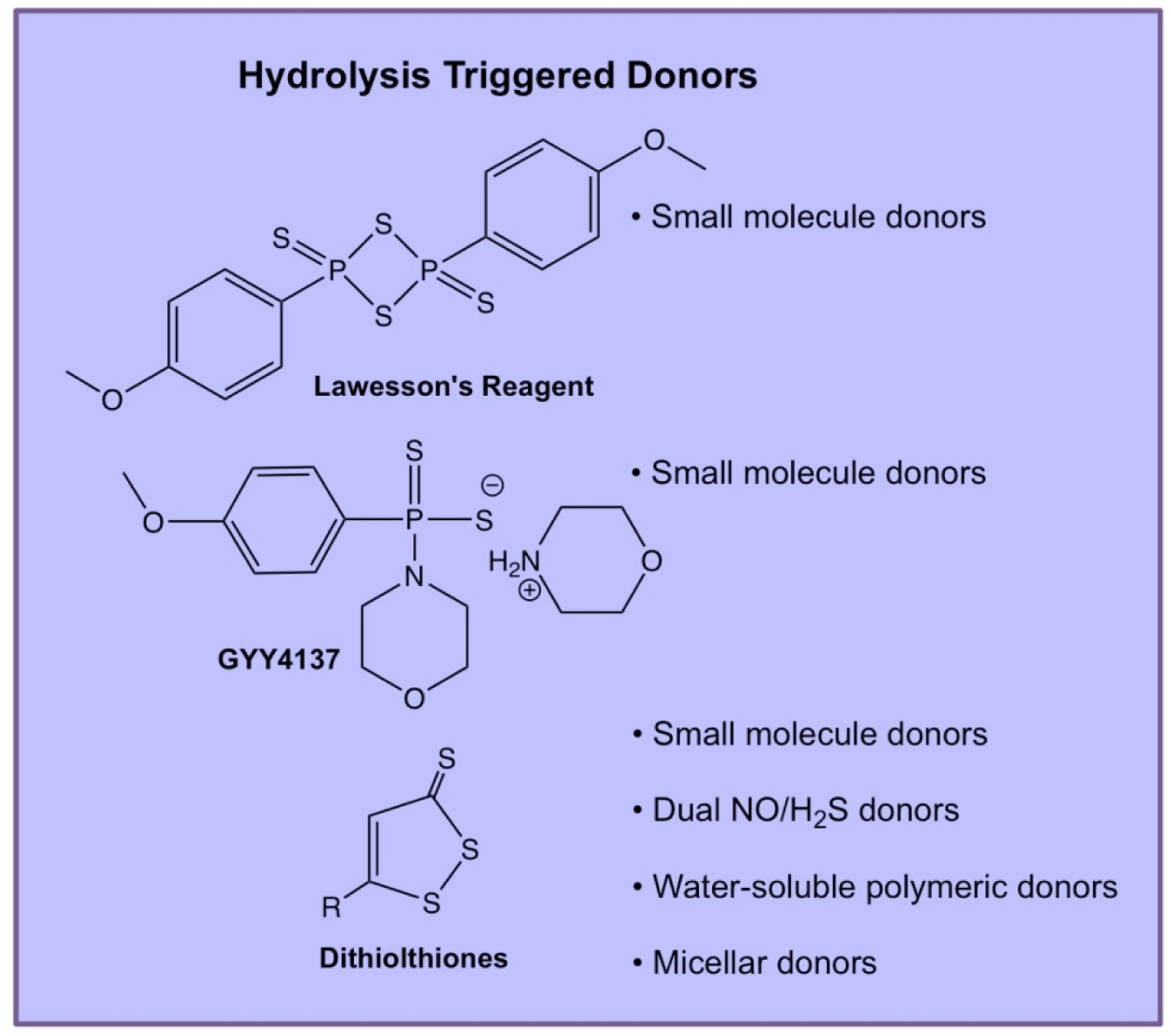
Chemical structures of selected hydrolysis-triggered H2S donors and descriptions of their use in various engineered delivery systems.
4.1.2.1. Lawesson’s Reagent and GYY4137
Lawesson’s reagent (LR) is a chemical reagent used widely for the thionation of amides, esters, and ketones to their corresponding sulfur analogs [48]. LR is commercially available, making it a popular choice for biologists studying H2S physiology. Importantly, LR releases H2S in aqueous media over a much longer period than sulfide salts, making it a useful early candidate for the evaluation of sustained H2S release. Much of the preliminary work in the field was performed with LR, but drawbacks, namely its low water solubility and lack of detailed kinetic analyses, led researchers to examine a water-soluble derivative of LR, called GYY4137. Synthesized via the reaction of morpholine and LR at room temperature, GYY4137 is easily prepared and purified before in vitro or in vivo administration. Many early studies on H2S biology applied GYY4137 as an H2S donor, but this compound also possesses drawbacks. Firstly, the preparation of GYY4137 yields the compound in a dichloromethane (DCM) complex, obfuscating biological data generated using the prodrug because DCM is metabolized into CO, another gasotransmitter with effects related to H2S [49]. Also, a lack of proper control compounds in many studies using GYY4137 further complicates the interpretation of the observed biological effects with exogenous GYY4137 delivery.
Xian et al. more recently developed a series of donors with structures related to GYY4137 in the form of phosphonamidothioates, denoted as JK donors [50]. The synthesis of these compounds was accomplished by combining Lawesson’s reagent with various amino acids, yielding a series of phosphonamidothioates analogous in structure to GYY4137. In their initial report, the authors found that in aqueous media at neutral and mildly basic pH, JK donors released low concentrations of H2S. In contrast, under mildly acidic conditions (pH ≤ 6.0), JK donors cyclized via nucleophilic addition of the carboxylic acid functionality of the amino acid, promoting H2S release by breaking the weak P–S bond. Across the series of JK donors, lower pH accelerated release rates, while a GYY4137 control showed no release profile variability at various pHs. H2S release profiles could also be tuned by altering the canonical R group substituent of the amino acid component of the donor. The authors observed that any substitution at the amino acid R group (i.e., R ≠ H) promoted cyclization, and thus showed faster release profiles at neutral and basic pH over the unsubstituted donor. The JK donors (25 and 50 μM) showed efficacy in reducing cellular damage resulting from anoxia/reoxygenation (A/R) treatment with H2O2 in vitro. JK-type donors were also successful in reducing infarct size per area-at-risk via intracardiac injection in mice in an I/R model. JK donors, specifically JK-1, have been used in several biological studies since the initial report. In one key example, Lefer showed that JK-1 exhibited protective effects in multiple organs by reducing oxidative stress, improving exercise capacity, and attenuating rene-angiotensin-aldosterone system activation [51]. Due to their modular nature and activity, we expect JK-type H2S donors to be a valuable chemical tool moving forward for investigating the cardioprotective effects of H2S.
4.1.2.2. Dithiolthiones
1,2-Dithiole-3-thiones (DTTs) are a group of compounds in the family of hydrolysis-triggered H2S donors. There is also evidence that they are triggered by intracellular enzymes, although the specific enzymes have not been identified [52]. Synthesized by the reaction of elemental sulfur and anethole, DTTs are easy to derivatize and can be readily attached to other molecules to make a wide variety of drugs and/or polymer-DTT conjugates. Substituted DTTs appear to hydrolyze cleanly, with the thione species being converted into a carbonyl [53]. However, in this study, complete hydrolysis required 48 h at 120 °C in a DMSO/H2O mixture. The authors noted that hydrolysis under physiological conditions was very slow and did not present any data at 37 °C. However, they did observe activity of several DTTs as COX-1 and COX-2 inhibitors, with less potency noted for the hydrolyzed DTTs against both targets. Therefore, DTTs may have bioactivity aside from their potential H2S-donating properties.
4.1.3. Thiol-Triggered H2S Donors
Thiol-triggered H2S donors are the most common class of non-hydrolysis-triggered synthetic donors. Free thiols are abundant nucleophiles in mammals and offer a platform from which thioldisulfide exchange can be used to accomplish H2S release after nucleophilic attack. A number of thiol-triggered H2S donors are shown in Figure 5.
Figure 5.
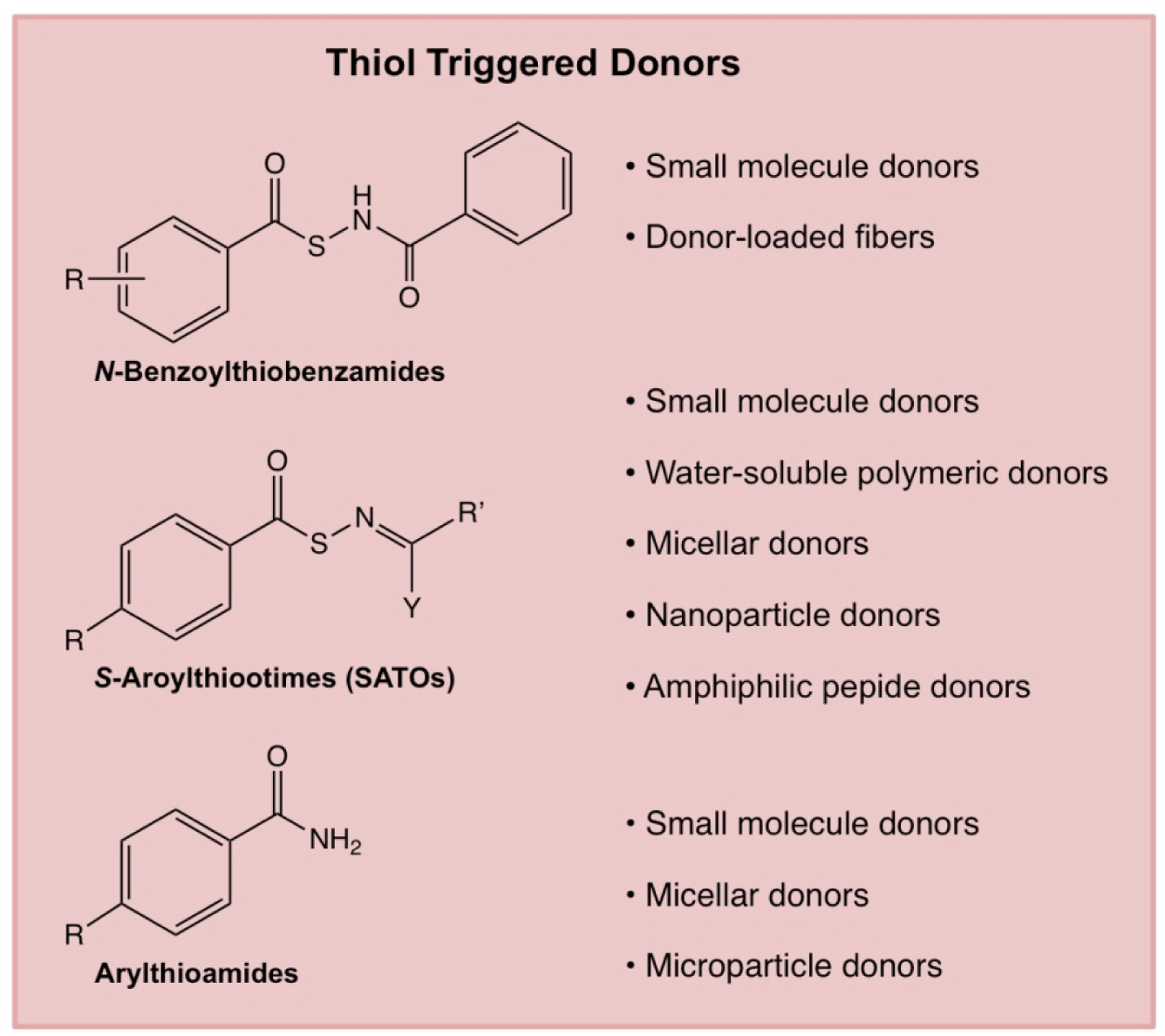
Chemical structures of selected thiol-triggered H2S donors and descriptions of their use in various engineered delivery systems.
4.1.3.1. N-Benzoylthiobenzamides
Among the first nucleophile-triggered H2S donors were the N-(benzoylthio)benzamides developed by Xian and coworkers [54]. Synthesized from substituted derivatives of thiobenzoic acid, a series of N-benzoylthiobenzamides were assessed for H2S release, with a range of different release rates observed. The H2S release mechanism was confirmed with the formation of N-acetylcysteine, cystine, and benzamide. In cell studies, a selected N-(benzoylthio)benzamide protected human keratinocytes against methylglyoxal (MGO)-induced cell damage, an issue prevalent in diabetics [55]. These donors have shown cardioprotective effects in animal models of myocardial I/R injury, displaying a reduction in infarct size over controls [56].
4.1.3.2. Arylthioamides
Arylthioamides (ArC(S)–NH2) are a class of donors that was first reported by Calderone and coworkers [57]. In this work, twelve arylthioamides were synthesized and evaluated for H2S release. All donors released H2S in response to cysteine. Release studies were conducted at relatively high concentrations of donor and thiol (1 mM and 4 mM, respectively), leading to rapid peak release time. The arylthioamides released only small amounts of H2S, exhibiting maximum concentrations between 3–21 μM from 1 mM donor concentrations. The fast rise to a steady state concentration suggests these donors are fast-releasing compounds; however, this quick rise to maximum concentration in solution is quite misleading as the peak H2S concentration represents a small fraction of the total available H2S. Some of the donors released H2S in the absence of a thiol trigger, indicating that they are not exclusively thiol-responsive. Alterations in ring electronics modulated release rates, but not in any clear pattern. One donor, p-hydroxybenzothioamide, was evaluated in a rat aortic ring contraction study and promoted vasodilation at 1 mM in the presence of noradrenaline (NA) without adding exogenous cysteine. Due to the sustained H2S release profile of p-hydroxybenzothioamide and ease of conjugation to other compounds, arylthioamides have been conjugated to a variety of drugs as conjugates, including NSAIDs. In one key example, the development of a naproxen-hydroxybenzothioamide conjugate, ATB-346, was described [58]. The efficacy of ATB-346 as an anticancer drug was investigated, revealing that it induced apoptosis in human melanoma cells in animal studies. ATB-346 has also shown efficacy in reducing gastrointestinal tract injury while maintaining chemopreventative activity against colorectal cancer when compared to naproxen controls [59]. Further studies on ATB-346 are underway by Antibe Therapeutics, where a phase II GI safety study was completed in 2017.
4.1.3.3. S-Aroylthiooximes
S-Aroylthiooximes (SATOs) are a class of thiol-triggered donors developed by Matson and coworkers [60]. SATOs (ArC(O)–S–N=CR2) are synthesized by condensation of an aryl aldehyde or ketone and an S-aroylthiohydroxylamine (SATHA, ArC(O)–S–NH2) in the presence of catalytic trifluoroacetic acid in a reaction analogous to oxime formation. A series of substituted small molecule SATOs were synthesized by varying both the substituent on the SATHA and the aldehyde or ketone. SATOs released H2S in the presence of cysteine and other thiols but did not show release in the presence of amines or water alone, suggesting SATOs possess stability in aqueous media. H2S release was measured with the methylene blue method as well as amperometrically using an H2S-sensitive electrode. A predictable electronics trend correlating the substituent on the SATHA ring with H2S release was observed by fitting release half-lives to a Hammett plot. Under the conditions tested, H2S release half-lives ranged from minutes to hours.
Although thiol-triggered H2S donors have shown efficacy in releasing H2S with well-defined release mechanisms, this subset of donors exhibits poor tissue targeting capabilities due to the ubiquitous nature of thiols inside cells. The design of thiol-triggered H2S donors in the future must incorporate targeting capabilities to mitigate potential off-target effects. Strategies researchers could use to accomplish this important task include the use of targeting peptides, macromolecular scaffolds for increased circulation, and innovative molecular design with the capability for selective thiol reactivity.
4.1.4. Light- and Enzyme-Triggered H2S Donors
Light-triggered prodrugs are useful tools for studies in vitro and hold promise as potential therapeutics due to the bioorthogonality of visible light as a trigger. Visible light possesses a unique advantage over other triggers because it enacts H2S release without major perturbation of native biochemical processes. However, light application only triggers H2S release in areas of the body where sufficient light penetration is possible. After prodrug administration, light of a particular wavelength can trigger release at the site of interest, potentially minimizing any off-target effects through direct spatiotemporal control over release.
Enzymes are of great importance to all living organisms and act on one or more specific biological substrates. In addition, utilizing enzymes as prodrug triggers often allows for specific targeting capabilities to a tissue of interest. Importantly, overexpression of enzymes is commonly symptomatic of many diseases, offering another layer of targeting capability to treat diseases with clever implementation of enzyme-triggered prodrugs. Several classes of light-triggered and enzyme-triggered H2S donors are shown in Figure 6.
Figure 6.
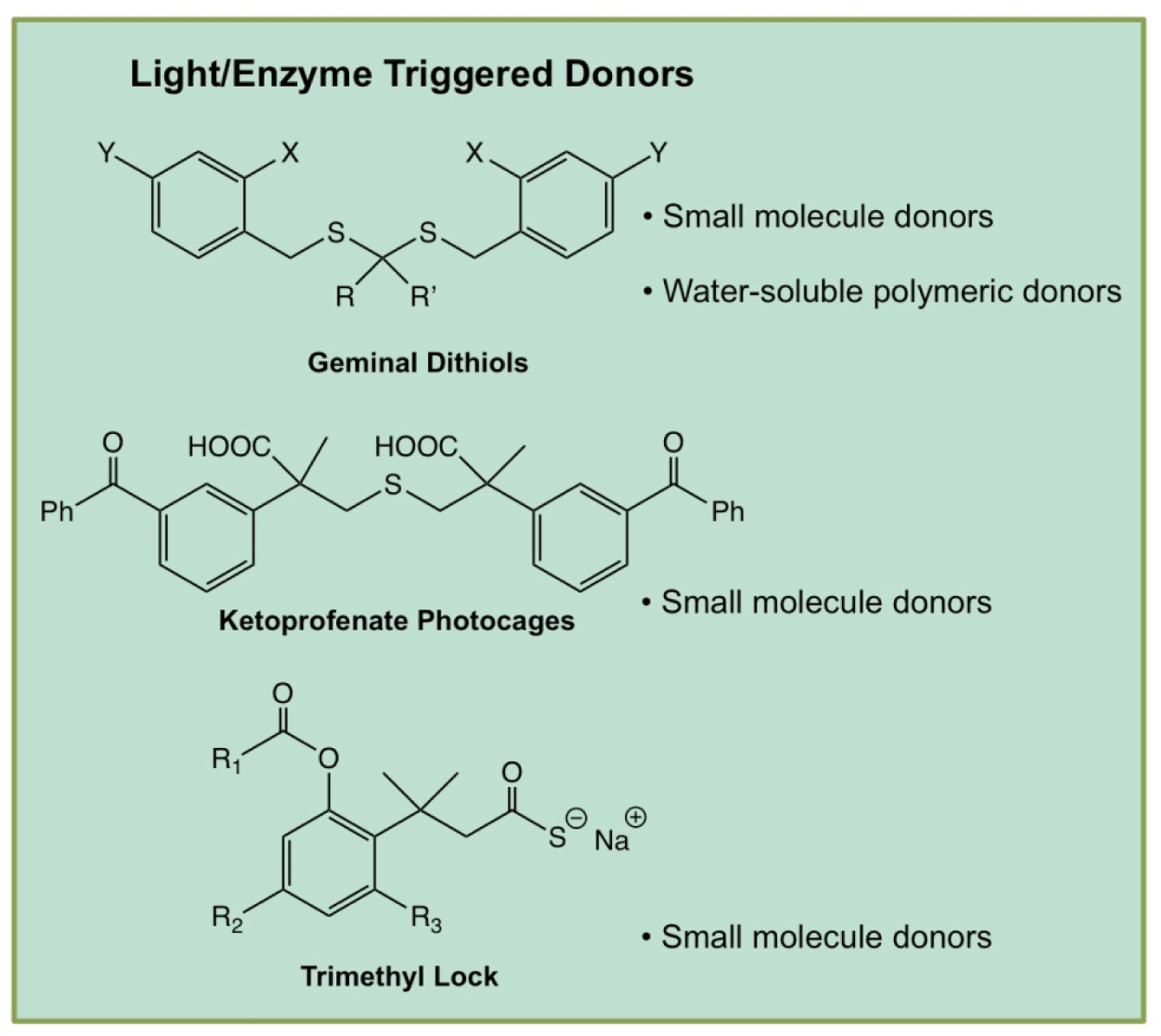
Chemical structures of selected light- and enzyme-triggered H2S donors and descriptions of their use in various engineered delivery systems.
4.1.4.1. Geminal-dithiols
Xian reported one of the first examples of light-triggered H2S donors in the form of geminal dithiols (ArCH2–S–C(CH3)2–S–CH2Ar).[61] The ortho-positioned nitro group photolyzed when irradiated with UV light (365 nm) to produce an unstable geminal dithiol intermediate. This intermediate hydrolyzed to yield H2S rapidly in aqueous media along with a benzyl alcohol byproduct. Xian’s donors released their full payload within ~30 min, with no H2S release being observed in the absence of UV light. Due to the acid-catalyzed hydrolysis mechanisms of gemdithiols, H2S release was accelerated at low pH as compared to slower release at higher pH. Modifications of the bridging groups in some of the compounds yielded trends in release rate, with bulky aromatic bridging groups leading to slower release compared to faster release from alkyl bridging groups. These differences in observed release rates were the result of alterations in sterics and electronics.
