1. Abstract
Early phase clinical trials are the first step in testing new medications and therapeutics developed by clinical and biomedical investigators. These trials aim to find a safe dose of a newly targeted drug (phase I) or find out more about the side effects and early signals of treatment efficacy (phase II). In a research institute, many biomedical investigators in oncology are encouraged to initiate such trials early in their careers as part of developing their research portfolio. These investigator-initiated trials (IITs) are funded internally by the University of Kansas Cancer Center or partially funded by pharmaceutical companies. As financial, administrative, and practical considerations play an essential role in the successful completion of IITs, it is imperative to efficiently allocate resources to plan, design, and execute these studies within the allotted time. This manuscript describes monitoring tools and processes to improve the efficiency, cost-effectivness, and reliability of IITs. The contributions of this team to processes such as: participant recruitment, feasibility analysis, clinical trial design, accrual monitoring, data management, interim analysis support, and final analysis and reporting are described in detail. This manuscript elucidates how, through the aid of technology and dedicated personnel support, the efficiency of IIT-related processes can be improved. Early results of these initiatives look promising, and the Biostatistics and Informatics team intends to continue fostering innovative methodologies to enhance cancer research by improving the efficiency of IITs.
Keywords: Clinical trial execution, Trial optimization, Data driven approach, Investigator initiated trial
1. Introduction
There are 70 National Cancer Institute (NCI) designated cancer centers in the United States and 50 of these centers have achieved “comprehensive cancer center status” [1]. Many cancer centers aspire to achieve and maintain the comprehensive designation, which provides them the opportunity to advance their research and clinical portfolio. This designation facilitates cutting-edge research as it allows the institution to meet the highest standards required for innovative drug discovery and treatment. Building a robust and comprehensive cancer center requires dynamic core programs that quickly adapt to the operational and scientific changes that occur at a rapid pace in the clinical trial industry. The Biostatistics and Informatics Shared Resource (BISR) is one of eight shared resources of the University of Kansas Cancer Center (KUCC) P30 grant. Researchers can conduct research studies known as Investigator Initiated Trials (IITs) through sponsorship by internal or external funding agencies. BISR ensures smooth conduct of IITs from the design and feasibility assessment to their completion. These elements play a vital role in the success of these studies. Most IITs conducted at the KUCC are phase I and phase II trials, which lay the foundation for successful drug discovery and potential cancer treatments [2]. While some IITs are industry-sponsored, others are sponsored internally by the institution or other competitive extramural funding agencies (e.g., NCI). Such institutionally-sponsored trials are often restricted by budgetary and administrative considerations, thereby limiting the study follow-up time and the overall scope of research. IITs should be efficiently designed and conducted pragmatically to achieve higher success rates. BISR has implemented a standardized procedure intended to help study investigators conduct efficient IITs to realize this objective.
The BISR is comprised of faculty and staff within the Department of Biostatistics & Data Science at the University of Kansas Medical Center with expertise that spans biostatistics, bioinformatics, informatics, and data science. The BISR collaborates with a wide array of disease working groups (DWG) in planning and conducting IITs for various types of cancer and is responsible for ensuring the smooth execution of IITs through all stages using a process-driven approach. As an initial step towards standardization, study investigators are instructed to contact the BISR as early as possible to enable streamlined and effective execution of their intended IITs. The purpose of this paper is to demonstrate how standardization of IIT-related processes improves the efficiency of early phase trials.
