Abstract
Objective:
Among older adult women with early-stage breast cancer who undergo lumpectomy, the benefits of radiotherapy vary according to tumor characteristics and life expectancy. We aimed to develop a risk calculator to predict individualized probability of long-term survival and local recurrence, accounting for these factors.
Methods
We developed a simulation model to estimate an individual patient’s risk of local recurrence and all-cause mortality according to age, comorbidities, functional status, tumor characteristics, and radiotherapy status. We integrated two existing prediction models, the Early Breast Cancer Trialist’s Collaborative Group prediction model for breast cancer specific outcomes and ePrognosis for life expectancy. An online risk calculator “Radiotherapy for Older Women (ROW)” was developed through an iterative multi-stage process, that included individual consultation and group meetings with an advisory committee (AC) comprised of patients, advocates, clinicians, and researchers.
Results
We developed the tool over 40 months and had 15 group meetings. The risk calculator developed as a simulation model with 16 factors (5 tumor-related, 3 demographic, 4 comorbidities, and 4 functional statuses). Across 56,700 simulated scenarios, the benefit of RT in terms of absolute 10-year local recurrence reduction, ranged from 0% to 34%, depending on individual characteristics. Based on feedback from the AC, overall survival and local recurrence were chosen as the output for ROW, with these outcomes displayed numerically (percentages and natural frequencies) and graphically (pictographs).
Conclusions
This tool “ROW” could facilitate shared decision making regarding receipt of radiotherapy for older women with early breast cancer. Additional studies to examine usability testing are underway.
Introduction
For older adult women with early-stage, estrogen receptor (ER) positive breast cancer who undergo breast-conserving surgery (BCS), two randomized controlled trials have demonstrated that adjuvant whole breast radiation therapy (WBRT, hereafter as RT) reduces local recurrence but does not improve overall survival. (1, 2) Based on these data, consensus guidelines recommend that omission of RT can safely be considered for women aged 70 or older with stage I disease who receive endocrine therapy (ET).(3) However, more than two-thirds of these women undergo RT.(4, 5) While RT does reduce local recurrence, it also requires travel to receive daily radiation for several weeks and poses risks of side effects, such as fatigue, breast pain, and pneumonitis.(6–10) Therefore, it is critical that women are well-informed when considering the receipt of RT.
Risk of local recurrence can vary significantly. Tumor size, tumor grade, margin width, ER status, and number of positive axillary nodes, for example, have all been associated with local recurrence.(11–13) Several risk calculators or nomograms have been developed to help RT decision-making by predicting outcomes with and without adjuvant RT.(12) However, these programs do not focus on older women, and do not incorporate competing mortality. It is well known that the benefits of an intervention decrease as life expectancy decreases.(14, 15) Older patients are more likely to have multiple comorbidities or impaired functional status, and to die of non-breast-cancer-related causes, and therefore might be less likely to benefit from RT. To our knowledge, there is no RT risk calculator that incorporates life expectancy into its prediction model, despite the substantive relationship between life expectancy and recurrence in this population.
Accordingly, we sought to develop an interactive, individualized, automated risk calculator “Radiotherapy for Older Women (ROW).” Our objective was to promote patient-centered treatment decisions by providing personalized risk estimates for older women with early-stage breast cancer. We proposed a novel model that integrates two published predictive models, ePrognosis (a survival prediction tool frequently used in geriatric assessment)(16, 17) and the Early Breast Cancer Trialist’s Collaborative Group (EBCTCG) prediction models.(13) We also convened an advisory committee (AC) to help develop ROW to be patient-centered and user-friendly. Through collaborative discussions, we presented individualized outcome estimates in an electronic format to facilitate provider-patient communication.
Methods
Study Design
The Human Investigation Committee of the authors’ institution approved this study. A synopsis of the study design is shown in Figure 1. In brief, this was a two-part study: (1) development of simulation models to estimate personalized outcomes, with and without RT; and (2) development of a risk calculator for older adult women deciding whether or not to undergo RT for early-stage breast cancer. As stakeholder engagement is a crucial component to the success of decision support development, patient clinician and researcher partners have been involved since inception. We organized an AC (Appendix A for detailed information about the committee members),(18) which included breast cancer survivors (n=6), advocates of breast cancer care and aging (n=7), oncology clinicians (n=4), and researchers (n=4). Our clinician partners consist of a multidisciplinary team, including a radiation oncologist, a medical oncologist, a surgical oncologist, and a geriatric oncologist. While researchers are from one single institute, the patient committee members were recruited from eight organizations across the state of Connecticut. Our patient committee members were also racially/ethnically diverse; five are African Americans and two are Hispanics. The AC plays a central role in guiding the calculator development, including but not limited to overviewing the assumptions of the simulation models and designing the layouts of the risk calculator. The iterative staged process occurred over 40 months through 15 group meetings and numerous individual consults. For instance, committee members served as a focus group in which the members’ perceptions, opinions, and attitudes toward the risk calculator were discussed during group meetings.
Figure 1.