4.1.4.2. α-Thioetherketones
Connal and coworkers developed a small molecule prodrug that incorporated a UV-responsive α-thioetherketone linkage with the ability to decompose into a thioaldehyde species and benzophenone, byproducts that are recognized as safe by the FDA [62]. The thioaldehyde generated H2S in the presence of an amine, yielding an imine byproduct. Possessing similar functionality to Connal’s thioetherketones, Singh and coworkers developed light-activated H2S donors using a p-hydroxyphenacyl phototrigger [63]. The methylene blue assay validated efficient H2S release from this family of donors with a maximum peaking concentration of 40 μM from 50 μM donor. Confocal microscopy confirmed Singh’s donors released both H2S and two equivalents of fluorophore in response to UV light (410 nm), providing a means of tracking H2S release in real time. More recently, the authors reported on a water-soluble derivative of this light-triggered donor which reached peak H2S concentrations of 45 μM in 30 min from 100 μM donor concentration, as measured by the methylene blue assay. Additionally, these donors exhibited no cytotoxicity towards HeLa cells at concentrations up to 20 μM both before and after photolysis [64]. This work exemplifies a step towards spatiotemporally controlled H2S release.
4.1.4.3. Enzyme-Triggered H2S Donors
Wang developed the first series of esterase-triggered H2S donors [65]. The release mechanism of these donors hinges upon a well-known lactonization reaction named “trimethyl lock” (TML), which has been widely used to release a variety of drugs [66]. Wang’s TML system first relies on the cleavage of a phenolic ester by an esterase, after which steric repulsion of three methyl groups places the resulting phenol in close proximity to a thioester, promoting lactonization. This cyclization reaction results in release of H2S from the thiocarbonyl group. The authors synthesized several TML derivatives in this study through variations of the phenolic ester moiety and addition or removal of the methyl substituents on the aromatic ring. Because three specifically placed methyl groups are required to drive lactonization after ester cleavage, Wang proposed that removing these substituents would offer a means of slowing H2S release in this system. As hypothesized, derivatives lacking aryl methyl groups exhibited longer release times, ranging from 45–99 min, while prodrugs containing the three methyl groups showed release rates ranging from 13–29 min. A variety of NSAID-TML hybrids were additionally synthesized and evaluated for their efficacy as anti-inflammatory agents, successfully inhibiting secretion of TNF-α.
In another example of enzyme-triggered H2S donors, Chakrapani and coworkers utilized a protected geminal dithiol as an H2S releasing moiety [67]. Instead of employing Xian’s photocleavable functionality, the authors utilized a para-nitro benzyl thioether as a protecting group for the geminal dithiols. The nitro group on the benzyl linker underwent reduction to an amine in the presence of E. coli nitroreductase (NR). The resulting unstable aniline then underwent 1,6-elimination to release the deprotected geminal dithiol, which in turn rapidly hydrolyzed to generate H2S and p-aminobenzyl alcohol as a byproduct. The donors showed H2S release out to 45 min using a fluorescent BODIPY probe, with peak instantaneous H2S concentrations reaching 30 μM in the presence of NR. In vitro studies using E. coli strains showed that the donor rescued the bacteria from oxidative stress caused by administration of common antibiotics, suggesting that H2S production in bacteria may possibly be a mechanism leading to antibiotic resistance. Not much is known about the interactions of H2S in prokaryote organisms, or about the function of H2S in related areas of human physiology such as the microbiome. To elucidate the role of H2S in these symbiotic systems and/or directly in prokaryotes, new varieties of chemical tools will need to be designed, synthesized, and tested.
5. Engineered H2S delivery platforms
While small molecule donors comprise the vast majority of H2S donors reported thus far, they possess several limitations when considering their use in biological systems. For example, many small molecule H2S donors are inherently hydrophobic, which limits solubility in aqueous environments and may result in low bioavailability. Additionally, the reactive nature of H2S donors incurs low stability in biological environments. Macromolecular donor systems offer a means to modulate the chemical, physical, and pharmacokinetic properties of a donor molecule without extensively changing its chemical nature. For example, hydrophobic donor molecules may be incorporated into hydrophilic polymers, either through covalent linkages or non-covalent sequestration, to improve solubility and circulation time. Importantly, the larger size of polymeric prodrugs allows for increased permeability in the leaky vasculature of tumors, providing additional targeting capabilities that small molecule donors usually do not possess [68]. Furthermore, the surrounding polymer structure may shield the incorporated donor motif against unintended degradation by biological nucleophiles. In addition, other materials such as peptides, nanoparticles, and inorganic assemblies may be used to encapsulate, bind, or covalently attach H2S donors, providing modular release platforms with multiple tuning handles. As such, there has been increased interest in macromolecular H2S donor systems; recent reviews provide an in-depth look at their development [69–71]. Here we provide a brief overview of some notable macromolecular H2S donor systems and their contributions as biological tools for delivering H2S. Of course, delivery of NO has many of the same challenges as H2S delivery, and similar engineered systems have been developed for NO delivery, several of which are highlighted graphically in Figure 7. NO delivery systems are discussed in section 7.
Figure 7.
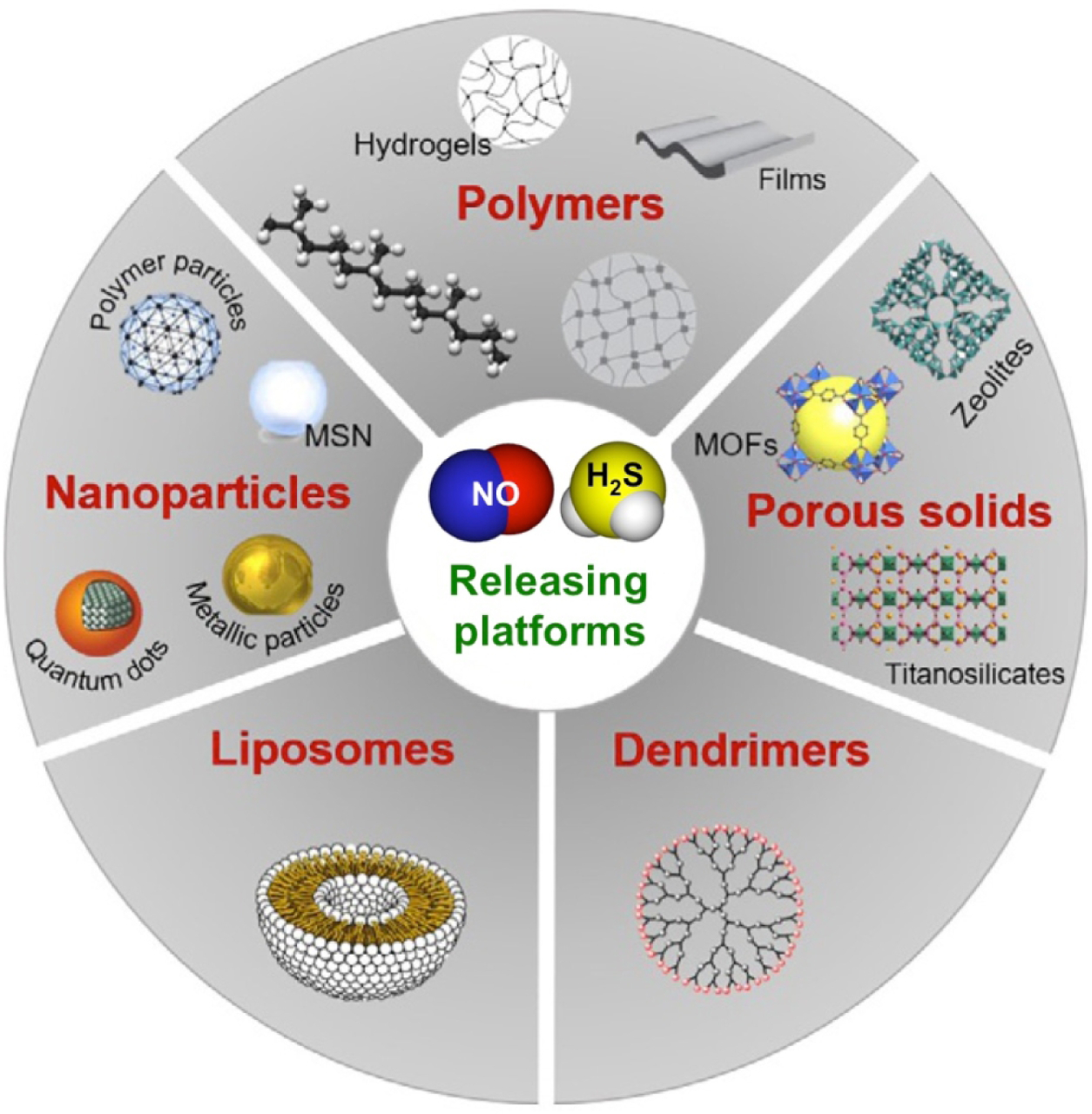
A schematic summary of the different platforms that have been engineered to store and release NO and H2S. Adapted from Therapeutic Application of Nitric Oxide in Cancer and Inflammatory Disorders, by Rosana Vieira Pinto and Moises Luzia Pinto, in Nanoporous Materials: New Generation of Nitric Oxide Donors, Pages 277–304. Copyright (2019), with permission from Elsevier”.
5.1. H2S-releasing polymers
5.1.1. ADT-Polymer conjugates
Perhaps the simplest strategy for creating macromolecular H2S donor systems is the covalent attachment of donor molecules to linear polymers. To this end, a variety of covalently attached macromolecular H2S donors have been developed. 5-(4-Hydroxyphenyl)-3H-1,2-dithiole-3-thione (ADT-OH) is a popular DTT that can be easily appended to polymers via common coupling chemistry. ADT-OH is the active metabolite in anethole trithione (a drug used in the treatment of dry mouth) and possesses bioactivity aside from releasing H2S, as noted above [72]. In addition, it lacks a clear mechanism of release, and the factors that affect release rate are not known. Despite these issues, ADT-OH has been conjugated onto several polymer systems, the first of which was reported by Hasegawa in 2014 [52]. In this work, ADT-OH was conjugated to the chain end of poly(ethylene glycol) (PEG), resulting in PEG-ADT. Conjugation to the polymer enhanced water solubility of the drug, significantly reducing toxicity in vitro in RAW-Blue macrophages. The PEG-ADT conjugates entered RAW cells through endocytosis, while small molecule ADT-OH predominantly diffused across the cell membrane. The authors attributed differences in cytotoxicity between small molecule and polymeric donors to a variance in intracellular distribution due to unique pathways of cellular entry. Furthermore, the slower release of H2S from PEG-ADT relative to ADT-OH resulted in enhanced potentiating effects on LPS-induced inflammation in RAW-blue macrophages. The same group also created polymer micelles containing ADT units [73]. Similar to PEG-ADT linear polymers, the micelles showed less cytotoxicity than small molecule ADT-OH in vitro. Unexpectedly, PEG-b-PADT micelles enhanced the inflammatory response in gardiquimode-stimulated murine macrophages, whereas ADT-OH slightly decreased the inflammatory response under similar conditions. These studies collectively show the ability of polymeric and micellar systems to attenuate certain toxic effects of H2S donors by controlling their entry into cells.
5.1.2. SATO-Polymer conjugates
In another example of macromolecular systems with covalently attached H2S donor molecules, Matson and coworkers developed water-soluble polymers conjugated with H2S releasing-SATO groups through a post-polymerization modification of pendant aldehydes [74]. In later work, the authors prepared amphiphilic block copolymers with SATO-functionalized hydrophobic segments, which readily formed spherical micelles in aqueous solutions [75]. Unlike small molecule and water-soluble polymeric SATO donors, H2S release from SATO-containing micelles was limited by diffusion of triggering cysteine molecules into the hydrophobic micelle core. As a result, SATO-containing micelles experienced drastically slower H2S release kinetics, exhibiting a 9-fold increase in release half-life relative to the small molecule SATO analog. Additionally, the polymer micelles showed greater efficacy in decreasing the viability of HCT116 colon carcinoma cells compared to small molecule H2S donors, highlighting the significance of H2S release rates in biological systems. To further expand this study, the authors developed a method for systematically tuning the H2S release rate from SATO-containing micelles through control of micelle core mobility [76]. In this work, a plasticizing comonomer was incorporated into the core-forming block of SATO-conjugated polymer amphiphiles to produce a series of SATO-containing micelles with varying amounts of micelle core mobility. The H2S release rate varied over 20-fold throughout the series of polymer micelles, signifying that diffusion of triggering cysteine molecules into the micelle core could be precisely controlled by tuning the chemical composition of the core-forming block. Altogether, these studies show the potential for macromolecular H2S donors in overcoming the challenges small molecule donors face in biological systems.
5.1.3. Arylthioamide-Polymer conjugates
In 2016, Davis et al developed amphiphilic block copolymers conjugated with H2S-releasing arylthiobenzamide groups through thionation of pendant benzonitrile groups [77]. The authors controlled the placement of thiobenzamide groups to form amphiphilic block copolymers with H2S-releasing groups in either the corona-forming or core-forming blocks. The polymer amphiphiles both formed micelles in aqueous buffered solutions. Faster H2S release from corona-functionalized versus core-functionalized micelles was attributed to the shielding effect of the micelle core limiting the rate of hydrolysis for thiobenzamide groups sequestered within it. Furthermore, H2S delivered from slow-releasing, core-functionalized polymer micelles produced a slow, sustained increase in cytosolic ERK signaling activity and a smaller but more rapid increase in plasma membrane-localized protein kinase C activity in HEK293 cells. These results demonstrate the potential for modifying specific cellular signaling pathways through release of H2S with spatiotemporal control.
5.1.4. Geminal Dithiol-Polymer conjugates
As mentioned above, light-triggered donors are promising tools for spatiotemporally controlled release of H2S in biological systems. As such, geminal dithiol donors were recently incorporated into macromolecular donor systems. Li reported a polymeric H2S donor system based on conjugation of 2-nitrobenzenemethanethiol to pendant ketones on a water-soluble polymethacrylate [78]. The rate of H2S release from the copolymer exhibited positive correlation with the UV light intensity, while in the absence of irradiation, no release was observed. Additionally, the water-soluble polymeric donor and its H2S-releasing photodegradation product exhibited no cytotoxicity towards human fibroblast cells at concentrations up to 1 mg/mL. While this work lacked a demonstration of light-triggered release at a site of interest in a biological environment, it represents a promising step forward in the development of H2S donors with spatiotemporally controlled release.
5.2. Microparticles and fibers
Macromolecular donors based on polymer assemblies (e.g. micelles or liposomes) and water-soluble polymers can circulate throughout the bloodstream, thereby delivering H2S systemically. In contrast, larger polymer assemblies or supramolecular structures (e.g. microparticles or hydrogels) persist in the area where they are implanted, leading to a localized release of H2S. Localized delivery can be particularly advantageous if the donor is implanted at a site of interest. Additionally, the larger size of these assemblies provides an increased shielding effect for donor moieties sequestered within them, leading to longer or sustained release of H2S.
5.2.1. Microparticles
In 2015, Bowden et al. reported polylactide microparticles system functionalized with thiobenzamide groups as a system for sustained delivery of H2S [79]. Ring-opening copolymerization of l-lactide and a 4-hydroxythiobenzamide-functionalized lactide monomer afforded polymers decorated with pendant thiobenzamide groups along the backbone. From the functionalized polylactides, two sets of spherical microparticles were generated with average diameters of 12 ± 4 and 0.5 ± 0.1 μm. The microparticles experienced 10% weight loss after four weeks at pH 7.4, suggesting the potential for prolonged H2S delivery. Degradation of the microparticles should result in increased exposure of H2S-releasing thiobenzamide groups, therefore a long timescale of degradation should elicit sustained delivery of H2S. However, the authors could not quantitatively measure H2S levels due to low thiobenzamide loadings in the microparticles, slow microparticle degradation, and rapid loss of H2S from aqueous solutions. Despite this limitation, this work demonstrates the potential for microparticle systems that can deliver H2S in a sustained manner.
5.2.2. Electrospun Fibers
Wang and co-workers reported the first electrospun H2S-releasing microfibers in 2015 based on a biodegradable polycaprolactone (PCL) polymer matrix.[80] A solution of PCL at different concentrations (6%, 8%, and 12%) and a thiol activated H2S-donor (NSHD1) were subjected to electrospinning to yield microfibers with diameters ranging from 0.5 to 1.5 μm. An increase in microfiber diameter was observed with increasing PCL concentration. H2S release half-lives for the microfibers were longer than NSHD-1 alone, with measurable H2S levels extending past 24 h. Release rate depended on fiber thickness, with thicker fibers releasing more slowly than thinner ones. Additionally, the H2S-releasing microfibers protected H9C2 cardiomyocytes subjected to oxidative stress by addition of H2O2. They also enhanced proliferation of 3T3 fibroblasts, which is potentially useful for wound healing. In later work, the authors incorporated JK-1, a hydrolysis-triggered H2S donor, into PCL fibers using a similar electrospinning technique [81]. The JK-1-doped PCL fibers showed an extended H2S release profile over the small molecule in solution, which is expected for a hydrolysis-triggered donor. Furthermore, one-time application of PCL-JK1 nanofibrous scaffolds to full-thickness cutaneous wound models in mice showed successful wound regeneration over 20 d with increased healing rates relative to control non-doped PCL fibers.
5.3. Hydrogels
Beyond use in PCL microfibers, the JK-1 H2S donor has also been encapsulated in a hydrogel system for the potential treatment of indivertible disc degeneration (IDD) [82]. Hydrogels are networks of polymer chains, held together by chemical or physical crosslinks, and expanded by water. In this work, JK1 was encapsulated within the porous network of a collagen hydrogel to generate Col-JK1 gel. The gel could be slowly degraded by MMP9, an enzyme that is overexpressed under IDD conditions. Degradation of the collagen gel caused release of JK1 molecules into the low pH environment of the inflamed tissue, which subsequently released H2S. As expected, the shielding effect of the surrounding hydrogel structure resulted in slower H2S release from Col-JK1 relative to the small molecule alone. Additionally, the presence of MMP9 led to increased degradation of the hydrogel structure, and accelerated release kinetics. Furthermore, Col-JK1 successfully inhibited apoptosis in nucleus pulposus cells and prevented degradation of extracellular matrix (ECM), indicating its potential for IDD treatment. While this system marks progress towards enzyme-triggered macromolecular donors, substantial release of H2S in the absence of MMP9 denotes the need for further development of these systems.
The robust SATO-forming reaction has also been leveraged to prepare H2S releasing amphiphilic peptide systems, some of which form hydrogels. For example, an amphiphilic peptide with the sequence IAVEEE was modified by attaching an aryl aldehyde to the N-terminus to form a SATO-based aromatic peptide amphiphile [83]. The SATO-containing peptide amphiphiles self-assembled in aqueous media to form nanofibers that gelled in the presence of calcium, affording hydrogels using 1 wt.% peptide, which exhibited sustained H2S release with a peaking time of ~120 min. In vitro studies using mouse brain endothelial cells showed minimal toxicity of the gels. More recently, a similar SATO-based aromatic peptide amphiphile system was testing for the treating occlusive diseases such as intimal hyperplasia (IH) [84]. The peptide gels inhibited vascular smooth muscle cell (VSMC) proliferation and IH in ex vivo human vein cultures. The peptide gels promoted HUVEC proliferation and transmigration, suggesting H2S donor gels such as these could aid in recovery after vascular intervention. Other peptide-based H2S-releasing SATO systems have also been recently reported [85–89]. We envision that SATO-peptide gels have a promising future for in vivo studies where localized H2S delivery is imperative to enact desired physiological effects.
6. NO and its applications
NO has a very diverse chemical biology and function; for example, it has a role in vascular relaxation [90], has anti-thrombolytic and anti-inflammatory effects [91], is involved in neurotransmission, immune-response facilitation, has antipathogenic response [92–94], has a central role in angiogenesis and is a mediator of the vascular endothelial growth factor (VEGF) [95], it displays antiatherosclerotic properties [96], and has a dichotomous role cancer biology [97]. Many of its actions follow a biphasic dose-response, which ranges from physiological, cytoprotective effects at relatively low concentrations to cytotoxic effects at much higher concentrations, reviewed in [3, 98–101]. In the following sections, we have reviewed some of the means by which NO is utilized to meet a particular clinical need and means by which its delivery is manipulated.
6.1. Classical synthetic NO donors
Before discussing synthetic NO donors, it is worthwhile to note that the FDA approved NO inhalation for treatments of patients with acute respiratory distress syndrome (ARDS) and for newborns with pulmonary hypertension in 1999 [102]. NO inhalation was shown to be beneficial in patients with post-cardiac arrest, extracorporeal membrane oxygenation (ECMO), cardiopulmonary bypass (CPB), sickle cell disease (SCD), acute chest syndrome (ACS), and lung and heart transplants [102]. However, NO inhalation as a means of drug therapy has potentially serious side effects. These include formation of nitrite (NO2−), which can react with the alveolar lining fluid producing nitric acid; formation of peroxynitrite (OONO−) if NO reacts with superoxide anions; reaction with oxyhemoglobin to yield methemoglobin leading to systemic hypoxemia, and others. These side effects limit the use of NO inhalation as a viable therapeutic modality, and caution must be exercised when doing so.
Nitrovasodilators (Fig 8), which include nitroglycerine (glycerol trinitrate), amyl nitrite, isosorbide mono- and dinitrate, erythrityl tetranitrate, and sodium nitroprusside are medications that are taken sublingually, orally, or subcutaneously for the treatment of angina pectoris and other coronary artery diseases. Nitroglycerine has been used effectively for over 100 years, and the other organic medicinal nitrates have been available since the 1930s. Because NO has a dichotomous role in cancer biology, with some reports suggesting that NO possesses anti-tumor properties, while others implicate NO in tumor promotion, in theory, these medications can inhibit or promote the development of cancer. For example, isosorbide mononitrate and dinitrate were shown to inhibit angiogenesis, tumor growth, and metastasis in mice [103], while feeding glyceryl trinitrate to F344 rats induced hepatocellular carcinomas [104]. A novel NO donor, 3-morpholino-sydnonimine (SIN-1, Fig 3) and its analog, a dual-acting NO-releasing and reactive oxygen-scavenging hybrid compound SA-2, were shown to lower elevated intraocular pressure that is associated with degeneration of the optic nerve and loss of retinal ganglion cells [105] by increasing superoxide dismutase enzyme activity. A seminal review on synthetic NO donors is given by Wang et al [106].