2. Materials and methods
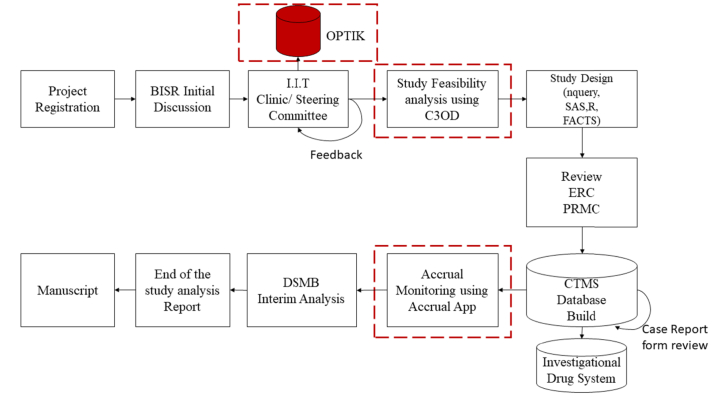
The BISR adopts a multifaceted process-driven approach to realize its main objectives, keeping in mind the critical aspects of overall design and workflow efficiency (Fig. 1). In this approach, attention is given to ensure that each component of the process is optimally executed while simultaneously securing robust functionality for the entire process. The process begins with the researcher registering their project with BISR, followed by an initial consultation with the statistician and the data manager to discuss the study design and database development. Researcher is then asked to present their idea at the IIT clinic which is held bi-weekly which involves KU Cancer Center leadership along with a panel of researchers who provide feedback and evaluate the idea before it could be approved. Specifically, a fundamental component of this approach is the BISR development of a Curated Cancer Clinical Outcomes Database (C3OD) and Organize & Prioritize Trends to Inform KU Cancer Center Members (OPTIK). C3OD is hosted on a secure server and enables oncologists to identify eligible patients based the inclusion and exclusion criteria defined in the study [3]. This database deploys advanced algorithms to combine data from electronic records with manually abstracted data, and provides a graphical user interface through which researchers can submit queries searching for patient availability. OPTIK is still in the development stage, but is being already utilized by the KU Cancer Center members to better understand the target population with in the KU Catchment Area. OPTIK corroborates data from multiple publicly available data with information of the population spread across the state and quantifies based on the demographics and socio economic status.
Fig. 1.
Investigator Initiated Trial workflow within BISR.
Once the study population is identified and a research team has prepared the clinical and biological components of their study proposal, the study design is discussed and critiqued at bi-weekly IIT Steering Committee meetings in which a BISR designated biostatistics faculty member provides an overview of the study feasibility and overall statistical design. The Steering Committee compiles a report providing feedback to the research team for further refining their research proposal. Once the research team is ready with a clearly defined hypothesis and the primary and secondary endpoints have been identified, a detailed face-to-face meeting is scheduled between the team of researchers and the lead biostatistician assigned to the study. The lead biostatistician is assigned based on the area of expertise that best matches with the unique characteristics of the study (Fig. 1). Next, the BISR proposes standard and innovative phase I and phase II designs after careful review of the study protocol, keeping in mind patient accrual rates and other administrative and financial considerations. Novel ideas consistent with newly developed statistical methods in the literature are encouraged, and corresponding documentation is provided in the statistical section of the protocol. Interim analysis plans for early detection of efficacy or futility may be provided. Likewise, sequential and adaptive designs are proposed in cases where they are deemed appropriate. Upon approval of the study protocol by both the Resourcing and Ethics Review Committees, the study progresses to research database development and management. At this stage, a senior research analyst uses the Clinical Trials Management System (CTMS) to build electronic case report forms (eCRFs) aimed at collecting study-specific information. To facilitate consistency and ease in performing this task, the analyst incorporates standardized eCRFs from a library developed specifically for this purpose. That is, the eCRFs in this library apply to all types of cancer IITs and can be readily deployed. In cases where the standard eCRFs do not suffice, the analyst develops new eCRFs specific to the study under consideration. Newly developed forms are then added to the library of standardized eCRFs, facilitating their use for future similar studies.
The study subsequently proceeds to recruitment following the study plan outlined in the protocol. Once recruitment begins, it is crucial to periodically keep track of whether the study is meeting recruitment goals. To this end, a web-based application, Accrual App, developed by the BISR, provides a key role in providing such information [[4], [5], [6]]. This application is updated daily and uses current enrollment information to generate posterior prediction intervals that convey potential delays in achieving recruitment targets. Additional features allow a user to sort and view the status of a clinical trial categorized by cancer type, researcher name, and study type, thereby providing critical feedback to the Clinical Trials Office (CTO) about the ongoing and predicted progress of an IIT. It also reminds the clinical investigators (who may be blinded) and the statistician when the study achieves the enrollment goal so that it can proceed to analysis.
