Abstract
Background
Lower extremity peripheral arterial disease (PAD) is a public health problem and many patients with PAD experience claudication despite adequate medical and/or surgical management. Mobilization of endogenous progenitor cells using Granulocyte-Macrophage Colony Stimulating Factor (GM-CSF) is a novel therapeutic option that has shown promising results in experimental models and phase I/IIA trials. The GPAD-3 trial will study the effect of two successive administrations of GM-CSF at 3-month interval for improving claudication among patients with lower extremity PAD.
Methods
We plan to recruit 176 patients in this ongoing randomized, double-blind, placebo-controlled Phase IIB trial. After screening for inclusion and exclusion criteria, eligible subjects undergo a 4-week screening phase where they perform subcutaneous placebo injections thrice weekly and walk at least three times a day until they develop claudication. After the screening phase, eligible subjects undergo baseline testing and are randomized 2:1 to receive 500 μg/day of GM-CSF subcutaneously thrice weekly for three weeks or placebo injections. After 3 months, follow-up endpoint testing is performed and subjects in the GM-CSF group receive the second administration of the drug for three weeks while subjects in placebo group receive matching placebo injections. All participants undergo endpoint testing at six-month and nine-month follow-up. The primary endpoint is change in 6-minute walk distance between baseline and 6-month follow-up.
Conclusion
GPAD-3 explores a novel approach to address the need for alternative therapies that can alleviate symptoms among patients with lower extremity PAD. If successful, this study will pave the way for a pivotal Phase III trial.
Keywords: peripheral artery disease, GM-CSF, angiogenesis, claudication
INTRODUCTION
Peripheral arterial disease (PAD) is characterized by an acute or chronic obstruction of one or more non-cardiac, non-intracranial conductance arteries.1 Lower extremity PAD is caused by obstruction of the aortoiliac, femoropopliteal, and/or infrapopliteal arteries that is most commonly caused by atherosclerosis.2 It is an important public health problem affecting nearly 8.5 million Americans and over 200 million individuals across the world.3, 4 Lower extremity arterial obstruction can lead to downstream ischemia that manifests as symptoms of intermittent claudication (leg discomfort with activity), and infrequently as critical limb ischemia and acute limb ischemia that can lead to tissue loss.5 However, only 10% of patients have the classic symptom of intermittent claudication, while approximately 40% do not have leg pain and the remaining 50% have a variety of symptoms that lead to walking impairment, but differ from classic claudication.6 Overall, these patients have a reduced quality of life related to functional impairment and loss of mobility,7 and are at a significant risk of early mortality and adverse cardiovascular events.8 Exercise therapy, arterial revascularization (endovascular or surgical), cilostazol, and statins have been shown to mitigate symptoms.9–12 However, several patients with PAD continue to suffer from lifestyle-limiting claudication despite appropriate medical and/or surgical management. Stem cell and progenitor cell therapies that promote neoangiogenesis are emerging treatment modalities to help mitigate symptoms of lower extremity PAD.13–16
RATIONALE
Progenitor cells (PCs), particularly those of endothelial origin, are involved in vascular repair and regeneration.17 They originate primarily from the bone marrow, differentiate into endothelial and other vascular cells in vivo and in vitro,18–20 and circulating PCs contribute to neovascularization during tissue repair by direct and paracrine mechanisms.21 Circulating PCs can be enumerated by flow cytometry and human hematopoietic stem cells have been isolated primarily through their expression of the marker CD34.22–24 CD34+ mononuclear cells from human bone marrow include distinct lineages of both hematopoietic (CD34+CD45med) and non-hematopoietic (mesenchymal) progenitors that lack the expression of CD45 and CD50 surface markers.25 CD133 is a 5-transmembrane antigen marker of primitive stem cells that is lost during maturation, and CD34− cells expressing CD133+ differentiate into CD34+ cells with greater proliferative activity and thus cells expressing both markers (CD34+/CD133+) may be further enriched for a vascular PC phenotype.26, 27 Co-expression of CXCR4 promotes homing of PCs to stromal derived factor (SDF)-enriched hypoxic environments for enhancing vascular repair, may also further characterize PCs with capacity for vascular regeneration.28 While additional expression of vascular endothelial growth factor receptor-2 (VEGFR2) has been proposed to identify more differentiated progenitor cell types,29 these sub-populations remain difficult to reproducibly quantify compared to other more abundant CD34+ populations.30
Endogenous, pharmacologically-stimulated, and exogenous PCs have been shown to contribute to reendothelialization of tissues after endothelial injury, attenuating progression to frank atherosclerosis.31–37 Granulocyte colony stimulating factor (G-CSF) and granulocyte-macrophage colony stimulating factor (GM-CSF) stimulate mobilization of hematopoietic and endothelial PCs and other precursors via specific receptors.38–43 Takahashi et al. in a seminal experiment demonstrated the potent stimulatory effect of GM-CSF on PC mobilization. In a rabbit model of hind limb ischemia, seven days of daily GM-CSF use augmented PC-enriched cell populations almost 2-fold, increased capillary density, and improved perfusion.44 In humans, GM-CSF can safely and effectively mobilize CD34+ cells from the bone marrow,45, 46 and pilot studies have observed possible benefit of cytokine therapy in patients with lower extremity PAD. A summary of these studies is provided in provided in Table 1.
