Summary
Sugars including lactose and fructose, or proteins (gluten), or biogenic amines (histamine), and combinations thereof may cause food intolerance/malabsorption. However, in usually asymptomatic patients with rare diseases, who present with functional, non-specific, non-allergic gastrointestinal (GI) complaints the etiologic factors of food intolerance/malabsorption need to be evaluated. We summarize patients with rare diseases, such as primary epiploic appendagitis, beta-thalassemias minor, Gullo syndrome and anomaly of the inferior vena cava, who presented functional, non-specific, non-allergic GI complaints. As conclusion, these GI symptoms in patients with otherwise asymptomatic, rare diseases were due to fructose malabsorption, histamine-, lactose intolerance and Helicobacter pylori (H.p.) infection. A registered and experienced dietician was employed to design an individually-tailored diet which ensured effective treatments and H.p. infection was accordingly eradicated.
Keywords: primary epiploic appendagitis; Gullo syndrome; beta-thalassemia minor; inferior vena cava anomaly; lactose; fructose, histamine
1. Introduction
Functional, non-specific, non-allergic gastrointestinal (GI) symptoms, including various abdominal complaints, are widespread and troublesome. Carbohydrates, including lactose and fructose, or proteins e.g. gluten, or biogenic amines, including histamine, and many of their combinations may cause food intolerance/malabsorption (1,2). However, in usually asymptomatic patients with rare diseases, who presented with functional, non-specific, non-allergic GI complaints all etiologic factors of food intolerance/malabsorption were evaluated and published. This included testing for fructose malabsorption, celiac disease, histamine-, and lactose intolerance, and Helicobacter pylori (H.p.) infection in each patient (3). Here, we review these patients with rare diseases, such as primary epiploic appendagitis, beta-thalassemias minor, Gullo syndrome and anomaly of the inferior vena cava, whose symptoms were caused by food intolerance/malabsorption and H.p. infection. As therapy a registered and experienced dietician was necessary to design an individually-tailored diet to ensure successful treatments and H.p. infection was subsequently eradicated.
2. Food intolerance/malabsorption
Mainly abdominal pain, bloating, postprandial fullness, diarrhea and/or obstipation, are functional, non-specific, non-allergic gastrointestinal (GI) symptoms and represent widespread, troublesome complaints. Adverse reactions to ingested foods are reported as food intolerance/ malabsorption and affect up to 20% of populations in westernized countries (1). Pathophysiology is still not well understood (4) but, food ingredients are described to cause various functional, non-specific, non-allergic GI symptoms and extra-intestinal complaints. Carbohydrates, mainly sugars including lactose and fructose, or proteins (gluten), biogenic amines (e.g. histamine), may cause food intolerance/malabsorption complaints (3). These widely used food components are not digested well and/or absorbed properly during GI passage and then influence the microbiome. Therefore, they may result in symptoms due to bacterial metabolism and fermentation (5,6).
Lactose intolerance is related to a deficiency of an enzyme, namely lactase, and causes gastrointestinal complaints with the consumption of dairy products. It was described with an estimated high global prevalence of 70 percent in the world's population (7). Depending on the ingested quantity of lactose and on the activity of lactase, patients with lactose intolerance may be asymptomatic or experience GI symptoms. If functional, non-specific, non-allergic GI complaints are present, then, besides other GI evaluations, a clinical diagnosis of lactose intolerance may usually be performed with a hydrogen breath test (8).
The increasing commercial use of high-fructose corn syrup, results in a growing occurrence of complaints caused by fructose malabsorption. GLUT-5 protein is the major fructose transporter in enterocytes of the small intestine and its limited absorption capacity causes malabsorption of fructose (9). This absorption capacity varies widely within populations, but it has been estimated that up to 50% of the U.S. population cannot absorb 25g of pure fructose. Then it was demonstrated, that up to 80% of healthy persons were unable to absorb 50g of fructose (10). Clinical diagnosis of fructose malabsorption is also performed with a hydrogen breath test (8).