In the next stage, a senior research analyst extracts the data in one of the following formats SAS, R, JSON, XML, CSV or EXCEL file and curates it further as per the specific requirements of the statistical analysis plan. The biostatistician analyzes the data with strict adherence to the study protocol and summarizes the results of his or her analyses. The analyst also prepares study reports for periodic assessment by the Data Safety Monitoring Board (DSMB), whose members monitor the IIT for issues related to safety and toxicity. For studies funded by the National Institutes of Health (NIH), the statistician and analyst together ensure that all rules and guidelines recommended by NIH are strictly adhered to during the discussion sessions with the DSMB [7]. Once the final analyses are completed and the research team discusses study results, the study moves on to the final stage where the study results are submitted to the funding agency and uploaded to the ClincalTrials.gov website. The research team then writes the manuscript and submits it to a suitable journal to share the study results with the scientific community.
3. Results
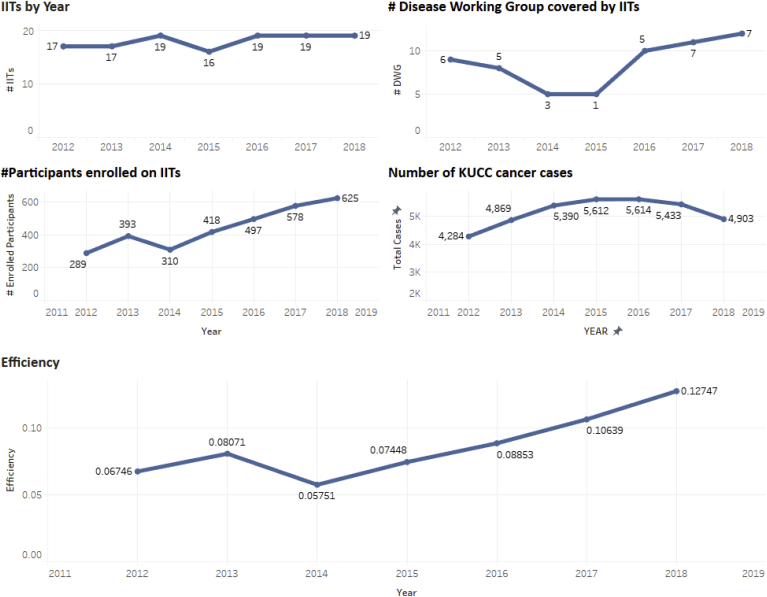
In order to evaluate the efficiency of the conduct of IIT conducted at the KU cancer Center, BISR has extracted data from the clinical trial management system starting from the year 2012 till 2018. Under Fig. 2, first graph illustrates the number of new IITs that were approved to begin between 2012 till 2018. Noticeably in 2015, we had the least number of IITs that were opened which was 15, and during the last three years we have been consistent with 19 new IITs approved to start enrollement. The second graph describes the number of unique disease working groups involved with IITs that were approved between 2012 till 2018. The number of cancer disease is representative of diverse area spanne by these IITs capturing – Breast, GI, GU, Gynecology, Head and Neck, Lung, Leukemia/Myeloid, Sarcoma/Melanoma and Multiple. Some of these IITs disease working group is classified as Multiple which means it can enroll participants with different diagnosis. The third graph on Fig. 2, represents the number of participants enrolled on to a theaurapatic clinical trial at the KU Cancer Center across the years since 2012 till 2018. Participants who are enrolled in 2012 are not counted towards the concecutive years. During 2018 we have enrolled 625 new participants on to the clinical trials, 2018 is also the year when we had 19 new IITs which were approved to open for enrollement, also keep in mind the previous years studies have also accrued which account towards the 625 particiapants enrollement during the year 2018. The fouth graph under Fig. 2 represents the number of new cancer cases that have been diagnosed at the KU Cancer Center across the years 2012 till 2018. We see that during the year 2015 and 2016 we have had highest number of new cancer cases, the source of this data is from our Tumor registry data, which is manually abstracted and verified by the cancer registry abstractors, For the calendar year 2018 the numbers are not complete as the abstractors are currently in the process of abstracting the 2018 cancer cases. While KU Cancer Center cares for approximately 6000 cancer patients per year, almost 15% of these patients are first seen at KU Cancer Center one or more years after they were diagnosed. For example, a patient may have been diagnosed in 2017 but they weren't seen at KUMC until early in 2018.