Table 1:
Summary of Clinical Trials evaluating the therapeutic role of GM-CSF in Peripheral Artery Disease
| Study | Design | Sample Size | GM-CSF dose | Results |
|---|---|---|---|---|
| START13 | Double-blinded, randomized, placebo-controlled trial | N=40 GM-CSF (N=20) Placebo (N=20) |
Subcutaneous injections 10 μg/kg/day On alternate days for 14 days (7 doses) |
Change in walking distance at 2 weeks: GM-CSF: 63±111 m vs. Placebo: 57±113 m (p=0.87) |
|
Change in pain-free walking distance at 2 weeks: GM-CSF: 28±49 m vs. Placebo: 30±43 m (p=0.89) | ||||
| G-PAD-I14 | Double-blinded, randomized, placebo-controlled trial | N=45 GM-CSF (N=29, three groups) Placebo (N=16) |
Subcutaneous injections 3 μg/kg/day (N=10), 6 μg/kg/day (N=9), 10 μg/kg/day (N=10) Three times a week for two weeks (6 doses) |
Change in brachial artery FMD at 12 weeks: GM-CSF: 2.9±06% to 4.6±0.6% (p=0.01) Placebo: 4.3±0.6% to 5.0±0.6% (p=0.50) |
|
Change in treadmill pain-free walking time at 12 weeks: GM-CSF: +38 s (p=0.008) Placebo: No significant change | ||||
|
Change in treadmill total walking time at 12 weeks: GM-CSF: +55 s (p=0.016) Placebo: No significant change | ||||
| GPAD-215 | Double-blinded, randomized, placebo-controlled trial | N=159 GM-CSF (N=80) Placebo (N=79) |
Subcutaneous injections 500 μg/day Three times a week for four weeks (12 doses) |
Change in treadmill peak walk time at 3 months: GM-CSF: 109 s (95% CI 67, 151) Placebo: 56 s (95% CI 14, 98) Difference: 53 s (95% CI −6, 112; p=0.08) |
|
Change in treadmill peak walk time at 6 months: GM-CSF: 112 s (95% CI 71, 153) Placebo: 77 s (95% CI 36, 117) Difference: 35 s (95% CI −26, 93; p=0.24) | ||||
|
Change in treadmill claudication onset time at 3 months: GM-CSF: 81 s (95% CI 52, 111) Placebo: 61 s (95% CI 32, 91) Difference: 20 s (95% CI −22, 62; p=0.35) | ||||
|
Change in treadmill claudication onset time at 6 months: GM-CSF: 93 s (95% CI 64, 122) Placebo: 61 s (95% CI 33, 89) Difference: 35 s (95% CI −8, 72; p=0.12) | ||||
| PROPEL16 | Double-blinded, randomized, placebo/attention-controlled trial with a 2 × 2 factorial design | N=210 GM-CSF + Supervised Exercise (N=53) Placebo + Supervised exercise (N=53) GM-CSF + Attention Control (N=53) Placebo + Attention Control (N=51) |
Subcutaneous injections 250 μg/m2/day Three times a week for two weeks (6 doses) |
Change in 6-minute walk distance at 12 weeks: GM-CSF + Exercise: 22.2 m (95% CI 5.4, 39.0) Placebo + Exercise: 28.5 m (95% CI 11.7, 45.4) Difference: −6.3 m (95% CI −30.2, 17.6; p=0.61) |
| GM-CSF + Attention Control: −6.4 m (95% CI −23.0, 10.1) Placebo + Attention Control: −5.0 m (95% CI −22.3, 12.2) Difference: −1.4 m (95% CI −25.2, 22.4; p=0.91) | ||||
|
Change in treadmill maximal walk time at 12 weeks: GM-CSF + Exercise: 3.5 min (95% CI 2.5, 4.5) Placebo + Exercise: 4.2 min (95% CI 3.2, 5.2) Difference: −0.7 min (95% CI −2.1, 0.8; p=0.35) | ||||
| GM-CSF + Attention Control: −0.1 min (95% CI −1.1, 0.9) Placebo + Attention Control: 0.5 min (95% CI −0.6, 1.6) Difference: −0.6 min (95% CI −2.1, 0.9; p=0.44) |
Abbreviations: GM-CSF = granulocyte-macrophage colony stimulating factor, m = meter, FMD = flow mediated dilation, s = second, min = minute.
In the START trial, GM-CSF or placebo were administered by seven subcutaneous injections over 14 days, and investigators found no changes in walking distance at 2 weeks in the GM-CSF and placebo groups.13 Notably, Doppler flow measurements in the placebo group declined significantly but remained unchanged in the treated group during the 3-month period.13 Based on the observations of improved neovascularization in experimental models with GM-CSF and the equivocal START trial, we previously completed a double-blind, placebo-controlled Phase I dose-escalation trial in 45 patients with symptomatic PAD (G-PAD-I).14 Patients received subcutaneous injections of either placebo or GM-CSF thrice weekly in escalating doses of 3, 6 or 10 μg/kg/day administered for two weeks. This trial demonstrated the safety of GM-CSF use, its ability to mobilize PCs into the circulation, and the adequacy of the dose range tested.14 At 12 weeks, patients receiving GM-CSF experienced improvements in brachial artery flow mediated dilation (FMD), pain-free treadmill walking time, and total treadmill walking time (Table 1). However, such improvements were not observed in the placebo group.14
The G-PAD-I study was followed by a larger double-blind, placebo-controlled Phase IIA trial, GPAD-2, where we tested the hypothesis that GM-CSF administration in patients with symptomatic PAD would result in improvement in treadmill peak walk time (PWT) at 3 months.15 A total of 159 patients were randomized 1:1 to receive four weeks of subcutaneous injections of GM-CSF or placebo.15 In the intention-to-treat analysis, the increase in PWT at 3 months in the GM-CSF group compared to placebo trended toward significance. (Table 1). In the per-protocol analysis, PWT increased in both groups, but the 113 second increase from baseline to 3 months, and the 122 second increase from baseline to 6 months in those receiving GM-CSF was significantly greater than the corresponding increase in the placebo group (44 seconds, p=0.02 and 57 seconds, p=0.02 at 3 and 6 months, respectively).15 GM-CSF increased the leucocyte and PC counts with peak mobilization at 2 weeks and patients with >100% increase in PCs had a greater increase in PWT compared to those with <100% increase (131 vs. 60 seconds, p=0.04).15
More recently, the Progenitor Cell Release Plus Exercise to Improve Functional Performance in PAD (PROPEL) trial randomized patients with symptomatic PAD in a 2 × 2 factorial design to GM-CSF, supervised exercise, or placebo.16 Patients received two weeks of subcutaneous injections of GM-CSF thrice-weekly or placebo along with supervised treadmill exercise sessions three times a week with an exercise physiologist or attention control.16 As compared to the placebo + exercise group, participants in the GM-CSF + exercise group did not have significantly different change in 6-minute walk distance or PWT at 12 weeks (Table 1).16 Additionally, patients in the GM-CSF + attention control group did not have significantly different change in 6-minute walk distance or PWT at 12 weeks as compared to placebo + attention control group (Table 1).16 However, PROPEL investigators were not able to recruit the pre-specified number of participants and importantly, did not test the potential impact of repeated GM-CSF treatment among patients with PAD.
