Abstract
Background:
Maternal inflammation in early pregnancy has been identified epidemiologically as a prenatal pathogenic factor for the offsprings’ later mental illness. The early manifestations in the newborn of the effects of maternal inflammation on fetal brain development are largely unknown.
Methods:
Maternal infection, depression, obesity, and other factors associated with inflammation were assessed at 16 weeks gestation. Maternal CRP and cytokines and maternal choline were measured then. Cerebral inhibition was assessed by inhibitory P50 sensory gating at one month of age, and infant behavior was assessed by maternal ratings at three months of age.
Results:
Maternal CRP diminished the development of cerebral inhibition in newborn males but paradoxically increased inhibition in females. Similar sex-dependent effects were seen in mothers’ assessment of their infant’s self-regulatory behaviors at three months of age. Higher maternal choline levels partly mitigated the effects of CRP in male offspring.
Conclusions:
The male fetal-placental unit appears to be more sensitive to maternal inflammation than females. Effects are particularly marked on cerebral inhibition. Deficits in cerebral inhibition one month after birth, similar to those observed in several mental illnesses, including schizophrenia, indicate fetal developmental pathways to later mental illness. Deficits in early infant behavior follow. Early intervention before birth, including prenatal vitamins, folate, and choline supplements, may help prevent fetal development of pathophysiological deficits that can have life-long consequences for mental health.
Keywords: Pregnancy, Prenatal Exposure Delayed Effects, Sensory gating, C-Reactive Protein, Choline, Child Development
Graphical Abstract

Introduction
Retrospective epidemiological studies established maternal infection during early second trimester as a risk factor for schizophrenia (Clarke 2009, Brown, 2010). Animal models identify maternal immune activation (MIA) with cytokine induction as a pathophysiological mechanism (WL Wu 2015). Maternal inflammation, often in response to respiratory and genitourinary infections in the early second trimester, decreases the development of inhibitory sensory gating and increases impulsivity (Meylbye 2004, Ghassabian 2018, Freedman 2019). Pathogenic effects of maternal cytokines on both the placenta and the brain have been proposed (Brown 2018). In addition to infection, maternal inflammation has been associated with obesity, maternal depression and anxiety, and environmental pollution (Madan 2009, Osborne 2013, van den Hooven 2012). Elevation in plasma C-reactive protein (CRP) at 16 weeks gestation and Interleukin-6 (IL-6) later in pregnancy in women whose offspring develop schizophrenia, adds molecular evidence for the pathogenic role of inflammation (Canetta 2014, Goldstein 2014). These epidemiological findings are now well-accepted, but limited information exists for which specific aspects of human fetal brain development are altered by maternal inflammation to produce mental illness in later life (Graham 2018, Spann 2018). The first pathophysiological signs of schizophrenia are apparent as early as birth (Erlenmeyer-Kimmling 1987, Walker 1994). RDoC provides a framework that can be applied prospectively to study the earliest development origins of schizophrenia by examining pathophysiological components of the illness that appear before diagnostic symptoms are apparent (Ross 2015).
This study assessed newborn cerebral inhibitory physiology as a primary outcome of fetal brain development using an auditory sensory gating measure, a biomarker of schizophrenia in adults, that first appears in newborns (Freedman 1979, Kisley 2003). Development of inhibitory neurons was characterized by sensory gating of the P50 cerebral evoked response in a paired-auditory stimulus paradigm (S1, S2). P50 amplitude is normally decremented in response to S2, because of inhibitory mechanisms activated by the response to S1. This inhibition is impaired in persons with schizophrenia (Adler 1982; Supplement A). P50 sensory gating is a physiological measure in the RDoC Cognition Domain, Perception Construct, Auditory Sub-Construct. P50 sensory gating has been used in adults with schizophrenia to detect familial or genetic risk (Freedman 1979, Hall 2011; Quednow 2012), but P50 inhibition can be recorded as soon as one month after birth (Kisley 2003). Diminished newborn P50 inhibition predicts early childhood problems in self-regulation, attention, and social function (Hutchison 2007, Ross 2016). These early childhood behaviors are recognized as early developmental symptoms in children who late develop psychosis (Rossi 2000, Rutter 2006; Pine 2015). Newborn P50 inhibitory deficits are increased if either parent has schizophrenia, a finding replicated by another group (Hunter 2011, E. Smith 2018).
Increased maternal choline promotes the development of newborn P50 inhibitory gating, and therefore, we also assessed the possible interaction of the maternal inflammatory response with choline in this study.
Methods
1. Maternal assessment and recruitment
Women were enrolled from a public safety-net prenatal clinic at 14–16 weeks gestation from July 2013 until July 2016. Gestational age was timed from the last menstrual period and by ultrasound. Exclusions were fetal anomaly and major maternal medical morbidity. The Colorado Multiple Institution Review Board approved the study; all participants gave informed consent. The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
Women were asked to participate in a prospective study of stress in pregnancy on their child’s development. Psychiatric diagnoses were made using the Structured Clinical Interview for DSM-IV Axis I Disorders with DSM-5 criteria. Self-ratings on Center for Epidemiological Studies of Depression-R (CESD-R), State-Trait Anxiety Inventory-State Version (STAI-S), and the Perceived Stress Scale (PSS) were acquired. Maternal sociodemographics and health, including infections, substance use, BMI, and prenatal vitamin use, were assessed. Labor, delivery, and neonatal parameters were recorded from the medical record. Investigators were blinded to maternal choline levels during assessments.
2. Assessment of maternal infection
The medical record for all prenatal care was reviewed. A mother’s self-report of infection was considered significant if it was entered as a problem in the medical record. Treatment was provided for all reported genitourinary infections. Most respiratory infections were viral and were therefore not treated. In addition, mothers had an in-person review of systems for symptoms of infection at 16 weeks by research personnel. The correlation between symptoms rated by the mother as moderate to severe on a custom-made scale from 1–8 in the interview and problems in the medical record is rs = 0.96, P < 0.001 (Freedman 2019).
3. Choline measurements
Maternal plasma was assayed for choline and its metabolite betaine at 16 weeks gestation by the Colorado Translational Research Center Metabolomics Laboratory using mass spectroscopy. Blood samples were obtained at least two hours after breakfast. Plasma was quickly separated by refrigerated centrifugation to prevent platelet phosphatidylcholine release.
Stable isotope standards for betaine (N,N,N-trimethylglycine, cat no D-3352) and choline (cat no D-2464) were purchased from CDN Isotopes. Serum samples were thawed on ice, then 20 μL was extracted with 480 μL of ice cold extraction buffer (5:3:2 MeOH:MeCN:H2O) containing 0.1 μM each of N,N,N-trimethylglycine-D9 (betaine) and [1,1,2,2-D4]choline. Extraction was performed by vigorous agitation at 40C for 30 min followed by centrifugation at 12,000 rpm, 40C for 10 min. A 100 μL aliquot of supernatant was transferred to a glass vial, dried under N2 flow, and resuspended in an equal volume of water containing 0.1% (v/v) formic acid. Aqueous extracts were analyzed by ultra high pressure liquid chromatography-mass spectrometry (UHPLC-MS) on a Thermo Vanquish UHPLC (San Jose, CA) coupled to a Thermo Q Exactive mass spectrometer (Bremen, Germany) via positive electrospray ionization. Solvents were water (phase A) and acetonitrile (phase B) supplemented with formic acid (0.1%) and flow rate was 0.25 mL/min. Metabolites were separated using a Kinetex C18 (Phenomenex, Torrance, CA) column (2.1 × 150 mm, 1.7 μm) with a 6 minute gradient of 0–2 min 2% B; 2–2.5 min increase to 25% B; 2.5–4 min hold at 25% B; 4–4.01 min decrease to 2% B; 4.01–6 min hold at 2% B. The Q Exactive mass spectrometer was operated in full scan mode over the range of 65–950 m/z. Samples were randomized and a quality control sample was injected every 10 runs. The coefficient of variation was < 10%. Data analysis was performed using Maven Metabolomic Analysis and Visualization Engine (Princeton University) following file conversion by MassMatrix (Case Western Reserve University). Absolute concentrations were obtained using the following equation:
where DF = dilution factor, in this case, 25 (i.e. 20μ of serum in a total 500μ volume).