Figure 8.
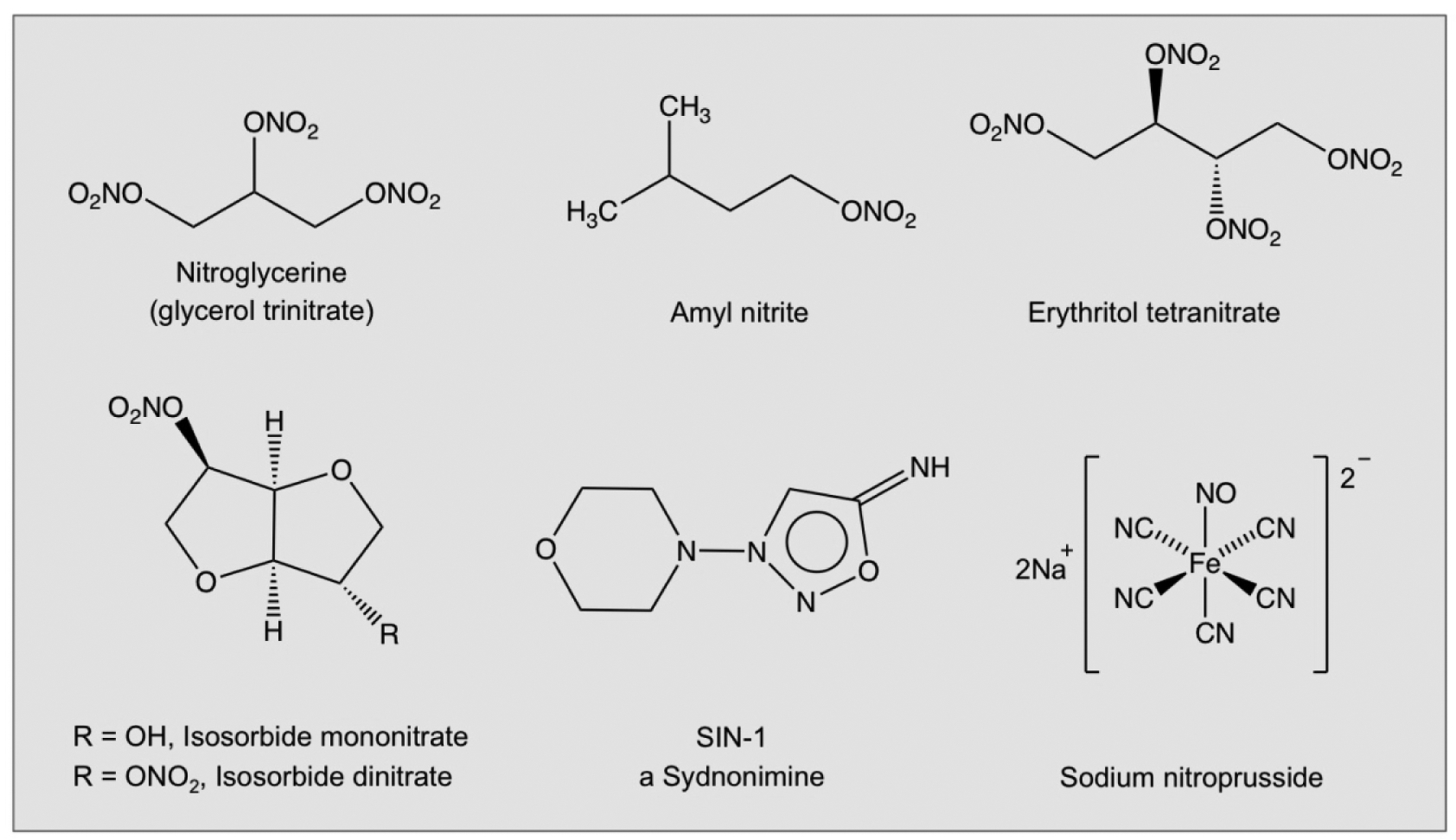
The chemical structures of some nitrovasodilators.
6.2. Classical NO-NSAID conjugates
Considerable epidemiological, interventional, and animal studies have established nonsteroidal anti-inflammatory drugs (NSAIDs) as the prototypical chemopreventive agents against many forms of cancer [3, 25, 100, 101, 107–109]. Chronic NSAID use eventually causes some degree of gastrointestinal (GI) erosions, which eventually may lead to ulcers, with most having cardiovascular (CV) and renal side effects. In order to overcome these potential side effects, nitric oxide-releasing NSAIDs (NO-NSAIDs), also known as COX-inhibiting nitric oxide donors (CINODs) were developed [110, 111]. The rationale for their development was essentially based on the observations that within the GI system, NO can enhance the local mucosal defense mechanisms, offsetting the decreases in prostaglandins (PGs) that come about due to cyclooxygenase (COX) inhibition following chronic NSAID use [112]. NO-NSAIDs have safer GI profiles compared to their corresponding parent NSAID in animals [113–120] and humans [121, 122].
NO-NSAIDs are traditional NSAIDs linked to a NO-releasing group via a chemical spacer. The three key structural components of this class of NO-NSAID are: the traditional NSAID moiety; the spacer, which can be either aliphatic or aromatic; and the NO-releasing group, which initially was a nitrate ester as shown in Fig 9 A and B. In evaluating these NO-NSAIDs in cell culture against a variety cancer cell lines, it was shown that positional isomerism greatly influenced all cell kinetic parameters that influence cellular mass. For example, the ortho and para positional isomers of NO-aspirin were significantly more potent than the meta isomer, and when the spacer was aliphatic the activity was considerably lower [123–125].
Figure 9.
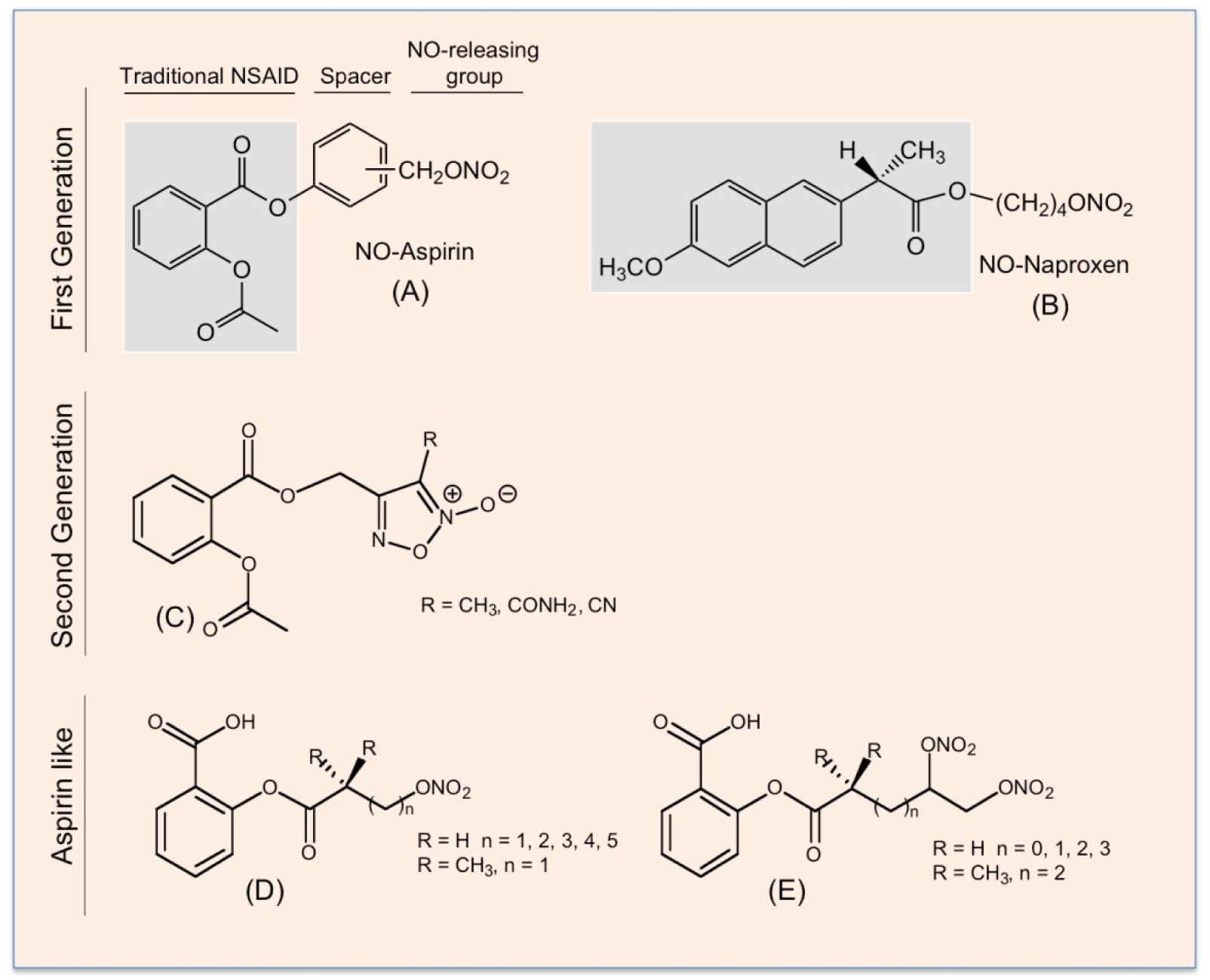
The chemical structures of first- and second-generation NO-NSAIDs and NO-donor “aspirin-like” compounds. The traditional NSAIDs, aspirin (A) and naproxen (B), are shown in the shaded boxes; the spacer molecule links the traditional NSAID to –ONO2, which can release NO. A second generation of NO-releasing aspirin in which a furoxan derivative is the NO donor (C). In the “aspirin-like” compounds, the acetyl group on the aspirin has been replaced by acyl groups containing nitroxy NO-releasing moieties, (D) and (E).
A second generation of NO-releasing aspirins uses furoxan derivatives as NO donors [126], (Fig 9–C). Unlike the nitrate esters which required enzymatic metabolism for NO release [127–129], the furoxan-based NSAID hybrids released NO in the presence of plasma, GSH, or albumin, that is through thiol-triggered mechanisms [130]. Another class of NO-releasing “aspirin-like” compounds have also been described where the acetyl group on the aspirin has been replaced by acyl groups containing nitroxy NO-releasing moieties (Fig 9 D and E). All these compounds have exhibited reduced GI toxicity compared to aspirin, and have strong anti-inflammatory properties [131].
6.3. NO-releasing coxibs
Selective cyclooxygenase-2 inhibitors (Coxibs) such as celecoxib, rofecoxib, and valdecoxib were developed to overcome the GI side effects of traditional nonselective NSAIDs [132], which are attributed to inhibition of COX-1. Overall, this class of compounds has a very good GI safety profile in the short term; however, this appears to be less robust with long-term use. However, total inhibition of COX-2 can lead to an eicosanoid imbalance by down-regulation of PGI2 and unaffected levels of TXA2 leading to increased chances of CV side effects events [133] as confirmed by several large-scale clinical trials, reviewed by [100, 134]. NO is cardioprotective in much the same way as PGI2 and it also inhibits both platelet aggregation and adhesion. Coxibs that release NO do exhibit a safer CV profile as exemplified by VA 694 showing significant improvement of coronary flow and a reduction of endothelial dysfunction [135]. Some examples include NO-celecoxib [136], NO-rofecoxib [137], NO-valdecoxib [138], VA 694 [135] (Fig 10 A–D, respectively); some others such as (pyrazoyl)benzenesulfonamides are derivatives of celecoxib [139] (Fig 10E), and a diazen-1-ium-1,2-diolate [140] (Fig 10F, an example of a NONO-coxib). There are a number of newly described NO-coxibs that are at various stages of preclinical development [141–148]; these compounds have enhanced solubility and appear to be more potent. Therapeutic applications of these prodrugs are diverse.
Figure 10.
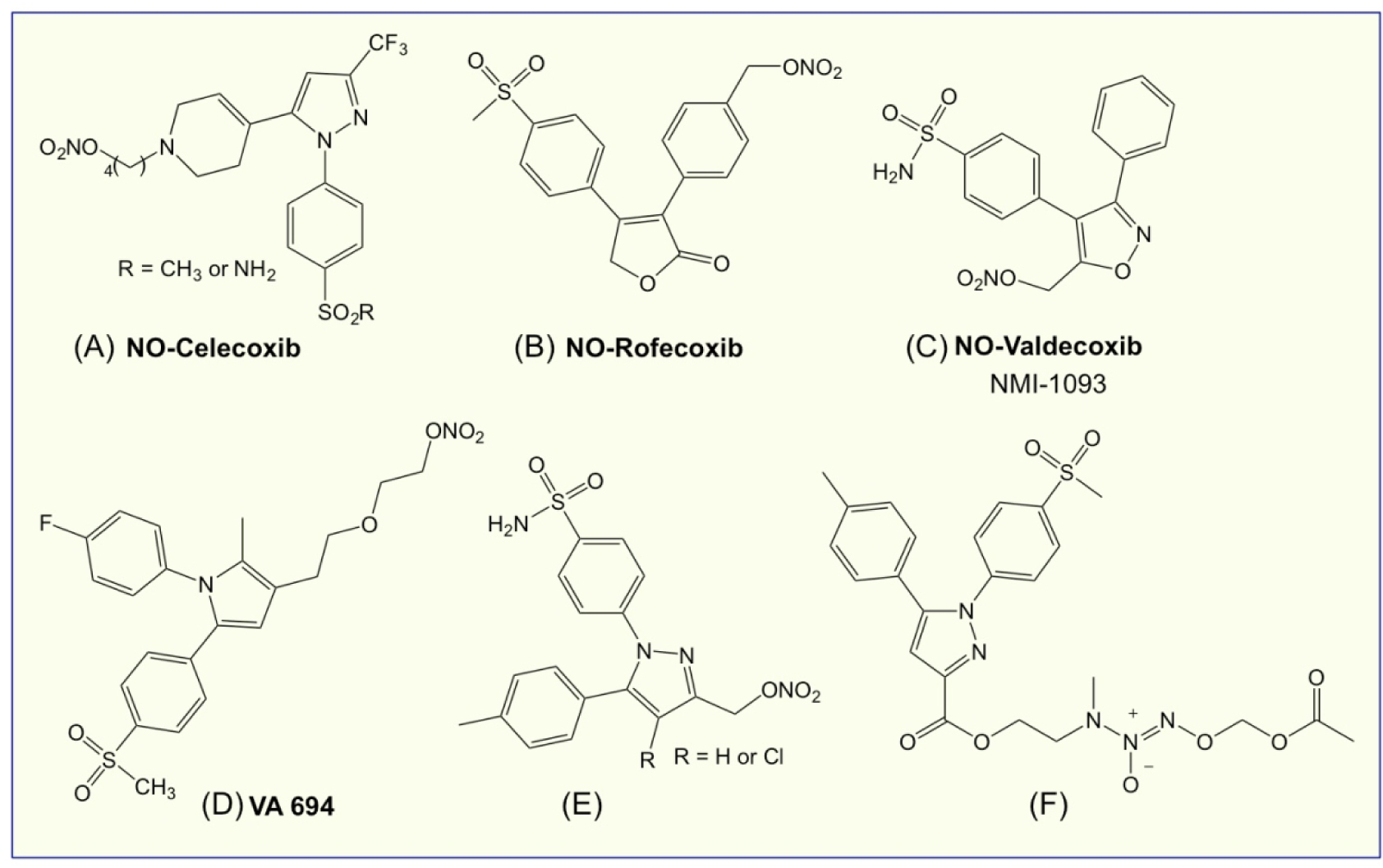
The chemical structures of some NO-coxibs.
6.4. Diazeniumdiolate-based NO-releasing compounds
Diazeniumdiolates (NONOates) are prodrugs that are revealed upon hydrolysis or metabolic activation form the parent NONOate anion, which further decomposes to release up to two moles of NO and the parent amine [149, 150] (Fig 11A). These prodrugs have an array of applications that are largely depend on the O-2 protecting group (‘R’, Fig 11A) and its mechanism of activation. Vinyl protected prodrug V-PYRRO/NO (Fig 11B) is activated by cytochrome P450 to release NO and shows hepatoprotective properties against a variety of toxins [151]. Glutathione (GSH)-activated arylated prodrug JS-K (Fig 11C) has anticancer activity [152–155]. Primary amine diazeniumdiolate prodrug AcOM-IPA/NO (Fig 11D) [156, 157] was reported to release nitroxyl (HNO) on protonation at N-2 (see Fig 11A for numbering); with possible applications in treating heart failure, alcohol abuse, and cancer [142, 158, 159]. Secondary amine diazeniumdiolate ions are protonated at N-3 to release NO [149].
Figure 11.
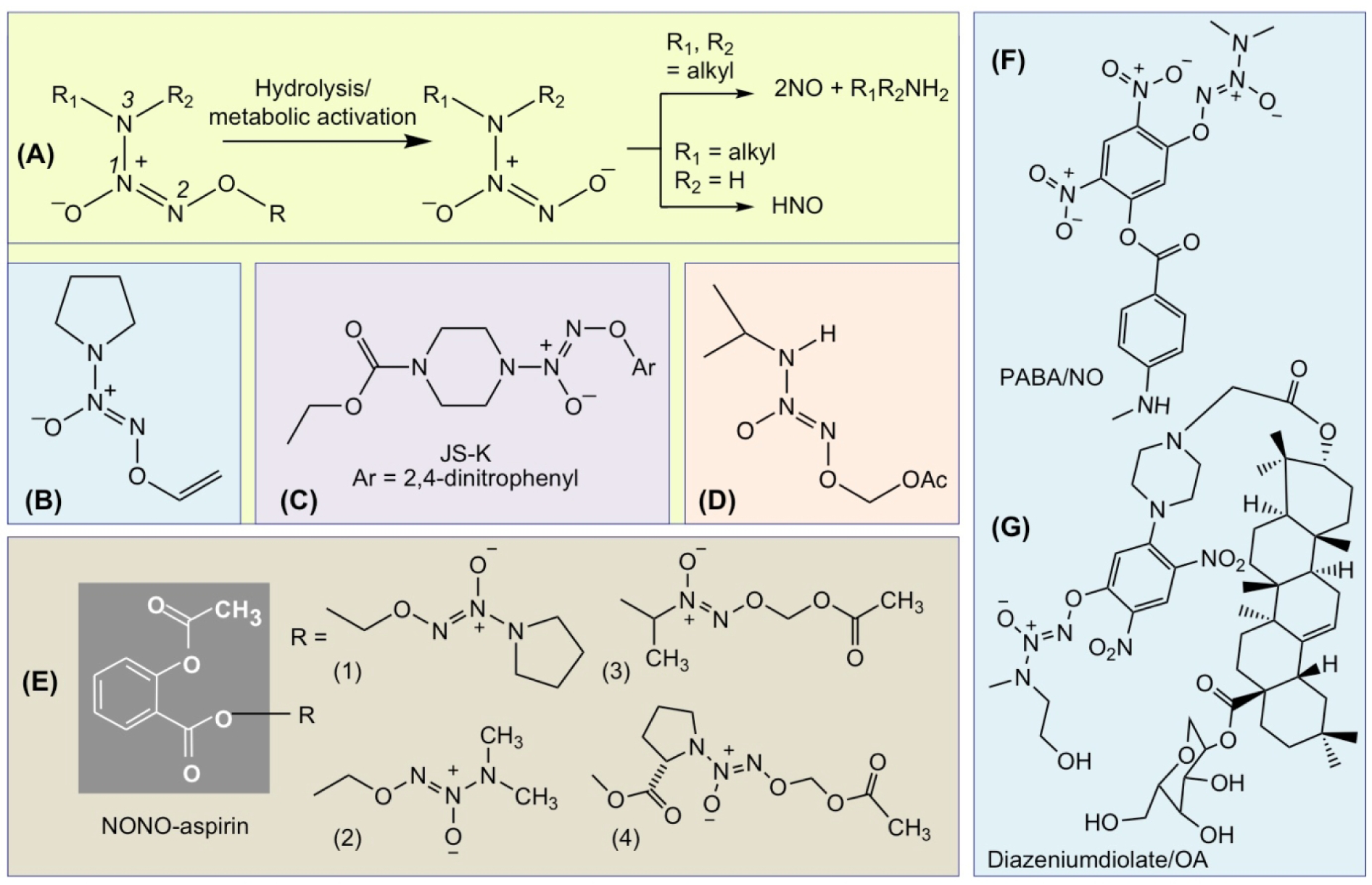
Activation of diazeniumdiolate prodrugs to release NO or HNO (A). Structures of V-PYRRO/NO (B), JS-K (C), and AcOM-IPA/NO (D). The chemical structures of NONO-aspirin (E); PABA/NO (F), and diazeniundiolate/OA hybrid (G).
6.5. NONO-NSAIDs
NONO-NSAIDs are based on linking a N-diazen-1-ium-1,2 diolate functional group to an NSAID and can potentially generate 2 equivalents of NO (Fig 11E). NONO-NSAIDs do not require redox activation before NO is released [160], whereas nitrate esters require a three-electron reduction [161]. The first agent reported in this class had a NONOate (O2-unsubstituted N-diazen-1-ium-1,2-diolate) attached via a one-carbon methylene spacer to the carboxylic acid group of a traditional NSAID (aspirin, ibuprofen, indomethacin) [162] (Fig 11A, R = (1) or (2)). The next series of NONO-NSAIDs (aspirin, ibuprofen, indomethacin) possessed an O2-acetoxymethyl-1-[N-(2-hydroxyethyl)-N-methylamino]diazen-1-ium-1,2-diolate moiety as the NO donor (2-HEMA/NO) [160] (Fig 11A, R = 3). Because in their synthesis a secondary dialkyamine was used, this led to a number of possible new NONO-NSAIDs. Close inspection revealed that upon hydrolysis it was possible to release one equiv of the corresponding nitrosoamine, a biologically toxic compound. To overcome this concern, a second-generation of O2-acetoxymethyl-protected (e.g., PROLI/NO) releasing NONO-NSAIDs was developed where a diazeniumdiolate ion obtained from an amine such L-proline, was used, the N-nitroso derivative of which is nontoxic [163] (Fig 11A, R = 4). As a class, all NONO-NSAIDs are reported to be devoid of GI toxicity, with no inhibitory effects on either COX-1 or COX-2, but have potent anti-inflammatory properties, consistent with acting as prodrugs requiring metabolic activation to release the parent NSAID.