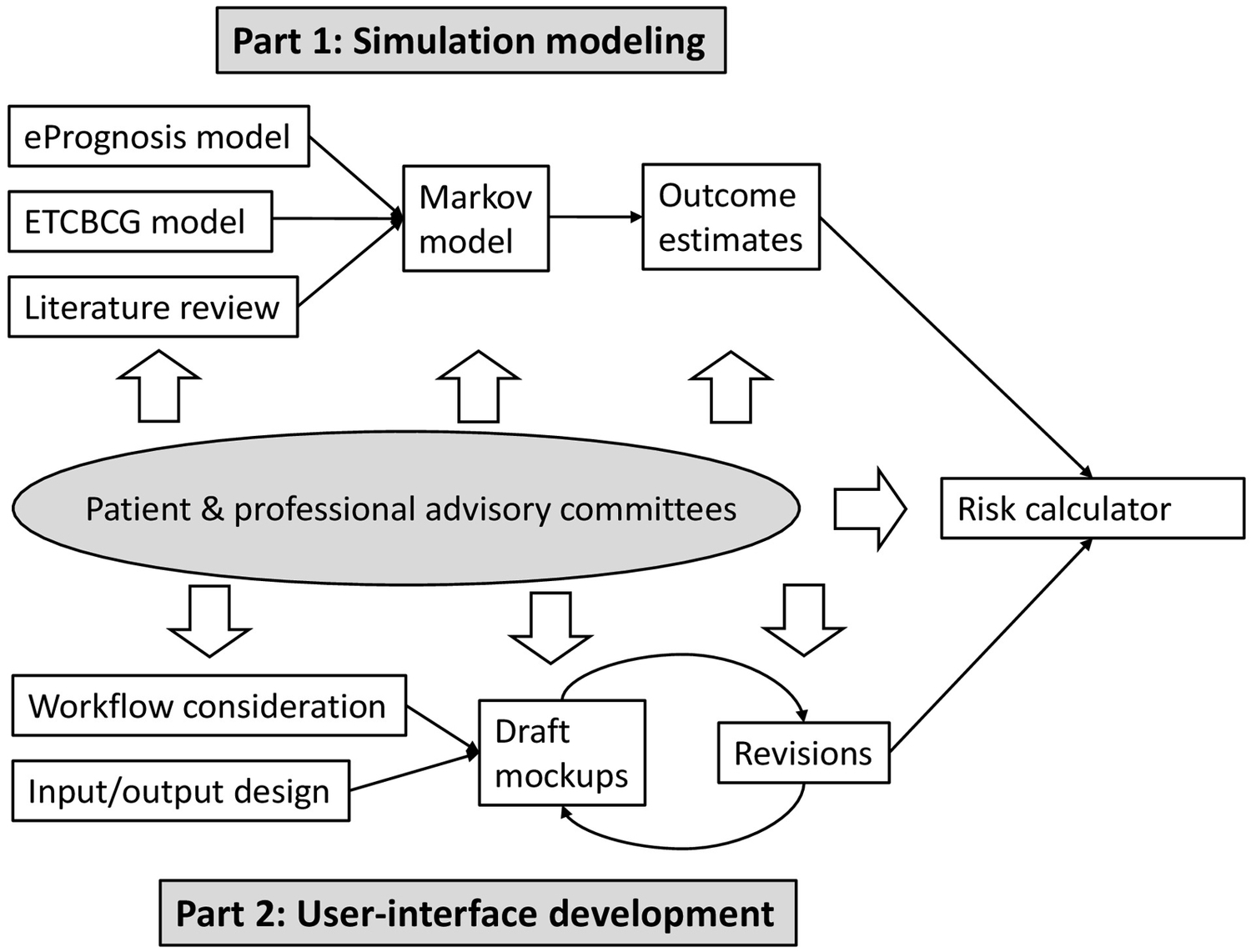
Overview of the development process
Simulation models
We constructed a prediction model that incorporated two main elements. First, we estimated 10-year mortality risks for older women without breast cancer by using ePrognosis, a validated prediction tool used by physicians to estimate life expectancy.(16) Based on patient age, sex, body mass index (BMI), smoking, functional status, and comorbidities, ePrognosis categorizes patients by the Lee index (ranging from 0 to 26) and predicts the risk of 10-year mortality for each stratum. Second, we estimated breast cancer specific outcomes using the EBCTCG prediction model regarding the efficacy of RT.(13) The EBCTCG model uses age, tumor size, tumor grade, and ER status to predict 10-year recurrence rates, with and without RT among women with lymph node (LN)-negative breast cancer. Using these two inputs, we constructed a Markov model, integrating the 10-year mortality from ePrognosis as the background mortality into the EBCTCG model. We incorporated the relative risks of recurrence attributed to LN status and margin status to estimate risks for the scenarios beyond the EBCTCG model.(19–21) This new model yielded individualized outcome estimates, including local breast cancer recurrence and overall survival, taking into account individual comorbidities, functional status, and tumor characteristics.
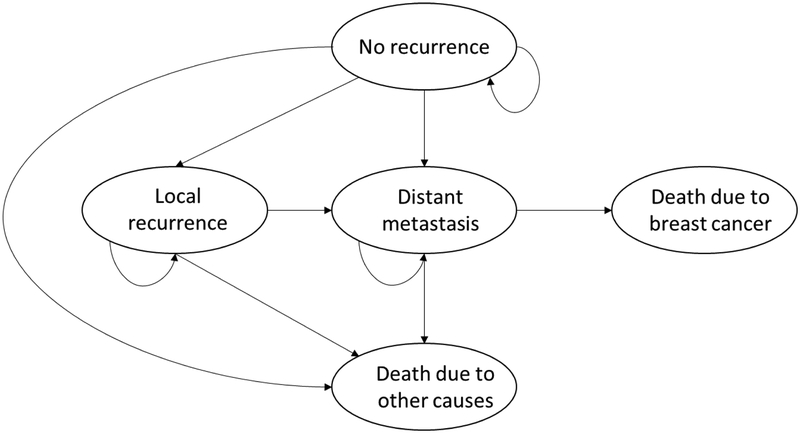
Our Markov model consisted of five health states, including no recurrence, local recurrence, distant metastasis, death due to other causes, and death due to breast cancer (Appendix B). Background mortality, the transition probability from state ‘no recurrence’, as well as state “local recurrence” and state “distant metastasis”, to state ‘death due to other disease’ was derived from the 10-year mortality based on the Lee index.(16) Our simulation was limited to female gender, and breast cancer mortality was excluded in the background mortality calculation (as we estimated breast cancer mortality separately).
The EBCTCG report provides the 10-year risks of any first recurrence (including both local recurrence and distant metastasis) according to age, tumor grade, tumor size, ER status, and RT status among LN-negative women who underwent BCS.(13) Using the proportion of local-regional recurrence vs. distant recurrence from the Cancer and Leukemia Group B (CALGB) C9343 trial,(22) we estimated the 10-year local-regional recurrence rate and distant metastasis rate separately. We incorporated the relative risks of LN positivity to estimate risks of recurrence for patients with LN-positive breast cancer (0–3 and ≥4).(23) Patients with local-regional recurrence were modeled with a higher probability of developing distant metastasis than those without local-regional recurrence.(24, 25) We assumed the annual rate of death due to metastatic breast cancer to be 0.243.(26) With the use of complementary data (19–21), we extended the risk estimations beyond the EBCTCGgroups to more specific subgroups by age, tumor size, and margin status. The detailed information of model assumptions and parameters are presented in Appendix C (online only).
We acknowledged that providers might have had other prognostic information beyond the variables included in our simulation models, such as factors related to overall health (other comorbidities or conditions) and tumor characteristics (Ki-67, lymphovascular invasion, potential size uncertainties, or genomic profiling results). Taking this into account, we conducted additional simulations which allowed adjustment of the risk estimates (more favorable, neutral [default], and less favorable for both health conditions and breast cancer characteristics). For example, when providers select a “less favorable” health condition because the patient has end-stage renal disease and undergoes maintenance hemodialysis, the tool will increase the Lee index by one to reflect the projected increase in mortality risk. Similarly, we assumed women with less favorable tumor characteristics were more likely to have recurrence than women with “neutral” tumor characteristics, with relative risk of 1.1. This was necessary because the current EBCTCG prediction did not include genomic profiling information or HER2 status. Thus, the final model considered 18 parameters (Table 1). We conducted 56,700 model simulations using each combination of 18 input parameters.
Table 1.