Therefore, in the ongoing randomized, placebo-controlled Phase IIB trial, GPAD-3, we are investigating whether repeat administration of GM-CSF injections (thrice a week for 3 weeks) three months after the initial treatment will increase the possible benefit of GM-CSF to alleviate claudication symptoms in patients with clinically stable PAD. GPAD-3 participants complete a 4-week screening phase before randomization where they are instructed to walk to symptom limitation at least 3 times a day which will help decrease the exercise training effect in our trial population.
The GPAD-3 trial addresses a compelling need for alternative therapy for patients with PAD that are symptomatic despite optimal medical management. We are investigating a unique and novel approach of delivering autologous cell therapy by employing endogenous PC mobilization and homing to the sites of ischemia in the lower extremities. If proven successful and effective, our results will likely provide an important therapeutic option for this population and pave the way for a pivotal Phase III trial.
METHODS
Major objective
The major objective of this study is to investigate the effects of mobilization of bone marrow PCs with two successive administrations of subcutaneous GM-CSF at three-month intervals in patients with atherosclerotic lower extremity PAD and walking impairment.
Hypothesis
Our hypothesis is that repeated GM-CSF use will lead to a sustained improvement in walking distance and quality of life in this patient population.
Endpoints
The primary endpoint of this trial is change in walking performance as measured using 6-minute walk distance at 6 months among participants receiving two treatments of GM-CSF (Group A) compared to change in distance among participants receiving placebo (Group B).
The secondary endpoints of this trial include:
Change in PWT during treadmill exercise at 6 months in Group A compared to change in Group B.
Comparison of single vs. two treatments of GM-CSF measured as change in (a) 6-minute walk distance and (b) PWT from 3 months to 6 months in Group A compared to change in Group B.
Comparison of single treatment with GM-CSF measured as: change in (a) 6-minute walk distance and (b) PWT from baseline to 3 months in Group A compared to change in Group B.
- In the group comparisons above, we will measure changes in the following endpoints:
- Walking Impairment Questionnaire (WIQ) sub-scores: walking distance, walking speed, and stair climbing domains
- Physical Health Scale Composite (PCS) score of the Short Form (SF)-36 questionnaire and its domains
- Claudication onset time (COT) during treadmill exercise
- Ankle-brachial index (ABI)
- Correlation between magnitude of change in 6-minute walk distance as well as PWT and the magnitude of change in circulating PC counts
- Long-term (1-year) persistence of changes in functional performance (determined using WIQ and SF-36 questionnaire)
- Adverse events data
Institutional Review Board
The GPAD-3 study protocol has been approved by the institutional review board at Emory University (Atlanta, Georgia).
Funding
The GPAD-3 study is funded by the National Heart, Lung, and Blood Institute of the National Institutes of Health grants 1R61HL138657–01, 4R33HL138657–02, and 5R33HL138657–03.
Study Design
We plan to recruit 176 adult patients with clinically stable, optimally treated atherosclerotic PAD and walking limitation. We started patient recruitment in December 2017 and have recruited 57 subjects thus far. The study has been approved to receive funding till July 2022. After screening for inclusion and exclusion criteria, eligible subjects are trained to perform subcutaneous injections and instructed to walk at least three times a day until they develop claudication or symptomatic limitation for four weeks. At the end of this 4-week period, subjects undergo baseline testing and are randomized 2:1 in a double-blinded manner to receive 500 μg/day of GM-CSF subcutaneously thrice weekly for 3 weeks (Group A) or placebo (Group B). We have used sex and diabetes status for performing stratified randomization. After 3 months, follow-up endpoint testing is performed and subjects in Group A receive the second administration of 500 μg/day of GM-CSF subcutaneously thrice weekly for another 3 weeks while Group B subjects receive matching placebo. All participants undergo endpoint testing two more time at 3-month intervals after the second drug administration (Figure 1). With this study design, we will be able to investigate whether repeat injections of GM-CSF three-months apart have a greater therapeutic effect compared to placebo, and whether the second dosing enhances the effects of GM-CSF. The key phases of the trial are described below.
Figure 1: GPAD-3 study design.
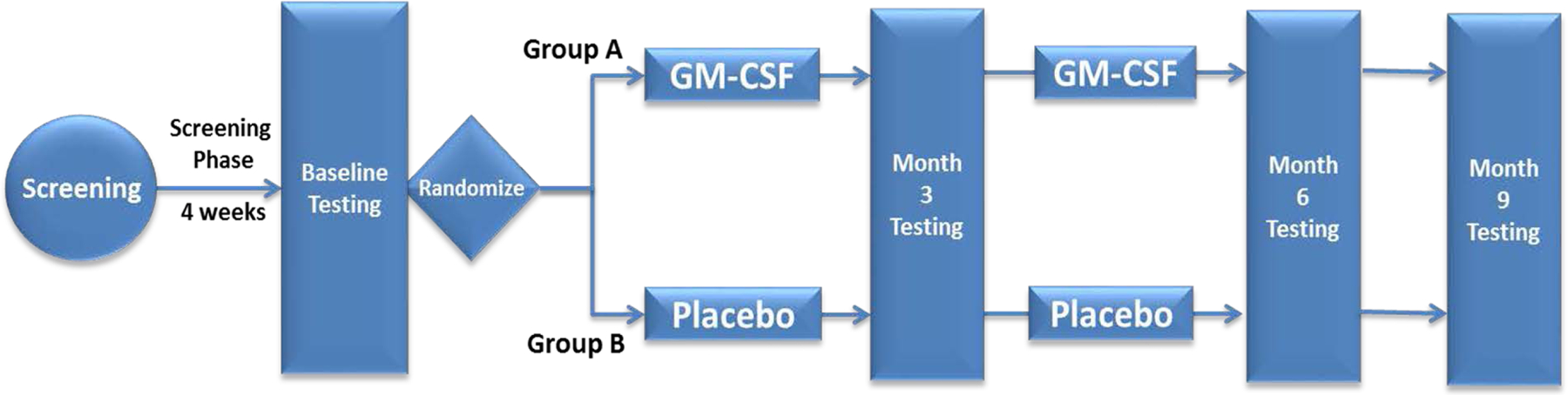
Screening phase:
After informed consent is obtained, subjects have inclusion and exclusion criteria evaluated and once they are deemed eligible, they undergo a 6-minute walk test and a treadmill exercise test using the Gardner or a modified Bruce protocol. The correct technique for subcutaneous injection administration is taught and all enrolled subjects start injecting placebo subcutaneously three times a week for the duration of the 4-week screening phase to familiarize themselves with subcutaneous injection use. They are also instructed to walk to symptom limitation at least 3 times a day during the screening phase while maintaining a daily electronic diary. Participant activity is monitored using an activity tracker, Fitbit Flex 2™ (Fitbit Inc., San Francisco, CA). This ensures that those who were very inactive before enrollment become familiar with walking exercise and any training effect from home exercise is achieved in all subjects before randomization. We are also testing subject compliance with the exercise regimen by tracking step count and physical activity at each visit.