6.6. HNO-NSAIDs
Decomposition of diazeniumdiolates can lead to formation of nitroxyl (HNO) and/or NO [149]. Potential actions of HNO are in overcoming heart failure [159], preconditioning against myocardial infarction [164], and treating alcohol abuse [158]. Using Angeli’s salt (Na2N2O3) to generate HNO, the first anticancer activity of HNO was reported in 2008 [165]. Recently two new NONO-NSAIDs were prepared by derivatizing both a primary and secondary amine diazeniumdiolate with aspirin to produce O2-(acetylsalicyloyloxymethyl)-1-(N-isopropylamino)-diazen-1-ium-1,2-diolate (IPA/NO-aspirin) and O2-(acetylsalicyloyloxymethyl)-1-(N,N-diethylamino)-diazen-1-ium-1,2-diolate (DEA/NO-aspirin) [166] (Fig 11A). Both have shown enhanced GI safety profiles, strong anti-inflammatory properties, and significantly enhanced cytotoxcity compared to either aspirin or the parent diazeniumdiolate toward nonsmall cell lung carcinoma cells (A549), but were not toxic toward endothelial cells (HUVECs) suggesting cancer-specific sensitivity.
6.7. JS-K and PABA/NO
The diazeniumdiolate prodrugs, JSK [O2-(2,4-dinitrophenyl)1-[(4-thoxycarbonyl)piperazin-1-yl]diazen-1-ium-1,2-diolate] (Fig 11C) and PABA/NO [O2-[2,4-dinitro-5-(N-methyl-N-4-carboxyphenylamino) phenyl] 1-N,N-dimethylamino)diazen-1-ium-1,2-diolate] (Fig 11F) were designed to be activated as anticancer agents by glutathione-S-transferase (GST)-induced release of NO [167]. The rationale for this was based on the observation that GST (specifically GST-π, a key phase II detoxification enzyme, is frequently over-expressed in cancer tissue [168, 169]. JS-K [155, 168, 170–173], and PABA/NO [174–176] have shown promise as anti-cancer agents. In order to improve the selectivity for cancer cells, hybrids of O2-(2,4-dinitrophenyl)diazeniumdiolates and oleanolic acid (OA) have been prepared [177], (Fig 11G). The rationale for these hybrids was based in part on the observation that OA imparts additional hepatic selectivity and a synergetic biological profile to the GSTπ-activated moiety [178], reviewed in [101].
6.8. RRx-001: an NO modulatory anticancer agent
RRx-001 (Fig 12B) also known as ABDNAZ (1-bromoacetyl-3,3-dinitroazetidine) is a novel aerospace-derived compound under active investigation as a chemo-, immuno-, and radiosensitizer. RRx-001 demonstrated antitumor activity and minimal toxicity in phase II clinical trials and has received clearance from the FDA and the EMA for phase III, multicenter studies in subjects with relapsed/refractory solid tumors (Clinical Trial registration: NCT03699956) [179–181]. This compound contains a unique, highly energetic organic nitro functional group called a gem dinitroazetidine that has not been used to date for medical and pharmaceutical applications. In an aerospace setting, compounds containing this energetic functionality, such as 1,3,3 trinitroazetidine, are designed to fragment explosively to propel rockets [182]. Modification of this structure by removing one of the nitro groups and substituting it with a bromoacetate group resulted in RRx-001, a nonexplosive that may be used to treat cancer [183], reviewed in [101]. RRx-001differs from other NO-donating compounds in that the molecule induces local, endogenous, and biphasic production or release of NO, rather than fragmenting to release NO systemically. This activity is closely linked to the metabolism of RRx-001; on infusion, the compound rapidly, irreversibly, and selectively binds to hemoglobin at a key NO binding site [184], and with glutathione [185, 186] in directly increasing oxidative stress [187]. While the RRx-001 glutathione adduct is rapidly excreted, RRx-001-bound hemoglobin remains in circulation for the duration of the lifetime of the red blood cell [188].
Figure 12.
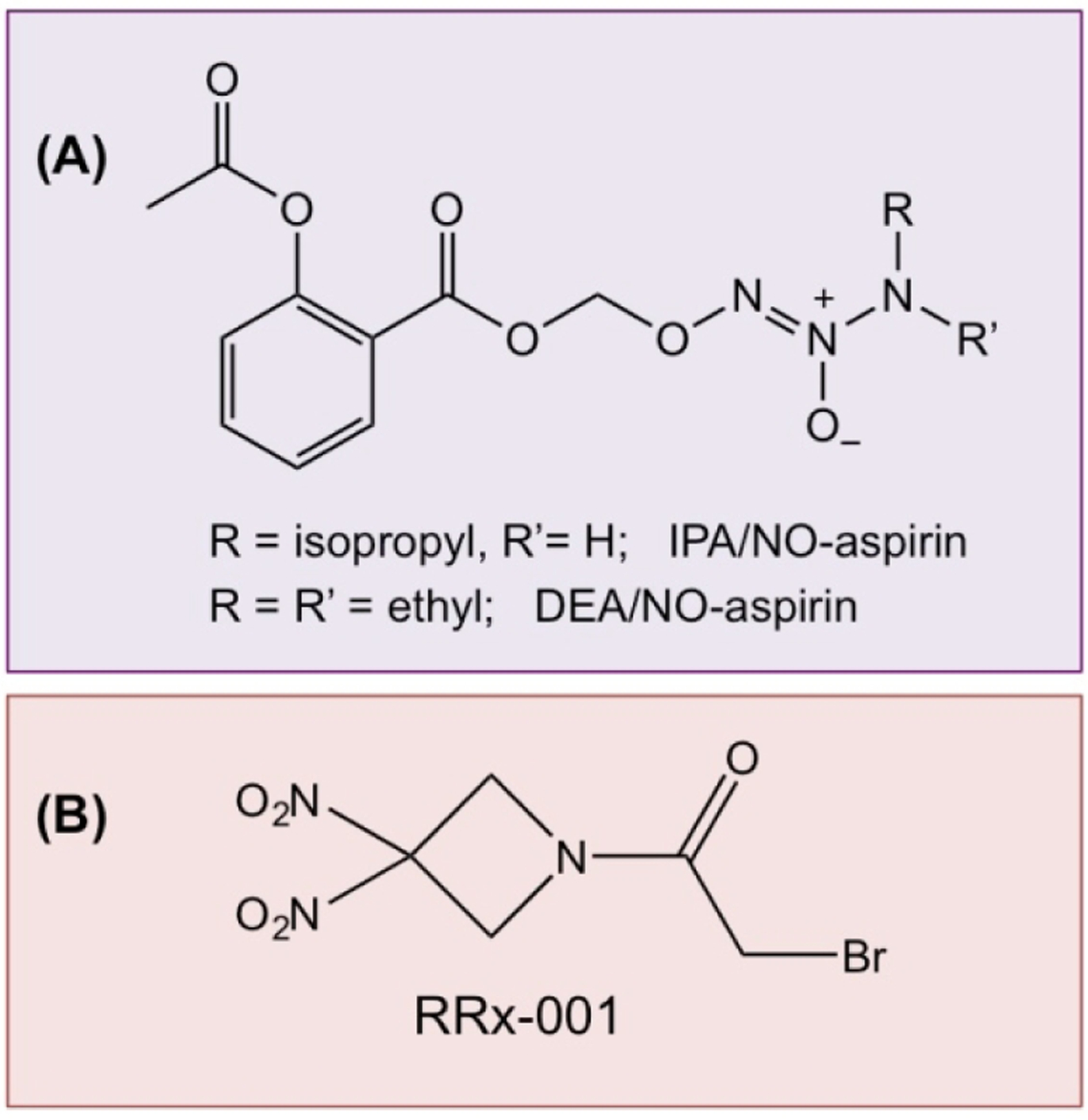
The chemical structures of IPA/NO-aspirin and DEA/NO-aspirin (A); RRx-001 (B).
6.9. Light triggered NO donors
Photodynamic therapy (PDT) is a novel approach for treatment of various pathologies including cancer and infectious diseases. The therapy is based on the interaction of a photosensitizer (PS), light and oxygen. None of these is individually toxic, but the combination produces a photochemical reaction that leads to the production of ROS and/or singlet oxygen (1O2) [189]. Cell death then occurs by apoptosis, autophagy or necrosis and the outcome depends on the PDT dose and localization of the PS [190, 191]. PDT is a 2-stage procedure. After the administration of a light-sensitive PS, tumor loci are irradiated with a light of appropriate wavelength that can be delivered to virtually any organ in the body by means of flexible fiber-optic devices [191]. Most PSs used in cancer therapy belong to the protoporphyrin family and are based on a tetrapyrrole structure [192]. An ideal sensitizer must have an absorption peak between 600 and 800 nm (red to deep red), higher wavelengths do not have enough penetration and do not excite oxygen to its singlet state, thus reducing generation of ROS that are required for cytotoxic effects [193].
The NO influence the response of the tumor cells to PDT [194, 195]. Activated PS can induce the production of NO by increasing the expression of constitutive NOS [196] or that of iNOS [197], and as discussed in this review and elsewhere [3, 100] NO has a dual role in cancer biology.
6.9.1. NO donors and PDT
This is an area of intense interest for medicinal chemists and its potential applications for cancer therapeutics. Many groups have synthesized NO donors to promote PDT-mediated anti-tumor cytotoxicity. Examples of photochemical NO releasing compounds incorporating various transition metals are, Roussin’s red salt anion [Fe2S2(NO)42−], and a ruthenium-nitrosyl complex where the NO is caged by coordination to the transition metal center; and a Cr(III) nitrito complex where the NO is caged by bonding to another oxygen, releasing NO by homolytic cleavage of the MO–NO bond, the chemistry of these compounds has been reviewed by Ford [198]. Another PS of interest is a silicon-phthalocyanine compound (Pc4) [196]. Recently, a series of photo responsive N-nitrosoaniline based NO donor polymers [amphiphilic diblock copolymers, PEO45-b-PoNBN25 (BP1), PEO45-b-PpNBN30 (BP2), and PEO45-b-PBN46 (BP3)] were synthesized by nitrosation of 4-aminobenzyl alcohol-based precursors [199]. Using appropriate probes, photo-mediated NO release from BP1 vesicles was confirmed in HeLa cells. Furthermore, BP1 was shown to be effective in a corneal wound-healing model.
6.9.2. Clinical applications of PDT
Ocular infection due to microbial contamination is one of the main risks associated with the wearing of contact lenses. Recently an NO-releasing soft contact lens that releases NO under daylight exposure was reported to be safe and have good activity against of Staphylococcus aureus [200]. PDT has been effectively used to treat Bowen’s Disease (BD), also known as squamous cell carcinoma in situ (SCCis), which most often is caused by exposure to ultraviolet light but may also occur as a result of Human Papillomavirus, arsenic exposure, or chronic radiation dermatitis [201]. Studies have shown that PDT is equally or more effective than conventional therapies such as 5-fluorouracil and cryotherapy in treating BD [202]. PDT has been used to treat cancers of the head and neck, prostate, bladder, lung, skin, gastrointestinal tumors, intraperitoneal malignancies, and others have, reviewed in [191].
6.10. Dual NO-H2S donors
Recently, a new class of anti-inflammatory pharmaceuticals were described in which an NO-releasing and an H2S-donating moiety were covalently attached to an NSAID backbone, thus releasing both NO and H2S; these chimeras have been termed NOSH-NSAIDs [203, 204] (Fig 13). The rationale for their development was based on the chemistry of NO and H2S, and the structural components of NO-NSAIDs and H2S-NSAIDs thus postulating that a hybrid capable of releasing both of these gasotransmeters might be more potent and effective than either one alone [203]. A number of these compounds have been reported; nitrate was used for NO release and this was attached to the parent NSAID through an aliphatic spacer, while one of the following moieties, 5-(4-hydroxyphenyl)-3H-1, 2-dithiole-3-thione (ADT-OH), or 4-hydroxy benzothiazamide (TBZ) or lipoic acid were used for H2S release. NOSH-NSAIDs displayed greater GI safety profiles compared to their parent counterparts and displayed strong antioxidant properties [205–207]. NOSH-aspirin, (NBS-1120) exhibited strong anti-inflammatory [203, 206], anti-pyretic, analgesic, and antiplatelet properties similar to its parent compound, aspirin [206]. NOSH-aspirin inhibited the growth of eleven different human cancer cell lines of six different histological subtypes with IC50s that were in the low to mid nanomolar ranges [203]. Using HT-29 colon cancer cells as a model, this growth inhibition was as a result of reductions in cell proliferation, G0/G1 cell cycle arrest, leading to increased apoptosis [208]. The efficacy of NOSH-aspirin at different concentrations was also compared to that of aspirin using an in vivo xenograft mouse model of colon cancer chemoprevention [206]. NOSH-aspirin dose-dependently inhibited tumor growth, and tumor mass and was at least 5-fold more potent than aspirin. Of note, NOSH-aspirin was also efficacious against established tumors in a xenograft model of colon cancer [208]. Clearly, as a chemo-preventive and chemotherapeutic agent, NOSH-aspirin is superior to aspirin both in terms of efficacy and safety. Qualitatively similar results have been reported for both NOSH-naproxen [207] and NOSH-sulindac [205]. With regards to efficacy of NOSH-naproxen in a xenograft model of colon cancer, it is important to note that while treatment of animals with NOSH-naproxen significantly reduced tumor growth and tumor mass with no overt sign of GI toxicity, naproxen-treated mice died due to GI bleeding [207]. Interestingly, when examining cell growth inhibition in the presence of the three individual components of NOSH aspirin (ADT-OH, a small molecule NO donor, and aspirin), the cocktail had an IC50 of 450 μM, a 9,000-fold difference compared with that of the intact NOSH-aspirin. These results indicate that cancer cell growth inhibition is influenced by more than simply delivering DTT and NO concurrently with aspirin, but the reasons for this synergy, although largely unknown, may have to do with generation of more potent entities such as persulfides.
Figure 13.
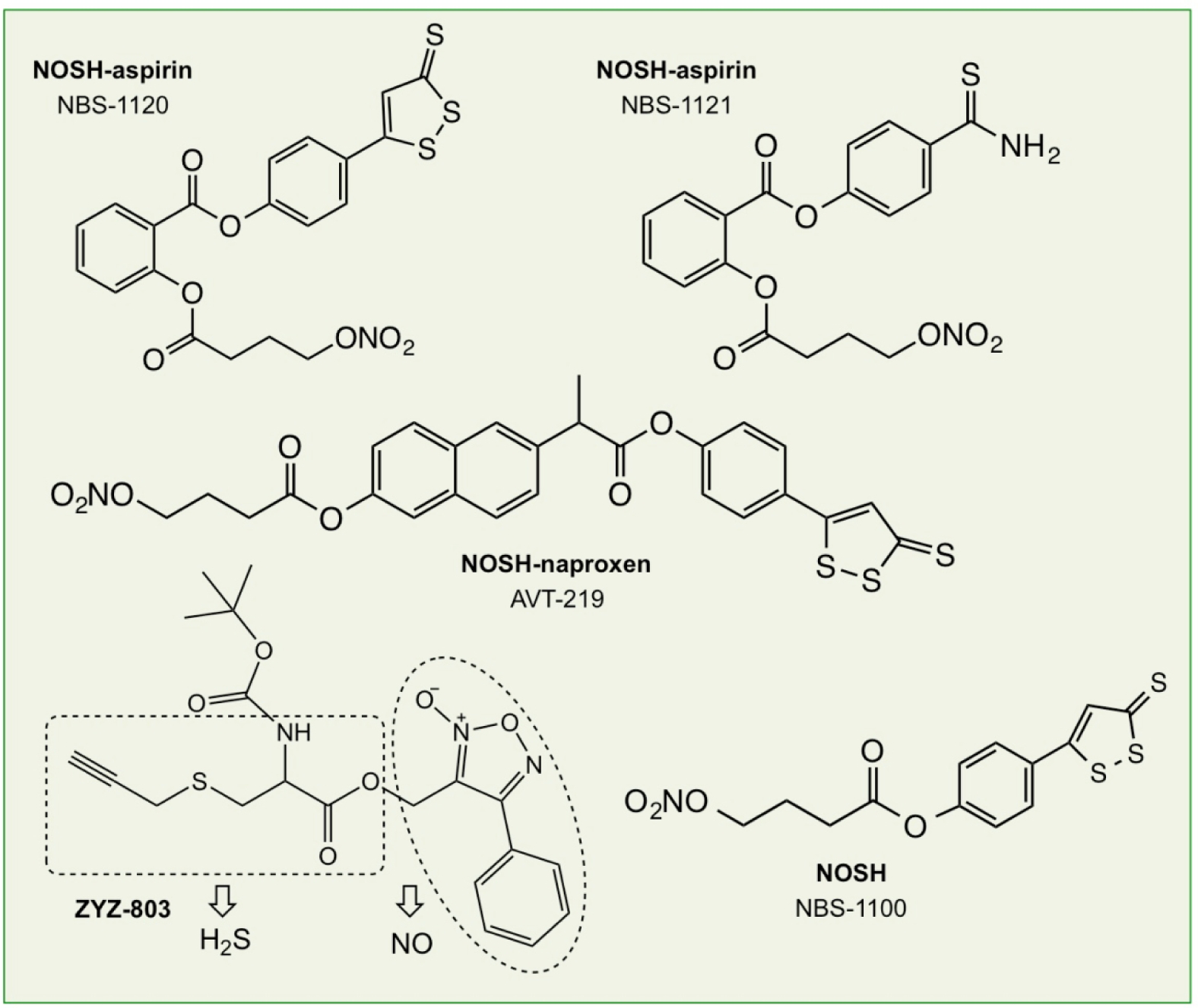
Structural components of NOSH-aspirin (NBS-1120 and NBS-1121), NOSH-naproxen (AVT-219), NOSH (NBS1100), and ZYZ-803.
Apart from being active against cancer and having potent anti-inflammatory properties, NOSH-aspirin (NBS-1120) and NOSH (NBS-1100, a molecule where butyl nitrate and ADT-OH are directly linked together, Fig 13) have protective effects in drought-stressed Medicago sativa L. Plants [209]. Plants were pre-treated with NOSH or NOSH-aspirin by foliar spraying and then exposed to moderate water deficit, while NO and H2S inhibitors (cPTIO and HT, respectively) were also employed. Phenotypic and physiological data showed that pre-treatment with the NOSH chimeras induced acclimation to subsequent drought stress and improved recovery following rewatering. This was accompanied by modified reactive oxygen and nitrogen species signaling and metabolism, as well as attenuation of cellular damage as evidenced by altered lipid peroxidation and proline accumulation levels. Furthermore, real-time RT-qPCR analysis revealed the differential regulation of multiple defense-related transcripts including enzymatic antioxidants [209].
Another H2S-NO hybrid molecule (ZYZ-803, Fig 13) has been shown to have efficacy in isoprenaline-induced heart failure [210]. The cardioprotective effect of ZYZ-803 was more potent than that of the H2S and/or NO donor alone. ZYZ-803 increased expression of CSE and eNOS activity. Blocking CSE and/or eNOS suppressed ZYZ-803-induced H2S and NO production and cardioprotection. ZYZ-803 increased VEGF and cGMP levels and also upregulated the endogenous antioxidants glutathione peroxidase (GPx) and heme oxygenase1 (HO-1). ZYZ-803 also induced angiogenesis in human umbilical vein endothelial cells (HUVECs) with STAT3 as well as CAMKII in mediating this effect [211].
7. Engineered NO delivery platforms
Delivering NO in a temporally and spatially controlled fashion can be challenging because of its potential release from the conjugated system in the first few minutes after administration. Of concern is also that some of the metabolic decomposition products can potentially be toxic [212]. To overcome these limitations and others, many different delivery platforms have been developed; some include polymers, nanoparticles, liposomes, dendrimers, and porous materials [213–219] (Figure 7).
7.1. Polymers
Bio-polymeric scaffolds should be non-toxic, biodegradable, and biocompatible. They can be either naturally occurring, such as sugar-based materials including chitosan, dextran, and hyaluronic acid [220–224], or synthetic polymers such as dendrimers. Polymer-based materials that are used for NO delivery could be used as coatings, films, or ointments. These polymers may contain RSNOs, nitrosamines, NONOates, or other NO-releasing entities, which overcome the limitations of their therapeutic use. This mode of NO delivery allows for controlled kinetic release, which may last for days [225] or even weeks [226]. The most common preparation strategy is based on dispersing thiol- or amine containing compounds in polymers, followed by exposure to NO gas to convert the parent polymer materials into NO donors [227]. Matrix structures have been used for functionalization with NO-releasing molecular systems because of their great biocompatibility with living tissues [228, 229].
Dendrimers are globular structures that consist of a central core surrounded by a highly branched corona with reactive surface groups; they are a particularly attractive class of synthetic polymers because of their multivalent surface and well-defined polymeric structure [230]. Because of their exterior functional groups encompassing a steric environment and hydrophobicity, multiple derivative structures are possible with varying NO payloads [231].