Mothers received information on diets higher in choline, but dietary intake was not estimated because of the low relationship of self-reported intake to maternal choline levels in pregnant women, r = 0.2 (BTF Wu 2012). The placental choline transporter CLT1 produces amniotic fluid levels approximately twice maternal plasma levels (Ilcol et al., 2002; Baumgartner et al., 2015). Uptake is proportional to maternal plasma concentration, which suggests that higher peak levels may be important determinants of amniotic fluid levels (Iwao et al., 2016). Maternal levels obtained in non-fasting conditions, as in the present study, can be elevated, but only after high-choline meals that exceed the recommended daily intake (Zeisel et al., 1980; Holm et al., 2003; Abratte et al., 2009). Only choline activates α7-nicotinic receptors (Alkondon et al., 1997). We found no effects of its metabolite betaine.
4. Maternal CRP and cytokines
Plasma CRP at 16 weeks gestation was assayed by the Beckman-Coulter high sensitivity assay at the Colorado Translational Research Center, Colorado Children’s Hospital. Tumor Necrosis Factor-alpha (TNFα) and Interleukin-6 (IL-6) and Interleukin-8 (IL-8) were assayed by R&D Systems high sensitivity assays at the CTRC Core Laboratory, Children’s Hospital of Colorado. Il-6 values above the detectable limit (1.9 pg/ml) were found for 9 of 91 samples from women without infection and 17 of 61 women with infection (Fisher’s exact test p = 0.007). TNF levels above detectable limits were observed in 81 of 91 women without infection and 60 of 61 women with infection (1.6 pg/ml). Levels below detectable limits were set to zero.
5. Neonatal physiological recording of cerebral inhibition
Newborns were studied one month (44 weeks) after birth adjusted for gestational age. Vertex electroencephalogram, electro-oculogram, submental electromyogram, and respiration were continuously recorded while infants napped (Kisley et al., 2003; Hunter et al., 2011). Recording of the cerebral auditory evoked potential P50, a positive EEG wave 50ms post stimulus, occurred in the second active sleep episode, the precursor of REM sleep, identified by low voltage desynchronized vertex activity with the absence of K-complexes, change in respiration, and large eye movements with submental atonia (Anders et al., 1971). The second active sleep episode was reached approximately 45 minutes after sleep onset. In adults, P50 inhibition in REM and waking are equivalent (Griffith and Freedman, 1995).
Two identical auditory stimuli are delivered 500 ms apart to elicit P50S1 and P50S2. P50 inhibition is often assessed as amplitude ratios P50 S2/P50 S1 or (P50S1-P50S2)/P50S1 (Adler et al., 1982). However, the skew inherent in ratios limits their power for correlation with risk factors. P50S2 amplitude, covaried for P50S1, which is normally distributed, has therefore also been used (DA Smith et al., 1994). A meta-analysis of 28 independent studies found that P50S2 amplitude, covaried for P50S1 distinguishes adults with schizophrenia from healthy controls with high effect size (d’ = 1.45, P<0.001, Supplement A). A recent study using magnetoencephalography also reported that the sensory gating defect in schizophrenia is robust to the measurement method (Schubring 2017). Regardless of the method used, higher P50S2 amplitudes indicate decreased inhibition. The assumption is that P50S1 variance is small, compared to P50S2 variance. In 151 newborns, effect sizes for P50S1 differences between newborns whose mothers had no known risk versus women with depression or schizophrenia ranged from 0–0.16. Effect sizes for the decrease in P50S2 amplitude were 0.21–0.50 (Hunter et al., 2011). The effect of maternal schizotypy on newborn P50 inhibition has been replicated by another group, who also found increased P50S2 amplitudes (E. Smith et al., 2018). Intraclass correlation between two newborn recordings 1 week apart is rICC = 0.84. Other technical aspects of recordings have been published (Hunter et al., 2008; Hunter et al., 2015).
6. Childhood behavioral assessments
Parents completed the Infant Behavior Questionnaire-Revised Short Form (IBQ-R) when the infant was three months of age (Gartstein and Rothbart, 2003; Putnam et al., 2014). The Parental Distress Subscale of the Parenting Stress Index was also completed as a possible covariate for parental bias (Abidin, 2012). The 91-item IBQ-R Short Form, commonly used to study behavior in children at this age, rates 14 aspects of child behavior, which the IBQ-R developers clustered into 3 indices by factor analysis: Surgency summarizes the child’s level of activity and positive affect; Negativity summarizes fearfulness and anxiety; and, Regulation summarizes duration of attention, responsiveness to parents, and enjoyment of quiet play. Two components of Regulation are also in Surgency, smiling and soothability, and the two indices are highly correlated in the present sample (r = 0.51, P <0.001). Covariation with Surgency isolates elements of Regulation that are more specific to the early development of attention and less attributable to the child’s general psychomotor activation. A similar covariance between Regulation and Surgency (0.80) has been documented by another group, who have also proposed revisions to the factor structure (Bosquet-Enlow et al., 2016). Lower IBQ-R Regulation at one year of age is associated with decreased reading readiness at age four years and decreased conscientiousness, organization, and increased distractibility at age 9 years of age (Putnam 2014, Slobodskaya 2016).
7. Statistical analyses
Neonatal P50S2 amplitude and childhood IBQ-R Regulation were the two principal outcomes, based on a previous work that found P50 inhibition was a biomarker of both infection and choline’s effects and that regulatory behaviors were the most affected outcome (Ross et al., 2016, Freedman et al., 2019). Kolmogorov-Smirnoff tests for each outcome did not find a significant deviation from normal distributions. General Linear Models analyzed CRP levels and choline levels as a continuous effect. For P50S2 amplitude, maternal smoking was a covariate because of its previously established effect on P50 inhibition (Hunter 2011). For IBQ-R analyses, maternal education was a covariate because of its correlation with both CRP levels (r = −0.169, P = 0.038) and IBQ-R Regulation (r = 0.196, P = 0.021). Follow-up analyses investigated the possible confounding effects of marijuana and alcohol use.
Choline’s effect size on P50 inhibition in a previous study was Cohen’s d’ = 0.7 (Ross 2013). We expected 20% of the women would have adequate choline levels and 30% attrition (BTF Wu 2012, Hoffman 2019). Therefore, we enrolled 200 women to have power 1-β > 0.95, α = 0.05, 1-tail to observe an overall choline effect.
Results
Mothers’ inflammatory response during early gestation
Of 316 mothers screened, 201 were enrolled by 16 weeks gestation, as dated from the last menstrual period and verified by ultrasound measurements. Of these, 162 brought their newborns to the one-month postnatal assessment, where the auditory evoked potentials were obtained (and as previously reported; Freedman et al, 2019). CRP levels were assessed in plasma samples obtained at 16 weeks gestation from 150 of the women (Figure 1). When the infants reached three months of age, 127 women brought them in for behavioral assessment. Attrition during the study after enrollment generally reflected mothers who moved from the pre-birth residence to their mothers’ homes to care for their infants and were then lost to follow-up. The significant differences between male and female babies were only in greater male birth weights and head circumferences (Table 1).
Figure 1.
Flow of mothers and offspring over the course of gestation and post-birth development.
Table 1.