Histamine intolerance (HIT) occurs with an unbalanced metabolism and through disproportionate amounts of histamine in the body. This is caused by a reduced ability of enzymes to degrade histamine and/or the consumption of histamine-containing foods. Within digestion, the enzyme diamine oxidase (DAO) is thought to be responsible for degradation of histamine (11). Due to the high variability of symptoms observed in many organs, the diagnosis of HIT is challenging. Standardized in vitro diagnostic tests for evaluation of HIT are not existing. Although, serum DAO values have not been established to reflect gastrointestinal DAO activity, the diagnosis of HIT may be supported with the measurement of DAO in serum. Patients with low serum DAO values (< 10 U/mL) (12), two or more GI symptoms described for HIT, and a reduction of symptoms due to a histamine-reduced diet may be diagnosed with HIT (13). Its exact prevalence is not known, but numbers of up to 3 percent in populations were estimated (14).
The absence of celiac disease (CD) or gluten malabsorption (15), and absence of a H.p. infection need to be confirmed also in all rare-disease patients with functional, non-specific, non-allergic GI symptoms. Although, the involvement of H.p. in patients with functional, nonspecific, non-allergic GI symptoms is controversial (16), an H.p. infection needs to be considered and, if H.p. is present then eradication therapy is required (17). Generally, in patients aged > 50 years GI endoscopy and ultrasound of the abdomen are valuable extra approaches for evaluation of functional, nonspecific, non-allergic GI symptoms (18).
3. Rare diseases and food intolerance/malabsorption
Appendices epiploicae are up to 100 subserosal fat pouches attached to the colon wall with a vascular stalk. Clinically, a primary epiploic appendagitis (PEA) is accompanied by localized pain, mainly in the lower left or right abdominal quadrant. PEA with its characteristic and diagnostic appearance has computed tomography (CT) as the diagnostic modality of choice (Figure 1) (19). However, clinicians are frequently unfamiliar with PEA (20). Currently, approximately 270 publications on appendagitis epiploica can be found in the U.S. National Library of Medicine PubMed. Because of our recent publications with descriptions of PEA, and due to the presence in the world-wide-web, our treatment centre was contacted by several patients with PEA during the last years (21). Usually, after a radiological PEA diagnosis these patients were asking for additional clinical treatment advice. In some of them anamnesis revealed, that they had had a CT-confirmed epiploic appendagitis months prior to presentation at our institution (22,23). These patients presented with various functional, non-specific, non-allergic GI symptoms, and were concerned about the possibility of continuing complaints due to PEA. Generally, PEA resolves within days to a few weeks without any medical or surgical treatment. However, months thereafter with evaluations of food malabsorption/intolerance the cause for their ongoing symptoms was found (Table 1).
Figure 1.
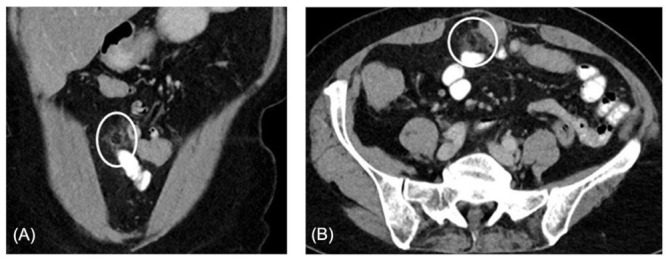
(A), Example of contrast enhanced longitudinal abdominal CT demonstrating a primary epiploic appendagitis adjacent to the transversal colon in a 68-year-old white female patient. The lesion, with a size of size 2.3 x 1.2cm, shows fat attenuation and surrounding inflammation. (B), In the same patient an axial abdominal CT with contrast enhancement showing primary epiploic appendagitis adjacent to the transversal colon in. The lesion demonstrates fat attenuation and surrounding inflammation.