Model inputs
| Input parameter | Possible values |
|---|---|
| Age | 65–69, 70–74, 75–79, 80–84, and ≥85 |
| Body mass index | <25 vs. ≥25 |
| Cigarette use | current user vs. former user or non-smoker |
| Chronic obstructive pulmonary disease | yes vs. no |
| Other cancer | yes vs. no |
| Congestive heart failure | yes vs. no |
| Diabetes | yes vs. no |
| Difficulty walking several blocks | yes vs. no |
| Difficulty managing finances | yes vs. no |
| Difficulty bathing | yes vs. no |
| Difficulty pushing/pulling large objects | yes vs. no |
| Tumor grade | low, intermediate, and high |
| Tumor size | 0–0.5cm, 0.6–1.0cm, 1.1–1.5cm, 1.6–2.0cm, and >2.0cm |
| Estrogen receptor status | positive vs. negative |
| Margin | positive, close, and negative |
| Lymph nodes | 0, 1–3, and ≥4 |
| Additional health conditions | neutral, more favorable, and less favorable |
| Additional breast cancer factors | neutral, more favorable, and less favorable |
User-interface development
Employing a user-centered approach, we designed the ROW web-based risk calculator. The development involved (1) integrating the risk calculator with the routine workflow, (2) specifying and designing input entries and output reports, and (3) designing and testing the user interface. To reduce clinical workload and engage patients, the ROW risk calculator allows patients to input demographics, comorbidities, and functional status independently before clinic visits. We require providers to input tumor characteristics. After entering tumor characteristics, providers and patients review the input summary together (see Figure 2). ROW allows physicians to correct any inaccuracies or “unknown” input. This final check can be completed directly from the summary screen; that is, providers do not need to return back to previous screens.
Figure 2.
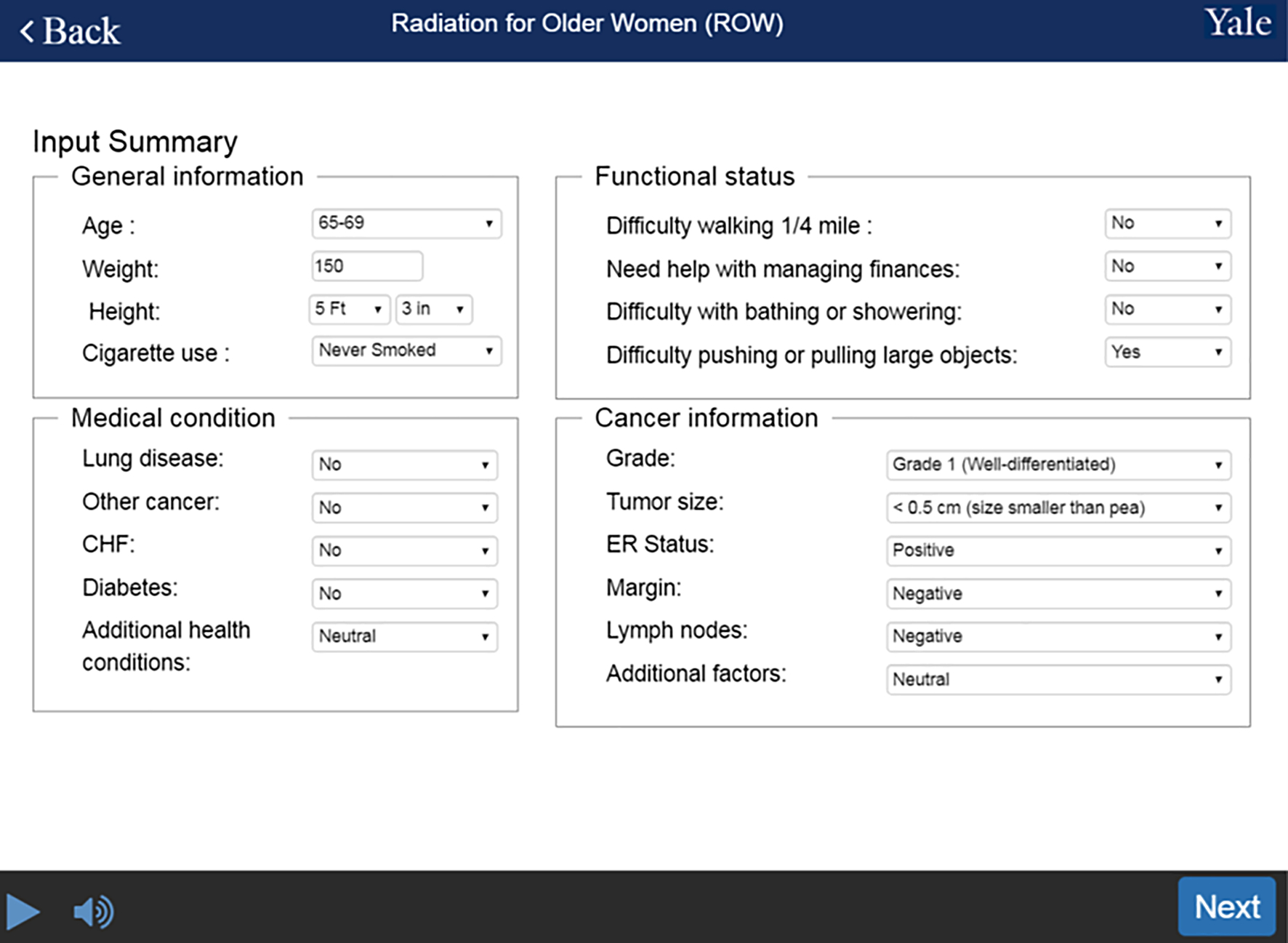
Input summary
To communicate the outcomes generated by our simulation modeling, we reviewed several mockups with the AC to determine the final design. ROW is designed to first provide the estimates of absolute risk for overall mortality, with and without undergoing RT. It then compares the risk of local breast cancer recurrence between RT and no RT. Additionally, ROW provides risk estimates in 5-year and 10-year intervals. The visual presentation of the calculated risk is described using both numerical (percentages and natural frequencies) and graphical (pictographs) presentations.
Results
Simulation results
Among 56,700 simulations, the mean 10-year local recurrence was 22.2% (range: 1–73%) for patients not undergoing RT, compared to 8.6% (range: 0–41%) for patients undergoing RT. The average absolute benefit of RT in 10-year local recurrence reduction across all scenarios was 13.5%, ranging from 0% to 34%. The mean 10-year all-cause mortality was 53.4% (range: 597%) for those not undergoing RT and 52.3% (range: 5–97%) if undergoing RT. The absolute mortality reduction attributable to the receipt of RT in our simulation was therefore 1.2% (range: 0–5%). Based on our estimation, RT reduces overall mortality when it reduces 10-year local recurrence risk by 6%.
The projected outcome estimates were consistent with clinically expected benefits of RT, as illustrated in four scenarios (Table 2). Each scenario represents a combination of two sets of ePrognosis variables (short vs. long life expectancy) and two sets of tumor characteristics (aggressive vs. non-aggressive breast cancer). Our simulation projected that RT would not improve overall survival except for women who had aggressive breast cancer and long life expectancy. Additionally, the model demonstrated that RT reduces local recurrence, especially for women with aggressive breast cancer and long life expectancy. For instance, the absolute risk reduction in 10-year local recurrence attributed to RT was 30% for patients with aggressive breast cancer and long life expectancy. However, the magnitude decreased to 17% for patients with same tumor characteristics but having multiple comorbidities and low functional status. Similarly, among patients with non-aggressive breast cancer, the projected absolute risk reduction was larger for patients with long life expectancy, compared with those with short life expectancy.
Table 2.