Fig. 2.
IITs conducted at the KU Cancer Center between 2012 till 2018.
The fifth graph under Fig. 2 is enrollment efficiency. Efficiency is defined as the number of participants that were enrolled on the IIT for a given year divided by the corresponding number of new cancer cases that have been diagnosed at the KU Cancer Center. Example: for the year 2012 the efficiency would be calculated as 289 divided by 4284 i.e. 0.0674 (or 6.74%). The efficiency percentage has doubled across the five year period and clearly the process and the tools such as the C3OD, OPTIK and Accrual App. Have provided an edge to the KU Cancer Center administration and the researchers to recruit, track and monitor every IIT.
4. Discussion
The standardization process for IITs appears to improve the efficiency of accrual and the likelihood of studies to meet at least 80% of the targeted sample size. The standardization and implementation of informatics tools help the study teams with their pre-study planning and feasibility analysis using C3OD through screening and identification of patients. Real-time accrual monitoring provides a current trajectory of accrual that can be intervened upon by study decision-makers. Due to the standardized procedures, the data are collected in a prescribed format leading to the streamlined generation of reports and data visualization for regulatory bodies. Moving forward, BISR continues to partner with Clinical Trials Office on building standard tools for internal review committees that provide feedback on feasibility of the trial and resources/budgetary feasibility. Any medical centers could easily adopt all the tools mentioned under this manuscript after understanding the methodology behind the statistical calculation and availability of the input data variables.
With the current implementation of a process-driven approach, BISR involvement has shown improved results in multiple aspects of the IIT design and development process. More innovative study designs help realize the study objectives and standardized data elements — along with study-specific data elements —create a faster and more efficient IIT. Tracking features in the Accrual App help monitor current patient enrollment as well as predict the date of study completion. For the monitoring tool to perform and help with decision making, the study team members have to make sure the data is entered in a timely manner. Considerable data has to be accumulated to build a rigorous monitoring tool. It has been four years since these tools have been implemented at our site; it is just going to help us improve as we conduct more studies in the future. When more data is collected, we can assess the performance of our process using more rigorous hypotheses and perform formal statistical tests to provide evidence in support of these hypotheses. We continue to fine-tune the tools to adapt to the dynamic landscape of clinical trial execution process.
Funding
This study was supported by the National Cancer Institute (NCI) Cancer Center Support Grant P30 CA168524 and used the Biostatistics and Informatics Shared Resource (BISR).
References
- 1.NCI-designated cancer centers: national cancer institute. https://www.cancer.gov/research/nci-role/cancer-centers [cited 2019 April 30, 2019]. Available from:
- 2.Piantadosi S. 2 ed. Wiley; Hoboken, New Jersey: 2005. Clinical Trials A Methodologic Perspective. [Google Scholar]
- 3.Mudaranthakam D.P., Thompson J., Hu J., Pei D., Chintala S.R., Park M. A Curated Cancer Clinical Outcomes Database (C3OD) for accelerating patient recruitment in cancer clinical trials. JAMIA Open. 2018;1(2):166–171. doi: 10.1093/jamiaopen/ooy023%JJAMIAOpen. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gajewski B.J., Simon S.D., Carlson S.E. Predicting accrual in clinical trials with Bayesian posterior predictive distributions. Stat. Med. 2008;27(13):2328–2340. doi: 10.1002/sim.3128. [DOI] [PubMed] [Google Scholar]
- 5.Jiang Y., Simon S., Mayo M.S., Gajewski B.J. Modeling and validating Bayesian accrual models on clinical data and simulations using adaptive priors. Stat. Med. 2015;34(4):613–629. doi: 10.1002/sim.6359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu J., Wick J.A., Mudaranthakam D.P., Jiang Y., Mayo M.S., Gajewski B.J. Accrual prediction program (APP): a web-based clinical trials tool for monitoring and predicting accrual for early phase cancer studies. Clin. Trials. 2019;16(6):657–664. doi: 10.1177/1740774519871474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.NIH Data and safety monitoring. April 30, 2019. https://grants.nih.gov/policy/humansubjects/policies-and-regulations/data-safety.htm Available from: