Abstract
Background
Oysters (Crassostrea gigas) are a popular marine product worldwide and have the advantage of nutritional benefits. This study aimed to investigate the effect of fermented oyster extract (FO) on growth promotion, including analysis of body size, bone microarchitecture, hematology and biochemistry in vivo.
Methods
The amount of nutrients and gamma aminobutyric acid (GABA) were determined. Sprague–Dawley rats were randomly divided into four groups: the control group, FO 50 group (FO 50 mg/kg), and FO 100 group (FO 100 mg/kg) were administered orally once daily and the recombinant human growth hormone (rhGH) group (200 μg/kg) was intraperitoneally injected once daily for 14 days.
Results
Oral administration of FO 100 significantly increased body length and had no effect on organ damage or hematological profiles. However, administration of rhGH significantly induced hypertrophy of the liver, kidney and spleen along with a marked increase in body length. Tibia length and the growth plate were increased, and bone morphometric parameters were slightly improved by FO and rhGH administration. Serum analysis showed that the levels of GH and insulin like growth factor-1 (IGF-1) were slightly upregulated by FO administration. Nevertheless, the protein expression of hepatic IGF-1 was markedly increased by FO 100 and rhGH administration.
Conclusions
FO have high content of GABA, and induced positive effects on body length, tibial length, growth-plate length and hepatic IGF-1 synthesis in SD rats with no toxicity or alterations of hematological profile. Therefore, these results suggest that GABA-enriched FO could be considered a potential alternative treatment for growth stimulation.
Keywords: Fermented oyster (FO), Recombinant human growth hormone (rhGH), Insulin like growth factor-1, Tibial growth plate, Gamma aminobutyric acid (GABA)
1. Introduction
Body growth is controlled by environmental factors and individual conditions, including nutritional, hormonal and genetic status, which interact to form a complex process across all organs.1, 2 Some therapies are available to improve growth, including recombinant human growth hormone (rhGH), insulin like growth factor-1 (IGF-1), aromatase inhibitors (anastrozole and letrozole) and bone lengthening surgery.2 rhGH therapy has been widely used for clinical purposes for more than fifty years.2, 3 Nevertheless, there are reported adverse effects of rhGH administration in children and adolescents, including edema, insulin resistance, progression of scoliosis, prepubertal gynecomastia, benign intracranial hypertension, etc.3 In this respect, aromatase inhibitors can be considered as alternative oral treatment strategies for short stature.4 However, several studies have found that aromatase inhibitors have toxicity, although they improved the predicted final height in children.5, 6 Therefore, there is a growing need to develop new and effective oral replacements that have low side effects and are safe for the treatment of growth failure.
Oysters are a popular marine product worldwide and have the advantage of nutritional benefits.7, 8 Oysters and its derivative have long been used as a traditional medicine in Southeast Asia for the treatment of acid indigestion, fatigue, hemorrhage and gastrointestinal troubles.9 Numerous studies have suggested that contents derived from oysters have bioactivity on bone health including osteogenic activity10 and improving bone growth mediators.11 We recently demonstrated that fermented oyster (Crassostrea gigas) extract (FO) prevents osteoclast differentiation, inhibits ovariectomy (OVX)-induced bone loss and decreasing of bone morphometric parameters,12, 13 and promotes bone formation.7 Although previous studies of the effects of FO on stimulation of osteogenesis and suppression of osteoclastogenesis, no studies have established the effect of FO as a replacement for growth failure. Therefore, the aim of this study was to evaluate the effect of FO on growth promotion, including an analysis of growth parameters, hematology and biochemistry in Sprague–Dawley rats.
2. Methods
2.1. Preparation of FO
FO used in this study was obtained from Marine Bioprocess Co. Ltd. (Busan, Republic of Korea). FO was prepared by fermentation with Lactobacillus brevis BJ20 as previously described with slight modifications,13 namely, glutamic acid and dextrin were used as a substitute for monosodium glutamate and an excipient, respectively. Prior to use in the experiments, the FO was diluted with distilled water to adjust the final treatment concentrations.
2.2. Chemical analyses of FO
The amounts of nutrients, such as carbohydrates, crude proteins and sugars, were determined by the methods of the Food Code of Korea.14 The content of gamma aminobutyric acid (GABA) was determined using a high-performance liquid chromatography (HPLC) system (Dionex U3000; Thermo Sci., Sunnyvale, CA, USA) equipped with a UV detector. Dionex Bonded Silica column (C18 5 μm 120 Å 4.6 × 250 mm) fitted with 4.0 × 3.0 mm i.d. guard column, both from Phenomenex (Torrance, CA), were used. Solvent A was 50 mM sodium acetate (pH 6.5), and solvent B consisted of 45% (v/v) acetonitrile, 45% (v/v) methanol and 10% (v/v) distilled water. The linear gradient was conducted for 30 min at 338 nm, and the injection volume was 20 μL. The solvent flow rate was 1.0 mL/min. All other chemicals used were of analytical grade, and were purchased from the Sigma–Aldrich Chemical Co.