Table 1. Patients with rare diseases and food intolerance/malabsorptions.
| Rare disease | Number of patients (Reference) | Food intolerance/malabsorption |
|---|---|---|
| Primary epiploic appendagitis | 1 (21) | LI, FM, H.p. |
| 1 (22) | FM, HI | |
| 1 (23) | FM | |
| Beta-thalassemia minor | 1 (26) | FM, HI |
| 1 (27) | LI | |
| Gullo syndrome | 1 (28) | LI, HI |
| Inferior vena cava anomaly | 1 (31) | LI |
Legend: FM, fructose malabsorption; HI, histamine intolerance; LI, lactose intolerance; H.p., Helicobacter pylori.
Beta-thalassemias minor, characterized by a reduced β-hemoglobin chain synthesis, are genetically heterogeneous autosomal recessive anemias (24). Due to mobility and migration, beta-thalassemias minor have spread from endemic areas like the Mediterranean, Africa and Asia, to northern Europe. Diagnosis of haemoglobinopathies is well defined, but there are poor data on the precise prevalence and trends of these diseases. Therefore, haemoglobinopathies are considered rare diseases in Central Europe (25). Usually, beta-thalassemias minor are asymptomatic in carriers of disease, although they may cause mild anaemia. We described food malabsorption/intolerance in two patients with beta-thalassaemias, which caused recurring functional, nonspecific, non-allergic GI symptoms (26).
Gullo syndrome is a rare, benign pancreatic hyperenzymemia. It is was described with a more than three-fold, above normal range, increase of serum pancreatic enzymes. This elevation of lipase and amylase occurs in the absence of a pancreatic disease. Diagnosis of Gullo syndrome is made when all evaluations, including radiologic examinations, for pancreatic diseases are without abnormality during the time period of at least one year. The additional diagnostic criterion is, that significant elevations and undulations of both pancreatic enzymes, lipase and amylase, occur on a day-to-day basis for five consecutive days (27). Only single patients are reported and a prevalence for Gullo syndrome is not known. Generally, patients with Gullo syndrome are asymptomatic. Although, we reported on a Gullo syndrome patient with recurring functional, nonspecific, non-allergic GI symptoms and these were found to be due to food malabsorption/intolerance (28).
Congenital anomalies of the inferior vena cava (IVC) are detected in young patients with unprovoked both-sided deep vein thrombosis in lower limbs. Occasionally, the discovery of a malformation of IVC is incidental during abdominal radiologic evaluations. Literature suggests that there exists a pro-thrombogenic effect in patients with these various congenital IVC anomalies. Generally, without thrombosis or without other associated additional, congenital defects, malformations of IVC are asymptomatic (29). The course and number of collateral veins in congenital anomaly or absence of the IVC are highly variable and if it is detected during abdominal surgery, then it may be detrimental for the patient. Although, anomalies of the IVC are well-recognized anatomic abnormalities they are considered rare diseases (30). Food malabsorption/intolerance may cause functional, nonspecific, non-allergic GI symptoms in otherwise asymptomatic congenital anomalies of the IVC (31).
4. Conclusion
However, in usually asymptomatic patients with rare diseases, who present with functional, non-specific, non-allergic GI complaints etiologic factors of food intolerance/malabsorption need be evaluated. This includes testing for fructose malabsorption, celiac disease, histamine-, and lactose intolerance, and H.p. infection in each patient. Studies have shown various combinations of these to occur in patients with functional, non-specific, non-allergic GI complaints (2,3).
The worldwide high prevalence of food intolerance/ malabsorption stresses the likelihood of co-occurrence in patients with rare diseases, too. In conclusion, functional, non-specific, non-allergic GI symptoms in these described patients with rare diseases, including primary epiploic appendagitis, beta-thalassemias minor, Gullo syndrome and anomaly of the inferior vena cava, were due to fructose malabsorption, histamine-, lactose intolerance and H.p. infection. A registered and experienced dietician was employed to design an individually-tailored diet which ensured effective treatments and H.p. infection was accordingly eradicated. Each patient's tolerance level was considered when recommending the dietary restrictions for long-term symptom reduction. Dietary advice ought to include nutritional variety, ensure alimentary adequacy and cause negligible impact on the gastrointestinal microbiome.