Projected benefits of radiotherapy, by life expectancy and breast cancer aggressiveness
| Aggressive breast cancer | Non-aggressive breast cancer | |||
|---|---|---|---|---|
| No RT | RT | No RT | RT | |
| 10-year overall survival | ||||
| Short life expectancy | 24% | 24% | 29% | 29% |
| Long life expectancy | 72% | 77% | 92% | 92% |
| 10-year local recurrence | ||||
| Short life expectancy | 28% | 11% | 3% | 1% |
| Long life expectancy | 52% | 22% | 7% | 2% |
Aggressive breast cancer: high grade, ≥2cm, estrogen receptor-negative, positive margin, ≥4 lymph node involvement
Non-aggressive breast cancer: low grade, 0.5–0.99cm, estrogen receptor-positive, negative margin, no lymph node involvement
Short life expectancy: age ≥85, BMI>25, never smoked, comorbidities of congestive heart failure and diabetes, and difficulty pushing or pulling large objects
Long life expectancy: age 65–69, BMI>25, never smoked, no comorbidities, and no functional impairment
The ROW risk calculator
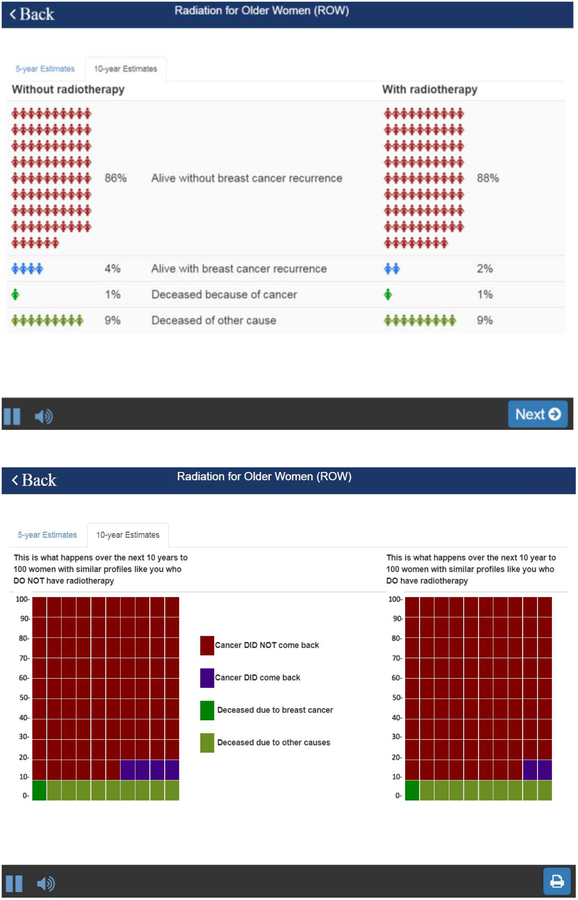
There are several important features of the interface: First, because our population is limited to women over age 65, each question is shown on one screen, enabling a large font size. Second, ROW has an audio function to and includes voice overs of all content. Third, the time to complete the task is short so as not to disrupt workflow. Patients can enter the required information within 10 minutes while waiting to be called. Finally, once all parameters are entered, ROW shows the projections of outcomes in numerical and graphical format (Figures 3A and 3B, details below). A beta-version of this risk calculator can be accessed online at https://rtbreastcancer.org.
Figure 3.
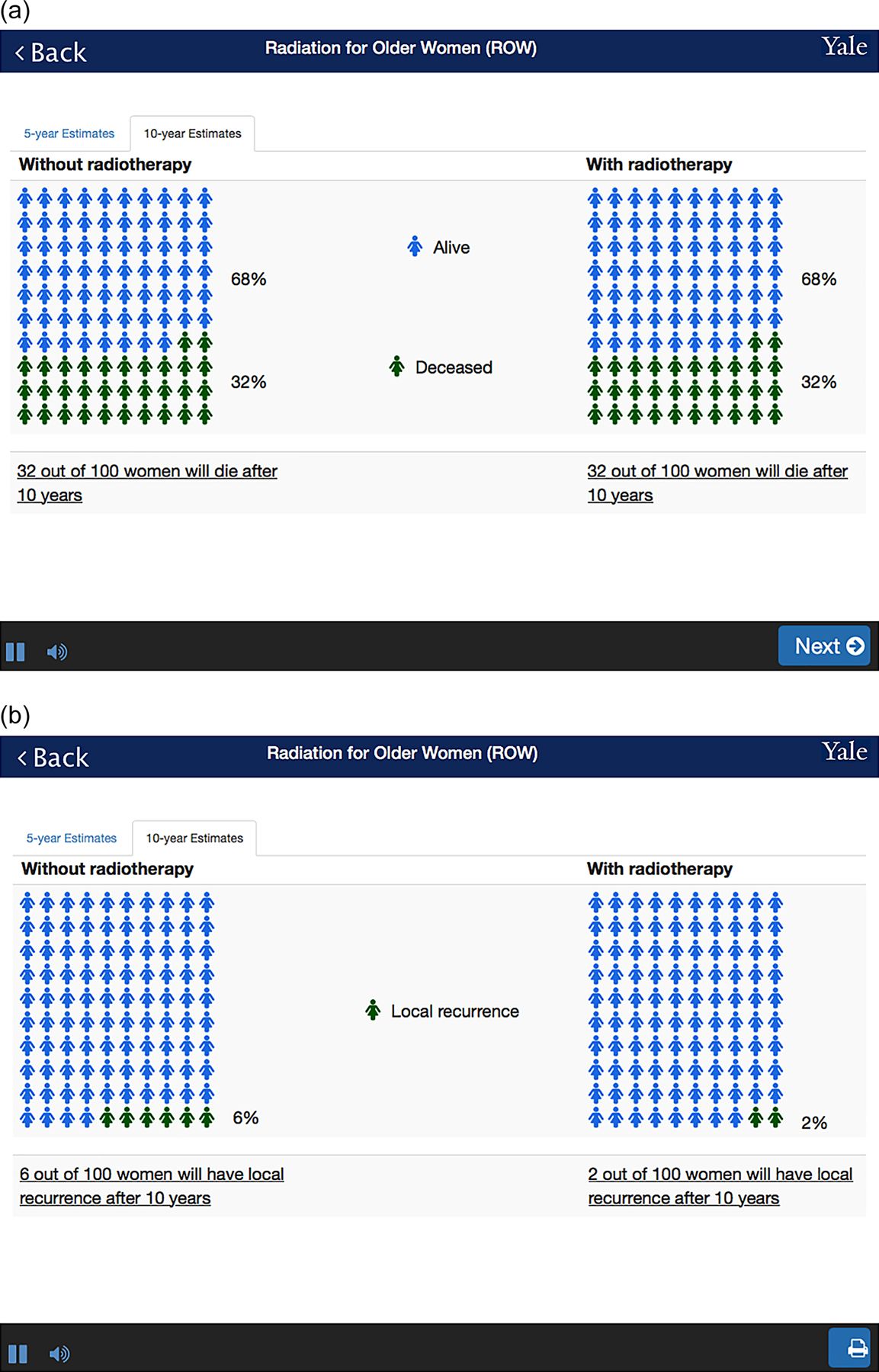
An output example: A. Overall survival; and B. Local recurrence
Pictographs are used to visually present the risks and benefits of RT. We provided both 5-year and 10-year estimates, reporting the frequencies of outcomes. First, we show the estimated overall survival with and without RT (Figure 3A). Based on our simulation, the estimates between two treatment strategies are identical, except in the setting of high risk breast cancer. Second, we show the estimated proportion of local recurrence with and without RT, as well as the frequencies of local recurrence (Figure 3B).