7.2. Nanomaterials
Due to their small size, nanomaterials have enhanced interaction and tissue penetration. Mesoporous silica nanoparticles (MSN) are biocompatible systems that have been used as NO carriers with a number of NO-releasing compounds including diazeniumdiolates [232]. Gold [233–235], silver [236, 237], and iron oxide nanoparticles [238–240], as well as quantum dots [241, 242], are other NO nanotechnology platforms that may be used for both diagnostic and therapeutic applications [227]. The preparation of these complexes is based on their surface functionalization with diazeniumdiolate or S-nitrosothiol groups [235, 238, 243]. Hollow polymeric nanoparticles made of synthetic polymers (e.g., polymethacrylate and polydopamine) have unique properties such as low density, optical scattering, and good flow capacity [244]. The large surface area of hollow polymeric nanoparticles facilitates NO donor functionalization on both the inner and outer surfaces, leading to larger NO payloads [245].
7.3. Liposomes
Liposomes are spherical vesicles composed of an inner aqueous core and a phospholipid bilayer outer shell, which can be either synthetic or natural, with an overall structure that mimics natural cell membranes. Both NO gas and NO donors (e.g., N-diazeniumdiolates, metal nitrosyls, and organic nitrites) have been encapsulated into liposomes to achieve controlled NO release [245–247]. The main techniques employed in forming liposomes are thin-film hydration, solvent injection, reverse-phase evaporation, membrane extrusion, and microfluidic technology [248, 249]. NO-releasing liposomes have great potential to be used for anticancer therapy [250, 251].
7.4. Porous materials
Porous materials for NO storage/release cover a large variety of structures that can be organic, inorganic, and inorganic-organic hybrids with varying pore sizes, percentage of porosity, and the presence (or absence) of interconnectivity between them [227, 252]. These include zeolites, titanosilicates, clays, and MOFs (metal organic frameworks). Chemisorption or physisorption is used to store NO with a wide range of metal ions within the pores, and NO is then released when water replaces NO on the metal centers and diffuses out of the porous structure. This methodology provides a highly efficient packing of NO within the solid and provides for controlled delivery to target tissues [227, 253, 254].
Zeolites are highly crystalline aluminosilicate microporous insoluble materials with a rigid three-dimensional open framework that may be of natural or synthetic origin [227]. Exposure to NO gas can result in both reversible and irreversible NO adsorptions, giving rise to different release kinetics. Also, the affinity for NO of the transition metals used in the various zeolites greatly affects the extent to which NO is adsorbed and released. Studies with zeolites Linde type A and Faujasite with Cu2+, Co2+, Ni2+, Mn2+, Zn2+ showed that Co had the highest storing capacity [255]. Topical application of NO-releasing zeolites (0.02 mL 33%, wt/wt) induced local vasodilatation and no significant inflammatory response [256]. Application of a topical ointment containing NO-loaded zinc-exchanged zeolites to wounds three times per week for 20 days in Zucker obese rats resulted in enhanced wound healing [257]. Furthermore, in vitro microbial studies showed activity against Escherichia coli, Acinetobacter baumannii, Staphylococcus epidermidis, methicillin-resistant S. aureus (MRSA), and Candida albicans fungus. An NO-releasing Zn2+-exchanged zeolite at a 50 wt.% composition in a polytetrafluoroethylene polymer showed activity against both Gram-negative Pseudomonas aeruginosa and Gram-positive methicillin-sensitive and methicillin-resistant S. aureus and Clostridium difficile [258].
Titanosilicates are microporous zeolite-type silicates possessing framework of unsaturated transition-metal centers; examples include ETS-4 [Na9Si12Ti5O38(OH)·xH2O], a titanosilicate that displays excellent NO adsorption capacity and slow releasing kinetics [254]. The use of this mode of NO delivery as applicable to biological systems needs to be investigated further.
Clays are already being used in the therapeutic field either as active substances or as excipients to other drugs [259]. They are very amenable due to their high mechanical, thermal, and chemical stability; they have regular structures and appreciable surface areas and thus have good adsorption and penetrability properties. NO-releasing mineral clays such as sepiolite and montmorillonite (MMT), synthetic clays (smectite clays with cobalt ions), and organo-clays (natural clays modified with l-histidine) have been developed and their activity evaluated in HeLa cells [260–262].
MOFs are porous hybrid inorganic-organic crystalline materials, built on metal ion oxoclusters connected by organic ligands, in a quasi-infinite array [263]. These are relatively new materials with varying toxicities due to the metals used and the amount of NO released. However, as a class, these are interesting and much more work is needed in order to make them viable/suitable for medical applications. A detailed review of these compounds is covered elsewhere [227, 263].
7.5. Hydrogels
As noted in section 5.3, gels are non-fluid polymer or colloidal networks that are expanded by a fluid. If the expanding fluid is water, the gel is then called a hydrogel. Hydrogels can absorb more than 90% of their dry weight in water, while chemical and physical crosslinkingof the polymeric chains make them insoluble in water [229]. NO-releasing hydrogels have been prepared that incorporate different NO-releasing moieties, such as S-nitrosothiols, S-nitrosoglutathione, diazeniumdiolates, sodium nitrite, and others; and varying polymeric matrices, for example pHEMA coated with polyurethane, pluronic F127, PVA functionalized with –SNO groups, hydroxyethyl cellulose etc., have many therapeutic applications ranging from bactericidal, topical vasodilation, and wound healing [264–267] (for detailed review see [229]). There are also NO donors that release NO exclusively under irradiation, and several photoactive metal–nitrosyl complexes using a polymerized matrix of poly(2-hydroxyethyl methacrylate) (pHEMA) have been developed [266].
8. Conclusions
Significant progress has been made in the fields of NO and H2S donor chemistry. Continued innovation from synthetic chemists will be a major factor in driving the NO~H2S research forward in the coming years, with an eye toward building NO and H2S donors that are clinically relevant therapeutics. Continued development of various delivery platforms for targeted therapy is of significant importance, with an additional focus towards further improving and developing platforms that are biocompatible in many contexts and degrade to form non-toxic metabolites. In this regard, the use of polymeric platforms is attractive due to low productions costs and ease by which different synthetic moieties can be incorporated. For example, polymeric particles can be functionalized with a poly(ethylene glycol) corona, giving rise to “stealth” properties which significantly increase circulation time and improved accumulation in tumors by the enhanced permeability and retention (EPR) effect [219]. While there are a considerable number of engineered platforms for the delivery of exogenous NO and H2S, not much has been done on materials that may respond to these endogenous gasotransmitters. Thus NO and H2S capturing materials may provide an unexplored area for clinical investigation.
PDT was the first drug-device combination approved by the FDA almost 3 decades ago, but it is underutilized clinically. The highly localized nature of PDT is one of its current limitations, because the treatment is ineffective against metastatic lesions, which are the most frequent cause of death in cancer patients. Ongoing research is focused on finding optimal PDT conditions to induce systemic immunity that may address this significant shortcoming [191]. For all of these efforts to flourish, it is important for chemists, pharmacologists, biologists, and physicians to work together. An era of precision medicine to improve individual outcomes is not too far in the future.
Acknowledgements
We are grateful to the National Institutes of Health (R01GM123508) for support of our work in this area. JBM also acknowledges the National Science Foundation (DMR-1454754) for supporting works on H2S-releasing peptide hydrogels.
Abbreviations:
- 3-MST
3-Mercaptopyruvate sulfurtransferase
- ADT-OH
5-(4-Hydroxyphenyl)-3H-1,2-dithiole-3-thione
- ARDS
Acute respiratory distress syndrome
- BODIPY
Boron-dipyrromethene
- CaMKII
Ca2+/CaM-dependent protein kinase II
- CAT
Cysteine aminotransferase
- CBS
Cystathionine β-synthase
- COX
Cyclooxygenase
- cGMP
Cyclic guanosine monophosphate
- CSE
Cystathionine γ-lyase
- DTT
1,2-Dithiole-3-thiones
- eNOS
Endothelial nitric oxide synthase
- GI
Gastrointestinal
- H2S
Hydrogen sulfide
- LPS
Lipopolysaccharides
- LR
Lawesson’s Reagent
- NF-κB
Nuclear factor kappa-light-chain-enhancer of activated B cells
- nNOS
Neuronal nitric oxide synthase
- NA
Noradrenaline
- NO
Nitric oxide
- NOS
Nitric oxide synthase
- NR
Nitroreductase
- Nrf2
Nuclear factor-like 2
- NSAIDs
Nonsteroidal anti-inflammatory drugs
- PDE5A
Phosphodiesterase 5A
- PGI2
Prostacyclin
- pHEMA
Poly(2-hydroxyethyl methacrylate)
- Pluronic F127
Poly(ethylene oxide)–poly(propylene oxide)–poly(ethylene oxide) triblock copolymer
- ROS
Reactive oxygen species
- RSNO
S-nitrosothiols
- RSSH
Persulfides
- SATO
S-aroylthiooxime
- sGC
Soluble guanylate cyclase
- SNAP
S-Nitroso-N-acetyl-penicillamine
- SNP
Sodium nitroprusside
- STAT3
Signal transducer and activator of transcription 3
- TBZ
4-Hydroxy benzothiazamide
- TML
Trimethyl lock
- TXA2
Thromboxane A2
- VEGF
Vascular endothelial growth factor
- VSMC
Vascular smooth muscle cells
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: The authors declare no conflict of interest.
References
- [1].Abe K, Kimura H, The possible role of hydrogen sulfide as an endogenous neuromodulator, The Journal of Neuroscience 16(3) (1996) 1066–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Wang R, Two’s company, three’s a crowd: can H2S be the third endogenous gaseous transmitter?, FASEB journal : official publication of the Federation of American Societies for Experimental Biology 16(13) (2002) 1792–8. [DOI] [PubMed] [Google Scholar]
- [3].Kashfi K, The dichotomous role of H2S in cancer cell biology? Deja vu all over again, Biochem Pharmacol 149 (2018) 205–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Benjamin N, O’Driscoll F, Dougall H, Duncan C, Smith L, Golden M, McKenzie H, Stomach NO synthesis, Nature 368(6471) (1994) 502. [DOI] [PubMed] [Google Scholar]
- [5].Gheibi S, Samsonov AP, Gheibi S, Vazquez AB, Kashfi K, Regulation of carbohydrate metabolism by nitric oxide and hydrogen sulfide: Implications in diabetes, Biochem Pharmacol (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kabil O, Banerjee R, Redox biochemistry of hydrogen sulfide, The Journal of biological chemistry 285(29) (2010) 21903–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Li L, Rose P, Moore PK, Hydrogen sulfide and cell signaling, Annual review of pharmacology and toxicology 51 (2011) 169–187. [DOI] [PubMed] [Google Scholar]
- [8].Gadalla MM, Snyder SH, Hydrogen sulfide as a gasotransmitter, Journal of Neurochemistry 113(1) (2010) 14–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Miles EW, Kraus JP, Cystathionine β-Synthase: Structure, Function, Regulation, and Location of Homocystinuria-causing Mutations, Journal of Biological Chemistry 279(29) (2004) 29871–29874. [DOI] [PubMed] [Google Scholar]
- [10].Pan LL, Liu XH, Gong QH, Yang HB, Zhu YZ, Role of Cystathionine γ-Lyase/Hydrogen Sulfide Pathway in Cardiovascular Disease: A Novel Therapeutic Strategy?, Antioxidants & Redox Signaling 17(1) (2011) 106–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Shibuya N, Tanaka M, Yoshida M, Ogasawara Y, Togawa T, Ishii K, Kimura H, 3-Mercaptopyruvate Sulfurtransferase Produces Hydrogen Sulfide and Bound Sulfane Sulfur in the Brain, Antioxidants & Redox Signaling 11(4) (2008) 703–714. [DOI] [PubMed] [Google Scholar]
- [12].Singh SB, Lin HC, Hydrogen Sulfide in Physiology and Diseases of the Digestive Tract, Microorganisms 3(4) (2015) 866–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Pan LL, Liu XH, Gong QH, Yang HB, Zhu YZ, Role of cystathionine gamma-lyase/hydrogen sulfide pathway in cardiovascular disease: a novel therapeutic strategy?, Antioxid Redox Signal 17(1) (2012) 106–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Moore PK, Whiteman M, Chemistry, Biochemistry and Pharmacology of Hydrogen Sulfide, Springer International Publishing; 2015. [Google Scholar]
- [15].Benavides GA, Squadrito GL, Mills RW, Patel HD, Isbell TS, Patel RP, Darley-Usmar VM, Doeller JE, Kraus DW, Hydrogen sulfide mediates the vasoactivity of garlic, Proceedings of the National Academy of Sciences of the United States of America 104(46) (2007) 17977–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Friebe A, Koesling D, Regulation of nitric oxide-sensitive guanylyl cyclase, Circulation research 93(2) (2003) 96–105. [DOI] [PubMed] [Google Scholar]
- [17].Lincoln T, Cornwell T, Komalavilas P, MacMillan-Crow LA, Boerth NJ, The nitric oxide-cyclic GMP signaling system in: M B (Ed.), Biochemistry of smooth muscle contraction, Academic Press, San Diego, 1996, pp. 257–268. [Google Scholar]
- [18].Dangel O, Mergia E, Karlisch K, Groneberg D, Koesling D, Friebe A, Nitric oxide-sensitive guanylyl cyclase is the only nitric oxide receptor mediating platelet inhibition, Journal of thrombosis and haemostasis : JTH 8(6) (2010) 1343–52. [DOI] [PubMed] [Google Scholar]
- [19].Lipton SA, Choi YB, Pan ZH, Lei SZ, Chen HS, Sucher NJ, Loscalzo J, Singel DJ, Stamler JS, A redox-based mechanism for the neuroprotective and neurodestructive effects of nitric oxide and related nitroso-compounds, Nature 364(6438) (1993) 626–32. [DOI] [PubMed] [Google Scholar]
- [20].Stamler JS, Redox signaling: nitrosylation and related target interactions of nitric oxide, Cell 78(6) (1994) 931–6. [DOI] [PubMed] [Google Scholar]
- [21].Stamler JS, Singel DJ, Loscalzo J, Biochemistry of nitric oxide and its redox-activated forms, Science (New York, N.Y.) 258(5090) (1992) 1898–902. [DOI] [PubMed] [Google Scholar]
- [22].Vannini F, Kashfi K, Nath N, The dual role of iNOS in cancer, Redox biology 6 (2015) 334–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Hess DT, Matsumoto A, Kim SO, Marshall HE, Stamler JS, Protein S-nitrosylation: purview and parameters, Nature reviews. Molecular cell biology 6(2) (2005) 150–66. [DOI] [PubMed] [Google Scholar]
- [24].Liu H, Colavitti R, Rovira II, Finkel T, Redox-dependent transcriptional regulation, Circulation research 97(10) (2005) 967–74. [DOI] [PubMed] [Google Scholar]
- [25].Kashfi K, Olson KR, Biology and therapeutic potential of hydrogen sulfide and hydrogen sulfide-releasing chimeras, Biochem Pharmacol 85(5) (2013) 689–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Zhao W, Zhang J, Lu Y, Wang R, The vasorelaxant effect of H(2)S as a novel endogenous gaseous K(ATP) channel opener, The EMBO journal 20(21) (2001) 6008–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Tang G, Wu L, Liang W, Wang R, Direct stimulation of K(ATP) channels by exogenous and endogenous hydrogen sulfide in vascular smooth muscle cells, Molecular pharmacology 68(6) (2005) 1757–64. [DOI] [PubMed] [Google Scholar]
- [28].Wallace JL, Wang R, Hydrogen sulfide-based therapeutics: exploiting a unique but ubiquitous gasotransmitter, Nat Rev Drug Discov 14(5) (2015) 329–345. [DOI] [PubMed] [Google Scholar]
- [29].Wang R, Signaling pathways for the vascular effects of hydrogen sulfide, Curr Opin Nephrol Hypertens 20(2) (2011) 107–12. [DOI] [PubMed] [Google Scholar]
- [30].Peers C, Bauer CC, Boyle JP, Scragg JL, Dallas ML, Modulation of ion channels by hydrogen sulfide, Antioxid Redox Signal 17(1) (2012) 95–105. [DOI] [PubMed] [Google Scholar]
- [31].Kanagy NL, Szabo C, Papapetropoulos A, Vascular Biology of Hydrogen Sulfide, Am J Physiol Cell Physiol (2017) ajpcell 00329 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Filipovic MR, Miljkovic J, Nauser T, Royzen M, Klos K, Shubina T, Koppenol WH, Lippard SJ, Ivanovic-Burmazovic I, Chemical characterization of the smallest S-nitrosothiol, HSNO; cellular cross-talk of H2S and S-nitrosothiols, Journal of the American Chemical Society 134(29) (2012) 12016–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Arp AJ, Childress JJ, Vetter RD, The sulphide-binding protein in the blood of the vestimentiferan tubeworm, Riftia pachyptila, is the extracellular hemoglobin, J. Exp. Biol 128 (1987) 139–158. [Google Scholar]
- [34].Grossi L, Hydrogen sulfide induces nitric oxide release from nitrite, Bioorganic & medicinal chemistry letters 19(21) (2009) 6092–4. [DOI] [PubMed] [Google Scholar]
- [35].Wang R, Shared signaling pathways among gasotransmitters, Proceedings of the National Academy of Sciences of the United States of America 109(23) (2012) 8801–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Lo Faro ML, Fox B, Whatmore JL, Winyard PG, Whiteman M, Hydrogen sulfide and nitric oxide interactions in inflammation, Nitric oxide : biology and chemistry / official journal of the Nitric Oxide Society 41 (2014) 38–47. [DOI] [PubMed] [Google Scholar]
- [37].Taoka S, Banerjee R, Characterization of NO binding to human cystathionine beta-synthase: possible implications of the effects of CO and NO binding to the human enzyme, Journal of inorganic biochemistry 87(4) (2001) 245–51. [DOI] [PubMed] [Google Scholar]
- [38].Kubo S, Doe I, Kurokawa Y, Nishikawa H, Kawabata A, Direct inhibition of endothelial nitric oxide synthase by hydrogen sulfide: contribution to dual modulation of vascular tension, Toxicology 232(1–2) (2007) 138–46. [DOI] [PubMed] [Google Scholar]
- [39].Kubo S, Kurokawa Y, Doe I, Masuko T, Sekiguchi F, Kawabata A, Hydrogen sulfide inhibits activity of three isoforms of recombinant nitric oxide synthase, Toxicology 241(1–2) (2007) 92–7. [DOI] [PubMed] [Google Scholar]
- [40].Zhao W, Wang R, H(2)S-induced vasorelaxation and underlying cellular and molecular mechanisms, American journal of physiology. Heart and circulatory physiology 283(2) (2002) H474–80. [DOI] [PubMed] [Google Scholar]
- [41].Jeong SO, Pae HO, Oh GS, Jeong GS, Lee BS, Lee S, Kim du Y, Rhew HY, Lee KM, Chung HT, Hydrogen sulfide potentiates interleukin-1beta-induced nitric oxide production via enhancement of extracellular signal-regulated kinase activation in rat vascular smooth muscle cells, Biochemical and biophysical research communications 345(3) (2006) 938–44. [DOI] [PubMed] [Google Scholar]
- [42].Predmore BL, Julian D, Cardounel AJ, Hydrogen sulfide increases nitric oxide production from endothelial cells by an akt-dependent mechanism, Frontiers in physiology 2 (2011) 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Kida M, Sugiyama T, Yoshimoto T, Ogawa Y, Hydrogen sulfide increases nitric oxide production with calcium-dependent activation of endothelial nitric oxide synthase in endothelial cells, European journal of pharmaceutical sciences : official journal of the European Federation for Pharmaceutical Sciences 48(1–2) (2013) 211–5. [DOI] [PubMed] [Google Scholar]