2.3. Animal and experimental procedures
This study was conducted in accordance with the animal experimentation guidelines of Dong-eui University, with approval of the Institutional Animal Care and Use Committee (No. R2019-002) for the use of animals in research. We purchased 79 Sprague–Dawley (SD) rats (female, postnatal day 21) from Samtako Bio Korea (Osan, Republic of Korea). After acclimatization for 7 days, all rats were randomly divided into four groups: the control group (n = 19, 100 μL of distilled water), FO 50 group (n = 20, 100 μL of 50 mg/kg/day), FO 100 group (n = 20, 100 μL of 100 mg/kg/day), and rhGH group (n = 20, 200 μg/kg/day). The control and FO groups were administered orally once per day in the morning for 14 days. The feeding dose of FO was determined based on the effective dosage of previously report.12 The rhGH group, as a positive control,15 was subcutaneously injected once daily for 14 days. rhGH was obtained from Dong-A ST Co, Ltd. (Growtropin®-II, Seoul, Republic of Korea). The body weight was measured weekly. All rats were sacrificed at day 14 after treatment, and the body length was defined separately as the length from the nose to the tail (N–T) and the length from the nose to the anus (N–A).
2.4. Collection of blood and tissue
Whole Blood was collected directly from the heart, placed in heparinized tube, allowed to clot for 30 min at room temperature. Thereafter the blood centrifuged to obtain the serum at 3000 rpm for 10 min at 4 °C, which was kept at −80 °C for subsequent analysis. After perfusion, organs were immediately surgically excised, including the thymus, heart, lung liver, kidney, spleen, uterus and ovary, weighed, and stored at −80 °C. The long bones (femurs and tibias) were dissected out and fixed in 4% paraformaldehyde.
2.5. Micro-CT and histomorphometric analysis
Bone morphometric parameters of rat tibias were evaluated using high-resolution micro-computed tomography (μCT, Skyscan 1272; Kontich, Belgium) with a source voltage of 80 kV, current of 125 μA and resolution of 12 μm. Bone volume per total volume (BV/TV), bone mineral density (BMD), trabecular separation (Tb. Sp.), and trabecular number (Tb. N.) were measured using CTAn software (Bruker; Kontich, Belgium) as reported previously.16, 17 For histomorphometric analysis, fixed tibias were decalcified in 12% ethylenediaminetetraacetic acid and embedded in paraffin. Longitudinal tissue sections were prepared using a microtome (Leica Biosystems, Nussloch, Germany) with 5 μm and stained with hematoxylin and eosin (H&E). For growth-plate analysis, mid regions of the tibia were selected, and the length of the upper and lower growth plate was measured using iSolution software (Daejeon, Korea) at 10× magnification. At least 10 regions of the growth plate were measured for each section.18
2.6. Hematology
Red blood cells (RBC), white blood cells (WBC), hematocrit, hemoglobin, mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), MCH concentration (MCHC) and platelet were analyzed with a Sysmex XN-9000 analyzer (Sysmex Corporation, Kobe City, Hyogo Prefecture, Japan).
2.7. Serum biochemistry
Serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), blood urea nitrogen (BUN), creatinine and calcium were measured using the Cobas 8000 C702 chemistry analyzer (Roche, Mannheim, Germany).
2.8. Serum GH, IGF-1 and IGFBP-3 levels
Serum GH (catalog No. EMIGFBP3), insulin like growth factor-1 (IGF-1, catalog No. OKBB00165) and insulin like growth factor binding protein-3 (IGFBP-3, catalog No. OKBB00172) levels were measured using enzyme-linked immunosorbent assay (ELISA) kits according to the manufacturer's instructions. The GH ELISA kit was obtained from Thermo Fisher Scientific (Waltham, MA, USA). IGF-1 and IGFBP-3 kits were purchased from AVIVA Systems Biology (San Diego, CA, USA).
2.9. Western blot analysis
Proteins from rat liver were extracted using the Bradford Protein assay kit (Bio-Rad Laboratories, Hercules, CA, USA). Total protein (50 μg) was separated by denaturing sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis. After electrophoresis, separated proteins were transferred onto polyvinylidene difluoride membranes (Schleicher & Schuell, Keene, NH, USA). The membranes were blocked with 5% skim milk in Tris-buffered saline containing 0.1% Triton X-100 (TBST) for 1 h at room temperature. The membranes were probed with mouse monoclonal anti-IGF-1 antibody (catalog No. sc-74116, Santa Cruz Biotechnology, Santa Cruz, CA, USA) at 4 °C overnight. After washing, the membranes were incubated with goat-anti-mouse IgG-HRP antibody (catalog No. sc-2005, Santa Cruz Biotechnology) for 1 h at room temperature as previously described.19 Protein expression of IGF-1 was detected by an enhanced chemiluminescence kit (GE Healthcare Life Sciences, Little Chalfont, UK) and visualized by a Fusion FX Image system (Vilber Lourmat, Torcy, France). All other chemicals used were of analytical grade, and were purchased from the Sigma–Aldrich Chemical Co.