ROW development
During our development phase, the risk calculator evolved as stakeholders provide feedback. Their suggestions led to changes in several interface elements such as font size, color, wording, and layout. At one point, ROW used two different ways to show the estimates of four outcomes, including survival without recurrence, survival with recurrence, breast cancer mortality, and other cause mortality (Appendix D). While the layout was comprehensive, it increased the complexity of the pictograph. Additionally, demonstrating the rates of distant metastasis and death from breast cancer in the radiation oncology encounter was felt to have the potential to undermine information given by other members of the multidisciplinary team. Because prognostication for distant metastasis and breast cancer-specific survival is best estimated by medical oncologist when making systematic therapy decisions, we removed these outcomes and redesigned the presentation format. Specifically, we included one screen for overall survival and one for local recurrence. Clinician stakeholders indicated that the revision was better aligned to how they communicate outcomes in clinical practice and patient stakeholders reported that graphical risk presentations facilitated understanding of radiotherapy benefits.
Discussion
Approximately half of all breast cancers in the United States occur in women over the age of 65.(27) For those with early disease, understanding the risks and benefits of RT is critical for treatment decision-making. There is, however, no risk calculator specific for the older adult population. As a majority of older adult women undergo RT despite a lack of survival benefit,(28) developing a patient-centered risk calculator to provide individualized risk estimates could help facilitate provider-patient communications.
The risk calculator not only can be used in clinical practice by itself but also could be integrated into a larger decision-support tool. The ability of ROW to present individualized benefits of RT has a unique advantage in decision aid development. Many decision aids have been developed to support shared decision-making processes: one of the major limitations of most existing decision aids is that they are often generic in nature, providing information on risk estimates by aggregating the patient outcomes. In practice, however, patients and providers need information that is individualized.(29) Prior literature has shown the benefits of individualized health information for patients and providers.(30–32) Compared with generic information, individualized information improved patient satisfaction and was more likely to lead to behavior change.(30) Additionally, lack of applicability due to patient characteristics has been identified as one of the most often reported barriers to implement shared decision-making in clinical practice,(33, 34) reflecting the importance to take individual situation into consideration.
Using simulation modeling, we were able to project outcome estimates, which were then used as the output of our risk calculator. The EBCTCG model has demonstrated that the benefit of RT in recurrence risk reduction varied across groups with different age and tumor characteristics. The model, however, does not include comorbidities and functional impairment, which are more prevalent in older adults and associated with decreased life expectancy. Building upon the EBCTCG model, we demonstrated that the benefit of RT in local recurrence reduction also differed greatly by individual life expectancy (as illustrated in Table 2). Thus, even if a radiation oncologist was able to use ePrognosis to estimate patient life expectancy, the RT benefit estimates derived from EBCTCG model for a patient with comorbidities or functional impairment could not be directly used. The provider needs to make some qualitative adjustment, which may be difficult for providers to discuss with patients. Availability of quantitative and personalized risk estimates as provided by ROW can help physicians communicate with their patients effectively, and empower patients to make an informed decision. The tool also has the potential to promote the use of geriatric assessments because information regarding comorbidities and functional status is required for risk estimation.
ROW was developed using early and consistent stakeholder engagement. Meeting with patients, advocates for breast cancer and aging care, and clinicians allowed us to integrate the simulation results into the risk calculator in a user-friendly fashion. The benefits of an iterative staged process ensured that the contents of ROW, such as information and layout, are responsive to the complexities of the decision context.
Our study has several limitations. First, our simulation applied a number of simplifying approximations to produce the estimates. For instance, the local recurrence estimates were derived from the EBCTCG data, which contains trials from decades ago. The estimates may not be generalizable to contemporary populations. Additionally, the estimates have not yet been validated. However, to validate the results presented by ROW is challenging because there are no population-based databases that have all information regarding comorbidities, functional status, tumor characteristic, and outcomes. We are planning to analyze patients in our institution to validate our estimates. Second, we did not account for compliance with endocrine therapy, as our risk calculator, based on the EBCTCG model, assumed that all ER-positive patients would receive endocrine therapy. Thus, for patients with ER-positive breast cancer, ROW indicates that the risk estimates apply to those who are taking hormone therapy. Given that non-compliance with endocrine therapy could definitively impact local control and survival, developing a risk calculator which can take endocrine therapy compliance into consideration is needed.
Additionally, the intervention strategy in our risk calculator was limited to whole breast RT, and therefore this tool is not appropriate for women making a decision about partial breast radiation. Fourth, a usability/feasibility study is still in progress: We are recruiting eligible patients from radiation oncology clinics and health volunteers from community to understand the experiences of using this tool. The findings from the ongoing usability and feasibility study could further improve the functionality of the risk calculator. Finally, we acknowledge that not all prognostic factors are included in our calculator. For example, HER2-receptor status and 21-gene assay result can provide important prognostic information. Although the tool has categories (such as “more favorable” and “less favorable”) for providers to modify the risk estimates, the magnitude of this adjustment has been clinically decided. Future research is needed to incorporate these factors in the prediction model.
In conclusion, we used simulation modeling to estimate local recurrence and overall survival for older women with early stage breast cancer, accounting for life expectancy and tumor characteristics. We used a patient-centered approach to design the user interface that presents individualized outcome estimates. Our study highlights the strength of multidisciplinary collaboration and stakeholder engagement. Our risk assessment tool could be ready for dissemination and implementation testing and has potential to help facilitate patient-specific RT decision-making.
Acknowledgement:
We appreciate Jazbel Wang for her assistance in website development.
Funding: This study was supported by grant 1K01HS023900-01 from the Agency for Healthcare Research and Quality (Dr. Wang), and by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, part of the National Institutes of Health, under Award Number AR060231-05 (Dr. Fraenkel). The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.
Financial Disclosures: Drs. Wang and Mougalian receive research support from Genentech. Dr. Killelea receives consulting fees from Genentech. Dr. Mougalian receives consulting fees from Eisai, Inc. Drs. Gross and Mougalian are on a grant sponsored by National Comprehensive Cancer Network/Pfizer. Drs. Evans and Gross receives research support from 21st Century Oncology. Dr. Gross receives research support from Johnson & Johnson. These sources of support were not used for any portion of the current manuscript. None of the other coauthors have conflicts to report.