- [44].Altaany Z, Yang G, Wang R, Crosstalk between hydrogen sulfide and nitric oxide in endothelial cells, J Cell Mol Med 17(7) (2013) 879–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Miyamoto R, Koike S, Takano Y, Shibuya N, Kimura Y, Hanaoka K, Urano Y, Ogasawara Y, Kimura H, Polysulfides (H2Sn) produced from the interaction of hydrogen sulfide (H2S) and nitric oxide (NO) activate TRPA1 channels, Scientific reports 7 (2017) 45995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Zhao W, Zhang J, Lu Y, Wang R, The vasorelaxant effect of H(2)S as a novel endogenous gaseous K(ATP) channel opener, The EMBO Journal 20(21) (2001) 6008–6016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Li T, Zhao B, Wang C, Wang H, Liu Z, Li W, Jin H, Tang C, Du J, Regulatory Effects of Hydrogen Sulfide on IL-6, IL-8 and IL-10 Levels in the Plasma and Pulmonary Tissue of Rats with Acute Lung Injury, Experimental Biology and Medicine 233(9) (2008) 1081–1087. [DOI] [PubMed] [Google Scholar]
- [48].Ozturk T, Ertas E, Mert O, Use of Lawesson’s Reagent in Organic Syntheses, Chemical Reviews 107(11) (2007) 5210–5278. [DOI] [PubMed] [Google Scholar]
- [49].Alexander BE, Coles SJ, Fox BC, Khan TF, Maliszewski J, Perry A, Pitak MB, Whiteman M, Wood ME, Investigating the generation of hydrogen sulfide from the phosphonamidodithioate slow-release donor GYY4137, MedChemComm 6(9) (2015) 1649–1655. [Google Scholar]
- [50].Kang J, Li Z, Organ CL, Park C-M, Yang C.-t., Pacheco A, Wang D, Lefer DJ, Xian M, pH-Controlled Hydrogen Sulfide Release for Myocardial Ischemia-Reperfusion Injury, Journal of the American Chemical Society 138(20) (2016) 6336–6339. [DOI] [PubMed] [Google Scholar]
- [51].Li Z, Organ CL, Kang J, Polhemus DJ, Trivedi RK, Sharp TE, Jenkins JS, Tao Y.-x., Xian M, Lefer DJ, Hydrogen Sulfide Attenuates Renin Angiotensin and Aldosterone Pathological Signaling to Preserve Kidney Function and Improve Exercise Tolerance in Heart Failure, JACC: Basic to Translational Science 3(6) (2018) 796–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Hasegawa U, van der Vlies AJ, Design and Synthesis of Polymeric Hydrogen Sulfide Donors, Bioconjugate Chem. 25(7) (2014) 1290–1300. [DOI] [PubMed] [Google Scholar]
- [53].Zanatta SD, Jarrott B, Williams SJ, Synthesis and Preliminary Pharmacological Evaluation of Aryl Dithiolethiones with Cyclooxygenase-2-Selective Inhibitory Activity and Hydrogen Sulfide-Releasing Properties, Australian Journal of Chemistry 63(6) (2010) 946–957. [Google Scholar]
- [54].Zhao Y, Wang H, Xian M, Cysteine-Activated Hydrogen Sulfide (H2S) Donors, Journal of the American Chemical Society 133(1) (2011) 15–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Yang CT, Zhao Y, Xian M, Li JH, Dong Q, Bai HB, Xu JD, Zhang MF, A novel controllable hydrogen sulfide-releasing molecule protects human skin keratinocytes against methylglyoxal-induced injury and dysfunction, Cellular physiology and biochemistry : international journal of experimental cellular physiology, biochemistry, and pharmacology 34(4) (2014) 1304–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Zhao Y, Yang C, Organ C, Li Z, Bhushan S, Otsuka H, Pacheco A, Kang J, Aguilar HC, Lefer DJ, Xian M, Design, Synthesis, and Cardioprotective Effects of N-Mercapto-Based Hydrogen Sulfide Donors, Journal of Medicinal Chemistry 58(18) (2015) 7501–7511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Martelli A, Testai L, Citi V, Marino A, Pugliesi I, Barresi E, Nesi G, Rapposelli S, Taliani S, Da Settimo F, Breschi MC, Calderone V, Arylthioamides as H2S Donors: l-Cysteine-Activated Releasing Properties and Vascular Effects in Vitro and in Vivo, ACS Medicinal Chemistry Letters 4(10) (2013) 904–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].De Cicco P, Panza E, Ercolano G, Armogida C, Sessa G, Pirozzi G, Cirino G, Wallace JL, Ianaro A, ATB-346, a novel hydrogen sulfide-releasing anti-inflammatory drug, induces apoptosis of human melanoma cells and inhibits melanoma development in vivo, Pharmacological research 114 (2016) 67–73. [DOI] [PubMed] [Google Scholar]
- [59].Elsheikh W, Blackler RW, Flannigan KL, Wallace JL, Enhanced chemopreventive effects of a hydrogen sulfide-releasing anti-inflammatory drug (ATB-346) in experimental colorectal cancer, Nitric Oxide 41(Supplement C) (2014) 131–137. [DOI] [PubMed] [Google Scholar]
- [60].Foster JC, Powell CR, Radzinski SC, Matson JB, S-Aroylthiooximes: A Facile Route to Hydrogen Sulfide Releasing Compounds with Structure-Dependent Release Kinetics, Organic Letters 16(6) (2014) 1558–1561. [DOI] [PubMed] [Google Scholar]
- [61].Devarie-Baez NO, Bagdon PE, Peng B, Zhao Y, Park C-M, Xian M, Light-Induced Hydrogen Sulfide Release from “Caged” gem-Dithiols, Organic Letters 15(11) (2013) 2786–2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Xiao Z, Bonnard T, Shakouri-Motlagh A, Wylie RAL, Collins J, White J, Heath DE, Hagemeyer CE, Connal LA, Triggered and Tunable Hydrogen Sulfide Release from Photogenerated Thiobenzaldehydes, Chem. Eur. J 23(47) (2017) 11294–11300. [DOI] [PubMed] [Google Scholar]
- [63].Venkatesh Y, Das J, Chaudhuri A, Karmakar A, Maiti TK, Singh NDP, Light triggered uncaging of hydrogen sulfide (H2S) with real-time monitoring, Chemical Communications 54(25) (2018) 3106–3109. [DOI] [PubMed] [Google Scholar]
- [64].Bera M, Maji S, Paul A, Ray S, Maiti TK, Singh NDP, A water soluble light activated hydrogen sulfide donor induced by an excited state meta effect, Organic & Biomolecular Chemistry 17(40) (2019) 9059–9064. [DOI] [PubMed] [Google Scholar]
- [65].Zheng Y, Yu B, Ji K, Pan Z, Chittavong V, Wang B, Esterase-Sensitive Prodrugs with Tunable Release Rates and Direct Generation of Hydrogen Sulfide, Angewandte Chemie (International ed. in English) 55(14) (2016) 4514–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Borchardt RT, Cohen LA, Stereopopulation control. III. Facilitation of intramolecular conjugate addition of the carboxyl group, Journal of the American Chemical Society 94(26) (1972) 9175–9182. [DOI] [PubMed] [Google Scholar]
- [67].Shukla P, Khodade VS, SharathChandra M, Chauhan P, Mishra S, Siddaramappa S, Pradeep BE, Singh A, Chakrapani H, “On demand” redox buffering by H2S contributes to antibiotic resistance revealed by a bacteria-specific H2S donor, Chemical Science 8(7) (2017) 4967–4972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Golombek SK, May J-N, Theek B, Appold L, Drude N, Kiessling F, Lammers T, Tumor targeting via EPR: Strategies to enhance patient responses, Adv Drug Deliv Rev 130 (2018) 17–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Urquhart MC, Ercole F, Whittaker MR, Boyd BJ, Davis TP, Quinn JF, Recent advances in the delivery of hydrogen sulfide via a macromolecular approach, Polymer Chemistry 9(35) (2018) 4431–4439. [Google Scholar]
- [70].Connal LA, The benefits of macromolecular hydrogen sulfide prodrugs, Journal of Materials Chemistry B 6(44) (2018) 7122–7128. [DOI] [PubMed] [Google Scholar]
- [71].Kaur K, Carrazzone R, Matson JB, The Benefits of Macromolecular/Supramolecular Approaches in H2S Delivery: A Review of Polymeric and Self-Assembled H2S Donors, Antioxidants & Redox Signaling doi: 10.1089/ars.2019.7864 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Hamada T, Nakane T, Kimura T, Arisawa K, Yoneda K, Yamamoto T, Osaki T, Treatment of Xerostomia with the Bile Secretion-Stimulating Drug Anethole Trithione: A Clinical Trial, The American Journal of the Medical Sciences 318(3) (1999) 146–151. [DOI] [PubMed] [Google Scholar]
- [73].Hasegawa U, van der Vlies AJ, Polymeric micelles for hydrogen sulfide delivery, MedChemComm 6(2) (2015) 273–276. [Google Scholar]
- [74].Foster JC, Matson JB, Functionalization of Methacrylate Polymers with Thiooximes: A Robust Postpolymerization Modification Reaction and a Method for the Preparation of H2S-Releasing Polymers, Macromolecules 47(15) (2014) 5089–5095. [Google Scholar]
- [75].Foster JC, Radzinski SC, Zou X, Finkielstein CV, Matson JB, H2S-Releasing Polymer Micelles for Studying Selective Cell Toxicity, Molecular Pharmaceutics 14(4) (2017) 1300–1306. [DOI] [PubMed] [Google Scholar]
- [76].Foster JC, Carrazzone RJ, Spear NJ, Radzinski SC, Arrington KJ, Matson JB, Tuning H2S Release by Controlling Mobility in a Micelle Core, Macromolecules 52(3) (2019) 1104–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Ercole F, Mansfeld FM, Kavallaris M, Whittaker MR, Quinn JF, Halls ML, Davis TP, Macromolecular Hydrogen Sulfide Donors Trigger Spatiotemporally Confined Changes in Cell Signaling, Biomacromolecules 17(1) (2016) 371–383. [DOI] [PubMed] [Google Scholar]
- [78].Li Z, Li D, Wang L, Lu C, Shan P, Zou X, Li Z, Photocontrollable water-soluble polymeric hydrogen sulfide (H2S) donor, Polymer 168 (2019) 16–20. [Google Scholar]
- [79].Long TR, Wongrakpanich A, Do A-V, Salem AK, Bowden NB, Long-term release of a thiobenzamide from a backbone functionalized poly(lactic acid), Polymer Chemistry 6(40) (2015) 7188–7195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Feng S, Zhao Y, Xian M, Wang Q, Biological thiols-triggered hydrogen sulfide releasing microfibers for tissue engineering applications, Acta Biomaterialia 27 (2015) 205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Wu J, Li Y, He C, Kang J, Ye J, Xiao Z, Zhu J, Chen A, Feng S, Li X, Xiao J, Xian M, Wang Q, Novel H2S Releasing Nanofibrous Coating for In Vivo Dermal Wound Regeneration, ACS Applied Materials & Interfaces 8(41) (2016) 27474–27481. [DOI] [PubMed] [Google Scholar]
- [82].Zheng Z, Chen A, He H, Chen Y, Chen J, Albashari AA, Li J, Yin J, He Z, Wang Q, Wu J, Wang Q, Kang J, Xian M, Wang X, Xiao J, pH and enzyme dual-responsive release of hydrogen sulfide for disc degeneration therapy, Journal of Materials Chemistry B 7(4) (2019) 611–618. [DOI] [PubMed] [Google Scholar]
- [83].Carter JM, Qian Y, Foster JC, Matson JB, Peptide-based hydrogen sulphide-releasing gels, Chemical Communications 51(66) (2015) 13131–13134. [DOI] [PubMed] [Google Scholar]
- [84].Longchamp A, Kaur K, Macabrey D, Dubuis C, Corpataux J-M, Déglise S, Matson JB, Allagnat F, Hydrogen sulfide-releasing peptide hydrogel limits the development of intimal hyperplasia in human vein segments, Acta Biomaterialia 97 (2019) 374–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Qian Y, Kaur K, Foster JC, Matson JB, Supramolecular Tuning of H2S Release from Aromatic Peptide Amphiphile Gels: Effect of Core Unit Substituents, Biomacromolecules 20(2) (2019) 1077–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Kaur K, Qian Y, Gandour RD, Matson JB, Hydrolytic Decomposition of S Aroylthiooximes: Effect of pH and N-Arylidene Substitution on Reaction Rate, The Journal of Organic Chemistry 83(21) (2018) 13363–13369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Wang Y, Kaur K, Scannelli SJ, Bitton R, Matson JB, Self-Assembled Nanostructures Regulate H2S Release from Constitutionally Isomeric Peptides, Journal of the American Chemical Society 140(44) (2018) 14945–14951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Wang Y, Matson JB, Supramolecular Nanostructures with Tunable Donor Loading for Controlled H2S Release, ACS Applied Bio Materials (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Zhou M, Qian Y, Zhu Y, Matson J, Elastase-triggered H2S delivery from polymer hydrogels, Chem Commun (Camb) (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Ignarro LJ, Buga GM, Wood KS, Byrns RE, Chaudhuri G, Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide, Proceedings of the National Academy of Sciences of the United States of America 84(24) (1987) 9265–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Ignarro LJ, Napoli C, Loscalzo J, Nitric oxide donors and cardiovascular agents modulating the bioactivity of nitric oxide: an overview, Circulation research 90(1) (2002) 21–8. [DOI] [PubMed] [Google Scholar]
- [92].Geller DA, Billiar TR, Molecular biology of nitric oxide synthases, Cancer metastasis reviews 17(1) (1998) 7–23. [DOI] [PubMed] [Google Scholar]
- [93].Quinn AC, Petros AJ, Vallance P, Nitric oxide: an endogenous gas, British journal of anaesthesia 74(4) (1995) 443–51. [DOI] [PubMed] [Google Scholar]
- [94].Bogdan C, Nitric oxide and the immune response, Nature immunology 2(10) (2001) 907–16. [DOI] [PubMed] [Google Scholar]
- [95].Luo JD, Chen AF, Nitric oxide: a newly discovered function on wound healing, Acta Pharmacol Sin 26(3) (2005) 259–64. [DOI] [PubMed] [Google Scholar]
- [96].Willmot MR, Bath PM, The potential of nitric oxide therapeutics in stroke, Expert Opin Investig Drugs 12(3) (2003) 455–70. [DOI] [PubMed] [Google Scholar]
- [97].Vannini F, Kashfi K, Nath N, The dual role of iNOS in cancer, Redox Biol 6 (2015) 334–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Szabo C, Gasotransmitters in cancer: from pathophysiology to experimental therapy, Nat Rev Drug Discov 15(3) (2016) 185–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Szabo C, Ransy C, Modis K, Andriamihaja M, Murghes B, Coletta C, Olah G, Yanagi K, Bouillaud F, Regulation of mitochondrial bioenergetic function by hydrogen sulfide. Part I. Biochemical and physiological mechanisms, Br J Pharmacol 171(8) (2014) 2099–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Kashfi K, Anti-inflammatory agents as cancer therapeutics, Adv Pharmacol 57 (2009) 31–89. [DOI] [PubMed] [Google Scholar]
- [101].Kashfi K, Duvalsaint PL, Nitric Oxide Donors and Therapeutic Applications in Cancer, in: Seabra AB (Ed.), Nitric Oxide Donors: Novel Biomedical Applications and Perspectives, Elsevier - Academic Press, Cambridge MA, USA, 2017, pp. 75–119. [Google Scholar]
- [102].Bhatraju P, Crawford J, Hall M, Lang JD Jr., Inhaled nitric oxide: Current clinical concepts, Nitric Oxide 50 (2015) 114–128. [DOI] [PubMed] [Google Scholar]
- [103].Pipili-Synetos E, Papageorgiou A, Sakkoula E, Sotiropoulou G, Fotsis T, Karakiulakis G, Maragoudakis ME, Inhibition of angiogenesis, tumour growth and metastasis by the NO-releasing vasodilators, isosorbide mononitrate and dinitrate, Br J Pharmacol 116(2) (1995) 1829–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Tamano S, Ward JM, Diwan BA, Keefer LK, Weghorst CM, Calvert RJ, Henneman JR, Ramljak D, Rice JM, Histogenesis and the role of p53 and K-ras mutations in hepatocarcinogenesis by glyceryl trinitrate (nitroglycerin) in male F344 rats, Carcinogenesis 17(11) (1996) 2477–86. [DOI] [PubMed] [Google Scholar]
- [105].Acharya S, Rogers P, Krishnamoorthy RR, Stankowska DL, Dias HV, Yorio T, Design and synthesis of novel hybrid sydnonimine and prodrug useful for glaucomatous optic neuropathy, Bioorg Med Chem Lett 26(5) (2016) 1490–4. [DOI] [PubMed] [Google Scholar]
- [106].Wang PG, Xian M, Tang X, Wu X, Wen Z, Cai T, Janczuk AJ, Nitric oxide donors: chemical activities and biological applications, Chem Rev 102(4) (2002) 1091–134. [DOI] [PubMed] [Google Scholar]
- [107].Thun MJ, Namboodiri MM, Heath C Jr., Aspirin use and reduced risk of fatal colon cancer, The New England Journal of Medicine 325(23) (1991) 1593–1596. [DOI] [PubMed] [Google Scholar]
- [108].Benamouzig R, Deyra J, Martin A, Girard B, Jullian E, Piednoir B, Couturier D, Coste T, Little J, Chaussade S, Daily soluble aspirin and prevention of colorectal adenoma recurrence: one-year results of the APACC trial, Gastroenterology 125(2) (2003) 328–36. [DOI] [PubMed] [Google Scholar]
- [109].Rothwell PM, Price JF, Fowkes FG, Zanchetti A, Roncaglioni MC, Tognoni G, Lee R, Belch JF, Wilson M, Mehta Z, Meade TW, Short-term effects of daily aspirin on cancer incidence, mortality, and non-vascular death: analysis of the time course of risks and benefits in 51 randomised controlled trials, Lancet (London, England) 379(9826) (2012) 1602–12. [DOI] [PubMed] [Google Scholar]
- [110].Wallace JL, Ignarro LJ, Fiorucci S, Potential cardioprotective actions of no-releasing aspirin, Nat Rev Drug Discov 1(5) (2002) 375–82. [DOI] [PubMed] [Google Scholar]
- [111].Wallace JL, Soldato PD, The therapeutic potential of NO-NSAIDs, Fundam Clin Pharmacol 17(1) (2003) 11–20. [DOI] [PubMed] [Google Scholar]
- [112].Wallace JL, Miller MJ, Nitric oxide in mucosal defense: a little goes a long way, Gastroenterology 119(2) (2000) 512–20. [DOI] [PubMed] [Google Scholar]
- [113].Davies NM, Roseth AG, Appleyard CB, McKnight W, Del Soldato P, Calignano A, Cirino G, Wallace JL, NO-naproxen vs. naproxen: ulcerogenic, analgesic and anti-inflammatory effects, Aliment Pharmacol Ther 11(1) (1997) 69–79. [DOI] [PubMed] [Google Scholar]
- [114].Wallace JL, Reuter B, Cicala C, McKnight W, Grisham M, Cirino G, A diclofenac derivative without ulcerogenic properties, Eur J Pharmacol 257(3) (1994) 249–55. [DOI] [PubMed] [Google Scholar]
- [115].Wallace JL, Reuter B, Cicala C, McKnight W, Grisham MB, Cirino G, Novel nonsteroidal anti-inflammatory drug derivatives with markedly reduced ulcerogenic properties in the rat, Gastroenterology 107(1) (1994) 173–9. [DOI] [PubMed] [Google Scholar]
- [116].Elliott SN, McKnight W, Cirino G, Wallace JL, A nitric oxide-releasing nonsteroidal anti-inflammatory drug accelerates gastric ulcer healing in rats, Gastroenterology 109(2) (1995) 524–30. [DOI] [PubMed] [Google Scholar]
- [117].Borhade N, Pathan AR, Halder S, Karwa M, Dhiman M, Pamidiboina V, Gund M, Deshattiwar JJ, Mali SV, Deshmukh NJ, Senthilkumar SP, Gaikwad P, Tipparam SG, Mudgal J, Dutta MC, Burhan AU, Thakre G, Sharma A, Deshpande S, Desai DC, Dubash NP, Jain AK, Sharma S, Nemmani KV, Satyam A, NO-NSAIDs. Part 3: nitric oxide-releasing prodrugs of non-steroidal anti-inflammatory drugs, Chemical & pharmaceutical bulletin 60(4) (2012) 465–81. [DOI] [PubMed] [Google Scholar]
- [118].Gund M, Gaikwad P, Borhade N, Burhan A, Desai DC, Sharma A, Dhiman M, Patil M, Sheikh J, Thakre G, Tipparam SG, Sharma S, Nemmani KV, Satyam A, Gastric-sparing nitric oxide-releasable ‘true’ prodrugs of aspirin and naproxen, Bioorg Med Chem Lett 24(24) (2014) 5587–92. [DOI] [PubMed] [Google Scholar]
- [119].Nemmani KV, Mali SV, Borhade N, Pathan AR, Karwa M, Pamidiboina V, Senthilkumar SP, Gund M, Jain AK, Mangu NK, Dubash NP, Desai DC, Sharma S, Satyam A, NO-NSAIDs: Gastric-sparing nitric oxide-releasable prodrugs of non-steroidal anti-inflammatory drugs, Bioorg Med Chem Lett 19(18) (2009) 5297–301. [DOI] [PubMed] [Google Scholar]
- [120].Pathan AR, Karwa M, Pamidiboina V, Deshattiwar JJ, Deshmukh NJ, Gaikwad PP, Mali SV, Desai DC, Dhiman M, Thanga Mariappan T, Sharma SD, Satyam A, Nemmani KV, Oral bioavailability, efficacy and gastric tolerability of P2026, a novel nitric oxide-releasing diclofenac in rat, Inflammopharmacology 18(4) (2010) 157–68. [DOI] [PubMed] [Google Scholar]
- [121].Fiorucci S, Santucci L, Gresele P, Faccino RM, Del Soldato P, Morelli A, Gastrointestinal safety of NO-aspirin (NCX-4016) in healthy human volunteers: a proof of concept endoscopic study, Gastroenterology 124(3) (2003) 600–7. [DOI] [PubMed] [Google Scholar]