Maternal status during gestation, labor, and delivery, neonatal outcomes stratified by offspring sex.
| Males = 76 Mean (SD) or N (%) | Females = 74 Mean (SD) or N (%) | P = (t-test or Fisher’s exact test) | |
|---|---|---|---|
| Maternal demographics | |||
| Maternal age yrs | 27.6 (6.3) | 29.8 (5.7) | 0.88 |
| Maternal education yrs | 13.7 (3.0) | 13.4 (3.1) | 0.54 |
| Pre-pregnancy BMI | 27.0 (6.7) | 27.6 (6.3) | 0.58 |
| Living with biological father N | 56 (69%) | 61 (76%) | 0.38 |
| Maternal mental illness and drug use | |||
| Bipolar Disorder N | 3 (4%) | 4 (5%) | 0.22 |
| Major Depression N | 14 (17%) | 10 (13%) | 0.51 |
| Anxiety Disorder N | 2 (2%) | 5 (6%) | 0.28 |
| Schizophrenia N | 0 | 2 (3%) | 0.24 |
| Alcohol use N | 14 (17%) | 8 (10%) | 0.25 |
| Cannabis use N | 16 (20%) | 9 (11%) | 0.19 |
| Current smoker N | 7 (9%) | 4 (5%) | 0.53 |
| Cocaine N | 7 (9%) | 5 (6%) | 0.27 |
| Opioids N | 2 (2%) | 2 (2%) | 0.89 |
| Obstetrical history | |||
| Gravidity N | 3.25 (1.87) | 2.88 (1.80) | 0.22 |
| Pre-term delivery N | 12 (15%) | 18 (23%) | 0.23 |
| Miscarriage, ectopic, aborted N | 56 (69%) | 54 (67%) | 0.87 |
| Living children N | 1.52 (1.42) | 1.20 (1.25) | 0.33 |
| Pregnancy, Labor, Delivery | |||
| Prenatal vitamins with folic acid N | 68 (89%) | 66 (89%) | 1.00 |
| Choline μM | 6.31 (1.78) | 6.49 (1.87) | 0.54 |
| Betaine μM | 11.1 (2.9) | 11.9 (4.2) | 0.18 |
| Pre-pregnancy BMI | 27.0 (6.7) | 27.6 (6.3) | 0.58 |
| Obesity BMI≥30 N | 19 (23%) | 28 (35%) | 0.12 |
| Common infections N | 35 (43%) | 30 (38%) | 0.52 |
| Infection severity | 2.05 (2.74) | 1.91 (2.70) | 0.75 |
| Hypertension N | 4 (5%) | 6 (8%) | 0.53 |
| Gestational diabetes N | 4 (5%) | 4 (5%) | 0.99 |
| Preeclampsia N | 7 (9%) | 6 (8%) | 0.99 |
| Premature labor N | 8 (10%) | 3 (4%) | 0.21 |
| Premature delivery <37 weeks N | 7 (7%) | 3 (4%) | 0.50 |
| Vaginal delivery N | 64 (67%) | 63 (66%) | 0.99 |
| Maternal self-ratings | |||
| Center for Epidemiological Studies of Depression-R | 14.5 (10.3) | 13.6 (8.6) | 0.53 |
| State-Trait Anxiety Inventory | 35.6 (12.2) | 35.9 (9.6) | 0.89 |
| Perceived Stress Scale | 23.4 (9.1) | 23.8 (6.8) | 0.75 |
| Parenting Stress Index 3 mos post birth | 26.8 (7.4) | 25.7 (7.2) | 0.81 |
| Neonatal Status | |||
| APGAR 5 min | 8.86 (0.45) | 8.69 (0.80) | 0.32 |
| Small for gestational age N | 3(4%) | 4 (5%) | 0.72 |
| Large for Gestational age N | 11 (14%) | 9 (71%) | 0.81 |
| Meconium fluid N | 16 (20%) | 20 (25%) | 0.57 |
| Nuchal cord N | 24 (30%) | 14 (66%) | 0.09 |
| Days in NICU>1 | 5 (6%) | 5 (6%) | 0.99 |
| Jaundice N | 32 (40%) | 36 (45%) | 0.52 |
| Gestational age at birth days | 273.8 (15.7) | 271.4 (18.6) | 0.38 |
| Birth weight g | 3266.9 (585.0) | 3049.4 (607.7) | 0.022 |
| Birth length cm | 49.7 (4.2) | 48.4 (5.3) | 0.086 |
| Birth head circumference cm | 35.0 (2.7) | 34.1 (2.7) | 0.045 |
| Maternal inflammation | |||
| CRP mg/ml | 7.75 (7.01) | 9.68 (8.00) | 0.12 |
| IL-6 pg/ml | 0.46 (1.6) | 0.63 (1.21) | 0.46 |
| IL-8 pg/ml | 1.18 (1.36) | 1.71 (2.38) | 0.09 |
| TNFα pg/ml | 3.32 (1.59 | 3.52 (1.95) | 0.48 |
| Newborn cerebral physiology 1 month post birth | |||
| P50S1 μV | 1.60 (0.67) | 1.81 (1.00) | 0.10 |
| P50S2μV | 0.83 (0.60) | 0.83 (0.65) | 0.96 |
| Infant behavior 3 months post birth | N = 72 | N = 65 | |
| Surgency | 4.19 (1.11) | 4.30 (1.12) | 0.58 |
| Negativity | 3.09 (0.89) | 3.13 (0.94) | 0.80 |
| Regulation | 5.26(0.68) | 5.21 (0.68) | 0.67 |
CRP levels correlated with maternal CESD-R self-ratings of depression, pre-pregnancy BMI, severity of gestational infection, and maternal education (Table 2). There was no correlation with infant sex. More educated women were older (r = 0.324, P < 0.001 and had lower self-ratings of depression (r = −0.190, P = 0.015) and lower BMI (r = −0.283, P < 0.001), which has been found in other populations (Bui 2018). CESD-R ratings correlated with both infection (r = 0.264, P = 0.001) and BMI (r = 0.210, P = 0.007). Multiple regression analysis found significant effects for CRP of infection severity (β = 0.202 (95% CI 0.047, 0.356) P = 0.011) and BMI (β = 0.329 (95% CI 0.176, 0.482) P < 0.001), but not depression or maternal education. If the ratings were dichotomized to identify mothers likely to come to clinical attention as depressed (CESD-R>15), obese (BMI > 30), or as having a significant infection (severity > 5), then only infection was a significant factor for CRP elevation (β = 0.237 (95% CI 0.076, 0.398) P = 0.004; Supplement Table 1).
Table 2.
Maternal factors associated with CRP elevation at 16 weeks gestation
| Parameters | Pearson r | P |
|---|---|---|
| Child sex | −.129 | 0.12 |
| Pre-pregnancy BMI | .358 | <0.001 |
| Infection severity rating 16 wks | .245 | 0.002 |
| Clinician-reported moderately severe infection 16 wks | .235 | 0.004 |
| CESD-R 16 wks | .175 | 0.03 |
| Maternal educations yrs | −.169 | 0.04 |
| Maternal age yrs | .051 | 0.53 |
| Bipolar disorder | −.049 | 0.55 |
| Depressive disorder | .078 | 0.34 |
| Anxiety disorder | −.103 | 0.21 |
| Schizophrenia | .045 | 0.58 |
| Nicotine use | .058 | 0.48 |
| Alcohol use | .036 | 0.66 |
| Cannabis use | .094 | 0.26 |
| CESD-R > 15 | .094 | 0.26 |
| Antidepressant | .107 | 0.19 |
| STAI 16 wks | .018 | 0.82 |
| PSS 16 wks | .102 | 0.22 |
| Antibiotic use | .022 | 0.79 |
| Obesity BMI≥30 | .030 | 0.71 |
| Other maternal cytokines | ||
| IL6 16 wks | .543 | <0.001 |
| IL8 wks | .198 | 0.02 |
| TNF wks | .186 | 0.02 |
CRP levels were not associated with either complications of labor and delivery, such as premature delivery, diabetes, or chorioamniotis, or any neonatal outcomes, such as lower APGAR scores or small size for gestational age (Table S1). CRP levels were correlated with other maternal cytokines, specifically IL-6, IL-8, and TNF-α (Table 2).
Newborn expression of cerebral inhibition
A paired-stimulus auditory paradigm (S1, S2) delivered during active sleep was used to assess the newborn’s development of cerebral inhibition, measured as the decrease in amplitude of P50S2, relative to P50S1 (Figure 2). There were no differences P50S1 or in P50S2 amplitude between male and female newborns (Table 1).
Figure 2.