2.10. Statistical analysis
The data are expressed as the means ± standard deviation (SD) and were analyzed using GraphPad Prism software (version 5.03; GraphPad Software, Inc., La Jolla, CA, USA). Differences between groups were assessed using analysis of variance followed by one-way ANOVA-Tukey's post hoc test, and p < 0.05 was considered to indicate a statistically significant difference.
3. Results
3.1. Proximate composition of FO
The proximate analysis showed that FO is composed of 46 g/100 g carbohydrate, 36 g/100 g crude protein, 6.3 g/100 g sugars and 114 mg/g GABA (Supplementary Table S1 and Figure S1).
3.2. Effect of FO on body weight and body length in SD rats
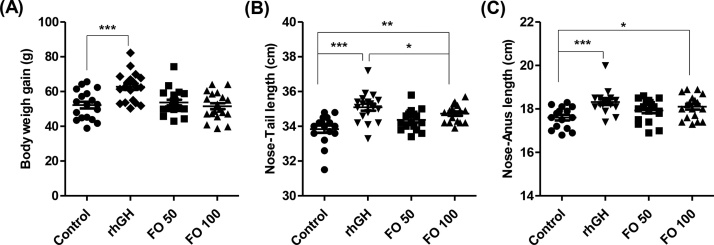
As shown in Fig. 1A, the body weight gains of the FO 50 (39.73 ± 3.46 g) and FO 100 (38.67 ± 4.90 g) groups were similar to those of the control group (37.91 ± 4.32 g). However, the body weight gain was significantly increased in the rhGH group (46.43 ± 5.53 g, p < 0.001) compared to that in the control group. In the FO 100 group, the N–T length and the N–A length were significantly increased to 34.73 ± 0.51 cm (p < 0.01) and 18.10 ± 0.54 cm (p < 0.05), respectively, compared to the control group (Fig. 1B and C). Furthermore, rhGH-treated rats showed marked increases in the N–T length (35.10 ± 0.85 cm, p < 0.001) and N–A length (18.32 ± 0.55 cm, p < 0.001) compared to control rats.
Fig. 1.
Effects of FO on body weight and body length in SD rats. (A) The body weight gain of each group after 2 weeks of treatment. (B and C) The length from the nose to tail and the nose to anus of each group after 2 weeks of treatment. Scatter plot graphs show the means ± standard deviation (SD, n = 19). Statistical analyses were conducted using analysis of ANOVA-Tukey's post hoc test between groups. *p < 0.05, **p < 0.01 and ***p < 0.001.
3.3. Effect of FO on organ weight in SD rats
The weights of selected organs were not significantly different between FO-treated groups and the control group (Table 1). Hypertrophies of the liver (1.30-fold of control, p < 0.05), kidney (1.22-fold of control, p < 0.05) and spleen (1.20-fold of control, p < 0.05) were significantly induced in rhGH-treated rats compared to control rats.
Table 1.
Changes in the Weight of Organs After 2 Weeks of Treatment
| Group | Weight of organs (g) |
||||||
|---|---|---|---|---|---|---|---|
| Thymus | Heart | Lung | Liver | Kidney | Spleen | Uterus Ovary | |
| Control | 0.46 ± 0.09 | 0.60 ± 0.05 | 0.88 ± 0.09 | 6.09 ± 0.50 | 1.40 ± 0.10 | 0.45 ± 0.08 | 0.39 ± 0.07 |
| rhGH | 0.47 ± 0.07 | 0.62 ± 0.06 | 0.93 ± 0.10 | 7.88 ± 1.61* | 1.63 ± 0.29* | 0.51 ± 0.06* | 0.41 ± 0.11 |
| FO 50 | 0.48 ± 0.06 | 0.61 ± 0.03 | 0.88 ± 0.08 | 6.60 ± 0.95 | 1.42 ± 0.11 | 0.44 ± 0.02 | 0.48 ± 0.14 |
| FO 100 | 0.38 ± 0.05 | 0.57 ± 0.05 | 0.85 ± 0.08 | 6.55 ± 1.02 | 1.46 ± 0.12 | 0.43 ± 0.08 | 0.38 ± 0.06 |
Mice were sacrificed at day 14 after treatment. Thymus, heart, lung, liver, kidney, spleen, uterus, and ovary were immediately surgically excised, and the weights were then measured. The data are expressed as the means ± standard deviation (SD, n = 19). The statistical analyses were conducted using analysis of ANOVA-Tukey's post hoc test between groups.
p < 0.05 compared to control.