- [122].Fiorucci S, Santucci L, Wallace JL, Sardina M, Romano M, del Soldato P, Morelli A, Interaction of a selective cyclooxygenase-2 inhibitor with aspirin and NO-releasing aspirin in the human gastric mucosa, Proceedings of the National Academy of Sciences of the United States of America 100(19) (2003) 10937–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Kashfi K, Ryan Y, Qiao LL, Williams JL, Chen J, Del Soldato P, Traganos F, Rigas B, Nitric oxide-donating nonsteroidal anti-inflammatory drugs inhibit the growth of various cultured human cancer cells: evidence of a tissue type-independent effect, The Journal of pharmacology and experimental therapeutics 303(3) (2002) 1273–82. [DOI] [PubMed] [Google Scholar]
- [124].Kashfi K, Borgo S, Williams JL, Chen J, Gao J, Glekas A, Benedini F, Del Soldato P, Rigas B, Positional isomerism markedly affects the growth inhibition of colon cancer cells by nitric oxide-donating aspirin in vitro and in vivo, The Journal of pharmacology and experimental therapeutics 312(3) (2005) 978–88. [DOI] [PubMed] [Google Scholar]
- [125].Nath N, Liu X, Jacobs L, Kashfi K, Flurbiprofen benzyl nitrate (NBS-242) inhibits the growth of A-431 human epidermoid carcinoma cells and targets beta-catenin, Drug design, development and therapy 7 (2013) 389–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Cena C, Lolli ML, Lazzarato L, Guaita E, Morini G, Coruzzi G, McElroy SP, Megson IL, Fruttero R, Gasco A, Antiinflammatory, gastrosparing, and antiplatelet properties of new NO-donor esters of aspirin, J Med Chem 46(5) (2003) 747–54. [DOI] [PubMed] [Google Scholar]
- [127].Grosser N, Schroder H, A common pathway for nitric oxide release from NO-aspirin and glyceryl trinitrate, Biochem Biophys Res Commun 274(1) (2000) 255–8. [DOI] [PubMed] [Google Scholar]
- [128].Carini M, Aldini G, Orioli M, Maffei Facino R, In vitro metabolism of a nitroderivative of acetylsalicylic acid (NCX4016) by rat liver: LC and LC-MS studies, J Pharm Biomed Anal 29(6) (2002) 1061–71. [DOI] [PubMed] [Google Scholar]
- [129].Gao J, Kashfi K, Rigas B, In vitro metabolism of nitric oxide-donating aspirin: the effect of positional isomerism, The Journal of pharmacology and experimental therapeutics 312(3) (2005) 989–97. [DOI] [PubMed] [Google Scholar]
- [130].Turnbull CM, Cena C, Fruttero R, Gasco A, Rossi AG, Megson IL, Mechanism of action of novel NO-releasing furoxan derivatives of aspirin in human platelets, Br J Pharmacol 148(4) (2006) 517–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Lazzarato L, Donnola M, Rolando B, Marini E, Cena C, Coruzzi G, Guaita E, Morini G, Fruttero R, Gasco A, Biondi S, Ongini E, Searching for new NO-donor aspirin-like molecules: a new class of nitrooxy-acyl derivatives of salicylic acid, J Med Chem 51(6) (2008) 1894–903. [DOI] [PubMed] [Google Scholar]
- [132].Solomon DH, Recommendations for use of selective and nonselective nonsteroidal antiinflammatory drugs: an American College of Rheumatology white paper, Arthritis and rheumatism 59(8) (2008) 1058–73. [DOI] [PubMed] [Google Scholar]
- [133].Funk CD, FitzGerald GA, COX-2 inhibitors and cardiovascular risk, Journal of cardiovascular pharmacology 50(5) (2007) 470–9. [DOI] [PubMed] [Google Scholar]
- [134].Kashfi K, Rigas B, Non-COX-2 targets and cancer: expanding the molecular target repertoire of chemoprevention, Biochem Pharmacol 70(7) (2005) 969–86. [DOI] [PubMed] [Google Scholar]
- [135].Martelli A, Testai L, Anzini M, Cappelli A, Di Capua A, Biava M, Poce G, Consalvi S, Giordani A, Caselli G, Rovati L, Ghelardini C, Patrignani P, Sautebin L, Breschi MC, Calderone V, The novel anti-inflammatory agent VA694, endowed with both NO-releasing and COX2-selective inhibiting properties, exhibits NO-mediated positive effects on blood pressure, coronary flow and endothelium in an experimental model of hypertension and endothelial dysfunction, Pharmacological research 78 (2013) 1–9. [DOI] [PubMed] [Google Scholar]
- [136].Chowdhury MA, Abdellatif KR, Dong Y, Yu G, Huang Z, Rahman M, Das D, Velazquez CA, Suresh MR, Knaus EE, Celecoxib analogs possessing a N-(4-nitrooxybutyl)piperidin-4-yl or N-(4-nitrooxybutyl)-1,2,3,6-tetrahydropyridin-4-yl nitric oxide donor moiety: synthesis, biological evaluation and nitric oxide release studies, Bioorg Med Chem Lett 20(4) (2010) 1324–9. [DOI] [PubMed] [Google Scholar]
- [137].Boschi D, Cena C, Di Stilo A, Rolando B, Manzini P, Fruttero R, Gasco A, Nitrooxymethyl-substituted analogues of rofecoxib: synthesis and pharmacological characterization, Chemistry & biodiversity 7(5) (2010) 1173–82. [DOI] [PubMed] [Google Scholar]
- [138].Dhawan V, Schwalb DJ, Shumway MJ, Warren MC, Wexler RS, Zemtseva IS, Zifcak BM, Janero DR, Selective nitros(yl)ation induced in vivo by a nitric oxide-donating cyclooxygenase-2 inhibitor: a NObonomic analysis, Free radical biology & medicine 39(9) (2005) 1191–207. [DOI] [PubMed] [Google Scholar]
- [139].Bechmann N, Kniess T, Kockerling M, Pigorsch A, Steinbach J, Pietzsch J, Novel (pyrazolyl)benzenesulfonamides with a nitric oxide-releasing moiety as selective cyclooxygenase-2 inhibitors, Bioorg Med Chem Lett 25(16) (2015) 3295–300. [DOI] [PubMed] [Google Scholar]
- [140].Abdellatif KR, Chowdhury MA, Dong Y, Knaus EE, Diazen-1-ium-1,2-diolated nitric oxide donor ester prodrugs of 1-(4-methanesulfonylphenyl)-5-aryl-1H-pyrazol-3-carboxylic acids: synthesis, nitric oxide release studies and anti-inflammatory activities, Bioorganic & medicinal chemistry 16(13) (2008) 6528–34. [DOI] [PubMed] [Google Scholar]
- [141].Abdellatif KR, Chowdhury MA, Velazquez CA, Huang Z, Dong Y, Das D, Yu G, Suresh MR, Knaus EE, Celecoxib prodrugs possessing a diazen-1-ium-1,2-diolate nitric oxide donor moiety: synthesis, biological evaluation and nitric oxide release studies, Bioorg Med Chem Lett 20(15) (2010) 4544–9. [DOI] [PubMed] [Google Scholar]
- [142].Basudhar D, Cheng RC, Bharadwaj G, Ridnour LA, Wink DA, Miranda KM, Chemotherapeutic potential of diazeniumdiolate-based aspirin prodrugs in breast cancer, Free radical biology & medicine 83 (2015) 101–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Abdellatif KR, Abdelgawad MA, Labib MB, Zidan TH, Synthesis, cyclooxygenase inhibition, anti-inflammatory evaluation and ulcerogenic liability of novel triarylpyrazoline derivatives as selective COX-2 inhibitors, Bioorg Med Chem Lett 25(24) (2015) 5787–91. [DOI] [PubMed] [Google Scholar]
- [144].Abdellatif KR, Elsaady MT, Abdel-Aziz SA, Abusabaa AH, Synthesis, cyclooxygenase inhibition and anti-inflammatory evaluation of new 1,3,5-triaryl-4,5-dihydro-1H-pyrazole derivatives possessing methanesulphonyl pharmacophore, Journal of enzyme inhibition and medicinal chemistry (2016) 1–11. [DOI] [PubMed] [Google Scholar]
- [145].Abdellatif KR, Fadaly WA, Ali WA, Kamel GM, Synthesis, cyclooxygenase inhibition, anti-inflammatory evaluation and ulcerogenic liability of new 1,5-diarylpyrazole derivatives, Journal of enzyme inhibition and medicinal chemistry (2016) 1–7. [DOI] [PubMed] [Google Scholar]
- [146].Bakr RB, Azouz AA, Abdellatif KR, Synthesis, cyclooxygenase inhibition, anti-inflammatory evaluation and ulcerogenic liability of new 1-phenylpyrazolo[3,4-d]pyrimidine derivatives, Journal of enzyme inhibition and medicinal chemistry (2016) 1–7. [DOI] [PubMed] [Google Scholar]
- [147].Biava M, Battilocchio C, Poce G, Alfonso S, Consalvi S, Di Capua A, Calderone V, Martelli A, Testai L, Sautebin L, Rossi A, Ghelardini C, Di Cesare Mannelli L, Giordani A, Persiani S, Colovic M, Dovizio M, Patrignani P, Anzini M, Enhancing the pharmacodynamic profile of a class of selective COX-2 inhibiting nitric oxide donors, Bioorganic & medicinal chemistry 22(2) (2014) 772–86. [DOI] [PubMed] [Google Scholar]
- [148].Gouda AM, Abdelazeem AH, Arafa el SA, Abdellatif KR, Design, synthesis and pharmacological evaluation of novel pyrrolizine derivatives as potential anticancer agents, Bioorganic chemistry 53 (2014) 1–7. [DOI] [PubMed] [Google Scholar]
- [149].Keefer LK, Fifty years of diazeniumdiolate research. From laboratory curiosity to broad-spectrum biomedical advances, ACS chemical biology 6(11) (2011) 1147–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Nandurdikar RS, Maciag AE, Cao Z, Keefer LK, Saavedra JE, Diazeniumdiolated carbamates: a novel class of nitric oxide donors, Bioorganic & medicinal chemistry 20(6) (2012) 2025–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Liu J, Saavedra JE, Lu T, Song JG, Clark J, Waalkes MP, Keefer LK, O(2)-Vinyl 1-(pyrrolidin-1-yl)diazen-1-ium-1,2-diolate protection against D-galactosamine/endotoxin-induced hepatotoxicity in mice: genomic analysis using microarrays, The Journal of pharmacology and experimental therapeutics 300(1) (2002) 18–25. [DOI] [PubMed] [Google Scholar]
- [152].Maciag AE, Saavedra JE, Chakrapani H, The nitric oxide prodrug JS-K and its structural analogues as cancer therapeutic agents, Anti-cancer agents in medicinal chemistry 9(7) (2009) 798–803. [DOI] [PubMed] [Google Scholar]
- [153].Tan G, Qiu M, Chen L, Zhang S, Ke L, Liu J, JS-K, a nitric oxide pro-drug, regulates growth and apoptosis through the ubiquitin-proteasome pathway in prostate cancer cells, BMC Cancer 17(1) (2017) 376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Qiu M, Chen L, Tan G, Ke L, Zhang S, Chen H, Liu J, JS-K promotes apoptosis by inducing ROS production in human prostate cancer cells, Oncol Lett 13(3) (2017) 1137–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Nath N, Chattopadhyay M, Pospishil L, Cieciura LZ, Goswami S, Kodela R, Saavedra JE, Keefer LK, Kashfi K, JS-K, a nitric oxide-releasing prodrug, modulates ss-catenin/TCF signaling in leukemic Jurkat cells: evidence of an S-nitrosylated mechanism, Biochem Pharmacol 80(11) (2010) 1641–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Andrei D, Salmon DJ, Donzelli S, Wahab A, Klose JR, Citro ML, Saavedra JE, Wink DA, Miranda KM, Keefer LK, Dual mechanisms of HNO generation by a nitroxyl prodrug of the diazeniumdiolate (NONOate) class, Journal of the American Chemical Society 132(46) (2010) 16526–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Salmon DJ, Torres de Holding CL, Thomas L, Peterson KV, Goodman GP, Saavedra JE, Srinivasan A, Davies KM, Keefer LK, Miranda KM, HNO and NO release from a primary amine-based diazeniumdiolate as a function of pH, Inorganic chemistry 50(8) (2011) 3262–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Nagasawa HT, Kawle SP, Elberling JA, DeMaster EG, Fukuto JM, Prodrugs of nitroxyl as potential aldehyde dehydrogenase inhibitors vis-a-vis vascular smooth muscle relaxants, J Med Chem 38(11) (1995) 1865–71. [DOI] [PubMed] [Google Scholar]
- [159].Paolocci N, Katori T, Champion HC, St John ME, Miranda KM, Fukuto JM, Wink DA, Kass DA, Positive inotropic and lusitropic effects of HNO/NO- in failing hearts: independence from beta-adrenergic signaling, Proceedings of the National Academy of Sciences of the United States of America 100(9) (2003) 5537–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Velazquez CA, Praveen Rao PN, Citro ML, Keefer LK, Knaus EE, O2-acetoxymethyl-protected diazeniumdiolate-based NSAIDs (NONO-NSAIDs): synthesis, nitric oxide release, and biological evaluation studies, Bioorganic & medicinal chemistry 15(14) (2007) 4767–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Thatcher GR, Nicolescu AC, Bennett BM, Toader V, Nitrates and NO release: contemporary aspects in biological and medicinal chemistry, Free radical biology & medicine 37(8) (2004) 1122–43. [DOI] [PubMed] [Google Scholar]
- [162].Velazquez C, Praveen Rao PN, Knaus EE, Novel nonsteroidal antiinflammatory drugs possessing a nitric oxide donor diazen-1-ium-1,2-diolate moiety: design, synthesis, biological evaluation, and nitric oxide release studies, J Med Chem 48(12) (2005) 4061–7. [DOI] [PubMed] [Google Scholar]
- [163].Velazquez CA, Chen QH, Citro ML, Keefer LK, Knaus EE, Second-generation aspirin and indomethacin prodrugs possessing an O(2)-(acetoxymethyl)-1-(2-carboxypyrrolidin-1-yl)diazenium-1,2-diolate nitric oxide donor moiety: design, synthesis, biological evaluation, and nitric oxide release studies, J Med Chem 51(6) (2008) 1954–61. [DOI] [PubMed] [Google Scholar]
- [164].Paolocci N, Saavedra WF, Miranda KM, Martignani C, Isoda T, Hare JM, Espey MG, Fukuto JM, Feelisch M, Wink DA, Kass DA, Nitroxyl anion exerts redox-sensitive positive cardiac inotropy in vivo by calcitonin gene-related peptide signaling, Proceedings of the National Academy of Sciences of the United States of America 98(18) (2001) 10463–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Norris AJ, Sartippour MR, Lu M, Park T, Rao JY, Jackson MI, Fukuto JM, Brooks MN, Nitroxyl inhibits breast tumor growth and angiogenesis, International journal of cancer 122(8) (2008) 1905–10. [DOI] [PubMed] [Google Scholar]
- [166].Basudhar D, Bharadwaj G, Cheng RY, Jain S, Shi S, Heinecke JL, Holland RJ, Ridnour LA, Caceres VM, Spadari-Bratfisch RC, Paolocci N, Velazquez-Martinez CA, Wink DA, Miranda KM, Synthesis and chemical and biological comparison of nitroxyl- and nitric oxide-releasing diazeniumdiolate-based aspirin derivatives, J Med Chem 56(20) (2013) 7804–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Keefer LK, Broad-Spectrum Anti-Cancer Activity of O-Arylated Diazeniumdiolates, Forum on immunopathological diseases and therapeutics 1(3) (2010) 205–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Laschak M, Spindler KD, Schrader AJ, Hessenauer A, Streicher W, Schrader M, Cronauer MV, JS-K, a glutathione/glutathione S-transferase-activated nitric oxide releasing prodrug inhibits androgen receptor and WNT-signaling in prostate cancer cells, BMC Cancer 12 (2012) 130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Bico P, Chen CY, Jones M, Erhardt J, Dirr H, Class pi glutathione S-transferase: Meisenheimer complex formation, Biochemistry and molecular biology international 33(5) (1994) 887–92. [PubMed] [Google Scholar]
- [170].Shami PJ, Saavedra JE, Wang LY, Bonifant CL, Diwan BA, Singh SV, Gu Y, Fox SD, Buzard GS, Citro ML, Waterhouse DJ, Davies KM, Ji X, Keefer LK, JS-K, a glutathione/glutathione S-transferase-activated nitric oxide donor of the diazeniumdiolate class with potent antineoplastic activity, Mol Cancer Ther 2(4) (2003) 409–17. [PubMed] [Google Scholar]
- [171].Shami PJ, Maciag AE, Eddington JK, Udupi V, Kosak KM, Saavedra JE, Keefer LK, JS-K, an arylating nitric oxide (NO) donor, has synergistic anti-leukemic activity with cytarabine (ARA-C), Leuk Res 33(11) (2009) 1525–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Ren Z, Kar S, Wang Z, Wang M, Saavedra JE, Carr BI, JS-K, a novel non-ionic diazeniumdiolate derivative, inhibits Hep 3B hepatoma cell growth and induces c-Jun phosphorylation via multiple MAP kinase pathways, J Cell Physiol 197(3) (2003) 426–34. [DOI] [PubMed] [Google Scholar]
- [173].Weyerbrock A, Osterberg N, Psarras N, Baumer B, Kogias E, Werres A, Bette S, Saavedra JE, Keefer LK, Papazoglou A, JS-K, a glutathione S-transferase-activated nitric oxide donor with antineoplastic activity in malignant gliomas, Neurosurgery 70(2) (2012) 497–510; discussion 510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Findlay VJ, Townsend DM, Saavedra JE, Buzard GS, Citro ML, Keefer LK, Ji X, Tew KD, Tumor cell responses to a novel glutathione S-transferase-activated nitric oxide-releasing prodrug, Molecular pharmacology 65(5) (2004) 1070–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Saavedra JE, Srinivasan A, Buzard GS, Davies KM, Waterhouse DJ, Inami K, Wilde TC, Citro ML, Cuellar M, Deschamps JR, Parrish D, Shami PJ, Findlay VJ, Townsend DM, Tew KD, Singh S, Jia L, Ji X, Keefer LK, PABA/NO as an anticancer lead: analogue synthesis, structure revision, solution chemistry, reactivity toward glutathione, and in vitro activity, J Med Chem 49(3) (2006) 1157–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Kogias E, Osterberg N, Baumer B, Psarras N, Koentges C, Papazoglou A, Saavedra JE, Keefer LK, Weyerbrock A, Growth-inhibitory and chemosensitizing effects of the glutathione-S-transferase-pi-activated nitric oxide donor PABA/NO in malignant gliomas, International journal of cancer 130(5) (2012) 1184–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Fu J, Liu L, Huang Z, Lai Y, Ji H, Peng S, Tian J, Zhang Y, Hybrid molecule from O2-(2,4-dinitrophenyl)diazeniumdiolate and oleanolic acid: a glutathione S-transferase pi-activated nitric oxide prodrug with selective anti-human hepatocellular carcinoma activity and improved stability, J Med Chem 56(11) (2013) 4641–55. [DOI] [PubMed] [Google Scholar]
- [178].Liu Y, Hartley DP, Liu J, Protection against carbon tetrachloride hepatotoxicity by oleanolic acid is not mediated through metallothionein, Toxicology letters 95(2) (1998) 77–85. [DOI] [PubMed] [Google Scholar]
- [179].Oronsky B, Reid TR, Larson C, Caroen S, Quinn M, Burbano E, Varner G, Thilagar B, Brown B, Coyle A, Ferry L, Abrouk N, Oronsky A, Scribner CL, Carter CA, REPLATINUM Phase III randomized study: RRx-001 + platinum doublet versus platinum doublet in third-line small cell lung cancer, Future Oncol 15(30) (2019) 3427–3433. [DOI] [PubMed] [Google Scholar]
- [180].Morgensztern D, Rose M, Waqar SN, Morris J, Ma PC, Reid T, Brzezniak CE, Zeman KG, Padmanabhan A, Hirth J, I.S. A, J.B. Trepel, S.K. Padda, RRx-001 followed by platinum plus etoposide in patients with previously treated small-cell lung cancer, Br J Cancer 121(3) (2019) 211–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Cabrales P, RRx-001 Acts as a Dual Small Molecule Checkpoint Inhibitor by Downregulating CD47 on Cancer Cells and SIRP-alpha on Monocytes/Macrophages, Transl Oncol 12(4) (2019) 626–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Sikder N, Sikder AK, Bulakh NR, Gandhe BR, 1,3,3-Trinitroazetidine (TNAZ), a melt-cast explosive: synthesis, characterization and thermal behaviour, Journal of hazardous materials 113(1–3) (2004) 35–43. [DOI] [PubMed] [Google Scholar]
- [183].Knox SJ, Cannizzo L, Warner K, Wardle R, Velarde S, Ning S, Cyclic nitro compounds, pharmaceutical compositions thereof and uses thereof., RadioRx, Inc., Alliant Techsystems Inc., United States, 2009. [Google Scholar]
- [184].Vitturi DA, Sun CW, Harper VM, Thrash-Williams B, Cantu-Medellin N, Chacko BK, Peng N, Dai Y, Wyss JM, Townes T, Patel RP, Antioxidant functions for the hemoglobin beta93 cysteine residue in erythrocytes and in the vascular compartment in vivo, Free radical biology & medicine 55 (2013) 119–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Scicinski J, Oronsky B, Cooper V, Taylor M, Alexander M, Hadar R, Cosford R, Fleischmann T, Fitch WL, Development of methods for the bioanalysis of RRx-001 and metabolites, Bioanalysis 6(7) (2014) 947–56. [DOI] [PubMed] [Google Scholar]
- [186].Scicinski J, Oronsky B, Taylor M, Luo G, Musick T, Marini J, Adams CM, Fitch WL, Preclinical evaluation of the metabolism and disposition of RRx-001, a novel investigative anticancer agent, Drug metabolism and disposition: the biological fate of chemicals 40(9) (2012) 1810–6. [DOI] [PubMed] [Google Scholar]
- [187].Ning S, Sekar TV, Scicinski J, Oronsky B, Peehl DM, Knox SJ, Paulmurugan R, Nrf2 activity as a potential biomarker for the pan-epigenetic anticancer agent, RRx-001, Oncotarget 6(25) (2015) 21547–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Scicinski J, Oronsky B, Ning S, Knox S, Peehl D, Kim MM, Langecker P, Fanger G, NO to cancer: The complex and multifaceted role of nitric oxide and the epigenetic nitric oxide donor, RRx-001, Redox biology 6 (2015) 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Rapozzi V, Della Pietra E, Zorzet S, Zacchigna M, Bonavida B, Xodo LE, Nitric oxide-mediated activity in anti-cancer photodynamic therapy, Nitric Oxide 30 (2013) 26–35. [DOI] [PubMed] [Google Scholar]