Randomization visit:
After the end of the screening phase patients undergo baseline testing and if they continue to meet the eligibility criteria, they are randomized. The 6-minute walk test and the treadmill exercise test are performed twice at the randomization visit to establish a stable and reproducible baseline. Treatment assignments are generated in random, permuted blocks (with block sizes of 3, 6, or 9) to ensure balance between the number of patients assigned to each of the two treatment arms and blinding is maintained by Investigational Pharmacy at the Emory University School of Medicine. Participants are stratified for diabetes so that a proportionate number of patients with diabetes (2:1) are randomized to each group.
First drug administration phase:
The first 3-week drug administration phase begins at the randomization visit. All subjects are evaluated every week by the study physician during this time period for any adverse effects and the peripheral blood count is checked. While the drug or placebo are given in a non-variable 500 μg dose, any modifications to the dose are made by an un-blinded investigator who receives the peripheral blood count results from the research labs and can alter the dose by informing the pharmacy and the subject. The drug dose can be modified if certain hematologic or constitutional toxic effects are observed as discussed later.
Three-month (12 week) visit:
Follow-up testing for study endpoints is performed at 3 months and Group A participants receive the second administration of GM-CSF 500μg subcutaneously thrice weekly for an additional 3 weeks, whereas Group B participants receive 3 weeks of placebo injections.
Second drug administration phase:
Similar to the first drug administration phase participants are evaluated each week for any adverse effects by the study physician and the peripheral blood count is checked.
Six-month (24 week) and Nine-month (36 week) visit:
All study endpoint tests done at the three-month visit are performed during the six-month and nine-month visits.
1, 2, and 3 years after enrollment:
Telephone follow-up is performed to acquire questionnaire and adverse event data.
Study Measurements
The study calendar detailing measurements performed during the study is depicted in Table 2.
Table 2:
GPAD-3 Trial Study Calendar
| Procedures | S | R | W 1 |
W 2 |
W 3 |
Month 3 |
W 14 |
W 15 |
W 16 |
Month 6 |
Month 9 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| History, physical | X | X | X | X | X | X | X | X | X | X | X |
| Blood Tests | |||||||||||
| CBC and Chemistry | X | X | X | X | X | X | X | X | X | X | X |
| Progenitor cells | X | X | X | X | X | X | |||||
| Functional measures | |||||||||||
| Treadmill exercise test | X | 2X | 2X | 2X | 2X | ||||||
| 6-minute walk test | X | 2X | 2X | 2X | 2X | ||||||
| ABI | X | X | X | X | X | ||||||
| Questionnaires | |||||||||||
| San Diego | X | ||||||||||
| SF-36 survey | X | X | X | X | |||||||
| WIQ | X | X | X | X | |||||||
| Adverse Effects/Events | X | X | X | X | X | X | X | X | X | ||
Abbreviations: S = screening visit, R = randomization visit, W = week, CBC = complete blood count, ABI = ankle brachial index, SF = short-from, WIQ = walking impairment questionnaire
Measures of GM-CSF response
The response to GM-CSF therapy is ascertained using 6-minute walk test, treadmill exercise test, ABI testing, and standardized questionnaires.
Six-minute walk test: participants are asked to walk up and down a 100-foot hallway for 6 minutes to cover the maximum distance possible. The distance completed after 6 minutes is recorded. The 6-minute walk distance is the primary outcome measure and the test is performed twice at randomization and the 3, 6, and 9-month visits.
Treadmill exercise test: graded treadmill exercise testing is performed using the Gardner protocol or the modified Bruce protocol and the PWT and COT are recorded. If a subject is unable to complete a minimum of 1 minute of the treadmill test or can walk >12 minutes on the modified Bruce protocol, they are considered a screen failure at the screening or randomization visit. Treadmill exercise testing is performed twice at randomization, and the 3, 6, and 9-month visits.
Doppler derived ABI: with the patient in supine position, bilateral brachial and ankle blood pressure cuffs are inflated about 30 mm Hg above the systolic pressure. Doppler flow signals are used to detect the reappearing perfusion while reducing the cuff pressure. The dorsalis pedis and posterior tibial pressures in each lower extremity are measured and the higher of the two is used to calculate the ABI.47 The highest brachial pressure of the two arms is used for calculating the ABI. ABI testing is performed before and after each treadmill exercise test.
Symptom assessment: functional status is assessed by the WIQ and SF-36 Health Survey to provide an index of change in symptoms during follow-up.
Physical activity assessment: physical activity is measured using the Fitbit Flex 2™ (Fitbit Inc., San Francisco, CA) device and these data are used to document the change in physical activity during the follow-up.