Appendix A
Advisory Committee Member
Dr. Abujarad
Researcher in computer science, Assistant Professor of Emergency Medicine, Yale University
Ms. Douglas
Breast cancer survivor; Patient advocate, Breast Cancer Alliance & National Lymphedema Network
Ms. Fedus
Gerontologist & Founder, Borrow My Glasses, LLC
Dr. Evans
Radiation oncologist, Associate Professor of Therapeutic Radiology, Yale University
Dr. Fraenkel
Researcher in decision science, Professor of Medicine (Rheumatology), Yale University
Ms. Freeman (through 2019)
Breast cancer survivor; Retired social worker and educator
Ms. Gilliland
Asst. Director, Clinical Program Development, VNA Community Healthcare of Guilford and Hamden
Ms. Gonzalez
Breast cancer survivor; Community Outreach Worker, CancerCare of Connecticut
Cary Gross
Researcher in cancer outcomes, Professor of Medicine (General Medicine), Yale University
Ms. Kidder
Director, Aging Resource Center, Agency on Aging of South Central Connecticut
Dr. Killelea
Surgical oncologist, Associate Professor of Surgery, Yale University
Dr. Mougalian
Medical oncologist, Assistant Professor of Medicine, Yale University
Ms. Nugent
Director of Regional Programs, CancerCare
Dr. Presley (through 2017)
Geriatric oncologist, Clinical Fellow in Medicine, Yale University
Ms. Pruitt
Aging Resource Center Coordinator, Agency on Aging of South Central Connecticut
Ms. Randolph
Breast cancer survivor; Representative, Sisters’ Journey
Ms. Roberts
Breast cancer survivor; Executive Director, Nubian Sisters Cancer Support Group
Ms. Santoro
Breast cancer survivor; Caregiver, Caregiver Support Network, VNA Community Healthcare
Ms. Sewell
Family member and supporter of cancer survivors, Nubian Sisters Cancer Support Group
Ms. Torres
Cancer Screening Coordinator/Referrals Supervisor, Fair Haven Community Health Center; Project Director, Komen Breast Cancer Project, Komen Connecticut
Appendix B
Schematic view of Markov model
Appendix C
Simulation Methods
We constructed a Markov model (Appendix B) to estimate individual outcomes, including breast cancer local recurrence, breast cancer specific mortality, and death due to other diseases, taking account for individual comorbidities, functional status, and tumor characteristics.
Input parameters
Background mortality rate
The transition probability from state ‘no recurrence’, as well as state “local recurrence” and state “distant metastasis”, to state ‘death due to other disease’ was derived from the 10-year mortality based on the Lee index.1 Based on patient age, sex, BMI, smoking, general health condition, functional status, and a variety of important comorbidities, the Lee index (ranging from 0 to 26) has been validated in predicting 10-year mortality rate (Appendix Table 1). To reflect our population, our tool was limited to female, and breast cancer mortality was excluded in background mortality calculation (we estimated breast cancer mortality separately, see below).
Local recurrence and distant metastasis rates
The EBCTCG report provides the 10-year risks of any first recurrence (including both local recurrence and distant metastasis) according to prognostic and other factors among node negative women allocated to BCS without RT.2 With this information, we obtained 10-year any recurrence rates for prognostic groups defined by combination of the factors including age (60–70, or 70+), tumor grade (low, intermediate, or high), tumor size (1–20 mm or 21–50 mm), and estrogen receptor status (“Lumpectomy, ER-poor” in original table as ER negative, “Lumpectomy, ER+ tam+” as ER positive) for the BCS alone group (Appendix Table 2). Note that the categorization of tumor grade and ER status fits in with our model.
We calibrated the risks by tumor size and age using 1) literature review reporting risk estimates of age and tumor size; and 2) distributions of age and tumor size. We first assumed the relative risk (RR) of recurrence for patients aged 70, compared to those aged 65 is equal to RR of recurrence for patients aged 75, compared to those aged 70. Using RR between age above 70 vs age 60–69 = 1.183 and the age distributions in the SEER-Medicare who received BCS, the RR of age 65 vs 70 was estimated at 1.07. Similarly, using the RR of tumor size from prior literature,4 we estimated the risks for tumor size 0–0.5cm, 0.6–1.0cm, 1.1–1.5cm, 1.6–2.0cm, and >2cm. Because some of the EBCTCG trials might include participants with positive margin, which would have a higher risk of recurrence, we applied the ECBTCG risk estimates to patients who had a close margin (0–2mm). Using estimates from a previous literature review,5 we estimated the 10-year any recurrence rates for other margin groups (positive margin and margin ≥2mm).
We incorporate the RRs of LN positivity to estimate risks of recurrence for patients with LN positive breast cancer (0–3 and ≥4).6 Lastly, we separated 10-year any recurrence rate to local-regional recurrence and distant metastasis using the information from the C9343 trial;7 that is, among patients with any recurrence, the proportion of local recurrence was estimated as 76.2%, and the proportion of distant metastasis was 23.8%.
To estimate risks for patients who received RT, we assumed RT is able to reduce local recurrence but not “distant metastasis only” recurrence. The RR of RT on local recurrence was estimated as 0.33, based on prior literature.8 We allowed that patients with local recurrence with higher probability of developing distant metastasis, depending on lymph node involvement.9,10 For instance, among patients with nodal negative breast cancer, annual probability of distant metastasis after local recurrence was 11% if local recurrence occurred less than 4 years, and was 6% if local recurrence occurred longer than 4 years.(12) The annual rate of death due to metastatic breast cancer is 0.243.11 The detailed information of relative risks we used were reported in Appendix Table 3.
References
1. Cruz M, Covinsky K, Widera EW, Stijacic-Cenzer I, Lee SJ. Predicting 10-year mortality for older adults. Jama. 2013;309(9):874–876.
2. Darby S, McGale P, Correa C, et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet. 2011;378(9804):1707–1716.
3. Sundquist M, Thorstenson S, Klintenberg C, Brudin L, Nordenskjold B. Indicators of loco-regional recurrence in breast cancer. The South East Swedish Breast Cancer Group. European journal of surgical oncology: the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology. 2000;26(4):357–362.
4. Veronesi U, Marubini E, Del Vecchio M, et al. Local recurrences and distant metastases after conservative breast cancer treatments: partly independent events. Journal of the National Cancer Institute. 1995;87(1):19–27.
5. Houssami N, Macaskill P, Marinovich ML, et al. Meta-analysis of the impact of surgical margins on local recurrence in women with early-stage invasive breast cancer treated with breast-conserving therapy. Eur J Cancer. 2010;46(18):3219–3232.
6. Truong PT, Jones SO, Kader HA, et al. Patients with t1 to t2 breast cancer with one to three positive nodes have higher local and regional recurrence risks compared with node-negative patients after breast-conserving surgery and whole-breast radiotherapy. International journal of radiation oncology, biology, physics. 2009;73(2):357–364.
7. Hughes KS, Schnaper LA, Bellon JR, et al. Lumpectomy plus tamoxifen with or without irradiation in women age 70 years or older with early breast cancer: long-term follow-up of CALGB 9343. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2013;31(19):2382–2387.
8. Vinh-Hung V, Verschraegen C. Breast-conserving surgery with or without radiotherapy: pooled-analysis for risks of ipsilateral breast tumor recurrence and mortality. Journal of the National Cancer Institute. 2004;96(2):115–121.