- [190].Dougherty TJ, An update on photodynamic therapy applications, J Clin Laser Med Surg 20(1) (2002) 3–7. [DOI] [PubMed] [Google Scholar]
- [191].Agostinis P, Berg K, Cengel KA, Foster TH, Girotti AW, Gollnick SO, Hahn SM, Hamblin MR, Juzeniene A, Kessel D, Korbelik M, Moan J, Mroz P, Nowis D, Piette J, Wilson BC, Golab J, Photodynamic therapy of cancer: an update, CA Cancer J Clin 61(4) (2011) 250–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Rapozzi V, Della Pietra E, Bonavida B, Dual roles of nitric oxide in the regulation of tumor cell response and resistance to photodynamic therapy, Redox biology 6 (2015) 311–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Juzeniene A, Nielsen KP, Moan J, Biophysical aspects of photodynamic therapy, J Environ Pathol Toxicol Oncol 25(1–2) (2006) 7–28. [DOI] [PubMed] [Google Scholar]
- [194].Korbelik M, Parkins CS, Shibuya H, Cecic I, Stratford MR, Chaplin DJ, Nitric oxide production by tumour tissue: impact on the response to photodynamic therapy, Br J Cancer 82(11) (2000) 1835–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Reeves KJ, Reed MW, Brown NJ, Is nitric oxide important in photodynamic therapy?, J Photochem Photobiol B 95(3) (2009) 141–7. [DOI] [PubMed] [Google Scholar]
- [196].Gupta S, Ahmad N, Mukhtar H, Involvement of nitric oxide during phthalocyanine (Pc4) photodynamic therapy-mediated apoptosis, Cancer Res 58(9) (1998) 1785–8. [PubMed] [Google Scholar]
- [197].Girotti AW, Upregulation of nitric oxide in tumor cells as a negative adaptation to photodynamic therapy, Lasers Surg Med 50(5) (2018) 590–598. [DOI] [PubMed] [Google Scholar]
- [198].Ford PC, Photochemical delivery of nitric oxide, Nitric Oxide 34 (2013) 56–64. [DOI] [PubMed] [Google Scholar]
- [199].Duan Y, Wang Y, Li X, Zhang G, Zhang G, Hu J, Light-triggered nitric oxide (NO) release from photoresponsive polymersomes for corneal wound healing, Chem Sci 11(1) (2020) 186–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Seggio M, Nostro A, Ginestra G, Quaglia F, Sortino S, Contact Lenses Delivering Nitric Oxide under Daylight for Reduction of Bacterial Contamination, Int J Mol Sci 20(15) (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].O’Connell KA, Okhovat JP, Zeitouni NC, Photodynamic therapy for Bowen’s Disease (squamous cell carcinoma in situ) current review and update, Photodiagnosis Photodyn Ther 24 (2018) 109–114. [DOI] [PubMed] [Google Scholar]
- [202].Fitzpatrick TB, Johnson RA, Wolff K, Suurmond D, Color atlas and synopsis of clinical dermatology: common and serious diseases, 4th ed. ed., McGraw-Hill; 2001. [Google Scholar]
- [203].Kodela R, Chattopadhyay M, Kashfi K, NOSH-Aspirin: A Novel Nitric Oxide-Hydrogen Sulfide-Releasing Hybrid: A New Class of Anti-inflammatory Pharmaceuticals, ACS medicinal chemistry letters 3(3) (2012) 257–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [204].Kodela R, Chattopadhyay M, Kashfi K, Synthesis and biological activity of NOSH-naproxen (AVT-219) and NOSH-sulindac (AVT-18A) as potent anti-inflammatory agents with chemotherapeutic potential, MedChemComm 4(11) (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].Kashfi K, Chattopadhyay M, Kodela R, NOSH-sulindac (AVT-18A) is a novel nitric oxide- and hydrogen sulfide-releasing hybrid that is gastrointestinal safe and has potent anti-inflammatory, analgesic, antipyretic, anti-platelet, and anti-cancer properties, Redox biology 6 (2015) 287–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [206].Kodela R, Chattopadhyay M, Velazquez-Martinez CA, Kashfi K, NOSH-aspirin (NBS-1120), a novel nitric oxide- and hydrogen sulfide-releasing hybrid has enhanced chemo-preventive properties compared to aspirin, is gastrointestinal safe with all the classic therapeutic indications, Biochemical pharmacology 98(4) (2015) 564–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [207].Chattopadhyay M, Kodela R, Duvalsaint PL, Kashfi K, Gastrointestinal safety, chemotherapeutic potential, and classic pharmacological profile of NOSH-naproxen (AVT-219) a dual NO- and H2S-releasing hybrid, Pharmacology research & perspectives 4(2) (2016) e00224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [208].Chattopadhyay M, Kodela R, Olson KR, Kashfi K, NOSH-aspirin (NBS-1120), a novel nitric oxide- and hydrogen sulfide-releasing hybrid is a potent inhibitor of colon cancer cell growth in vitro and in a xenograft mouse model, Biochemical and biophysical research communications 419(3) (2012) 523–8. [DOI] [PubMed] [Google Scholar]
- [209].Antoniou C, Xenofontos R, Chatzimichail G, Christou A, Kashfi K, Fotopoulos V, Exploring the potential of nitric oxide- and hydrogen-sulfide releasing (NOSH) chimeras as novel priming agents against drought stress in Medicago sativa plants, Biomolecules 10(120) (2020) 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [210].Wu D, Hu Q, Xiong Y, Zhu D, Mao Y, Zhu YZ, Novel H2S-NO hybrid molecule (ZYZ-803) promoted synergistic effects against heart failure, Redox Biol 15 (2018) 243–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [211].Xiong Y, Chang LL, Tran B, Dai T, Zhong R, Mao YC, Zhu YZ, ZYZ-803, a novel hydrogen sulfide-nitric oxide conjugated donor, promotes angiogenesis via cross-talk between STAT3 and CaMKII, Acta Pharmacol Sin (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [212].Miller MR, Megson IL, Recent developments in nitric oxide donor drugs, Br J Pharmacol 151(3) (2007) 305–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [213].Brisbois EJ, Bayliss J, Wu J, Major TC, Xi C, Wang SC, Bartlett RH, Handa H, Meyerhoff ME, Optimized polymeric film-based nitric oxide delivery inhibits bacterial growth in a mouse burn wound model, Acta Biomater 10(10) (2014) 4136–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [214].Macherla C, Sanchez DA, Ahmadi MS, Vellozzi EM, Friedman AJ, Nosanchuk JD, Martinez LR, Nitric oxide releasing nanoparticles for treatment of Candida albicans burn infections, Front Microbiol 3 (2012) 193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [215].Riccio DA, Schoenfisch MH, Nitric oxide release: part I. Macromolecular scaffolds, Chem Soc Rev 41(10) (2012) 3731–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [216].Sung YC, Jin PR, Chu LA, Hsu FF, Wang MR, Chang CC, Chiou SJ, Qiu JT, Gao DY, Lin CC, Chen YS, Hsu YC, Wang J, Wang FN, Yu PL, Chiang AS, Wu AY, Ko JJ, Lai CP, Lu TT, Chen Y, Delivery of nitric oxide with a nanocarrier promotes tumour vessel normalization and potentiates anti-cancer therapies, Nat Nanotechnol 14(12) (2019) 1160–1169. [DOI] [PubMed] [Google Scholar]
- [217].Pinto RV, Fernandes AC, Antunes F, Lin Z, Rocha J, Pires J, Pinto ML, New generation of nitric oxide-releasing porous materials: Assessment of their potential to regulate biological functions, Nitric Oxide 90 (2019) 29–36. [DOI] [PubMed] [Google Scholar]
- [218].Lee YJ, Kim YR, Jeong WY, Lee S, Shin JH, Lee GJ, Potential Protective Effect of Nitric Oxide-Releasing Nanofibers in Hypoxia/Reoxygenation-Induced Cardiomyocyte Injury, J Nanosci Nanotechnol 19(10) (2019) 6539–6545. [DOI] [PubMed] [Google Scholar]
- [219].Quinn JF, Whittaker MR, Davis TP, Delivering nitric oxide with nanoparticles, J Control Release 205 (2015) 190–205. [DOI] [PubMed] [Google Scholar]
- [220].Di Meo C, Capitani D, Mannina L, Brancaleoni E, Galesso D, De Luca G, Crescenzi V, Synthesis and NMR characterization of new hyaluronan-based NO donors, Biomacromolecules 7(4) (2006) 1253–60. [DOI] [PubMed] [Google Scholar]
- [221].Damodaran VB, Place LW, Kipper MJ, Reynolds MM, Enzymatically degradable nitric oxide releasingS-nitrosated dextranthiomers for biomedical applications, J. Mater. Chem (22) (2012) 23038–23048. [Google Scholar]
- [222].Lu Y, Shah A, Hunter RA, Soto RJ, Schoenfisch MH, S-Nitrosothiol-modified nitric oxide-releasing chitosan oligosaccharides as antibacterial agents, Acta Biomater 12 (2015) 62–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [223].Lutzke A, Pegalajar-Jurado A, Neufeld BH, Reynolds MM, Nitric oxide-releasing S-nitrosated derivatives of chitin and chitosan for biomedical applications, J. Mater. Chem. B (2) (2014) 7449–7458. [DOI] [PubMed] [Google Scholar]
- [224].Lu Y, Slomberg DL, Schoenfisch MH, Nitric oxide-releasing chitosan oligosaccharides as antibacterial agents, Biomaterials 35(5) (2014) 1716–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [225].Zhao Q, Zhang J, Song L, Ji Q, Yao Y, Cui Y, Shen J, Wang PG, Kong D, Polysaccharide-based biomaterials with on-demand nitric oxide releasing property regulated by enzyme catalysis, Biomaterials 34(33) (2013) 8450–8. [DOI] [PubMed] [Google Scholar]
- [226].Lowe A, Bills J, Verma R, Lavery L, Davis K, Balkus KJ Jr., Electrospun nitric oxide releasing bandage with enhanced wound healing, Acta Biomater 13 (2015) 121–30. [DOI] [PubMed] [Google Scholar]
- [227].Pinto RV, Pinto ML, Nanoporous Materials: New Generation of Nitric Oxide Donors, in: Bonavida L.M.a.B. (Ed.), Therapeutic Application of Nitric Oxide in Cancer and Inflammatory Disorders, Elsevier - Academic Press, San Diego, CA, USA, 2019, pp. 277–305. [Google Scholar]
- [228].Smith DJ, Chakravarthy D, Pulfer S, Simmons ML, Hrabie JA, Citro ML, Saavedra JE, Davies KM, Hutsell TC, Mooradian DL, Hanson SR, Keefer LK, Nitric oxide-releasing polymers containing the [N(O)NO]- group, J Med Chem 39(5) (1996) 1148–56. [DOI] [PubMed] [Google Scholar]
- [229].Champeau M, Seabra AB, de Oliveira MG, Hydrogels for topical nitric oxide delivery, in: Seabra AB (Ed.), Nitric oxide donors, Academic Press; 2017, pp. 313–330. [Google Scholar]
- [230].Stasko NA, Schoenfisch MH, Dendrimers as a scaffold for nitric oxide release, J Am Chem Soc 128(25) (2006) 8265–71. [DOI] [PubMed] [Google Scholar]
- [231].Lu Y, Sun B, Li C, Schoenfisch MH, Structurally Diverse Nitric Oxide-Releasing Poly(propylene Imine) Dendrimers, Chem Mater 23(18) (2011) 4227–4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [232].Shin JH, Schoenfisch MH, Inorganic/Organic Hybrid Silica Nanoparticles as a Nitric Oxide Delivery Scaffold, Chem Mater 20(1) (2008) 239–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [233].Nam J, Won N, Bang J, Jin H, Park J, Jung S, Jung S, Park Y, Kim S, Surface engineering of inorganic nanoparticles for imaging and therapy, Adv Drug Deliv Rev 65(5) (2013) 622–48. [DOI] [PubMed] [Google Scholar]
- [234].Polizzi MA, Stasko NA, Schoenfisch MH, Water-soluble nitric oxide-releasing gold nanoparticles, Langmuir 23(9) (2007) 4938–43. [DOI] [PubMed] [Google Scholar]
- [235].Rothrock AR, Donkers RL, Schoenfisch MH, Synthesis of nitric oxide-releasing gold nanoparticles, J Am Chem Soc 127(26) (2005) 9362–3. [DOI] [PubMed] [Google Scholar]
- [236].Gehring J, Trepka B, Klinkenberg N, Bronner H, Schleheck D, Polarz S, Sunlight-Triggered Nanoparticle Synergy: Teamwork of Reactive Oxygen Species and Nitric Oxide Released from Mesoporous Organosilica with Advanced Antibacterial Activity, J Am Chem Soc 138(9) (2016) 3076–84. [DOI] [PubMed] [Google Scholar]
- [237].Manosalva N, Tortella G, Cristina Diez M, Schalchli H, Seabra AB, Duran N, Rubilar O, Green synthesis of silver nanoparticles: effect of synthesis reaction parameters on antimicrobial activity, World J Microbiol Biotechnol 35(6) (2019) 88. [DOI] [PubMed] [Google Scholar]
- [238].Zhang XF, Mansouri S, Mbeh DA, Yahia L, Sacher E, Veres T, Nitric oxide delivery by core/shell superparamagnetic nanoparticle vehicles with enhanced biocompatibility, Langmuir 28(35) (2012) 12879–85. [DOI] [PubMed] [Google Scholar]
- [239].Molina MM, Seabra AB, de Oliveira MG, Itri R, Haddad PS, Nitric oxide donor superparamagnetic iron oxide nanoparticles, Mater Sci Eng C Mater Biol Appl 33(2) (2013) 746–51. [DOI] [PubMed] [Google Scholar]
- [240].Singh N, Sallem F, Mirjolet C, Nury T, Sahoo SK, Millot N, Kumar R, Polydopamine Modified Superparamagnetic Iron Oxide Nanoparticles as Multifunctional Nanocarrier for Targeted Prostate Cancer Treatment, Nanomaterials (Basel) 9(2) (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [241].Neuman D, Ostrowski AD, Mikhailovsky AA, Absalonson RO, Strouse GF, Ford PC, Quantum dot fluorescence quenching pathways with Cr(III) complexes. photosensitized NO production from trans-Cr(cyclam)(ONO)2+, J Am Chem Soc 130(1) (2008) 168–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [242].Tan L, Wan A, Li H, Ag2S quantum dots conjugated chitosan nanospheres toward light-triggered nitric oxide release and near-infrared fluorescence imaging, Langmuir 29(48) (2013) 15032–42. [DOI] [PubMed] [Google Scholar]
- [243].Reynolds MM, Frost MC, Meyerhoff ME, Nitric oxide-releasing hydrophobic polymers: preparation, characterization, and potential biomedical applications, Free Radic Biol Med 37(7) (2004) 926–36. [DOI] [PubMed] [Google Scholar]
- [244].Park D, Kim J, Lee YM, Park J, Kim WJ, Polydopamine Hollow Nanoparticle Functionalized with N-diazeniumdiolates as a Nitric Oxide Delivery Carrier for Antibacterial Therapy, Adv Healthc Mater 5(16) (2016) 2019–24. [DOI] [PubMed] [Google Scholar]
- [245].Yang L, Feura ES, Ahonen MJR, Schoenfisch MH, Nitric Oxide-Releasing Macromolecular Scaffolds for Antibacterial Applications, Adv Healthc Mater 7(13) (2018) e1800155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [246].Elnaggar MA, Subbiah R, Han DK, Joung YK, Lipid-based carriers for controlled delivery of nitric oxide, Expert Opin Drug Deliv 14(12) (2017) 1341–1353. [DOI] [PubMed] [Google Scholar]
- [247].Suchyta DJ, Schoenfisch MH, Encapsulation of N-Diazeniumdiolates within Liposomes for Enhanced Nitric Oxide Donor Stability and Delivery, Mol Pharm 12(10) (2015) 3569–74. [DOI] [PubMed] [Google Scholar]
- [248].Mazur F, Bally M, Stadler B, Chandrawati R, Liposomes and lipid bilayers in biosensors, Adv Colloid Interface Sci 249 (2017) 88–99. [DOI] [PubMed] [Google Scholar]
- [249].Pattni BS, Chupin VV, Torchilin VP, New Developments in Liposomal Drug Delivery, Chem Rev 115(19) (2015) 10938–66. [DOI] [PubMed] [Google Scholar]
- [250].Suchyta DJ, Schoenfisch MH, Anticancer potency of nitric oxide-releasing liposomes, RSC Adv 7(84) (2017) 53236–53246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [251].Seabra AB, de Lima R, Calderon M, Nitric oxide releasing nanomaterials for cancer treatment: current status and perspectives, Curr Top Med Chem 15(4) (2015) 298–308. [DOI] [PubMed] [Google Scholar]
- [252].Morris RE, Wheatley PS, Gas storage in nanoporous materials, Angewandte Chemie (International ed. in English) 47(27) (2008) 4966–81. [DOI] [PubMed] [Google Scholar]
- [253].Pinto RV, Antunes F, Pires J, Graca V, Brandao P, Pinto ML, Vitamin B3 metal-organic frameworks as potential delivery vehicles for therapeutic nitric oxide, Acta Biomater 51 (2017) 66–74. [DOI] [PubMed] [Google Scholar]
- [254].Pinto ML, Rocha J, Gomes JR, Pires J, Slow release of NO by microporous titanosilicate ETS-4, J Am Chem Soc 133(16) (2011) 6396–402. [DOI] [PubMed] [Google Scholar]
- [255].Wheatley PS, Butler AR, Crane MS, Rossi AG, Megson IL, Morris RE, Zeolites for storage and delivery of nitric oxide in human physiology, in: Čejka NŽJ, Nachtigall P (Ed.), Molecular Sieves: From Basic Research to Industrial Applications, Elsevier; 2005, pp. 2033–2040. [Google Scholar]
- [256].Mowbray M, Tan X, Wheatley PS, Rossi AG, Morris RE, Weller RB, Topically applied nitric oxide induces T-lymphocyte infiltration in human skin, but minimal inflammation, J Invest Dermatol 128(2) (2008) 352–60. [DOI] [PubMed] [Google Scholar]
- [257].Neidrauer M, Ercan UK, Bhattacharyya A, Samuels J, Sedlak J, Trikha R, Barbee KA, Weingarten MS, Joshi SG, Antimicrobial efficacy and wound-healing property of a topical ointment containing nitric-oxide-loaded zeolites, J Med Microbiol 63(Pt 2) (2014) 203–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [258].Fox S, Wilkinson TS, Wheatley PS, Xiao B, Morris RE, Sutherland A, Simpson AJ, Barlow PG, Butler AR, Megson IL, Rossi AG, NO-loaded Zn(2+)-exchanged zeolite materials: a potential bifunctional anti-bacterial strategy, Acta Biomater 6(4) (2010) 1515–21. [DOI] [PubMed] [Google Scholar]
- [259].Viseras C, Cerezo P, Sanchez R, Salcedo I, Aguzzi C, Current challenges in clay minerals for drug delivery, Applied Clay Science 48(3) (2010) 291–295. [Google Scholar]
- [260].Fernandes AC, Pinto ML, Antunes F, Pires J, Clay based materials for storage and therapeutic release of nitric oxide, J. Mater. Chem. B (1) (2013) 3287–3294 [DOI] [PubMed] [Google Scholar]
- [261].Fernandes AC, Pinto ML, Antunes F, Pires J, L-Histidine-based organoclays for the storage and release of therapeutic nitric oxide, J. Mater. Chem. B 3(17) (2015) 3556–3563. [DOI] [PubMed] [Google Scholar]
- [262].Fernandes AC, Pinto ML, Antunes F, Pires J, Synthetic cobalt clays for the storage and slow release of therapeutic nitric oxide, RSC Adv 6(47) (2016) 41195–41203. [Google Scholar]
- [263].Maurin G, Serre C, Cooper A, Ferey G, The new age of MOFs and of their porous-related solids, Chem Soc Rev 46(11) (2017) 3104–3107. [DOI] [PubMed] [Google Scholar]
- [264].Schanuel FS, Raggio Santos KS, Monte-Alto-Costa A, de Oliveira MG, Combined nitric oxide-releasing poly(vinyl alcohol) film/F127 hydrogel for accelerating wound healing, Colloids Surf B Biointerfaces 130 (2015) 182–91. [DOI] [PubMed] [Google Scholar]
- [265].Marcilli RH, de Oliveira MG, Nitric oxide-releasing poly(vinyl alcohol) film for increasing dermal vasodilation, Colloids Surf B Biointerfaces 116 (2014) 643–51. [DOI] [PubMed] [Google Scholar]
- [266].Halpenny GM, Steinhardt RC, Okialda KA, Mascharak PK, Characterization of pHEMA-based hydrogels that exhibit light-induced bactericidal effect via release of NO, J Mater Sci Mater Med 20(11) (2009) 2353–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [267].Gao J, Zheng W, Zhang J, Guan D, Yang Z, Kong D, Zhao Q, Enzyme-controllable delivery of nitric oxide from a molecular hydrogel, Chem Commun (Camb) 49(80) (2013) 9173–5. [DOI] [PubMed] [Google Scholar]
- [268].Ondrias K, Stasko A, Cacanyiova S, Sulova Z, Krizanova O, Kristek F, Malekova L, Knezl V, Breier A, H(2)S and HS(−) donor NaHS releases nitric oxide from nitrosothiols, metal nitrosyl complex, brain homogenate and murine L1210 leukaemia cells, Pflugers Archiv : European journal of physiology 457(2) (2008) 271–9. [DOI] [PubMed] [Google Scholar]
- [269].Cortese-Krott MM, Kuhnle GG, Dyson A, Fernandez BO, Grman M, DuMond JF, Barrow MP, McLeod G, Nakagawa H, Ondrias K, Nagy P, King SB, Saavedra JE, Keefer LK, Singer M, Kelm M, Butler AR, Feelisch M, Key bioactive reaction products of the NO/H2S interaction are S/N-hybrid species, polysulfides, and nitroxyl, Proceedings of the National Academy of Sciences of the United States of America (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [270].Cortese-Krott MM, Kuhnle GG, Dyson A, Fernandez BO, Grman M, DuMond JF, Barrow MP, McLeod G, Nakagawa H, Ondrias K, Nagy P, King SB, Saavedra JE, Keefer LK, Singer M, Kelm M, Butler AR, Feelisch M, Key bioactive reaction products of the NO/H2S interaction are S/N-hybrid species, polysulfides, and nitroxyl, Proc Natl Acad Sci U S A 112(34) (2015) E4651–60. [DOI] [PMC free article] [PubMed] [Google Scholar]