Measures of mechanisms and determinants of GM-CSF response
The mechanisms and determinants of GM-CSF response will be determined by measuring complete blood count, including white blood cell count, at each visit and by measuring circulating PCs at randomization, end of first and second drug administration phases, and at the 3, 6, and 9-month visits. The numbers of circulating mononuclear cells expressing the PC specific epitopes (CD34, CD133, CXCR4, and VEGF2R) is being counted in peripheral blood samples collected in EDTA tubes using fluorescent activated sorting. Blood samples are prepared within 4 hours of collection and incubated with fluorochrome-labeled monoclonal antihuman mouse antibodies. We incubate 300 μl of peripheral blood with 7 μl of FITC-CD34 (BD Biosciences), PerCP-CD45 (BD Biosciences), PE-VEGFR2 (R&D system), 5 microL APC-CD133 (Miltenyi), and 3 microL PE-Cy7-conjugated anti-CXCR4 (EBioscience, clone 12G5) in the dark for 15 minutes.48 Then 1.5 mL ammonium chloride lysing buffer is added to lyse red blood cells, following which 1.5 mL staining medium (PBS with 3% heat-inactivated serum and 0.1% sodium azide) is added to stop the lysing reaction.48 Prior to flow cytometry, 100 microL of AccuCheck Counting Beads (Invitrogen, Cat#: PCB100) are added to act as an internal standard for direct estimation of the concentration of target cell subsets.48 At least 2.5 million events are acquired from the cytometer. Flow cytometry data is analyzed with Flowjo software (Treestar, Inc.) and circulating PC populations (CD34+, CD34+/CD133+, CD34+/CXCR4+, and CD34+/VEGF2R+) will be reported as cell counts per mL.48
Study Population and Sites
We plan to recruit 176 patients in the trial with the expectation that at least 150 will complete the entire study. This sample size was derived based on power calculations that are described later. Each patient is carefully assessed to determine eligibility and ensure compliance with the inclusion and exclusion criteria listed in Table 3. Subjects are asked to fill out a screening questionnaire including the San Diego questionnaire for assessment of walking limitation/claudication and a full physical examination is performed by a study physician at the screening and randomization visit. Participants are being recruited from clinics and hospitals affiliated with the Emory University School of Medicine.
Table 3:
GPAD-3 Inclusion and Exclusion Criteria
| Inclusion Criteria | Exclusion Criteria |
|---|---|
|
|
Abbreviations: PAD = peripheral arterial disease, PWT = peak walk time, ABI = ankle-brachial index, TBI = toe-brachial index, NYHA = New York heart association, HbA1c = glycated hemoglobin, AST = aspartate aminotransferase, ALT = alanine aminotransferase
Safety and Adverse Events
Subjects are closely monitored for any adverse events during the entire study and for dose-limiting toxicity during the drug administration phases. These adverse events are reported by the principal investigator (Arshed Quyyumi, MD) to the Emory University Institutional Review Board, the Georgia Clinical and Translational Science Alliance, and Data Safety Monitoring Board in accordance with the National Cancer Institute Common Terminology Criteria for Adverse Events. The GM-CSF dose being used in GPAD-3 (500 μg/day, thrice weekly) is the same as the GPAD-2 trial, albeit with a different duration of treatment. GM-CSF was generally well tolerated in GPAD-2;15 and the adverse effects of GM-CSF use, and dose-limiting toxicities are described below.
A serious adverse event is characterized by at least one of the following:
A fatal or life-threatening event
An event that renders the patient permanently disabled
An event requiring in-patient hospitalization
An unexpected adverse event is this trial is defined as an event which:
Has not been previously reported with GM-CSF use
Is symptomatically and pathophysiologically related to a known toxicity but differs because of greater severity or increased frequency among trial participants
If significant serious or unexpected adverse effects occur as defined above, the principal investigator will break the code and inform the Institutional Review Board, Data Safety Monitoring Board and the Food and Drug Administration within 48 hours for death and within 7 days for other events. The subject will be removed from the study and the protocol will be reviewed to make appropriate changes.
GM-CSF has the following known adverse effects:
Constitutional: fever, asthenia, malaise, headache, allergic or anaphylactic reaction
Pulmonary: dyspnea, pleural effusion, capillary leak syndrome
Hematopoietic: blood dyscrasia, neutralizing antibodies against GM-CSF
Cardiovascular: edema, pericardial effusion, transient supraventricular arrhythmias
Gastrointestinal: nausea, worsening of pre-existing hepatic disease
Renal: worsening of pre-existing renal disease
Skin: rash at the injection site
During the drug administration phases participants are monitored for GM-CSF dose-limiting toxicity:
Hematological: elevation of leukocyte counts (>35,000/ml) or depression of platelet counts (<75,000/ml). The complete blood count data is reviewed only by an un-blinded investigator.
Constitutional: a skin rash involving >25% body surface area, pain not responding to at least 4 doses of non-steroidal anti-inflammatory drugs per day, or documented fever >38.5°C
Significant splenic enlargement
Adverse events: major cardiovascular events include death, myocardial infarction, cerebrovascular events (stroke and transient ischemic attacks), and amputations. All events include major cardiovascular events mentioned above, and angina admissions, coronary or peripheral revascularization, and progression of symptoms to critical limb ischemia
If any of these toxicities are observed, the un-blinded investigator contacts the Investigational Pharmacy and/or the subjects to discontinue the drug by switching to placebo or decrease the subsequent doses to half (250 μg) depending on the severity of dose-limiting toxicity. However, such subjects will remain in the study, receive a placebo injection when appropriate, and continue to be followed for efficacy endpoints and late toxicity.
Statistical Methods
Sample size and power
Power calculations for GPAD-3 were conducted based on the preliminary data from the GPAD-2 and GOALS trials assuming a Type I error α=0.05.15, 49 For the primary endpoint of change in 6-minute walk distance at 6 months, a sample size of 150 (N=100 in the GM-CSF treatment Group A) and N=50 in the placebo (Group B) will detect differences of 46.5 m and 53.7 m between the groups with 80% and 90% power, respectively. Both differences are smaller than the previously reported treatment effect (e.g., an estimate of 58 m difference between the treatment intervention and control group in GOALS trial).49 For the secondary endpoint of change in PWT, a sample size of 150 (N=100 in Group A and N=50 in Group B) will detect differences of 112.8 seconds and 130.6 seconds between the groups with 80% and 90% power, respectively. While these minimal detectable differences are slightly greater than the treatment effects reported in the GPAD-2 study at 3 months,15 they are considered feasible, given that the proposed study includes a run-in period aimed at reducing the exercise training effect we observed in the GPAD-2 placebo group.15 Given that the dropout rate in the GPAD-2 study was 6.3% and there will be a longer follow-up time in GPAD-3 due to two treatment periods, we conservatively plan to recruit a total of 176 subjects (117 in Group A and 59 in Group) to allow for a 15% dropout rate.