9. Wapnir IL, Anderson SJ, Mamounas EP, et al. Prognosis after ipsilateral breast tumor recurrence and locoregional recurrences in five National Surgical Adjuvant Breast and Bowel Project node-positive adjuvant breast cancer trials. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2006;24(13):2028–2037.
10. Anderson SJ, Wapnir I, Dignam JJ, et al. Prognosis after ipsilateral breast tumor recurrence and locoregional recurrences in patients treated by breast-conserving therapy in five National Surgical Adjuvant Breast and Bowel Project protocols of node-negative breast cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2009;27(15):2466–2473.
11. SEER cancer statistics review. National Cancer Institute (1975–2005). Accessed September 27, 2008, at http://seer.cancer.gov/csr/1975_2005/results_merged/sect_04_breast.pdf).
Appendix Table 1.
Lee index to predict mortality due to other diseases
| Point score | 10-year mortality |
|---|---|
| 0 | 2.80% |
| 1 | 4.00% |
| 2 | 6.00% |
| 3 | 9.10% |
| 4 | 14% |
| 5 | 21% |
| 6 | 30% |
| 7 | 40% |
| 8 | 52% |
| 9 | 62% |
| 10 | 71% |
| 11 | 81% |
| 12 | 85% |
| 13 | 89% |
| 14 | 95% |
Appendix Table 2.
10-year risk of recurrence, women with nodal negative breast cancer receiving BCS without RT
| Tumor Size | ER | Tumor Grade | Age | 10-year risk of recurrence (local or distant metastasis) |
|---|---|---|---|---|
| 1–20 mm | Negative | 1 | 60–70 | 25% |
| 1–20 mm | Negative | 1 | >70 | 23% |
| 1–20 mm | Positive | 1 | 60–70 | 11% |
| 1–20 mm | Positive | 1 | >70 | 10% |
| 1–20 mm | Negative | 2 | 60–70 | 37% |
| 1–20 mm | Negative | 2 | >70 | 34% |
| 1–20 mm | Positive | 2 | 60–70 | 19% |
| 1–20 mm | Positive | 2 | >70 | 16% |
| 1–20 mm | Negative | 3 | 60–70 | 45% |
| 1–20 mm | Negative | 3 | >70 | 40% |
| 1–20 mm | Positive | 3 | 60–70 | 32% |
| 1–20 mm | Positive | 3 | >70 | 27% |
| 21–50 mm | Negative | 1 | 60–70 | 37% |
| 21–50 mm | Negative | 1 | >70 | 34% |
| 21–50 mm | Positive | 1 | 60–70 | 17% |
| 21–50 mm | Positive | 1 | >70 | 15% |
| 21–50 mm | Negative | 2 | 60–70 | 53% |
| 21–50 mm | Negative | 2 | >70 | 49% |
| 21–50 mm | Positive | 2 | 60–70 | 28% |
| 21–50 mm | Positive | 2 | >70 | 24% |
| 21–50 mm | Negative | 3 | 60–70 | 61% |
| 21–50 mm | Negative | 3 | >70 | 56% |
| 21–50 mm | Positive | 3 | 60–70 | 45% |
| 21–50 mm | Positive | 3 | >70 | 38% |
Appendix Table 3.
Relative risks
| Reference Group | Comparison Group | Relative Risk | Source | |
|---|---|---|---|---|
| Age | for each 5-year increase | 1.07 | Calibration; 3 | |
| Tumor size | <=0.5cm | 0.6–1.0cm | 1.70 | Calibration; 4 |
| <=0.5cm | 1.1–1.5cm | 1.90 | ||
| <=0.5cm | 1.6–2.0cm | 2.09 | ||
| <=0.5cm | >2.0cm | 3.16 | ||
| Surgical margin | Close | Positive | 1.34 | 5 |
| Close | Negative | 0.56 | ||
| Lymph nodes | negative | 1–3 | 1.85 | 6 |
| negative | ≥4 | 2 | ||
| RT | No RT | RT | 0.33 | 8 |
Appendix D.
Examples of early output during the development phase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: Drs. Wang and Mougalian receive research support from Genentech. Dr. Killelea receives consulting fees from Genentech. Dr. Mougalian receives consulting fees from Eisai and Celgene. Drs. Gross and Mougalian are on a grant sponsored by National Comprehensive Cancer Network/Pfizer. Dr. Gross receives research support from Johnson & Johnson, and support for travel from Flatiron, Inc. These sources of support were not used for any portion of the current manuscript. None of the other coauthors have conflicts to report.
References
- 1.Hughes KS, Schnaper LA, Berry D, Cirrincione C, McCormick B, Shank B, et al. Lumpectomy plus tamoxifen with or without irradiation in women 70 years of age or older with early breast cancer. N Engl J Med. 2004;351(10):971–7. [DOI] [PubMed] [Google Scholar]
- 2.Kunkler IH, Williams LJ, Jack WJ, Cameron DA, Dixon JM, investigators PI. Breast-conserving surgery with or without irradiation in women aged 65 years or older with early breast cancer (PRIME II): a randomised controlled trial. Lancet Oncol. 2015;16(3):266–73. [DOI] [PubMed] [Google Scholar]
- 3.NCCN. NNCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). 2017; http://www.nccn.org/patients. Accessed July 17, 2017.