3.4. Effect of FO on hematological and biochemical profiles in SD rats
An analysis of RBC, WBC, hematocrit, hemoglobin, MCV, MCH, MCHC and platelets showed no differences among the groups (Table 2). Furthermore, no biochemical abnormalities, including the levels of serum ALT, AST, BUN, creatinine and calcium, were observed among the groups.
Table 2.
Changes in the Hematological and Biochemical Profiles After 2 Weeks of Treatment
| Parameter (units) | Group |
|||
|---|---|---|---|---|
| Normal | rhGH | FO 50 | FO 100 | |
| RBC (106/μL) | 6.67 ± 0.39 | 6.60 ± 0.19 | 6.79 ± 0.15 | 6.92 ± 0.30 |
| WBC (103/μL) | 3.18 ± 0.45 | 2.85 ± 0.42 | 2.66 ± 0.96 | 3.75 ± 0.96 |
| Hematocrit (%) | 48.81 ± 2.86 | 48.69 ± 1.26 | 49.46 ± 1.41 | 50.08 ± 2.29 |
| Hemoglobin (g/dL) | 14.24 ± 0.66 | 14.04 ± 0.28 | 14.31 ± 0.49 | 14.21 ± 0.59 |
| MCV (fL) | 73.16 ± 1.52 | 73.70 ± 1.93 | 72.90 ± 1.40 | 72.56 ± 1.40 |
| MCH (pg) | 21.34 ± 0.48 | 21.36 ± 0.59 | 21.06 ± 0.63 | 20.94 ± 0.34 |
| MCHC (g/dL) | 29.16 ± 0.40 | 28.88 ± 0.44 | 28.89 ± 0.57 | 28.99 ± 0.31 |
| Platelet (103/μL) | 1101.38 ± 257.49 | 1219.63 ± 130.72 | 1222.88 ± 218.25 | 1360.38 ± 181.24 |
| ALT (U/L) | 40.70 ± 7.09 | 39.50 ± 6.87 | 35.20 ± 6.89 | 37.90 ± 6.81 |
| AST (U/L) | 100.20 ± 11.42 | 92.40 ± 14.18 | 105.10 ± 11.42 | 101.70 ± 10.14 |
| BUN (mg/dL) | 14.36 ± 2.93 | 12.52 ± 1.38 | 13.51 ± 1.62 | 12.76 ± 1.26 |
| Creatinine (mg/dL) | 0.29 ± 0.05 | 0.28 ± 0.02 | 0.29 ± 0.03 | 0.29 ± 0.03 |
| Calcium (mg/dL) | 11.45 ± 0.59 | 11.44 ± 0.31 | 11.14 ± 0.25 | 11.22 ± 0.27 |
At day 14 after treatment, whole blood and serum were analyzed for hematological and biochemical evaluation. The data are expressed as the means ± standard deviation (SD, n = 19). The statistical analyses were conducted using analysis of ANOVA-Tukey's post hoc test between groups. All data showed that there is no statistically significant difference between groups. ALT, alanine aminotransferase; AST, aspartate aminotransferase; BUN, blood urea nitrogen, MCV, mean corpuscular volume; MCH, mean corpuscular hemoglobin; MCHC, MCH concentration; RBC, red blood cells; WBC, white blood cells.
3.5. Effect of FO on tibial trabecular bone microarchitecture in SD rats
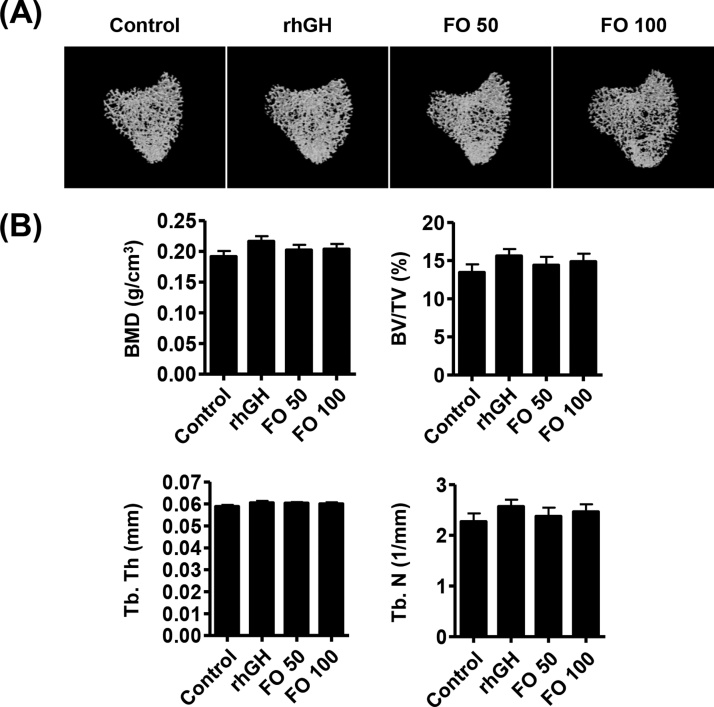
The results of μCT analysis showed that BMD, BV/TV and Tb.N were slightly increased in the rhGH and FO groups, but there was no statistically significant difference compared with the control group (Fig. 2A and B). In addition, there was no predominated concentration-dependent effect between FO 50 and FO 100 administration.