Statistical analysis
Trial participant data are being entered onto paper case report forms and then entered into the main study database in REDCap (Vanderbilt University, Nashville, TN). Descriptive statistics will be performed initially by calculating means, standard deviation, range, descriptive plots and histograms for each variable to determine distribution, outliers, and homogeneity of variance across groups as well as detect potential selection bias due to missing data and dropout. In the presence of missing data or dropouts, sensitivity analysis will be conducted as needed.50 All primary analyses will be based on the intention-to-treat principle for the estimation of effectiveness. In the presence of noncompliance, we will also use statistical approaches including instrumental variable to account for noncompliance.51
The primary endpoint is change in the 6-minute walk distance between baseline and 6-month follow-up. Secondary endpoints include change in PWT; WIQ sub-scores: the walking distance, walking speed, and stair climbing domains; PCS score of the SF-36; COT during treadmill exercise; and ABI. To assess the treatment effect of double administration of GM-CSF (and single administration of GM-CSF) on each endpoint, two sample t-tests or Wilcoxon tests will be used to compare change from baseline to 6 months (and change from baseline to 3 months) between Groups A and B. As a secondary analysis to explore if double administration is more effective than single administration of GM-CSF, linear mixed models (LMMs) or generalized linear mixed models (GLMMs) will be used to assess treatment effects over time (i.e., whether group difference at 6 months is greater than 3 month) by including an interaction term between groups and time. Spearman correlation will be used to assess association between the observed change in each endpoint and the improvement in circulating PCs at each time point stratified by treatment groups. LMMs or GLMMs will be used to assess association between the observed improvement in each endpoint and the improvement in circulating PCs over time, while controlling for treatment groups and other potential confounders. In all regression analyses, Akaike Information Criterion (AIC) will be used for model selection.52
Interim analysis
We will conduct one interim analysis when 50% of the entire sample completes 6-month follow-up. To preserve the overall Type I error rate for effectiveness at the 0.05 significance level, we will employ the Lan-DeMets alpha spending function method with O’Brien-Fleming type boundaries to determine group sequential boundaries for the interim analysis of the accumulated data.53 The critical value for the upper and lower bounds for the interim analysis (50% completion) and the final analysis (100% completion) will be 2.9626 (corresponding α=0.0015) and 1.9686 (corresponding α=0.0245), respectively. In addition to assessing treatment efficacy, the interim analysis report will also include a summary of monthly and cumulative accrual, patient characteristics and status (for participants off treatment), assessment of participant adherence to the treatment regimen, adverse events, and protocol violations. Study enrollment will continue during the interim analysis as long as no safety issues are identified. Unblinded investigators will perform the interim analysis, while the principal investigator and study personnel performing study measurements and entering data will remain blinded.
Anticipated results and challenges in interpretation
We anticipate that GM-CSF use will lead to improvement in primary and secondary endpoints of the trial at 6-month follow-up. We also anticipate that two doses of GM-CSF will produce greater improvement in trial endpoints as compared with single dosing, and that this improvement will persist till 9 months. Additionally, we expect to observe a correlation between the magnitude of PC mobilization into the circulation with improvement in endpoints. Lastly, we expect that GM-CSF therapy use will be safe. However, we are not exploring mechanisms of GM-CSF response beyond PC mobilization in this trial which would be a potential challenge in interpreting our results.
SUMMARY
The GPAD-3 study is an ongoing randomized, placebo-controlled Phase IIB trial that is investigating the utility of mobilizing bone marrow progenitor cells with two successive administrations of subcutaneous GM-CSF at three-month intervals in patients with atherosclerotic lower extremity PAD and walking impairment. This trial explores a novel approach to address the compelling need for alternative therapy among patients with symptomatic PAD. If the trial is successful and effective, our results will help provide an important therapeutic option for these patients and pave the way for a pivotal Phase III trial.
Study funding
The GPAD-3 study is funded by the National Heart, Lung, and Blood Institute of the National Institutes of Health grants 1R61HL138657-01, 4R33HL138657-02, and 5R33HL138657-03. A.M., and D.S.D. have been supported by the Abraham J. & Phyllis Katz Foundation (Atlanta, GA). A.M. is supported by American Heart Association postdoctoral fellowship award 19POST34400057. A.Q. is also supported by NIH grants 1P20HL113451-01, 1P30DK111024-03S1, 5R01HL095479-08, 3RF1AG051633-01S2, 5R01AG042127-06, 2P01HL086773-08, U54AG062334-01, 1R01HL141205-01, 5P01HL101398-02, 1P20HL113451-01, 5P01HL086773-09 1RF1AG051633-01, R01 NS064162-01, R01 HL89650-01, HL095479-01, 1DP3DK094346-01, 2P01HL086773, and American Heart Association grant 15SFCRN23910003.