Top: Examples of P50S1 and P50S2 auditory evoked responses recorded 1 month after birth in a female newborn. P50 amplitude is measured from the most positive peak relative to the preceding negativity. The mother’s 16-week gestation CRP was 15.3mg/ml and her choline was 5.87μM. P50S1 was 1.50μV and P50S2 was 0.27μV. Bottom: Effects of CRP on P50S2 for male and female infants.
Maternal CRP levels had a sex-dependent effect on P50S2 amplitude (Fdf1 = 4.597, P = 0.034; Table S2); P50S1 was unaffected. CRP levels correlated with increased P50S2 amplitude in males (β = 0.205 (95% CI 0.023, 0.387) P = 0.03), but decreased P50S2 amplitude in females (β = −0.214 (95% CI −0.390,−0.038) P = 0.016). Only CRP levels were associated with this effect on P50 amplitudes; the other cytokine levels had no significant association (Table S3). Cigarette, marijuana, and alcohol use during early pregnancy did not affect the significance of the effects of CRP on P50S2 amplitude (Table S4).
Infant behavior at three months of age
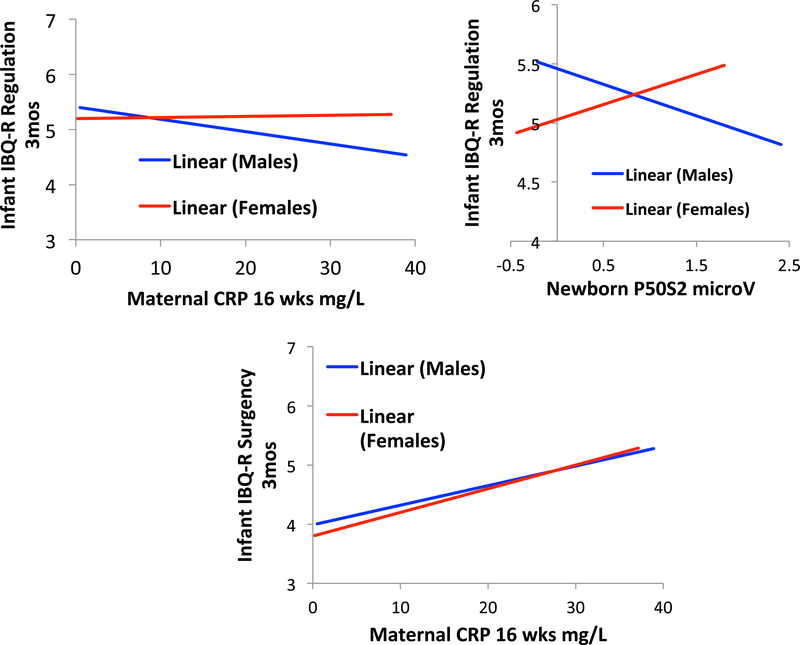
Maternal ratings of their infants’ behavior showed no differences between male and female infants on the three principal indices, Surgency, Regulation, and Negativity (Table 1). Multivariate analysis of effects on CRP on the three IBQ-R indices found that maternal CRP increased 3-month IBQ-R Surgency regardless of the sex of the infant (Fdf1 =5.088, P = 0.026; β = 0.207 (95% CI 0.036, 0.378). The two most affected components of this scale were Vocalizations (Wald χ2df1 = 6.299 P = 0.012) and High Intensity Pleasure (Wald χ2df1 = 7.478, P = 0.006). The effects of CRP on IBQ-R Regulation were sex-dependent (CRP*sex: Fdf1 = 4.244, P = 0.042; Figure 3; Table S5). There were no significant effects of CRP on IBQ-R Negativity. In males, CRP decreased Regulation (β = −0.209 (95% CI −0.429, 0) P = 0.049), but there was no significant effect in females (β = 0.062, P = 0.6). The two most affected IBQ-R components were Soothability (CRP*sex: Wald χ2df1 = 4.842, P = 0.042) and Cuddliness (CRP*sex: Wald χ2df1 = 6.043, P = 0.014). Cigarette, marijuana, and alcohol use during early pregnancy did not affect the significance of the effects of CRP on IBQ-R Regulation (Table S6).
Figure 3.
Top left: Effects of maternal CRP on infant IBQ-R Regulation in male and female infants. Top right: Relation of newborn P50S2 amplitude to infant IBQ-R Regulation in males and females. Bottom: Effects of maternal CRP on infant IBQ-R Surgency.
Multiple regression found that newborn P50S2 amplitude was related to 3-month old infant Regulation, also in a sex-dependent manner (P50S2*sex: Wald χ2df1 = 7.052, P = 0.008; Figure 3, Table S7).
IL-6 levels correlated with infant higher Negativity across both sexes (r= 0.229, P = 0.009).
Interaction of maternal choline levels and prenatal vitamins with the maternal inflammatory response
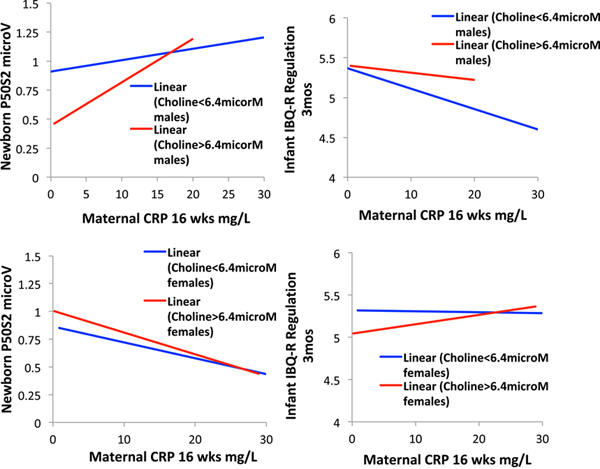
Maternal choline levels at 16 weeks gestation did not directly affect CRP levels, but maternal choline levels decreased the effect of CRP levels on P50S2 amplitude. Multiple regression found effects of maternal choline for all newborns ((β = −0.121 (95% CI −0.227, −0.015) Wald χ2df1 = 2.017, P = 0.029) and sex specific effects also (Wald χ2df1 =7.888, P = 0.019; Figure 5, Table S8). In males, choline significantly diminished P50S2 amplitude (β = −0.216 (95% CI −0.297, −0.035) P = 0.019); the effect of CRP did not change appreciably (β = 0.195, P = 0.032, compared to P = 0.201, P = 0.020) when choline was not considered). In females, the effect of choline was small and not significant (β = 0.010, P = 0.9). To demonstrate the 3-way interaction of choline with CRP and child sex, choline levels were dichotomized based on the mean value in the sample 6.4 μM. For males whose mothers had choline levels > 6.4 μM, the effect of CRP on P50 S2 amplitude was considerably decremented, until CRP levels rose above 15 mg/ml (Figure 4).
Figure 4.
Top: Effects of maternal choline levels and maternal CRP on male newborn P50S2 (left) and IBQ-R Regulation (right). Bottom: Effects of maternal choline levels and maternal CRP on female newborn P50S2 (left) and IBQ-R Regulation (right).
Prenatal vitamins with folic acid decreased P50S2 amplitude in both sexes, but the effects did not reach significance (in males, β = −0.168, P = 0.07; in females, β = −0.148, P = 0.09).
Multivariate analysis of the 3 IBQ-R indices found a significant interaction of child sex, CRP level, and choline level for Regulation (Fdf1 = 3.186, P = 0.045) and no effects on the other indices (Table S9). For males whose mothers had choline levels below 6.4 μM, the effect of CRP on Regulation was β = 0.352 (95% CI 0.044, 0.660) P = 0.028. If the mother had choline levels above 6.4 μM, the effect of CRP levels became non-significant (β = 0.093, P = 0.6; Figure 5). The effect of CRP on Regulation was non-significant for females regardless of maternal choline level.