- 4.Rutter CE, Lester-Coll NH, Mancini BR, Corso CD, Park HS, Yeboa DN, et al. The evolving role of adjuvant radiotherapy for elderly women with early-stage breast cancer. Cancer. 2015;121(14):2331–40. [DOI] [PubMed] [Google Scholar]
- 5.Soulos PR, Yu JB, Roberts KB, Raldow AC, Herrin J, Long JB, et al. Assessing the impact of a cooperative group trial on breast cancer care in the medicare population. J Clin Oncol. 2012;30(14):1601–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davidoff AJ, Erten M, Shaffer T, Shoemaker JS, Zuckerman IH, Pandya N, et al. Out-of-pocket health care expenditure burden for Medicare beneficiaries with cancer. Cancer. 2013;119(6):1257–65. [DOI] [PubMed] [Google Scholar]
- 7.Whelan TJ, Levine M, Julian J, Kirkbride P, Skingley P. The effects of radiation therapy on quality of life of women with breast carcinoma: results of a randomized trial. Ontario Clinical Oncology Group. Cancer. 2000;88(10):2260–6. [PubMed] [Google Scholar]
- 8.Omarini C, Thanopoulou E, Johnston SR. Pneumonitis and pulmonary fibrosis associated with breast cancer treatments. Breast Cancer Res Treat. 2014;146(2):245–58. [DOI] [PubMed] [Google Scholar]
- 9.Demirci S, Nam J, Hubbs JL, Nguyen T, Marks LB. Radiation-induced cardiac toxicity after therapy for breast cancer: interaction between treatment era and follow-up duration. Int J Radiat Oncol Biol Phys. 2009;73(4):980–7. [DOI] [PubMed] [Google Scholar]
- 10.Yi M, Cormier JN, Xing Y, Giordano SH, Chai C, Meric-Bernstam F, et al. Other primary malignancies in breast cancer patients treated with breast conserving surgery and radiation therapy. Ann Surg Oncol. 2013;20(5):1514–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Albert JM, Liu DD, Shen Y, Pan IW, Shih YC, Hoffman KE, et al. Nomogram to predict the benefit of radiation for older patients with breast cancer treated with conservative surgery. J Clin Oncol. 2012;30(23):2837–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sanghani M, Balk E, Cady B, Wazer D. Predicting the risk of local recurrence in patients with breast cancer: an approach to a new computer-based predictive tool. Am J Clin Oncol. 2007;30(5):473–80. [DOI] [PubMed] [Google Scholar]
- 13.Darby S, McGale P, Correa C, Taylor C, Arriagada R, Clarke M, et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet. 2011;378(9804):1707–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gross CP, McAvay GJ, Krumholz HM, Paltiel AD, Bhasin D, Tinetti ME. The effect of age and chronic illness on life expectancy after a diagnosis of colorectal cancer: implications for screening. Annals of internal medicine. 2006;145(9):646–53. [DOI] [PubMed] [Google Scholar]
- 15.Ravdin PM, Siminoff LA, Davis GJ, Mercer MB, Hewlett J, Gerson N, et al. Computer program to assist in making decisions about adjuvant therapy for women with early breast cancer. J Clin Oncol. 2001;19(4):980–91. [DOI] [PubMed] [Google Scholar]
- 16.Cruz M, Covinsky K, Widera EW, Stijacic-Cenzer I, Lee SJ. Predicting 10-year mortality for older adults. Jama. 2013;309(9):874–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.ePrognosis. Available at https://eprognosis.ucsf.edu/leeschonberg.php.
- 18.Wang SY, Kelly G, Gross C, Killelea BK, Mougalian S, Presley C, et al. Information Needs of Older Women With Early-Stage Breast Cancer When Making Radiation Therapy Decisions. Int J Radiat Oncol Biol Phys. 2017;98(4):733–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sundquist M, Thorstenson S, Klintenberg C, Brudin L, Nordenskjold B. Indicators of loco-regional recurrence in breast cancer. The South East Swedish Breast Cancer Group. European journal of surgical oncology: the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology. 2000;26(4):357–62. [DOI] [PubMed] [Google Scholar]
- 20.Veronesi U, Marubini E, Del Vecchio M, Manzari A, Andreola S, Greco M, et al. Local recurrences and distant metastases after conservative breast cancer treatments: partly independent events. Journal of the National Cancer Institute. 1995;87(1):19–27. [DOI] [PubMed] [Google Scholar]
- 21.Houssami N, Macaskill P, Marinovich ML, Dixon JM, Irwig L, Brennan ME, et al. Meta analysis of the impact of surgical margins on local recurrence in women with early-stage invasive breast cancer treated with breast-conserving therapy. Eur J Cancer. 2010;46(18):3219–32. [DOI] [PubMed] [Google Scholar]
- 22.Hughes KS, Schnaper LA, Bellon JR, Cirrincione CT, Berry DA, McCormick B, et al. Lumpectomy plus tamoxifen with or without irradiation in women age 70 years or older with early breast cancer: long-term follow-up of CALGB 9343. J Clin Oncol. 2013;31(19):2382–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Truong PT, Jones SO, Kader HA, Wai ES, Speers CH, Alexander AS, et al. Patients with t1 to t2 breast cancer with one to three positive nodes have higher local and regional recurrence risks compared with node-negative patients after breast-conserving surgery and whole-breast radiotherapy. Int J Radiat Oncol Biol Phys. 2009;73(2):357–64. [DOI] [PubMed] [Google Scholar]
- 24.Wapnir IL, Anderson SJ, Mamounas EP, Geyer CE Jr., Jeong JH, Tan-Chiu E, et al. Prognosis after ipsilateral breast tumor recurrence and locoregional recurrences in five National Surgical Adjuvant Breast and Bowel Project node-positive adjuvant breast cancer trials. J Clin Oncol. 2006;24(13):2028–37. [DOI] [PubMed] [Google Scholar]
- 25.Anderson SJ, Wapnir I, Dignam JJ, Fisher B, Mamounas EP, Jeong JH, et al. Prognosis after ipsilateral breast tumor recurrence and locoregional recurrences in patients treated by breast-conserving therapy in five National Surgical Adjuvant Breast and Bowel Project protocols of node-negative breast cancer. J Clin Oncol. 2009;27(15):2466–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.SEER cancer statistics review. National Cancer Institute (1975–2005). Accessed September 27, 2008, at http://seer.cancer.gov/csr/1975_2005/results_merged/sect_04_breast.pdf).
- 27.Holmes CE, Muss HB. Diagnosis and treatment of breast cancer in the elderly. CA Cancer J Clin. 2003;53(4):227–44. [DOI] [PubMed] [Google Scholar]
- 28.Hurria A, Lichtman SM, Gardes J, Li D, Limaye S, Patil S, et al. Identifying vulnerable older adults with cancer: integrating geriatric assessment into oncology practice. J Am Geriatr Soc. 2007;55(10):1604–8. [DOI] [PubMed] [Google Scholar]
- 29.Kane HL, Halpern MT, Squiers LB, Treiman KA, McCormack LA. Implementing and evaluating shared decision making in oncology practice. CA Cancer J Clin. 2014;64(6):377–88. [DOI] [PubMed] [Google Scholar]
- 30.Jimison H, Gorman P, Woods S, Nygren P, Walker M, Norris S, et al. Barriers and drivers of health information technology use for the elderly, chronically ill, and underserved. Evid Rep Technol Assess (Full Rep). 2008(175):1–1422. [PMC free article] [PubMed] [Google Scholar]
- 31.Group EBCC Group ER, Bijker N, Meijnen P, Peterse JL, Bogaerts J, et al. Breast-conserving treatment with or without radiotherapy in ductal carcinoma-in-situ: ten-year results of European Organisation for Research and Treatment of Cancer randomized phase III trial 10853—a study by the EORTC Breast Cancer Cooperative Group and EORTC Radiotherapy Group. J Clin Oncol. 2006;24(21):3381–7. [DOI] [PubMed] [Google Scholar]
- 32.Strecher VJ, Shiffman S, West R. Moderators and mediators of a web-based computer-tailored smoking cessation program among nicotine patch users. Nicotine Tob Res. 2006;8 Suppl 1:S95–101. [DOI] [PubMed] [Google Scholar]
- 33.Graham ID, Logan J, O’Connor A, Weeks KE, Aaron S, Cranney A, et al. A qualitative study of physicians’ perceptions of three decision aids. Patient Educ Couns. 2003;50(3):279–83. [DOI] [PubMed] [Google Scholar]
- 34.Gravel K, Legare F, Graham ID. Barriers and facilitators to implementing shared decision-making in clinical practice: a systematic review of health professionals’ perceptions. Implement Sci. 2006;1:16. [DOI] [PMC free article] [PubMed] [Google Scholar]