Footnotes
Competing interests: no authors have any competing interests
Clinical trial registration
The GPAD-3 trial has been registered on ClinicalTrials.gov under ID# NCT03304821
REFERENCES
- 1.Kullo IJ and Rooke TW. CLINICAL PRACTICE. Peripheral Artery Disease. N Engl J Med 2016; 374: 861–871. 2016/03/11. DOI: 10.1056/NEJMcp1507631. [DOI] [PubMed] [Google Scholar]
- 2.Ouriel K Peripheral arterial disease. Lancet 2001; 358: 1257–1264. 2001/10/25. DOI: 10.1016/S0140-6736(01)06351-6. [DOI] [PubMed] [Google Scholar]
- 3.Allison MA, Ho E, Denenberg JO, et al. Ethnic-specific prevalence of peripheral arterial disease in the United States. Am J Prev Med 2007; 32: 328–333. 2007/03/27. DOI: 10.1016/j.amepre.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 4.Fowkes FG, Rudan D, Rudan I, et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet 2013; 382: 1329–1340. 2013/08/07. DOI: 10.1016/s0140-6736(13)61249-0. [DOI] [PubMed] [Google Scholar]
- 5.Members WC, Gerhard-Herman MD, Gornik HL, et al. 2016 AHA/ACC Guideline on the Management of Patients With Lower Extremity Peripheral Artery Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2017; 135: e686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hirsch AT, Criqui MH, Treat-Jacobson D, et al. Peripheral Arterial Disease Detection, Awareness, and Treatment in Primary Care. JAMA 2001; 286: 1317–1324. DOI: 10.1001/jama.286.11.1317 %J JAMA. [DOI] [PubMed] [Google Scholar]
- 7.McDermott MM, Greenland P, Liu K, et al. Leg symptoms in peripheral arterial disease: associated clinical characteristics and functional impairment. Jama 2001; 286: 1599–1606. 2001/10/05. [DOI] [PubMed] [Google Scholar]
- 8.Fowkes FG, Murray GD, Butcher I, et al. Ankle brachial index combined with Framingham Risk Score to predict cardiovascular events and mortality: a meta-analysis. Jama 2008; 300: 197–208. 2008/07/10. DOI: 10.1001/jama.300.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Malgor RD, Alahdab F, Elraiyah TA, et al. A systematic review of treatment of intermittent claudication in the lower extremities. Journal of vascular surgery 2015; 61: 54S–73S. 2015/02/28. DOI: 10.1016/j.jvs.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 10.Murphy TP, Cutlip DE, Regensteiner JG, et al. Supervised exercise, stent revascularization, or medical therapy for claudication due to aortoiliac peripheral artery disease: the CLEVER study. J Am Coll Cardiol 2015; 65: 999–1009. 2015/03/15. DOI: 10.1016/j.jacc.2014.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bedenis R, Stewart M, Cleanthis M, et al. Cilostazol for intermittent claudication. Cochrane Database Syst Rev 2014: CD003748 2014/11/02. DOI: 10.1002/14651858.CD003748.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mohler ER 3rd, Hiatt WR and Creager MA. Cholesterol reduction with atorvastatin improves walking distance in patients with peripheral arterial disease. Circulation 2003; 108: 1481–1486. 2003/09/04. DOI: 10.1161/01.CIR.0000090686.57897.F5. [DOI] [PubMed] [Google Scholar]
- 13.van Royen N, Schirmer SH, Atasever B, et al. START Trial: a pilot study on STimulation of ARTeriogenesis using subcutaneous application of granulocyte-macrophage colony-stimulating factor as a new treatment for peripheral vascular disease. Circulation 2005; 112: 1040–1046. 2005/08/10. DOI: 10.1161/CIRCULATIONAHA.104.529552. [DOI] [PubMed] [Google Scholar]
- 14.Subramaniyam V, Waller EK, Murrow JR, et al. Bone marrow mobilization with granulocyte macrophage colony-stimulating factor improves endothelial dysfunction and exercise capacity in patients with peripheral arterial disease. Am Heart J 2009; 158: 53–60 e51. 2009/06/23. DOI: 10.1016/j.ahj.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 15.Poole J, Mavromatis K, Binongo JN, et al. Effect of progenitor cell mobilization with granulocyte-macrophage colony-stimulating factor in patients with peripheral artery disease: a randomized clinical trial. JAMA 2013; 310: 2631–2639. 2013/11/20. DOI: 10.1001/jama.2013.282540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McDermott MM, Ferrucci L, Tian L, et al. Effect of Granulocyte-Macrophage Colony-Stimulating Factor With or Without Supervised Exercise on Walking Performance in Patients With Peripheral Artery Disease: The PROPEL Randomized Clinical Trial. JAMA 2017; 318: 2089–2098. 2017/11/16. DOI: 10.1001/jama.2017.17437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Asahara T, Murohara T, Sullivan A, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science 1997; 275: 964–967. 1997/02/14. [DOI] [PubMed] [Google Scholar]
- 18.Asahara T, Masuda H, Takahashi T, et al. Bone Marrow Origin of Endothelial Progenitor Cells Responsible for Postnatal Vasculogenesis in Physiological and Pathological Neovascularization. Circ Res 1999; 85: 221–228. [DOI] [PubMed] [Google Scholar]
- 19.Lin Y, Weisdorf DJ, Solovey A, et al. Origins of circulating endothelial cells and endothelial outgrowth from blood. Journal of Clinical Investigation January 2000; 105: 71–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Urbich C and Dimmeler S. Endothelial progenitor cells: characterization and role in vascular biology. Circ Res 2004; 95: 343–353. [DOI] [PubMed] [Google Scholar]
- 21.Asahara T, Masuda H, Takahashi T, et al. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res 1999; 85: 221–228. 1999/08/07. DOI: 10.1161/01.res.85.3.221. [DOI] [PubMed] [Google Scholar]
- 22.Krause DS, Fackler MJ, Civin CI, et al. CD34: structure, biology, and clinical utility [see comments]. Blood 1996; 87: 1–13. [PubMed] [Google Scholar]
- 23.Berenson RJ. Transplantation of CD34+ hematopoietic precursors: clinical rationale. Transplantation Proceedings 1992; 24: 3032–3034. [PubMed] [Google Scholar]
- 24.Baum CM, Weissman IL, Tsukamoto AS, et al. Isolation of a candidate human hematopoietic stem-cell population. Proceedings of the National Academy of Sciences of the United States of America 1992; 89: 2804–2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Waller EK, Olweus J, Lund-Johansen F, et al. The “common stem cell” hypothesis reevaluated: human fetal bone marrow contains separate populations of hematopoietic and stromal progenitors. Blood 1995; 85: 2422–2435. Comparative Study 1995/05/01. [PubMed] [Google Scholar]
- 26.Gehling UM, Ergun S, Schumacher U, et al. In vitro differentiation of endothelial cells from AC133-positive progenitor cells Blood 2000; 95: 3106–3112. Research Support, Non-U.S. Gov’t; 2000/05/16. [PubMed] [Google Scholar]