Fig. 2.
Effects of FO on tibial trabecular bone microarchitecture in SD rats. Bone morphometric parameters were analyzed using high-resolution micro-computed tomography (μCT, Skyscan 1272; Kontich, Belgium). (A) Micro-CT images of tibial trabecular bone. (B) Analysis of bone morphometric parameters, such as bone volume per total volume (BV/TV), bone mineral density (BMD), trabecular thickness (Tb. Th), and trabecular number (Tb. N). The data are expressed as the means ± standard deviation (SD, n = 19).
3.6. Effect of FO on proximal tibial growth plate in SD rats
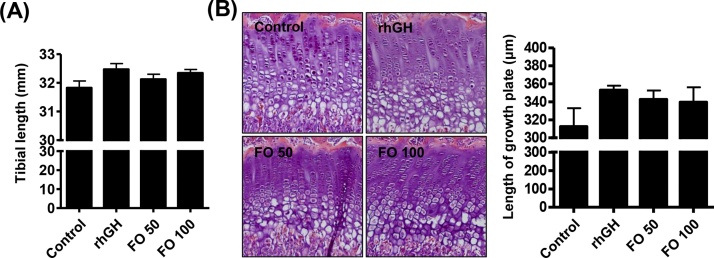
No statistically significant difference was observed in the rhGH group compared with the control group, although proximal tibial length was slightly increased. Proximal tibial length was partially lengthened in the FO 100 group (32.34 ± 0.43 mm, Fig. 3A). The effect of FO on the increased proximal tibial length was dose dependent. The length of the proximal tibial growth plate gradually increased with FO 50 (342.73 ± 14.02 μm) and FO 100 (and 339.55 ± 28.63 μm) administration, and these increasing levels were similar to those in the rhGH group (352.97 ± 6.87 μm) (Fig. 3B).
Fig. 3.
Effects of FO on the proximal tibial growth plate in SD rats. (A) Tibial length. (B, left) Representative photographs of H&E-stained chondrocytes of the proximal tibial growth plate in SD rats. (B, right) Length of the proximal tibial growth plate. The data are expressed as the means ± standard deviation (SD, n = 19).
3.7. Effect of FO on growth parameters in SD rats
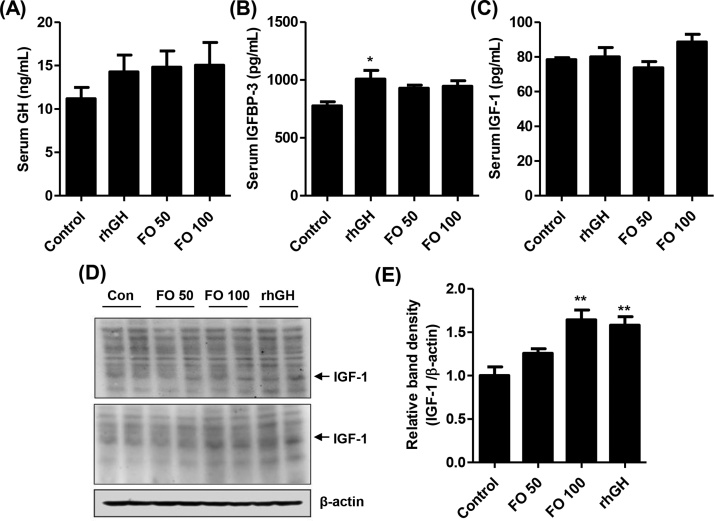
The ELISA results for serum GH levels indicated that, although FO 50, FO 100 and rhGH groups showed a growing trend after 2 weeks of administration, there were no significant differences among the groups (Fig. 4A). In addition, serum IGFBP-3 levels slightly increased with FO administration, but those levels were not significantly different compared with those in the control group (Fig. 4B). However, rhGH treatment gradually upregulated serum IGFBP-3 levels (1.30-fold that of control, p < 0.05) in SD rats. Serum IGF-1 levels were highest in the FO 100 group (88.56 ± 17.73 pg/mL, Fig. 4C). However, there was no significant difference in serum IGF-1 levels between control and rhGH-treated rats (Fig. 4C). The protein expressions of hepatic IGF-1 were markedly upregulated by FO 100 (1.64-folds of control, p < 0.01) and rhGH administration (1.58 folds of control, p < 0.01, Fig. 4C).