Discussion
Increased maternal CRP levels in early gestation were associated with decreased development of P50 cerebral inhibition in males but not females. The decrease in P50 sensory inhibitory gating was further reflected in decreased infant Regulation behaviors rated on the IBQ-R at three months of age. CRP levels were increased by maternal infections, obesity, and depression, with infection being predominant (Freedman et al, 2019). Unlike inhibitory gating and Regulation, increases in both infant Surgency, also related to elevated CRP, and infant Negativity, related to elevated IL-6, were observed in both sexes. Effects on both the placenta’s integrity and neuronal development have been investigated in the maternal immune response (Brown and Meyer 2018). The sex-specific effects on inhibition in male fetuses suggest that there may be specific effects of maternal inflammation on the development of inhibitory interneurons, particularly since their development appears to be facilitated in females by the inflammatory response. Other aspects of early behavior, Surgency and Negativity, are similarly affected in both sexes, however, suggesting that there may be both sex-dependent and sex-independent mechanisms affecting fetal brain development.
In animal models, cerebral interneurons are specifically sensitive to maternal inflammation and hypoxia (Lacaille 2019). Parvalbumin neurons of the hippocampus are specifically affected (Canetta 2016). A pathophysiological mechanism involving the astroglia response has been proposed (Sobue 2018). Male mice have a greater reduction in hippocampal volume and more chronic macrophage infiltration than females after maternal immune activation (Dada 2015). Male mice are also more sensitive to other insults, such as prenatal radiation, with greater loss of hippocampal pyramidal and interneurons (Granapathi 2017). However, female mice are more sensitive to the apoptotic effects of prenatal dexamethasone (Zuloaga 2012), and effects on NMDA receptors in the hippocampus after prenatal glucocorticoids are more marked in females than males (Owen 2014). A second hit has also been proposed as a pathophysiological mechanism. Animals exposed to maternal immune activation during gestation are more likely to show deficits after pubertal stressors. This combination reduced expression of parvalbumin inhibitory interneurons, but the analyses were performed only in male mice (Giovanali 2013).
In humans the retrospective epidemiological data are not as clear. Maternal CRP levels at about 16 weeks gestation are associated with increased risk of schizophrenia and autism spectrum disorder in a Finnish cohort (Canetta 2014; Brown 2014), but decreased autism spectrum disorder in a California cohort (Zerbo 2016). In a Dutch cohort, higher maternal CRP levels at 13 weeks gestation were related to autism traits in the general population (Koks 2016). There were no sex-specific effects in any of these cohorts, despite the preponderance of schizophrenia and autism disorders in males. In a New England cohort, however, mothers of males with schizophrenia had higher Il-6 levels in early third trimester (Goldstein 2014). The double prenatal-adolescent hit is also apparently pathogenic in humans. In a Danish cohort, females had increased risk for schizophrenia after prenatal exposure to infection compared to males, but additional pubertal trauma resulted in males having a markedly increased risk (Debost 2017). Like animal models, human females are also more sensitive to the effects of corticosteroids released naturally when mothers become depressed (DJ Kim 2017). Thus, there may be a second pathway by which female fetuses are affected because the mother’s infection is often accompanied by depression (Freedman 2019).
CRP does not generally cross from the maternal circulation to directly affect the fetus (Malek 2006). A possible mechanism of the effects of maternal immune activation is cytokine-mediated macrophage attack of the placenta, which the mother’s immune system may treat as a foreign body. Placental cytokines are increased in the placenta in animal models of stress during pregnancy, specifically in males (Bronson 2014). In humans, elevated CRP in early gestation is associated with chronic placental villitis (Ernst 2013). Maternal CRP is deposited in the human placenta during pregnancy, where increased levels are associated with chorioamnionitis, pre-eclampsia, and preterm delivery (EN Kim 2015). Genes expressed in the placenta are associated with schizophrenia in the subset of patients who had prenatal complications, including significant infection. The expression of these genes in the placenta is specifically upregulated in males compared to females (Ursini 2018). Maternal immune activation of umbilical vein macrophages is also greater in males than in females (Kim-Fine 2012).
This maternal immune attack on the placenta, although not directly transmitted to the fetus, triggers a reaction in the fetus itself that directly affects fetal brain development, including interneuron migration specifically (Oskvig 2012). These interneurons are responsible for inhibition of the cerebral auditory evoked response (Miller 1995). The timing of measurement of maternal CRP levels, early in pregnancy versus closer to term, is critical for assessing pathogenic versus normal effects. Cerebral interneurons are in a critical stage of development at 16 weeks gestation, when we found effects in this study and when effects are also seen on the later risk for schizophrenia (Bayatti 2008, Zecevic 2011, Canetta 2014). Later in pregnancy, inflammation and CRP are involved in the normal parturition process. The placenta at term synthesizes CRP (Malek 2006). Increased inflammatory cytokines in the last trimester are associated with enhanced brain development (Spann 2018, Graham 2018). Maternal IL-6 levels at 16 weeks gestation, which we found associated with Infant Negativity, were not similarly associated when levels across the entire pregnancy were considered (Rudolph 2018).
The effects of choline on the development of cerebral inhibition are mediated by activation of α7-nicotinic receptors (Alkondon 1997, Ross 2013). α7-nicotinic receptors on hippocampal neurons are required for the induction of the chloride ion gradient necessary for neuronal inhibition (Liu 2006). From the graph in Figure 5, the effects of choline on inhibition appear to be competitive with the effects of maternal inflammation in males. In an animal model, deletion of CHRNA7, the gene associated with α7-nicotinic receptors, completely blocks the effects of choline on the development of inhibition (Stevens 2014). Direct comparison of CHRNA7 deletion with prenatal maternal immune activation found similar effects of both insults on the offsprings’ development (Giovanoli 2018), providing further evidence for the possible competing effects of α7-nicotinic receptor activation and maternal inflammatory effects on brain development. α7-nicotinic receptor activation mediates the anti-inflammatory action of the vagus nerve (Wang 2003). In pregnant wild-type C57BL/6N mice subjected to maternal immune activation, choline supplementation did not change IL-6 levels in the placenta, but did lower IL-6 levels in the fetal brain, suggesting no general anti-inflammatory effect but rather a specific effect in the fetus itself. Effects were seen in both males and females (WL Wu 2015). Effects of choline on maternal cytokine levels were observed in a rat gestational model with nearly 5-fold dietary choline supplementation (Zhang 2018), but there were no effects on mothers’ cytokine levels observed in our study with normal human diets. A study of 2-fold choline supplementation on the expression of placental angiogenic factors and pro-inflammatory factors in a mouse model with a hemizygous Dlx3+/− gene deletion model of placental insufficiency found effects of supplementation in early gestation were greater in male placentas and in later gestation, greater in females (King 2019). Thus, like maternal immune activation, the sites of choline’s effects appear to be complex, with different but interacting effects in mother, placenta, and fetal brain.
Effects of choline in the human infants in this study were most marked on the development of cerebral inhibition and behavioral Regulation where sex-dependent effects of inflammation were also seen. Sex-specific effects of choline have not been generally observed in other human studies of choline’s effects (Ross 2013, Ross 2016). Nonetheless, for the more vulnerable male fetus in particular higher choline levels would seem to be a useful intervention to prevent the initial steps in the pathophysiology of later mental illness from occurring in utero, when the mother experiences inflammation.
Supplementary Material
Table 3.
Effects of maternal factors on CRP levels at 16 weeks gestation.
| Maternal factors | Standardized Coefficients Beta |
95% Wald Confidence Interval | Wald Chi-Square df1 | P | |
|---|---|---|---|---|---|
| CESD-R 16 weeks | 0.033 | −0.125 | 0.191 | .177 | 0.679 |
| Infection severity 16 weeks | 0.202 | 0.047 | 0.356 | 6.841 | 0.011 |
| Pre-pregnancy BMI | 0.329 | 0.176 | 0.482 | 18.537 | <0.001 |
| Maternal education yrs | −0.050 | −0.208 | 0.108 | −.635 | 0.527 |
Acknowledgements
This study was conceived and initiated by the late Randal G. Ross, M.D. Viridiana Galicia-Rodriguez participated in the research effort.
The study was supported by the Institute for Children’s Mental Disorders; The Anschutz Foundation; National Institutes of Health NIH/NCATS grant number UL1 TR001082 (all authors); NICHD grant number K12HD001271-11 (Dr. Hoffman).
Footnotes
The authors report no conflict of interest.
Data from the study are available by request.
References
- Abidin RR (2012). Parenting Stress Index. 3rd edn. Odessa, FL: Psychological Assessment Resources. [Google Scholar]
- Abratte CM, Wang W, Li R, Axume J, Moriarty DJ, Caudill MA (2009). Choline status is not a reliable indicator of moderate changes in dietary choline consumption in premenopausal women. The Journal of Nutritional Biochemistry 20, 62–69. [DOI] [PubMed] [Google Scholar]
- Adler LE, Pachtman E, Franks RD, Pecevich M, Waldo MC, Freedman R (1982). Neurophysiological evidence for a defect in neuronal mechanisms involved in sensory gating in schizophrenia. Biological Psychiatry 17, 639–654. [PubMed] [Google Scholar]
- Alkondon M, Pereira EFR, Cortes WS, Maelicke A, Albuquerque EX (1997). Choline is a selective agonist at alpha7 nicotinic acetylcholine receptors in rat brain neurons. European Journal of Neuroscience 9, 2734–2742. [DOI] [PubMed] [Google Scholar]
- Anders T, Emde R, Parmelee A (1971). A manual of standardized terminology, techniques and criteria for scoring of states of sleep and wakefulness in newborn infants. Los Angeles: UCLA Brain Information Service, NINDS Neurological Information Network. [Google Scholar]
- Baumgartner HK, Trinder KM, Galimanis CE, Post A, Phang T, Ross RG, Winn VD (2015). Characterization of choline transporters in the human placenta over gestation. Placenta. NIH Public Access; 36, 1362–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayatti N, Moss JA, Sun, Ambrose P, Ward JFH, Lindsay L, Clowry GJ (2008). A molecular neuroanatomical study of the developing human neocortex from 8 to 17 postconceptional weeks revealing the early differentiation of the subplate and subventricular zone. Cerebral Cortex 18,1536–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosquet-Enlow M, White MT, Hails K, Cabrera I, Wright RJ (2016). The Infant Behavior Questionnaire-Revised: Factor structure in a culturally and sociodemographically diverse sample in the United States. Infant Behavior and Development. NIH Public Access; 43, 24–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheatham CL, Goldman BD, Fischer LM, da Costa K-AA, Reznick JS, Zeisel SH (2012). Phosphatidylcholine supplementation in pregnant women consuming moderate-choline diets does not enhance infant cognitive function: a randomized, double-blind, placebo-controlled trial. The American Journal of Clinical Nutrition 96, 1465–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman R, Hunter SK, Law AJ, Wagner BD, D’Allesandro A, Christians U, Noonan KQ, Wywra A, Hoffman MC (2019). Higher gestational choline levels in maternal infection are protective for infant brain development. Journal of Pediatrics 208,198–206e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartstein MA, Putnam S, Kliewer R (2016). Do Infant Temperament Characteristics Predict Core Academic Abilities in Preschool-Aged Children? Learning and individual differences. NIH Public Access; 45, 299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartstein MA, Rothbart MK (2003). Studying infant temperament via the Revised Infant Behavior Questionnaire. Infant Behavior and Development 26, 64–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith JM, Freedman R (1995). Normalization of the auditory P50 gating deficit of schizophrenic patients after non-REM but not REM sleep. Psychiatry Research 56, 271–278. [DOI] [PubMed] [Google Scholar]
- Hoffman MC, Olincy A, D’Alessandro A, Reisz JA, Hansen KC, Hunter SK, Freedman R, Ross RG (2019). Effects of phosphatidylcholine and betaine supplements on women’s serum choline. Journal of Nutrition & Intermediary Metabolism. 10.1016/j.jnim.2019.100094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm PI, Ueland PM, Kvalheim G, Lien EA (2003). Determination of choline, betaine, and dimethylglycine in plasma by a high-throughput method based on normal-phase chromatography-tandem mass spectrometry. Clinical Chemistry 49, 286–294. [DOI] [PubMed] [Google Scholar]
- Hunter SK, Corral N, Ponicsan H, Ross RG (2008). Reliability of P50 auditory sensory gating measures in infants during active sleep. Neuroreport 19, 79–82. [DOI] [PubMed] [Google Scholar]
- Hunter SK, Gillow SJ, Ross RG (2015). Stability of P50 auditory sensory gating during sleep from infancy to 4 years of age. Brain Cognition 94, 4–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter SK, Kisley MA, McCarthy L, Freedman R, Ross RG (2011). Diminished cerebral inhibition in neonates associated with risk factors for schizophrenia: Parental psychosis, maternal depression, and nicotine use. Schizophrenia Bulletin 37, 1200–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilcol YO, Uncu G, Ulus IH (2002). Free and phospholipid-bound choline concentrations in serum during pregnancy, after delivery, and in newborns. Archives of Physiology and Biochemistry 110, 393–399. [DOI] [PubMed] [Google Scholar]
- Ilcol YO, Yilmaz Z, Ulus IH (2003). Serum free and phospholipid-bound choline decrease and surgery and methylprednisolone administration in dogs. Neuroscience Letters 339, 195–198. [DOI] [PubMed] [Google Scholar]
- Iwao B, Yara M, Hara N, Kawai Y, Yamanaka T, Nishihara H, Inoue T, Inazu M (2016). Functional expression of choline transporter like-protein 1 (CTL1) and CTL2 in human brain microvascular endothelial cells. Neurochemistry International 93, 40–50. [DOI] [PubMed] [Google Scholar]
- Jacobson SW, Carter RC, Molteno CD, Stanton ME, Herbert JS, Lindinger NM, Lewis CE, Dodge NC, Hoyme HE, Zeisel SH, Meintjes EM, Duggan CP, Jacobson JL (2018). Efficacy of maternal choline supplementation during pregnancy in mitigating adverse effects of prenatal alcohol exposure on growth and cognitive function: A randomized, double-blind, placebo-controlled clinical trial. Alcoholism: Clinical and Experimental Research 42, 1327–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisley MA, Polk SD, Ross RG, Levisohn PM, Freedman R (2003). Early postnatal development of sensory gating. Neuroreport. Schizophrenia Research 14, 693–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Neff RA, Berg DK (2006). Sequential Interplay of Nicotinic and GABAergic Signaling Guides Neuronal Development. Science 314, 1610–1613. [DOI] [PubMed] [Google Scholar]
- Miller CL, Freedman R (1995). The activity of hippocampal interneurons and pyramidal cells during the response of the hippocampus to repeated auditory stimuli. Neuroscience 69, 371–381. [DOI] [PubMed] [Google Scholar]
- Pine DS, Fox N A (2015). Childhood antecedents and risk for adult mental disorders. Annual Review of Psychology 66, 459–485. [DOI] [PubMed] [Google Scholar]
- Putnam SP, Helbig AL, Gartstein MA, Rothbart MK, Leerkes E (2014). Development and assessment of short and very short forms of the infant behavior questionnaire-revised. Journal of Personality Assessment 96, 445–458. [DOI] [PubMed] [Google Scholar]
- Ross RG, Hunter SK, Hoffman MC, McCarthy L, Chambers BM, Law AJ, Leonard S, Zerbe GO, Freedman R (2016). Perinatal phosphatidylcholine supplementation and early childhood behavior problems: Evidence for CHRNA7 moderation. American Journal of Psychiatry 173, 509–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross RG, Hunter SK, McCarthy L, Beuler J, Hutchison AK, Wagner BD, Leonard S, Stevens KE, Freedman R (2013). Perinatal choline effects on neonatal pathophysiology related to later schizophrenia risk. American Journal of Psychiatry 170, 290–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter M, Kim-Cohen J, Maughan B (2006). Continuities and discontinuities in psychopathology between childhood and adult life. Journal of Child Psychology and Psychiatry 47, 276–295. [DOI] [PubMed] [Google Scholar]
- Slobodskaya HR, Kozlova EA (2016). Early temperament as a predictor of later personality. Personality and Individual Differences 99, 127–132. [Google Scholar]
- Smith DA, Boutros NN, Schwarzkopf SB (1994). Reliability of P50 auditory event-related potential indices of sensory gating. Psychophysiology 31, 495–502. [DOI] [PubMed] [Google Scholar]
- Smith E, Crawford T, Thomas M, Reid V (2018). Schizotypy and sensory gating: A 6-month-old EEG study. Schizophrenia Bulletin 44, S301–S302. [Google Scholar]
- Stevens KE, Choo KS, Stitzel JA, Marks MJ, Adams CE (2014). Long-term improvements in sensory inhibition with gestational choline supplementation linked to α7 nicotinic receptors through studies in Chrna7 null mutation mice. Brain Research 1552, 26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Yu M, Ochani M, Amelia CA, Tanovic M, Susaria S, et al. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature 2003;421:384–8. [DOI] [PubMed] [Google Scholar]
- Wu BTF, Dyer RA, King DJJ, Richardson KJ, Innis SM (2012). Early second trimester maternal plasma choline and betaine are related to measures of early cognitive development in term infants. PLoS ONE 7, e43448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zecevic N, Hu F, Jakovcevski I (2011). Interneurons in the developing human neocortex. Developmental Neurobiology 7, 18–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeisel SH, Growden JH, Wurtman RJ, Magil SG, Logue M (1980). Normal plasma choline responses to ingested lecithin. Neurology 30, 1226–1229. [DOI] [PubMed] [Google Scholar]
- van den Hooven EH; de Kluizenaar Y; Pierik FH; Hofman A van Ratingen SW; Zandveld PY; Lindemans J; Russcher H; Steegers EA; Miedema HM; Jaddoe VW. Chronic air pollution exposure during pregnancy and maternal and fetal C-reactive protein levels: the Generation R Study. Environmental Health Perspectives. 120(5):746–51, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham AM, Rasmussen JM, Rudolph MD et al. Maternal systemic Interleukin-6 during pregnancy is associated with newborn amygdala phenotypes and subsequent behavior at 2 years of age. Biological Psychiatry 2018; 83:109–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghassabian A, Albert PS, Hornig M et al. Gestational cytokine concentrations and neurocognitive development at 7 years. Translational Psychiatry 2018; 8:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spann MN, Monk C, Scheinost D, Peterson BS. Maternal immune activation during the third trimester Is associated with neonatal functional connectivity of the salience network and fetal to toddler behavior. J Neuroscience. 2018;38:2877–2886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerbo O, Traglia M, Yoshida C, Heuer LS, Ashwood P, Delorenze GN, Hansen RL, Kharrazi M, Van de Water JRH, Yolken RM, Weiss LA, Croen LA. Maternal mid-pregnancy C-reactive protein and risk of autism spectrum disorders: the early markers for autism study. Transl Psychiatry (2016) 6, e783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koks N, Ghassabian A, Greaves-Lord K, Hofman A, Jaddoe VWV, Verhulst F, Tiemeier H. Maternal C-Reactive Protein concentration in early pregnancy and child autistic traits in the general population. Ped Perinatal Epidemiol 30(2):181–9, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim-Fine S, Regnault TR, Lee JS, Gimbel SA, Greenspoon JA, Fairbairn J, Summers K, de Vrijer B. Male gender promotes an increased inflammatory response to lipopolysaccharide in umbilical vein blood. J Maternal-Fetal and Neonatal Med 2012; 25:2470–2474. [DOI] [PubMed] [Google Scholar]
- Goldstein JM, Cherkerzian S, Seidman LJ, Donatelli JA, Remington AG, Tsuang MT, Hornig M, Buka SL, 2014. Prenatal maternal immune disruption and sex dependent risk for psychoses. Psychol. Med. 44, 3249–3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debost JP, Larsen JT, Munk-Olsen T, Mortensen PB, Meyer U, Petersen L. Joint Effects of Exposure to Prenatal Infection and Peripubertal Psychological Trauma in Schizophrenia. Schizophr Bull. 2017. January;43(1):171–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AS, Sourander A, Hinkka-Yli-Salomäki S, et al. : Elevated maternal C-reactive protein and autism in a national birth cohort. Mol Psychiatry 2014; 19:259–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bui Q, Miller CC. The age that women have babies: how a gap divides America. NY Times, https://www.nytimes.com/interactive/2018/08/04/upshot/up-birth-age-gap.html. [Accessed 6 November 2018]. [Google Scholar]
- Schubring D, Tzvetan P, Miller GA, Rockstroh B. Consistency of abnormal sensory gating in first-admission and chronic schizophrenia across quantification methods. Psychophysiology 2018;55:e13006. [DOI] [PubMed] [Google Scholar]
- Ursini G, Punzi Gm Qiang C et al. Convergence of placenta biology and genetic risk for schizophrenia. Nature Med 2018;24:792–801 [DOI] [PubMed] [Google Scholar]
- Lacaille H; Vacher CM; Bakalar D; O’Reilly JJ; Salzbank J; Penn AA. Impaired interneuron development in a novel model of neonatal brain injury. eNeuro. 2019;6. doi: 10.1523/ENEURO.0300-18.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobue A; Ito N; Nagai T; Shan W; Hada K; Nakajima A; Murakami Y; Mouri A; Yamamoto Y; Nabeshima T; Saito K; Yamada K. Astroglial major histocompatibility complex class I following immune activation leads to behavioral and neuropathological changes. GLIA. 66(5):1034–1052, 2018. 05.) [DOI] [PubMed] [Google Scholar]
- Dada T, Rosenzweig JM, Al Shammary M, Firdaus W, Al Rebh S, Borbiev T, Tekes A, Zhang J, Alqahtani E, Mori S, Pletnikov MV, Johnston MV, Irina Burd I. Mouse model of intrauterine inflammation: Sex-specific differences in long-term neurologic and immune sequelae. Brain, Behavior, and Immunity 38 (2014) 142–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganapathi R; Manda K. Later life changes in hippocampal neurogenesis and behavioral functions after low-dose prenatal irradiation at early organogenesis stage. International Journal of Radiation Oncology, Biology, Physics. 98(1):63–74, 2017. [DOI] [PubMed] [Google Scholar]
- Zuloaga DG; Carbone DL; Quihuis A; Hiroi R; Chong DL; Handa RJ. Perinatal dexamethasone-induced alterations in apoptosis within the hippocampus and paraventricular nucleus of the hypothalamus are influenced by age and sex. Journal of Neuroscience Research. 90(7):1403–12, 2012. [DOI] [PubMed] [Google Scholar]
- Owen D; Setiawan E; Li A; McCabe L; Matthews SG. Regulation of N-methyl-D-aspartate receptor subunit expression in the fetal guinea pig brain. Biology of Reproduction. 71(2):676–83, 2004. [DOI] [PubMed] [Google Scholar]
- Kim EN, Yoon BH, Jeon EJ, Lee JB, Hong JS, Lee JY, Hwang D, Kim KC, Kim JS, Kim CJ. Placental deposition of C-reactive protein is a common feature of human pregnancy. Placenta 36 (2015) 704–707. [DOI] [PubMed] [Google Scholar]
- Ernst LM; Grobman WA; Wolfe K; Huang MH; McDade TW; Holl JL; Borders AE. Buss C; Lord C; Wadiwalla M; Hellhammer DH; Lupien SJ; Meaney MJ; Pruessner JC. Biological markers of stress in pregnancy: associations with chronic placental inflammation at delivery. American Journal of Perinatology. 30(7):557–64, 2013. August. [DOI] [PubMed] [Google Scholar]
- Malek A, Bersinger NA, Di Santo S, Mueller MD, Sager R, Schneider H, Ghezzi F, Karousou E, Passi A, DeLuca G, Raio L. C-reactive protein production in term human placental tissue. Placenta 2006;27: 619–625. [DOI] [PubMed] [Google Scholar]
- Canetta1S, Bolkan S, Padilla-Coreano N, Song LJ, R Sahn R, Harrison NL, Gordon JA, Brown A, Kellendonk C. Maternal immune activation leads to selective functional deficits in offspring parvalbumin interneurons. Molecular Psychiatry (2016) 21, 956–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronson SL, Bale TL. Prenatal stress-induced increases in placental inflammation and offspring hyperactivity are male-specific and ameliorated by maternal anti-inflammatory treatment. Endocrinology 155, 2635–2646 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melbye H, Hvidsten D, Holm A, Nordbø SA, Brox J. The course of C-reactive protein response in untreated upper respiratory tract infection. Br J Gen Pract 2004;54:653–658. [PMC free article] [PubMed] [Google Scholar]
- Madan JC, Davis JM, Craig WY, Collins M, Allan W, Quinn R, Dammann O. Maternal obesity and markers of inflammation in pregnancy. Cytokine 2009;47:61–64. [DOI] [PubMed] [Google Scholar]
- Osborne LM, Monk C. Perinatal depression--the fourth inflammatory morbidity of pregnancy?: Theory and literature review. Psychoneuroendocrinology. 2013;38(10):1929–1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AS, Meyer U. Maternal immune activation and neuropsychiatric illness: a translational research perspective. Am J Psychiatry 2018;175:1073–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AS, Sourander A, Hinkka-Yli-Salomäki S, McKeague IW, Sundvall J, Surcel HM. Elevated maternal C-reactive protein and autism in a national birth cohort. Mol Psychiatry. 2014;19(2):259–264. doi: 10.1038/mp.2012.197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlenmeyer-Kimling L, Cornblatt B. The New York High-Risk Project: a follow-up report. Schizophr Bull. 1987;13(3):451–61. [DOI] [PubMed] [Google Scholar]
- Walker EF, Savoie T, Davis D: Neuromotor precursors of schizophrenia. Schizophr Bull 1994; 20:441–451 [DOI] [PubMed] [Google Scholar]
- Brown AS; Derkits EJ. Prenatal infection and schizophrenia: a review of epidemiologic and translational studies. American Journal of Psychiatry. 167(3):261–80, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu WL; Adams CE; Stevens KE; Chow KH; Freedman R; Patterson PH. The interaction between maternal immune activation and alpha 7 nicotinic acetylcholine receptor in regulating behaviors in the offspring. Brain, Behavior, & Immunity. 46:192–202, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canetta S; Sourander A; Surcel HM; Hinkka-Yli-Salomaki S; Leiviska J; Kellendonk C; McKeague IW; Brown AS Elevated maternal C-reactive protein and increased risk of schizophrenia in a national birth cohort. American Journal of Psychiatry. 171(9):960–8, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi A, Pollice R, Daneluzzo E, Marinangeli MG, Stratt P: Behavioral neurodevelopmental abnormalities and schizophrenic disorder: a retrospective evaluation with the Child Behavior Checklist (CBCL). Schizophrenia Res 2000; 44:121–128 [DOI] [PubMed] [Google Scholar]
- Giovanoli S, Engler H, Engler A et al. Stress in puberty unmasks latent neuropathological consequences of prenatal immune activation in mice. Science 2013; 339:1095–1099. [DOI] [PubMed] [Google Scholar]
- Clarke MC, Tanskanen A, Huttunen M, Whittaker JC, Cannon M. Evidence for an interaction between familial liability and prenatal exposure to infection in the causation of schizophrenia. Am J Psychiatry. 2009;166(9):1025–30. [DOI] [PubMed] [Google Scholar]
- Andersson NW; Goodwin RD; Okkels N; Gustafsson LN; Taha F; Cole SW; Munk-Jorgensen P Depression and the risk of severe infections: prospective analyses on a nationwide representative sample. International Journal of Epidemiology. 45(1):131–9, 2016 [DOI] [PubMed] [Google Scholar]
- Freedman R, Coon H, Myles-Worsley M, Orr-Urtreger A, Olincy A, Davis A, Polymeropoulos M, Holik J, Hopkins J, Hoff M, Rosenthal J, Waldo MC, Reimherr F, Wender P, Yaw J, Young DA, Breese CR, Adams C, Patterson D, Adler LE, Kruglyak L, Leonard S, Byerley W. Linkage of a neurophysiological deficit in schizophrenia to a chromosome 15 locus. Proc Natl Acad Sci U S A. 1997;94(2):587–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman R, Hunter SK, Law AJ, Wagner BD, D’Alessandro A, Christians U, Noonan K, Wyrwa A, Hoffman MC. Higher gestational choline levels in maternal infection protect infant brain development. Journal of Pediatrics 2019;208:198–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oskvig DB, Elkahloun AG, Johnson KR, Phillips TM, Herkenham M. Maternal immune activation by LPS selectively alters specific gene expression profiles of interneuron migration and oxidative stress in the fetus without triggering a fetal immune response. Brain Behav Immun 2012;26:623–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph MD, Graham AM, Feczko E, Miranda-Dominguez O, Rasmussen JM, Nardos R et al. (2018). Maternal IL-6 during pregnancy can be estimated from newborn brain connectivity and predicts future working memory in offspring. Nat. Neurosci. 21, 765–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison AK, Hunter SK, Wagner BD, Calvin EA, Zerbe GO, Ross RG. Diminished infant P50 sensory gating predicts increased 40-month-old attention, anxiety/depression, and externalizing symptoms. J Atten Disord 2007;21:209–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quednow BB, Brinkmeyer J, Mobascher A, Nothnagel M, Musso F, Gründer G, Savary N, Petrovsky N, Frommann I, Lennertz L, Spreckelmeyer KN, Wienker TF, Dahmen N, Thuerauf N, Clepce M, Kiefer F, Majic T, Mössner R, Maier W, Gallinat J, Diaz-Lacava A, Toliat MR, Thiele H, Nürnberg P, Wagner M, Winterer G. Schizophrenia risk polymorphisms in the TCF4 gene interact with smoking in the modulation of auditory sensory gating. Proc Natl Acad Sci U S A. 2012;109(16):6271–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall MH, Taylor G, Salisbury DF, Levy DL. Sensory gating event-related potentials and oscillations in schizophrenia patients and their unaffected relatives. Schizophr Bull. 2011;37(6):1187–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith E, Crawford T, Thomas M, Reid V. Schizotypy and sensory gating: a 6-month-old EEG study. Schizophr Bull 2018;44:S301–S302. [Google Scholar]
- 80. Hunter SK, Gillow SJ, Ross RG. Stability of P50 auditory sensory gating during sleep from infancy to 4 years of age. Brain Cognition 2015;94:4–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D-J, Davis EP, Sandman CA et al. Prenatal maternal cortisol has sex-specific associations with child brain network properties. Cerebral Cortex 2017;27:5230–5241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovanoli S, Werge TM, Mortensen PB, Didriksen, Meyer U. Interactive effects between hemizygous 15q13.3 microdeletion and peripubertal stress on adult behavioral functions. Neuropsychopharmacology (2019) 44:703–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Han X, Bao J, Yang J, Shi SQ, Garfield RE, Liu H. Choline supplementation during pregnancy protects against gestational lipopolysaccharide-induced inflammatory responses. Reprod Sci. 2018. January;25(1):74–85. [DOI] [PubMed] [Google Scholar]
- King JH, Kwan STC, Yan J, Jiang X, Fomin VG, Levine SP, Wei E, Roberson MS, Caudill MA. Maternal choline supplementation modulates placental markers of inflammation, angiogenesis, and apoptosis in a mouse model of placental insufficiency. Nutrients. 2019. February 12;11(2). pii: E374. doi: 10.3390/nu11020374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim-Fine S, Regnault TR, Lee JS, Gimbel SA, Greenspoon JA, Fairbairn J, Summers K, de Vrijer B. Male gender promotes an increased inflammatory response to lipopolysaccharide in umbilical vein blood. J Maternal-Fetal and Neonatal Med 2012; 25:2470–2474. [DOI] [PubMed] [Google Scholar]
- Makinson R, Lloyd K, Rayasam A et al. Intrauterine inflammation induces sex-specific effects on neuroinflammation, white matter, and behavior. Brain, Behavior, and Immunity 66 (2017) 277–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross RG, Freedman R. Endophenotypes in Schizophrenia for the Perinatal Period: Criteria for Validation. Schizophr Bull. 2015. July;41(4):824–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.