- 27.Yin AH, Miraglia S, Zanjani ED, et al. AC133, a novel marker for human hematopoietic stem and progenitor cells. Blood 1997; 90: 5002–5012. [PubMed] [Google Scholar]
- 28.Seeger FH, Rasper T, Koyanagi M, et al. CXCR4 expression determines functional activity of bone marrow-derived mononuclear cells for therapeutic neovascularization in acute ischemia Arteriosclerosis, thrombosis, and vascular biology 2009; 29: 1802–1809. Comparative Study Research Support, Non-U.S. Gov’t; 2009/08/22. DOI: 10.1161/ATVBAHA.109.194688. [DOI] [PubMed] [Google Scholar]
- 29.Alaiti MA, Ishikawa M and Costa MA. Bone marrow and circulating stem/progenitor cells for regenerative cardiovascular therapy. Transl Res 2010; 156: 112–129. 2010/08/31. DOI: 10.1016/j.trsl.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 30.Patel RS, Li Q, Ghasemzadeh N, et al. Circulating CD34+ progenitor cells and risk of mortality in a population with coronary artery disease. Circ Res 2015; 116: 289–297. DOI: 10.1161/CIRCRESAHA.116.304187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shi Q, Rafii S, Wu MH, et al. Evidence for circulating bone marrow-derived endothelial cells. Blood 1998; 92: 362–367. [PubMed] [Google Scholar]
- 32.Peichev M, Naiyer AJ, Pereira D, et al. Expression of VEGFR-2 and AC133 by circulating human CD34+ cells identifies a population of functional endothelial precursors. Blood 2000; 95: 952–958. [PubMed] [Google Scholar]
- 33.Fujiyama S, Amano K, Uehira K, et al. Bone marrow monocyte lineage cells adhere on injured endothelium in a monocyte chemoattractant protein-1-dependent manner and accelerate reendothelialization as endothelial progenitor cells. Circ Res 2003; 93: 980–989. [DOI] [PubMed] [Google Scholar]
- 34.Griese DP, Ehsan A, Melo LG, et al. Isolation and transplantation of autologous circulating endothelial cells into denuded vessels and prosthetic grafts: implications for cell-based vascular therapy. Circulation 2003; 108: 2710–2715. [DOI] [PubMed] [Google Scholar]
- 35.Werner N, Priller J, Laufs U, et al. Bone marrow-derived progenitor cells modulate vascular reendothelialization and neointimal formation: effect of 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibition. Arterioscler Thromb Vasc Biol 2002; 22: 1567–1572. [DOI] [PubMed] [Google Scholar]
- 36.Rauscher FM, Goldschmidt-Clermont PJ, Davis BH, et al. Aging, progenitor cell exhaustion, and atherosclerosis. Circulation 2003; 108: 457–463. [DOI] [PubMed] [Google Scholar]
- 37.Gunsilius E, Duba H-C, Petzer AL, et al. Evidence from a leukaemia model for maintenance of vascular endothelium by bone-marrow-derived endothelial cells. The Lancet 2000; 355: 1688–1691. [DOI] [PubMed] [Google Scholar]
- 38.Bussolino F and al. e. Granulocyte- granulocyte-macrophage-colony stimulating factors induce human endothelial cells to migrate and proliferate. Nature 1989; 337: 471–473. [DOI] [PubMed] [Google Scholar]
- 39.Bussolino F and al. e. In vitro and in vivo activation of endothelial cells by colony-stimulating factors. Journal of Clinical Investigation 1991; 87: 986–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Soldi R and al. e. Activation of JAK2 in human vascular endothelial cells by granulocyte-macrophage colony-stimulating factor. Blood 1997; 89: 863–872. [PubMed] [Google Scholar]
- 41.Aglietta M, Piacibello W, Sanavio F, et al. Kinetics of human hemopoietic cells after in vivo administration of granulocyte-macrophage colony-stimulating factor. Journal of Clinical Investigation 1989; 83: 551–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Takeshita S, Zheng L, Brogi E, et al. Therapeutic angiogenesis. A single intraarterial bolus of vascular endothelial growth factor augments revascularization in a rabbit ischemic hind limb model. Journal of Clinical Investigation 1994; 93: 662–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Seiler C, Pohl T, Wustmann K, et al. Promotion of collateral growth by granulocyte-macrophage colony-stimulating factor in patients with coronary artery disease: a randomized, double-blind, placebo-controlled study. Circulation 2001; 104: 2012–2017. [DOI] [PubMed] [Google Scholar]
- 44.Takahashi T, Kalka C, Masuda H, et al. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nature Medicine 1999; 5: 434–438. [DOI] [PubMed] [Google Scholar]
- 45.Schwaab Thomas CPGT Jennifer J. Gibson, Cole Bernard F., Schned Alan R., Harris Robert, Fisher Jan L., Crosby Nancy, Stempkowski Laura M., Heaney John A., Ernstoff Marc S.. Tumor-related immunity in prostate cancer patients treated with human recombinant granulocyte monocyte-colony stimulating factor (GM-CSF). The Prostate 2006; 66: 667–674. [DOI] [PubMed] [Google Scholar]
- 46.Lane TA, Ho AD, Bashey A, et al. Mobilization of blood-derived stem and progenitor cells in normal subjects by granulocyte-macrophage- and granulocyte-colony-stimulating factors. Transfusion 1999; 39: 39–47. [DOI] [PubMed] [Google Scholar]
- 47.Aboyans V, Criqui MH, Abraham P, et al. Measurement and interpretation of the ankle-brachial index: a scientific statement from the American Heart Association. Circulation 2012; 126: 2890–2909. 2012/11/20. DOI: 10.1161/CIR.0b013e318276fbcb. [DOI] [PubMed] [Google Scholar]
- 48.Mahar EA, Mou L, Hayek SS, et al. Flow cytometric data analysis of circulating progenitor cell stability. Data Brief 2017; 10: 346–348. 2016/12/23. DOI: 10.1016/j.dib.2016.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McDermott MM, Liu K, Guralnik JM, et al. Home-based walking exercise intervention in peripheral artery disease: a randomized clinical trial. JAMA 2013; 310: 57–65. 2013/07/04. DOI: 10.1001/jama.2013.7231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Little RJ, D’Agostino R, Cohen ML, et al. The prevention and treatment of missing data in clinical trials. N Engl J Med 2012; 367: 1355–1360. 2012/10/05. DOI: 10.1056/NEJMsr1203730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Little RJ, Long Q and Lin X. A comparison of methods for estimating the causal effect of a treatment in randomized clinical trials subject to noncompliance Biometrics 2009; 65: 640–649. Comparative Study Evaluation Studies Research Support, N.I.H., Extramural; 2008/05/31. DOI: 10.1111/j.1541-0420.2008.01066.x. [DOI] [PubMed] [Google Scholar]
- 52.Akaike H A new look at the statistical model identification. Automatic Control,. IEEE transactions on bio-medical engineering 1974; 19(6): 716–723. [Google Scholar]
- 53.DeMets DL and Lan KK. Interim analysis: the alpha spending function approach. Stat Med 1994; 13: 1341–1352; discussion 1353–1346. 1994/07/15. DOI: 10.1002/sim.4780131308. [DOI] [PubMed] [Google Scholar]