Fig. 4.
Effects of FO on growth parameters in SD rats. (A–C) Serum GH, IGFBP-3 and IGF-1 levels. The serum growth parameters were measured using ELISA kits. The data are expressed as the means ± standard deviation (SD, n = 19). The statistical analyses were conducted using analysis of ANOVA-Tukey's post hoc test. *p < 0.05 compared to control. (D) The protein expression of hepatic IGF-1. β-actin served as the loading control. (E) Bar graphs indicate the relative band density of the IGF-1 in western blot analysis. The statistical analyses were conducted using analysis of ANOVA-Tukey's post hoc test. **p < 0.01 compared to control.
4. Discussion
In the present study, we investigated the effect of FO on growth promotion, including analyses of body size, bone microarchitecture, hematology and biochemistry in SD rats. Our results show that oral administration of FO 100 significantly increased body lengths, including N–T and N–A. These results are meaningful in that those body lengths are the first criteria used to determine the growth in height. Additionally, our data indicate that BMD, BV/TV and Tb.N were slightly increased in the FO groups compared with the control group. Furthermore, in the FO 100 group, the lengths of the proximal tibia and proximal tibial growth plate gradually increased. Meanwhile, administration of rhGH, as a positive control, also improved the N–A, N–T, proximal tibia and proximal tibial growth plate lengths in SD rats. However, the administration of rhGH increased body length and proximal tibial growth-plate length but also induced body weight increases and organ hypertrophy. These are consistent with previous human and animal studies.3, 20, 21, 22, 23, 24 Based on the present study, administration of FO induced increased body length and proximal tibial growth-plate length without organ damage in SD rats. Additionally, our results show that the levels of serum GH and IGFBP-3 are slightly upregulated by FO administration. Nevertheless, the protein expression of hepatic IGF-1 was markedly increased by FO 100 and rhGH administration. These results suggest that, although administration of FO did not affect circulating serum IGF-1 levels, IGF-1 synthesis occurred in the liver.
GABA has been directly implicated in the regulation of muscle tone25 and stimulated osteoblastogenesis by upregulating bone formation genes in ovariectomized rats.26 Recently, one study showed that GABA-enriched fermented sea tangle (FST) significantly increased human growth hormone and IGF-1 levels27 and muscle-related growth factors.28 Our previous report found that GABA is efficiently produced through biotransformation from glutamic acid during fermentation with L. brevis BJ20.29 From these results, it is estimated that FO, including GABA, can enhance growth parameters, such as hepatic IGF-1 synthesis as well as body lengths, in SD rats.
In current study, our findings shown that that FO have high content of GABA, and increases body length and hepatic IGF-1 synthesis in SD rats without toxicity. Nevertheless, the limitations of this study are no statistical significance although bone morphometric parameters and serum growth factors were slightly upregulated by FO administration. Therefore, further studies are needed to assess the efficacy of FO at high doses and in long-term animal models.
Author contributions
Conceptualization: B-JL, GYK, EKP and YHC. Methodology: JSN, J-HP and B-JL. Validation: J-HP and B-JL. Formal Analysis: MW, Y-SK and Y-CC. Investigation: HL, HH, SYJ, MYK, SYK, G-YK and EKP. Writing - Original Draft: HL and YHC. Writing - Review & Editing: YHC. Visualization: JSN. Supervision: YHC. Funding Acquisition: GYK and Y-JJ.
Conflict of interest
The authors declare no conflicts of interest.
Funding
This research was part of the project titled ‘Development of functional food products with natural materials derived from marine resources (20170285),’ funded by the Ministry of Oceans and Fisheries of South Korea.
Ethical statement
This study was conducted in accordance with the animal experimentation guidelines of Dong-eui University, with approval of the Institutional Animal Care and Use Committee (No. R2019-002).
Data availability
Data and materials will be available from the authors upon request.
Footnotes
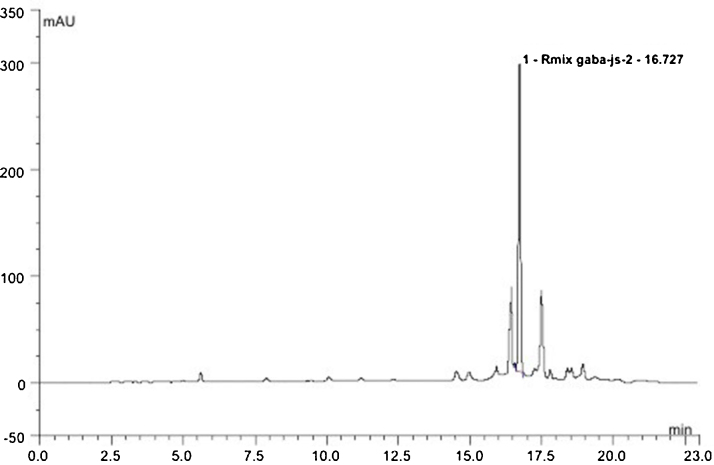
Supplementary Fig. S1. HPLC chromatogram for γ-amino butyric acid of fermented oyster extract and Supplementary Table S1 Proximate composition of FO can be found in the online version at doi:10.1016/j.imr.2020.100412.
Supplementary materials
The following are Supplementary Fig, S1 and Suppleentary Table S1.
References
- 1.Rosenfeld R.G. Insulin-like growth factors and the basis of growth. N Engl J Med. 2003;349:2184–2186. doi: 10.1056/NEJMp038156. [DOI] [PubMed] [Google Scholar]
- 2.Argente J. Challenges in the management of short stature. Horm Res Paediatr. 2016;85:2–10. doi: 10.1159/000442350. [DOI] [PubMed] [Google Scholar]
- 3.Souza F.M., Collett-Solberg P.F. Adverse effects of growth hormone replacement therapy in children. Arq Bras Endocrinol Metab. 2011;55:559–565. doi: 10.1590/s0004-27302011000800009. [DOI] [PubMed] [Google Scholar]
- 4.Linardi A., Damiani D., Longui C.A. The use of aromatase inhibitors in boys with short stature: what to know before prescribing? Arch Endocrinol Metab. 2017;61:391–397. doi: 10.1590/2359-3997000000284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sini V., Botticelli A., Lunardi G., Gori S., Marchetti P. Pharmacogenetics and aromatase inhibitor induced side effects in breast cancer patients. Pharmacogenomics. 2017;18:821–830. doi: 10.2217/pgs-2017-0006. [DOI] [PubMed] [Google Scholar]
- 6.Foglietta J., Inno A., de Iuliis F., Sini V., Duranti S., Turazza M. Cardiotoxicity of aromatase inhibitors in breast cancer patients. Clin Breast Cancer. 2017;17:11–17. doi: 10.1016/j.clbc.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 7.Molagoda I.M.N., Karunarathne W.A.H.M., Choi Y.H., Park E.K., Jeon Y.J., Lee B.J. Fermented oyster extract promotes osteoblast differentiation by activating the Wnt/β-catenin signaling pathway, leading to bone formation. Biomolecules. 2019;9 doi: 10.3390/biom9110711. pii: E711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coringa R., de Sousa E.M., Botelho J.N., Diniz R.S., de Sá J.C., da Cruz M.C.F.N. Bone substitute made from a Brazilian oyster shell functions as a fast stimulator for bone-forming cells in an animal model. PLoS ONE. 2018;13:e0198697. doi: 10.1371/journal.pone.0198697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim S.K., Pallela R. Medicinal foods from marine animals: current status and prospects. Adv Food Nutr Res. 2012;65:1–9. doi: 10.1016/B978-0-12-416003-3.00001-9. [DOI] [PubMed] [Google Scholar]
- 10.Oliveira D.V., Silva T.S., Cordeiro O.D., Cavaco S.I., Simes D.C. Identification of proteins with potential osteogenic activity present in the water-soluble matrix proteins from Crassostrea gigas nacre using a proteomic approach. Sci World J. 2012;2012:765909. doi: 10.1100/2012/765909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moon P.D., Kim M.H., Lim H.S., Oh H.A., Nam S.Y., Han N.R. Taurine a major amino acid of oyster, enhances linear bone growth in a mouse model of protein malnutrition. Biofactors. 2015;41:190–197. doi: 10.1002/biof.1213. [DOI] [PubMed] [Google Scholar]
- 12.Ihn H.J., Kim J.A., Lim S., Nam S.H., Hwang S.H., Lim J. Fermented oyster extract prevents ovariectomy-induced bone loss and suppresses osteoclastogenesis. Nutrients. 2019;11 doi: 10.3390/nu11061392. pii: E1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jeong J.W., Choi S.H., Han M.H., Kim G.Y., Park C., Hong S.H. Protective effects of fermented oyster extract against RANKL-induced osteoclastogenesis through scavenging ROS generation in RAW 264.7 cells. Int J Mol googlSci. 2019;20 doi: 10.3390/ijms20061439. pii: E1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ministry of Food and Drug Safety . 2019. General analysis methods, Food Code, Cheongju-si, Republic of Korea. https://www.mfds.go.kr/eng/index.do. [Google Scholar]
- 15.Lee D., Lee S.H., Lee Y.H., Song J., Kim H. Astragalus extract mixture HT042 increases longitudinal bone growth rate by upregulating circulatory IGF-1 in rats. Evid Based Complement Altern Med. 2017;2017:6935802. doi: 10.1155/2017/6935802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jung S.W., Oh S.H., Lee I.S., Byun J.H., Lee J.H. In situ gelling hydrogel with anti-bacterial activity and bone healing property for treatment of osteomyelitis. Tissue Eng Regen Med. 2019;16:479–490. doi: 10.1007/s13770-019-00206-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lim S., Kim J.A., Lee T., Lee D., Nam S.H., Lim J. Stimulatory effects of KPR-A148 on osteoblast differentiation and bone regeneration. Tissue Eng Regen Med. 2019;16:405–413. doi: 10.1007/s13770-019-00200-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choudhary D., Pandey A., Adhikary S., Ahmad N., Bhatia C., Bhambhani S. Genetically engineered flavonol enriched tomato fruit modulates chondrogenesis to increase bone length in growing animals. Sci Rep. 2016;6:21668. doi: 10.1038/srep21668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park J.W., Kim J.E., Kang M.J., Choi H.J., Bae S.J., Hwang D.Y. Compensatory role of C3 convertase on the strain difference for C3 protein expression in FVB/N, C3H/HeN and C57BL/6N mice. Lab Anim Res. 2020;36:4. doi: 10.1186/s42826-020-0036-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Voss S.C., Giraud S., Alsayrafi M., Bourdon P.C., Schumacher Y.O., Saugy M. The effect of a period of intensive exercise on the isoform test to detect growth hormone doping in sports. Growth Horm IGF Res. 2013;23:105–108. doi: 10.1016/j.ghir.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 21.Palabiyik O., Tastekin E., Doganlar Z.B., Tayfur P., Dogan A., Vardar S.A. Alteration in cardiac PI3K/Akt/mTOR and ERK signaling pathways with the use of growth hormone and swimming, and the roles of miR21 and miR133. Biomed Rep. 2019;0:1–10. doi: 10.3892/br.2018.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kopchick J.J., Bellush L.L., Coschigano K.T. Transgenic models of growth hormone action. Annu Rev Nutr. 1999;19:437–461. doi: 10.1146/annurev.nutr.19.1.437. [DOI] [PubMed] [Google Scholar]
- 23.Penney D.G., Dunbar J.C., Baylerian M.S. Cardiomegaly and hemodynamics in rats with a transplantable growth hormone-secreting tumor. Cardiovasc Res. 1985;19:270–277. doi: 10.1093/cvr/19.5.270. [DOI] [PubMed] [Google Scholar]
- 24.Bryant J., Baxter L., Cave C.B., Milne R. Recombinant growth hormone for idiopathic short stature in children and adolescents. Cochrane Database Syst Rev. 2007;3:CD004440. doi: 10.1002/14651858.CD004440.pub2. [DOI] [PubMed] [Google Scholar]
- 25.Watanabe M., Maemura K., Kanbara K., Tamayama T., Hayasaki H. GABA and GABA receptors in the central nervous system and other organs. Int Rev Cytol. 2002;213:1–47. doi: 10.1016/s0074-7696(02)13011-7. [DOI] [PubMed] [Google Scholar]
- 26.Muhammad S.I., Maznah I., Mahmud R., Zuki A.B., Imam M.U. Upregulation of genes related to bone formation by γ-amino butyric acid and γ-oryzanol in germinated brown rice is via the activation of GABAB-receptors and reduction of serum IL-6 in rats. Clin Interv Aging. 2013;8:1259–1271. doi: 10.2147/CIA.S45943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Choi W.C., Reid S.N.S., Ryu J.K., Kim Y., Jo Y.H., Jeon B.H. Effects of γ-aminobutyric acid-enriched fermented sea tangle (Laminaria japonica) on brain derived neurotrophic factor-related muscle growth and lipolysis in middle aged women. Algae. 2016;31:175–187. [Google Scholar]
- 28.Reid S.N.S., Ryu J.K., Kim Y., Jeon B.H. The effects of fermented Laminaria japonica on short-term working memory and physical fitness in the elderly. Evid Based Complement Alternat Med. 2018;2018:8109621. doi: 10.1155/2018/8109621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee B.J., Kim J.S., Kang Y.M., Lim J.H., Kim Y.M., Lee M.S. Antioxidant activity and γ-aminobutyric acid (GABA) content in sea tangle fermented by Lactobacillus brevis BJ20 isolated from traditional fermented foods. Food Chem. 2010;122:271–276. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data and materials will be available from the authors upon request.