References
- 1. Tuck CJ, Biesiekierski JR, Schmid-Grendelmeier P, Pohl D. Food intolerances. Nutrients. 2019, 11:1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wilder-Smith CH, Materna A, Wermelinger C, Schuler J. Fructose and lactose intolerance and malabsorption testing the relationship with symptoms in functional gastrointestinal disorders. Aliment Pharmacol Ther. 2013; 37:1074-1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Enko D, Meinitzer A, Mangge H, Kriegshäuser G, Halwachs-Baumann G, Reininghaus EZ, Bengesser SA, Schnedl WJ. Concomitant prevalence of low serum diamine oxidase activity and carbohydrate malabsorption. Can J Gastroenterol Hepatol. 2016; 2016:4893501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schiller LR. Evaluation of chronic diarrhea and irritable bowel syndrome with diarrhea in adults in the era of precision medicine. Am J Gastroenterol. 2018; 113:660-669. [DOI] [PubMed] [Google Scholar]
- 5. Schink M, Konturek PC, Tietz E, Dieterich W, Pinzer TC, Wirtz S, Neurath MF, Zopf Y. Microbial patterns in patients with histamine intolerance. J Physiol Pharmacol. 2018; 69:579-593. [DOI] [PubMed] [Google Scholar]
- 6. Albenberg LG, Wu GD. Diet and the intestinal microbiome: Associations, functions and implications for health and disease. Gastroenterology. 2014; 146:1564-1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Storhaug CL, Fosse SK, Fadnes LT. Country, regional, and global estimates for lactose malabsorption in adults: A systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2017; 2:738-746. [DOI] [PubMed] [Google Scholar]
- 8. Rezaie A, Buresi M, Lembo A, Lin H, McCallum R, Rao S, Schmulson M, Valdovinos M, Zakko S, Pimentel M. Hydrogen and methane-based breath testing in gastrointestinal disorders: The North American consensus. Am J Gastroenterol. 2017; 112:775-784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gibson PR, Newnham E, Barrett JS, Shepherd SJ, Muir JG. Review article: Fructose malabsorption and the bigger picture. Aliment Pharm Ther. 2007; 25:349-63. [DOI] [PubMed] [Google Scholar]
- 10. Latulippe ME, Skoog SM. Fructose malabsorption and intolerance: effects of fructose with and without simultaneous glucose ingestion. Crit Rev Food Sci Nutr. 2011; 51:583-592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Reese I, Ballmer-Weber B, Beyer K, Fuchs T, Kleine- Tebbe J, Klimek L, Lepp U, Niggemann B, Saloga J, Schäfer C, Werfel T, Zuberbier T, Worm M. German guideline for the management of adverse reactions to ingested histamine: Guideline of the German Society for Allergology and Clinical Immunology (DGAKI), the German Society for Pediatric Allergology and Environmental Medicine (GPA), the German Association of Allergologists (AeDA), and the Swiss Society for Allergology and Immunology (SGAI). Allergo J Int. 2017; 26:72-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mušič E, Korošec P, Šilar M, Adamič K, Košnik M, Rijavec M. Serum diamine oxidase activity as a diagnostic test for histamine intolerance. Wien Klin Wochenschr. 2013; 125:239-43. [DOI] [PubMed] [Google Scholar]
- 13. San Mauro Martin I, Brachero S, Garicano Vilar E. Histamine intolerance and dietary management: A complete review. Allergol Immunopathol (Madr). 2016; 44:475-483. [DOI] [PubMed] [Google Scholar]
- 14. Pinzer TC, Tietz E, Waldmann E, Schink M, Neurath MF, Zopf Y. Circadian profiling reveals higher histamine plasma levels and lower diamine oxidase serum activities in 24% of patients with suspected histamine intolerance compared to food allergy and controls. Allergy. 2018; 73:949-957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mustalahti K, Catassi C, Reunanen A, Fabiani E, Heier M, McMillan S, Murray L, Metzger MH, Gasparin M, Bravi E, Mäki M; Coeliac EU Cluster, Project Epidemiology. The prevalence of celiac disease in Europe: Results of a centralized, international mass screening project. Ann Med. 2010; 42:587-595. [DOI] [PubMed] [Google Scholar]
- 16. Franceschi F, Annalisa T, Teresa DR, Giovanna D, Ianiro G, Franco S, Viviana G, Valentina T, Riccardo LL, Antonio G. Role of Helicobacter pylori infection on nutrition and metabolism. World J Gastroenterol. 2014; 20:12809-12817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tomita T, Oshima T, Miwa H. New approaches to diagnosis and treatment of functional dyspepsia. Curr Gastroenterol Rep. 2018; 20:55. [DOI] [PubMed] [Google Scholar]
- 18. Bai T, Wang WF, Zhang L, Wang H, Qian W, Song J, Hou XH. Positive endoscopic and ultrasonographic findings in symptom-diagnosed functional gastrointestinal disorder patients: Data from a Chinese cross-sectional study. J Dig Dis. 2018; 19:759-765. [DOI] [PubMed] [Google Scholar]
- 19. Giambelluca D, Cannella R, Caruana G, Salvaggio L, Grassedonio E, Galia M, Midiri M, Salvaggio G. CT imaging findings of epiploic appendagitis: an unusual cause of abdominal pain. Insights Imaging. 2019; 10:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Almeida AT, Melão L, Viamonte B, Cunha R, Pereira JM. Epiploic appendagitis: An entity frequently unknown to clinicians - diagnostic imaging, pitfalls, and look-alikes. Am J Roentgenol. 2009; 193:1243-1251. [DOI] [PubMed] [Google Scholar]
- 21. Schnedl WJ, Kalmar P, Mangge H, Krause R, Wallner- Liebmann SJ. Co-occur rence of carbohydrate malabsorption and primary epiploic appendagitis. World J Gastroenterol. 2015; 21:10242-10254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schnedl WJ, Reittner P, Enko D, Mangge H. Food malabsorption/intolerance complaints triggered by primary epiploic appendagitis. EXCLI J. 2019; 18:746-749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schnedl WJ, Lipp RW, Wallner-Liebmann SJ, Kalmar P, Szolar DH, Mangge H. Primary epiploic appendagitis and fructose intolerance. Europ J Clin Nutr. 2014; 68:1359-61. [DOI] [PubMed] [Google Scholar]
- 24. Origa R. β-thalassemia. Genet Med. 2017; 19:609-619. [DOI] [PubMed] [Google Scholar]
- 25. Martinez PA, Angastiniotis M, Eleftheriou A, Gulbis B, Mañú Pereira MdelM, Petrova-Benedict R, Corrons JLV. Haemoglobinopathies in Europe: Health & migration policy perspectives. Orphanet J Rare Dis. 2014; 9:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schnedl WJ, Schenk M, Lackner S, Holasek SJ, Mangge H. β-thalassemia minor, carbohydrate malabsorption and histamine intolerance. J Comm Hosp Int Med Persp. 2017; 7:227-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gullo L. Day-to-day variations of serum pancreatic enzymes in benign pancreatic hyperenzymemia. Clin Gastroenterol Hepatol. 2007; 5:70-74. [DOI] [PubMed] [Google Scholar]
- 28. Schnedl WJ, Enko D, Mangge H, Schenk M, Lackner S, Holasek SJ. Benigne pancreatic hyperenzymemia (Gullo syndrome), histamine intolerance, and carbohydrate malabsorption. Proc (Bayl Univ Med Cent). 2017; 30:177-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Oliveira JD, Martins I. Congenital systemic venous return anomalies to the right atrium review. Insights Imaging. 2019; 10:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hostiuc S, Minoiu C, Negoi I, Rusu MC, Hostiuc M. Duplication and transposition of inferior vena cava: A meta-analysis of prevalence. J Vasc Surg Venous Lymphat Disord. 2019; 7:742-755. [DOI] [PubMed] [Google Scholar]
- 31. Schnedl WJ, Kalmar P, Wallner-Liebmann SJ, Reiter G, Mangge H, Lipp RW. Anomaly of the inferior vena cava and lactose malabsorption. Phlebologie. 2016; 45:35-39. [Google Scholar]


